Swelling Behaviour of Sulfate Soil Treated with Lime–Metakaolin at Different Curing Ages
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Kaolin
2.1.2. Sulfate
2.1.3. Binder
2.2. Mix Compositions
2.3. Specimens’ Preparation
2.4. Testing Method
3. Results and Discussions
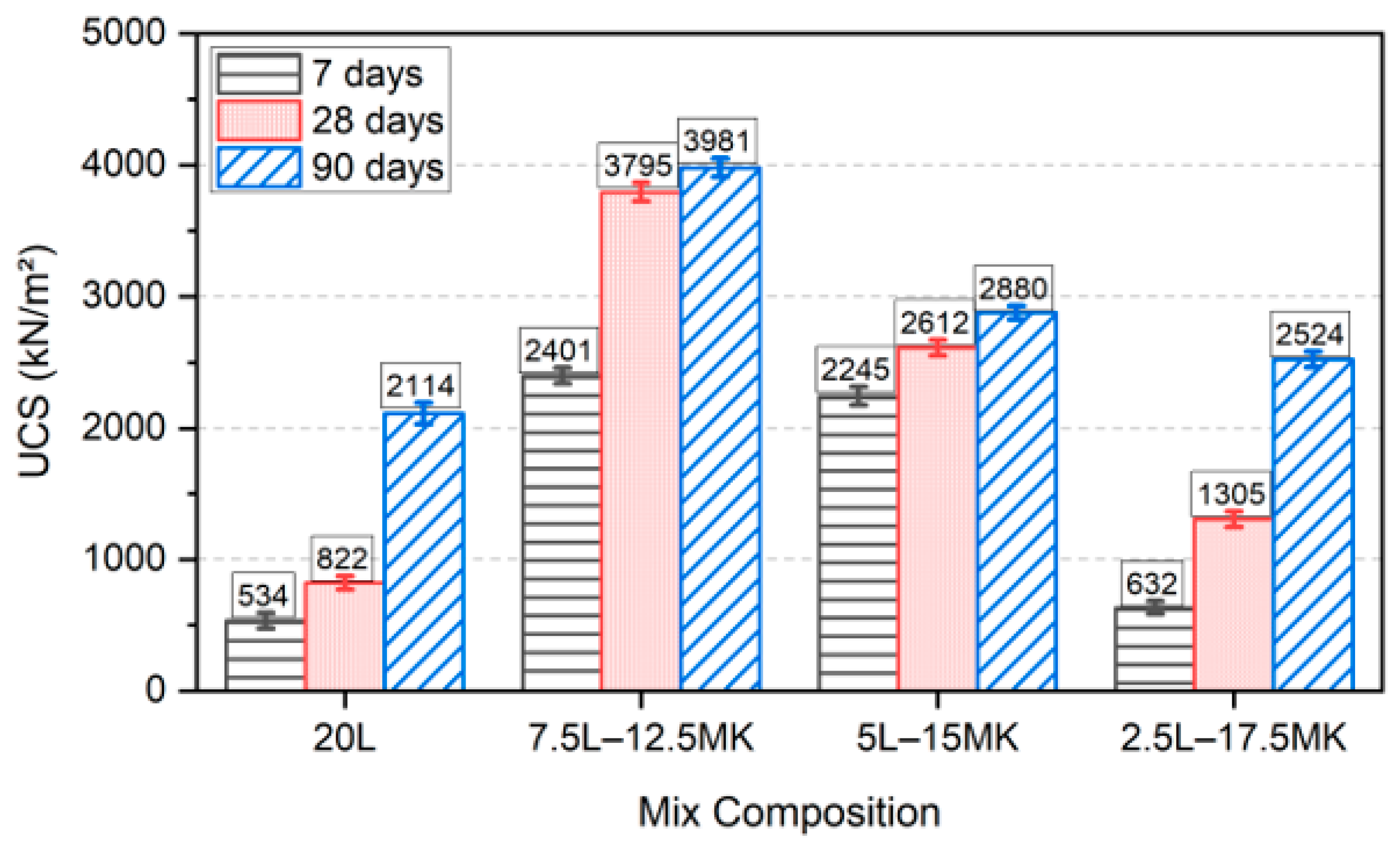
3.1. UCS Development
3.2. UCS of Soaked Specimens
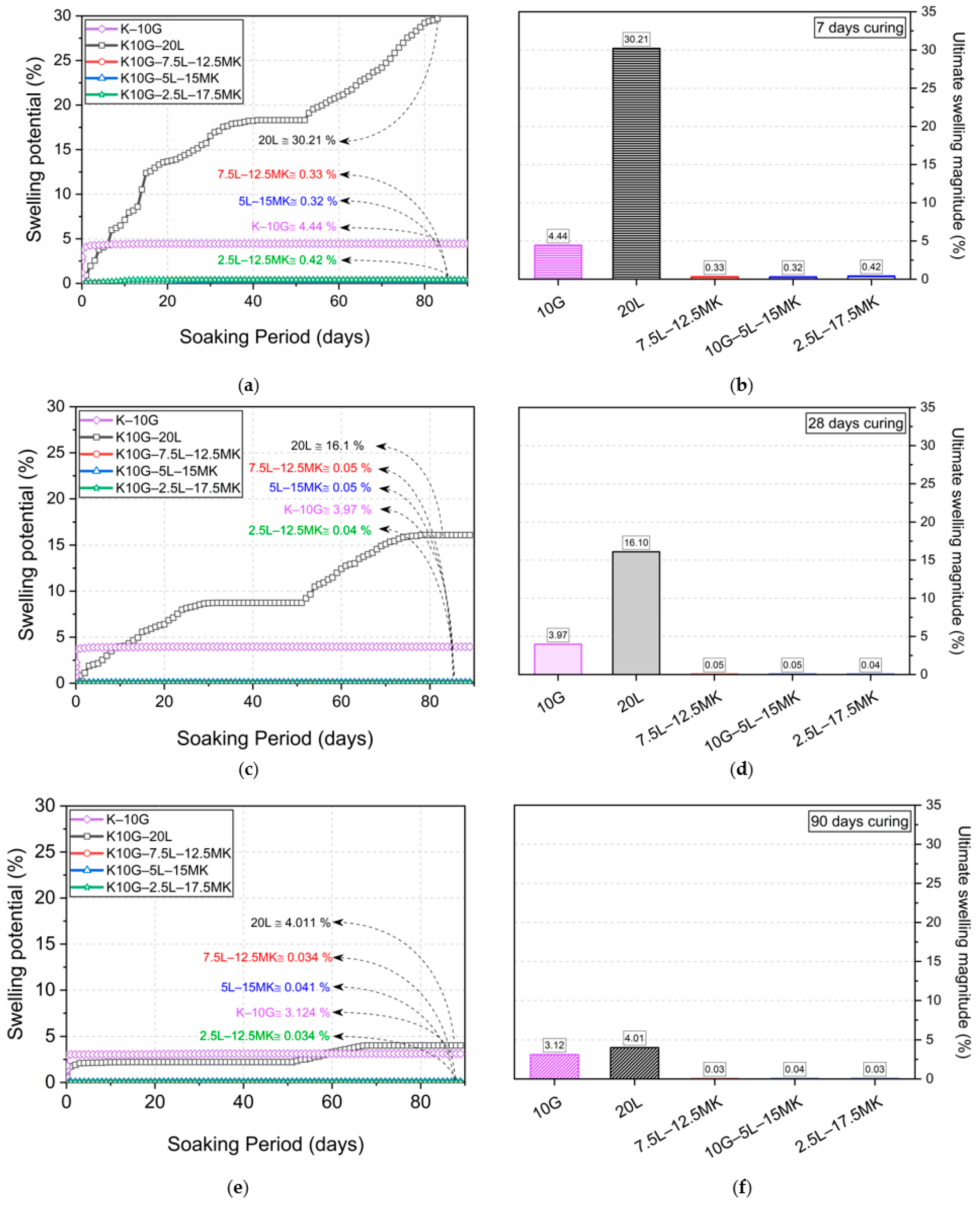
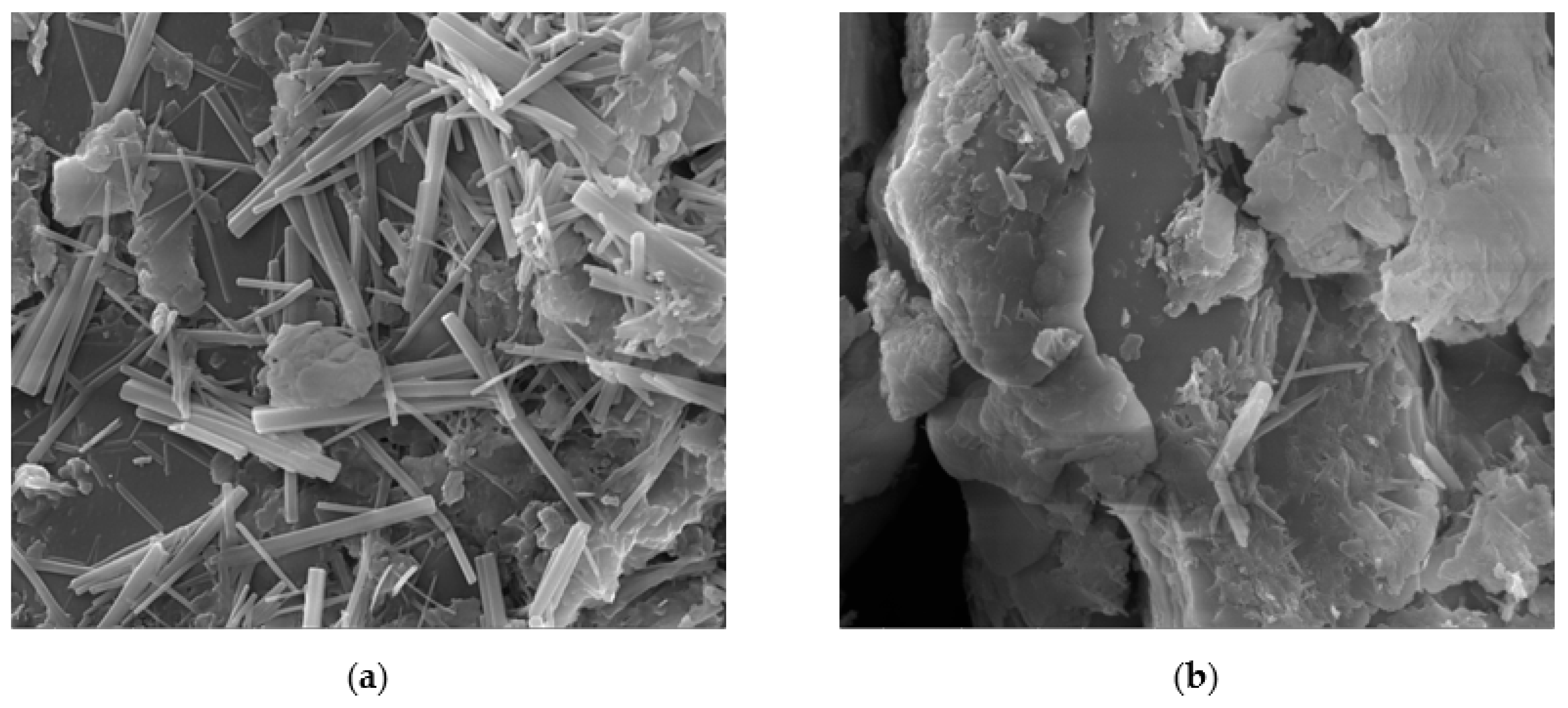
3.3. Swelling Behaviour
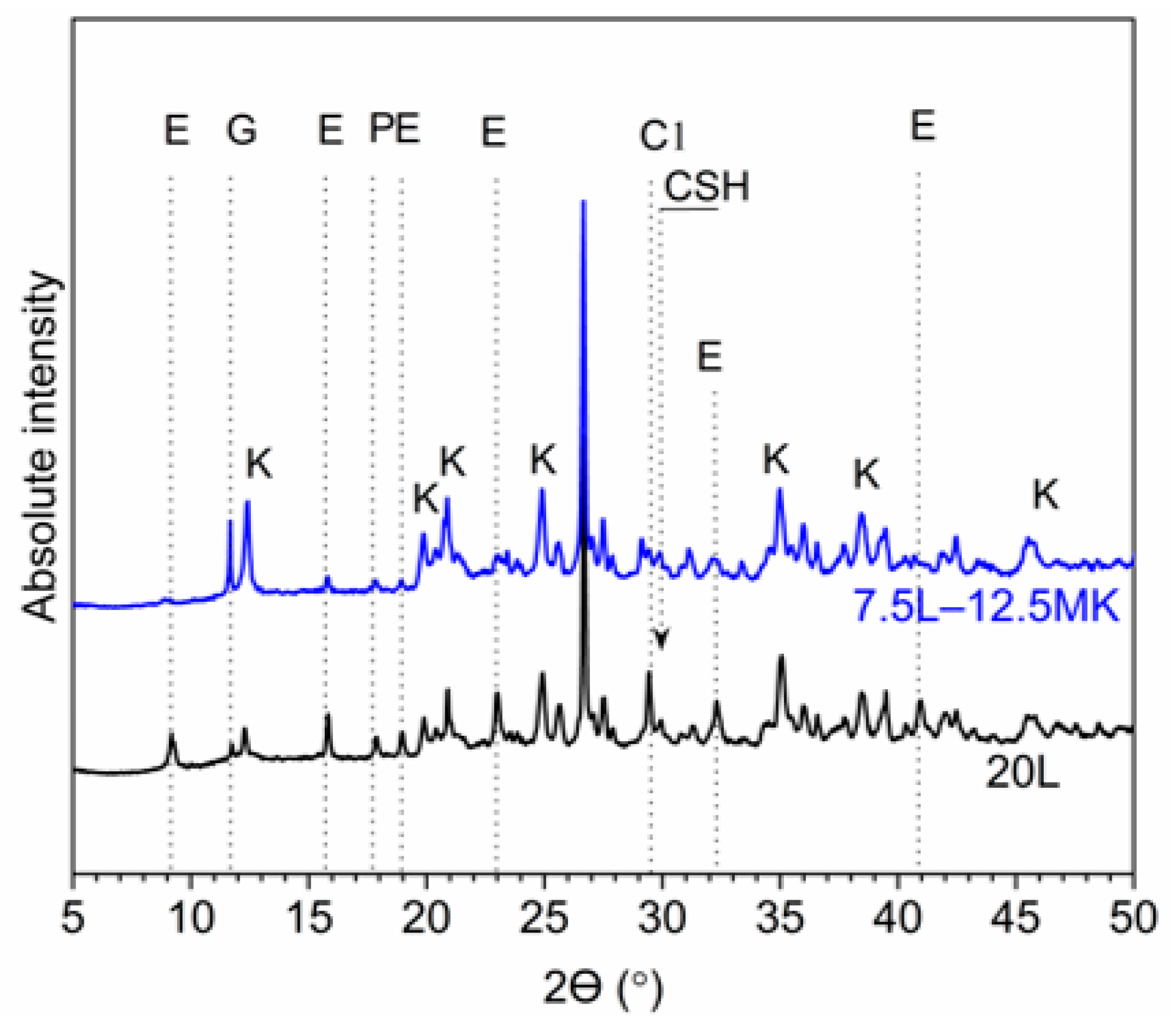
3.4. Analytical Analysis
4. Conclusions
- Replacement of lime with metakaolin remarkably improved the unconfined compressive strength and significantly suppressed the swelling potential of sulfate soil. This is probably due to its fineness, its high reactivity, and its significant capability in the decalcification of calcium hydroxide, which consequently promotes the restriction of ettringite and the neoformation of further hydrated compounds.
- The physico-mechanical assessment identified 7.5L–12.5MK as the optimal blend for sulfate soil stabilisation, specifically increasing the strength to about fourfold and suppressing the swelling to near-zero (0.33%).
- A high substitution level of lime with metakaolin suppresses the swelling potential of sulfate soil, but also induces a compromise on strength, probably because the faster depletion of lime negatively affects the fabric modification; thus, it is not recommended for better strength performance.
- The limitations of this research study, which may influence the authenticity of the findings, are the use of an artificial laboratory-blended sulfate soil and the use of a single calcination degree (700–750 °C) for the production of metakaolin from kaolin.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UCS | Unconfined Compressive Strength |
| CSH | Calcium Silicate Hydrate |
| CAH | Calcium Aluminate Hydrate |
| K | Kaolin |
| G | Gypsum |
| L | Lime |
| MK | Metakaolin |
References
- Li, H.; Radwan, A.E.; Yin, S.; Al-Gharbawi, A.S.A.; Najemalden, A.M.; Fattah, M.Y. Expansive Soil Stabilization with Lime, Cement, and Silica Fume. Appl. Sci. 2023, 13, 436. [Google Scholar] [CrossRef]
- Bazarbekova, A.; Shon, C.S.; Kissambinova, A.; Zhang, D.; Kim, J. Evaluating the Efficacy of Limestone Powder as a Partial Replacement of Ordinary Portland Cement for the Sustainable Stabilization of Sulfate-Bearing Saline Soil. Sustainability 2024, 16, 9224. [Google Scholar] [CrossRef]
- Khadka, S.D.; Jayawickrama, P.W.; Senadheera, S.; Segvic, B. Stabilization of Highly Expansive Soils Containing Sulfate Using Metakaolin and Fly Ash Based Geopolymer Modified with Lime and Gypsum. Transp. Geotech. 2020, 23, 100327. [Google Scholar] [CrossRef]
- Oti, J.E.; Kinuthia, J.M. Stabilised Unfired Clay Bricks for Environmental and Sustainable Use. Appl. Clay Sci. 2012, 58, 52–59. [Google Scholar] [CrossRef]
- Oti, J.E.; Kinuthia, J.M.; Bai, J. Compressive Strength and Microstructural Analysis of Unfired Clay Masonry Bricks. Eng. Geol. 2009, 109, 230–240. [Google Scholar] [CrossRef]
- Abdelbaset, A.M.; Katunský, D.; Zeleňáková, M.; El-Feky, M.H. Mechanical Properties Stabilization of Low Plasticity Kaolin Soil Using Fly Ash and Hydrated Lime. Case Stud. Constr. Mater. 2024, 21, e03662. [Google Scholar] [CrossRef]
- Diniz, B.C.; Fedrigo, W.; Kleinert, T.R.; dos Santos Batista, G.; Núñez, W.P.; Correa, B.M.; Brito, L.A.T. Lime Stabilization of Tropical Soil for Resilient Pavements: Mechanical, Microscopic, and Mineralogical Characteristics. Materials 2024, 17, 4720. [Google Scholar] [CrossRef] [PubMed]
- Negawo, W.J.; Di Emidio, G.; Bezuijen, A.; Verastegui Flores, R.D.; François, B. Lime-Stabilisation of High Plasticity Swelling Clay from Ethiopia. Eur. J. Environ. Civ. Eng. 2017, 23, 504–514. [Google Scholar] [CrossRef]
- Baldovino, J.A.; Moreira, E.B.; Izzo, R.L.d.S.; Rose, J.L. Empirical Relationships with Unconfined Compressive Strength and Split Tensile Strength for the Long Term of a Lime-Treated Silty Soil. J. Mater. Civ. Eng. 2018, 30, 06018008. [Google Scholar] [CrossRef]
- Li, W.; Yang, K.; Cheng, Y.; Huang, K.; Hu, Y.; Liu, L.; Li, X.; Li, W.; Yang, K.; Cheng, Y.; et al. Dynamic Mechanical Performance of Sulfate-Bearing Soils Stabilized by Magnesia-Ground Granulated Blast Furnace Slag. Sustainability 2024, 16, 4313. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Zhang, P.; Chen, Z. Strength Performance and Stabilization Mechanism of Fine Sandy Soils Stabilized with Cement and Metakaolin. Sustainability 2023, 15, 3431. [Google Scholar] [CrossRef]
- Seco, A.; Del Castillo, J.M.; Perlot, C.; Marcelino-Sádaba, S.; Prieto, E.; Espuelas, S. Experimental Study of the Valorization of Sulfate Soils for Use as Construction Material. Sustainability 2022, 14, 6609. [Google Scholar] [CrossRef]
- Ebailila, M.; Kinuthia, J.; Oti, J. Suppression of Sulfate-Induced Expansion with Lime–Silica Fume Blends. Materials 2022, 15, 2821. [Google Scholar] [CrossRef]
- Wu, Z.; Deng, Y.; Liu, S.; Liu, Q.; Chen, Y.; Zha, F. Strength and Micro-Structure Evolution of Compacted Soils Modified by Admixtures of Cement and Metakaolin. Appl. Clay Sci. 2016, 127–128, 44–51. [Google Scholar] [CrossRef]
- Ma, X.; Wei, P.; Jiang, P.; Xu, H.; Li, N.; Qian, S.; Wang, W.; Mei, G. Frost Resistance and Micro Mechanism of Metakaolin and Polypropylene Fiber Modified Coastal Cement Soil. J. Mater. Res. Technol. 2025, 35, 3058–3072. [Google Scholar] [CrossRef]
- Umar, I.H.; Abubakar, S.; Lin, H.; Hassan, J.I. Metakaolin as a Soil Stabilizing Admixture: A Comprehensive Analysis of California Bearing Ratio and Consolidation Behavior Using Experimental and Machine Learning Approaches. Earth Sci. Inform. 2025, 18, 200. [Google Scholar] [CrossRef]
- Dumpa, V.; Vipparty, R.; Mantripragada, A.K.; Raju, G.V.R.P. Evaluating the Strength Characteristics of Lime and Metakaolin Stabilized Expansive Soil. Lect. Notes Civ. Eng. 2019, 14, 239–248. [Google Scholar] [CrossRef]
- Huang, K.; Tang, H.; Cai, G.; Ma, D.; Huang, K.; Liu, L.; Wang, F. Research on the Dynamic Fracture Characteristics and Constitutive Model of Cement-Metakaolin Stabilized Soil under Freeze-Thaw Cycle Conditions. Constr. Build. Mater. 2025, 481, 141641. [Google Scholar] [CrossRef]
- Hayder, M.R.; Ziari, H.; Shaban, A.M. Characterizing Geotechnical Properties of Sand Subgrade Soils Stabilized with Geopolymer Based on Metakaolin. Case Stud. Constr. Mater. 2025, 22, e04389. [Google Scholar] [CrossRef]
- Sakr, M.A.; Azzam, W.R.; Meguid, M.A.; Hassan, A.F.; Ghoneim, H.A. Evaluation of Micro-Metakaolin and Ferric Chloride Solution in Stabilising Expansive Soils. Proc. Inst. Civ. Eng.—Ground Improv. 2022, 177, 103–115. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Cheng, Y.; Zhang, Y.; Bai, X. Effects of Coal-Bearing Metakaolin on the Compressive Strength and Permeability of Cemented Silty Soil and Mechanisms. Constr. Build. Mater. 2018, 186, 174–181. [Google Scholar] [CrossRef]
- Muhammad, A.; Yusuf, A.; Umar, M. Assessment of Lateritic Soil Stabilized Using Metakaolin. J. Geotech. Stud. 2020, 5, 15–26. [Google Scholar] [CrossRef]
- Xu, G.; Liu, S.; Ni, J.; Dai, D.; Li, M. Mechanical Properties and Durability of Lime–Metakaolin-Treated Saline Soil. J. Mater. Civ. Eng. 2025, 37, 04025369. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, R.; Guo, J.; Cai, G.; Li, Y. Study on Mechanical Properties and Microstructure of Metakaolin-Slag-Calcium Carbide Residue Synergistic Solidifying Waste Engineering Mud. Constr. Build. Mater. 2024, 438, 137135. [Google Scholar] [CrossRef]
- Dao, P.L.; Bui, V.D.; Onyelowe, K.C.; Ebid, A.M.; Le, V.D.; Ahaneku, I.E. Effect of Metakaolin on the Mechanical Properties of Lateritic Soil. Geotech. Res. 2022, 9, 211–218. [Google Scholar] [CrossRef]
- Hassannezhad, K.; Akyol, Y.; Dursun, M.C.; Ow-Yang, C.W.; Gulgun, M.A. Effect of Metakaolin and Lime on Strength Development of Blended Cement Paste. Constr. Mater. 2022, 2, 297–313. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Zhao, C.; Zhang, Z.; Kou, J.; Jing, Z. Experimental Study on Engineering Characteristics of Cement-Metakaolin Composite Modified Expansive Soil. Acad. J. Archit. Geotech. Eng. 2024, 6, 62–69. [Google Scholar] [CrossRef]
- Deng, T.; Deng, Y.; Yue, X.; Cui, Y. Deterioration of Marine Soft Clay at East China Solidified by Cementmetakaolin Composite. Environ. Geotech. 2021, 10, 57–65. [Google Scholar] [CrossRef]
- BS EN ISO 17892-4:2016; Geotechnical Investigation and Testing—Laboratory Testing of Soil—Determination of Particle Size Distribution. BSI Standards Limited: London, UK, 2016.
- Ehwailat, K.I.A.; Ismail, M.A.M.; Ezreig, A.M.A. Novel Approach for Suppression of Ettringite Formation in Sulfate-Bearing Soil Using Blends of Nano-Magnesium Oxide, Ground Granulated Blast-Furnace Slag and Rice Husk Ash. Appl. Sci. Artic. 2021, 11, 6618. [Google Scholar] [CrossRef]
- Ebailila, M.; Kinuthia, J.; Oti, J. Role of Gypsum Content on the Long-Term Performance of Lime-Stabilised Soil. Materials 2022, 15, 5099. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Recommended Practice for Stabilization of Sulfate-Rich Subgrade Soils; The National Academies Press: Washington, DC, USA, 2009; ISBN 9780309429825. [Google Scholar]
- Ehwailat, K.I.A.; Ismail, M.A.M.; Ezreig, A.M.A. Novel Approach to the Treatment of Gypseous Soil-Induced Ettringite Using Blends of Non-Calcium-Based Stabilizer, Ground Granulated Blast-Furnace Slag, and Metakaolin. Materials 2021, 14, 5198. [Google Scholar] [CrossRef]
- BS EN 13286-2:2010; Unbound and Hydraulically Bound Mixtures Part 2: Test Methods for Laboratory Reference Density and Water Content—Proctor Compaction. BSI Standards Ltd.: London, UK, 2012.
- BS EN ISO 17892-7:2018; Geotechnical Investigation and Testing-Laboratory Testing of Soil—Part 7: Unconfined Compression Test. BSI Standards Limited: London, UK, 2018; ISBN 178927:2017.
- Jha, A.K.; Sivapullaiah, P.V. Role of Gypsum on Microstructure and Strength of Soil. Environ. Geotech. 2016, 3, 78–89. [Google Scholar] [CrossRef]
- Ebailila, M. Sulfate Soil Stabilisation with Silica Fume-Based Binders. Ph.D. Thesis, University of South Wales, Pontypridd, UK, 2022. [Google Scholar]
- Zhang, J.; Deng, A.; Jaksa, M. Enhancing Mechanical Behavior of Micaceous Soil with Jute Fibers and Lime Additives. J. Rock Mech. Geotech. Eng. 2021, 13, 1093–1100. [Google Scholar] [CrossRef]
- Oluremi, J.R.; Ishola, K. Compaction and Strength Characteristics of Lead Contaminated Lateritic Soil Treated with Eco-Friendly Biopolymer for Use as Road Foundation Material. Hybrid Adv. 2024, 5, 100158. [Google Scholar] [CrossRef]
- BS EN 14227-15:2015; Hydraulically Bound Mixtures—Specifications, Part 15: Hydraulically Stabilized Soils. BSI Standards Limited: London, UK, 2015.
- BS 9227:2019; Hydraulically Bound Materials for Civil Engineering Purposes—Specification for Production and Installation in Pavements. BSI Standards Limited: London, UK, 2019.
- Rao, K.S.S.; Tripathy, S. Effect of Aging on Swelling and Swell-Shrink Behavior of a Compacted Expansive Soil. Geotech. Test. J. 2003, 26, 36–46. [Google Scholar] [CrossRef]
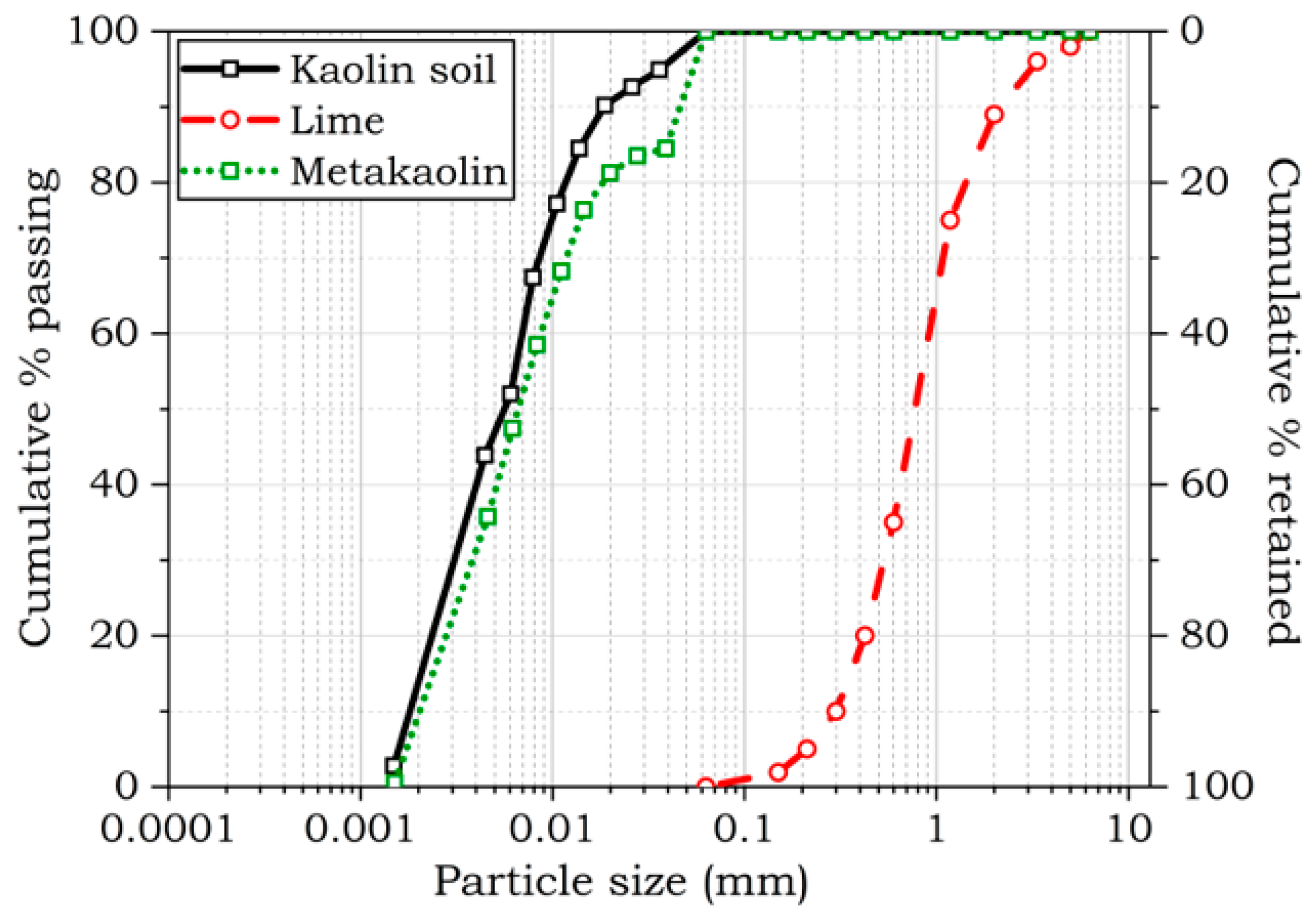



| Oxides and Elements | Compositions as Weight Percentages (%) | |||
|---|---|---|---|---|
| Kaolin | Gypsum | Lime | Metakaolin | |
| CaO | - | - | - | 0.2 |
| Ca(OH)2 | - | - | 92 | - |
| MgO | - | - | 3.5 | 0.1 |
| SiO2 | 58 | - | 2.5 | 52 |
| Al2O3 | 38 | - | 0.9 | 36 |
| Ca2SO4 | - | 99 | 0.1 | - |
| Fe | - | 0.005 | 0.06 | 8 |
| SO3 | - | - | - | - |
| LOI | 11–14 | 0.99 | 0.24 | 3.7 |
| Properties | Kaolin | Lime | Metakaolin |
|---|---|---|---|
| Plasticity index (%) | 19.7 | ||
| Swelling (%) | 4.8 | ||
| Specific gravity (mg/m3) | 2.5 | 2.2 | 2.3 |
| pH | 5 | 12 | 7 |
| Colour | White | White | White |
| Mix Code | Mix Compositions (%) | |||||
|---|---|---|---|---|---|---|
| Soil Material (%) | Water (%) | Stabiliser (%) | Stabiliser in % by Soil | |||
| Kaolin | Gypsum | Lime | Metakaolin | |||
| K10G–20L | 90 | 10 | 29.4 | 20 | 20 | - |
| K10G–2.5L–17.5MK | 90 | 10 | 29 | 20 | 2.5 | 17.5 |
| K10G–5L–15MK | 90 | 10 | 28.6 | 20 | 5 | 15 |
| K10G–7.5L–12.5MK | 90 | 10 | 28 | 20 | 7.5 | 12.5 |
| Mix Code | Compaction Parameters | |
|---|---|---|
| OMC (%) | MDD (Mg/m3) | |
| K–10G | 29 | 1.32 |
| K10G–20L | 29.4 | 1.27 |
| K10G–2.5L–17.5MK | 29 | 1.28 |
| K10G–5L–15MK | 28.6 | 1.31 |
| K10G–7.5L–12.5MK | 28 | 1.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebailila, M.; Ehwailat, K.; Oti, J. Swelling Behaviour of Sulfate Soil Treated with Lime–Metakaolin at Different Curing Ages. Ceramics 2025, 8, 133. https://doi.org/10.3390/ceramics8040133
Ebailila M, Ehwailat K, Oti J. Swelling Behaviour of Sulfate Soil Treated with Lime–Metakaolin at Different Curing Ages. Ceramics. 2025; 8(4):133. https://doi.org/10.3390/ceramics8040133
Chicago/Turabian StyleEbailila, Mansour, Khaled Ehwailat, and Jonathan Oti. 2025. "Swelling Behaviour of Sulfate Soil Treated with Lime–Metakaolin at Different Curing Ages" Ceramics 8, no. 4: 133. https://doi.org/10.3390/ceramics8040133
APA StyleEbailila, M., Ehwailat, K., & Oti, J. (2025). Swelling Behaviour of Sulfate Soil Treated with Lime–Metakaolin at Different Curing Ages. Ceramics, 8(4), 133. https://doi.org/10.3390/ceramics8040133







