Longleaf Pine Seedlings Are Extremely Resilient to the Combined Effects of Experimental Fire and Drought
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
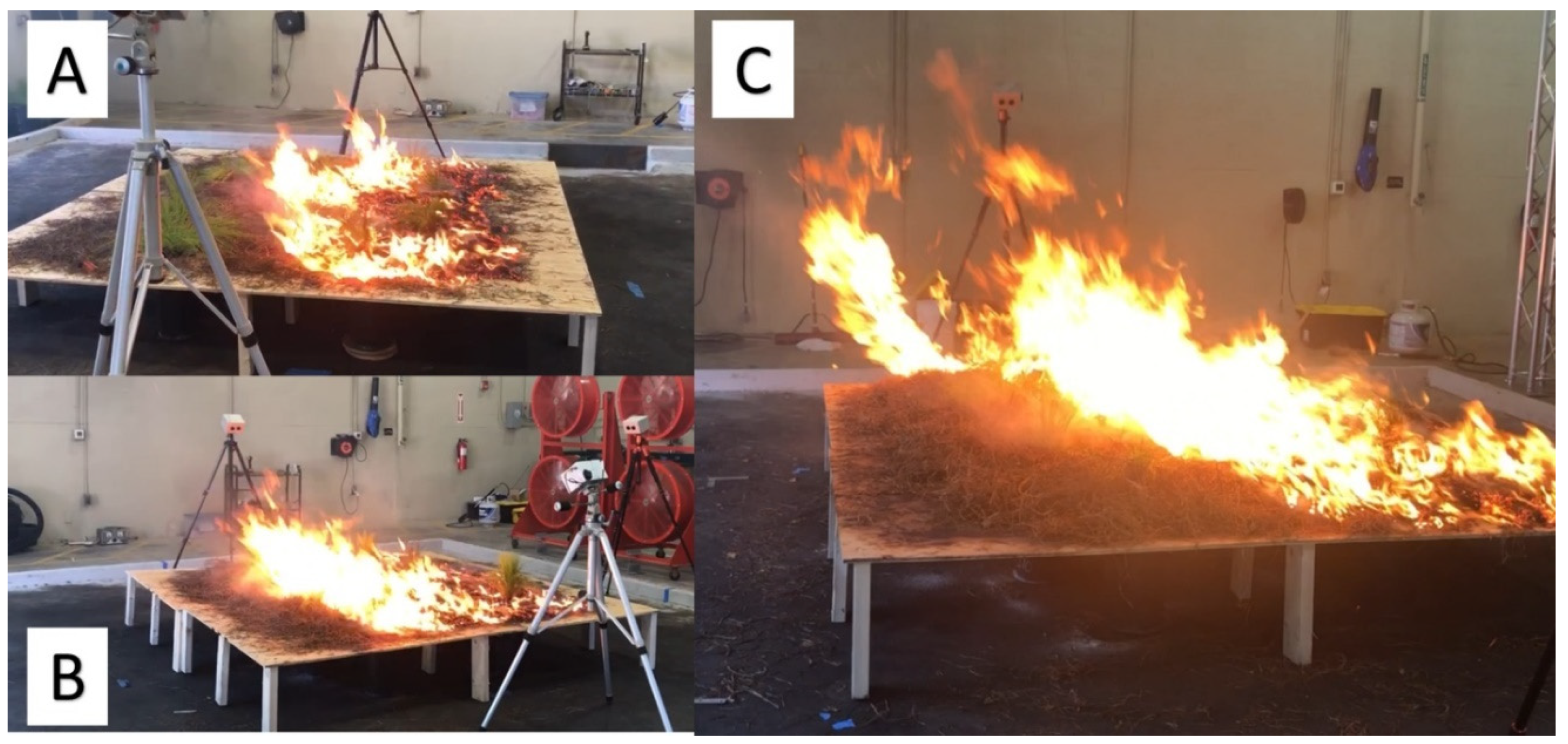
2.2. Experimental Design & Treatment Application
2.3. Plant Imaging and Tracking of Recovery
2.4. Physiological Measurements
2.5. Analyses
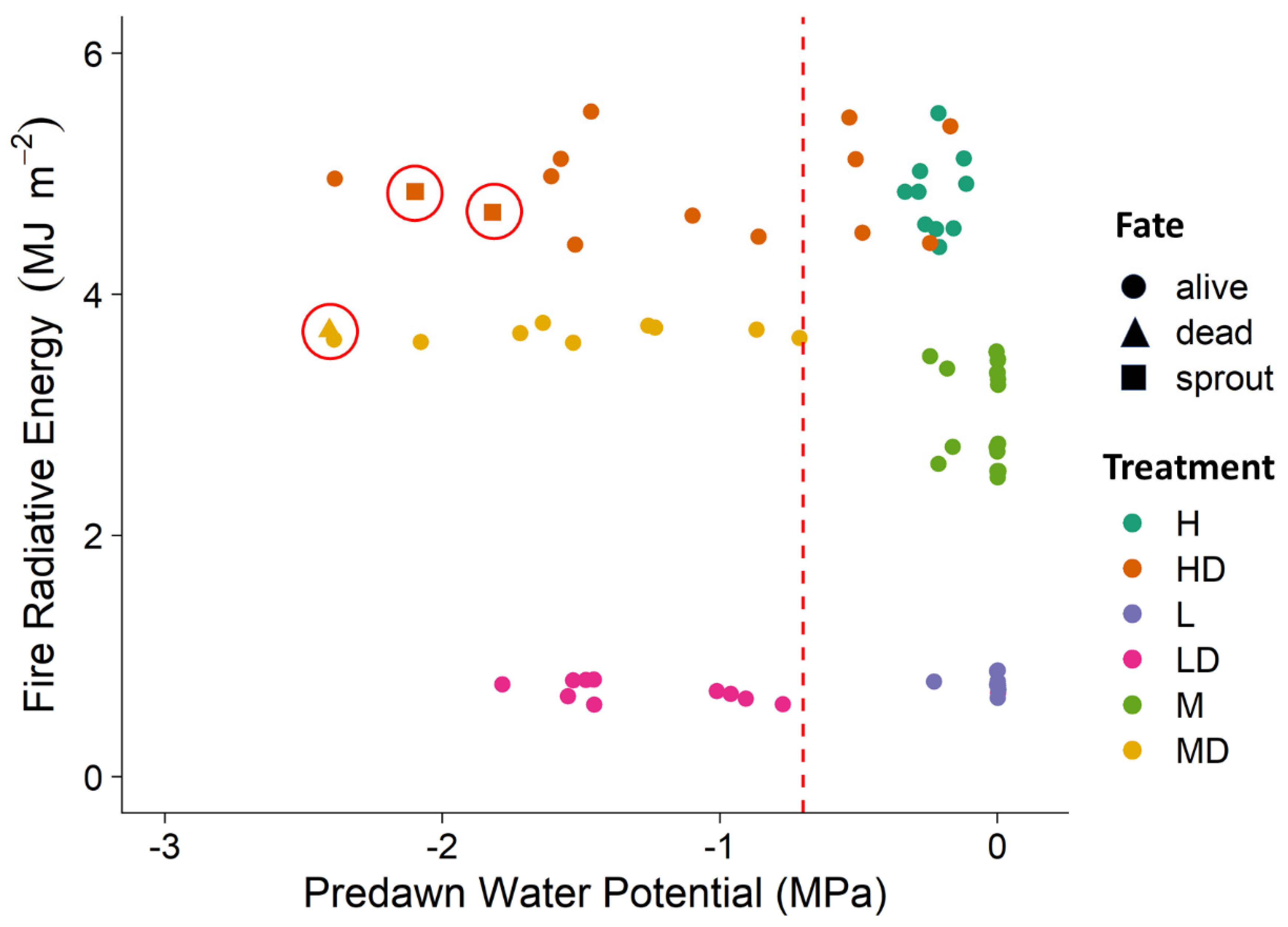
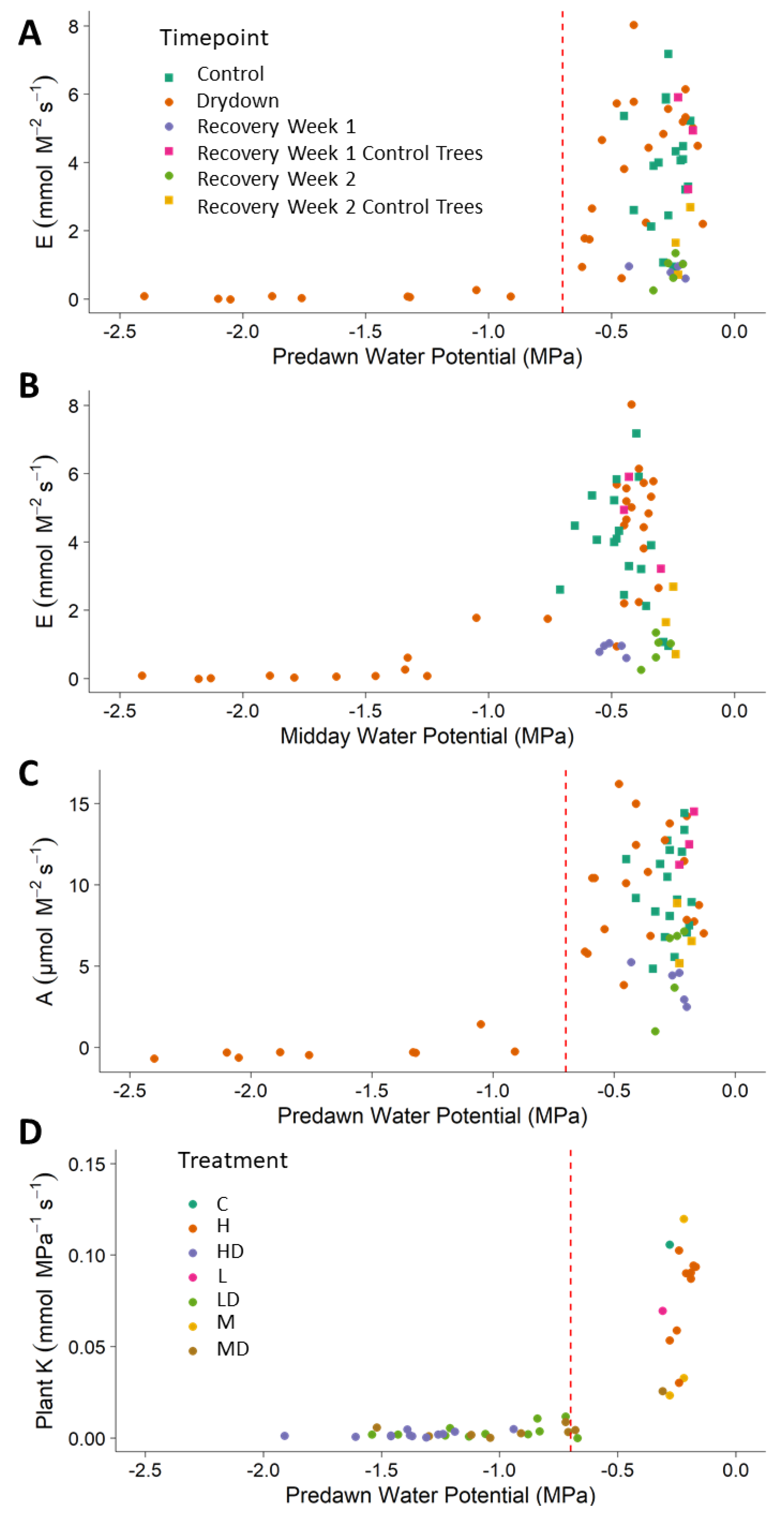
3. Results
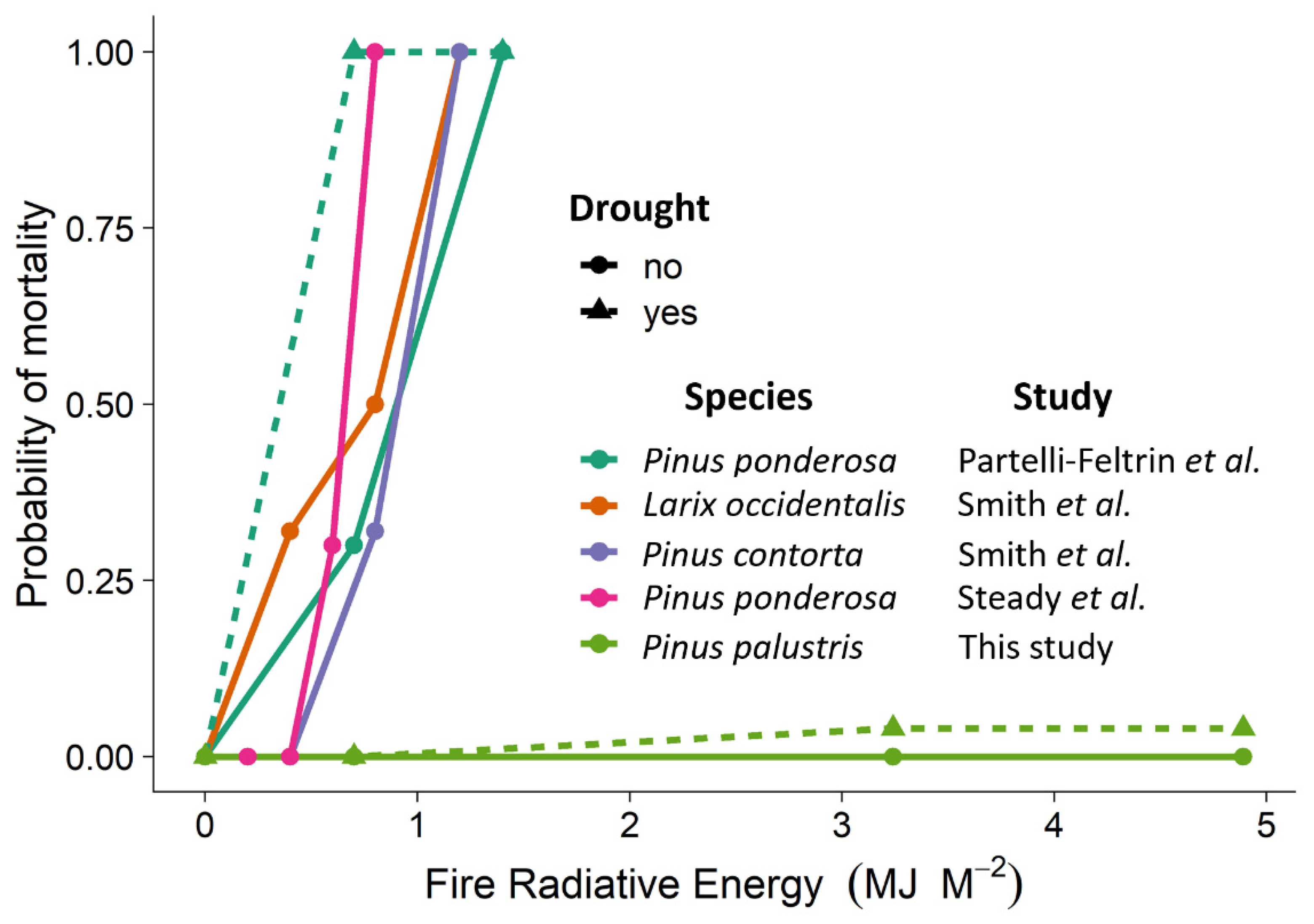
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2014–Impacts, Adaptation and Vulnerability: Part A: Global and Sectoral Aspects: Working Group II Contribution to the IPCC Fifth Assessment Report: Volume 1: Global and Sectoral Aspects. Cambridge University Press, Cambridge. 2014. Available online: https://www.cambridge.org/core/books/climate-change-2014-impacts-adaptation-and-vulnerability-part-a-global-and-sectoral-aspects/1BE4ED76F97CF3A75C64487E6274783A (accessed on 15 January 2022).
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- IPCC. Climate Change 2022: Impacts, Adaptation, and Vulnerability; Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Hartmann, H.; Moura, C.F.; Anderegg, W.R.L.; Ruehr, N.K.; Salmon, Y.; Allen, C.D.; Arndt, S.K.; Breshears, D.D.; Davi, H.; Galbraith, D. Research frontiers for improving our understanding of drought-induced tree and forest mortality. New Phytol. 2018, 218, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Woolman, A.M.; Coop, J.D.; Shaw, J.D.; DeMarco, J. Extent of recent fire-induced losses of ponderosa pine forests of Arizona and New Mexico, USA. For. Ecol. Manag. 2022, 520, 120381. [Google Scholar] [CrossRef]
- Smith, M.D. The ecological role of climate extremes: Current understanding and future prospects. J. Ecol. 2011, 99, 651–655. [Google Scholar] [CrossRef]
- Seneviratne, S.I.; Nicholls, N.; Easterling, D.; Goodess, C.M.; Kanae, S.; Kossin, J.; Luo, Y.; Marengo, J.; McInnes, K.; Rahimi, M.; et al. Changes in climate extremes and their impacts on the natural physical environment. In Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation. A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change (IPCC); Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Easterling, D.R.; Kunkel, K.E.; Arnold, J.R.; Knutson, T.; LeGrande, A.N.; Leung, L.R.; Vose, R.S.; Waliser, D.E.; Wehner, M.F. Precipitation Change in the United States. In Climate Science Special Report: Fourth National Climate Assessment, 1; (GSFC-E-DAA-TN49608); U.S. Global Change Research Program: Washington, DC, USA, 2017. [Google Scholar]
- Mitchell, R.J.; Liu, Y.; O’Brien, J.J.; Elliott, K.J.; Starr, G.; Miniat, C.F.; Hiers, J.K. Future climate and fire interactions in the southeastern region of the United States. For. Ecol. Manag. 2014, 327, 316–326. [Google Scholar] [CrossRef]
- Bowman, D.M.J.S.; Williamson, G.J.; Abatzoglou, J.T.; Kolden, C.A.; Cochrane, M.A.; Smith, A.M.S. Human exposure and sensitivity to globally extreme wildfire events. Nat. Ecol. Evol. 2017, 1, 58. [Google Scholar] [CrossRef] [PubMed]
- Ahlgren, C.E. Some Effects of Fire on Reproduction and Growth of Vegetation in Northeastern Minnesota. Ecology 1960, 41, 431–445. [Google Scholar] [CrossRef]
- Gisborne, H.T. Measuring Forest-Fire Danger in Northern Idaho. No. 29; United States, Department of Agriculture: Washington, DC, USA, 1928. [Google Scholar]
- Brethauer, D.K.; Sharma, A.; Vogel, J.G.; Miller, D.L.; van Santen, E. Longleaf pine seedling growth and survival: Effects of season and intensity of simulated prescribed burning. For. Ecol. Manag. 2021, 502, 119719. [Google Scholar] [CrossRef]
- Smith, A.M.S.; Sparks, A.M.; Kolden, C.A.; Abatzoglou, J.T.; Talhelm, A.F.; Johnson, D.M.; Boschetti, L.; Lutz, J.A.; Apostol, K.G.; Yedinak, K.M.; et al. Towards a new paradigm in fire severity research using dose–response experiments. Int. J. Wildland Fire 2016, 25, 158–166. [Google Scholar] [CrossRef]
- Smith, A.M.S.; Talhelm, A.F.; Johnson, D.M.; Sparks, A.M.; Kolden, C.A.; Yedinak, K.M.; Apostol, K.G.; Tinkham, W.T.; Abatzoglou, J.T.; Lutz, J.A.; et al. Effects of fire radiative energy density dose on Pinus contorta and Larix occidentalis seedling physiology and mortality. Int. J. Wildland Fire 2017, 26, 82–94. [Google Scholar] [CrossRef]
- Dickinson, M.B.; Butler, B.W.; Hudak, A.T.; Bright, B.C.; Kremens, R.L.; Klauberg, C. Inferring energy incident on sensors in low-intensity surface fires from remotely sensed radiation and using it to predict tree stem injury. Int. J. Wildland Fire 2019, 28, 230. [Google Scholar] [CrossRef]
- Partelli-Feltrin, R.; Johnson, D.M.; Sparks, A.M.; Adams, H.D.; Kolden, C.A.; Nelson, A.S.; Smith, A.M.S. Drought Increases Vulnerability of Pinus ponderosa Saplings to Fire-Induced Mortality. Fire 2020, 3, 56. [Google Scholar] [CrossRef]
- Partelli-Feltrin, R.; Smith, A.M.S.; Adams, H.D.; Kolden, C.A.; Johnson, D.M. Short- and long-term effects of fire on stem hydraulics in Pinus ponderosa saplings. Plant Cell Environ. 2021, 44, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Ruswick, S.K.; O’Brien, J.J.; Aubrey, D.P. Carbon starvation is absent regardless of season of burn in Liquidambar styraciflua L. For. Ecol. Manag. 2021, 479, 118588. [Google Scholar] [CrossRef]
- Wooster, M.J.; Roberts, G.J.; Giglio, L.; Roy, D.P.; Freeborn, P.H.; Boschetti, L.; Justice, C.; Ichoku, C.; Schroeder, W.; Davies, D.; et al. Satellite remote sensing of active fires: History and current status, applications and future requirements. Remote Sens. Environ. 2021, 267, 112694. [Google Scholar] [CrossRef]
- Steady, W.D.; Partelli Feltrin, R.; Johnson, D.M.; Sparks, A.M.; Kolden, C.A.; Talhelm, A.F.; Lutz, J.A.; Boschetti, L.; Hudak, A.T.; Nelson, A.S.; et al. The Survival of Pinus ponderosa Saplings Subjected to Increasing Levels of Fire Behavior and Impacts on Post-Fire Growth. Fire 2019, 2, 23. [Google Scholar] [CrossRef]
- Jolly, W.M.; Johnson, D.M. Pyro-Ecophysiology: Shifting the Paradigm of Live Wildland Fuel Research. Fire 2018, 1, 8. [Google Scholar] [CrossRef]
- Melvin, M.A. National Prescribed Fire Use Report. Tech. Bull. 2020, 4–20. Available online: https://www.nwfirescience.org/sites/default/files/publications/2020-Prescribed-Fire-Use-Report-1.pdf (accessed on 15 January 2022).
- Keeley, J.E. Ecology and evolution of pine life histories. Ann. For. Sci. 2012, 69, 445–453. [Google Scholar] [CrossRef]
- Wahlenberg, W.G. Longleaf Pine: Its Use, Ecology, Regeneration, Protection, Growth, and Management; US Forest Service: Washington, DC, USA, 1946. [Google Scholar]
- O’Brien, J.J.; Hiers, J.K.; Callaham, M.A.; Mitchell, R.J.; Jack, S.B. Interactions among Overstory Structure, Seedling Life-history Traits, and Fire in Frequently Burned Neotropical Pine Forests. Ambio 2008, 37, 542–547. [Google Scholar] [CrossRef]
- Frost, C. History and future of the longleaf pine ecosystem. In The Longleaf Pine Ecosystem; Springer: Berlin/Heidelberg, Germany, 2007; pp. 9–48. [Google Scholar]
- Boyer, W.D. Pinus palustris Mill. longleaf pine. Silv. N. Am. 1990, 1, 405–412. [Google Scholar]
- Aubrey, D.P. Grass(stage)root movement to ensure future resilience of longleaf pine ecosystems. New For. 2021. [Google Scholar] [CrossRef]
- Fonda, R.W. Burning Characteristics of Needles from Eight Pine Species. For. Sci. 2001, 47, 390–396. [Google Scholar]
- Mitchell, R.J.; Hiers, J.K.; O’Brien, J.; Starr, G. Ecological forestry in the Southeast: Understanding the ecology of fuels. J. For. 2009, 107, 391–397. [Google Scholar]
- Wiggers, M.S.; Kirkman, L.K.; Boyd, R.S.; Hiers, J.K. Fine-scale variation in surface fire environment and legume germination in the longleaf pine ecosystem. For. Ecol. Manag. 2013, 310, 54–63. [Google Scholar] [CrossRef]
- Pivovaroff, A.L.; Emery, N.; Sharifi, M.R.; Witter, M.; Keeley, J.E.; Rundel, P.W. The Effect of Ecophysiological Traits on Live Fuel Moisture Content. Fire 2019, 2, 28. [Google Scholar] [CrossRef]
- Cochard, H.; Bréda, N.; Granier, A. Whole tree hydraulic conductance and water loss regulation in Quercus during drought: Evidence for stomatal control of embolism? Ann. For. Sci. 1996, 53, 197–206. [Google Scholar] [CrossRef]
- Dichio, B.; Montanaro, G.; Sofo, A.; Xiloyannis, C. Stem and whole-plant hydraulics in olive (Olea europaea) and kiwifruit (Actinidia deliciosa). Trees 2013, 27, 183–191. [Google Scholar] [CrossRef]
- Tsuda, M.; Tyree, M.T. Plant hydraulic conductance measured by the high pressure flow meter in crop plants. J. Exp. Bot. 2000, 51, 823–828. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with Image. J. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- O’Brien, J.J.; Loudermilk, E.L.; Hornsby, B.; Hudak, A.T.; Bright, B.C.; Dickinson, M.B.; Hiers, J.K.; Teske, C.; Ottmar, R.D. High-resolution infrared thermography for capturing wildland fire behaviour: RxCADRE 2012. Int. J. Wildland Fire 2015, 25, 62–75. [Google Scholar] [CrossRef]
- Wilke, C. cowplot: Streamlined Plot Theme and Plot Annotations for ’ggplot2. 2019. Available online: https://CRAN.R-project.org/package=cowplot (accessed on 15 January 2022).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- O’Brien, J.J.; Loudermilk, E.L.; Hiers, J.K.; Pokswinski, S.M.; Hornsby, B.; Hudak, A.T.; Strother, D.; Rowell, E.; Bright, B.C. Canopy-Derived Fuels Drive Patterns of In-Fire Energy Release and Understory Plant Mortality in a Longleaf Pine (Pinus palustris) Sandhill in Northwest Florida, USA. Can. J. Remote Sens. 2016, 42, 489–500. [Google Scholar] [CrossRef]
- Hammond, W.M.; Yu, K.; Wilson, L.A.; Will, R.E.; Anderegg, W.R.; Adams, H.D. Dead or dying? Quantifying the point of no return from hydraulic failure in drought-induced tree mortality. New Phytol. 2019, 223, 1834–1843. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Moule, B.; Yu, D.; Wang, G.G. Fire Survival of Longleaf Pine (Pinus palustris) Grass Stage Seedlings: The Role of Seedling Size, Root Collar Position, and Resprouting. Forests 2019, 10, 1070. [Google Scholar] [CrossRef]
- Grace, S.L.; Platt, W.J. Effects of Adult Tree Density and Fire on the Demography of Pregrass Stage Juvenile Longleaf Pine (Pinus palustris Mill.). J. Ecol. 1995, 83, 75–86. [Google Scholar] [CrossRef]
- Jack, S.B.; Hiers, J.K.; Mitchell, R.J.; Gagnon, J.L. Fuel loading and fire intensity-effects on longleaf pine seedling survival. In Proceedings of the 14th Biennial Southern Silvicultural Research Conference, Athens, Georgia, 26 February–1 March 2007; Gen. Tech. Rep. SRS–121; Stanturf, J.A., Ed.; US Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2010; pp. 275–279. Available online: https://www.fs.usda.gov/treesearch/pubs/35825 (accessed on 15 January 2022).
- Knapp, B.O.; Pile, L.S.; Walker, J.L.; Geoff Wang, G. Fire effects on a fire-adapted species: Response of grass stage longleaf pine seedlings to experimental burning. Fire Ecol. 2018, 14, 2. [Google Scholar] [CrossRef]
- Addington, R.N.; Hudson, S.J.; Hiers, J.K.; Hurteau, M.D.; Hutcherson, T.F.; Matusick, G.; Parker, J.M. Relationships among wildfire, prescribed fire, and drought in a fire-prone landscape in the south-eastern United States. Int. J. Wildland Fire 2015, 24, 778–783. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, L.A.; Spencer, R.N.; Aubrey, D.P.; O’Brien, J.J.; Smith, A.M.S.; Thomas, R.W.; Johnson, D.M. Longleaf Pine Seedlings Are Extremely Resilient to the Combined Effects of Experimental Fire and Drought. Fire 2022, 5, 128. https://doi.org/10.3390/fire5050128
Wilson LA, Spencer RN, Aubrey DP, O’Brien JJ, Smith AMS, Thomas RW, Johnson DM. Longleaf Pine Seedlings Are Extremely Resilient to the Combined Effects of Experimental Fire and Drought. Fire. 2022; 5(5):128. https://doi.org/10.3390/fire5050128
Chicago/Turabian StyleWilson, Luke A., Robert N. Spencer, Doug P. Aubrey, Joseph J. O’Brien, Alistair M. S. Smith, Ream W. Thomas, and Daniel M. Johnson. 2022. "Longleaf Pine Seedlings Are Extremely Resilient to the Combined Effects of Experimental Fire and Drought" Fire 5, no. 5: 128. https://doi.org/10.3390/fire5050128
APA StyleWilson, L. A., Spencer, R. N., Aubrey, D. P., O’Brien, J. J., Smith, A. M. S., Thomas, R. W., & Johnson, D. M. (2022). Longleaf Pine Seedlings Are Extremely Resilient to the Combined Effects of Experimental Fire and Drought. Fire, 5(5), 128. https://doi.org/10.3390/fire5050128







