Ignition of Fuel Beds by Cigarettes: A Conceptual Model to Assess Fuel Bed Moisture Content and Wind Velocity Effect on the Ignition Time and Probability
Abstract
:1. Introduction
2. Physical Processes and Conceptual Model
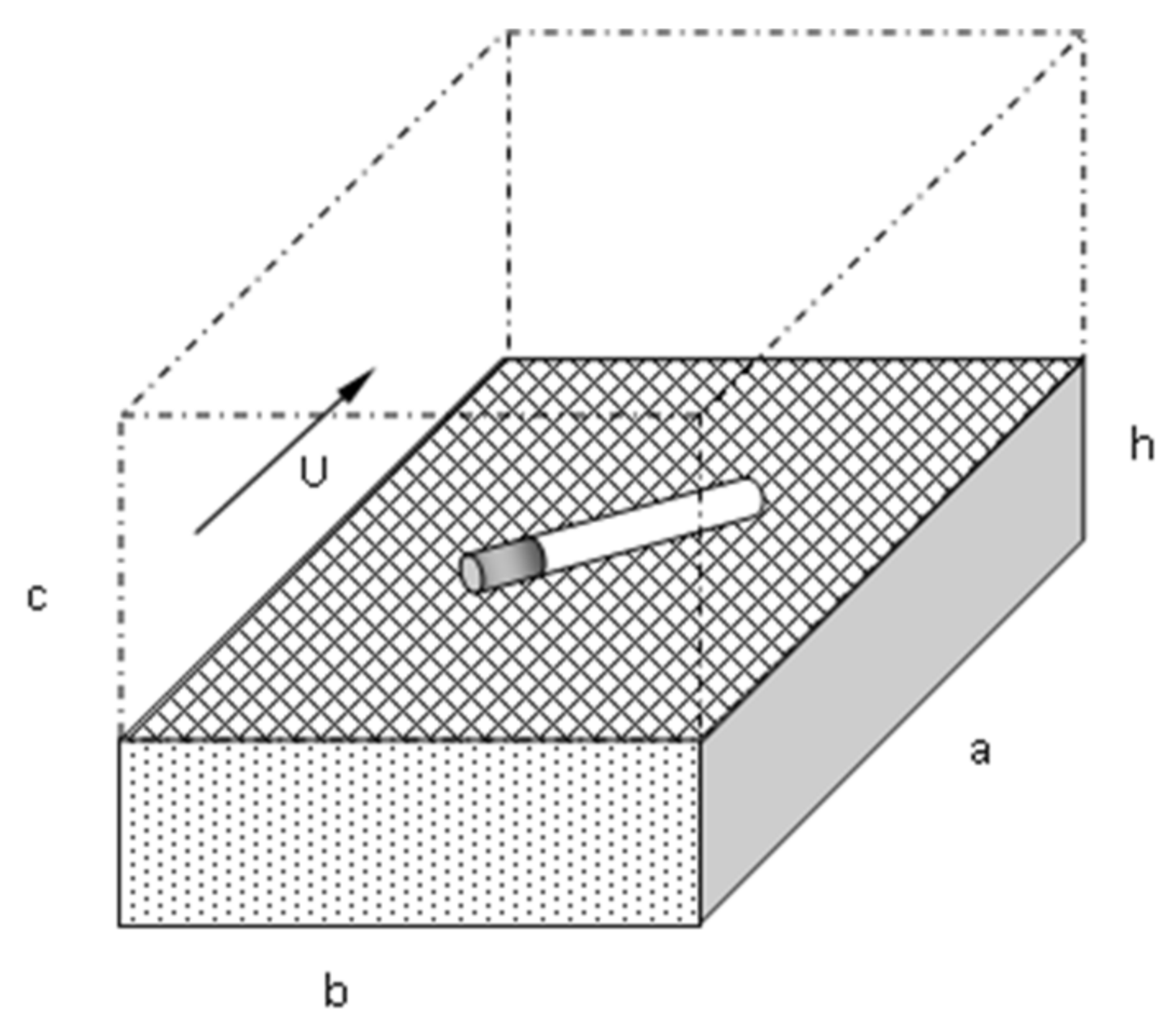
2.1. Definition of the Problem
2.2. Physical Processes of Cigarette Burning and Fuel Bed Ignition
- The global energy balance of the system described above;
- The process of burning of an individual cigarette without suction or puffing, in various positions, under the influence of a flow of varying velocity and direction;
- The heating of a portion of the fuel bed by a cigarette, producing its dehydration (Phase 1); after this process, we have either process 4 or 5:
- Process of cigarette or fuel bed combustion extinction (Phase 2a);
- Process of pyrolysis and partial combustion of the fuel bed leading to its ignition as flaming combustion (Phase 2b).
2.3. Energy Balance
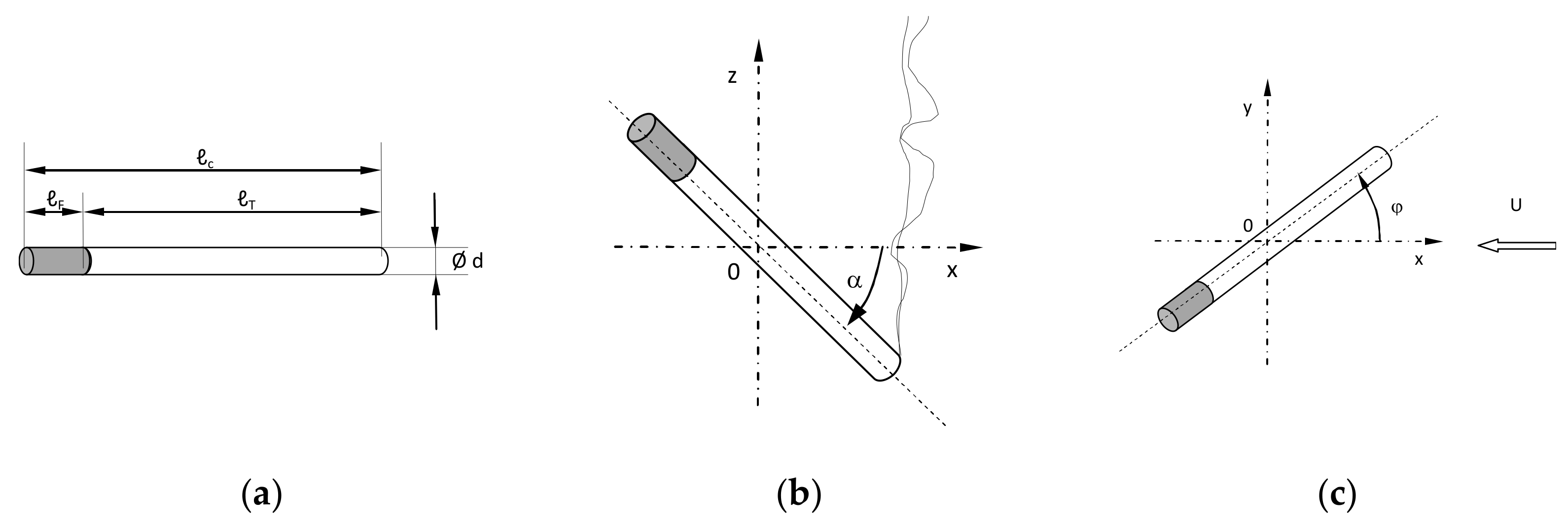
2.4. Cigarette Burning
- -
- Apparent density (tobacco mass per unit volume) ρT, given by:
- -
- Linear density (tobacco mass per unit length) γT, given by:
2.5. Fuel Bed Properties
2.6. Time to Ignition
2.7. Probability of Ignition
- Fuel bed moisture content—we assume that there is a critical value x1 above which ignition is not possible because of the damping caused by fuel moisture that will extinguish the cigarette.
- Wind velocity—we assume that there is a critical value Uc above which cigarette combustion will be extinguished and therefore ignition is not possible.
- Cigarette burnout—as was explained above cigarette combustion may be extinguished before the dehydration process is concluded corresponding to and therefore ignition will not occur.
- Moisture damping—water vapor released during the dehydration and pyrolysis processes may damp or even extinguish cigarette or fuel bed combustion hampering fuel bed ignition. In this case, we have .
3. Materials and Methods
3.1. Cigarette Properties
3.2. Rate of Combustion
3.3. Ignition Tests
4. Results and Discussion
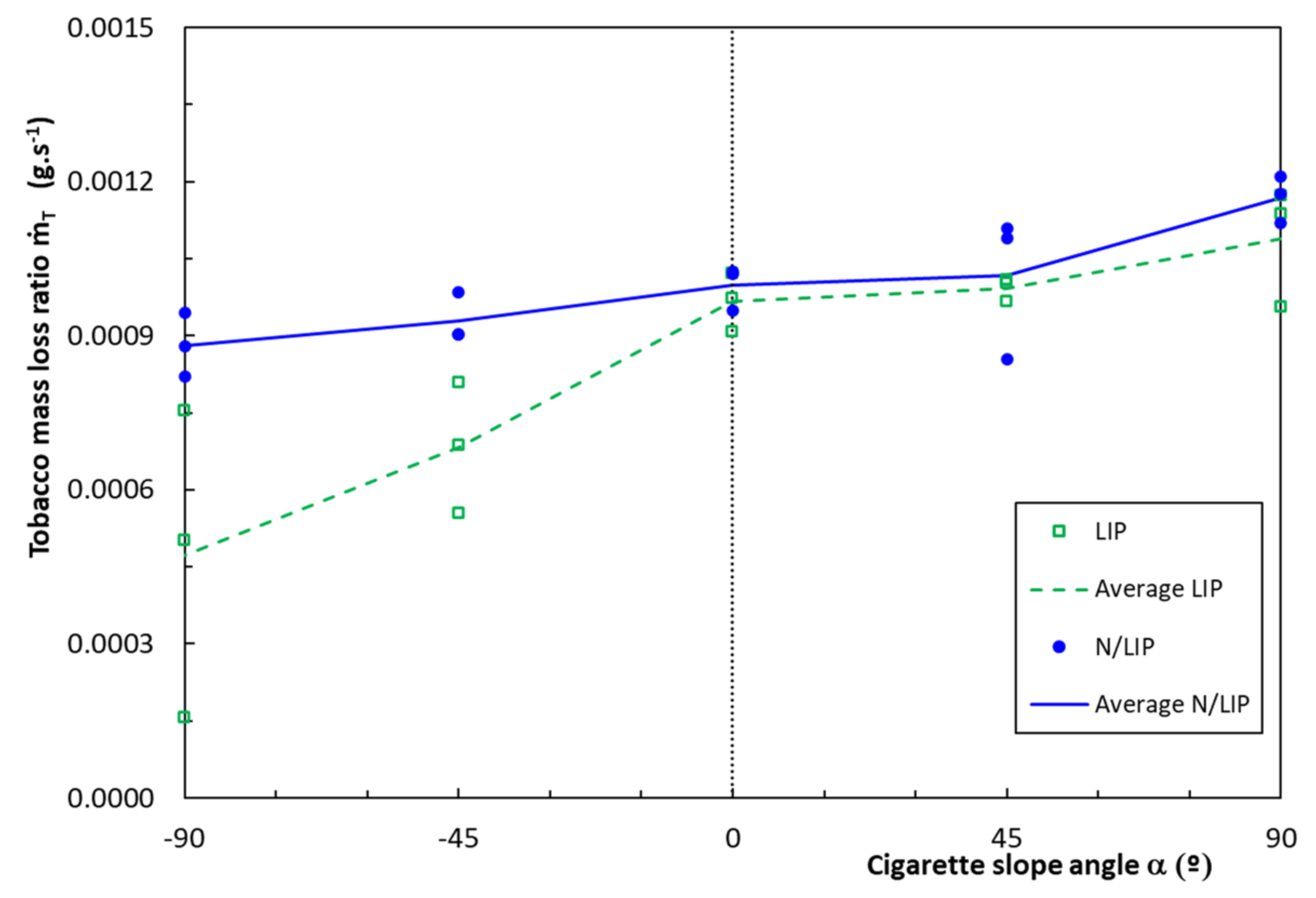
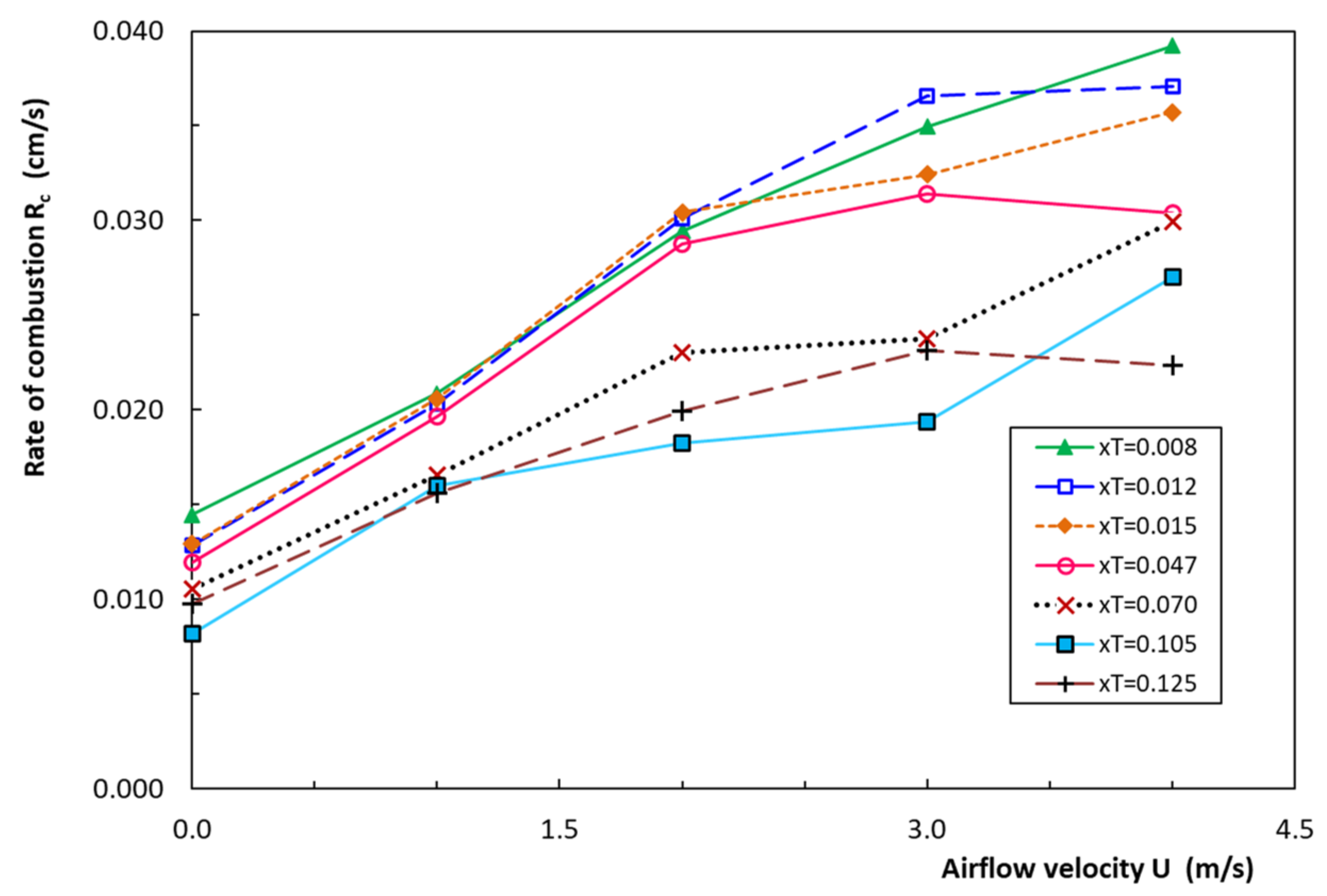
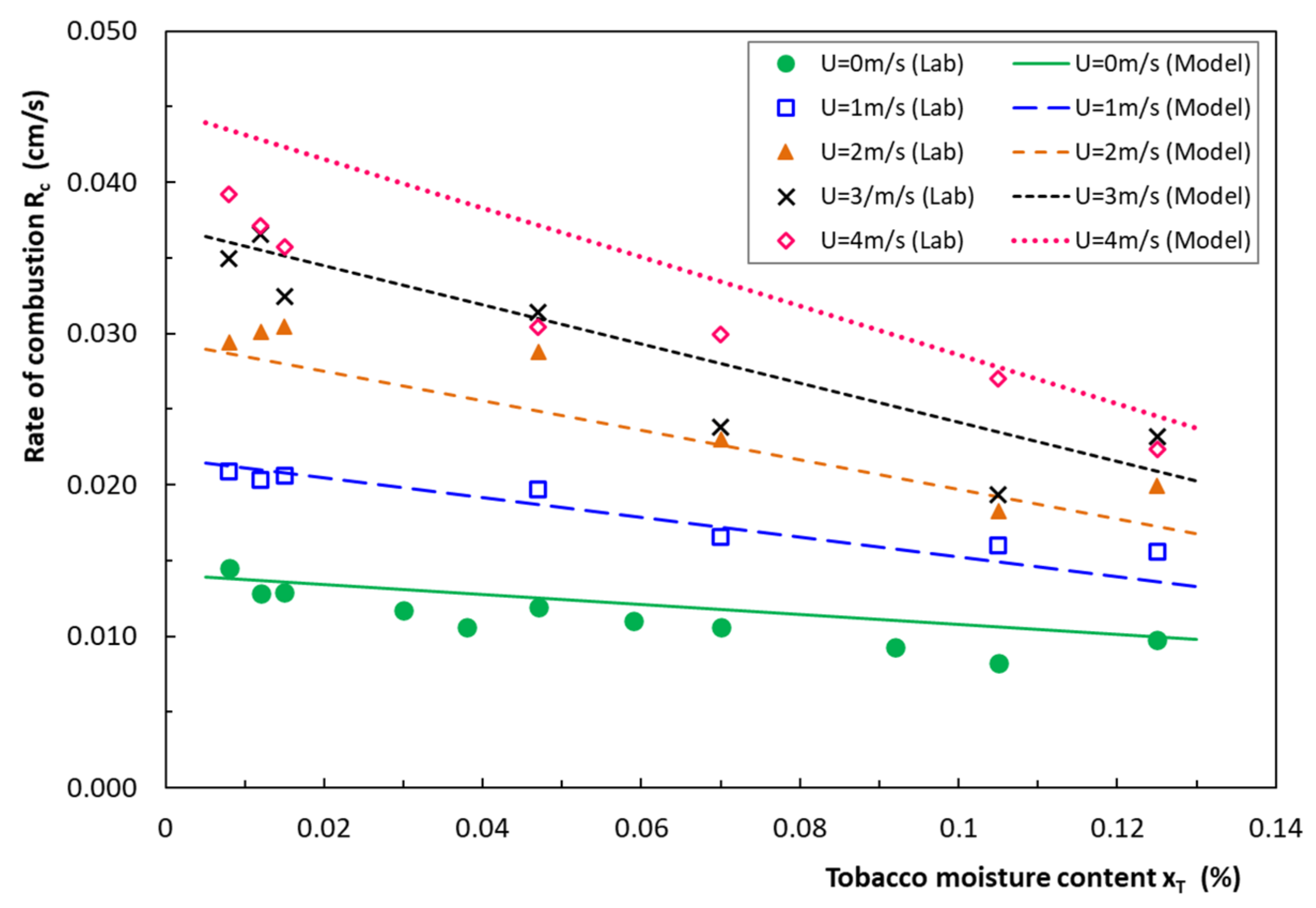
4.1. Rate of Combustion of Cigarettes
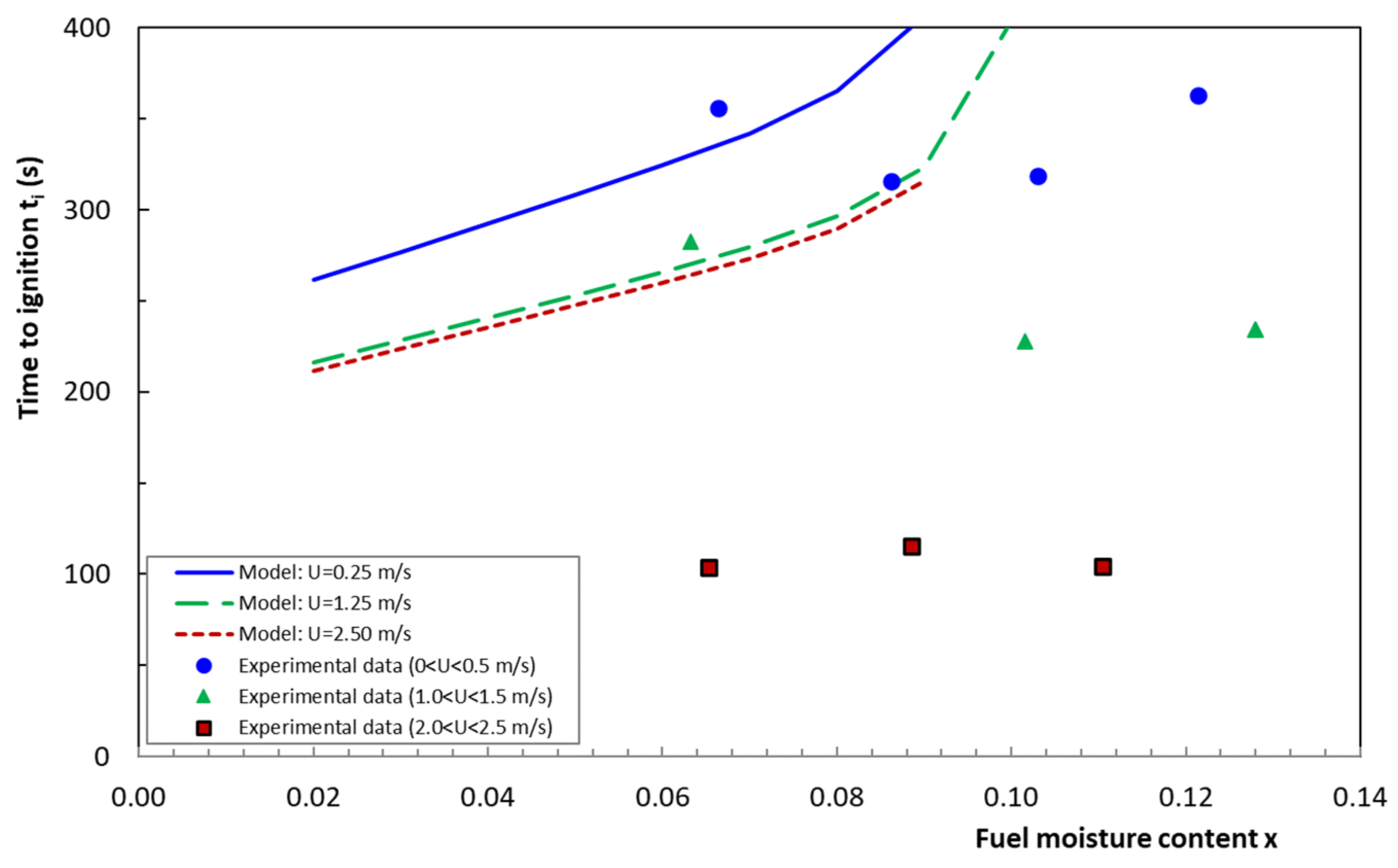
4.2. Model Validation
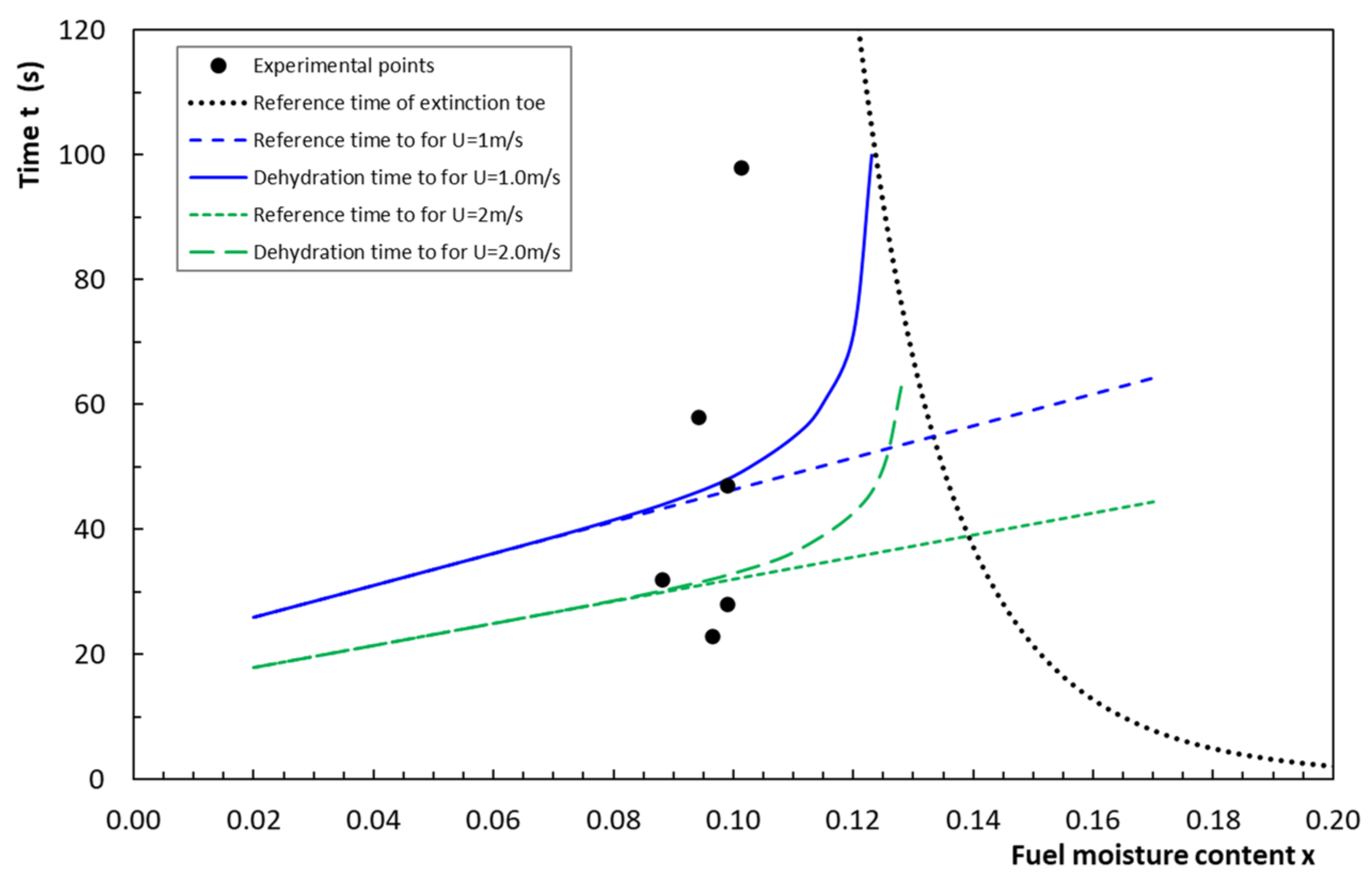
4.3. Dehydration Time
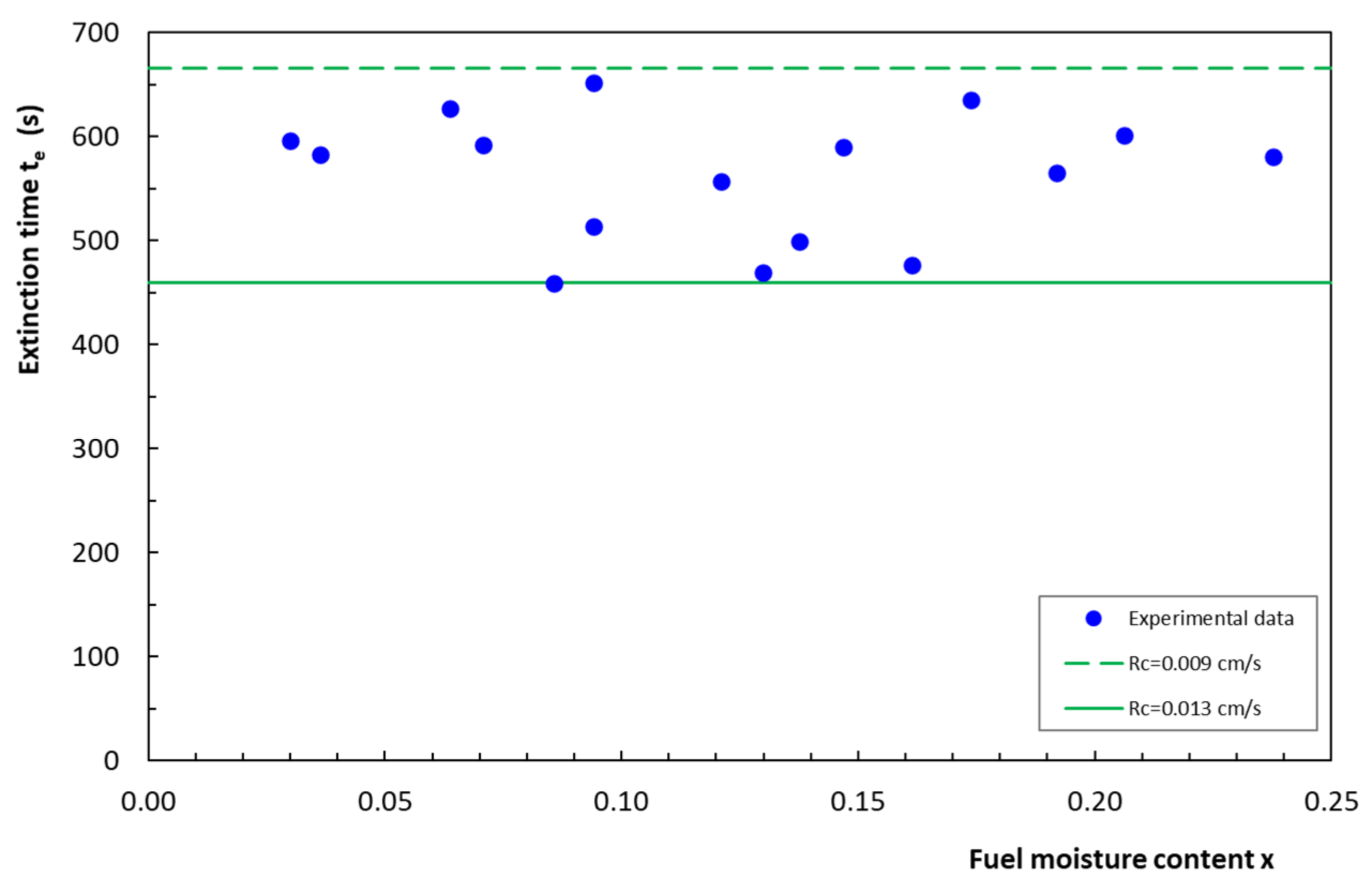
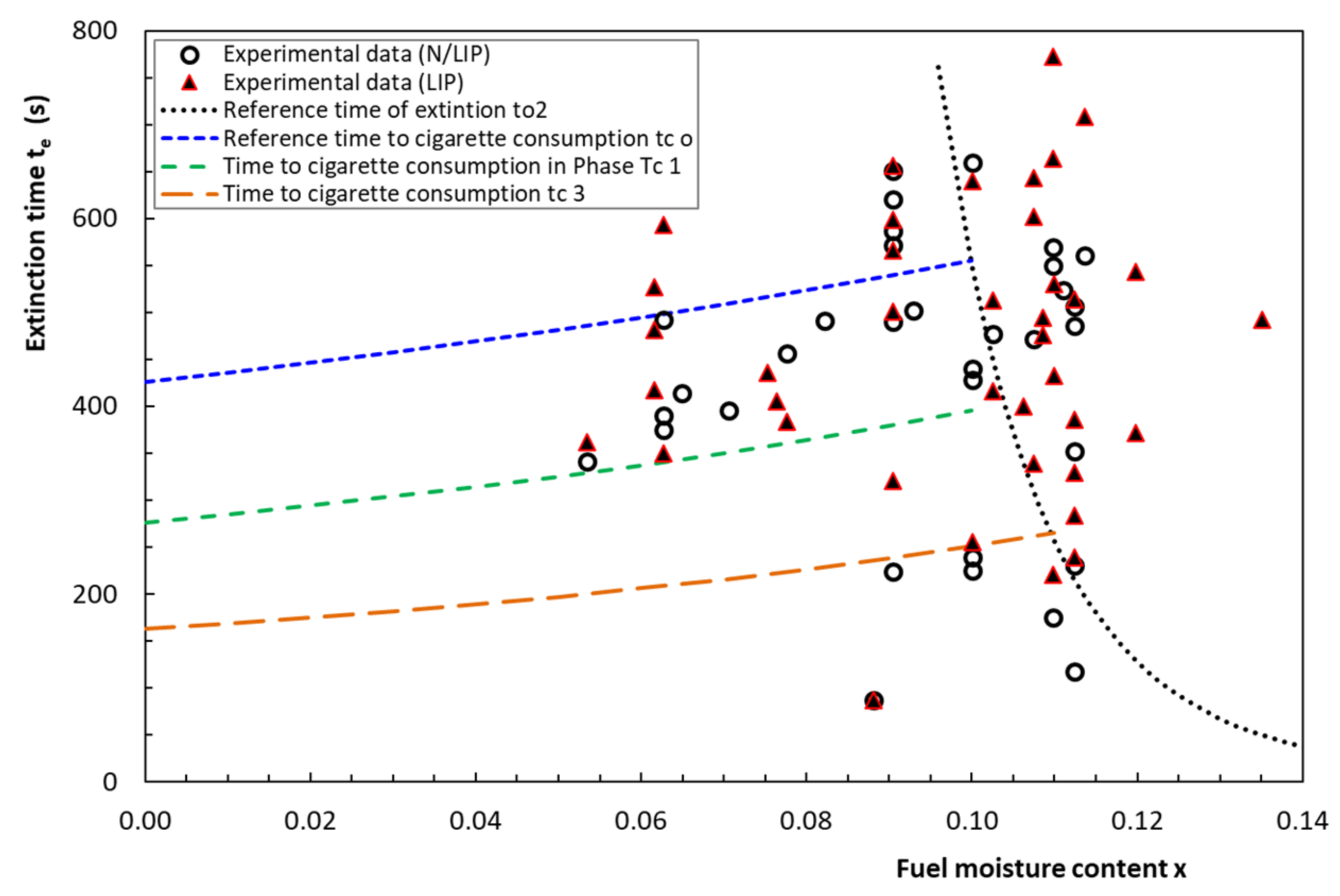
4.4. Extinction Time
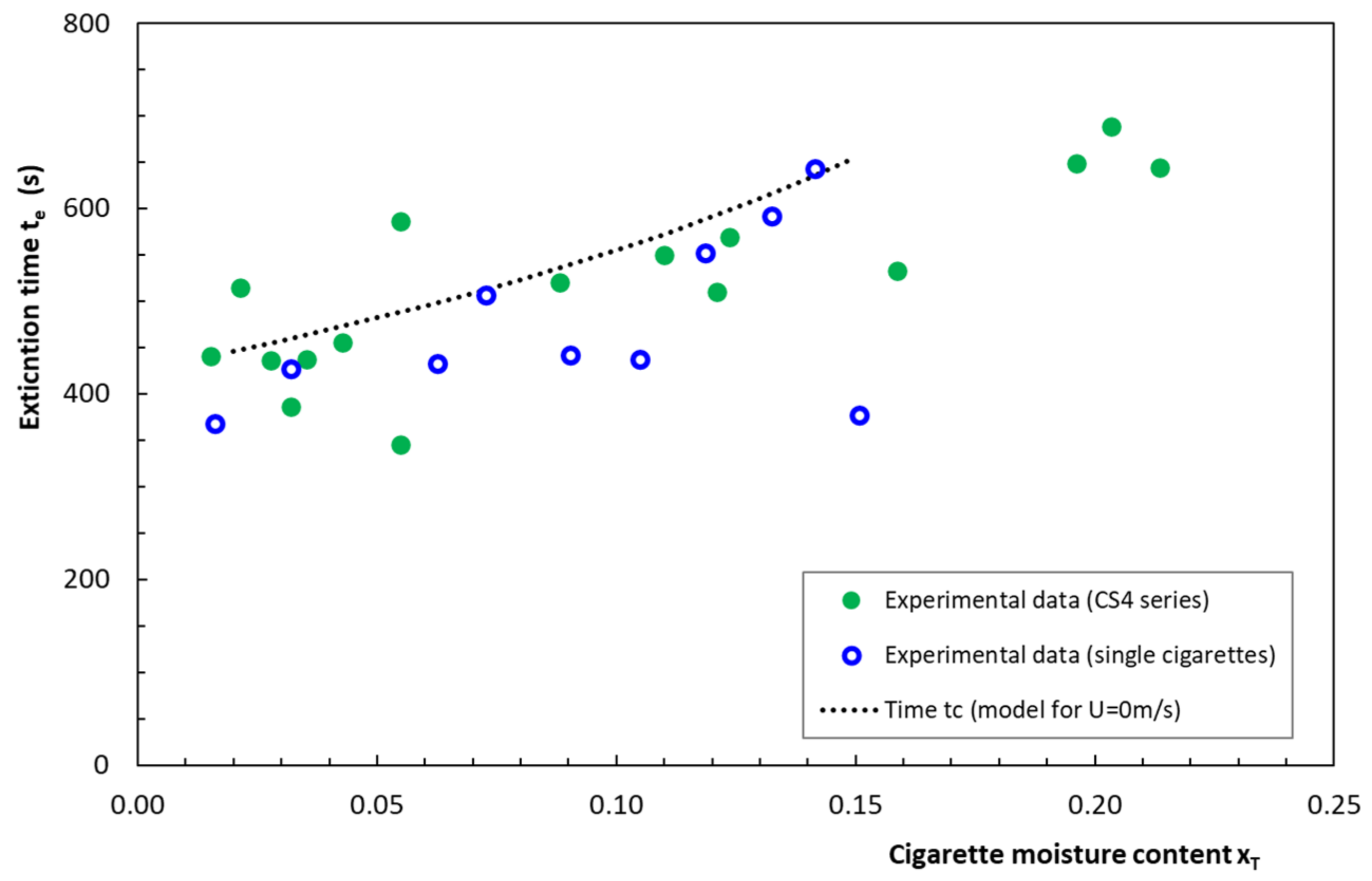
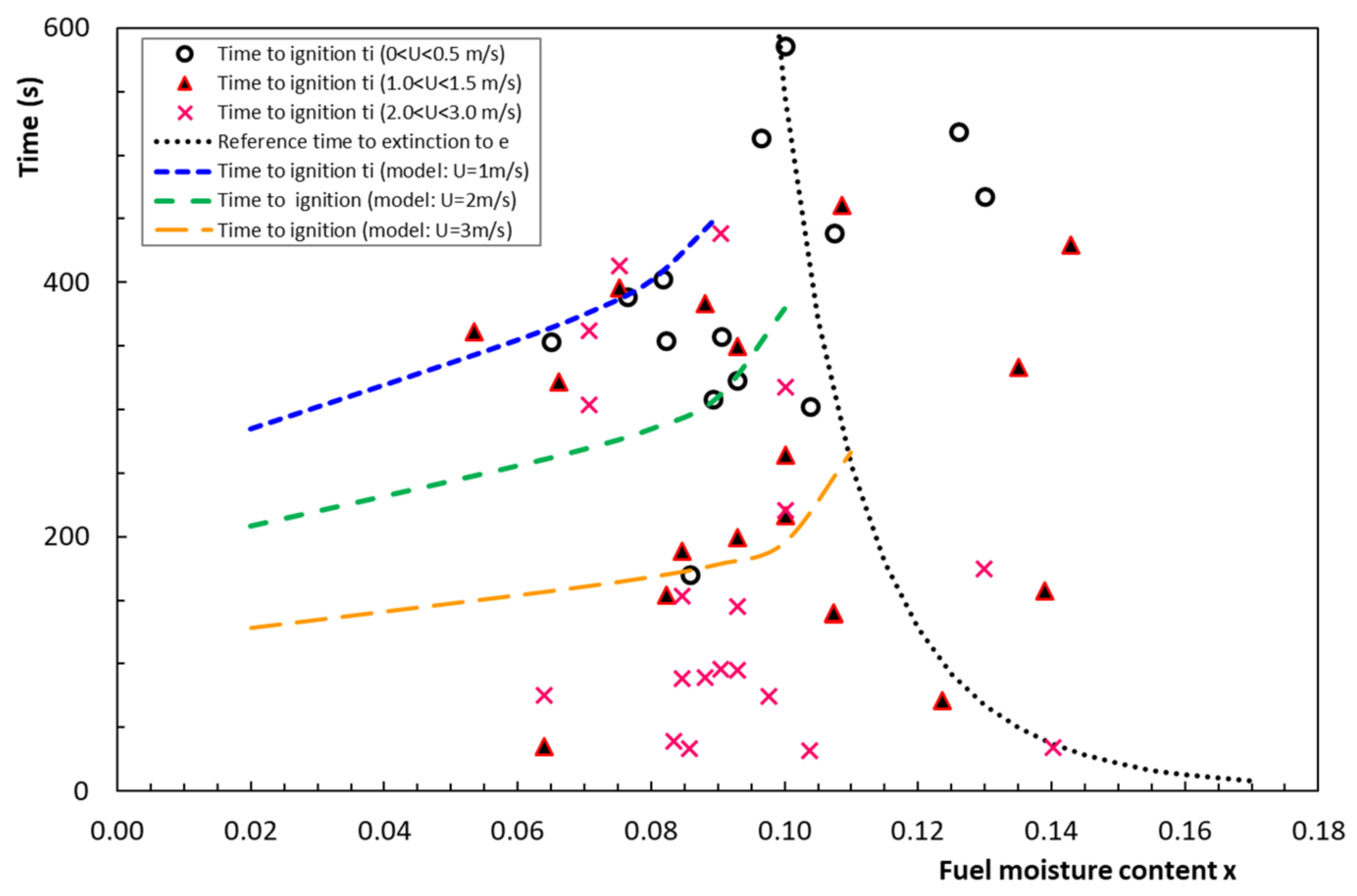
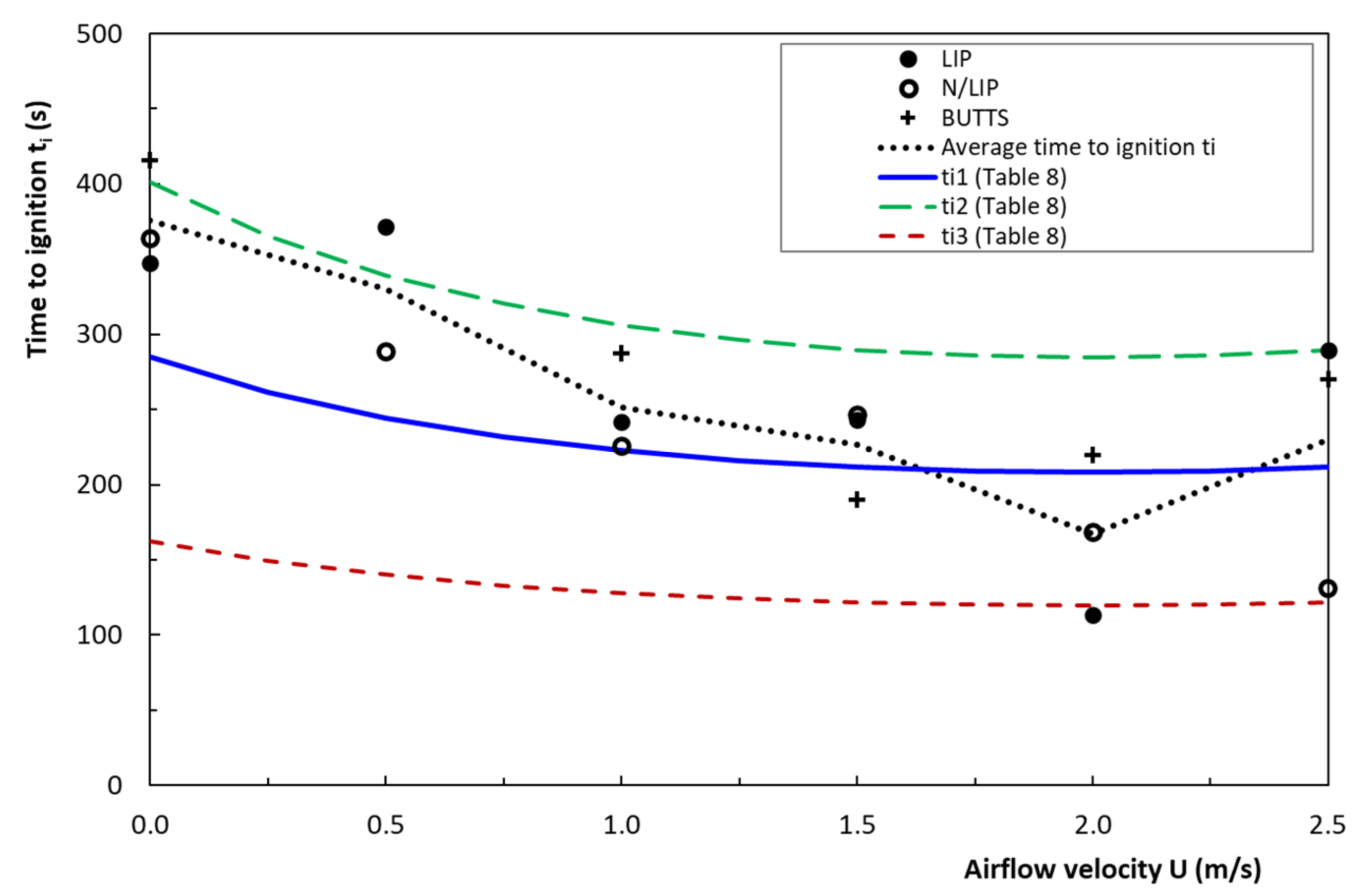
4.5. Time to Ignition
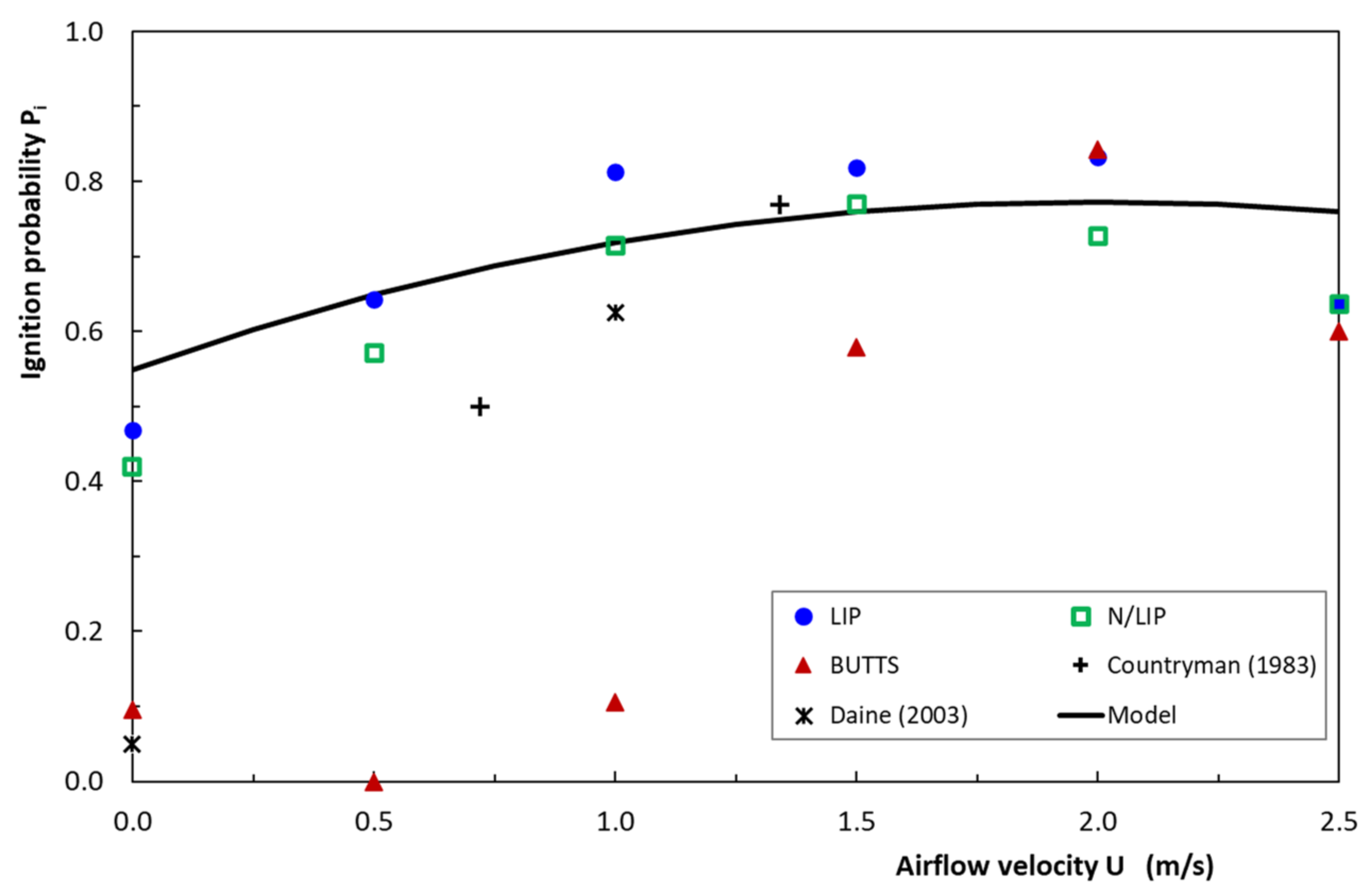
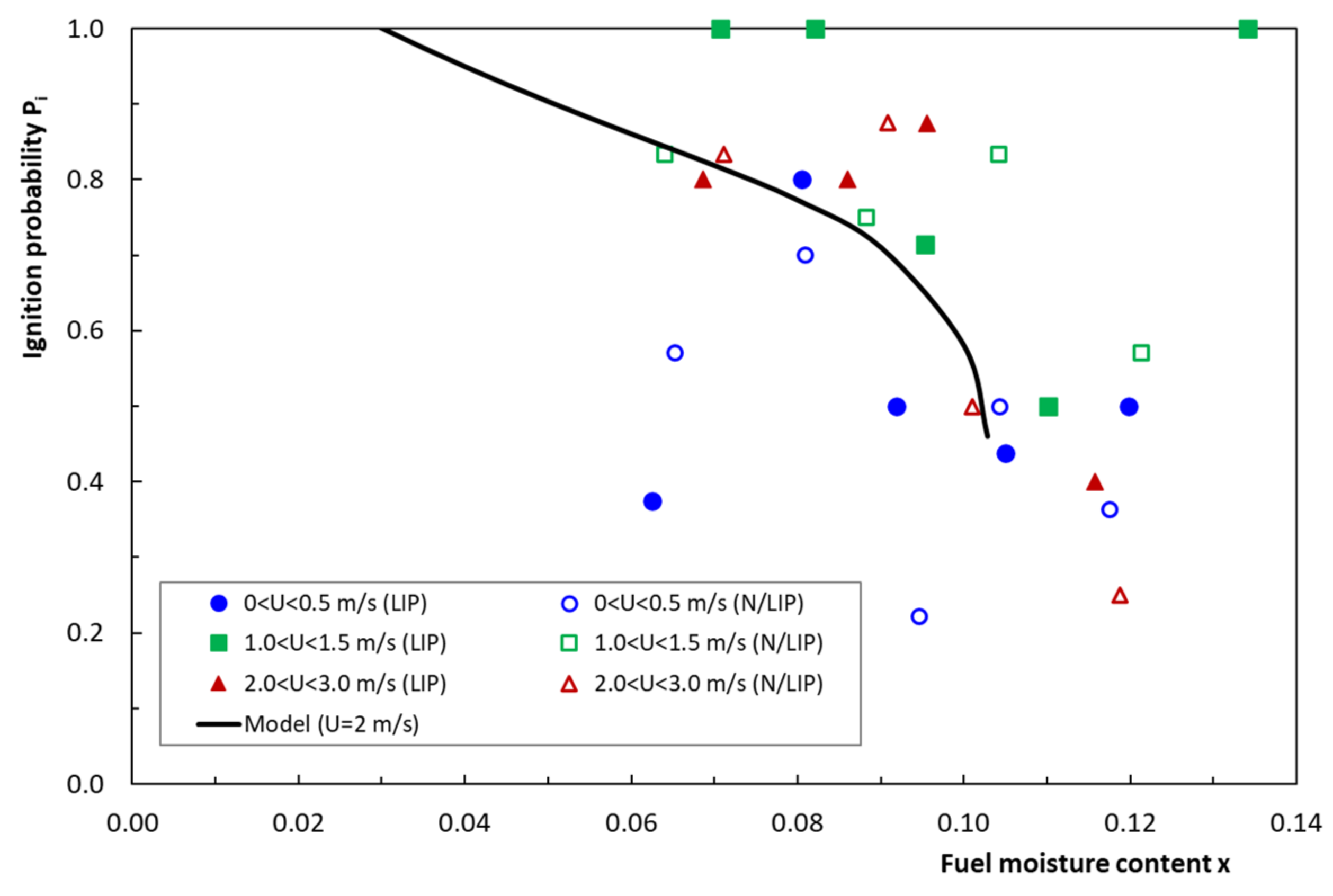
4.6. Ignition Probability
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Symbols
| Symbol | Units | Description |
| φ | - | Angle of cigarette in relation to wind velocity |
| α | - | Angle of cigarette with horizontal plane |
| αo | s−1 | Damping coefficient in Equation (22) |
| α1 | s−1 | Coefficient in Equation (23) |
| α2 | - | Exponent in Equation (23) |
| β | - | Coefficient in Equation (32) |
| β1 | s−1 | Time constant in fuel combustion process |
| γT | kg.m−1 | Mass of tobacco and paper per unit of length |
| ΔTi | K | Characteristic temperature elevation for particle ignition |
| ΔTw | K | Characteristic temperature elevation for water evaporation |
| τw | Pa | Wall shear stress |
| ρT | kg.m−3 | Apparent density of the cigarette |
| ∅ | Flux of energy | |
| a | m | Horizontal dimension of control volume |
| ac | J.s−1 | Parameter in Equation (2) |
| aG | J.s−1 | Parameter in Equation (7) |
| as | J.s−1 | Parameter in Equation (3) |
| au | J.s.m−2 | Parameter in Equation (4) |
| b | m | Horizontal dimension of control volume |
| bc | J.m−1 | Parameter in Equation (2) |
| br | J.s−1 | Parameter in Equation (3) |
| c | m | Vertical dimension of control volume |
| Cp | J.g−1.K−1 | Heat capacity of fuelbed particles |
| d | m | Diameter of the cigarette |
| E | J | Energy of combustion of fuelbed |
| E1 | J | Energy absorbed by fuelbed in Phase 1 |
| E2 | J | Energy absorbed by fuelbed in Phase 2a |
| EG | J | Total enthalpy in the control volume |
| J.s−1 | Gain of energy due to combustion | |
| J.s−1 | Global energy balance | |
| J.s−1 | Modified energy balance during Phase 1 | |
| J.s−1 | Modified energy balance | |
| J.s−1 | Modified energy balance during Phase 2a | |
| J.s−1 | Modified energy balance during Phase 1 | |
| J.s−1 | Modified energy balance during Phase 2a | |
| J.s−1 | Loss of energy due to radiation | |
| J.s−1 | Loss of energy due to convection | |
| hf | m | Fuelbed height |
| HT | J.kg−1 | combustion yield of tobacco |
| Hw | J.g−1 | Latent heat of evaporation of water in the fuelbed particles |
| m | Length of the filter | |
| m | Total length of cigarette | |
| m | Length of the tobacco filled part of the cigarette | |
| LIP | - | Lower ignition propensity |
| k | s−1 | Parameter in Equation (40) |
| m1 | kg | Effective mass of the fuelbed affected by the cigarette in Phase 1 |
| m2 | kg | Effective mass of the fuelbed affected by the cigarette in Phase 2a |
| Mc | kg | Initial mass of the cigarette |
| Mf | g.m−2 | Fuelbed load |
| MF | g | Mass of the filter of the cigarette |
| MKS | - | System of units based on the three mechanical units meter, kilogram, and second |
| MT | kg | Mass of tobacco and paper contents of the cigarette |
| MTS | kg | Mass of tobacco and paper without water content |
| kg.s−1 | Mass loss rate of cigarette | |
| N/LIP | - | No LIP (Lower ignition propensity) |
| Pi | - | Probability of ignition of the fuelbed by a cigarette |
| Rc | m.s−1 | Rate of combustion of the cigarette |
| RAD | Average relative deviation | |
| RIP | - | Reduced ignition propensity |
| S | m−2 | Surface |
| Sc | m−2 | Surface of the control volume |
| t | s | Time measured since drop of cigarette on fuelbed |
| To | K | Ambient gas temperature |
| to1 | s | Reference time in Phase 1 |
| to2 | s | Reference time in Phase 2 |
| toe | s | Reference time of extinction |
| t1 | s | Time required to end Phase 1 |
| t2 | s | Time required since the end of Phase 1 to achieve ignition |
| tc | s | Time to cigarette consumption or burn out |
| te | s | Time to cigarette and overall combustion extinction |
| ti | s | Time to fuelbed ignition |
| ti2 | s | Time to fuelbed ignition in Phase 2a |
| T0 | K | Initial value of ambient temperature |
| Tw | K | Temperature of water evaporation |
| Ti | K | Temperature of particle ignition |
| T’i | K | Characteristic temperature of particle ignition |
| T’w | K | Characteristic temperature of water evaporation |
| U | m.s−1 | Reference wind or flow velocity |
| U1 | m.s−1 | Flow velocity that maximizes heat release |
| Uc | m.s−1 | Critical flow velocity |
| uτ | m.s−1 | Friction velocity |
| V | m3 | Volume |
| Vc | m3 | Volume control |
| x | - | Fraction of water in the fuelbed referred to its dry mass |
| x1 | - | Fraction of water in the fuelbed referred to its dry mass after Phase 1 |
| xT | - | Fraction of water in the cigarette referred to its dry mass |
References
- Chapman, S.; Balmain, A. Time to legislate for fire-safe cigarettes in Australia. Med. J. Aust. 2004, 181, 292–293. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, M. Ignição de Combustíveis Florestais por Partículas com Elevada Temperatura. Master’s Thesis, University of Coimbra, 2008. Available online: https://www.academia.edu/44971705/_Igni%C3%A7%C3%A3o_de_Combust%C3%ADveis_Florestais_por_Part%C3%ADculas_com_Elevada_Temperatura_por_M%C3%A1rio_Loureiro_UC_Igni%C3%A7%C3%A3o_de_Combust%C3%ADveis_Florestais_por_Part%C3%ADculas_com_Elevada_Temperatura (accessed on 19 May 2021). (In Portuguese).
- Galante, M. As Causas dos Incêndios Florestais em Portugal Continental. In Proceedings of the 5th Congresso Florestal Nacional, Lisboa, Portugal, 16–19 May 2005; Galante, M., Ed.; Direcção-Geral dos Recursos Florestais, Divisão de Defesa da Floresta Contra Incêndios, DGRF: Lisboa, Portugal, 2005. (In Portuguese). [Google Scholar]
- Fire Program Statistics. U.S. Fish and Wildlife Service. Available online: www.fws.gov/fire/program_statistics/ (accessed on 19 May 2021).
- Seccion de Estadisticas Historicas CONAF (Chile). Available online: http://www.conaf.cl/proteccion/seccion-estadisticas-historicas.html (accessed on 19 May 2021).
- Kim, D.H.; Lee, M.B.; Viegas, D.X. Ignition of Surface Fuels by Cigarette in Forest Fire. In VI International Conference on Forest Fire Research, 1st ed.; Viegas, D.X., ADAI-CEIF, Eds.; ADAI-CEIF; University of Coimbra: Coimbra, Portugal, 2010; ISBN 978-989-20-2157-7. [Google Scholar]
- Countryman, C.M. Cigarette Temperatures and Burning Rates; USDA Forest Service; Pacific Southwest Forest; Range Experiment Station; US Forest Service: Riverside, CA, USA, 1982. Available online: http://legacy.library.ucsf.edu/tid/kvr81b00 (accessed on 19 May 2021).
- Countryman, C.M. Ignition of Grass Fuels by Cigarettes, Fire Management Notes; USDA Forest Service; Pacific Southwest Forest; Range Experiment Station: Riverside, CA, USA, 1983; Volume 44, pp. 3–7. Available online: http://books.google.pt/books/about/Ignition_of_Grass_Fuels_by_Cigarettes.html?id=8w3oHAAACAAJ&redir_esc=yAuthor (accessed on 19 May 2021).
- Dainer, J. Can Cigarette Butts Start Bushfires? Ph.D. Thesis, Forensic Science, University of Technology, Sydney, Australia, 2003. [Google Scholar]
- Galtié, J.F. Quantifying the propensity of cigarettes to initiate wildland fire in Mediterranean environment. For. Ecol. Manag. 2006, 234, S237–S265. [Google Scholar] [CrossRef]
- Xanthopoulos, G.; Ghosn, D.; Kazakis, G. Investigation of the wind speed threshold above which discarded cigarettes are likely to be moved by the wind. Int. J. Wildland Fire 2006, 15, 567–576. [Google Scholar] [CrossRef]
- Project Fire Paradox. D3.1–5 Probability of Ignition Modelling in Forest Fuels—First Results. 2006. Available online: http://fireintuition.efi.int/metadata-browser.fire?i=6181ea5733188d27624eeae341b96ff7 (accessed on 19 May 2021).
- Sun, P.; Zhang, Y.; Sun, L.; Hu, H.; Guo, F.; Wang, G.; Zhang, H. Influence of Fuel Moisture Content, Packing Ratio and Wind Velocity on the Ignition Probability of Fuel Beds Composed of Mongolian Oak Leaves via Cigarette Butts. Forests 2018, 9, 507. [Google Scholar] [CrossRef] [Green Version]
- McRae, D.D.; Kellogg, D.S.; Waymack, B.E.; Fournier, L.G.; Dwyer, R.W. Modification of the NIST Test for measuring the Ignition Propensity of Cigarettes. J. Fire Sci. 2000, 18, 215–241. [Google Scholar] [CrossRef]
- Baker, R.R.; Coburn, S.; Liu, C.; McAdam, K.G. The science behind the development and performance of reduced ignition propensity cigarettes. Fire Sci. Rev. 2016, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Viegas, D.X.; Neto, L.P. Wall shear-stress as a Parameter to Correlate the Rate of Spread of a Wind Induced Forest Fire. Int. J. Wildland Fire 1991, 1, 177–188. [Google Scholar] [CrossRef]
- Costa, F.S. Natural Smouldering of Cigarettes. In Proceedings of the HT2003 ASME Summer Heat Transfer Conference, Las Vegas, NV, USA, 21–23 July 2003. [Google Scholar] [CrossRef]
- Viegas, D.X.; Gabbert, W.; Figueiredo, A.R.; Almeida, M.A.; Reva, V.; Ribeiro, L.M.; Viegas, M.T.; Oliveira, R.; Raposo, J.R. Relatório do Incêndio Florestal de Tavira/São Brás de Alportel, 18–22 Julho 2012; Centro de Estudos sobre Incêndios Florestais, ADAI/LAETA, Universidade de Coimbra: Coimbra, Portugal, 2012. Available online: http://www.portugal.gov.pt/pt/os-ministerios/ministerio-da-administracao-interna/documentos-oficiais/20121010-rel-incendio-florestal-tavira-jul2012.aspx (accessed on 19 May 2021).
- Almeida, M.A.; Viegas, D.X.; Miranda, A.I.; Reva, V. Effect of particle orientation and of flow velocity on the combustibility of Pinus pinaster and Eucalyptus globulus firebrand material. Int. J. Wildland Fire 2011, 20, 946–962. [Google Scholar] [CrossRef]


| Country | Total Number of Wildfires | Fires Caused by Smoking | Percentage (%) |
|---|---|---|---|
| Portugal [3] | 611,111 | 784 | 2.27 |
| USA [4] | 5041 | 91 | 17.73 |
| Chile [5] | 53,347 | 299 | 5.01 |
| Republic of S. Korea [6] | 570 | 51 | 9.8 |
| Ref. | Description | (mm) | (mm) | Diameter d (mm) | Initial Mass Mc (g) | Tobacco Mass MT (g) |
|---|---|---|---|---|---|---|
| 1 | NO LIP | 82 | 27 | 8 | 0.885 | 0.660 |
| 2 | LIP | 82 | 27 | 8 | 0.815 | 0.615 |
| 3 | BUTTS | 40 | 27 | 8 | 0.390 | 0.195 |
| Series | Cig. Type | Number of Tests | α (º) | ϕ (º) | U (m.s−1) | xT - | tc (s) | Rc (cm.s−1) | mc (g.s−1) |
|---|---|---|---|---|---|---|---|---|---|
| C1 | LIP | 17 | 0 | 0 | 0 | 0.02/0.15 | 351/667 | 0.007/0.13 | 0.4/0.8 |
| C2 | LIP | 15 | −90/90 | 0 | 0 | - | 405/672 | - | 0.4/1.1 |
| C3 | N/LIP | 15 | −90/90 | 0 | 0 | - | 368/618 | - | 0.9/1.2 |
| C4 | LIP | 39 | 0 | 0 | 0/4 | 0.01/0.13 | 130/530 | 0.08/0.4 | - |
| C5 | LIP | 12 | 0 | 0/180 | 0/3 | 0.05/0.11 | 160–400 | 0.08/0.36 | - |
| Series | Cig. Type | Number of Tests | U (m.s−1) | xT - | x - | ti (s) | te (s) | Pi - |
|---|---|---|---|---|---|---|---|---|
| CS1 | LIP | 104 | 0/2.5 | - | 0.05/0.14 | 32/659 | 31.5/772 | 0.69 |
| CS2 | N/LIP | 84 | 0/2.5 | - | 0.05/0.14 | 34/627 | 33.6/659 | 0.62 |
| CS3 | LIP Butts | 115 | 0/2.5 | - | 0.06/0.15 | 48/350 | 48/499 | 0.37 |
| CS4 | LIP | 16 | 0 | 0.01/0.17 | 0.095 | ∞ | 246/688 | 0 |
| CS5 | LIP | 16 | 0 | 0.095 | 0.03/0.23 | ∞ | 459/652 | 0 |
| Case | U (m.s−1) | ĖG′ (J.s−1) | m1 (g) |
|---|---|---|---|
| to a and t1 a | 1 | 0.0192 | 0.0020 |
| to b and t1 a | 2 | 0.0208 | 0.0015 |
| Case | U (m.s−1) | ĖG’ (J. s−1) | m1 (g) |
|---|---|---|---|
| ti 1 | 0 | 0.0142 | 0.01 |
| ti 2 | 2 | 0.0208 | 0.01 |
| ti 3 | 3 | 0.0192 | 0.005 |
| Case | U (m.s−1) | ĖG’ (J.s−1) | m1 (g) |
|---|---|---|---|
| ti 1 | 0.25 | 0.0157 | 0.006 |
| ti 2 | 1.25 | 0.0199 | 0.006 |
| ti 3 | 2.25 | 0.0207 | 0.004 |
| Case | x | m1 (g) |
|---|---|---|
| ti1 | 0.02 | 0.010 |
| ti2 | 0.08 | 0.010 |
| ti3 | 0.02 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viegas, D.X.; Oliveira, R.; Almeida, M.; Kim, D. Ignition of Fuel Beds by Cigarettes: A Conceptual Model to Assess Fuel Bed Moisture Content and Wind Velocity Effect on the Ignition Time and Probability. Fire 2021, 4, 35. https://doi.org/10.3390/fire4030035
Viegas DX, Oliveira R, Almeida M, Kim D. Ignition of Fuel Beds by Cigarettes: A Conceptual Model to Assess Fuel Bed Moisture Content and Wind Velocity Effect on the Ignition Time and Probability. Fire. 2021; 4(3):35. https://doi.org/10.3390/fire4030035
Chicago/Turabian StyleViegas, Domingos Xavier, Ricardo Oliveira, Miguel Almeida, and Donghyun Kim. 2021. "Ignition of Fuel Beds by Cigarettes: A Conceptual Model to Assess Fuel Bed Moisture Content and Wind Velocity Effect on the Ignition Time and Probability" Fire 4, no. 3: 35. https://doi.org/10.3390/fire4030035
APA StyleViegas, D. X., Oliveira, R., Almeida, M., & Kim, D. (2021). Ignition of Fuel Beds by Cigarettes: A Conceptual Model to Assess Fuel Bed Moisture Content and Wind Velocity Effect on the Ignition Time and Probability. Fire, 4(3), 35. https://doi.org/10.3390/fire4030035








