1. Introduction
The rapid advancement of nanotechnology has created a growing demand for nanomaterials with unique physical and chemical properties. Among the methods used to produce nanosized materials, chemical approaches (such as sol–gel synthesis and chemical deposition) and electrochemical techniques are widely employed [
1]. Physical methods, which involve substance evaporation followed by nucleation, condensation, and coagulation, are also of significant interest. In particular, laser ablation, low-pressure plasma torch synthesis, and electrical explosion of conductors have attracted considerable attention for the production of nanomaterials [
2,
3,
4].
Nanoscale oxide materials are widely employed across various fields including chemical industry, medicine, agriculture, and microelectronics. Iron oxide nanoparticles have demonstrated particularly broad applications in biomedicine and agriculture, being utilized for medical imaging and diagnostics, cancer therapy through magnetic hyperthermia, targeted drug delivery, and plant growth stimulation [
5,
6]. These nanoparticles exhibit antibacterial properties while maintaining good biocompatibility. Their ability to enhance plant growth is attributed to improved metabolic processes, increased nutrient translocation to aerial plant parts, and activation of photosynthetic pigment synthesis.
Recent years have seen growing interest in atmospheric-pressure low-temperature plasma discharges for nanomaterial synthesis [
7,
8,
9,
10]. This approach offers the advantages of simple implementation, high efficiency, and numerous adjustable parameters. Two primary methodologies exist for nanoparticle synthesis in liquids using such plasma systems: the treatment of metal salt solutions, where cations serve as nanoparticle precursors [
11,
12], and electrode erosion, where nanoparticles form from electrode material through plasma-induced evaporation and localized high temperatures [
13,
14]. By varying electrode materials and incorporating stabilizers containing thiol, amine, or carboxyl groups, researchers can control particle characteristics including composition (even producing bimetallic particles) and size distribution [
1,
15]. The synthesis of particles via electrode material erosion is advantageous as it eliminates the need for additional chemical reagents, such as precursors and reducing agents. However, the particles produced by this method are prone to rapid agglomeration in aqueous environments, necessitating the use of stabilizers to achieve controlled particle sizes.
Various plasma discharge configurations are employed for nanoparticle synthesis, including dielectric barrier discharge (DBD), microwave (RF) discharges, glow discharges, systems with liquid-submerged electrodes, and spark and arc discharges. The arc discharge technique, for instance, is utilized for nanomaterial synthesis both in the gas phase [
16,
17] and in liquid [
18,
19], primarily through the evaporation of electrode material.
While this method demonstrates high efficiency in particle generation—for example, yielding a powder formation rate of 97 mg/min for CuO/Cu
2O at a current of 4 A [
19]—the resulting particles often exhibit a relatively large size distribution (e.g., TiO
2~164.51 nm [
16]). Furthermore, the introduction of additional chemical reagents into a high-current DC arc system to control agglomeration presents a significant challenge, as the intense thermal conditions can lead to the decomposition of such stabilizers.
A comparative study of a spark discharge (f = 10 kHz, U = 10 kV) and a DBD (f = 1 kHz, U = 6 kV) for synthesizing silver nanoparticles in argon revealed distinct characteristics [
20]. Particle size measurements, conducted using a scanning mobility particle sizer on the outlet gas stream, showed that the DBD produced nanoparticles with smaller sizes, improved monodispersity, and lower number concentrations compared to the spark discharge. The number concentration of DBD-generated particles decreased significantly at elevated voltage and frequency, whereas it increased for spark-generated particles under the same conditions.
Alternative methods include nanoparticle synthesis via glow discharge plasma electrolysis using salt solutions [
21]. RF discharges are also used, though they typically require low-pressure conditions and specific precursors [
22,
23], thereby adding complexity to the process.
Notably, a literature survey conducted by the authors revealed a lack of studies on the synthesis of chelate compounds using plasma technologies. This presents a significant research gap, particularly considering the potential applications in the agro-industrial sector. Chelated forms of trace elements are known for their enhanced bioavailability and can serve as highly effective fertilizers for various crops.
When aqueous solutions are exposed to low-temperature plasma discharges in air, the process generates both short-lived and long-lived reactive oxygen and nitrogen species alongside electrode erosion [
24,
25,
26]. The reactions of primary species lead to the formation of hydrogen peroxide, nitrites, and nitrates that remain stable in solution. Numerous recent reviews have documented applications of plasma technology in agriculture, demonstrating that plasma-activated solutions can stimulate seed germination, accelerate root development, and increase biomass production [
27,
28,
29]. These effects are mediated through the activation of plant growth regulators, changes in phytohormone levels, and the induction of stress resistance mechanisms.
Stabilizing agents with thiol, amine, or carboxyl groups in the receiving liquid are known to promote the formation of smaller nanoparticles with narrower size distributions [
1]. Ethylenediaminetetraacetic acid and its disodium salt are widely employed as key additives in the synthesis of inorganic nanoparticles, serving several critical functions. EDTA is particularly noteworthy as it serves dual roles in nanoparticle synthesis as both a reducing agent [
30] and a size/structure controller [
31,
32,
33]. The disodium salt form presents additional interest due to its potential metal ion absorption capacity from plasma-treated solutions.
Furthermore, novel anti-cancer agents [
34] are being developed based on iron–EDTA complexes, which catalyze the Fenton reaction to significantly enhance the production of reactive oxygen species (ROS) within tumor cells.
Beyond their primary metal-chelating function, agents like EDTA can perform auxiliary roles in plasma environments. For instance, the antibacterial effect of surface micro-discharge (SMD) plasma was enhanced by the addition of EDTA [
35]. In a separate application, chelating agents were shown to improve the dispersion of Ni species in a catalyst, thereby exposing more active sites. This improved dispersion, achieved using a dielectric barrier discharge (DBD) reactor, enhanced the catalyst’s reactivity in the dry reforming of methane reaction [
36].
This study investigates the effects of an underwater plasma discharge on solutions containing ethylenediaminetetraacetic acid (EDTA) and its disodium salt. The research explores two primary objectives: (1) the controlled synthesis of metallic nanoparticles through electrode erosion, and (2) evaluating the potential of the treated solutions as liquid fertilizers, owing to their content of chelated iron and hydrogen peroxide. To achieve this, a discharge was generated within bubbles of argon injected between stainless-steel electrodes [
26,
37,
38,
39]. Multiple electrode pairs were employed simultaneously to enhance the production of reactive oxygen species (ROS) and the metal content in the liquid. By varying the concentrations of EDTA and its disodium salt, we aim to identify the key physicochemical processes and determine the optimal conditions for both nanoparticle synthesis and fertilizer production, exploring previous research [
40].
2. Materials and Methods
The hydrodynamic radius of the particles was determined by dynamic light scattering (Zetasizer ULTRA (Malvern Panalytical Ltd., Malvern, UK)). In the study, a spectrophotometer HACH LANGE DR-5000 (HACH LANGE GmbH, Düsseldorf, Germany) was used for recording absorbance spectra. AvaSpec-DUAL spectrometers (spectral ranges 219–381 and 379–521 nm, average optical resolution 0.12 and 0.1 nm), StarLine AvaSpec-ULS2048CL (spectral range 517–748 nm, average optical resolution 0.3 nm) were used to record emission spectra. The conductivity and pH of the medium were determined using a SevenExcellence multichannel meter (Mettler Toledo, Greifensee, Switzerland). A JEM 2100 transmission electron microscope (TEM) (JEOL, Tokyo, Japan) was used to study the structure of eroded particles. The accelerating voltage of 200 kV was applied. Scanning electron microscopy was performed using a JSM-6480LV (JEOL, Tokyo, Japan).
The concentration of +3 metal ions was determined by the absorption spectrum of orange solution at a wavelength of 560 nm, using XO (xylenol orange) reagent [
41].
The concentration of H
2O
2 was determined by the reaction of H
2O
2 and titanium sulfate Ti(SO
4)
2, using a spectrophotometer. The concentration of H
2O
2 could be determined from the absorbance at a wavelength of 410 nm of the yellow solution of H
2TiO
4 [
42].
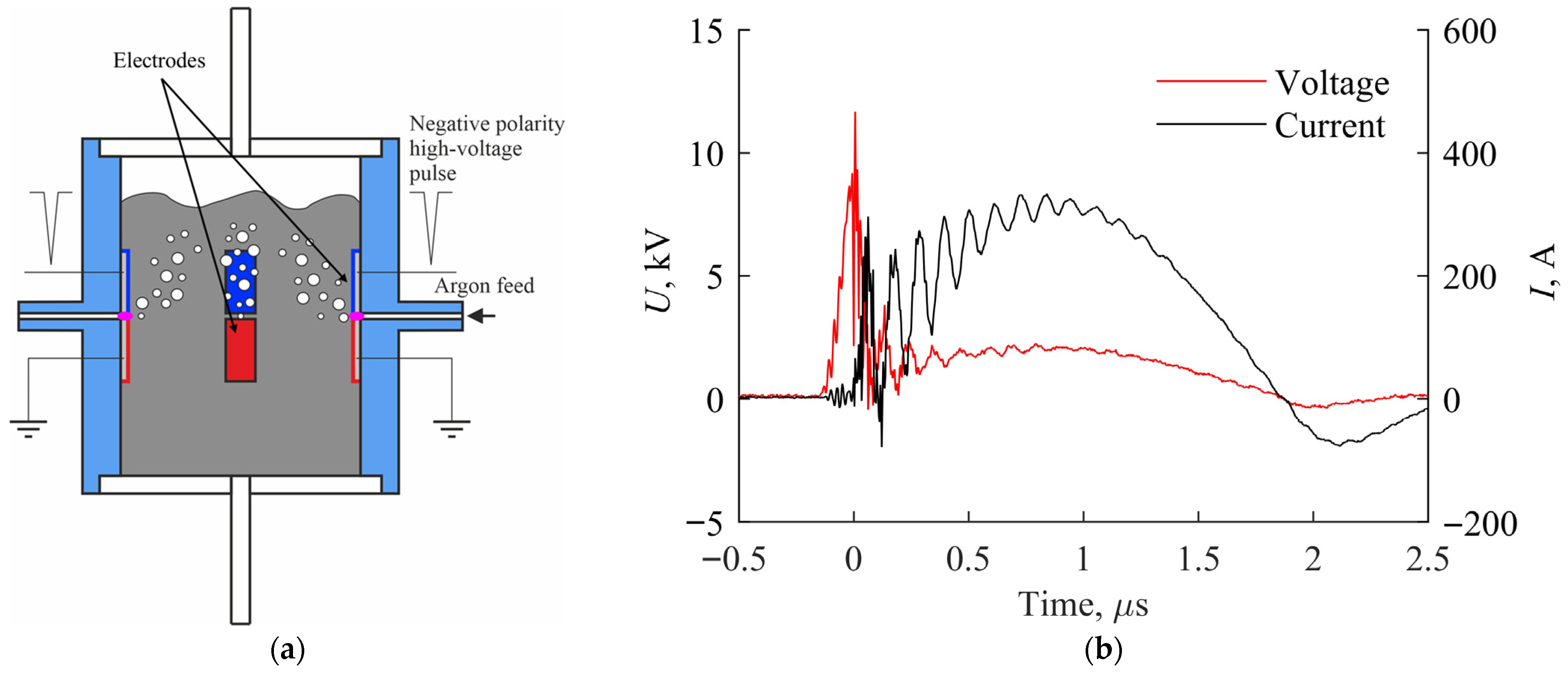
The study employed a high-voltage pulsed-periodic multielectrode ring discharge system in liquid with gas injection between electrodes, capable of delivering total power up to 1000 W [
26,
37,
38]. The discharge chamber consists of a dielectric ring-shaped tube housing four electrode pairs arranged circumferentially, with a 2 mm interelectrode gap for each pair. The high-energy pulses (up to 1 J, ~2 μs duration) enable efficient energy transfer to the liquid, facilitating rapid reaction kinetics and significant alterations in the thermodynamic and chemical parameters of aqueous solutions. The system in
Figure 1a operates with an applied voltage amplitude U~20 kV, while during established spark channel conditions U~2 kV with current I~300 A at a pulse repetition frequency f = 50 Hz (
Figure 1b).
The experiments utilized stainless-steel electrodes with high iron content (>66%), alloyed with Cr (17–19%), Ni (9–11%), and trace amounts (<1%) of Si, Cu, Ti, C, P, Mn, and S. Argon served as the injected gas, with a 6 L/min flow.
The modular design allows for straightforward scaling, capable of processing up to 600 mL liquid simultaneously—each chamber holds 120 mL, with provisions for connecting up to five chambers or implementing continuous flow operation. By selecting appropriate electrode materials and injected gases, the system can be tailored to produce specific chemical compositions for diverse applications.
For particle composition variation, experiments employed a single discharge chamber to treat 120 mL aqueous solutions for 10 min. Test solutions included EDTA (0.3 and 3 mM) and disodium EDTA salt (0.3, 3, and 30 mM), along with deionized water as the control.
3. Results
The experimental spectra were acquired by averaging over at least 50 discharge events in frequency-resolved mode. The emission spectra in
Figure 2 display characteristic atomic lines of Fe I, Fe II, Ni I, Cr I, Cr II, Ar I, and Ar II, indicating the presence of metal vapors from the electrode material, which are subsequently introduced into the liquid. The absence of molecular bands is consistent with rapid molecular dissociation, a phenomenon reported in previous studies under similar discharge conditions [
43,
44].
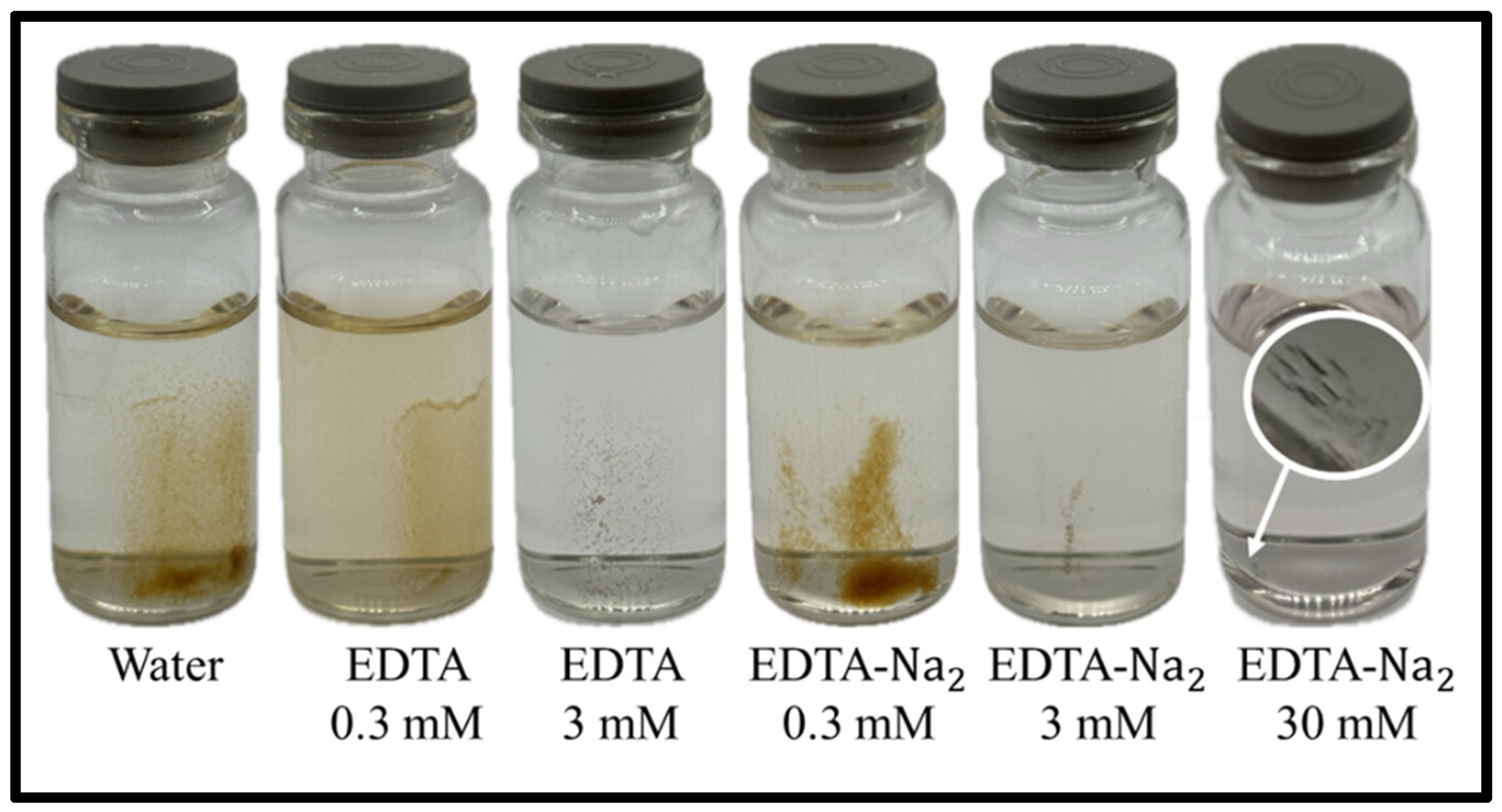
Figure 3 displays the samples obtained in this study, which visually exhibit different precipitate morphologies and quantities. The circled area on the right sample is a magnified view of the particles pointed to by the arrow.
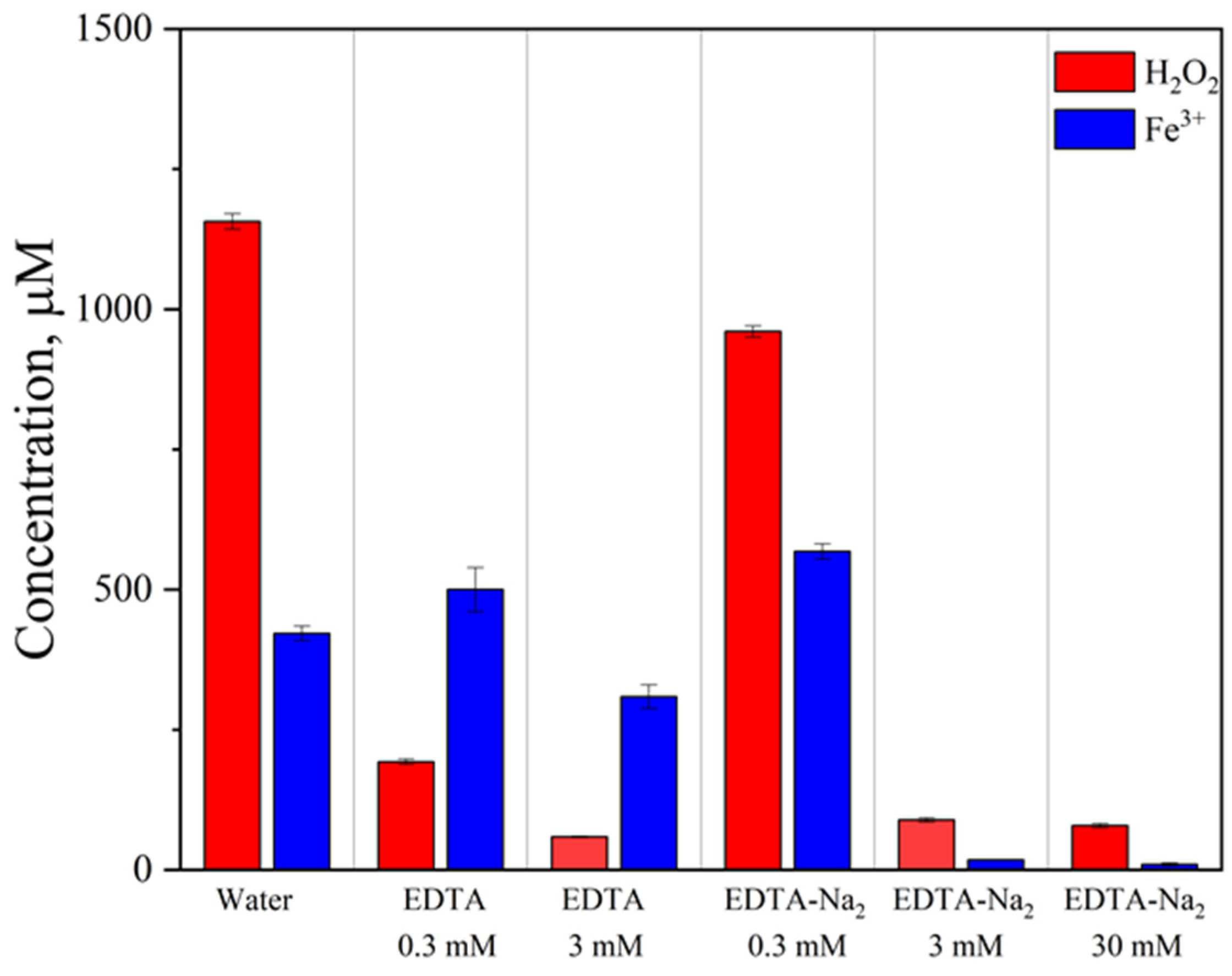
The hydrogen peroxide concentration generated in deionized water reached 1200 μM. The formation of H
2O
2 is primarily attributed to the recombination of hydroxyl radicals (•OH + •OH → H
2O
2). When using EDTA and EDTA-Na
2 solutions, the hydrogen peroxide concentration decreased with increasing solute concentration. This reduction is likely due to either competing oxidation reactions (e.g., Fe(II)-EDTA + H
2O
2 → Fe(III)-EDTA + OH
− + •OH) or the formation of Fe(II)-EDTA-H
2O
2 complexes [
45].
Notably, in a 30 mM EDTA-Na
2 solution, the detectable H
2O
2 concentration was significantly lower, suggesting either the formation of a stable complex with strong intermolecular bonds or the catalytic decomposition of hydrogen peroxide. The increase in EDTA-Na
2 concentration also resulted in decreased concentrations of detectable Fe
3+ ions (
Figure 4), which is consistent with the formation of stable Fe-EDTA complexes. The resulting iron chelate is highly stable, preventing the detection of free Fe
3+ ions using xylenol orange (XO) [
46].
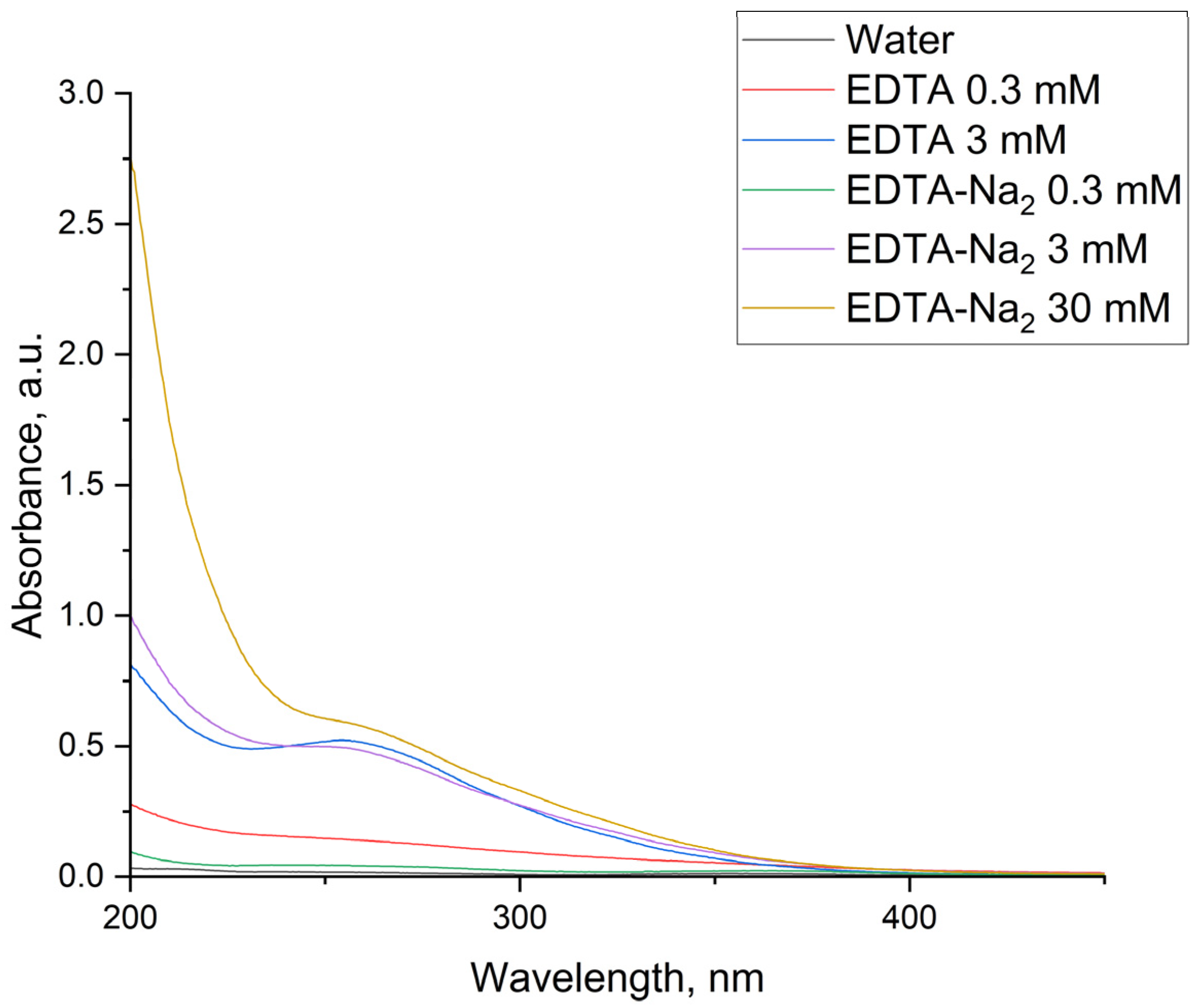
Figure 5 presents the absorption spectra of treated liquids, with all samples diluted 8-fold. A distinct absorption band at 260 nm is clearly visible. According to [
47], this spectral feature is characteristic of Fe(II)-EDTA/Fe(III)-EDTA complex formation. Notably, this complex only forms in high-concentration solutions (3 mM EDTA and 3/30 mM EDTA-Na
2).
Table 1 shows the change in pH and conductivity of treated solutions (number 1 means samples before plasma treatment, number 2 means samples after plasma treatment). The observed increase in the specific electrical conductivity of deionized water during treatment is associated with the introduction of iron ions (Fe
2+/Fe
3+) into the solution due to the erosion of a steel electrodes.
When EDTA and EDTA-Na2 solutions are used, the contribution of electrochemical erosion becomes more substantial. This is facilitated by the high initial ionic strength of these solutions, whose conductivity ranged from ~50 to ~3500 μS/cm. A key finding is the decrease in solution conductivity after treatment: significant for the acidic form of EDTA, and slight for its disodium salt.
In the case of the acid, this fact can be observed due to the chelation reaction Fe2+ + H4EDTA → [Fe·EDTA]2− + 4H+, which does not lead to a net release of mobile H+ ions. Instead, the protons are buffered by the EDTA system, protonating other EDTA species. These complexes replace highly mobile ions (H+) with larger, less mobile complex ions, resulting in a net decrease in ionic mobility and overall conductivity. Moreover, hydrogen ions can absorb hydroxyl ions formed by plasma exposure with the formation of water.
In the case of Na
2H
2EDTA, the chelation of iron ions proceeds via an exchange reaction. The reaction can be described as follows:
In exchange for the metal ion bound in the complex, sodium ions are released, due to which the conductivity of the solution decreases slightly, which also indirectly confirms the formation of chelate compounds.
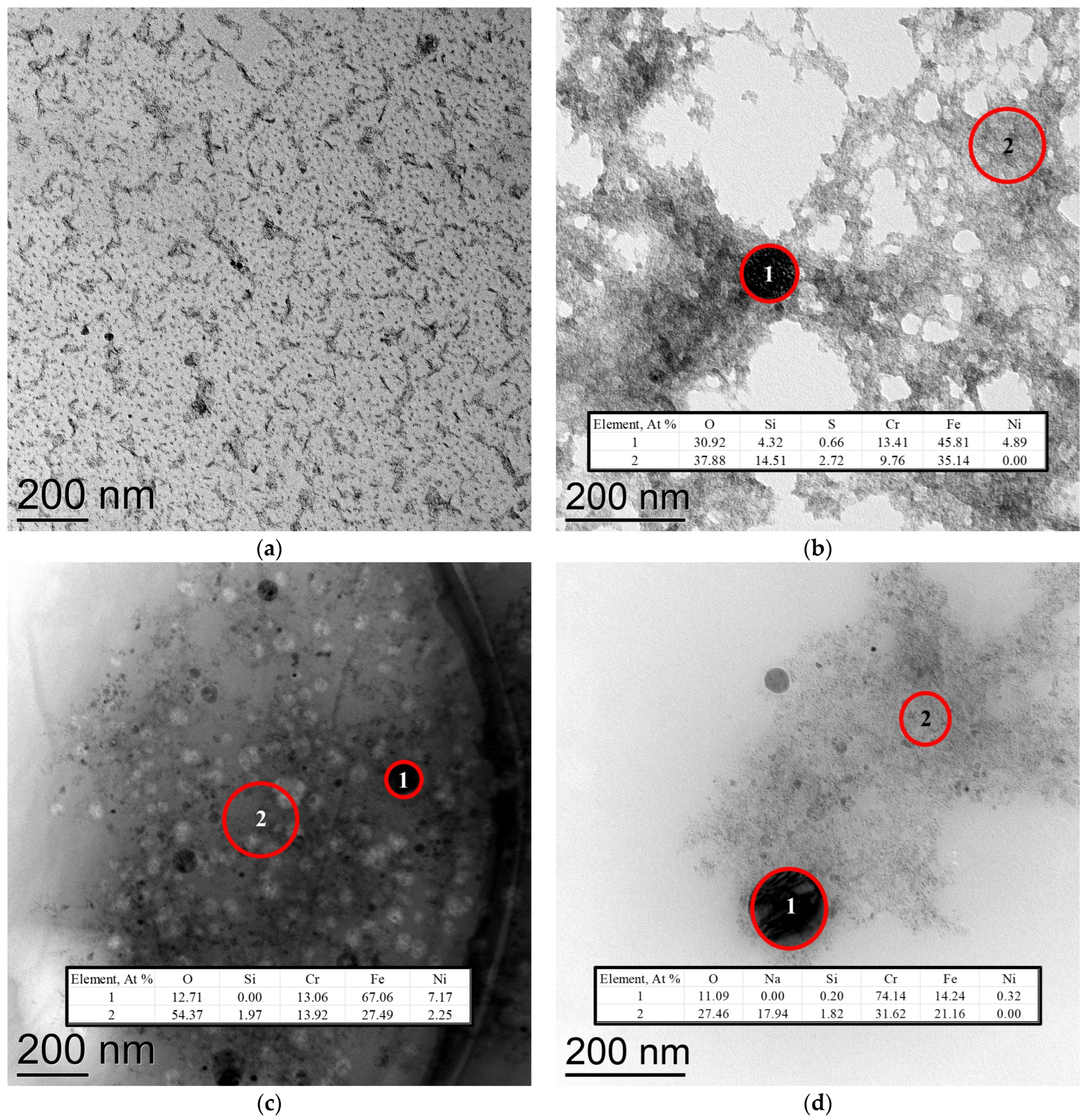
Figure 6 presents transmission electron microscopy (TEM) images. The micrograph of the sample obtained in the case of the deionized water treatment (
Figure 6a) shows particles visually similar to Fe(OH)
3, as reported in [
48]. Energy-dispersive X-ray spectroscopy (EDX) data are also provided for cases
Figure 6b–d.
On the corresponding EDX spectra for samples c and d, spurious impurity signals (Mo, Zn, K, Cl), which cannot be present in the plasma–liquid system, have been removed. It is important to note that this analysis technique is not highly reliable for light elements.
Nevertheless, the formation of spherical dark particles is clearly observed upon treatment with all three solutions. In the cases of exposure to 0.3 mM EDTA-Na2 and 3 mM EDTA, these particles contain a significant amount of iron. For the EDTA sample, white particles are also visible in the background, likely residues of undissolved EDTA.
In contrast, after treatment with 30 mM EDTA-Na2, the dark aggregates consist primarily of chromium with a low iron concentration (perhaps, composite particle). This suggests the most efficient chelation of iron ions, preventing their precipitation and leading to the predominance of chromium-based phases.
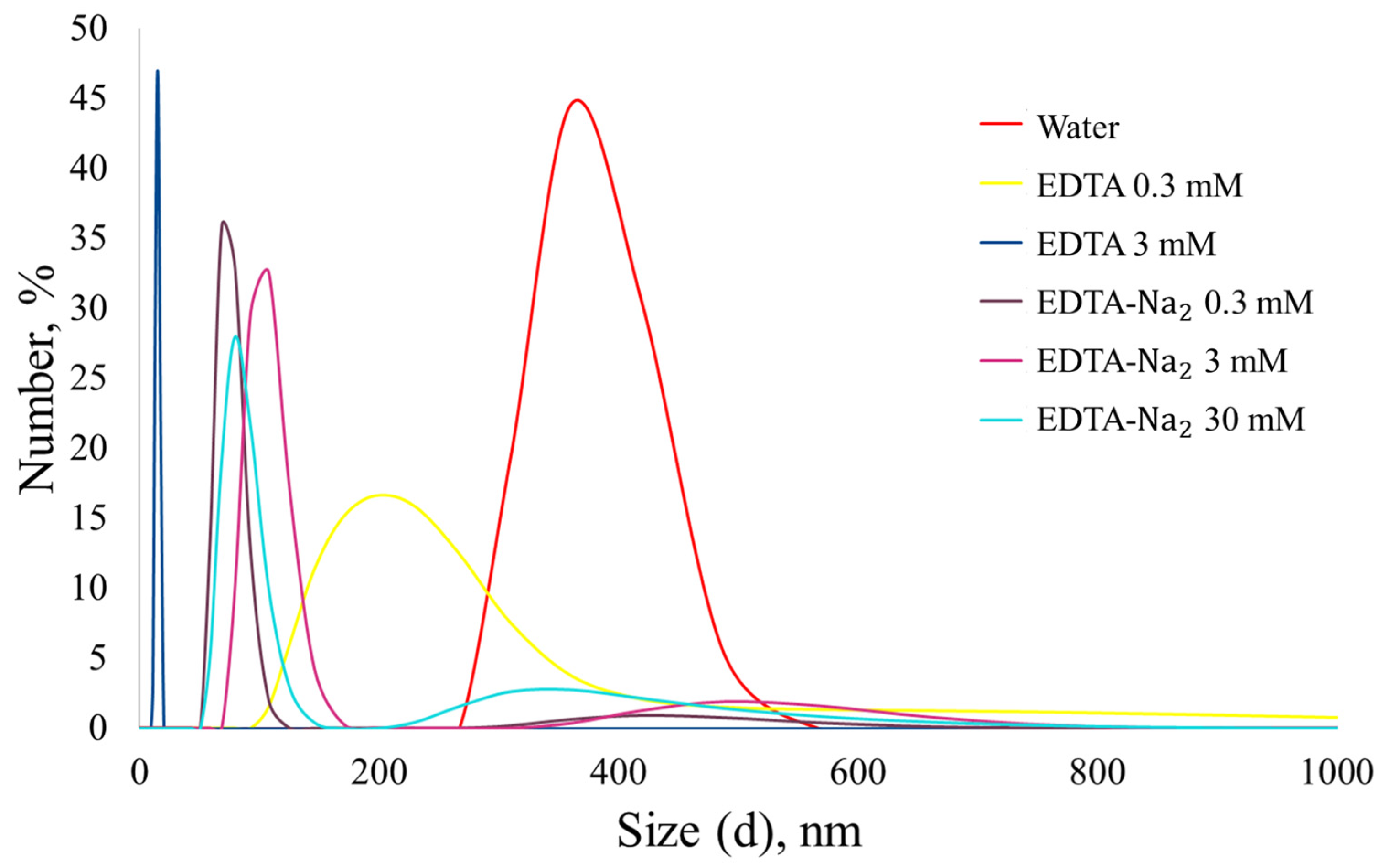
Figure 7 shows the particle size distributions obtained after preliminary ultrasonic treatment. It is worth noting that the smallest particle size was achieved using 3 mM EDTA; however, a previous study [
40] without the use of ultrasound reported a different result, which may indicate poor stabilization of nanoparticles when only using EDTA.
Furthermore, the precipitates obtained from the water-treated sample and the magnetic fraction (separated using a neodymium magnet) from the 3 mM EDTA-treated sample were investigated using scanning electron microscopy (SEM). The results presented in
Figure 8b indicate that the magnetic fraction from the sample treated with 3 mM EDTA mostly consists of spherical particles. Such spherical particles are also present in
Figure 8a in the discharge-treated sample of deionized water, but they are smaller and scarce. In each sample, a small fraction of the precipitate exhibits a very rapid response to a strong neodymium magnet.
4. Discussion
Typical erosion rate for the stainless-steel electrode system (for the whole set of four pairs or total eight pieces) is 1.5 mg/min for the treatment of deionized water. For the 60 min total time of sample treatment in this study, this results in a total mass loss of 90 mg. For typical AISI 304 stainless steel densities of 8 g/cm3 and with each electrode surface measuring 2 × 8 mm2, this erosion corresponds to a ~0.18 mm change in the electrode gap length for each electrode pair. It should be noted that the change in electrode shape is not in the form of a flat layer but rather manifests as more material eroded in the middle of the frontal face. However, such changes should not drastically affect the discharge parameters.
Further questions arise: Is it valid to use erosion rates obtained in the case of deionized water treatment for the case of EDTA or EDTA-Na2 solutions? How could the presence of EDTA or EDTA-Na2 in the liquid possibly change the parameters of the discharge so that the erosion rate would be different from that for deionized water treatment?
(1) EDTA and EDTA-Na2 create elevated electrical conductivity that has previously been found to affect the breakdown delay for the multispark discharge system: although the discharge is believed to run between electrodes inside a gas bubble, there are seemingly currents through liquid which can lead to energy losses and electrolysis.
(2) Discharge in contact with liquid can introduce solvated species (or products of their destruction) into the gas phase, thus changing gas composition and discharge parameters.
The effect of electrical conductivity on the breakdown delay or even on the breakdown voltage would likely not significantly affect the erosion rate, as it relates mainly to the short breakdown phase rather than to the 1.5 μs spark phase, when most of the energy from the power source is delivered. The spark phase becomes more important, and the breakdown phase less so, if we assume that the main mechanism of erosion is thermal—due to the Ohmic heating of localized spots where the spark channel is in contact with the electrode surface (where current density is maximal) and due to heat flow from the hot gas in the discharge channel. The maximum current is 300 A, and the peak gas temperature is achieved during the spark stage rather than during breakdown.
The possible contribution of electrolysis to the total erosion rate is a more uncontrollable factor but is definitely more relevant for the samples with the highest electrical conductivity, in our case, the 30 mM EDTA-Na
2 solution and electrical conductivity 3515 μS/cm. As was shown for various discharges where electrodes are in contact with liquid, the electrolysis can make a significant contribution to the total erosion [
49]. However, in the multispark discharge configuration the electrode gap is just after the gas line orifice, and thus most of the open frontal surface of each electrode is presumably not in contact with liquid. It would be worthwhile in further experiments to check the total erosion rate via mass measurements of electrodes, at least for the 30 mM EDTA-Na
2 sample, in order to confirm or reject the role of electrolysis.
It was found for discharges with a liquid cathode that solvated species move into the gas phase, including in the discharge zone [
50]. Ion acceleration in the cathode layer and following sputtering of the liquid surface is a proposed mechanism. It would also not be surprising to find originally solvated species or products of their decomposition in the gas phase for other types of discharges where plasma is in contact with liquid. However, in the case of the multispark discharge configuration, the spark channel is between the electrodes in a flow of argon and not in direct contact with liquid. Thus, another mechanism should be proposed, e.g., the action of shockwaves during breakdown on the liquid surface, resulting in aerosol formation. At the same time, gas in the electrode gap is renewed between subsequent high-voltage pulses: a 6 L/min argon flow rate branched into four channels, assuming each bubble diameter is in the range of 5–10 mm, yields 1–7 subsequent bubbles formed between neighboring high-voltage pulses. This does not allow continuous accumulation of originally solvated species in the gas phase, but does not fully exclude their presence. If these species are present in the discharge zone anyway then they should be fully dissociated—as mentioned above, there are no molecular bands in the optical emission spectra of the discharge (
Figure 2), and this is typical for the spark stage of high current discharges [
44]. So, the plasma will be enriched with new elements from EDTA or EDTA-Na
2 such as C, N, and Na (H and O are already present in the plasma from water molecules). The change in gas composition can affect plasma/gas temperatures and/or discharge current. Thus, it is definitely necessary that the emission spectra specifically for the cases of EDTA and EDTA-Na
2 solutions treatment be registered in a dedicated experiment, at least to establish the presence or absence of C, N, Na lines.
The high intensity of the H
α line (
Figure 2b) indicates higher concentrations of water molecules in the discharge region than would be expected from the initial water vapor content in argon or from the concentration of water vapor due to its diffusion from the liquid–gas interface against the argon flow direction (directly into the discharge channel). Two mechanisms are likely responsible for the increased water concentrations in the discharge region: (1) intense liquid evaporation enhanced by the plasma heat flux during the developed spark stage; and (2) the action of shockwaves generated during the breakdown stage on the liquid surface, leading to aerosol formation and its subsequent introduction into the spark channel. It should be noted that the exact distance from the spark channel to the liquid surface is not constant. It can vary from pulse to pulse, both due to the displacement of the liquid–gas interface during bubble formation and due to the shifting of the spark channel contact points with the initially flat electrode surface.
In the 30 mM EDTA-Na2 solution, the precipitate consisted of the single fraction that moved rapidly under the action of a strong neodymium magnet. In the EDTA 3 mM discharge-treated water, the precipitate consisted of two distinct fractions: one moved rapidly under the action of a strong neodymium magnet (exactly the same as in the EDTA-Na2 30 mM sample) and the other was a somewhat blurry, grayish fraction that moved slowly.
Let us assume that the second “blurry, grayish” fraction in the EDTA 3 mM treated sample is Fe-Cr hydroxide (Fe
x, Cr
1−x)(OH)
3. Fe(III) and Cr(III) are able to form hydroxides in water [
51,
52,
53]. This hydroxide has very low solubility and precipitates easily. If the Fe/Cr ratio is strongly in favor of Fe, then at least a “slow” reaction on the neodymium magnet would not be surprising. But at least some reaction would be observed, rather than in the case of just a non-magnetic hydrolyzed Cr(III). H
2EDTA
2− from the dissolution of the disodium salt of EDTA in water and at pH 5 has faster kinetics of Cr(III) complexation than H
4EDTA from the dissolution of EDTA itself at pH 3 [
54]. The same is true for Fe(III) and means that there are less possible reagents for Fe-Cr hydroxide formation. That is why in the discharge-treated EDTA-Na
2 3 and 30 mM such a fraction is not present. But if Cr(III) is not complexed by EDTA quickly, then stable Fe-Cr hydroxide is formed and for H
4EDTA, even at pH 3.0, the kinetics of Fe(III) complexation will be very slow. But long-term, it will ultimately undergo complexation with EDTA. When reexamination of the samples was performed after a few months of storage, there was only a single fraction of precipitate—the fraction with the strong reaction on the magnet.
This strong magnetic fraction is likely to be the solidified droplets of Fe-Cr-Ni alloy, and the following facts are in favor of this:
(1) In 30 mM EDTA-Na2, only such solid micro-particles of zero valent metal could remain non-complexed by the ligand and have such a magnetic response;
(2) EDX data for the micrograph in
Figure 8b do not contradict this, as they show that all three metals in the probing spots have a ratio close to the initial AISI 304 stainless steel but slightly different;
(3) The spherical form of particles in
Figure 8b is characteristic of melt droplets, the formation of which is common for high-current discharges;
(4) Such magnetic particles are present in all samples (small spherical particles can be seen also in
Figure 8a), implying a possible universal physical rather than chemical process of origin.
Some questions arise as to why the magnetic fraction was not dissolved and metals from it were not complexed after a few months, e.g., for the EDTA 3 mM sample, where the pH was still around 3 even after discharge treatment, and for EDTA-Na2 30 mM, where EDTA was evidently in excess.
All of the above allows us to assume that metals enter the liquids via two major pathways: from the gas—atoms, clusters, or solid nanoparticles (spectra in
Figure 2 strongly suggest this); or from the molten material of the electrodes in the form of micro- or even nano-droplets. While the role of the former in complex formation and the Fenton reaction is already understood, the potential role of neutral metal particles (Fe-Cr-Ni alloy) is not as straightforward. Surface reactions on neutral iron particles are possible, involving their oxidation to Fe(II) and the formation of superoxide radicals, followed by a Fenton reaction [
55]. The generation of OH radicals in the Fenton cycle may lead to the degradation of both free EDTA molecules and pre-formed iron–EDTA chelates. However, the Fenton reaction proceeds at substantial rates optimally at pH 2–3. Therefore, this effect is likely most significant for the 3 mM EDTA samples, where the Fenton reaction involving Fe(II)-EDTA is already feasible.
The composite Fe-Cr-Ni oxides–hydroxides were also obtained in a discharge with two electrodes submerged in an aqueous tetracycline solution, where one electrode was iron and the second was made of a nickel–chromium alloy. The resulting Fe
2O
3-Ni-Cr layered double-hydroxide composite exhibited magnetic properties [
56].
Another factor potentially influencing the result is the photoreduction of Fe(III) to Fe(II) by UV radiation, followed by a photo-Fenton reaction. This process may also include the reduction of iron within Fe(III)-EDTA complexes [
57]. Furthermore, the generation of UV radiation in a multispark discharge has already been demonstrated [
58].
The reaction Fe(II)-EDTA + H
2O
2 → Fe(III)-EDTA + OH
− + •OH is the Fenton reaction and a part of the cycle, along with the reaction Fe(III)-EDTA + H
2O
2 → Fe(II)-EDTA + HO
2•. The hydroxyl radical •OH is a non-selective strong oxidant that oxidizes most organic compounds. Thus, in the absence of targeted organic compounds •OH will degrade EDTA or Fe-EDTA complexes into low molecular weight acids and CO
2 [
55].
This process of EDTA degradation, along with the irreversible consumption of H
2O
2, will work to stop the cycle. However, the Fenton reaction has faster kinetics at a pH of lower than 4, and should be more important for the EDTA 0.3 and 3 mM samples than for any EDTA-Na
2 samples. This could possibly explain the much lower H
2O
2 concentration in the EDTA 0.3 mM sample than in the deionized water sample or in the EDTA-Na
2 0.3 mM sample (
Figure 4). Another channel of EDTA degradation could be •OH radicals generated by the multispark discharge itself. Then, less •OH radicals would be available for the reaction •OH + •OH → H
2O
2. The estimation of the maximal concentration of metals in the Fe-Cr-Ni ratio of the treated samples for AISI 304 and at a typical erosion rate for the AISI 304 stainless-steel electrodes yields
CFe = 1477 μM,
CCr = 457 μM, and
CNi = 234 μM. Thus, 3 mM concentrations of EDTA and EDTA-Na
2 should be enough to bind all metal ions, but the unknown EDTA degradation rate by the •OH radicals makes this uncertain. In the case of EDTA solutions, most of the Fe should be in the Fe(III) state, as the initial pH favors the Fenton reaction, which tends to convert all Fe(II) into Fe(III) given enough H
2O
2. The contribution of dissolved oxygen in water to the oxidation of Fe should be negligible, since preliminary sparging with argon is performed before the discharge starts and continuously during the treatment. The lowest values of free Fe(III) in the EDTA-Na
2 3 and 30 mM samples mean the highest values of formed Fe-EDTA complexes, confirmed by UV-vis absorption spectra (
Figure 5) and indirectly by the pH increase that is expected at complexation. A possible explanation is the higher activity of H
2EDTA
2− as a ligand in the EDTA-Na
2 water solution rather than H
4EDTA in the water solutions of EDTA itself.
The study demonstrates the fundamental possibility of varying the ratios of metal fractions formed during electrode erosion by adding chelate compounds.
This may be useful both for the purpose of obtaining a dominant fraction for nanoparticle synthesis and for considering the formation of metal ion complexes with EDTA for agriculture, with a reduction in solid compounds.
Numerous research groups have successfully utilized plasma-based techniques for the synthesis of nanoparticles, both through the direct treatment of inorganic metal salts and via electrode erosion in liquid and gaseous environments. These nanoparticles are further employed in the creation of functional composite materials [
59,
60,
61].
However, a common observation across these studies is the formation of a broad spectrum of phase compositions. For instance, when using iron electrodes, the resulting products typically consist of a mixture of various iron oxides alongside metallic (zerovalent) iron. A significant and ongoing challenge in this field lies in achieving precise control over the erosion process to selectively produce a dominant fraction of a desired phase. Furthermore, effective strategies are required to stabilize the synthesized nanoparticles and inhibit their agglomeration.
Crucially, this control can be exerted by carefully managing the plasma parameters and discharge conditions. For example, the influence of an external magnetic field on an impulse underwater discharge and its subsequent effect on the synthesis of iron oxides was investigated in [
62]. The authors demonstrated that not only were the discharge properties altered, but the orientation of the applied magnetic field directly influenced the resulting crystal structure, leading to the preferential formation of different Fe
2O
3 polymorphs (synergies).
In a complementary study [
63], control was achieved by varying the discharge current in an underwater system. The authors reported a distinct phase transition: at a lower current of 0.25 A, the predominant phases were FeO and magnetite Fe
3O
4, whereas at a higher current of 0.8 A, the phase composition shifted towards various Fe
2O
3 polymorphs. This further underscores the potential of tuning discharge parameters to steer the phase outcome of plasma-assisted synthesis.
Another critical aspect of nanoparticle synthesis via gas discharge processing is the influential role of short- and long-lived reactive oxygen and nitrogen species (RONS). These species, generated in the plasma plume, can participate in reactions directly in the gas phase or dissolve into the liquid to subsequently interact with dissolved metal precursors. The chemical environment of the solution, such as its pH, is a key factor governing these processes. For instance, pH has been shown to significantly affect gold nanoparticle formation by modulating the reduction in precursor ions caused by both solvated electrons and hydrogen peroxide (H
2O
2), ultimately determining the final particle size [
64].
Beyond the chemistry of the solution, the reduction mechanism itself is critically dependent on the interplay of various plasma-generated agents. Recent research has demonstrated the paramount importance of short-lived reducing radicals in conjunction with H
2O
2 for an efficient reduction in tetrachloroaurate ions (AuCl
4−) [
12]. The proposed reaction model suggests that H
2O
2 enables an autocatalytic surface growth mechanism. This process, occurring on a timescale of approximately one second, significantly enhances the overall reduction rate compared to systems relying solely on direct reduction by solvated electrons [
12].
5. Conclusions
We have developed a rapid one-pot process based on a multispark discharge for the synthesis of iron chelates in aqueous solutions. The resulting products simultaneously contain reactive oxygen species (ROS) and metal-containing nanoparticles, using only EDTA and EDTA-Na2 as chemical reagents.
The investigation reveals two primary pathways for metal delivery into the solution: (i) via the gas phase as atoms, clusters, or solid nanoparticles, confirmed by optical emission spectroscopy; and (ii) through the erosion of electrode material, forming micron- and submicron-sized droplets of the Fe-Cr-Ni alloy. While the role of ionic metal species in complex formation and Fenton reactions is well-established, the presence of neutral, magnetic metal particles introduces an additional, complex factor to the system’s chemistry. These particles may act as surfaces for catalytic reactions, potentially initiating the oxidation of iron and the generation of superoxide radicals, thereby contributing to the Fenton cycle.
The composition and stability of the resulting products are highly dependent on the solution conditions. The use of free acid EDTA at a low pH (2–3) favors the Fenton reaction, leading to the significant generation of hydroxyl radicals. These radicals, in turn, contribute to the degradation of both free EDTA molecules and pre-formed complexes, as well as the consumption of H2O2, explaining the lower observed peroxide concentrations in these samples. In contrast, solutions of EDTA-Na2 at a higher pH promote more efficient complexation due to the higher ligand activity of H2EDTA2−, resulting in higher yields of stable Fe-EDTA complexes and a single, magnetic fraction of precipitate.
The identified magnetic fraction, consistent across all samples, is attributed to solidified droplets of the stainless-steel electrode material. Its spherical morphology, elemental composition, and strong magnetic response point to a physical erosion mechanism (melting and sputtering) inherent to the high-current spark discharge, rather than a chemical precipitation process. The persistence of this fraction, even in the presence of excess ligand, underscores its chemical stability and potential role as a long-term catalytic agent.
Key factors influencing the process’s efficiency and chemical pathways include the electrical conductivity of the solution, which affects breakdown initiation and electrolysis; the introduction of solvent-derived species (C, N, Na) into the plasma, altering its properties; and the intense evaporation and aerosol formation driven by plasma heat flux and shockwaves, ensuring the transport of water and reactants into the discharge zone. Furthermore, the confirmed generation of UV radiation in such discharges suggests the potential contribution of photo-Fenton reactions, adding another layer to the complex reaction network.
To summarize, EDTA and EDTA-Na2 addition allows us to control the ratios of iron chelates and iron oxide nanoparticles in the treatment of water solutions with underwater gas discharge in injected argon.