Plasma Electrolytic Polishing of Porous Nitinol Structures
Abstract
1. Introduction
2. Nitinol Samples and Experimental Methodology
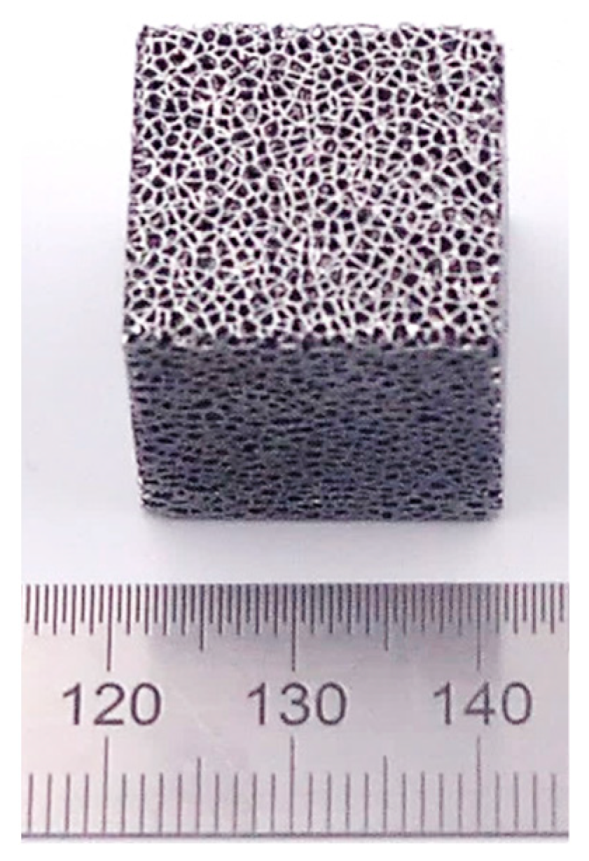
2.1. Nitinol Samples
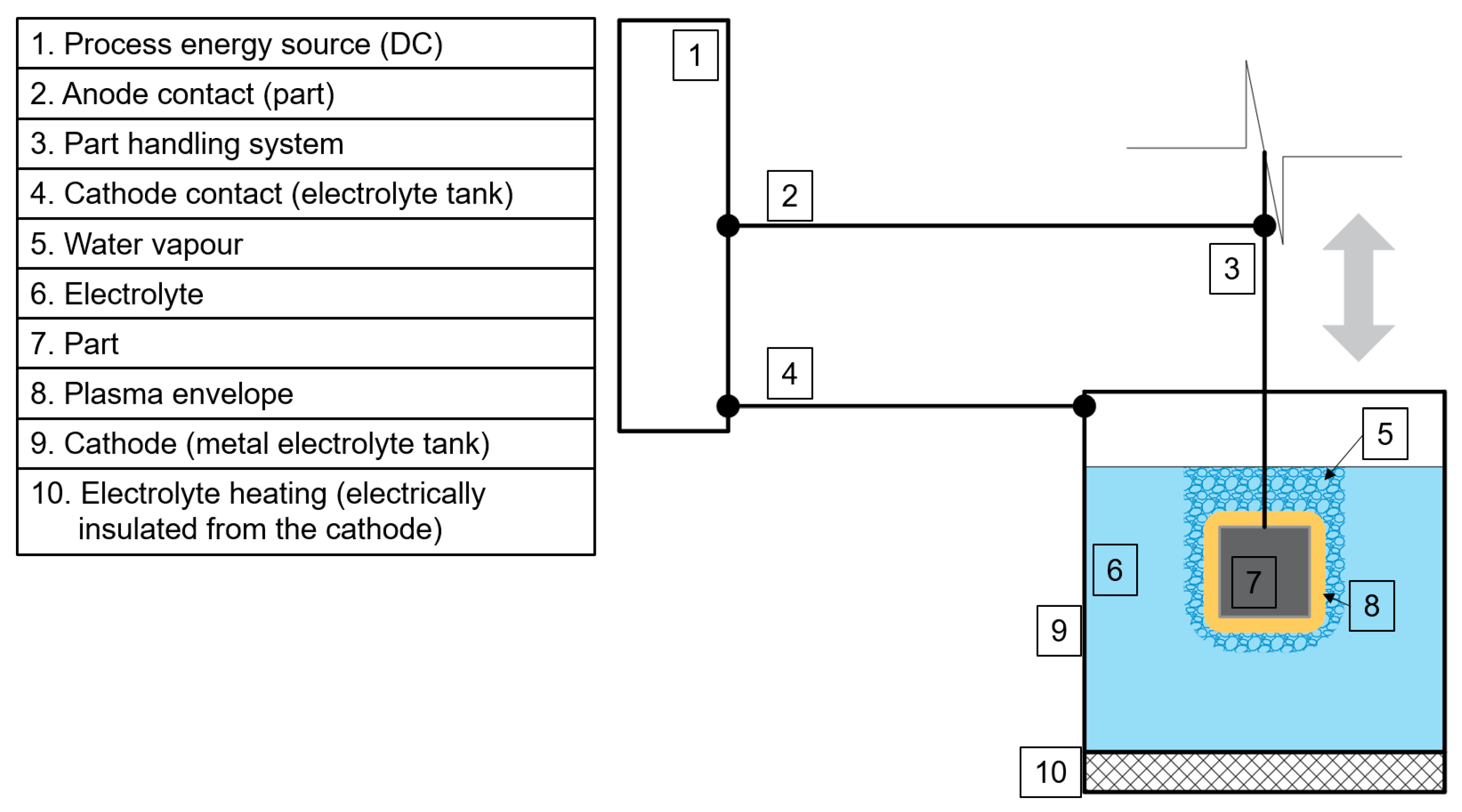
2.2. Plasma Electrolytic Polishing
2.3. Energy Dispersive X-ray Spectroscopy
2.4. Differential Scanning Calorimetry
3. Results and Discussion
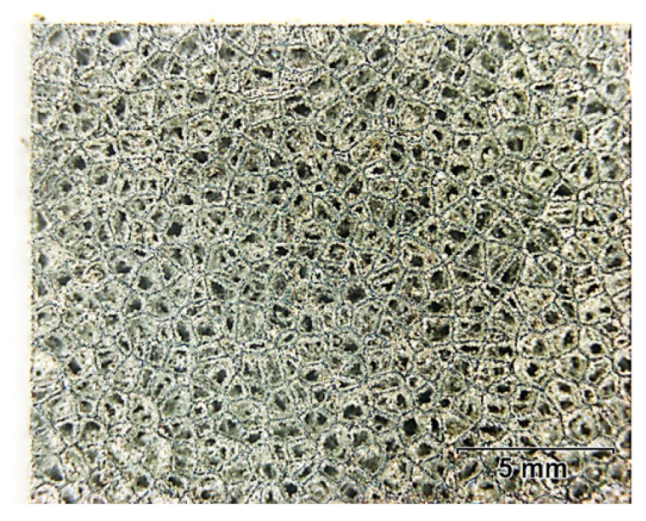
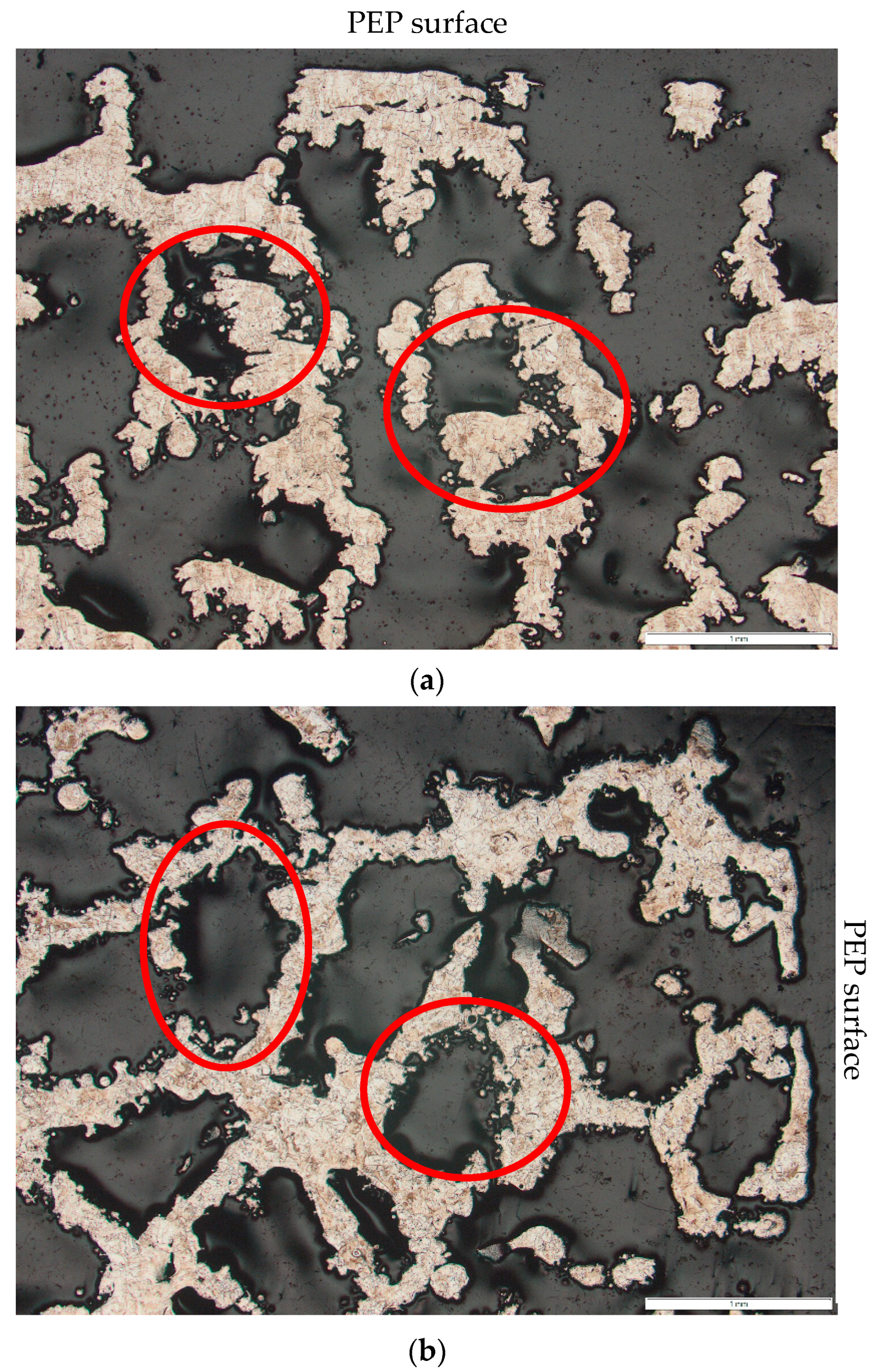
3.1. The Depth of the PEP
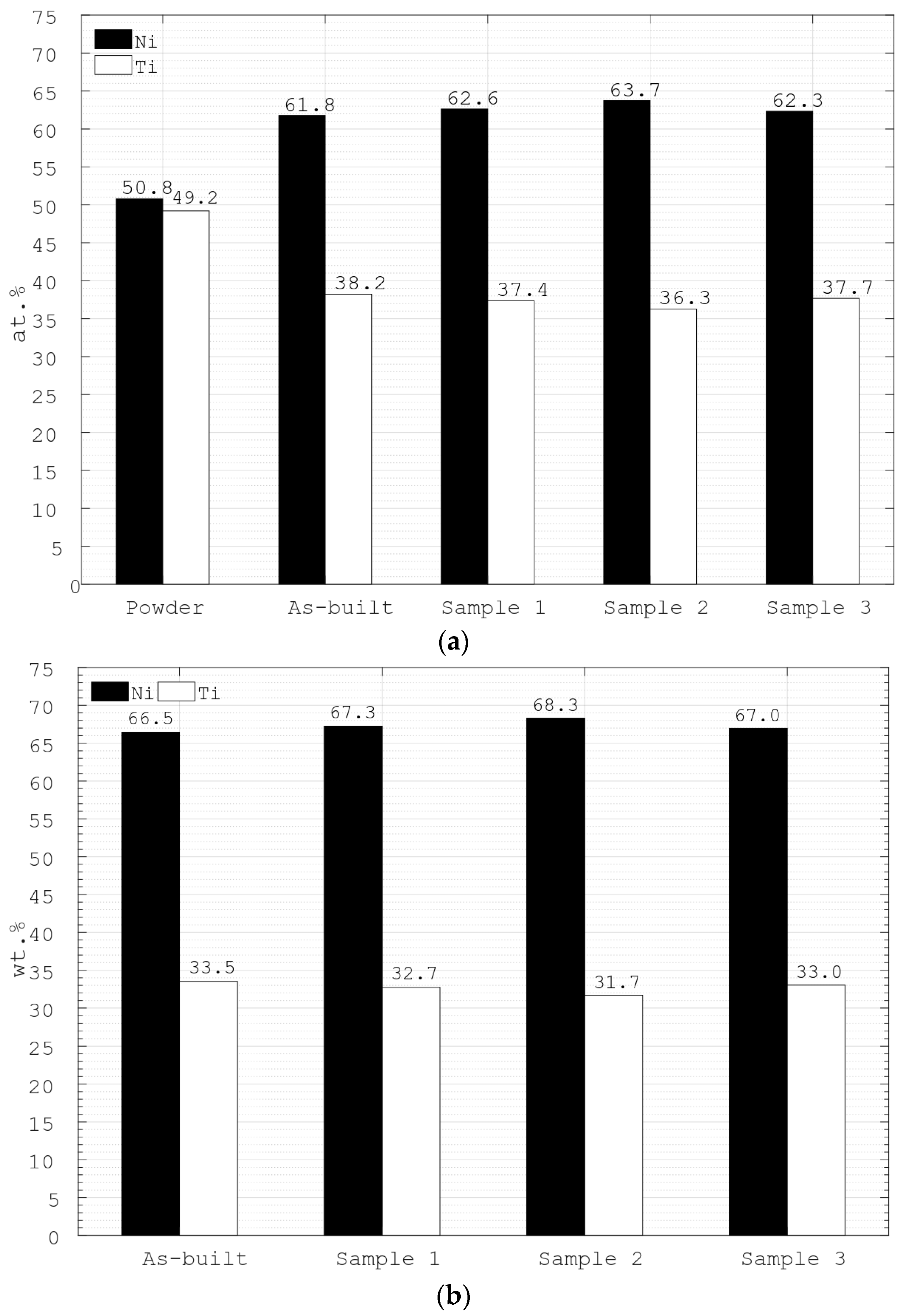
3.2. Energy Dispersive X-ray Spectroscopy
3.3. Differential Scanning Calorimetry
4. Conclusions
5. Limitations of This Study
- Due to the construction of the test set-up, the maintenance of constant electrolyte temperature during the PEP process was not possible;
- Due to the resolution of the built-in ampermeter, no precise readings of the resulting process current could be obtained;
- Due to the nature of the samples chosen for this study, no qualitative surface roughness measurement could be conducted.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kauffman, G.B.; Mayo, I. The Story of Nitinol: The Serendipitous Discovery of the Memory Metal and Its Applications. Chem. Educ. 1997, 2, 1–21. [Google Scholar] [CrossRef]
- Kabirifar, P.; Žerovnik, A.; Ahčin, Ž.; Porenta, L.; Brojan, M.; Tušek, J. Elastocaloric Cooling: State-of-the-art and Future Challenges in Designing Regenerative Elastocaloric Devices. Stroj. Vestn.-J. Mech. Eng. 2019, 65, 615–630. [Google Scholar] [CrossRef]
- Duerig, T.; Pelton, A.; Stöckel, D. An overview of nitinol medical applications. Mater. Sci. Eng. A 1999, 273–275, 149–160. [Google Scholar] [CrossRef]
- Biscarini, A.; Mazzolai, G.; Tuissi, A. Enhanced Nitinol Properties for Biomedical Applications. Recent Pat. Biomed. Eng. 2010, 1, 180–196. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.-K.; Kim, H.K.; Unnithan, A.R.; Kim, C.S.; Park, C.H. Optimization of Electropolishing on NiTi Alloy Stents and Its Influence on Corrosion Behavior. J. Nanosci. Nanotechnol. 2017, 17, 2333–2339. [Google Scholar] [CrossRef]
- Engelbrecht, K.; Tušek, J.; Eriksen, D.; Lei, T.; Lee, C.-Y.; Tušek, J.; Pryds, N. A regenerative elastocaloric device: Experimental results. J. Phys. D Appl. Phys. 2017, 50, 424006. [Google Scholar] [CrossRef]
- Tušek, J.; Engelbrecht, K.; Millán-Solsona, R.; Mañosa, L.; Vives, E.; Mikkelsen, L.P.; Pryds, N. The Elastocaloric Effect: A Way to Cool Efficiently. Adv. Energy Mater. 2015, 5, 1500361. [Google Scholar] [CrossRef]
- Stepputat, V.; Zeidler, H.; Safranchik, D.; Strokin, E.; Böttger-Hiller, F. Investigation of Post-Processing of Additively Manufactured Nitinol Smart Springs with Plasma-Electrolytic Polishing. Materials 2021, 14, 4093. [Google Scholar] [CrossRef]
- Weinert, K.; Petzoldt, V.; Kötter, D. Turning and Drilling of NiTi Shape Memory Alloys. CIRP Ann. 2004, 53, 65–68. [Google Scholar] [CrossRef]
- Mwangi, J.W.; Weisheit, L.; Bui, V.D.; Zanjani, M.Y.; Schubert, A. Influence of Micro-EDM on the Phase Transformation Behaviour of Medical-Grade Nitinol. Shape Mem. Superelasticity 2018, 4, 450–461. [Google Scholar] [CrossRef]
- Andani, M.T.; Haberland, C.; Walker, J.; Elahinia, M. An Investigation of Effective Process Parameters on Phase Transfor-mation Temperature of Nitinol Manufactured by Selective Laser Melting. In Proceedings of the ASME 2014 Conference on Smart Materials, Adaptive Structures and Intelligent Systems, Newport, RI, USA, 8–10 September 2014; pp. 1–5. [Google Scholar]
- Walker, J.; Andani, M.T.; Haberland, C.; Elahinia, M. Additive Manufacturing of Nitinol Shape Memory Alloys to Overcome Challenges in Conventional Nitinol Fabrication. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Montreal, QC, Canada, 14–20 November 2014. [Google Scholar] [CrossRef]
- Haberland, C.; Elahinia, M.; Walker, J.; Meier, H. Visions, Concepts and Strategies for Smart Nitinol Actuators and Complex Nitinol Structures Produced by Additive Manufacturing. In Proceedings of the ASME 2013 Conference on Smart Materials, Adaptive Structures and Intelligent Systems, Snowbird, UT, USA, 16–18 September 2013. [Google Scholar] [CrossRef]
- Jahadakbar, A.; Nematollahi, M.; Safaei, K.; Bayati, P.; Giri, G.; Dabbaghi, H.; Dean, D.; Elahinia, M. Design, Modeling, Additive Manufacturing, and Polishing of Stiffness-Modulated Porous Nitinol Bone Fixation Plates Followed by Thermomechanical and Composition Analysis. Metals 2020, 10, 151. [Google Scholar] [CrossRef]
- Bram, M.; Bitzer, M.; Buchkremer, H.P.; Stover, D. Reproducibility Study of NiTi Parts Made by Metal Injection Molding. J. Mater. Eng. Perform. 2012, 21, 2701–2712. [Google Scholar] [CrossRef]
- Hou, H.; Simsek, E.; Stasak, D.; Al Hasan, N.; Qian, S.; Ott, R.; Cui, J.; Takeuchi, I. Elastocaloric cooling of additive manufactured shape memory alloys with large latent heat. J. Phys. D Appl. Phys. 2017, 50, 404001. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; Zhang, W. Additive manufacturing of metallic components—Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Navickaitė, K.; Liang, J.; Bahl, C.; Wieland, S.; Buchenau, T.; Engelbrecht, K. Experimental characterization of active magnetic regenerators constructed using laser beam melting technique. Appl. Therm. Eng. 2020, 174, 115297. [Google Scholar] [CrossRef]
- Navickaitė, K.; Nestler, K.; Kain, M.; Tosello, G.; Pedersen, D.B.; Penzel, M.; Böttger-Hiller, F.; Zeidler, H. Effective polishing of inner surfaces of additive manufactured inserts for polymer extrusion using Plasma Electrolytic Polishing. In Proceedings of the RapidTech3D Additive Manufacturing Hub, Erfurt, Germany, 17–19 May 2022; pp. 1–10. [Google Scholar]
- Hansen, A.W.; Beltrami, L.V.R.; Antonini, L.M.; Villarinho, D.J.; das Neves, J.C.K.; Marino, C.E.B.; Malfatti, C.D.F. Oxide Formation on NiTi Surface: Influence of the Heat Treatment Time to Achieve the Shape Memory. Mater. Res. 2015, 18, 1053–1061. [Google Scholar] [CrossRef]
- Engelbrecht, K.; Tušek, J.; Sanna, S.; Eriksen, D.; Mishin, O.V.; Bahl, C.R.H.; Pryds, N. Effects of surface finish and mechanical training on Ni-Ti sheets for elastocaloric cooling. APL Mater. 2016, 4, 064110. [Google Scholar] [CrossRef]
- Bormann, T.; Schumacher, R.; Müller, B.; Mertmann, M.; de Wild, M. Tailoring Selective Laser Melting Process Parameters for NiTi Implants. J. Mater. Eng. Perform. 2012, 21, 2519–2524. [Google Scholar] [CrossRef]
- Kapoor, D. Nitinol for Medical Applications: A Brief Introduction to the Properties and Processing of Nickel Titanium Shape Memory Alloys and their Use in Stents. Johns. Matthey Technol. Rev. 2017, 61, 66–76. [Google Scholar] [CrossRef]
- Mwangi, J.W.; Zeidler, H.; Schubert, A.; Wohlschloegel, M.; Braeuner, C.; Wohlschlögel, M.; Bräuner, C. Heat affected zone analysis for laser and micro-electrical discharge machined nitinol. In Proceedings of the International Conference on Shape Memory and Superelastic Technologies SMST, San Diego, CA, USA, 15–19 May 2017; pp. 179–180. [Google Scholar]
- Kashapov, L.N.; Kashapov, N.F.; Denisov, D.G. Plasma electrolytic treatment of products after selective laser melting. J. Phys. Conf. Ser. 2016, 669, 012029. [Google Scholar] [CrossRef]
- Zeidler, H.; Nestler, K.; Boettger-Hiller, F.; Schubert, A.; Previtali, B.; Gökhan, A.D.; Demir, A.G. Finishing of la-ser-machined coronary stents by plasma electrolytic polishing Henning. In Proceedings of the Euspen’s 16th International Conference & Exhibition, Bedfordshire, UK, 30 May–3 June 2016; Volume 49, pp. 83–87. [Google Scholar]
- Belkin, P.N.; Silkin, S.A.; D’Yakov, I.G.; Burov, S.V.; Kusmanov, S.A. Influence of Plasma Electrolytic Polishing Conditions on Surface Roughness of Steel. Surf. Eng. Appl. Electrochem. 2019, 55, 55–62. [Google Scholar] [CrossRef]
- Rokicki, R.; Hryniewicz, T. Nitinol surface finishing by magnetoelectropolishing. Trans. IMF 2008, 86, 280–285. [Google Scholar] [CrossRef]
- Barbosa, F.O.G.; Gomes, J.A.D.C.P.; de Araújo, M.C.P. Influence of Electrochemical Polishing on the Mechanical Properties of K3 Nickel-Titanium Rotary Instruments. J. Endod. 2008, 34, 1533–1536. [Google Scholar] [CrossRef] [PubMed]
- Nestler, K.; Böttger-Hiller, F.; Adamitzki, W.; Glowa, G.; Zeidler, H.; Schubert, A. Plasma Electrolytic Polishing—An Overview of Applied Technologies and Current Challenges to Extend the Polishable Material Range. Procedia CIRP 2016, 42, 503–507. [Google Scholar] [CrossRef]
- Navickaitė, K.; Nestler, K.; Böttger-Hiller, F.; Matias, C.; Diskin, A.; Golan, O.; Garkun, A.; Strokin, E.; Biletskiy, R.; Safranchik, D.; et al. Efficient polishing of additive manufactured titanium alloys. Procedia CIRP 2022, 108, 346–351. [Google Scholar] [CrossRef]
- Navickaitė, K.; Ianniciello, L.; Tušek, J.; Engelbrecht, K.; Bahl, C.R.H.; Penzel, M.; Nestler, K.; Böttger-Hiller, F.; Zeidler, H. Plasma Electrolytic Polishing of Nitinol: Investigation of Functional Properties. Materials 2021, 14, 6450. [Google Scholar] [CrossRef]
- Belkin, P.; Kusmanov, S.; Parfenov, E. Mechanism and technological opportunity of plasma electrolytic polishing of metals and alloys surfaces. Appl. Surf. Sci. Adv. 2020, 1, 100016. [Google Scholar] [CrossRef]
- Zatkalíková, V.; Podhorský, Š.; Štrbák, M.; Liptáková, T.; Markovičová, L.; Kuchariková, L. Plasma Electrolytic Polishing—An Ecological Way for Increased Corrosion Resistance in Austenitic Stainless Steels. Materials 2022, 15, 4223. [Google Scholar] [CrossRef]
- Quitzke, S.; Martin, A.; Schubert, A. Correlation between current density and ablation rate of Jet-PeP. In Proceedings of the 16th International Symposium on Electrochemical Machining Technology INSECT, Chemnitz, Germany, 24–25 November 2020; Volume 2020, pp. 181–188. [Google Scholar]
- Kranhold, C.; Schulze, H.P.; Kröning, O.; Zeidler, H. Classification and evaluation of optical phenomena in Jet-PeP with respect to the characteristic of plasma electrolytic polishing. In Proceedings of the International Symposium on Electrochemical Machining Technology INSECT, Saabrücken, Germany, 24–25 November 2019; pp. 153–158. [Google Scholar]
- Quitzke, S.; Martin, A.; Schubert, A. Influence of ignition and polishing phase on jet shape and electric current in Jet- Plasma electrolytic Polishing. In Proceedings of the Euspen’s 21st International Conference & Exhibition, Cambridge, UK, 7–10 June 2021. [Google Scholar]
- Parfenov, E.; Farrakhov, R.; Mukaeva, V.; Gusarov, A.; Nevyantseva, R.; Yerokhin, A. Electric field effect on surface layer removal during electrolytic plasma polishing. Surf. Coat. Technol. 2016, 307, 1329–1340. [Google Scholar] [CrossRef]
- Myкаева, Е.В.; Пaрфенов, М.В.; Гoрбатков; Нeвьянцева, Р.Р. Optimal process control of electrolyte-plasma polishing on the basis of control of the state of the object by impedance spectra. Вестник Уфимского Государственного Авиационного Технического Университета 2014, 3, 150–157. [Google Scholar]
- Cornelsen, M.; Deutsch, C.; Seitz, H. Electrolytic Plasma Polishing of Pipe Inner Surfaces. Metals 2017, 8, 12. [Google Scholar] [CrossRef]
- Cornelsen, M.; Deutsch, C.; Seitz, H. Influence of the Velocity and the Number of Polishing Passages on the Roughness of Electrolytic Plasma Polished Pipe Inner Surfaces. Metals 2018, 8, 330. [Google Scholar] [CrossRef]
- Voo, L.; Armand, M.; Kleinberger, M. Stress fracture risk analysis of the human femur based on computational biomechanics. Johns Hopkins APL Tech. Dig. (Appl. Phys. Lab.) 2004, 25, 223–230. [Google Scholar]
- Ivanova, H.P.; Sinkevic, Y.V.; Sheleg, V.K.; Jankovskii, I.N. ИССЛЕДОВАНИЕ МОРФОЛОГИИ И ХИМИЧЕСКОГО СОСТАВА ЭЛЕКТРОИМПУЛЬСНО ПОЛИРОВАННОЙ ПОВЕРХНОСТИ УГЛЕРОДИСТЫХ И КОРРОЗИОННОСТОЙКИХ СТАЛЕЙ (Investigation of morphology and chemical composition of carbon steel and stainless steel polished using electric impuls). Sci. Technol. 2012, 3–10. [Google Scholar]


| Parameter | Value |
|---|---|
| Machine | EOS M290 |
| Hatch Spacing, h | 70 µm |
| Laser power, P | 100 W |
| Laser speed, v | 620 mm/s |
| Layer thickness, σ | 40 µm |
| Particle size distribution | 16–45 µm |
| Sample Name | Exp. No. | Time, t, s | Electrolyte Temp., Θ, °C | Voltage, U, V | Current, I, A | Electric Charge, Q, A min | Mass before PEP, m, g | Mass after PEP, m, g | Difference, Δm, g | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Start | End | Start | Middle | ||||||||
| 1 | 1 | 60 | 76.0 | 82.7 | 333 | 10 | 6 | 7 | 19.202 | 18.771 | 0.431 |
| 2 | 60 | 75.8 | 82.0 | 332 | 9 | 6 | 7 | ||||
| 2 | 1 | 120 | 76.3 | 86.2 | 330 | 9 | 6 | 13 | 19.138 | 18.369 | 0.769 |
| 2 | 120 | 75.5 | 85.0 | 332 | 9 | 6 | 14 | ||||
| 3 | 1 | 240 | 75.8 | 80.4 | 330 | 10 | 7 | 29 | 19.145 | 18.027 | 1.118 |
| 2 | 240 | 76.3 | 80.7 | 331 | 9 | 6 | 28 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navickaitė, K.; Roßmann, K.; Nestler, K.; Böttger-Hiller, F.; Penzel, M.; Grund, T.; Lampke, T.; Zeidler, H. Plasma Electrolytic Polishing of Porous Nitinol Structures. Plasma 2022, 5, 555-568. https://doi.org/10.3390/plasma5040039
Navickaitė K, Roßmann K, Nestler K, Böttger-Hiller F, Penzel M, Grund T, Lampke T, Zeidler H. Plasma Electrolytic Polishing of Porous Nitinol Structures. Plasma. 2022; 5(4):555-568. https://doi.org/10.3390/plasma5040039
Chicago/Turabian StyleNavickaitė, Kristina, Karl Roßmann, Klaus Nestler, Falko Böttger-Hiller, Michael Penzel, Thomas Grund, Thomas Lampke, and Henning Zeidler. 2022. "Plasma Electrolytic Polishing of Porous Nitinol Structures" Plasma 5, no. 4: 555-568. https://doi.org/10.3390/plasma5040039
APA StyleNavickaitė, K., Roßmann, K., Nestler, K., Böttger-Hiller, F., Penzel, M., Grund, T., Lampke, T., & Zeidler, H. (2022). Plasma Electrolytic Polishing of Porous Nitinol Structures. Plasma, 5(4), 555-568. https://doi.org/10.3390/plasma5040039








