Ambrosia Beetle Attacks in Mediterranean Cork Oak Forests Following Fire: Which Factors Drive Host Selection?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
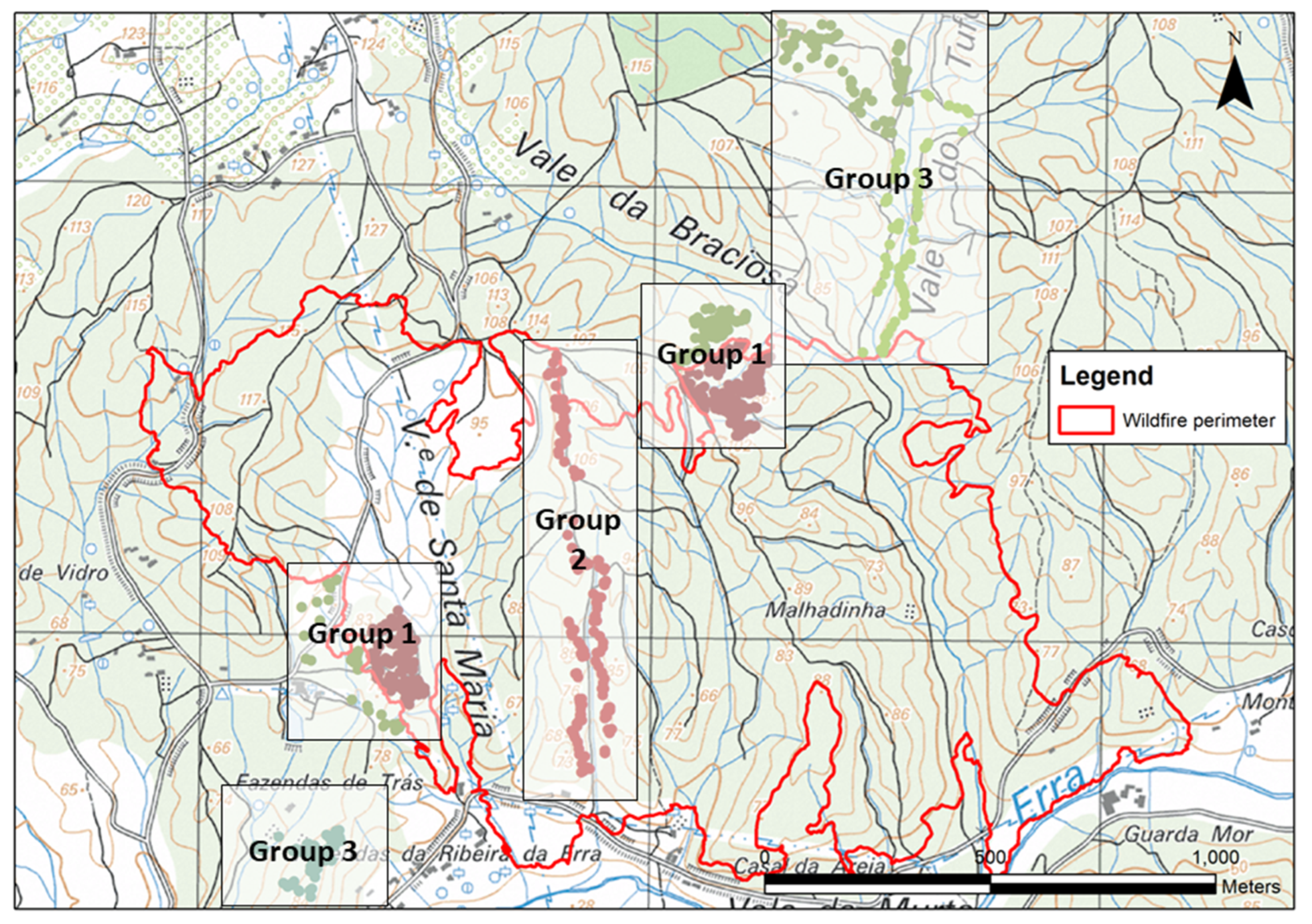
2.2.1. Assessing Early Ambrosia Beetle Attacks in Burned and Unburned Cork Oaks—Group 1
2.2.2. Assessing Ambrosia Beetle Attacks along a Distance Gradient to the Unburned Area—Group 2
2.2.3. Assessing Beetle Attacks in Unburned Oaks Debarked in Different Years Following the Wildfire—Group 3
2.3. Data Analyses
3. Results
3.1. Drivers of Early Ambrosia Beetle Attacks in Burned and Unburned Cork Oaks—Group 1
3.2. Ambrosia Beetle Attacks along a Distance Gradient to the Unburned Area—Group 2
3.3. Ambrosia Beetle Attacks in Unburned Oaks Debarked in Different Years after Fire—Group 3
4. Discussion
4.1. Major Drivers of Ambrosia Beetle Attacks in Mediterranean Oak Forests Following Fire
4.2. Wildfire and Fire-Related Factors
4.3. Distance to the Unburned Area
4.4. Crown Physiological Condition (Volume of Green Crown)
4.5. Tree Size, Bark Thickness, and Cork Exploitation
4.6. Are Neighboring Unburned Forests at Higher Risk from Ambrosia Beetle Attacks Following Wildfire?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- San-Miguel-Ayanz, J.; Durrant, T.; Boca, R.; Maianti, P.; Liberta, G.; Artes Vivancos, T.; Jacome Felix Oom, D.; Branco, A.; De Rigo, D.; Ferrari, D.; et al. Forest Fires in Europe, Middle East and North Africa 2020; EUR 30862 EN; Publications Office of the European Union: Luxembourg, 2021; ISBN 978-92-76-42350-8. [Google Scholar] [CrossRef]
- Bär, A.; Michaletz, S.T.; Mayr, S. Fire effects on tree physiology. New Phytol. 2019, 223, 1728–1741. [Google Scholar] [CrossRef] [PubMed]
- Menges, E.S.; Deyrup, M.A. Postfire survival in south Florida slash pine: Interacting effects of fire intensity, fire season, vegetation, burn size, and bark beetles. Int. J. Wildland Fire 2001, 10, 53. [Google Scholar] [CrossRef]
- Wylie, F.R.; Peters, B.; DeBaar, M.; King, J.; Fitzgerald, C. Managing attack by bark and ambrosia beetles (Coleoptera: Scolytidae) in fire-damaged Pinus plantations and salvaged logs in Queensland, Australia. Aust. For. 1999, 62, 148–153. [Google Scholar] [CrossRef]
- Kirkendall, R.L.; Biedermann, P.H.W.; Jordal, B.H. Chapter 3—Evolution and Diversity of Bark and Ambrosia Beetles. In Bark Beetles; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 85–156. [Google Scholar]
- Ebeling, W. Wood-Destroying Insects and Fungi. In Urban Entomology; L.A. University of California Division of Agricultural Sciences: Berkeley, CA, USA, 1978; pp. 167–216. [Google Scholar]
- Hulcr, J.; Black, A.; Prior, K.; Chen, C.-Y.; Li, H.-F. Studies of Ambrosia Beetles (Coleoptera: Curculionidae) in Their Native Ranges Help Predict Invasion Impact. Fla. Entomol. 2017, 100, 257–261. [Google Scholar] [CrossRef]
- Balachowsky, A. Faune de France, Coléoptères Scolytides, T. 50; Librairie de la Faculté des Sciences: Paris, France, 1949. [Google Scholar]
- Mendel, Z.; Protasov, A.; Sharon, M.; Zveibil, A.; Ben Yehuda, S.; Odonnell, K.; Rabaglia, R.J.; Wysoki, M.; Freeman, S. Asian ambrosia beetle Euwallacea fornicatus and its novel symbiotic fungus Fusarium sp. pose a serious threat to the Israeli avocado industry. Phytoparasitica 2012, 40, 235–238. [Google Scholar] [CrossRef]
- Paap, T.; Wingfield, M.J.; De Beer, Z.W.; Roets, F. Lessons from a major pest invasion: The polyphagous shot hole borer in South Africa. S. Afr. J. Sci. 2020, 116, 8575. [Google Scholar] [CrossRef]
- Kubono, T.; Ito, S. Raffaelea quercivora sp. nov. associated with mass mortality of Japanese oak, and the ambrosia beetle (Platypus quercivorus). Mycoscience 2002, 43, 0255–0260. [Google Scholar] [CrossRef]
- Sousa, E.; Inácio, M.L. New aspects of Platypus cylindrus Fab. (Coleoptera: Platypodidae): Life history on cork oak stands in Portugal. In Entomological Research in Mediterranean Forest Ecosystems; Lieutier, F., Ghaioule, D., Eds.; INRA Editions: Paris, France, 2005; pp. 147–168. [Google Scholar]
- Tiberi, R.; Branco, M.; Bracalini, M.; Croci, F.; Panzavolta, T. Cork oak pests: A review of insect damage and management. Ann. For. Sci. 2016, 73, 219–232. [Google Scholar] [CrossRef]
- Muñoz-Adalia, E.J.; Ahmed, J.; Colinas, C. Microclimatic conditions drive summer flight phenology of Platypus cylindrus in managed cork oak stands. J. Appl. Entomol. 2022, 146, 964–974. [Google Scholar] [CrossRef]
- Belhoucine, L.; Bouhraoua, R.T.; Meijer, M.; Houbraken, J.; Harrak, M.J.; Samson, R.A.; Equihua-Martínez, A.; Pujade-Villar, J. Mycobiota associated with Platypus cylindrus (Coleoptera: Curculionidae, Platypodidae) in cork oak stands of North West Algeria, Africa. Afr. J. Microbiol. Res. 2011, 5, 4411–4423. [Google Scholar] [CrossRef]
- APCOR. Cork 2020; Associação Portuguesa de Cortiça: Santa Maria de Lamas, Portugal, 2020; p. 68. Available online: https://www.apcor.pt/en/portfolio-posts/apcor-year-book-2020/ (accessed on 10 June 2022).
- Pereira, H. Cork: Biology, Production and Uses; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Catry, F.X.; Branco, M.; Sousa, E.; Caetano, J.; Naves, P.; Nóbrega, F. Presence and dynamics of ambrosia beetles and other xylophagous insects in a Mediterranean cork oak forest following fire. For. Ecol. Manag. 2017, 404, 45–54. [Google Scholar] [CrossRef]
- Catry, F.; Respício, J.; Branco, M. Monitoring ambrosia beetle species in cork oak trees in central Portugal. IOBC-WPRS Bull. 2020, 152, 49–50. [Google Scholar]
- Catry, F.X.; Pausas, J.; Moreira, F.; Fernandes, P.M.; Rego, F. Post-fire response variability in Mediterranean Basin tree species in Portugal. Int. J. Wildland Fire 2013, 22, 919–932. [Google Scholar] [CrossRef]
- Catry, F.X.; Moreira, F.; Pausas, J.; Fernandes, P.; Rego, F.C.; Cardillo, E.; Curt, T. Cork oak vulnerability to fire: The role of bark harvesting, tree characteristics and abiotic factors. PLoS ONE 2012, 7, e39810. [Google Scholar] [CrossRef] [PubMed]
- Natividade, J.V. Direcção-Geral dos Serviços Florestais e Aquícolas; Subericultura: Lisboa, Portugal, 1950. [Google Scholar]
- Oliveira, G.; Costa, A. How resilient is Quercus suber L. to cork harvesting? A review and identification of knowledge gaps. For. Ecol. Manag. 2012, 270, 257–272. [Google Scholar] [CrossRef]
- Sousa, E.M.R.; Debouzie, D. Distribution spatio-temporelle des attaques de Platypus cylindrus F. (Coleoptera: Platypodidae) dans des peuplements de Chênes-lièges au Portugal. Integr. Prot. Oak For. IOBC 1999, 22, 47–58. [Google Scholar]
- APA. Atlas do Ambiente—Temperatura. 2020. Available online: https://sniambgeoportal.apambiente.pt/ (accessed on 30 May 2020).
- Catry, F.; Rego, F.; Moreira, F.; Fernandes, P.; Pausas, J. Post-fire tree mortality in mixed forests of central Portugal. For. Ecol. Manag. 2010, 260, 1184–1192. [Google Scholar] [CrossRef]
- McHugh, C.W.; Kolb, T.E. Ponderosa pine mortality following fire in northern Arizona. Int. J. Wildland Fire 2003, 12, 7–22. [Google Scholar] [CrossRef]
- Ryan, K. Techniques for assessing fire damage to trees. In Proceedings of the Symposium: Fire Its Field Effects, Jackson, WY, USA, 19–21 October 1982; Lotan, J.E., Ed.; Intermountain Fire Council: Missoula, MT, USA, 1982; pp. 1–11. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Naimi, B.; Hamm, N.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Venables, W.; Ripley, B. Modern Applied Statistics with S; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Ploetz, R.C.; Hulcr, J.; Wingfield, M.J.; de Beer, Z.W. Destructive tree diseases associated with ambrosia and bark beetles: Black swan events in tree pathology? Plant. Dis. 2013, 97, 856–872. [Google Scholar] [CrossRef]
- Gugliuzzo, A.; Criscione, G.; Siscaro, G.; Russo, A.; Garzia, G.T. First data on the flight activity and distribution of the ambrosia beetle Xylosandrus compactus (Eichhoff) on carob trees in Sicily. EPPO Bull. 2019, 49, 340–351. [Google Scholar] [CrossRef]
- Beh, M.M.; Metz, M.R.; Seybold, S.J.; Rizzo, D.M. The novel interaction between Phytophthora ramorum and wildfire elicits elevated ambrosia beetle landing rates on tanoak, Notholithocarpus densiflorus. For. Ecol. Manag. 2014, 318, 21–33. [Google Scholar] [CrossRef]
- Gara, R.I.; Arnold, P.; Peters, J.; Montesdeoca, J. The Isabela fire: Galapagos Islands. Turrialba 1987, 37, 53–57. [Google Scholar]
- Wylie, F.R.; Shanahan, P.J. Insect attack in fire-damaged plantation trees at bulolo in papua New Guinea. Aust. J. Entomol. 1976, 14, 371–382. [Google Scholar] [CrossRef]
- Fonseca-González, J.; de los Santos-Posadas, H.M.; Rodríguez-Ortega, A.; Laguna, R.R. Effect of wildfire and bark beetle damage on pinus patula schl. et cham. mortality at hidalgo, México. Agrociencia 2014, 48, 103–113. [Google Scholar]
- Hanula, J.L.; Meeker, J.R.; Miller, D.R.; Barnard, E.L. Association of wildfire with tree health and numbers of pine bark beetles, reproduction weevils and their associates in Florida. For. Ecol. Manag. 2002, 170, 233–247. [Google Scholar] [CrossRef]
- Hood, S.M.; Smith, S.L.; Cluck, D.R. Predicting mortality for five California conifers following wildfire. For. Ecol. Manag. 2010, 260, 750–762. [Google Scholar] [CrossRef]
- Tabacaru, C.A.; McPike, S.M.; Erbilgin, N. Fire-mediated interactions between a tree-killing bark beetle and its competitors. For. Ecol. Manag. 2015, 356, 262–272. [Google Scholar] [CrossRef]
- Sullivan, B.T.; Fettig, C.J.; Otrosina, W.J.; Dalusky, M.J.; Berisford, C. Association between severity of prescribed burns and subsequent activity of conifer-infesting beetles in stands of longleaf pine. For. Ecol. Manag. 2003, 185, 327–340. [Google Scholar] [CrossRef]
- Ranger, C.M.; Reding, M.E.; Schultz, P.B.; Oliver, J.B. Influence of flood-stress on ambrosia beetle host-selection and implications for their management in a changing climate. Agric. For. Entomol. 2013, 15, 56–64. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Westlind, D.J. Physiological stress and ethanol accumulation in tree stems and woody tissues at sublethal temperatures from fire. Bioscience 2017, 67, 443. [Google Scholar] [CrossRef]
- Kelsey, R.G. Ethanol synthesis in Douglas-fir logs felled in November, January, and March and its relationship to ambrosia beetle attack. Can. J. For. Res. 1994, 24, 2096–2104. [Google Scholar] [CrossRef]
- Rassati, D.; Contarini, M.; Ranger, C.M.; Cavaletto, G.; Rossini, L.; Speranza, S.; Faccoli, M.; Marini, L. Fungal pathogen and ethanol affect host selection and colonization success in ambrosia beetles. Agric. For. Entomol. 2020, 22, 1–9. [Google Scholar] [CrossRef]
- Ranger, C.M.; Reding, M.E.; Gandhi, K.J.K.; Oliver, J.B.; Schultz, P.B.; Cañas, L.; Herms, D.A. Species dependent influence of (-)-alpha-pinene on attraction of ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) to ethanol-baited traps in nursery agroecosystems. J. Econ. Entomol. 2011, 104, 574–579. [Google Scholar] [CrossRef]
- Ytsma, G. Colonization of southern beech byPlatypus caviceps (Coleoptera: Platypodidae). J. Chem. Ecol. 1989, 15, 1171–1176. [Google Scholar] [CrossRef]
- Rudinsky, J.A.; Daterman, G.E. Field Studies on Flight Patterns and Olfactory Responses of Ambrosia Beetles in Douglas-fir Forests of Western Oregon. Can. Entomol. 2012, 96, 1339–1352. [Google Scholar] [CrossRef]
- Seo, M.; Martini, X.; Rivera, M.J.; Stelinski, L.L. Flight Capacities and Diurnal Flight Patterns of the Ambrosia Beetles, Xyleborus glabratus and Monarthrum mali (Coleoptera: Curculionidae). Environ. Entomol. 2017, 46, 729–734. [Google Scholar] [CrossRef]
- Ávalos, J.A.; Martí-Campoy, A.; Soto, A. Study of the flying ability of Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) adults using a computer-monitored flight mill. Bull. Entomol. Res. 2014, 104, 462–470. [Google Scholar] [CrossRef]
- Okada, R.; Pham, D.L.; Ito, Y.; Yamasaki, M.; Ikeno, H. Measuring the Flight Ability of the Ambrosia Beetle, Platypus Quercivorus (Murayama), Using a Low-Cost, Small, and Easily Constructed Flight Mill. J. Vis. Exp. 2018, 138, e57468. [Google Scholar] [CrossRef]
- Borden, J.H.; Chong, L.J.; Savoie, A.; Wilson, I.M. Responses to green leaf volatiles in two biogeoclimatic zones by striped ambrosia beetle, Trypodendron lineatum. J. Chem. Ecol. 1997, 23, 2479–2491. [Google Scholar] [CrossRef]
- Pham, D.L.; Ito, Y.; Okada, R.; Ikeno, H.; Kazama, H.; Mori, N.; Yamasaki, M. Platypus quercivorus ambrosia beetles use leaf volatiles in host selection. Entomol. Exp. Et Appl. 2020, 168, 928–939. [Google Scholar] [CrossRef]
- Bellahirech, A.; Branco, M.; Catry, F.X.; Bonifácio, L.; Sousa, E.; Ben Jamâa, M.L. Site- and tree-related factors affecting colonization of cork oaks Quercus suber L. by ambrosia beetles in Tunisia. Ann. For. Sci. 2019, 76, 45. [Google Scholar]
- Maner, M.L.; Hanula, J.L.; Braman, S.K. Gallery Productivity, Emergence, and Flight Activity of the Redbay Ambrosia Beetle (Coleoptera: Curculionidae: Scolytinae). Environ. Entomol. 2013, 42, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Zach, P.; Topp, W.; Kulfan, J.; Simon, M. Colonization of two alien ambrosia beetles (Coleoptera, Scolytidae) on debarked spruce logs. Biologia 2001, 56, 175–181. [Google Scholar]
- Fernández Fernández, M.M. Colonization of fire-damaged trees by Ips sexdentatus (Boerner) as related to the percentage of burnt crown. Entomol. Fenn. 2006, 17, 381–386. [Google Scholar] [CrossRef]
- Ryan, K.C.; Amman, G.D. Bark beetle activity and delayed tree mortality in the Greater Yellowstone Area following the 1988 fires. Bark Beetles Fuels Fire Bibliogr. 1996, 15, 151–158. [Google Scholar]
- McPherson, B.A.; Erbilgin, N.; Bonello, P.; Wood, D.L. Fungal species assemblages associated with Phytophthora ramorum-infected coast live oaks following bark and ambrosia beetle colonization in northern California. For. Ecol. Manag. 2013, 291, 30–42. [Google Scholar] [CrossRef]
- Reding, M.E.; Ranger, C.M.; Schultz, P.B. Colonization of Trees by Ambrosia Beetles (Coleoptera: Curculionidae: Scolytinae) Is Influenced by Duration of Flood Stress. J. Econ. Entomol. 2021, 114, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Santolamazza-Carbone, S.; Pestaña, M.; Vega, J.A. Post-fire attractiveness of maritime pines (Pinus pinaster Ait.) to xylophagous insects. J. Pest. Sci. 2011, 84, 343–353. [Google Scholar] [CrossRef]

| Groups | Group 1 | Group 2 | Group 3 | |
|---|---|---|---|---|
| Characteristics | ||||
| Sampled Trees | n | 491 | 140 | 210 |
| Sampling time after fire | months | 5–8 | 12 | 39 |
| Variables assessed | Units | |||
| Burned/Unburned (B/U) | n | 369B/122U | 140B | 210U |
| Presence of beetles (0–2 m) | holes | x | x | x |
| Density of beetles (0–2 m) | holes/m2 | x | x | x |
| Location of beetles in the trunk | cat. | x | x | x |
| Tree size (DBH, height) | cm/m | x | x | x |
| Exploited/Unexploited (E/U) | n | 391E/100U | 113E/27U | 210E |
| Last debarking year | year | 2008/2012 | 2012 | 2014/2015/2016 |
| Debarking intensity | m/m2 | x | ||
| Bark thickness | mm | x | ||
| Stem wounds | cat./n | x | x | |
| Tree status (live/dead) | cat. | x | x | x |
| Crown status | cat. | x | x | x |
| Tree regeneration (crown/basal) | cat. | x | x | |
| Fire severity (depth/height) | cat./% | x | x | |
| Distance to burned/unburned | m | x | x | x |
| Vegetation cover (trees/shrubs) | % | x | ||
| Basal area (trees/shrubs) | m2/ha | x | ||
| Aspect | cat. | x | ||
| Slope | % | x | ||
| Other pests/diseases | cat./n | x |
| Groups | Group 1 | Group 2 | ||||
|---|---|---|---|---|---|---|
| Burned | Unburned | Burned | ||||
| Number of Sampled Trees | 369 | 122 | 140 | |||
| Exploited/Unexploited (E/U) | 298E/71U | 93E/29U | 113E/27U | |||
| Variable | Mean (SE) | Range | Mean (SE) | Range | Mean (SE) | Range |
| DBH 1 | 23.0 (0.4) | 6–57 | 25.9 (0.9) | 6–50 | 28.6 (3.8) | 9–56 |
| Bark thickness 2 | 17.8 (0.5) | 5–61 | 20.6 (0.7) | 9–46 | - | - |
| Debarking height 3 | 1.7 (0.5) | 0–6 | 1.8 (0.1) | 0–4 | - | - |
| Stem wounds 4 | 0.1 (0.0) | 0–1 | 0.1 (0.0) | 0–1 | - | - |
| Green crown volume 5 | 5.1 (0.5) | 0–60 | 78.4 (1.5) | 20–100 | - | - |
| Maximum char height 6 | 91.2 (0.8) | 21–100 | - | - | - | - |
| Trunk char depth class 1 7 | 2.2 (0.4) | 0–60 | - | - | 6.4 (1.5) | 0–100 |
| Trunk char depth class 3 8 | 49.9 (2.0) | 0–100 | - | - | 20.1 (1.6) | 0–80 |
| Number of ambrosia 9 | 9.3 (0.7) | 0–40 | 0.0 (0.0) | 0 | 9.2 (1.2) | 0–89 |
| Density of ambrosia 10 | 40.2 (3.4) | 0–367 | 0.0 (0.0) | 0 | 28.4 (3.8) | 0–285 |
| Model | Variables | Coefficients | p-Value | AIC |
|---|---|---|---|---|
| Presence of attacks (n = 369) | β0 1 | −24.013 ± 997 | - | - |
| Trunk diameter 2 | 0.268 ± 0.04 | <0.001 | 342.6 | |
| High fire severity 3 | 0.028 ± 0.01 | <0.001 | 309.6 | |
| Green crown volume 4 | −0.113 ± 0.03 | <0.001 | 295.4 | |
| Exploited (yes) 5 | 18.028 ± 997 | <0.001 | 290.1 | |
| Distance to unburned 6 | −0.023 ± 0.01 | <0.001 | 284.6 | |
| Intensity of attacks (n = 174) | β0 1 | 2.698 ± 0.24 | - | - |
| Distance to unburned 6 | −0.011 ± 0.00 | <0.001 | 1350.8 | |
| Bark thickness 7 | 0.050 ± 0.01 | <0.001 | 1349.7 | |
| Debarking height 8 | 0.236 ± 0.07 | <0.001 | 1334.5 | |
| Green crown volume 4 | −0.040 ± 0.01 | <0.01 | 1332.8 |
| Model | Variables | Coefficients | p-Value | AIC |
|---|---|---|---|---|
| Presence of attacks (n = 140) | β0 1 | 0.267 ± 0.04 | - | - |
| Trunk diameter 2 | 0.186 ± 0.04 | <0.001 | 168.0 | |
| Live crown 3 | −1.560 ± 0.51 | <0.01 | 141.9 | |
| Low fire severity 4 | −0.004 ± 0.01 | <0.01 | 141.8 | |
| Intensity of attacks (n = 78) | β0 1 | 3.661 ± 0.18 | - | - |
| Distance to unburned 5 | −0.002 ± 0.00 | 0.01 | 589.0 |
| Cork Oak Stand Debarking Year | ||||||
|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | ||||
| Sampled trees 1 | 70 | 70 | 70 | |||
| Attacked trees 2 | 1 | 14 | 0 | |||
| Variable | Mean (SE) | Range | Mean (SE) | Range | Mean (SE) | Range |
| DBH 3 | 35.2 (1.0) | 20–60 | 30.0 (0.9) | 15–51 | 28.5 (1.1) | 14–60 |
| Green crown 4 | 83.5 (1.5) | 30–95 | 77.4 (2.3) | 0–95 | 86.6 (1.1) | 40–95 |
| Dry crown 5 | 0.7 (0.0) | 0–2 | 1.7 (1.1) | 0–60 | 0.7 (0.0) | 0–0 |
| Stem wounds 6 | 1.5 (0.1) | 0–3 | 1.7 (0.1) | 0–3 | 1.0 (0.1) | 0–3 |
| Distance to burned 7 | 214 (18) | 7–527 | 591 (8) | 440–696 | 322 (6) | 237–413 |
| Number of ambrosia 8 | 0.2 (0.2) | 0–17 | 3.6 (1.6) | 0–84 | 0.0 (0.0) | 0–0 |
| Density of ambrosia 9 | 1.0 (1.0) | 0–69 | 11.5 (5.1) | 0–283 | 0.0 (0.0) | 0–0 |
| Coefficients | p-Value | AIC | |
|---|---|---|---|
| β0 1 | −2.258 ± 1.766 | - | - |
| Stem wounds 2 | 1.487 ± 0.498 | <0.001 | 88.55 |
| Green crown 3 | −0.048 ± 0.016 | <0.01 | 83.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catry, F.X.; Branco, M.; Moreira, F.; Sousa, E.; Rego, F. Ambrosia Beetle Attacks in Mediterranean Cork Oak Forests Following Fire: Which Factors Drive Host Selection? Fire 2022, 5, 115. https://doi.org/10.3390/fire5040115
Catry FX, Branco M, Moreira F, Sousa E, Rego F. Ambrosia Beetle Attacks in Mediterranean Cork Oak Forests Following Fire: Which Factors Drive Host Selection? Fire. 2022; 5(4):115. https://doi.org/10.3390/fire5040115
Chicago/Turabian StyleCatry, Filipe X., Manuela Branco, Francisco Moreira, Edmundo Sousa, and Francisco Rego. 2022. "Ambrosia Beetle Attacks in Mediterranean Cork Oak Forests Following Fire: Which Factors Drive Host Selection?" Fire 5, no. 4: 115. https://doi.org/10.3390/fire5040115
APA StyleCatry, F. X., Branco, M., Moreira, F., Sousa, E., & Rego, F. (2022). Ambrosia Beetle Attacks in Mediterranean Cork Oak Forests Following Fire: Which Factors Drive Host Selection? Fire, 5(4), 115. https://doi.org/10.3390/fire5040115







