Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) often causes serious infections in hospitals. Vancomycin is widely accepted as the standard therapy for MRSA infection, but its widespread use has resulted in the generation of vancomycin-resistant S. aureus (VRSA). To reduce the potential risk of MRSA and VRSA emergence in aquatic environments, we investigated the degradation of methicillin and vancomycin by cold atmospheric pressure plasma jet (APPJ) irradiation using N2, O2, and CO2 gases. The concentrations of methicillin and vancomycin in distilled water were decreased in a time-dependent manner by the plasma jet irradiation; that is, compared with the pre-treatment levels, the concentrations of methicillin and vancomycin were reduced by 20 to 50% after plasma jet irradiation for 10 s. No methicillin and vancomycin signals were detected after 300 s irradiation. Reactive species generated from the plasma jet electrophilically attacked and fragmented the antibiotic molecules. The present method realizes direct plasma ignition in a solution, and therefore, the reactive species can easily react with antibiotic molecules. In addition, plasma can be generated from various gas species that are abundant in the atmosphere. Therefore, cold APPJ irradiation can be a powerful, cost-effective, and environmentally friendly means for the treatment of antibiotics in aqueous samples.
1. Introduction
The discovery of penicillin has paved the way for the isolation of a huge number of antibiotics from microorganisms and their synthesis in an effort to overcome infectious diseases. However, the indiscriminate use of antibiotics has resulted in the emergence of antimicrobial-resistant microorganisms in the body and the environment. Methicillin-resistant Staphylococcus aureus (MRSA), which shows resistance to β-lactam antibiotics, has been isolated worldwide [1]. Vancomycin, a glycopeptide antibiotic, is the standard therapy for MRSA infection. Recently, however, the emergence of MRSA less susceptible to vancomycin (vancomycin-intermediate Staphylococcus aureus; VISA) or resistant to vancomycin (vancomycin-Resistant Staphylococcus aureus; VRSA) has been reported [2]. The spread of infections caused by such antimicrobial-resistant bacteria in hospitals [3] or their release from hospitals into the environment [4,5] has become a serious public health concern. Diwan et al. have reported that the amount of new quinolone antibiotic ciprofloxacin used in hospitals is clearly correlated with its concentration in wastewater [6]. Zhang et al. have reported a positive correlation between the number of antimicrobial-resistant Escherichia coli (E. coli) and the antimicrobial concentrations in a river [7]. Those findings indicate that the release of antibiotics into the environment has a potential risk of generating antimicrobial-resistant microorganisms. In addition, horizontal gene transfer may lead to the emergence of bacteria with multiple antibiotic resistance genes. Indeed, it is reported that 86% of bacteria detected in wastewater containing antibiotics have more than 20 antibiotic resistance genes, and 95% of bacteria contain at least one mobile genetic element [8]. In a WHO report published in 2014, the increase in the number of deaths associated with antimicrobial resistance (AMR) was seriously considered, and coordinated global actions to mitigate AMR were established [9]. Thus, AMR is an urgent and serious public health threat that should be addressed in the 21st century, where infectious diseases abound.
Antibiotics are released into the environment, such as surface water and soils, through human and animal excreta, effluents and wastes from pharmaceutical manufacturing plants [10,11,12,13], and the use of antimicrobial pesticides in agriculture [14] and aquaculture [15]. Despite the global use of antibiotics, few wastewater treatment plants (WWTPs) are available. Furthermore, it has been reported that the removal efficiency of antibiotics was inadequate in a WWTP [16]. As a result, various kinds of antibiotics have been detected in the aquatic ecosystem, such as surface water [17,18], groundwater [19,20], seawater [21], and drinking water [22,23]. The half-lives of antibiotics in aquatic environments range from a few minutes to a few dozens of days [24]. However, a constant amount is present in the environment because of the continuous discharge of antibiotics, resulting in a high risk of developing resistant strains.
Several treatment processes, such as biodegradation, filtration, coagulation, flocculation, sedimentation, adsorption, and oxidative degradation, have been developed to minimize the release of antibiotics into the environment [25]. Biodegradation in activated sludge is effective for the treatment of organic matter in wastewater [26]. However, the presence of high concentrations of contaminants, including antibiotics, can easily affect the biological activity of degrading antibiotics. On the other hand, filtration, coagulation, flocculation, sedimentation, and adsorption include a potential disadvantage of generating solid waste containing undegraded antibiotics [25]. Oxidation by chlorine dioxide or chlorine is effective in degrading β-lactam antibiotics [27,28]. However, the reactivity may decrease as the matrix of the effluent becomes more complex [27]. In addition, chlorinated byproducts, which may be highly toxic to organisms, are formed by the oxidation treatment with chlorine compounds [27,29]. Oxidation by radical species is a powerful technique that completely degrades organic molecules into CO2 and H2O. Radical species, including hydroxyl radicals with a high oxidation potential (E0 = 2.8 V), are generated through ozonation [30,31], the Fenton/photo-Fenton reaction [32,33], photolysis [34,35], semiconductor photocatalysis [36,37], and an electrochemical reaction [38,39]. The formation of hydroxyl radicals by ozonation is pH-dependent [40], and the mass transfer rate of ozone from the gas phase to the liquid phase limits effective oxidation. The Fenton reaction with ferrous ions is a robust oxidization reaction; however, a low pH is preferable to avoid precipitation of ferrous oxyhydroxide [41]. Ultraviolet radiation generates ferrous ions from ferric hydroxide and accelerates the oxidation [42]. In this photo-Fenton reaction, the turbidity of water inhibits light penetration to adversely affect the oxidation efficiency in the same manner as that observed in photolysis and semiconductor photocatalysis. In the case of an electrochemical reaction, the oxidation of antibiotics occurs at the anode. Therefore, a low flow rate of water is desirable to increase the oxidation efficiency while decreasing the volume of water to be treated. These conventional treatment processes have both advantages and disadvantages and therefore, it is necessary to develop an efficient antibiotic degradation method that combines several of these treatment processes or other innovative methods.
In this study, we applied a cold atmospheric pressure plasma jet (APPJ) irradiation technique that is widely used in materials and biomedical research [43,44]. Reactive species are supplied to a solution irradiated with AAPJ, and reactions between the reactive species and solvent molecules produce other types of reactive species [45]. Therefore, the oxidative degradation of antibiotics by the reactive species generated from APPJ is possible. We previously reported the degradation of tetrodotoxin, a pufferfish toxin, by plasma jet irradiation for 600 s [46]. In addition to purified simple gaseous species, ambient air is also applied as a plasma source in this technique [45]. Hence, the present APPJ technique is a powerful, cost-effective, and environmentally friendly means for the treatment of wastewater containing antibiotics.
2. Materials and Methods
2.1. Reagents
Methicillin sodium salt was purchased from Toronto Research Chemicals Inc. (North York, ON, Canada). Vancomycin hydrochloride was purchased from FUJIFILM Wako Pure Chemicals (Osaka, Japan). The chemical structures of these two antibiotics are depicted in Figure 1. As the mobile phase in liquid chromatography, acetonitrile (ACN, LC-MS grade), distilled water (LC-MS grade), formic acid (FA, 98–100%), and 1 M ammonium formate were purchased from Kanto Chemical (Tokyo, Japan). Milli-Q water with a specific resistance of 18.2 MΩ cm (Merck Millipore, Burlington, MA, USA) was used for the preparation of methicillin and vancomycin solutions.
Figure 1.
Chemical structure of (a) methicillin and (b) vancomycin.
2.2. Cold Atmospheric Pressure Plasma Jet Irradiation
Both methicillin and vancomycin were dissolved in and diluted with Milli-Q water to make 32 μg/mL, and 1 mL of each of the solutions was dispensed into a well of a 24-well plate for gas bubbling treatment and plasma jet irradiation. Plasma jet was generated from high-purity nitrogen (N2), oxygen (O2), and carbon dioxide (CO2) gases using an in-house plasma generator [45]. Plasma gas was supplied at a flow rate of 3.0 L/min, and a high voltage of 9 kV with a frequency of 16 kHz was applied to generate a plasma of approximately 10 W between the two electrodes. The plasma generator was placed 15 mm above the surface of the antibiotic solutions in the well, and a plasma jet was irradiated for 10, 60, and 300 s. We also prepared samples bubbled with N2, O2, and CO2 gases for 300 s. All samples were collected in a microtube and stored at −25 °C before measurements.
2.3. Determination of Methicillin and Vancomycin by Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry (LC-Q/TOF-MS)
The concentrations of methicillin and vancomycin were determined by liquid chromatography (Prominence UFLC, Shimadzu, Kyoto, Japan) quadrupole time-of-flight mass spectrometry (LC-Q/TOF-MS; X500R QTOF, AB Sciex, Foster City, CA, USA) hyphenated technique. For the separation of antibiotics, an Intrada Organic Acid column (150 × 2 mm, 3 µm; Imtakt Corp., Kyoto, Japan) was used under gradient elution with a mobile phase including 0.1% FA, 10% ACN (A) and 100 mM ammonium formate, 10% ACN (B). The gradient conditions were as follows: 0 to 1 min: 0% B and 1 to 7 min: 0–100% B. The flow rate was 0.2 mL/min, and the injection volume was 5 μL. Methicillin and vancomycin were analyzed in the negative ion mode, and the signals at m/z 379.10 ± 0.01 and 1446.42 ± 0.01 were monitored, respectively (Figure 2). Details of the operational settings and parameters are summarized in Table 1. The concentrations were determined from the regression curves obtained from the standard solutions of methicillin and vancomycin, having concentrations of 1.6, 3.2, and 6.4 μg/mL in 80% ACN. The plasma jet-irradiated sample solutions were diluted 10 times with 80% ACN, and the maximum concentration was 3.2 μg/mL. Measurement of the sample solutions was repeated three times. The recovery of methicillin and vancomycin from the plasma-irradiated or gas-bubbled solutions was calculated by comparing their concentrations with those of control solutions.
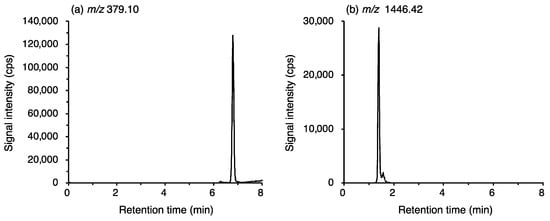
Figure 2.
Elution profiles at m/z corresponding to the negative ions of (a) methicillin ([M-H]−, 379.10) and (b) vancomycin ([M-H]−, 1446.42).

Table 1.
Instrumentation and operational settings.
3. Results
Methicillin and vancomycin were detected by Q/TOF-MS after separation by LC. The signals at m/z 379.10 and 1446.42 were monitored. Strong peaks detected at the retention times of 6.8 min and 1.4 min were assigned to methicillin and vancomycin, respectively (Figure 2).
The peak areas were calculated to determine the concentrations of the antibiotics in the sample solutions. The resulting amounts of the antibiotics in the solutions irradiated with APPJ or bubbled with gases were calculated from the concentration and volume of the solution and are shown in Figure 3. For both methicillin and vancomycin, the 10 s plasma jet irradiation degraded the antibiotics to 50 to 80% of their original amounts. The remaining antibiotics in the solutions were completely degraded within 60 s for methicillin and 300 s for vancomycin; their concentrations were below the detection limit (0.33 μg/mL for both compounds). On the other hand, bubbling with each gas used as a plasma source did not degrade the antibiotics. These results suggest that the reactive species generated from the plasma jet reacted with the antibiotics in the solutions, resulting in a time-dependent decrease in the antibiotic concentration in the solutions.
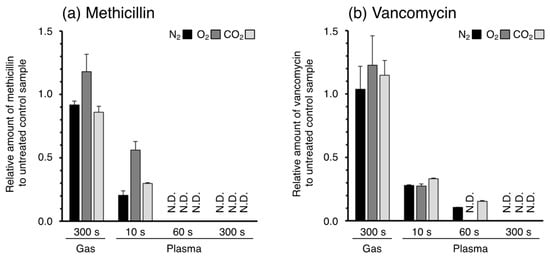
Figure 3.
Relative amounts of (a) methicillin and (b) vancomycin in the solution after plasma or gas treatment. The values represent the relative amount of antibiotics in plasma (10 s, 60 s, and 300 s) and gas-treated solution to that in untreated control sample. The results of N2, O2, and CO2 plasma were depicted as black, gray, and pale gray bars, respectively. Data represent mean value of repeated measurements (S.D., n = 3), and the data for which the signals on the corresponding m/z were not detected are expressed as N.D.
The degradation rates of the antibiotics by the different gas species slightly varied: methicillin was more rapidly degraded by N2 and CO2 plasma than O2 plasma at 10 s, whereas vancomycin was more rapidly degraded by O2 plasma than N2 and CO2 plasma at 60 s. To confirm the repeatability of the plasma jet irradiation, the irradiation of each plasma jet for 10 s was repeated three times, and the collected samples were analyzed. The repeatability of the plasma jet irradiation was <5% in each condition (Figure 4). Hence, the difference in the degradation rate of the antibiotics is dependent on the gas species. However, the average degradation rate was markedly different from those obtained in the first experiment (Figure 3). Because large amounts of the antibiotics were degraded within 10 s, slight differences in the analytical conditions would lead to large day-to-day variation (i.e., reproducibility). This has hindered efforts to elucidate the plasma degradation potential of the gas species.
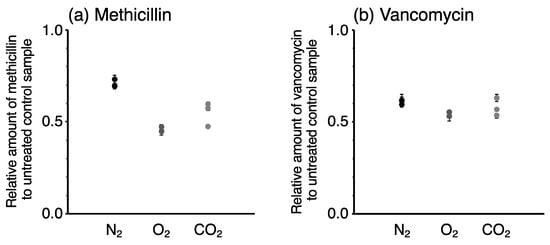
Figure 4.
Repeatability of plasma treatment for 10 s. Three independent solutions of (a) methicillin and (b) vancomycin were treated with plasma jet generated from N2, O2, and CO2. The amounts of antibiotics in each sample were measured three times, and analytical uncertainties were expressed as error bars on each symbol.
The amounts and kinds of reactive oxygen species vary depending on the gas species used as a plasma source [45,46,47]. In our previous research, we found that N2 plasma produced more hydroxyl radicals than the plasma generated with other gases [45]. The reactive species in CO2 plasma are singlet oxygen and hydrogen peroxide [45]. More ozone is produced with O2 plasma than with N2 and CO2 plasma [47]. We initially speculated that hydroxyl radicals having a high oxidation potential (E0 = 2.8 V) are responsible for the degradation. However, despite the high production of hydroxyl radicals, no clear superiority of N2 plasma for the degradation of antibiotics was observed in this study. This contradictory result is partly attributed to differences in the plasma conditions from our previous research (e.g., plasma gas flow rate, height of plasma irradiation from liquid surface, and irradiation time). The amounts of reactive species are dramatically changed by the distance from the plasma nozzle [48,49] and the irradiation time [48,50]. The composition of reactive species also varies significantly among the gas phase, the interface region, and the liquid phase, owing to their lifetimes and Henry’s constant [48]. Moreover, not only reactive oxygen species such as hydroxyl radical, hydrogen peroxide, singlet oxygen, and ozone but also substantial amounts of reactive nitrogen species such as nitric oxide, nitric acid, nitrous acid, and peroxynitrite are produced by the interaction with N2 in the atmosphere [51,52]. In our previous study, the level of nitric oxide was below the detection limit when N2, O2, and CO2 plasma was irradiated into water [45]. Some of the reactive oxygen and nitrogen species, which are mainly radicals, are short-lived molecules [52]. This has hindered efforts to determine the precise concentrations of the reactive species and to perform highly reproducible plasma irradiation. Therefore, it is difficult to quantitatively elucidate the contribution of specific reactive species to antibiotic degradation. However, it should be noted that N2, O2, and CO2 plasma jet irradiation generated reactive species that resulted in the rapid degradation of the antibiotics in this study. Hence, the APPJ technique can greatly contribute to minimizing the occurrence of infectious diseases caused by antimicrobial-resistant organisms.
4. Conclusions
We investigated the degradation of two antibiotics, methicillin and vancomycin, by APPJ irradiation. Both methicillin and vancomycin were degraded in a time-dependent manner. Compared with the pre-treatment levels, the amounts of methicillin and vancomycin were decreased by 20 to 50% after plasma jet irradiation for 10 s. Methicillin and vancomycin were completely degraded after plasma jet irradiation for 60 s and 300 s, respectively. The present AAPJ technique can generate reactive species in solution, and the reactive species can easily react with the antibiotics in the solution. In addition, APPJ can be generated from ambient air. Therefore, the AAPJ technique is a powerful, cost-effective, and environmentally friendly means of degrading antibiotics contaminating the hydrosphere.
Author Contributions
Conceptualization, Y.-k.T.; methodology, Y.-k.T., Y.Y. and A.O.; validation, Y.-k.T., T.O. and Y.Y.; formal analysis, Y.-k.T., T.O. and Y.Y.; investigation, Y.-k.T.; data curation, Y.-k.T.; writing—original draft preparation, Y.-k.T.; writing—review and editing, A.O. and Y.O.; supervision, A.O. and Y.O.; funding acquisition, Y.-k.T., Y.Y., A.O. and Y.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI, Grant Numbers 20K18978, 21H04920, 22H04973, 22K15260, and 22K19044. A part of this research is based on the Cooperative Research Project of the Research Center for Biomedical Engineering, Japan, Grant Numbers 1030(2022) and 1006(2023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hackbarth, C.J.; Chambers, H.F. Methicillin-resistant staphylococci: Genetics and mechanisms of resistance. Antimicrob. Agents Chemother. 1989, 33, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Shariati, A.; Dadashi, M.; Moghadam, M.T.; van Belkum, A.; Yaslianifard, S.; Darban-Sarokhalil, D. Global prevalence and distribution of vancomycin resistant, vancomycin intermediate and heterogeneously vancomycin intermediate Staphylococcus aureus clinical isolates: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 12689. [Google Scholar] [CrossRef] [PubMed]
- Kotay, S.; Chai, W.; Guilford, W.; Barry, K.; Mathers, A.J. Spread from the Sink to the Patient: In Situ Study Using Green Fluorescent Protein (GFP)-Expressing Escherichia coli To Model Bacterial Dispersion from Hand-Washing Sink-Trap Reservoirs. Appl. Environ. Microbiol. 2017, 83, E03327. [Google Scholar] [CrossRef] [PubMed]
- Hocquet, D.; Muller, A.; Bertrand, X. What happens in hospitals does not stay in hospitals: Antibiotic-resistant bacteria in hospital wastewater systems. J. Hosp. Infect. 2016, 93, 395–402. [Google Scholar] [CrossRef]
- Varela, A.R.; Ferro, G.; Vredenburg, J.; Yanık, M.; Vieira, L.; Rizzo, L.; Lameiras, C.; Manaia, C.M. Vancomycin resistant enterococci: From the hospital effluent to the urban wastewater treatment plant. Sci. Total Environ. 2013, 450–451, 155–161. [Google Scholar] [CrossRef]
- Diwan, V.; Tamhankar, A.J.; Khandal, R.K.; Sen, S.; Aggarwal, M.; Marothi, Y.; Iyer, R.V.; Sundblad-Tonderski, K.; Stålsby Lundborg, C. Antibiotics and antibiotic-resistant bacteria in waters associated with a hospital in Ujjain, India. BMC Public Health 2010, 10, 414. [Google Scholar] [CrossRef]
- Zhang, Q.; Jia, A.; Wan, Y.; Liu, H.; Wang, K.; Peng, H.; Dong, Z.; Hu, J. Occurrences of Three Classes of Antibiotics in a Natural River Basin: Association with Antibiotic-Resistant Escherichia coli. Environ. Sci. Technol. 2014, 48, 14317–14325. [Google Scholar] [CrossRef]
- Marathe, N.P.; Regina, V.R.; Walujkar, S.A.; Charan, S.S.; Moore, E.R.B.; Larsson, D.G.J.; Shouche, Y.S. A Treatment Plant Receiving Waste Water from Multiple Bulk Drug Manufacturers Is a Reservoir for Highly Multi-Drug Resistant Integron-Bearing Bacteria. PLoS ONE 2013, 8, e77310. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Larsson, D.G.J. Pollution from drug manufacturing: Review and perspectives. Philos. Trans. R. Soc. 2014, 369, 20130571. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; de Pedro, C.; Paxeus, N. Effluent from drug manufactures contains extremely high levels of pharmaceuticals. J. Hazard. Mater. 2007, 148, 751–755. [Google Scholar] [CrossRef]
- Li, D.; Yang, M.; Hu, J.; Ren, L.; Zhang, Y.; Li, K. Determination and fate of oxytetracycline and related compounds in oxytetracycline production wastewater and the receiving river. Environ. Toxicol. Chem. 2008, 27, 80–86. [Google Scholar] [CrossRef]
- Lübbert, C.; Baars, C.; Dayakar, A.; Lippmann, N.; Rodloff, A.C.; Kinzig, M.; Sörgel, F. Environmental pollution with antimicrobial agents from bulk drug manufacturing industries in Hyderabad, South India, is associated with dissemination of extended-spectrum beta-lactamase and carbapenemase-producing pathogens. Infection 2017, 45, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.A.; Ferreira, J.P.; LeJeune, J.T. Antimicrobial Use and Resistance in Plant Agriculture: A One Health Perspective. Agriculture 2022, 12, 289. [Google Scholar] [CrossRef]
- Schar, D.; Klein, E.Y.; Laxminarayan, R.; Gilbert, M.; Van Boeckel, T.P. Global trends in antimicrobial use in aquaculture. Sci. Rep. 2020, 10, 21878. [Google Scholar] [CrossRef]
- Zhou, L.J.; Ying, G.G.; Liu, S.; Zhao, J.L.; Yang, B.; Chen, Z.F.; Lai, H.J. Occurrence and fate of eleven classes of antibiotics in two typical wastewater treatment plants in South China. Sci. Total Environ. 2013, 452–453, 365–376. [Google Scholar] [CrossRef]
- Kairigo, P.; Ngumba, E.; Sundberg, L.-R.; Gachanja, A.; Tuhkanen, T. Contamination of Surface Water and River Sediments by Antibiotic and Antiretroviral Drug Cocktails in Low and Middle-Income Countries: Occurrence, Risk and Mitigation Strategies. Water 2020, 12, 1376. [Google Scholar] [CrossRef]
- Anh, H.Q.; Le, T.P.Q.; Da Le, N.; Lu, X.X.; Duong, T.T.; Garnier, J.; Rochelle-Newall, E.; Zhang, S.; Oh, N.-H.; Oeurng, C.; et al. Antibiotics in surface water of East and Southeast Asian countries: A focused review on contamination status, pollution sources, potential risks, and future perspectives. Sci. Total Environ. 2021, 764, 142865. [Google Scholar] [CrossRef]
- Fu, C.; Xu, B.; Chen, H.; Zhao, X.; Li, G.; Zheng, Y.; Qiu, W.; Zheng, C.; Duan, L.; Wang, W. Occurrence and distribution of antibiotics in groundwater, surface water, and sediment in Xiong’an New Area, China, and their relationship with antibiotic resistance genes. Sci. Total Environ. 2022, 807, 151011. [Google Scholar] [CrossRef]
- Twinomucunguzi, F.R.B.; Nyenje, P.M.; Semiyaga, S.; Kebirungi, P.; Kulabako, R.N.; Kansiime, F. Antibiotics in shallow groundwater underlying urban informal settlements in developing countries: Influence of on-site sanitation practices and risk assessment. Urban Water J. 2022, 1–13. [Google Scholar] [CrossRef]
- Hernández, F.; Calısto-Ulloa, N.; Gómez-Fuentes, C.; Gómez, M.; Ferrer, J.; González-Rocha, G.; Bello-Toledo, H.; Botero-Coy, A.M.; Boıx, C.; Ibáñez, M.; et al. Occurrence of antibiotics and bacterial resistance in wastewater and sea water from the Antarctic. J. Hazard. Mater. 2019, 363, 447–456. [Google Scholar] [CrossRef]
- Wang, H.; Wang, N.; Wang, B.; Zhao, Q.; Fang, H.; Fu, C.; Tang, C.; Jiang, F.; Zhou, Y.; Chen, Y.; et al. Antibiotics in Drinking Water in Shanghai and Their Contribution to Antibiotic Exposure of School Children. Environ. Sci. Technol. 2016, 50, 2692–2699. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Cheng, W.; Wan, T.; Wang, M.; Ren, J.; Li, Y.; Huang, C. Occurrence of antibiotics in rural drinking water and related human health risk assessment. Environ. Technol. 2021, 42, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Harrower, J.; McNaughtan, M.; Hunter, C.; Hough, R.; Zhang, Z.; Helwig, K. Chemical Fate and Partitioning Behavior of Antibiotics in the Aquatic Environment—A Review. Environ. Toxicol. Chem. 2021, 40, 3275–3298. [Google Scholar] [CrossRef] [PubMed]
- Homem, V.; Santos, L. Degradation and removal methods of antibiotics from aqueous matrices—A review. J. Environ. Manag. 2011, 92, 2304–2347. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T. Biodegradation and Adsorption of Antibiotics in the Activated Sludge Process. Environ. Sci. Technol. 2010, 44, 3468–3473. [Google Scholar] [CrossRef]
- Adams, C.; Wang, Y.; Loftin, K.; Meyer, M. Removal of Antibiotics from Surface and Distilled Water in Conventional Water Treatment Processes. J. Environ. Eng. 2002, 128, 253–260. [Google Scholar] [CrossRef]
- Navalon, S.; Alvaro, M.; Garcia, H. Reaction of chlorine dioxide with emergent water pollutants: Product study of the reaction of three β-lactam antibiotics with ClO2. Water Res. 2008, 42, 1935–1942. [Google Scholar] [CrossRef]
- Li, W.; Liu, K.; Min, Z.; Li, J.; Zhang, M.; Korshin, G.V.; Han, J. Transformation of macrolide antibiotics during chlorination process: Kinetics, degradation products, and comprehensive toxicity evaluation. Sci. Total Environ. 2023, 858, 159800. [Google Scholar] [CrossRef]
- Yargeau, V.; Leclair, C. Potential of ozonation for the degradation of antibiotics in wastewater. Water Sci. Technol. 2007, 55, 321–326. [Google Scholar] [CrossRef]
- Iakovides, I.C.; Michael-Kordatou, I.; Moreira, N.F.F.; Ribeiro, A.R.; Fernandes, T.; Pereira, M.F.R.; Nunes, O.C.; Manaia, C.M.; Silva, A.M.T.; Fatta-Kassinos, D. Continuous ozonation of urban wastewater: Removal of antibiotics, antibiotic-resistant Escherichia coli and antibiotic resistance genes and phytotoxicity. Water Res. 2019, 159, 333–347. [Google Scholar] [CrossRef]
- Elmolla, E.; Chaudhuri, M. Optimization of Fenton process for treatment of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution. J. Hazard. Mater. 2009, 170, 666–672. [Google Scholar] [CrossRef]
- Yi, H.; Lai, C.; Huo, X.; Qin, L.; Fu, Y.; Liu, S.; Li, L.; Zhang, M.; Chen, M.; Zeng, G. H2O2-free photo-Fenton system for antibiotics degradation in water via the synergism of oxygen-enriched graphitic carbon nitride polymer and nano manganese ferrite. Environ. Sci. Nano 2022, 9, 815–826. [Google Scholar] [CrossRef]
- Baena-Nogueras, R.M.; González-Mazo, E.; Lara-Martín, P.A. Photolysis of Antibiotics under Simulated Sunlight Irradiation: Identification of Photoproducts by High-Resolution Mass Spectrometry. Environ. Sci. Technol. 2017, 51, 3148–3156. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, W.; Liang, B.; Han, J.; Cheng, H.; Haider, M.R.; Wang, H.; Liu, W.; Liu, S.; Wang, A. UV photolysis as an efficient pretreatment method for antibiotics decomposition and their antibacterial activity elimination. J. Hazard. Mater. 2020, 392, 122321. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Nguyen, V.; Jiang, Z.; Wang, D.; Zhu, Z.; Wang, W.-N. Highly-oriented one-dimensional MOF-semiconductor nanoarrays for efficient photodegradation of antibiotics. Catal. Sci. Technol. 2018, 8, 2117–2123. [Google Scholar] [CrossRef]
- Kutuzova, A.; Dontsova, T.; Kwapinski, W. Application of TiO2-Based Photocatalysts to Antibiotics Degradation: Cases of Sulfamethoxazole, Trimethoprim and Ciprofloxacin. Catalysts 2021, 11, 728. [Google Scholar] [CrossRef]
- Haidar, M.; Dirany, A.; Sirés, I.; Oturan, N.; Oturan, M.A. Electrochemical degradation of the antibiotic sulfachloropyridazine by hydroxyl radicals generated at a BDD anode. Chemosphere 2013, 91, 1304–1309. [Google Scholar] [CrossRef]
- Yuan, Q.; Qu, S.; Li, R.; Huo, Z.-Y.; Gao, Y.; Luo, Y. Degradation of antibiotics by electrochemical advanced oxidation processes (EAOPs): Performance, mechanisms, and perspectives. Sci. Total Environ. 2023, 856, 159092. [Google Scholar] [CrossRef]
- Arslan-Alaton, I.; Dogruel, S. Pre-treatment of penicillin formulation effluent by advanced oxidation processes. J. Hazard. Mater. 2004, 112, 105–113. [Google Scholar] [CrossRef]
- Jung, Y.S.; Lim, W.T.; Park, J.Y.; Kim, Y.H. Effect of pH on Fenton and Fenton-like oxidation. Environ. Technol. 2009, 30, 183–190. [Google Scholar] [CrossRef]
- Ghaly, M.Y.; Härtel, G.; Mayer, R.; Haseneder, R. Photochemical oxidation of p-chlorophenol by UV/H2O2 and photo-Fenton process. A comparative study. Waste Manag. 2001, 21, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M.; Lu, X.; Keidar, M. Perspective: The physics, diagnostics, and applications of atmospheric pressure low temperature plasma sources used in plasma medicine. J. Appl. Phys. 2017, 122, 020901. [Google Scholar] [CrossRef]
- Adamovich, I.; Baalrud, S.D.; Bogaerts, A.; Bruggeman, P.J.; Cappelli, M.; Colombo, V.; Czarnetzki, U.; Ebert, U.; Eden, J.G.; Favia, P.; et al. The 2017 Plasma Roadmap: Low temperature plasma science and technology. J. Phys. D Appl. Phys. 2017, 50, 323001. [Google Scholar] [CrossRef]
- Takamatsu, T.; Uehara, K.; Sasaki, Y.; Miyahara, H.; Matsumura, Y.; Iwasawa, A.; Ito, N.; Azuma, T.; Kohno, M.; Okino, A. Investigation of reactive species using various gas plasmas. RSC Adv. 2014, 4, 39901–39905. [Google Scholar] [CrossRef]
- Takamatsu, T.; Miyahara, H.; Azuma, T.; Okino, A. Decomposition of tetrodotoxin using multi-gas plasma jet. J. Toxicol. Sci. 2014, 39, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, Y.; Takamatsu, T.; Aizawa, T.; Moriya, S.; Matsumura, Y.; Iwasawa, A.; Okino, A. Influence of Controlling Plasma Gas Species and Temperature on Reactive Species and Bactericidal Effect of the Plasma. Appl. Sci. 2021, 11, 11674. [Google Scholar] [CrossRef]
- Verlackt, C.C.W.; Van Boxem, W.; Bogaerts, A. Transport and accumulation of plasma generated species in aqueous solution. Phys. Chem. Chem. Phys. 2018, 20, 6845–6859. [Google Scholar] [CrossRef]
- Ellerweg, D.; von Keudell, A.; Benedikt, J. Unexpected O and O3 production in the effluent of He/O2 microplasma jets emanating into ambient air. Plasma Sources Sci. Technol. 2012, 21, 034019. [Google Scholar] [CrossRef]
- Hamaguchi, S. Chemically reactive species in liquids generated by atmospheric-pressure plasmas and their roles in plasma medicine. AIP Conf. Proc. 2013, 1545, 214–222. [Google Scholar]
- Chauvin, J.; Judée, F.; Yousfi, M.; Vicendo, P.; Merbahi, N. Analysis of reactive oxygen and nitrogen species generated in three liquid media by low temperature helium plasma jet. Sci. Rep. 2017, 7, 4562. [Google Scholar] [CrossRef]
- Gorbanev, Y.; Privat-Maldonado, A.; Bogaerts, A. Analysis of Short-Lived Reactive Species in Plasma–Air–Water Systems: The Dos and the Do Nots. Anal. Chem. 2018, 90, 13151–13158. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).