Improving of Thermoelectric Efficiency of Layered Sodium Cobaltite Through Its Doping by Different Metal Oxides
Abstract
1. Introduction
2. Materials and Methods
= 3 Na0.89Co0.90Me0.10O2 + 1.335 CO2
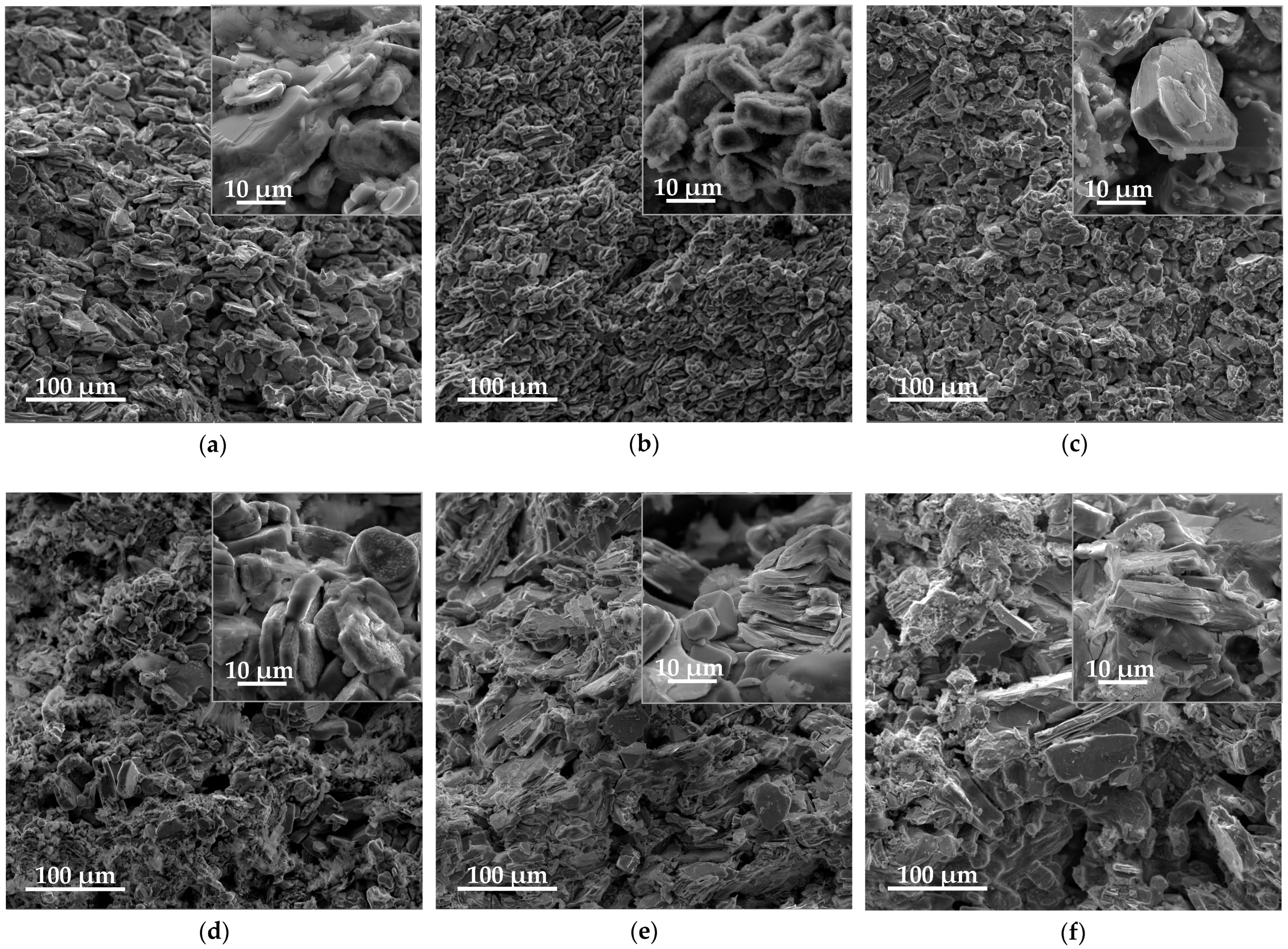
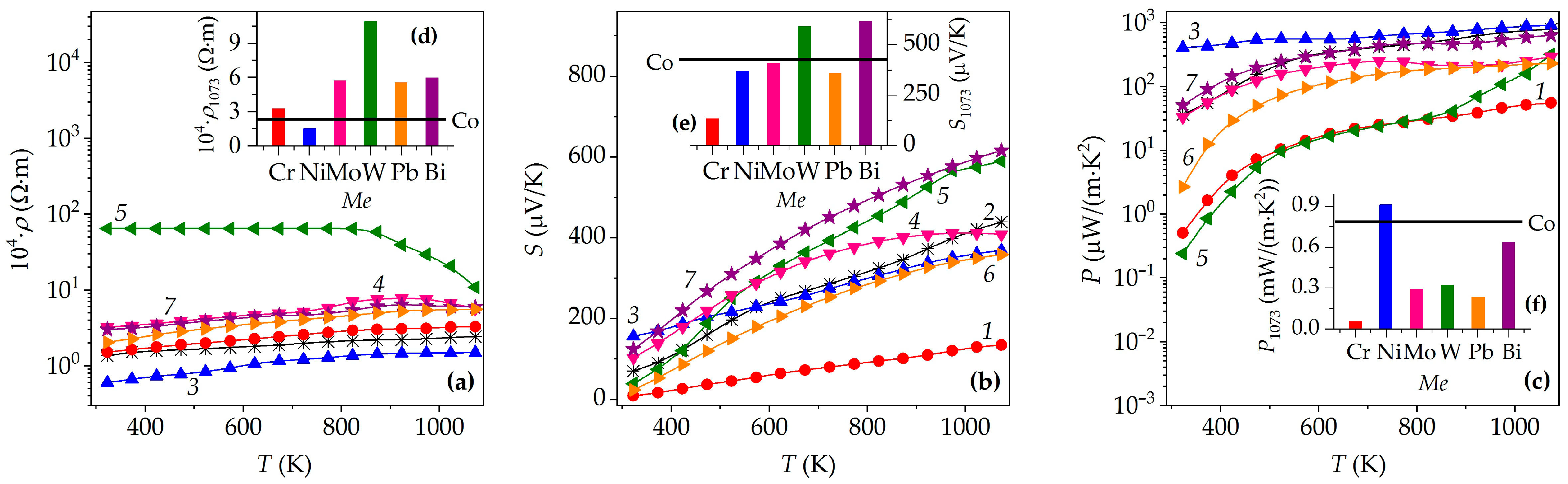
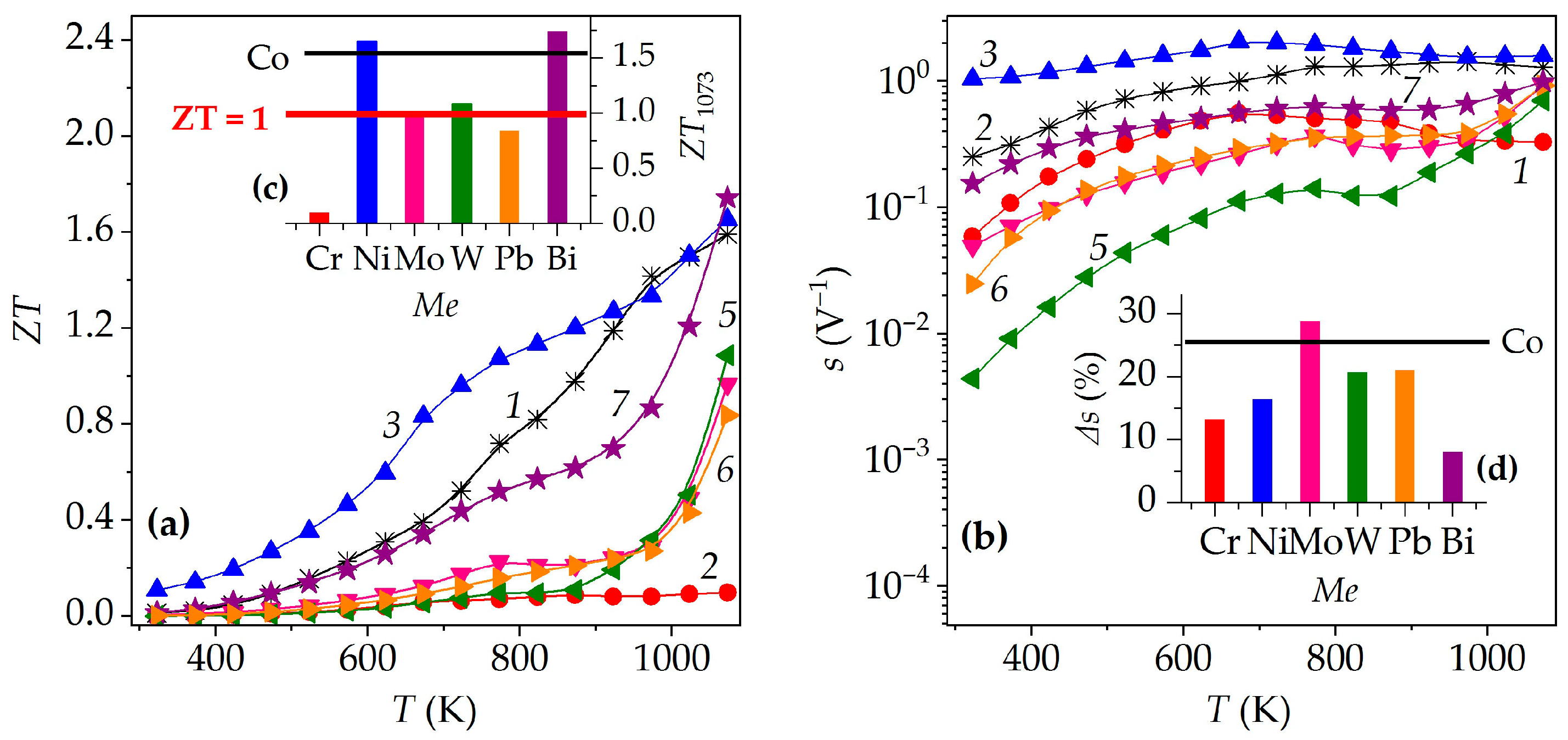
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klyndyuk, A.I.; Kharytonau, D.S.; Matsukevich, I.V.; Chizhova, E.A.; Lenčéš, Z.; Socha, R.P.; Zimowska, M.; Hanzel, O.; Janek, M. Effect of cationic nonstoichiometry on thermoelectric properties of layered calcium cobaltite obtained by field assisted sintering technology (FAST). Ceram. Int. 2024, 50, 30970–30979. [Google Scholar] [CrossRef]
- Polevik, A.O.; Sobolev, A.V.; Glazcova, I.S.; Presniakov, I.A.; Verchenko, V.Y.; Link, J.; Stern, R.; Shevelkov, A.V. Interplay between Fe(II) and Fe(III) and Its Impact on Thermoelectric Properties of Iron-Substituted Colusites Cu26−xFexV2Sn6S32. Compounds 2023, 3, 348–364. [Google Scholar] [CrossRef]
- Yu, J.; Chen, K.; Azough, F.; Alvarez-Ruiz, D.T.; Reece, M.I.; Freer, R. Enhancing the thermoelectric performance of calcium cobaltite ceramics by tuning composition and processing. ACS Appl. Mater. Interfaces 2020, 12, 47634–47646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, K.; Liu, J.; Wang, J.; Yan, K.; Liu, P.; Wang, Y. Influence of the phase transformation in NaxCoO2 ceramics on thermoelectric properties. Ceram. Int. 2018, 44, 17251–17257. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, C.; Xie, Y. Layered thermoelectric materials: Structure, bonding, and performance mechanisms. Appl. Phys. Rev. 2020, 9, 011303. [Google Scholar] [CrossRef]
- Kuzanyan, A.S.; Margani, N.G.; Zhghamadze, V.V.; Mumladze, G.; Badalyan, G.R. Impact of Sr(BO2)2 Dopant on Power Factor of Bi2Sr2Co1.8Oy Thermoelectric. J. Contemp. Phys. 2021, 56, 146–149. [Google Scholar] [CrossRef]
- Oopathumpa, C.; Boonthuma, D.; Smith, S.M. Effect of Poly(Vinyl Alcohol) on Thermoelectric Properties of Sodium Cobalt Oxide. Key Eng. Mater. 2019, 798, 304–309. [Google Scholar] [CrossRef]
- Mallick, M.M.; Vitta, V. Enhancing Thermoelectric Figure-of-Merit of Polycrystalline NayCoO2 by a Combination of Non-stoichiometry and Co-substitution. J. Electron. Mater. 2018, 47, 3230–3237. [Google Scholar] [CrossRef]
- Yu, J.; Chang, Y.; Jakubczyk, E.; Wang, B.; Azough, F.; Dorey, R.; Freer, R. Modulation of electrical transport in calcium cobaltite ceramics and thick films through microstructure control and doping. J. Eur. Ceram. Soc. 2021, 41, 4859–4869. [Google Scholar] [CrossRef]
- Noudem, J.G.; Xing, Y. Overview of Spark Plasma Texturing of Functional Ceramics. Ceramics 2021, 4, 97–107. [Google Scholar] [CrossRef]
- Jansen, M.; Hoppe, R. Notiz zur Kenntnis der Oxocobaltate des Natriums. Z. Für Anorg. Und Allg. Chem. 1974, 408, 104–106. [Google Scholar] [CrossRef]
- Terasaki, I.; Sasago, Y.; Uchinokura, K. Large thermoelectric power in NaCo2O4 single crystals. Phys. Rev. B 1997, 56, R12685–R12687. [Google Scholar] [CrossRef]
- Koumoto, K.; Terasaki, I.; Funahashi, R. Complex Oxide Materials for Potential Thermoelectric Applications. MRS Bull. 2006, 31, 206–210. [Google Scholar] [CrossRef]
- Viciu, L.; Huang, Q.; Cava, R.J. Stoichiometric oxygen content in NaxCoO2. Phys. Rev. B 2006, 73, 212107. [Google Scholar] [CrossRef]
- Han, Y.; Ruan, Y.; Xue, M.; Shi, M.; Song, Z.; Zhou, Y.; Teng, J. Effect of Annealing Time on the Cyclic Characteristics of Ceramic Oxide Thin Film Thermocouples. Micromachines 2022, 13, 1970. [Google Scholar] [CrossRef]
- Krasutskaya, N.S.; Klyndyuk, A.I.; Evseeva, L.E.; Gundilovich, N.N.; Chizova, E.A.; Paspelau, A.V. Enhanced thermoelectric performance of Na0.55CoO2 ceramics doped by transition and heavy metal oxides. Solids 2024, 5, 267–277. [Google Scholar] [CrossRef]
- Molenda, J.; Baster, D.; Milewska, A.; Świerczek, K.; Bora, D.K.; Braun, A.; Tobola, J. Electronic origin of difference in discharge curve between LixCoO2 and NaxCoO2 cathodes. Solid State Ion. 2014, 271, 15–27. [Google Scholar] [CrossRef]
- Pohle, B.; Gorbunov, M.; Lu, Q.; Bahrami, A.; Nielsch, K.; Mikhailova, D. Structural and electrochemical properties of layered P2-Na0.8Co0.8Ti0.2O2 cathode in sodium-ion batteries. Energies 2022, 15, 3371. [Google Scholar] [CrossRef]
- Nguyen, L.M.; Nguyen, V.H.; Nguyen, D.M.N.; Le, M.K.; Tran, V.M.; Le, M.L.P. Evaluating electrochemical properties of layered NaxMn0.5Co0.5O2 obtained at different calcined temperatures. Chemengineering 2023, 7, 33. [Google Scholar] [CrossRef]
- Baster, D.; Zając, W.; Kondracki, Ł.; Hartman, F.; Molenda, J. Improvement of electrochemical performance of Na0.7Co1−yMnyO2–cathode material for rechargeable sodium-ion batteries. Solid State Ion. 2016, 288, 213–218. [Google Scholar] [CrossRef]
- Banobre-López, M.; Rivadulla, F.; Caudillo, R.; López-Quintela, M.A.; Rivas, J.; Goodenough, J.B. Role of doping and Dimensionality in the Superconductivity of NaxCoO2. Chem. Mater. 2005, 17, 1965–1968. [Google Scholar] [CrossRef]
- Akram, R.; Khan, J.; Rafique, S.; Hussain, M.; Maqsood, A.; Naz, A.A. Enhanced thermoelectric properties of single phase Na doped NaxCoO2 thermoelectric material. Mater. Lett. 2021, 300, 130180. [Google Scholar] [CrossRef]
- Assadi, M.H.N.; Katayama-Yoshida, H. Restoration of long range order of Na ions in NaxCoO2 at high temperatures by sodium site doping. Comput. Mater. Sci. 2015, 109, 308–311. [Google Scholar] [CrossRef]
- Lee, M.; Viciu, L.; Li, L.; Wang, Y.; Foo, M.L.; Watauchi, S.; Pascal, R.A., Jr.; Cava, R.J.; Ong, N.P. Enhancement of thermopower in NaxCoO2 in the large-x regime (x ≥ 0.75). Phys. B 2008, 403, 1564–1568. [Google Scholar] [CrossRef]
- Krasutskaya, N.S.; Klyndyuk, A.I.; Evseeva, L.E.; Tanaeva, S.A. Synthesis and properties of NaxCoO2 (x = 0.55, 0.89) oxide thermoelectric. Inorg. Mater. 2016, 52, 393–399. [Google Scholar] [CrossRef]
- Plewa, A.; Kulka, A.; Kondracki, Ł.; Lu, L.; Molenda, J. Phase diagram of NaFeyCo1−yO2 and evolution of its physico- and electrochemical properties with changing iron content. J. Power Sources 2019, 419, 42–51. [Google Scholar] [CrossRef]
- Devi Meena, J.; Gayathri, N.; Bharathi, A.; Ramachandran, K. Effect of nickel substitution on thermal properties of Na0.9CoO2. Bull. Mater. Sci. 2007, 30, 345–348. [Google Scholar] [CrossRef]
- Zhou, R.; Li, J.; Wei, W.; Li, X.; Luo, M. Atomic substituents effect on boosting desalination performances of Zn-doped NaxCoO2. Desalination 2020, 496, 114695. [Google Scholar] [CrossRef]
- Ono, Y.; Kato, N.; Miyazaki, Y.; Kajitani, T. Transport Properties of Ca-doped γ-NaxCoO2. J. Ceram. Soc. Jpn. 2004, 5, S626–S628. [Google Scholar] [CrossRef]
- Bos, J.W.G.; Hertz, J.T.; Morosan, E.; Cava, R.J. Magnetic and thermoelectric properties of layered LixNayCoO2. J. Chemisrtry State Chem. 2007, 180, 3211–3217. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Liu, J.; Hu, Z. Power factor enhancement in NaxCoO2 doped by Bi. J. Alloys Compd. 2014, 582, 59–63. [Google Scholar] [CrossRef]
- Seetawan, T.; Amornkitbamburg, V.; Burinprakhon, T.; Maensiri, S.; Kurosaki, K.; Muta, H.; Uno, M.; Yamanaka, S. Thermoelectric power and electrical resistivity of Ag-doped Na1.5Co2O4. J. Alloys Compd. 2006, 407, 314–317. [Google Scholar] [CrossRef]
- Hussein, M.; Assadi, N. Hf doping for enhancing the thermoelectric performance in layered Na0.75CoO2. Mater. Today Proc. 2021, 42, 1542–1546. [Google Scholar] [CrossRef]
- Nakhowong, R. Effect of reduced graphene oxide on the enhancement of thermoelectric power factor of γ-NaxCo2O4. Mater. Sci. Eng. B 2020, 261, 114679. [Google Scholar] [CrossRef]
- Ito, M.; Nagira, T.; Furumoto, D.; Katsuyama, S.; Nagai, H. Synthesis of NaxCoO2 thermoelectric oxides by the polymerized complex method. Scr. Mater. 2003, 48, 403–408. [Google Scholar] [CrossRef]
- Suan, M.S.M.; Farhan, M.A.; Lau, K.T.; Munawar, R.F.; Ismail, S. Structural and Electrical Properties of NaxCoO2 Thermoelectric Synthesized via Citric-Nitrate Auto-Combustion Reaction. J. Phys. Conf. Ser. 2018, 1082, 012033. [Google Scholar] [CrossRef]
- Itahara, H.; Fujita, K.; Sugiyama, J.; Nakamura, K.; Tani, T. Highly Textured NaxCoO2–δ Ceramics Fabricated by Both Templated Grain Growth and Reactive Templated Grain Growth Methods Using Single-Crystalline Particles as Templates. J. Ceram. Soc. Jpn. 2003, 111, 227–231. [Google Scholar] [CrossRef]
- Park, K.; Jang, K.U.; Know, H.-C.; Kim, J.-G.; Cho, W.-S.Y. Influence of partial substitution of Cu for Co on the thermoelectric properties of NaCo2O4. J. Alloys Compd. 2006, 419, 213–219. [Google Scholar] [CrossRef]
- Terasaki, I.; Tsukada, I.; Iguchi, Y. Impurity-induced transition and impurity-enhanced thermopower in the thermoelectric oxide NaCo2−xCuxO4. Phys. Rev. B 2002, 65, 195106. [Google Scholar] [CrossRef]
- Park, K.; Choi, J.W.; Lee, G.W.; Kim, S.-J.; Lim, Y.-S.; Choi, S.-M.; Seo, W.-S.; Lim, S.M. Thermoelectric Properties of Solution-Combustion-Processed Na(Co1−xNix)2O4. Mater. Lett. 2012, 18, 1061–1065. [Google Scholar] [CrossRef]
- Park, K.; Jang, K.U. Improvement in High-Temperature Properties of NaCo2O4 through Partial Substitution of Ni for Co. Mater. Lett. 2006, 60, 1106–1110. [Google Scholar] [CrossRef]
- Park, K.; Lee, J.H. Enhanced Thermoelectric Properties of NaCo2O4 by Adding Zn. Mater. Lett. 2008, 62, 2366–2368. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, X.; Liu, J.; Lin, Z.; Hu, Y.; Wu, Y.; Han, C.; Hu, Z. Power factor enhancement induced by Bi and Mn co-substitution in NaxCoO2 thermoelectric materials. J. Alloys Compd. 2016, 661, 161–167. [Google Scholar] [CrossRef]
- Shin, W.; Murayama, N.; Ikeda, K.; Sago, S.; Terasaki, I. Thermoelectric Device of Na(Co,Cu)2O4 and (Ba,Sr)PbO3. J. Ceram. Soc. Jpn. 2002, 110, 727–730. [Google Scholar] [CrossRef]
- Klyndyuk, A.I.; Krasutskaya, N.S.; Chizhova, E.A.; Evseeva, L.E.; Tanaeva, S.A. Synthesis and properties of Na0.55Co0.9M0.1O2 (M =Sc, Ti, Cr-Zn, Mo, W, Pb, Bi) solid solutions. Glass Phys. Chem. 2016, 42, 100–107. [Google Scholar] [CrossRef]
- Shannon, D.; Prewitt, C.T. Effective ionic radii in oxides and fluorides, Acta Crystallographica Section B: Structural Science. Cryst. Eng. Mater. 1969, 25, 925–946. [Google Scholar]
- Greenstein, G.R. The Merck index: An Encyclopedia of Chemicals, Drugs, and Biologicals. Ref. Rev. 2025, 21, 2746. [Google Scholar]
- Klyndyuk, A.I.; Krasutskaya, N.S.; Dyatlova, E.M. Influence of sintering temperature on the properties of NaxCoO2 ceramics. Proc. BSTU 2010, XVIII, 99–102. (In Russian) [Google Scholar]
- Pyatnitsky, I.V. Analitical Chemistry of Cobalt; Science: Moscow, Russia, 1965; 292p. (In Russian) [Google Scholar]
- Sopicka-Lizer, M.; Kozłowska, K.; Bobrowska-Grzesik, E.; Plewa, J.; Altenburg, H. Assessment of Co(II), Co(III) and Co(IV) content in thermoelectric cobaltites. Arh. Metall. Mater. 2009, 54, 881–888. [Google Scholar]
- Azad, S.; Kumar, N.; Chand, S. Structural, morphological, optical and dielectric properties of Ti1−xFexO2 nanoparticles sythesized using sol-gel method. J. Sol-Gel Sci. Technol. 2022, 105, 163–175. [Google Scholar] [CrossRef]
- Marwah, T.J. Using the size strain plot method to specity lattice parameters. Haitham J. Pure Appl. Sci. 2023, 36, 123–129. [Google Scholar] [CrossRef]
- Lotgering, F.K. Topotactical reactions with ferrimagnetic oxides having hexagonal crystal structures. J. Inorg. Nucl. Chem. 1959, 9, 113–123. [Google Scholar] [CrossRef]
- Klyndyuk, A.I.; Chizhova, E.A.; Latypov, R.S.; Shevchenko, S.V.; Kononovich, V.M. Effect of the addition of copper particles on the thermoelectric properties of the Ca3Co4O9+δ ceramics produced by two-step sintering. Inorg. Mater. 2022, 67, 237–244. [Google Scholar] [CrossRef]
- Klyndyuk, A.I.; Petrov, G.S.; Poluyan, A.F.; Bashkirov, L.A. Structure and Physicochemical Properties of Y2Ba1−xMxCuO5 (M = Sr, Ca) Solid Solutions. Inorg. Mater. 1999, 35, 512–516. [Google Scholar] [CrossRef]
- Zorkovská, A.; Feher, A.; Sébek, J.; Šantavá, E.; Bradaric, I. Non-Fermi-liquid behavior in the layered NaxCoO2. Low Temp. Phys. 2007, 33, 944–947. [Google Scholar] [CrossRef]
- Snyder, G.J.; Ursell, T.S. Thermoelectric Efficiency and Compatibility. Phys. Rev. Lett. 2003, 91, 148301. [Google Scholar] [CrossRef]
- Delmas, C.; Braconnier, J.-J.; Fouassier, C.; Hagenmuller, P. Electrochemical intertcalation of sodium in NaxCoO2 bronzes. Solid State Ion. 1981, 3, 165–169. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Cui, Y.; Zhang, H.; Xiao, F.; Wang, L.; Nakano, H. High temperature electrical conductivity and thermoelectric power of NaxCoO2. Solid State Ion. 2008, 179, 2308–2312. [Google Scholar] [CrossRef]
- Matsubara, I.; Zhou, Y.; Takeuchi, T.; Funahashi, R.; Shikano, M.; Murayama, N.; Shin, W.; Izu, N. Thermoelectric properties of spark-plasma-sintered Na1+xCo2O4 ceramics. J. Ceramic. Soc. Jpn. 2003, 111, 238–241. [Google Scholar] [CrossRef]
- Li, N.; Jiang, Y.; Li, G.; Wang, C.; Shi, J.; Yu, D. Self-ignition route to Ag-doped Na1.7Co2O4 and its thermoelectric properties. J. Alloys Compd. 2009, 467, 444–449. [Google Scholar] [CrossRef]
- Lei, Y.; Li, X.; Liu, L.; Ceder, G. Synthesis and Stochiometry of Different Layered Sodium Cobalt Oxides. Chem. Mater. 2014, 26, 5288–5296. [Google Scholar] [CrossRef]
- Klyndyuk, A.I.; Matsukevich, I.V. Synthesis, structure and properties of Ca3Co3.85M0.15O9+δ (M–Ti–Zn, Mo, W, Pb, Bi). Russ. J. Inorg. Chem. 2015, 51, 1025–1031. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Lan, J.; Shen, Z.; Liu, Y.; Nan, C.-W.; Li, J.-F. High-temperature electrical transport behaviors in textured Ca3Co4O9-based polycrystalline ceramics. Appl. Phys. Lett. 2009, 94, 072107. [Google Scholar] [CrossRef]
- Pan, L.; Mitra, S.; Zhao, L.-D.; Shen, Y.; Wang, Y.; Fesel, C.; Berardan, D. The Role of Ionized Impurity Scattering on the Thermoelectric Performances of Rock Salt AgPbmSnSe2+m. Adv. Funct. Mater. 2016, 26, 5149–5157. [Google Scholar] [CrossRef]
- Snyder, G.J.; Snyder, A.H.; Wood, M.; Gurunathan, R.; Snyder, B.H.; Niu, C. Weighted Mobility. Adv. Mater. 2020, 32, 2001537. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, S.; Liu, F.; Yan, G.; Chen, J.; Li, H.; Wang, J.; Yu, W.; Fu, G. Laser-induced voltage effects in c-axis inclined NaxCoO2 thin films. Appl. Surf. Sci. 2012, 258, 7330–7333. [Google Scholar] [CrossRef]
- Liu, W.; Tang, X.; Sharp, J. Low-temperature solid state synthesis and thermoelectric properties of high-performance and low-cost Sb-doped Mg2Si0.6Sn0.4. J. Phys. D Appl. Phys. 2010, 43, 085406. [Google Scholar] [CrossRef]
- Fergus, J.W. Oxide material for high temperature thermoelectric energy conversion. J. Eur. Ceram. Soc. 2012, 32, 525–540. [Google Scholar] [CrossRef]
- Krόlicka, A.K.; Piersa, M.; Mirowska, A.; Michalska, M. Effect of sol-gel and solid state synthesis techniques on structural, morphological and thermoelectric performancs of Ca3Co4O9. Ceram. Int. 2018, 44, 13736–13743. [Google Scholar] [CrossRef]
- Klyndyuk, A.I.; Krasutskaya, N.S.; Khort, A.A. Synthesis and Properties of Ceramics Based on a Layered Bismuth Calcium Cobaltite. Inorg. Mater. 2018, 54, 509–514. [Google Scholar] [CrossRef]
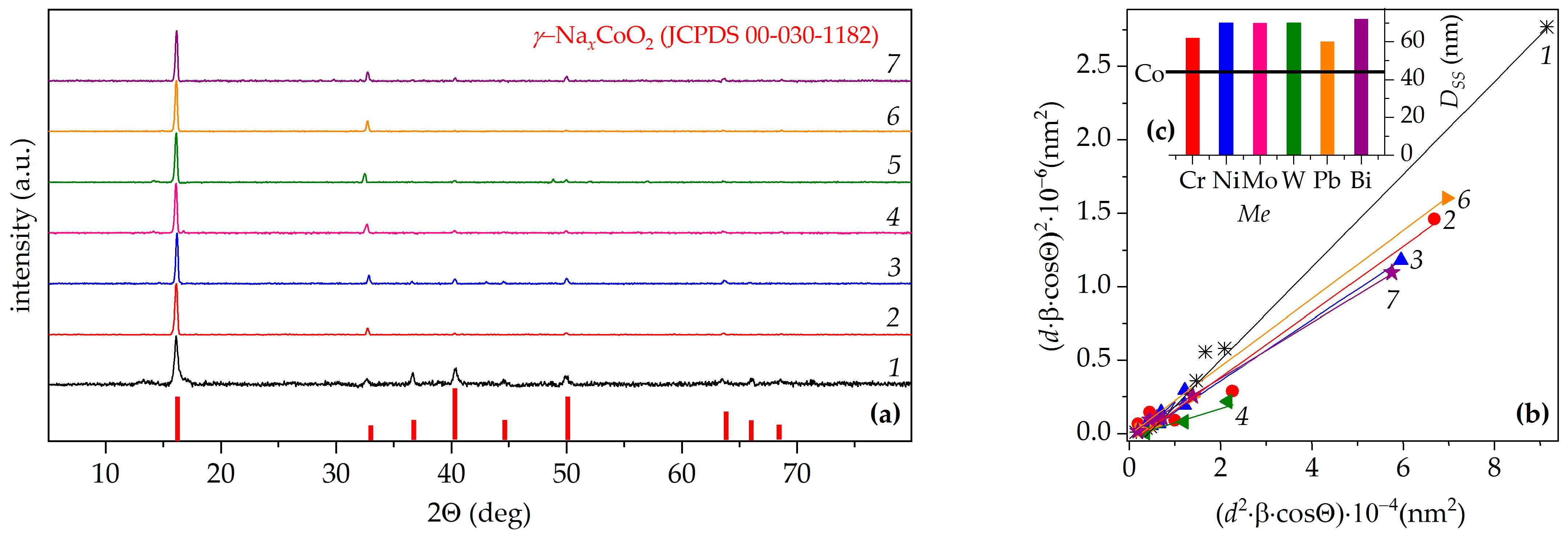

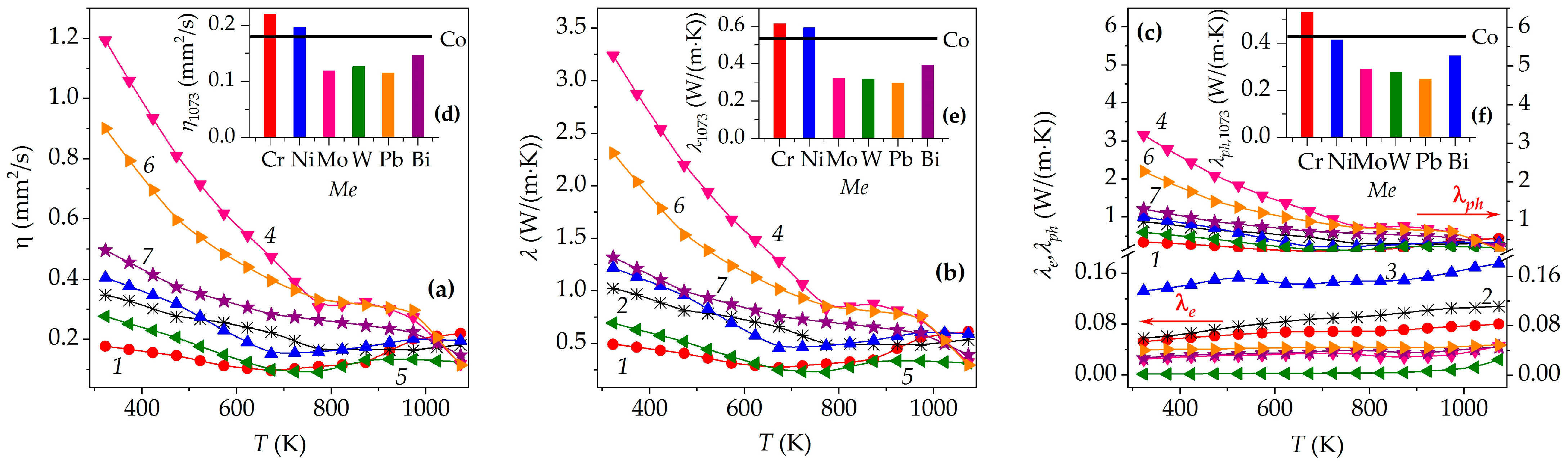
| Me | xNa | Co+Z | a, Å | c, Å | c/a | V, Å3 | f | Ds, nm | DSS, nm | ε × 104 | dXRD, g/cm3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cr | 0.893 | 3.11 | 2.825 | 10.93 | 3.870 | 75.53 | 0.90 | 69 | 62 | 3.43 | 4.86 |
| Co | 0.891 | 3.11 | 2.826 | 10.94 | 3.872 | 75.71 | 0.31 | 63 | 45 | 4.56 | 4.98 |
| Ni | 0.889 | 3.23 | 2.831 | 10.92 | 3.856 | 75.75 | 0.69 | 70 | 70 | 2.61 | 4.88 |
| Mo | 0.894 | 2.78 | 2.822 | 10.96 | 3.883 | 75.57 | 0.87 | 73 | 70 | 1.41 | 5.06 |
| W | 0.889 | 2.78 | 2.825 | 10.97 | 3.884 | 75.80 | 0.92 | 67 | 69 | 1.50 | 5.43 |
| Pb | 0.888 | 3.01 | 2.825 | 10.94 | 3.873 | 75.64 | 0.92 | 79 | 60 | 3.22 | 5.55 |
| Bi | 0.894 | 2.90 | 2.823 | 10.93 | 3.872 | 75.45 | 0.76 | 77 | 72 | 2.49 | 5.56 |
| M | dEXP, g/cm3 | Πt, % | Πo, % | Πc, % | 105 × α, K−1 | 104 × ρ1073, Ω·m | S1073, μV/K | P1073, mW/(m·K2) | λ1073, W/(m·K) | ZT1073 |
|---|---|---|---|---|---|---|---|---|---|---|
| Cr | 3.18 | 35 | 16 | 19 | 1.68 | 3.28 | 134 | 0.055 | 0.613 | 0.10 |
| Co | 3.38 | 32 | 19 | 13 | 1.34 | 2.43 | 439 | 0.794 | 0.536 | 1.59 |
| Ni | 3.46 | 29 | 22 | 7 | 1.42 | 1.50 | 369 | 0.910 | 0.591 | 1.65 |
| Mo | 3.22 | 36 | 19 | 17 | 1.47 | 5.73 | 408 | 0.291 | 0.323 | 0.97 |
| W | 3.20 | 41 | 17 | 24 | 1.39 | 10.9 | 519 | 0.320 | 0.316 | 1.09 |
| Pb | 3.34 | 40 | 18 | 22 | 1.26 | 5.58 | 358 | 0.230 | 0.295 | 0.84 |
| Bi | 3.47 | 38 | 18 | 20 | 1.25 | 5.97 | 616 | 0.636 | 0.392 | 1.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasutskaya, N.S.; Chizhova, E.A.; Zizika, J.A.; Buka, A.V.; Wang, H.; Klyndyuk, A.I. Improving of Thermoelectric Efficiency of Layered Sodium Cobaltite Through Its Doping by Different Metal Oxides. Ceramics 2025, 8, 86. https://doi.org/10.3390/ceramics8030086
Krasutskaya NS, Chizhova EA, Zizika JA, Buka AV, Wang H, Klyndyuk AI. Improving of Thermoelectric Efficiency of Layered Sodium Cobaltite Through Its Doping by Different Metal Oxides. Ceramics. 2025; 8(3):86. https://doi.org/10.3390/ceramics8030086
Chicago/Turabian StyleKrasutskaya, Natalie S., Ekaterina A. Chizhova, Julia A. Zizika, Alexey V. Buka, Hongchao Wang, and Andrei I. Klyndyuk. 2025. "Improving of Thermoelectric Efficiency of Layered Sodium Cobaltite Through Its Doping by Different Metal Oxides" Ceramics 8, no. 3: 86. https://doi.org/10.3390/ceramics8030086
APA StyleKrasutskaya, N. S., Chizhova, E. A., Zizika, J. A., Buka, A. V., Wang, H., & Klyndyuk, A. I. (2025). Improving of Thermoelectric Efficiency of Layered Sodium Cobaltite Through Its Doping by Different Metal Oxides. Ceramics, 8(3), 86. https://doi.org/10.3390/ceramics8030086