Changes in Morphology Caused by Mass Transfer Phenomenon
Abstract
1. Introduction
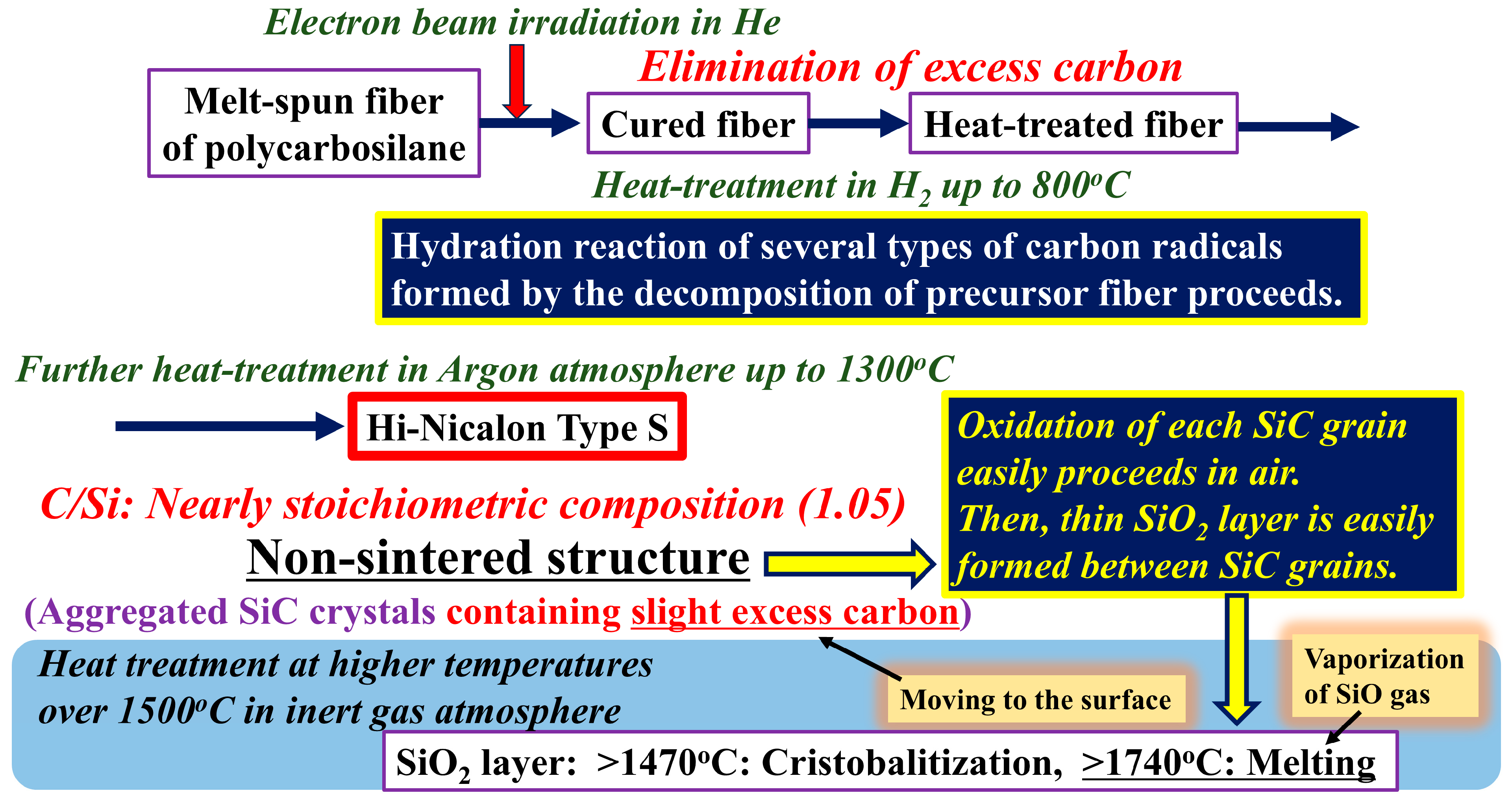
2. Materials and Methods
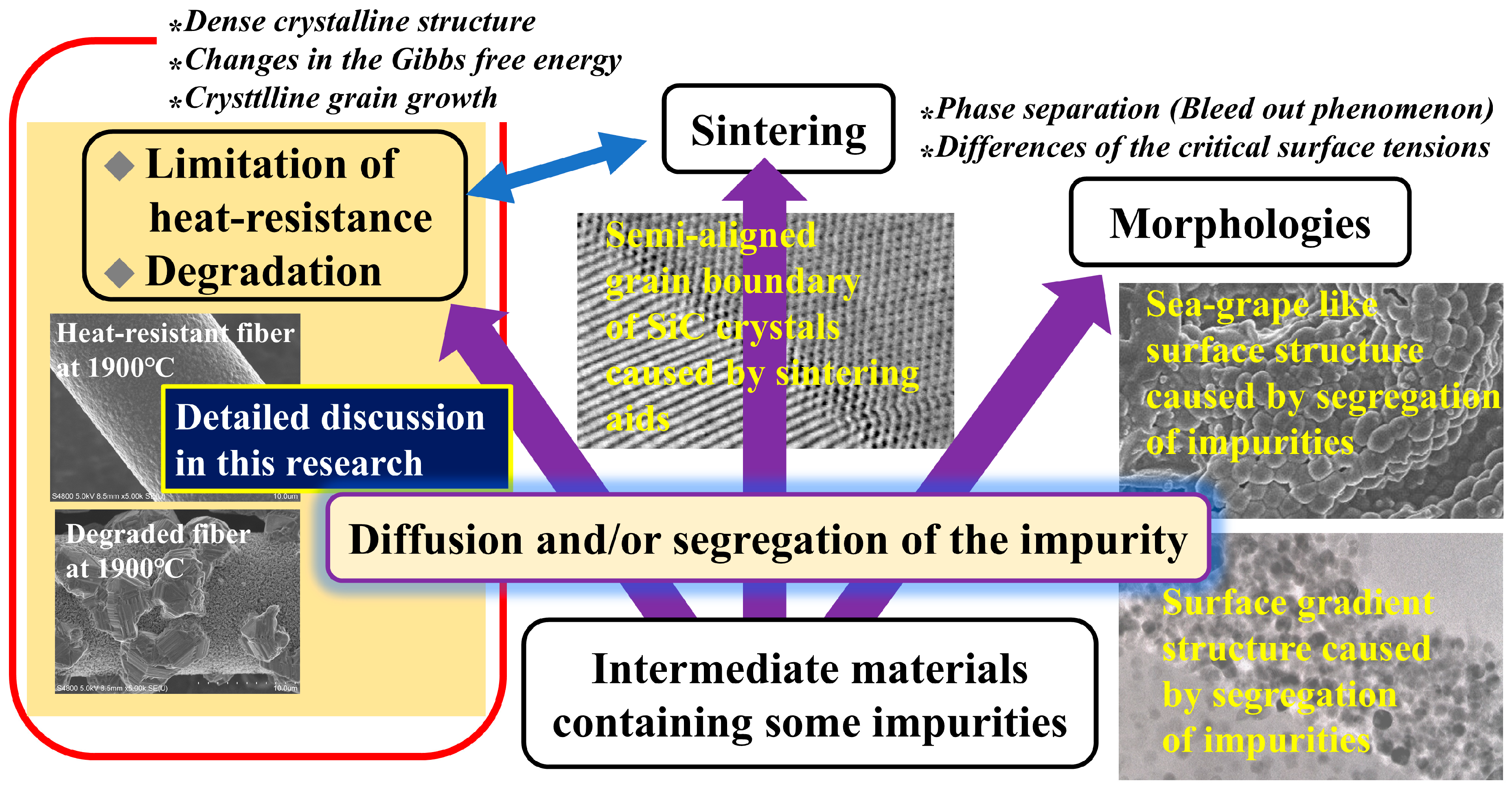
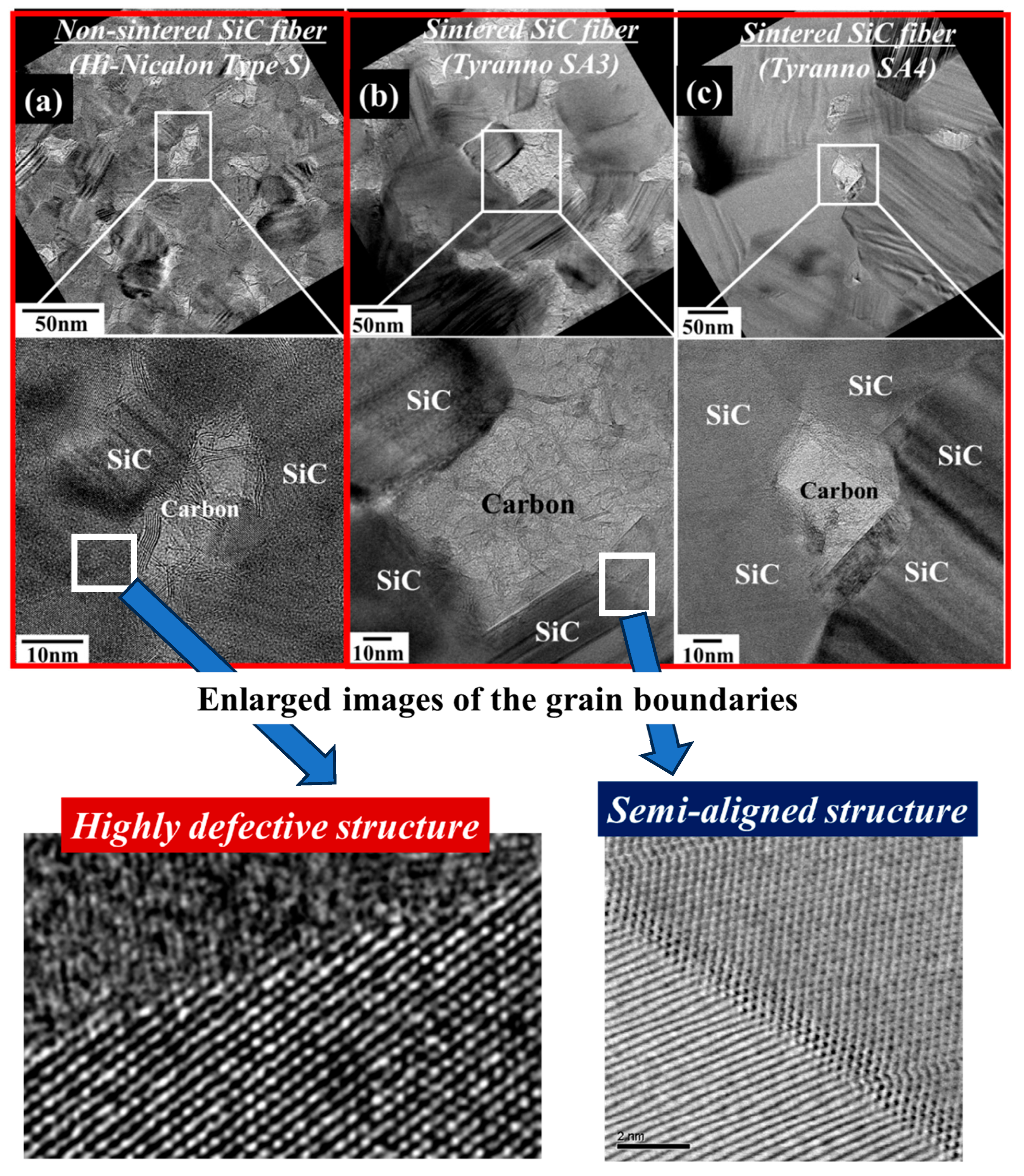
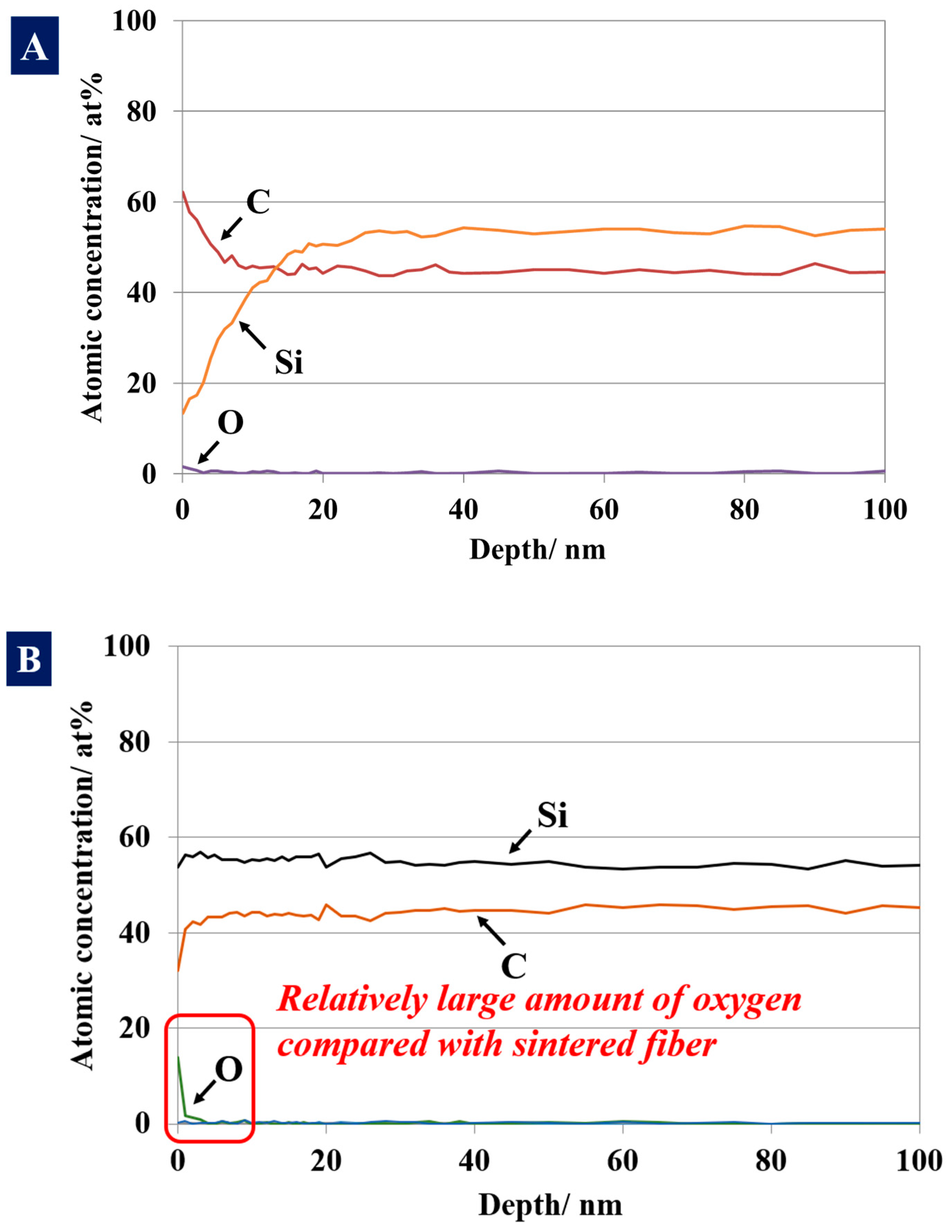
3. Results and Discussion
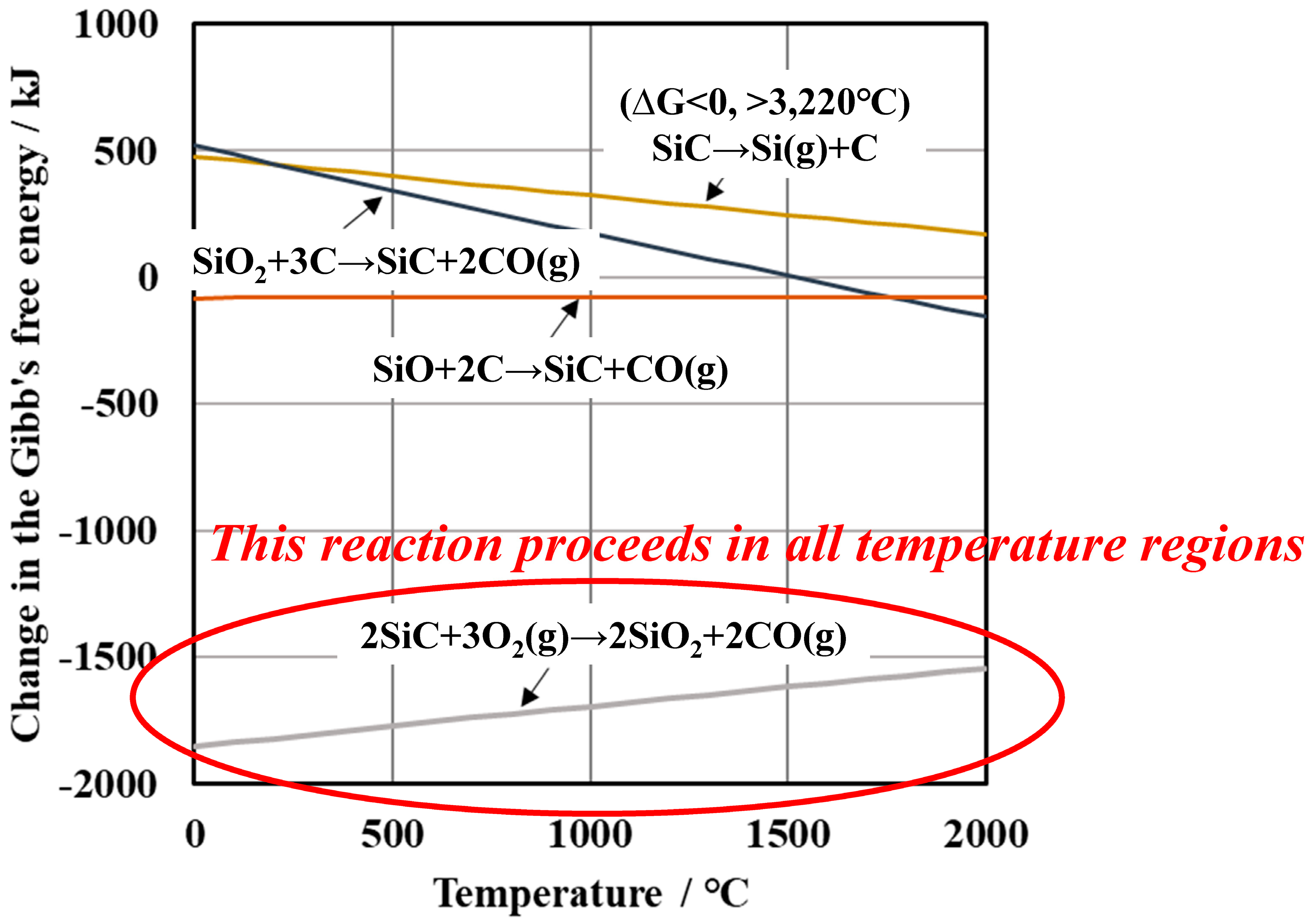
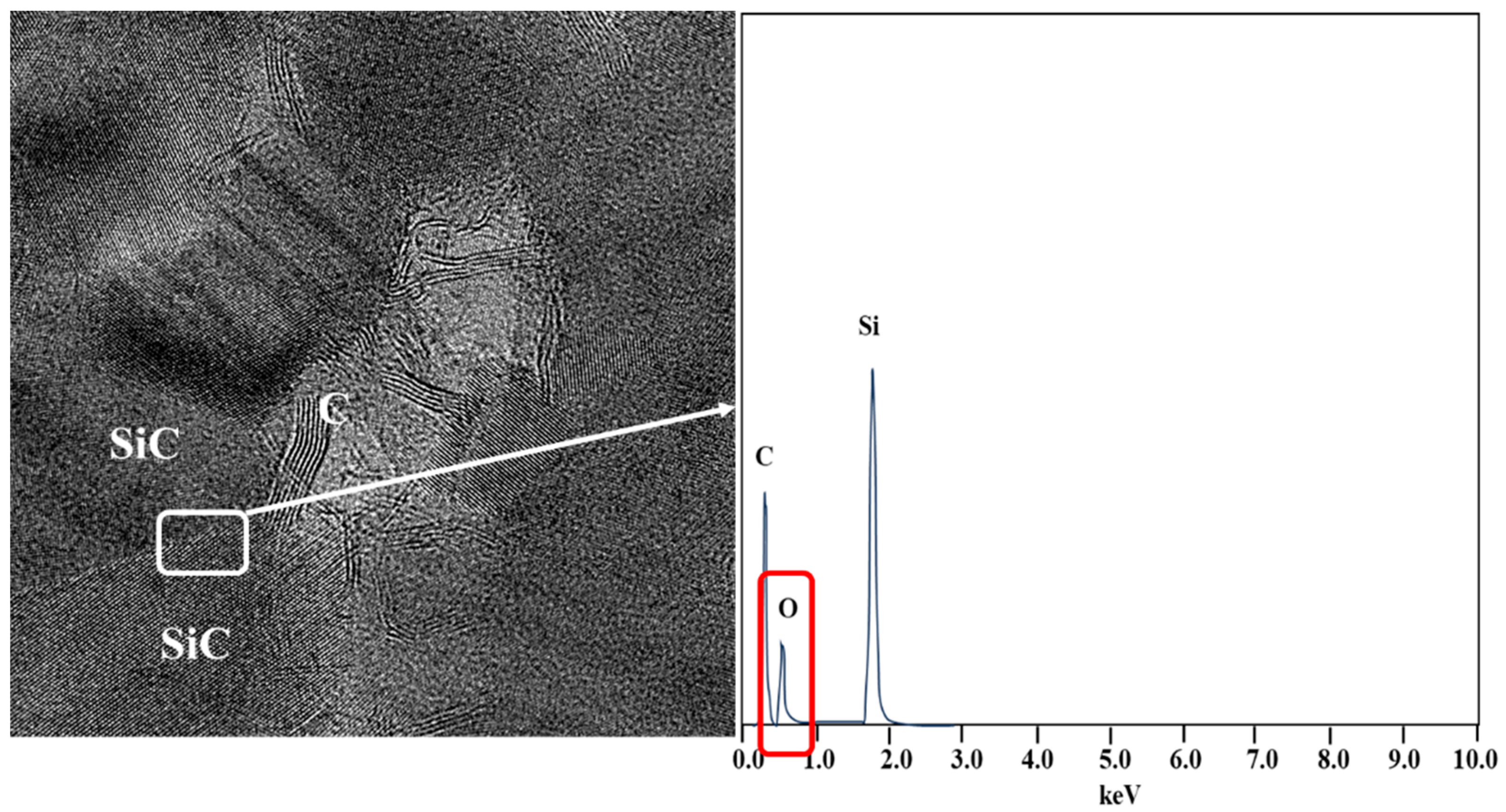
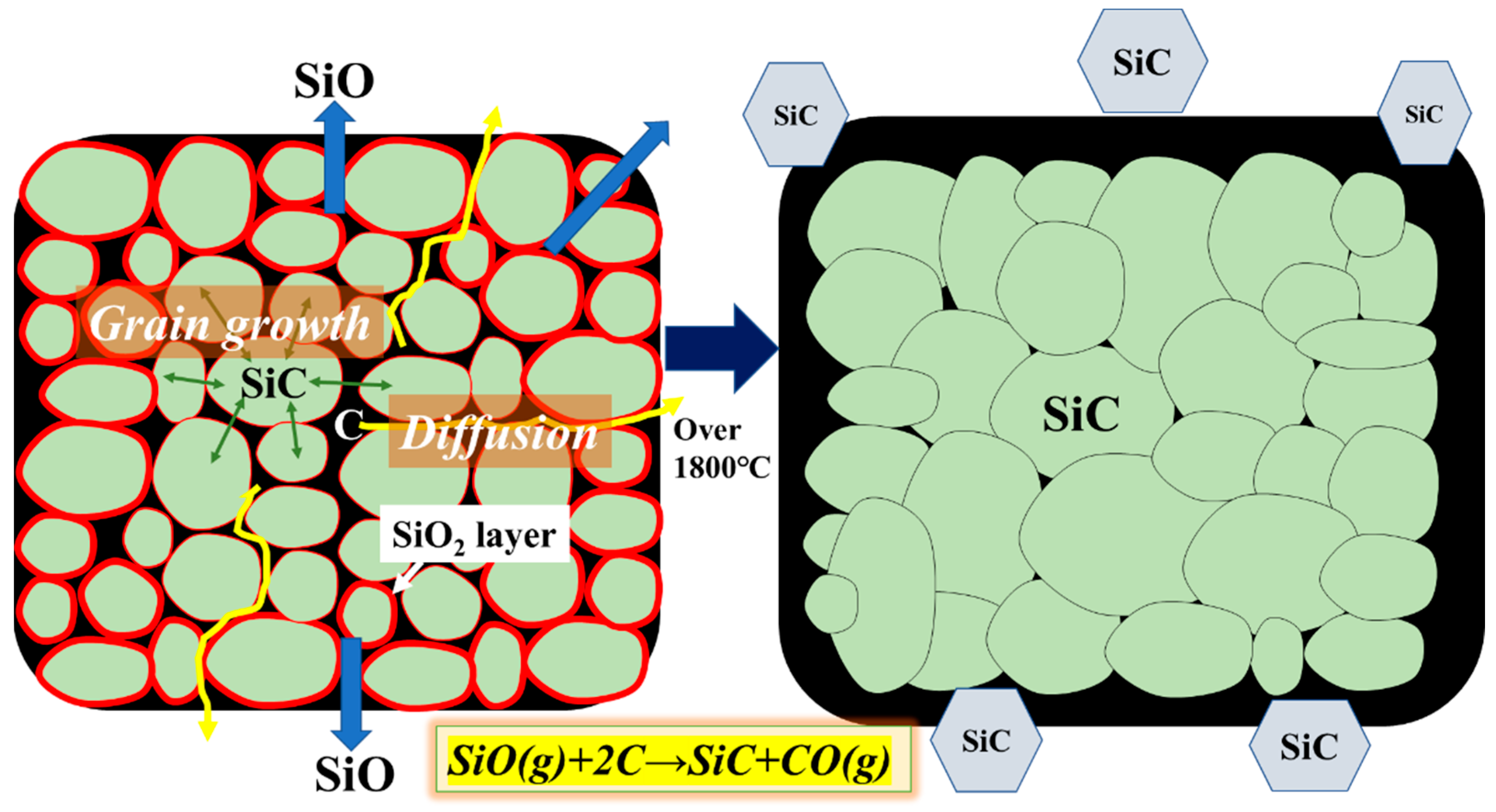
3.1. Differences in the Fine Structure and Oxygen Content of Polycristalline Fibers
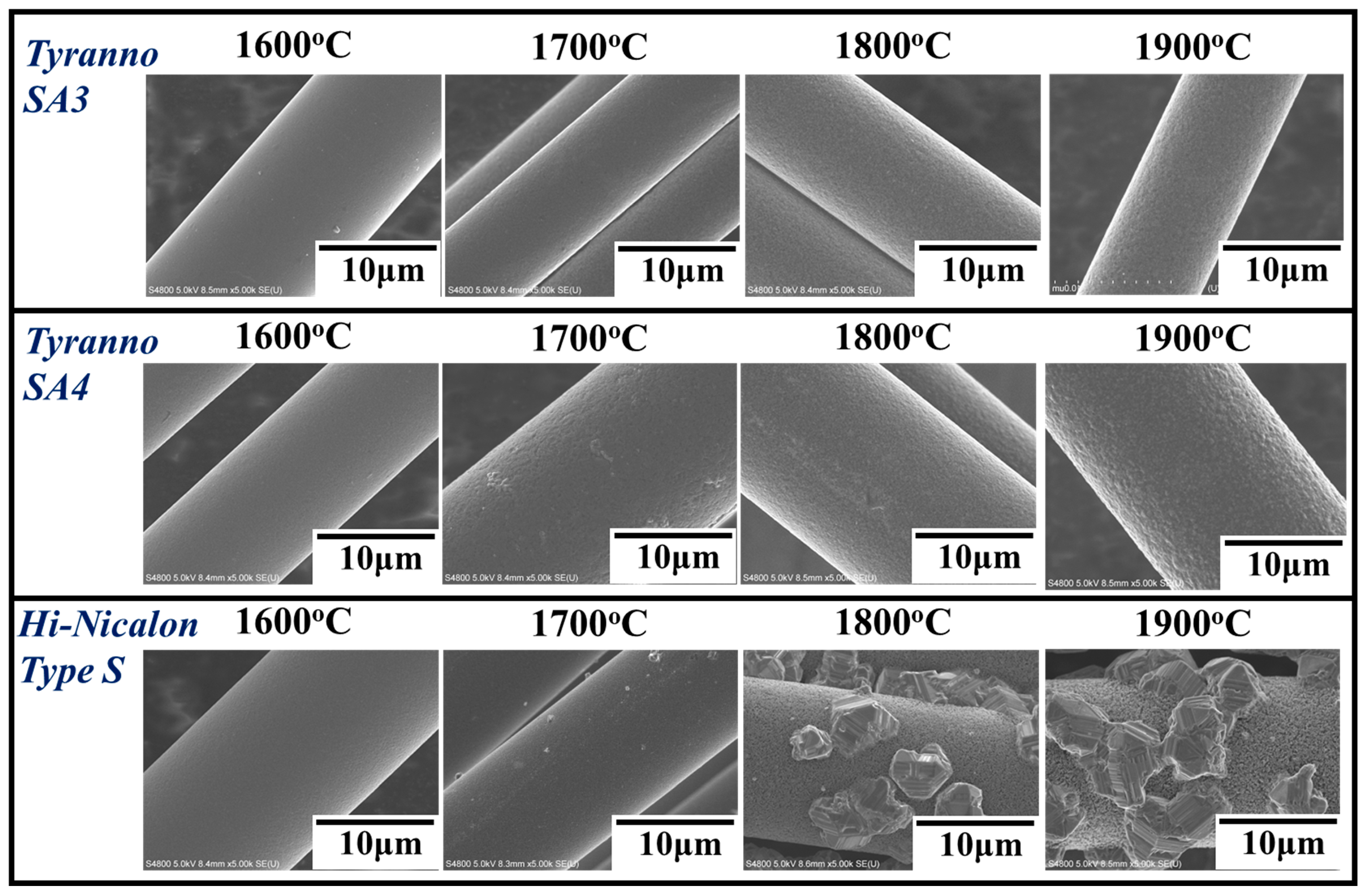
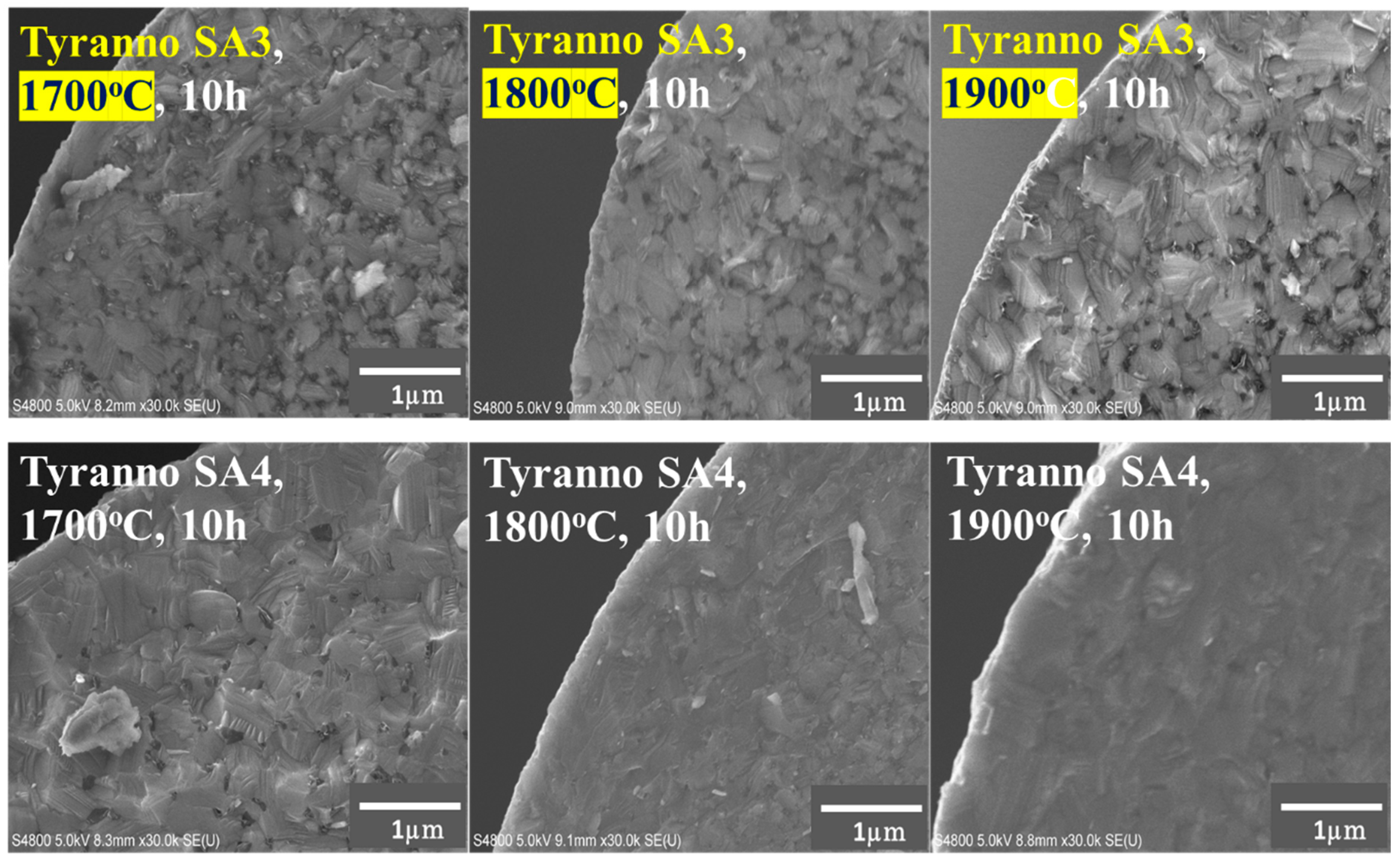
3.2. Differences in the Heat Resistance of These Fibers
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yajima, S.; Okamura, K.; Hayashi, J.; Omori, M. Synthesis of Continuous SiC Fibers with High Tensile Strength. J. Am. Ceram. Soc. 1976, 59, 324–327. [Google Scholar] [CrossRef]
- Munch, E.; Launey, M.E.; Alsem, D.H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Tough, Bio-Inspired Hybrid Materials. Science 2008, 322, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S. Graded Materials for Resistance to Contact Deformation and Damage. Science 2001, 292, 2447–2451. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rui, J.; Li, Y.; Qiu, Y.; Hongli, L.; Tian, H. Zr-doped SiOC ceramics fibers and the high-temperature thermal performance. Int. J. Appl. Ceram. Technol. 2023, 20, 2438–2448. [Google Scholar] [CrossRef]
- Shimoda, K.; Hinoki, T. Effect of BN nanoparticle content in SiC matrix on microstructure and mechanical properties of SiC/SiC composites. Int. J. Appl. Ceram. Technol. 2023, 20, 2466–2477. [Google Scholar] [CrossRef]
- Terrani, K.; Jolly, B.; Trammell, M. 3D printing of high-purity silicon carbide. J. Am. Ceram. Soc. 2020, 103, 1575–1581. [Google Scholar] [CrossRef]
- Chen, Y.; Ai, S.; Wang, P.; Fang, D. A physically based thermos-elastoplastic constitutive model for braided CMCs-SiC at ultra-high temperature. J. Am. Ceram. Soc. 2022, 105, 2196–2208. [Google Scholar] [CrossRef]
- Barbosa, L.C.M.; Lorrette, C.; Bras, S.L.; Baranger, E.; Lamon, J. Tensile strength analysis and fractography on single nuclear grade SiC fibers at room temperature. J. Nucl. Mater. 2023, 576, 154256. [Google Scholar] [CrossRef]
- Eckel, Z.C.; Zhou, C.; Martin, J.H.; Jacobsen, A.J.; Carter, W.B.; Schaedler, T.A. Additive manufacturing of polymer-derived ceramics. Science 2016, 351, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gou, Y.; Wang, H.; Wang, Y. Revealing the formation mechanism of the skin-core structure in nearly stoichiometric SiC fiber. J. Euro. Ceram. Soc. 2020, 40, 2295–2305. [Google Scholar]
- Ishikawa, T.; Kohtoku, Y.; Kumagawa, K.; Yamamura, T.; Nagasawa, T. High-strength alkali-resistant sintered SIC fibre stable to 2200 °C. Nature 1998, 391, 773–775. [Google Scholar] [CrossRef]
- Ishikawa, T.; Yamaoka, H.; Harada, Y.; Fujii, T.; Nagasawa, T. A general process for in situ formation of functional surface layers on ceramics. Nature 2002, 416, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Lipowitz, J.; Barnard, T.; Bujalski, D.; Rabe, J.; Zank, G.; Zangvil, A.; Xu, Y. Fine-diameter polycrystalline SiC fibers. Compos. Sci. Technol. 1994, 51, 167–171. [Google Scholar]
- Ishikawa, T.; Kajii, S.; Matsunaga, K.; Hogami, T.; Kohtoku, Y.; Nagasawa, T. A tough, thermally conductive silicon carbide composite with high strength up to 1600 °C in air. Science 1998, 282, 1295–1297. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Urano, A.; Sakamoto, J.; Imai, Y. Microstructure and Oxidation Behavior of Silicon Carbide Fibers Derived from Polycarbosilane. J. Am. Ceram. Soc. 2000, 83, 1171–1176. [Google Scholar] [CrossRef]
- Yajima, S.; Hayashi, J.; Omori, M.; Okamura, K. Development of a silicon carbide fibre with high tensile strength. Nature 1976, 261, 683–685. [Google Scholar] [CrossRef]
- Ichikawa, H. Polymer-derived ceramic fibers. Annu. Rev. Mater. Res. 2016, 46, 335–356. [Google Scholar] [CrossRef]
- Rahaman, M.N. Sintering of Ceramics (Taylor & Francis Group, 2008); CRC Press: Boca Raton, FL, USA, 2007; pp. 27–28. [Google Scholar]
- Braun, J.; Sauder, C. Mechanical behavior of SiS/SiC composites reinforced with new Tyranno SA4 fibers: Effect of interface thickness and comparison with Tyranno SA3 and Hi-Nicalon S reinforced composites. J. Nucl. Mater. 2022, 558, 153367. [Google Scholar] [CrossRef]
- Bhatt, R.T.; Halbig, M.C. Creep properties of melt infiltrated SiC/SiC composites with Sylramic-iBN and Hi-Nicalon S fibers. Int. J. Appl. Ceram. Tech. 2022, 19, 1074–1091. [Google Scholar] [CrossRef]
- Lamon, J. Handbook of Ceramic Composites; Springer: Berlin/Heidelberg, Germany, 2005; pp. 55–76. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishikawa, T. Changes in Morphology Caused by Mass Transfer Phenomenon. Ceramics 2025, 8, 120. https://doi.org/10.3390/ceramics8040120
Ishikawa T. Changes in Morphology Caused by Mass Transfer Phenomenon. Ceramics. 2025; 8(4):120. https://doi.org/10.3390/ceramics8040120
Chicago/Turabian StyleIshikawa, Toshihiro. 2025. "Changes in Morphology Caused by Mass Transfer Phenomenon" Ceramics 8, no. 4: 120. https://doi.org/10.3390/ceramics8040120
APA StyleIshikawa, T. (2025). Changes in Morphology Caused by Mass Transfer Phenomenon. Ceramics, 8(4), 120. https://doi.org/10.3390/ceramics8040120





