Clinical Advances in Calcium Phosphate for Maxillomandibular Bone Regeneration: From Bench to Bedside
Abstract
1. Introduction
2. Method
3. Calcium Phosphate
3.1. Tricalcium Phosphate
3.2. Alpha-Tricalcium Phosphate (α-TCP)
3.3. Beta-Tricalcium Phosphate (β-TCP)
3.4. Tetracalcium Phosphate (TTCP)
3.5. Dicalcium Phosphate Dehydrate
3.6. Dicalcium Phosphate Anhydrous (Monetite)
3.7. Octacalcium Phosphate (OCP)
3.8. HA with Low Crystallinity
3.9. Biphasic CP
3.10. Calcium Silicate
3.11. Bioactive Surface
3.11.1. Bioglass and Glass-Ceramics
3.11.2. Bioactivity
3.11.3. Biodegradability
3.11.4. Porosity
4. The Role of Calcium Phosphate in Osteogenesis
5. Bioceramics for Bone Tissue Regeneration in Clinical Trials
6. Future Directions
7. Limitations
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shimizu, S.; Tsuchiya, S.; Hirakawa, A.; Kato, K.; Ando, M.; Mizuno, M.; Osugi, M.; Okabe, K.; Katagiri, W.; Hibi, H. Design of a Randomized Controlled Clinical Study of tissue-engineered osteogenic materials using bone marrow-derived mesenchymal cells for Maxillomandibular bone defects in Japan: The TEOM study protocol. BMC Oral Health 2019, 19, 69. [Google Scholar] [CrossRef]
- Probst, F.A.; Fliefel, R.; Burian, E.; Probst, M.; Eddicks, M.; Cornelsen, M.; Riedl, C.; Seitz, H.; Aszódi, A.; Schieker, M.; et al. Bone regeneration of minipig mandibular defect by adipose derived mesenchymal stem cells seeded tri-calcium phosphate- poly(D,L-lactide-co-glycolide) scaffolds. Sci. Rep. 2020, 10, 2062. [Google Scholar] [CrossRef]
- Bonardi, J.P.; Pereira, R.d.S.; Mourão, C.F.; Mendes, B.C.; Lowenstein, A.; Montemezzi, P.; Giubilato, F.; Okamoto, R.; Hochuli-Vieira, E. Clinical Assessment of Biphasic Calcium Phosphate in Granules and Paste Forms in Human Maxillary Sinus Bone Augmentation: A Randomized, Split-Mouth Clinical Trial. Materials 2023, 16, 1059. [Google Scholar] [CrossRef]
- Kretlow, J.D.; Young, S.; Klouda, L.; Wong, M.; Mikos, A.G. Injectable biomaterials for regenerating complex craniofacial tissues. Adv. Mater. 2009, 21, 3368–3393. [Google Scholar] [CrossRef]
- Lin, H.K.; Pan, Y.H.; Salamanca, E.; Lin, Y.T.; Chang, W.J. Prevention of Bone Resorption by HA/β-TCP + Collagen Composite after Tooth Extraction: A Case Series. Int. J. Environ. Res. Public Health 2019, 16, 4616. [Google Scholar] [CrossRef]
- Chuang, K.-W.; Liu, Y.-C.; Balaji, R.; Chiu, Y.-C.; Yu, J.; Liao, Y.-C. Enhancing Stability of High-Concentration β-Tricalcium Phosphate Suspension for Biomedical Application. Materials 2022, 16, 228. [Google Scholar] [CrossRef]
- Shaffer, C.D.; App, G.R. The use of plaster of paris in treating infrabony periodontal defects in humans. J. Periodontol. 1971, 42, 685–690. [Google Scholar] [CrossRef]
- Couri, C.J.; Maze, G.I.; Hinkson, D.W.; Collins, B.H., III; Dawson, D.V. Medical grade calcium sulfate hemihydrate versus expanded polytetrafluoroethylene in the treatment of mandibular class II furcations. J. Periodontol. 2002, 73, 1352–1359. [Google Scholar] [CrossRef]
- Bădilă, A.E.; Rădulescu, D.M.; Niculescu, A.-G.; Grumezescu, A.M.; Rădulescu, M.; Rădulescu, A.R. Recent Advances in the Treatment of Bone Metastases and Primary Bone Tumors: An Up-to-Date Review. Cancers 2021, 13, 4229. [Google Scholar] [CrossRef]
- Kim, J.H.; Oh, J.H.; Han, I.; Kim, H.-S.; Chung, S.W. Grafting using injectable calcium sulfate in bone tumor surgery: Comparison with demineralized bone matrix-based grafting. Clin. Orthop. Surg. 2011, 3, 191–201. [Google Scholar] [CrossRef]
- Cheah, C.W.; Al-Namnam, N.M.; Lau, M.N.; Lim, G.S.; Raman, R.; Fairbairn, P.; Ngeow, W.C. Synthetic Material for Bone, Periodontal, and Dental Tissue Regeneration: Where Are We Now, and Where Are We Heading Next? Materials 2021, 14, 6123. [Google Scholar] [CrossRef]
- Millan, C.; Vivanco, J.F.; Benjumeda-Wijnhoven, I.M.; Bjelica, S.; Santibanez, J.F. Mesenchymal Stem Cells and Calcium Phosphate Bioceramics: Implications in Periodontal Bone Regeneration. Adv. Exp. Med. Biol. 2018, 1107, 91–112. [Google Scholar]
- Lauritano, D.; Limongelli, L.; Moreo, G.; Favia, G.; Carinci, F. Nanomaterials for Periodontal Tissue Engineering: Chitosan-Based Scaffolds. A Systematic Review. Nanomaterials 2020, 10, 605. [Google Scholar] [CrossRef]
- Pérez-Cortez, J.E.; Sánchez-Rodríguez, V.H.; Gallegos-Martínez, S.; Chuck-Hernández, C.; Rodriguez, C.A.; Álvarez, M.M.; Santiago, G.T.-D.; Vázquez-Lepe, E.; Martínez-López, J.I. Low-Cost Light-Based GelMA 3D Bioprinting via Retrofitting: Manufacturability Test and Cell Culture Assessment. Micromachines 2022, 14, 55. [Google Scholar] [CrossRef]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef]
- Fu, P.-S.; Wang, J.-C.; Lai, P.-L.; Liu, S.-M.; Chen, Y.-S.; Chen, W.-C.; Hung, C.-C. Biodegradable Hydrogel Beads Combined with Calcium Phosphate Bone Cement for Bone Repair: In Vitro and In Vivo Characterization. Polymers 2022, 14, 505. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Lode, A.; Richter, R.F.; Pradel, W.; Franke, A.; Rauner, M.; Stadlinger, B.; Lauer, G.; Gelinsky, M.; Korn, P. Toward Biofabrication of Resorbable Implants Consisting of a Calcium Phosphate Cement and Fibrin—A Characterization In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 1218. [Google Scholar] [CrossRef]
- Köse, S.; Kankilic, B.; Gizer, M.; Ciftci Dede, E.; Bayramli, E.; Korkusuz, P.; Korkusuz, F. Stem Cell and Advanced Nano Bioceramic Interactions. Adv. Exp. Med. Biol. 2018, 1077, 317–342. [Google Scholar]
- Bellucci, D.; Sola, A.; Cannillo, V. Hydroxyapatite and tricalcium phosphate composites with bioactive glass as second phase: State of the art and current applications. J. Biomed. Mater. Res. A 2016, 104, 1030–1056. [Google Scholar] [CrossRef]
- Terzioğlu, P.; Öğüt, H.; Kalemtaş, A. Natural calcium phosphates from fish bones and their potential biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 899–911. [Google Scholar] [CrossRef]
- Pazarçeviren, A.E.; Tezcaner, A.; Keskin, D.; Kolukısa, S.T.; Sürdem, S.; Evis, Z. Boron-doped Biphasic Hydroxyapatite/β-Tricalcium Phosphate for Bone Tissue Engineering. Biol. Trace Elem. Res. 2021, 199, 968–980. [Google Scholar] [CrossRef]
- Carrodeguas, R.G.; De Aza, S. α-Tricalcium phosphate: Synthesis, properties and biomedical applications. Acta Biomater. 2011, 7, 3536–3546. [Google Scholar] [CrossRef]
- Tarafder, S.; Dernell, W.S.; Bandyopadhyay, A.; Bose, S. SrO- and MgO-doped microwave sintered 3D printed tricalcium phosphate scaffolds: Mechanical properties and in vivo osteogenesis in a rabbit model. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 679–690. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Chen, I.-C.; Su, C.-Y.; Tsai, H.-H.; Young, T.-H.; Fang, H.-W. Development of Injectable Calcium Sulfate and Self-Setting Calcium Phosphate Composite Bone Graft Materials for Minimally Invasive Surgery. Int. J. Mol. Sci. 2022, 23, 7590. [Google Scholar] [CrossRef]
- Moseke, C.; Gbureck, U. Tetracalcium phosphate: Synthesis, properties and biomedical applications. Acta Biomater. 2010, 6, 3815–3823. [Google Scholar] [CrossRef]
- Golfeshan, F.; Mosaddad, S.A.; Ghaderi, F. The Effect of Toothpastes Containing Natural Ingredients Such As Theobromine and Caffeine on Enamel Microhardness: An In Vitro Study. Evid. Based Complement. Altern. Med. 2021, 2021, 3304543. [Google Scholar] [CrossRef]
- Nimmawitt, P.; Sittikornpaiboon, P.; Jaemsuwan, S.; Arunjaroensuk, S.; Wang, J.-C.; Hung, C.-C.; Kaboosaya, B.; Pimkhaokham, A. Pimkhaokham, The stability of tetracalcium phosphate/titanium implants: A short-term follow-up study. J. Dent. Sci. 2022, 17, 1030–1034. [Google Scholar] [CrossRef]
- Safronova, T.; Kiselev, A.; Selezneva, I.; Shatalova, T.; Lukina, Y.; Filippov, Y.; Toshev, O.; Tikhonova, S.; Antonova, O.; Knotko, A. Knotko, Bioceramics Based on β-Calcium Pyrophosphate. Materials 2022, 15, 3105. [Google Scholar] [CrossRef]
- Moussa, H.; Jiang, W.; Alsheghri, A.; Mansour, A.; El Hadad, A.; Pan, H.; Tang, R.; Song, J.; Vargas, J.; McKee, M.D.; et al. High strength brushite bioceramics obtained by selective regulation of crystal growth with chiral biomolecules. Acta Biomater. 2020, 106, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, L.; Gbureck, U.; Bhaduri, S.B.; Sikder, P. Monetite, an important calcium phosphate compound-Its synthesis, properties and applications in orthopedics. Acta Biomater. 2021, 127, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, F.; Le Nihouannen, D.; Eimar, H.; Sheikh, Z.; Komarova, S.; Barralet, J.; Tamimi, F. The effect of autoclaving on the physical and biological properties of dicalcium phosphate dihydrate bioceramics: Brushite vs. monetite. Acta Biomater. 2012, 8, 3161–3169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; You, J.; Wang, Z.; Gu, Y.; Chen, D.; Lin, B.; Zhao, X.; Lin, J.; Lin, J.; Liu, W. 3D printing monetite-coated Ti-6Al-4V surface with osteoimmunomodulatory function to enhance osteogenesis. Biomater. Adv. 2022, 134, 112562. [Google Scholar] [CrossRef]
- Crane, N.J.; Popescu, V.; Morris, M.D.; Steenhuis, P.; Ignelzi, M.A., Jr. Raman spectroscopic evidence for octacalcium phosphate and other transient mineral species deposited during intramembranous mineralization. Bone 2006, 39, 434–442. [Google Scholar] [CrossRef]
- Suzuki, O.; Nakamura, M.; Miyasaka, Y.; Kagayama, M.; Sakurai, M. Maclura pomifera agglutinin-binding glycoconjugates on converted apatite from synthetic octacalcium phosphate implanted into subperiosteal region of mouse calvaria. Bone Min. 1993, 20, 151–166. [Google Scholar] [CrossRef]
- Honda, Y.; Anada, T.; Kamakura, S.; Morimoto, S.; Kuriyagawa, T.; Suzuki, O. The effect of microstructure of octacalcium phosphate on the bone regenerative property. Tissue Eng. Part A 2009, 15, 1965–1973. [Google Scholar] [CrossRef]
- Liu, X.; Shiwaku, Y.; Hamai, R.; Tsuchiya, K.; Takahashi, T.; Suzuki, O. Effect of Octacalcium Phosphate Crystals on the Osteogenic Differentiation of Tendon Stem/Progenitor Cells In Vitro. Int. J. Mol. Sci. 2023, 24, 1235. [Google Scholar] [CrossRef]
- Yokoi, T.; Shimabukuro, M.; Kawashita, M. Octacalcium phosphate with incorporated carboxylate ions: A review. Sci. Technol. Adv. Mater. 2022, 23, 434–445. [Google Scholar] [CrossRef]
- Orii, Y.; Masumoto, H.; Honda, Y.; Anada, T.; Goto, T.; Sasaki, K.; Suzuki, O. Enhancement of octacalcium phosphate deposition on a titanium surface activated by electron cyclotron resonance plasma oxidation. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 93, 476–483. [Google Scholar] [CrossRef]
- Suzuki, O.; Kamakura, S.; Katagiri, T.; Nakamura, M.; Zhao, B.; Honda, Y.; Kamijo, R. Bone formation enhanced by implanted octacalcium phosphate involving conversion into Ca-deficient hydroxyapatite. Biomaterials 2006, 27, 2671–2681. [Google Scholar] [CrossRef]
- Thrivikraman, G.; Athirasala, A.; Twohig, C.; Boda, S.K.; Bertassoni, L.E. Bertassoni, Biomaterials for Craniofacial Bone Regeneration. Dent. Clin. N. Am. 2017, 61, 835–856. [Google Scholar] [CrossRef] [PubMed]
- Takami, M.; Mochizuki, A.; Yamada, A.; Tachi, K.; Zhao, B.; Miyamoto, Y.; Anada, T.; Honda, Y.; Inoue, T.; Nakamura, M.; et al. Osteoclast differentiation induced by synthetic octacalcium phosphate through receptor activator of NF-kappaB ligand expression in osteoblasts. Tissue Eng. Part A 2009, 15, 3991–4000. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, O.; Shiwaku, Y.; Hamai, R. Octacalcium phosphate bone substitute materials: Comparison between properties of biomaterials and other calcium phosphate materials. Dent. Mater. J. 2020, 39, 187–199. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. Engl. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Szpalski, C.; Barr, J.; Wetterau, M.; Saadeh, P.B.; Warren, S.M. Cranial bone defects: Current and future strategies. Neurosurg. Focus. 2010, 29, E8. [Google Scholar] [CrossRef]
- Inagaki, M.; Kameyama, T. Phase transformation of plasma-sprayed hydroxyapatite coating with preferred crystalline orientation. Biomaterials 2007, 28, 2923–2931. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, S.; Wang, Y.; Yu, Z.; Ao, H.; Zhang, H.; Qin, L.; Guillaume, O.; Eglin, D.; Richards, R.G.; et al. Anti-infective efficacy, cytocompatibility and biocompatibility of a 3D-printed osteoconductive composite scaffold functionalized with quaternized chitosan. Acta Biomater. 2016, 46, 112–128. [Google Scholar] [CrossRef]
- Gonçalves, E.M.; Oliveira, F.J.; Silva, R.F.; Neto, M.A.; Fernandes, M.H.; Amaral, M.; Vallet-Regí, M.; Vila, M. Three-dimensional printed PCL-hydroxyapatite scaffolds filled with CNTs for bone cell growth stimulation. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1210–1219. [Google Scholar] [CrossRef]
- Zhu, Q.; Jiao, H.; Zhao, X.; Tang, Y.; Zhao, K.; Gou, X. Hydroxyapatite formation in biomimetic synthesis with the interface of a pDA@SIS membrane. RSC Adv. 2022, 12, 13209–13219. [Google Scholar] [CrossRef]
- Le Nihouannen, D.; Duval, L.; Lecomte, A.; Julien, M.; Guicheux, J.; Daculsi, G.; Layrolle, P. Interactions of total bone marrow cells with increasing quantities of macroporous calcium phosphate ceramic granules. J. Mater. Sci. Mater. Med. 2007, 18, 1983–1990. [Google Scholar] [CrossRef]
- Witek, L.; Smay, J.; Silva, N.R.F.A.; Guda, T.; Ong, J.L.; Coelho, P.G. Sintering effects on chemical and physical properties of bioactive ceramics. J. Adv. Ceram. 2013, 2, 274–284. [Google Scholar] [CrossRef]
- Wu, S.-C.; Hsu, H.-C.; Hsu, S.-K.; Wang, W.-H.; Ho, W.-F. Preparation and characterization of four different compositions of calcium phosphate scaffolds for bone tissue engineering. Mater. Charact. 2011, 62, 526–534. [Google Scholar] [CrossRef]
- Zhao, N.; Wang, Y.; Qin, L.; Guo, Z.; Li, D. Effect of composition and macropore percentage on mechanical and in vitro cell proliferation and differentiation properties of 3D printed HA/β-TCP scaffolds. RSC Adv. 2017, 7, 43186–43196. [Google Scholar] [CrossRef]
- Lu, J.; Blary, M.-C.; Vavasseur, S.; Descamps, M.; Anselme, K.; Hardouin, P. Relationship between bioceramics sintering and micro-particles-induced cellular damages. J. Mater. Sci. Mater. Med. 2004, 15, 361–365. [Google Scholar] [CrossRef]
- Lei, W.; Wu, Y.; He, P.; Wu, J.; Chen, J.; Liu, Y.; Zhang, H.; de Bruijn, J.D.; Bao, C.; Li, Y.; et al. Osteoclastogenesis-characterized osteoinductive biphasic calcium phosphate ceramic for bone regeneration in rabbit maxillary sinus lift. J. Mater. Chem. B 2025, 13, 5880–5897. [Google Scholar] [CrossRef]
- Xu, S.; Lin, K.; Wang, Z.; Chang, J.; Wang, L.; Lu, J.; Ning, C. Reconstruction of calvarial defect of rabbits using porous calcium silicate bioactive ceramics. Biomaterials 2008, 29, 2588–2596. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Z.; Han, J.; Jiang, X.; Lei, L.; Yang, X.; Sun, W.; Gou, Z.; Chen, L. Modularized bioceramic scaffold/hydrogel membrane hierarchical architecture beneficial for periodontal tissue regeneration in dogs. Biomater. Res. 2022, 26, 68. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Ma, B.; Zhu, H.; Huan, Z.; Ma, N.; Chang, J. 3D-Printed Bioactive Ca3SiO5 Bone Cement Scaffolds with Nano Surface Structure for Bone Regeneration. ACS Appl. Mater. Interfaces 2017, 9, 5757–5767. [Google Scholar] [CrossRef]
- Pan, H.; Deng, L.; Huang, L.; Zhang, Q.; Yu, J.; Huang, Y.; Chen, L.; Chang, J. Chang, 3D-printed Sr2ZnSi2O7 scaffold facilitates vascularized bone regeneration through macrophage immunomodulation. Front. Bioeng. Biotechnol. 2022, 10, 1007535. [Google Scholar] [CrossRef]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive Glasses: Where Are We and Where Are We Going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef]
- Schepers, E.J.G.; Ducheyne, P. Bioactive glass particles of narrow size range for the treatment of oral bone defects: A 1–24 month experiment with several materials and particle sizes and size ranges. J. Oral Rehabil. 1997, 24, 171–181. [Google Scholar] [CrossRef]
- Mariano, L.C.; Fernandes, M.H.R.; Gomes, P.S. Antimicrobial Biomaterials for the Healing of Infected Bone Tissue: A Systematic Review of Microtomographic Data on Experimental Animal Models. J. Funct. Biomater. 2022, 13, 193. [Google Scholar] [CrossRef]
- Vitale-Brovarone, C.; Baino, F.; Verné, E. High strength bioactive glass-ceramic scaffolds for bone regeneration. J. Mater. Sci. Mater. Med. 2009, 20, 643–653. [Google Scholar] [CrossRef]
- Varanasi, V.G.; Owyoung, J.B.; Saiz, E.; Marshall, S.J.; Marshall, G.W.; Loomer, P.M. The ionic products of bioactive glass particle dissolution enhance periodontal ligament fibroblast osteocalcin expression and enhance early mineralized tissue development. J. Biomed. Mater. Res. A 2011, 98, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Subbaiah, R.; Thomas, B. Efficacy of a bioactive alloplast, in the treatment of human periodontal osseous defects-a clinical study. Med. Oral Patol. Oral Cir. Bucal 2011, 16, e239–e244. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.S.; Narula, S.C.; Sharma, R.K.; Tewari, S.; Yadav, R. Clinical evaluation of guided tissue regeneration combined with autogenous bone or autogenous bone mixed with bioactive glass in intrabony defects. J. Oral Sci. 2011, 53, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, P.; Büttner, T.; Pacheco, V.M.; Boccaccini, A.R. Boron-containing bioactive glasses in bone and soft tissue engineering. J. Eur. Ceram. Soc. 2018, 38, 855–869. [Google Scholar] [CrossRef]
- Crider, K.S.; Williams, J.L.; Qi, Y.P.; Gutman, J.; Yeung, L.F.; Mai, C.T.; Finkelstein, J.L.; Mehta, S.; Pons-Duran, C.; Menéndez, C.; et al. Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. Cochrane Database Syst. Rev. 2022, 2022, CD014217. [Google Scholar] [CrossRef]
- Sadiasa, A.; Sarkar, S.K.; Franco, R.A.; Min, Y.K.; Lee, B.T. Bioactive glass incorporation in calcium phosphate cement-based injectable bone substitute for improved in vitro biocompatibility and in vivo bone regeneration. J. Biomater. Appl. 2014, 28, 739–756. [Google Scholar] [CrossRef]
- Douglas, T.E.L.; Piwowarczyk, W.; Pamula, E.; Liskova, J.; Schaubroeck, D.; Leeuwenburgh, S.C.G.; Brackman, G.; Balcaen, L.; Detsch, R.; Declercq, H.; et al. Injectable self-gelling composites for bone tissue engineering based on gellan gum hydrogel enriched with different bioglasses. Biomed. Mater. 2014, 9, 045014. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; Arcos, D.; Vallet-Regí, M. Upgrading calcium phosphate scaffolds for tissue engineering applications. Key Eng. Mater. 2008, 377, 19–42. [Google Scholar] [CrossRef]
- Kuboki, Y.; Jin, Q.; Takita, H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J. Bone Jt. Surg. 2001, 83, S105–S115. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Zhou, L.; Chen, C.; Chen, G. Sequential release of vascular endothelial growth factor-A and bone morphogenetic protein-2 from osteogenic scaffolds assembled by PLGA microcapsules: A preliminary study in vitro. Int. J. Biol. Macromol. 2023, 232, 123330. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, M.; Selvam, R.E. Review of Physical, Mechanical, and Biological Characteristics of 3D-Printed Bioceramic Scaffolds for Bone Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2022, 8, 5060–5093. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Liu, Y. Mesenchymal Stromal/Stem Cell (MSC)-Based Vector Biomaterials for Clinical Tissue Engineering and Inflammation Research: A Narrative Mini Review. J. Inflamm. Res. 2023, 16, 257–267. [Google Scholar] [CrossRef]
- Gou, Y.; Qi, K.; Wei, Y.; Gu, Z.; Xie, H. Advances of calcium phosphate nanoceramics for the osteoinductive potential and mechanistic pathways in maxillofacial bone defect repair. Nano TransMed 2024, 3, 100033. [Google Scholar] [CrossRef]
- Peng, Y.; Zhuang, Y.; Liu, Y.; Le, H.; Li, D.; Zhang, M.; Liu, K.; Zhang, Y.; Zuo, J.; Ding, J. Bioinspired gradient scaffolds for osteochondral tissue engineering. Exploration 2023, 3, 20210043. [Google Scholar] [CrossRef]
- Jiang, Y.; Qin, H.; Wan, H.; Yang, J.; Yu, Q.; Jiang, M.; Yu, B. Asprin-loaded strontium-containing α-calcium sulphate hemihydrate/nano-hydroxyapatite composite promotes regeneration of critical bone defects. J. Cell. Mol. Med. 2020, 24, 13690–13702. [Google Scholar] [CrossRef]
- Nie, Z.; Hu, Z.; Guo, X.; Xiao, Y.; Liu, X.; de Bruijn, J.D.; Bao, C.; Yuan, H. Genesis of osteoclasts on calcium phosphate ceramics and their role in material-induced bone formation. Acta Biomater. 2023, 157, 625–638. [Google Scholar] [CrossRef]
- Dehkord, E.S.; De Carvalho, B.; Ernst, M.; Albert, A.; Lambert, F.; Geris, L. Influence of physicochemical characteristics of calcium phosphate-based biomaterials in cranio-maxillofacial bone regeneration. A systematic literature review and meta-analysis of preclinical models. Mater. Today Bio 2024, 26, 101100. [Google Scholar]
- Zhou, Y.; You, D.; Xu, M.; Shao, Y.; Hu, X.; Xie, Y.; Ma, H.; Lan, R.; Shen, Y.; Mao, Y.; et al. Bionic repair strategy for craniomaxillofacial bone tissue reconstruction. Transl. Dent. Res. 2025, 1, 100037. [Google Scholar] [CrossRef]
- Schulz, M.C.; Holtzhausen, S.; Nies, B.; Heinemann, S.; Muallah, D.; Kroschwald, L.; Paetzold-Byhain, K.; Lauer, G.; Sembdner, P. Sembdner Three-Dimensional Plotted Calcium Phosphate Scaffolds for Bone Defect Augmentation—A New Method for Regeneration. J. Pers. Med. 2023, 13, 464. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Kamakura, S.; Matsui, K.; Fukuda, M.; Takano, H.; Iino, M.; Ishikawa, S.; Kawana, H.; Soma, T.; Imamura, E.; et al. Clinical study of octacalcium phosphate and collagen composite in oral and maxillofacial surgery. J. Tissue Eng. 2020, 11, 2041731419896449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, P.; Li, X.; Wang, Y.; Qin, Z.; Zhang, C.; Li, J. Reconstruction of mandibular bone defects using biphasic calcium phosphate bone substitutes with simultaneous implant placement in mini-swine: A pilot in vivo study. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2071–2079. [Google Scholar] [CrossRef]
- Mahmoud, E.M.; Sayed, M.; Mansour, T.S.; Naga, S.M. Biodegradable ceramic materials for orthopedic and dentistry applications. Discov. Appl. Sci. 2025, 7, 990. [Google Scholar] [CrossRef]
- Miri, Z.; Haugen, H.J.; Loca, D.; Rossi, F.; Perale, G.; Moghanian, A.; Ma, Q. Review on the strategies to improve the mechanical strength of highly porous bone bioceramic scaffolds. J. Eur. Ceram. Soc. 2024, 44, 23–42. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Apostolova, M.D. Advances in Multifunctional Bioactive Coatings for Metallic Bone Implants. Materials 2022, 16, 183. [Google Scholar] [CrossRef]
- Beltrán, A.M.; Trueba, P.; Borie, F.; Alcudia, A.; Begines, B.; Rodriguez-Ortiz, J.A.; Torres, Y. 12—Bioactive ceramic composite material stability, characterization, and bonding to bone. In Fundamental Biomaterials: Ceramics; Thomas, S., Balakrishnan, P., Sreekala, M.S., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 273–296. [Google Scholar]
- de Carvalho, A.B.G.; Rahimnejad, M.; Oliveira, R.L.M.S.; Sikder, P.; Saavedra, G.S.F.A.; Bhaduri, S.B.; Gawlitta, D.; Malda, J.; Kaigler, D.; Trichês, E.S.; et al. Personalized bioceramic grafts for craniomaxillofacial bone regeneration. Int. J. Oral Sci. 2024, 16, 62. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Mishchenko, O.; Yanovska, A.; Kosinov, O.; Maksymov, D.; Moskalenko, R.; Ramanavicius, A.; Pogorielov, M. Pogorielov Synthetic Calcium–Phosphate Materials for Bone Grafting. Polymers 2023, 15, 3822. [Google Scholar] [CrossRef]
- Tripathi, R.; Samadi, F.M.; Kumar, S. Treatment of cystic lesion of mandible using combination of modified bone granules and calcium phosphate bone cement: A preliminary report. J. Oral Biol. Craniofac. Res. 2016, 6 (Suppl. 1), S33–S38. [Google Scholar] [CrossRef]
- Hosseini, S. A Review of Bone Cements as Injectable Materials for Treatment of Bone-Related Diseases: Current Status and Future Developments. J. Res. Orthop. Sci. 2022, 9, 1–14. [Google Scholar] [CrossRef]
- De Pace, R.; Molinari, S.; Mazzoni, E.; Perale, G. Perale Bone Regeneration: A Review of Current Treatment Strategies. J. Clin. Med. 2025, 14, 1838. [Google Scholar] [CrossRef]
- Hayashi, C.; Kinoshita, A.; Oda, S.; Mizutani, K.; Shirakata, Y.; Ishikawa, I. Injectable Calcium Phosphate Bone Cement Provides Favorable Space and a Scaffold for Periodontal Regeneration in Dogs. J. Periodontol. 2006, 77, 940–946. [Google Scholar] [CrossRef]
- Huang, X.; Lou, Y.; Duan, Y.; Liu, H.; Tian, J.; Shen, Y.; Wei, X. Biomaterial scaffolds in maxillofacial bone tissue engineering: A review of recent advances. Bioact. Mater. 2024, 33, 129–156. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, N.; Ivanov, S.; Peev, S.; Dikova, T. Types of Bone Substitutes and Their Application in Regenerative Medicine: A Systematic Review. J. Funct. Biomater. 2025, 16, 341. [Google Scholar] [CrossRef] [PubMed]
- Donos, N.; Akcali, A.; Padhye, N.; Sculean, A.; Calciolari, E. Bone regeneration in implant dentistry: Which are the factors affecting the clinical outcome? Periodontology 2000 2023, 93, 26–55. [Google Scholar] [CrossRef]
- Pina, S.; Rebelo, R.; Correlo, V.M.; Oliveira, J.M.; Reis, R.L. Bioceramics for Osteochondral Tissue Engineering and Regeneration. Adv. Exp. Med. Biol. 2018, 1058, 53–75. [Google Scholar] [PubMed]
- Khandelwal, A.; Jose, J.; Teja, K.V.; Palanivelu, A. Comparative evaluation of postoperative pain and periapical healing after root canal treatment using three different base endodontic sealers—A randomized control clinical trial. J. Clin. Exp. Dent. 2022, 14, e144–e152. [Google Scholar]
- Dimitrova-Nakov, S.; Uzunoglu, E.; Ardila-Osorio, H.; Baudry, A.; Richard, G.; Kellermann, O.; Goldberg, M. In vitro bioactivity of Bioroot™ RCS, via A4 mouse pulpal stem cells. Dent. Mater. 2015, 31, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Borlina, S.C.; de Souza, V.; Holland, R.; Murata, S.S.; Gomes-Filho, J.E.; Junior, E.D.; Marion, J.J.d.C.; Neto, D.d.A. Influence of apical foramen widening and sealer on the healing of chronic periapical lesions induced in dogs’ teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 932–940. [Google Scholar] [CrossRef]
- Machado, R.; Comparin, D.; Ignácio, S.A.; Neto, U.X.d.S. Postoperative pain after endodontic treatment of necrotic teeth with large intentional foraminal enlargement. Restor. Dent. Endod. 2021, 46, e31. [Google Scholar] [CrossRef]
- Habibzadeh, S.; Ghoncheh, Z.; Kabiri, P.; Mosaddad, S.A. Diagnostic efficacy of cone-beam computed tomography for detection of vertical root fractures in endodontically treated teeth: A systematic review. BMC Med. Imaging 2023, 23, 68. [Google Scholar] [CrossRef]
- Li, Q.; Liao, W.; Fu, G.; Liao, J.; Zhang, R.; Li, M.; Yang, Y.; Ma, Y.; Zheng, M.; Zheng, Q. Combining autologous bone marrow buffy coat and angioconductive bioceramic rod grafting with advanced core decompression improves short-term outcomes in early avascular necrosis of the femoral head: A prospective, randomized, comparative study. Stem Cell Res. Ther. 2021, 12, 354. [Google Scholar] [CrossRef]
- Gómez-Barrena, E.; Padilla-Eguiluz, N.; Rosset, P.; Gebhard, F.; Hernigou, P.; Baldini, N.; Rouard, H.; Sensebé, L.; Gonzalo-Daganzo, R.-M.; Giordano, R.; et al. Early efficacy evaluation of mesenchymal stromal cells (MSC) combined to biomaterials to treat long bone non-unions. Injury 2020, 51 (Suppl. 1), S63–S73. [Google Scholar] [CrossRef]
- Malzoni, C.M.d.A.; Gonçalves, V.; Possari, J.; Junior, E.M. The use of 3D ceramic block graft compared with autogenous block graft for rehabilitation of the atrophic maxilla: A randomized controlled clinical trial. Trials 2022, 23, 903. [Google Scholar] [CrossRef]
- Han, C.B.; An, S.C. Injectable bioactive glass in the restoration of oral bone defect. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1665–1668. [Google Scholar] [PubMed]
- Mistry, S.; Roy, R.; Kundu, B.; Datta, S.; Kumar, M.; Chanda, A.; Kundu, D. Clinical Outcome of Hydroxyapatite Coated, Bioactive Glass Coated, and Machined Ti6Al4V Threaded Dental Implant in Human Jaws: A Short-Term Comparative Study. Implant. Dent. 2016, 25, 252–260. [Google Scholar] [CrossRef]
- Akhlaghi, F.; Hesami, N.; Rad, M.R.; Nazeman, P.; Fahimipour, F.; Khojasteh, A. Improved bone regeneration through amniotic membrane loaded with buccal fat pad-derived MSCs as an adjuvant in maxillomandibular reconstruction. J. Craniomaxillofac Surg. 2019, 47, 1266–1273. [Google Scholar] [CrossRef]
- Palarie, V.; Bicer, C.; Lehmann, K.M.; Zahalka, M.; Draenert, F.G.; Kämmerer, P.W. Early outcome of an implant system with a resorbable adhesive calcium-phosphate coating--a prospective clinical study in partially dentate patients. Clin. Oral Investig. 2012, 16, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Marcacci, M.; Kon, E.; Moukhachev, V.; Lavroukov, A.; Kutepov, S.; Quarto, R.; Mastrogiacomo, M.; Cancedda, R. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007, 13, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Hu, Y.; Li, J.; Zeng, Y.; Ren, H. Comparison of the outcome of different bone grafts combined with modified core decompression for the treatment of ARCO II stage femoral head necrosis. Int. Orthop. 2022, 46, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Rauschmann, M.; Vogl, T.; Verheyden, A.; Pflugmacher, R.; Werba, T.; Schmidt, S.; Hierholzer, J. Bioceramic vertebral augmentation with a calcium sulphate/hydroxyapatite composite (Cerament SpineSupport): In vertebral compression fractures due to osteoporosis. Eur. Spine J. 2010, 19, 887–892. [Google Scholar] [CrossRef]
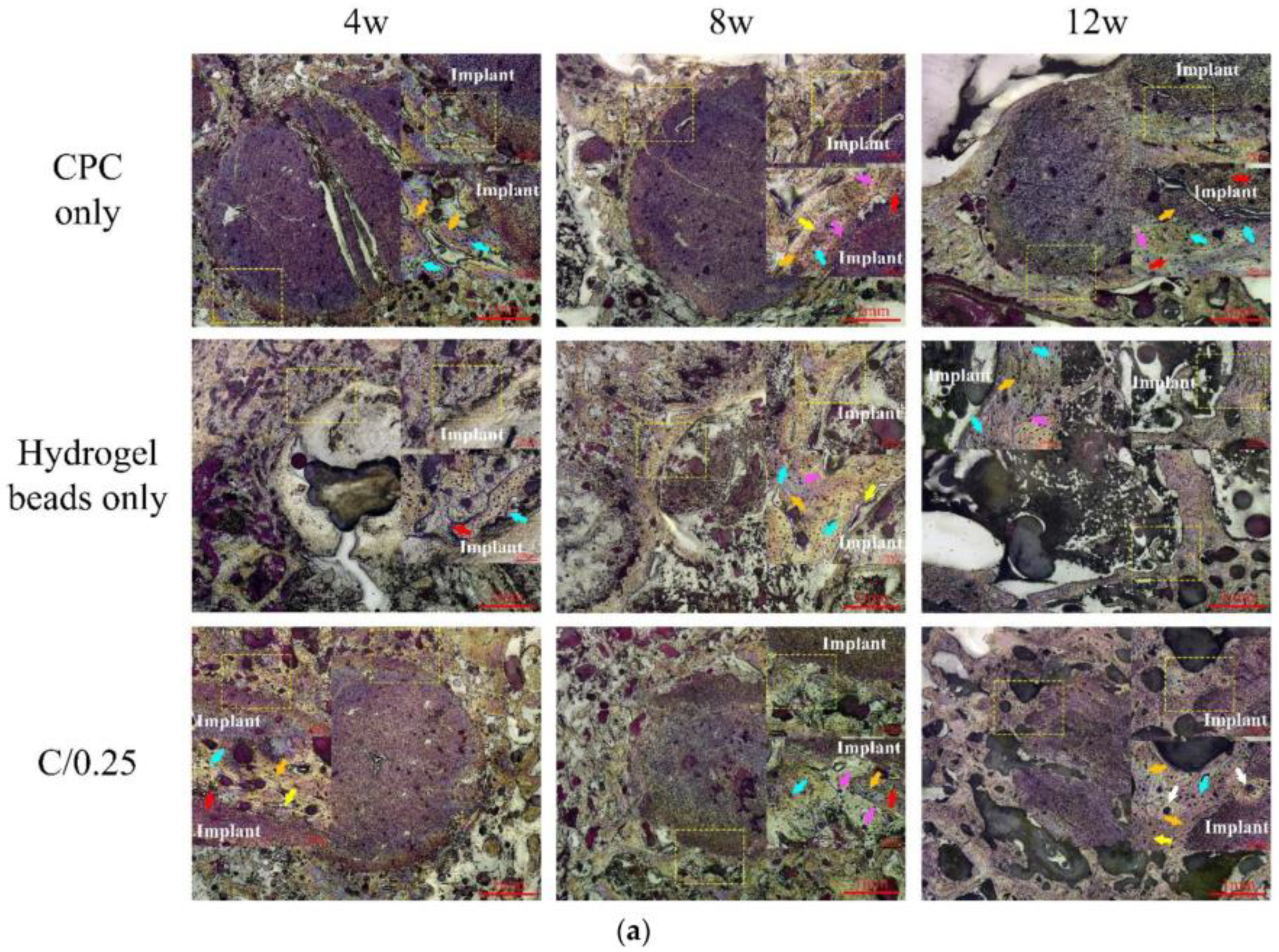
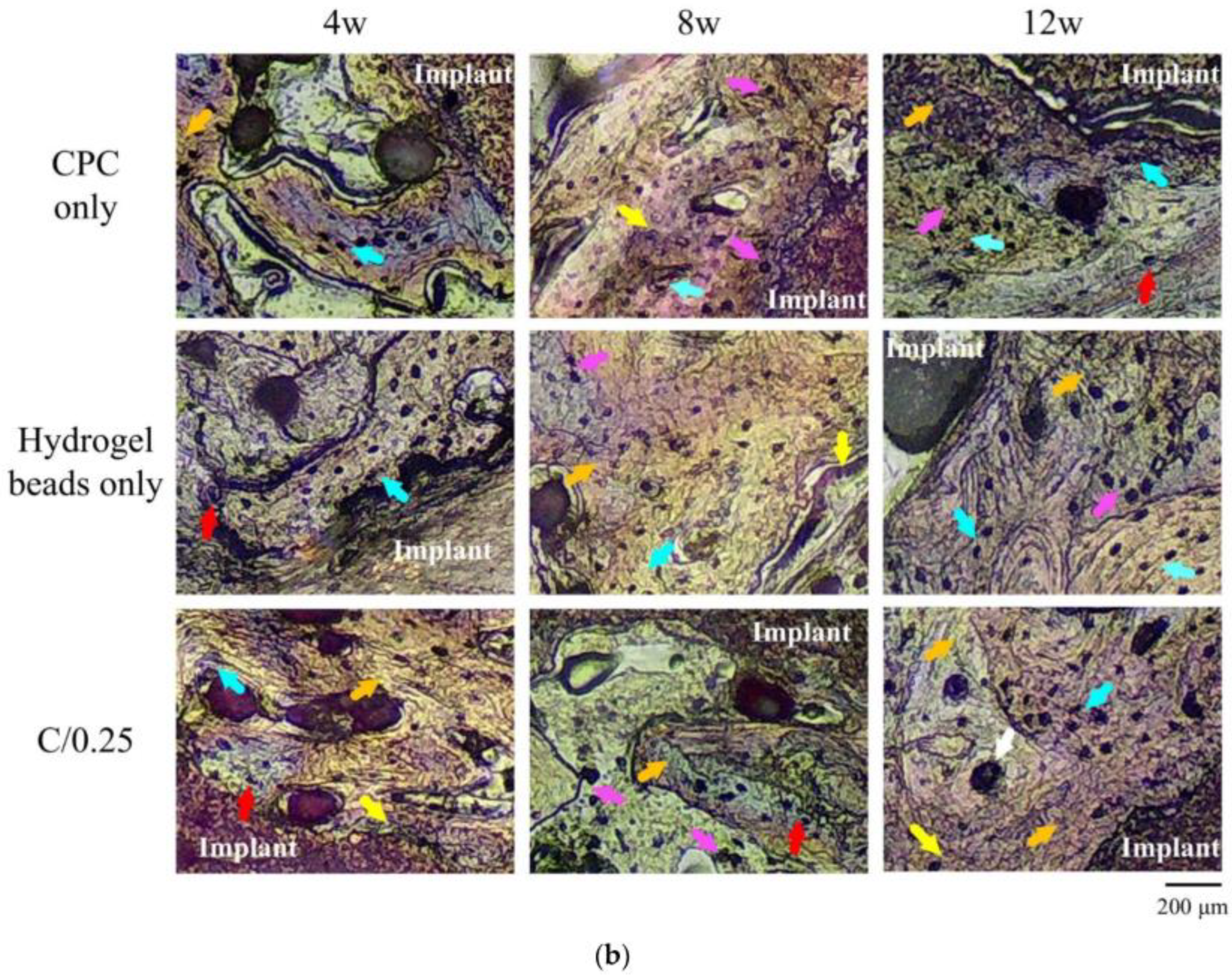
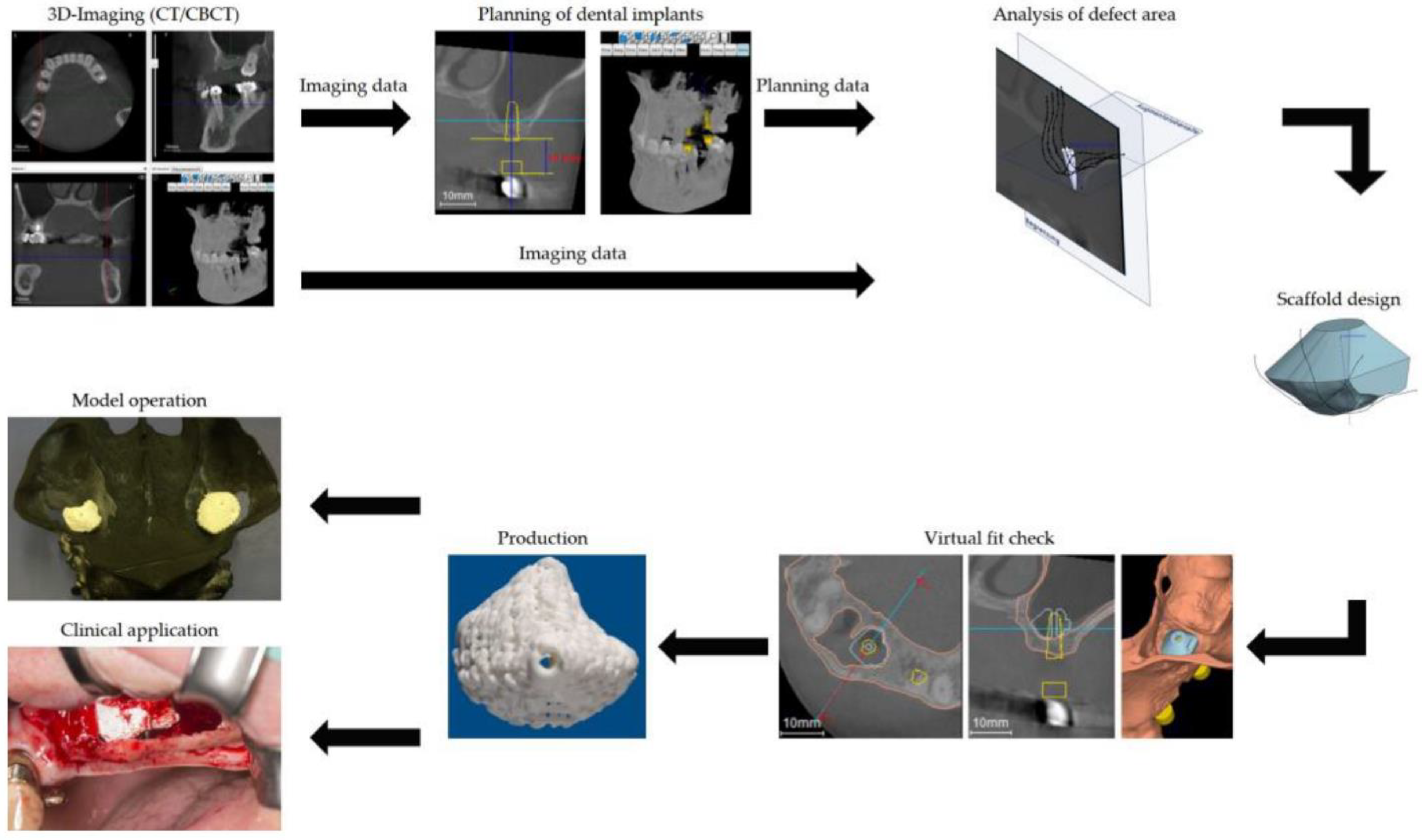
| Biomaterial | Date and Status (Follow-Up) | Method | Ages (Years) | Results | Ref |
|---|---|---|---|---|---|
| β-TCP | from 2015 to 2020 | ABC and ABR grafts with ACD will be evaluated for the effects of early ANFH. | 18–60 | Autologous bone marrow buffy coats and bioceramic rods, combined with advanced core decompression, are effective treatments for early ANFH. | [108] |
| biphasic calcium phosphate (BCP) | 6-month | The study compared biphasic calcium phosphate (BCP) in two forms: granules and paste, as well as histomorphometric measurements and osteocalcin immunolabeling. | 52 to 64 years | Bone calcification was demonstrated in both groups by immunolabeling for osteocalcin. Implant placement with both biomaterials, therefore, presents satisfactory results. | [3] |
| HA/β-TCP + collagen | 9 months | Fifty-seven extraction sockets were located in the posterior regions of the mandibles and maxillas of 51 patients. HA/β-TCP + collagen was inserted into all dental sockets and covered with flaps immediately after extraction. The patient was followed up three months after extraction with radiographs and stents. | 20 and 89 years | After three months, bone height was maintained, indicating good performance of the HA/β-TCP + collagen graft. | [5] |
| bioactive calcium-phosphate (CP) coating | 12-month | Using dental implants coated with bioactive calcium-phosphate (CP) in partially edentulous patients with varied clinical indications, this study evaluated the outcomes early after early and delayed prosthetic loading. | 41.44 years (18–62 years) | Study implants with CP coating performed better than conventional implants without a specific coating after 1 year of use. A one-year evaluation of implants coated with CP was not possible. The long-term effects require further study. | [114] |
| HA and ß-TCP | 12-month | Clinical and radiological evaluation of the effectiveness of long bone consolidation in healing delayed unions and non-unions | 39 ± 13 years | Following surgery, biopsies confirmed bone formation surrounding the bioceramic granules with expanded MSCs attached. | [109] |
| β-TCP | 8 months | compare the performance of an alloplastic graft, Plenum® Oss 3Dβ fit, a 3D- β-TCP to autograft. | over 18 | Using this synthetic bone substitute to repair bone defects poses an alternative to using autogenous bone grafts due to its osteoconductive properties. | [110] |
| Porous HA | 6.5 years follow-up | A new TE approach was used to treat patients with large diaphysis defects and inadequate therapeutic alternatives. In culture, cells were expanded on HA ceramic scaffolds that matched the size and shape of the bone deficit. | 16–41 | Porous bioceramics combined with culture-expanded osteoprogenitor cells reduced critical-sized defects in long bones significantly. | [115] |
| β-TCP | followed up for 42 to 48 (44.62 ± 1.81) months | A comparison can be made between four groups that received different types of bone grafts at various times. | Bioceramics grafts have shorter operation times and less blood loss than other bone grafts. Early treatment of osteonecrosis of the femoral head may involve this bone graft. | [116] | |
| HA and BG | 6-month and 12-month following treatment | Analyze how oral bone defects heal. | As compared to HA bioceramics, injectable BG performed better in restoring oral bone defects. | [111] | |
| calcium sulfate and HA | 18-month follow-up | Partially resorbable calcium sulfate and HA composite: effectiveness and safety. | over 50 years | When osteoporotic patients with vertebral compression fractures experience sustained pain relief for 18 months, fracture healing can occur. | [117] |
| HA/BG | 12-month follow-up | In the human jawbone, evaluate the clinical outcome of titanium alloy threaded dental implants with HA coating, BG coating, and machined titanium alloy coating. | 18–58 years | As an alternative coating material for dental implants, BG-coated implants can achieve osseointegration just as effectively as HA-coated and machined titanium implants. | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafavi Moghaddam, S.A.; Mojtahedi, H.; Bahador, A.; Kamali Hakim, L.; Tebyaniyan, H. Clinical Advances in Calcium Phosphate for Maxillomandibular Bone Regeneration: From Bench to Bedside. Ceramics 2025, 8, 129. https://doi.org/10.3390/ceramics8040129
Mostafavi Moghaddam SA, Mojtahedi H, Bahador A, Kamali Hakim L, Tebyaniyan H. Clinical Advances in Calcium Phosphate for Maxillomandibular Bone Regeneration: From Bench to Bedside. Ceramics. 2025; 8(4):129. https://doi.org/10.3390/ceramics8040129
Chicago/Turabian StyleMostafavi Moghaddam, Seyed Ali, Hamid Mojtahedi, Amirhossein Bahador, Lotfollah Kamali Hakim, and Hamid Tebyaniyan. 2025. "Clinical Advances in Calcium Phosphate for Maxillomandibular Bone Regeneration: From Bench to Bedside" Ceramics 8, no. 4: 129. https://doi.org/10.3390/ceramics8040129
APA StyleMostafavi Moghaddam, S. A., Mojtahedi, H., Bahador, A., Kamali Hakim, L., & Tebyaniyan, H. (2025). Clinical Advances in Calcium Phosphate for Maxillomandibular Bone Regeneration: From Bench to Bedside. Ceramics, 8(4), 129. https://doi.org/10.3390/ceramics8040129








