Effect of Ni2+ Doping on the Crystal Structure and Properties of LiAl5O8 Low-Permittivity Microwave Dielectric Ceramics
Abstract
1. Introduction
2. Materials and Methods
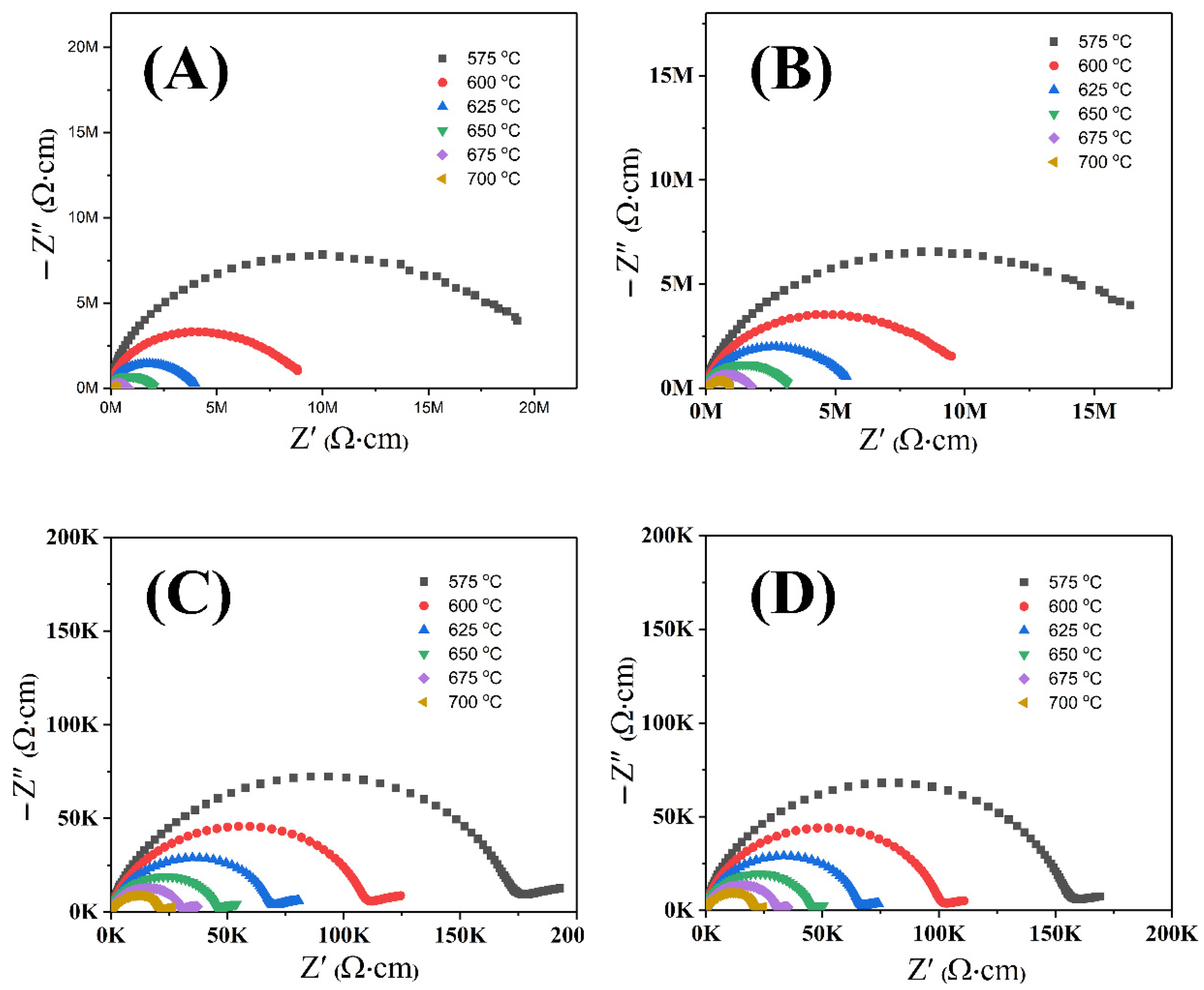
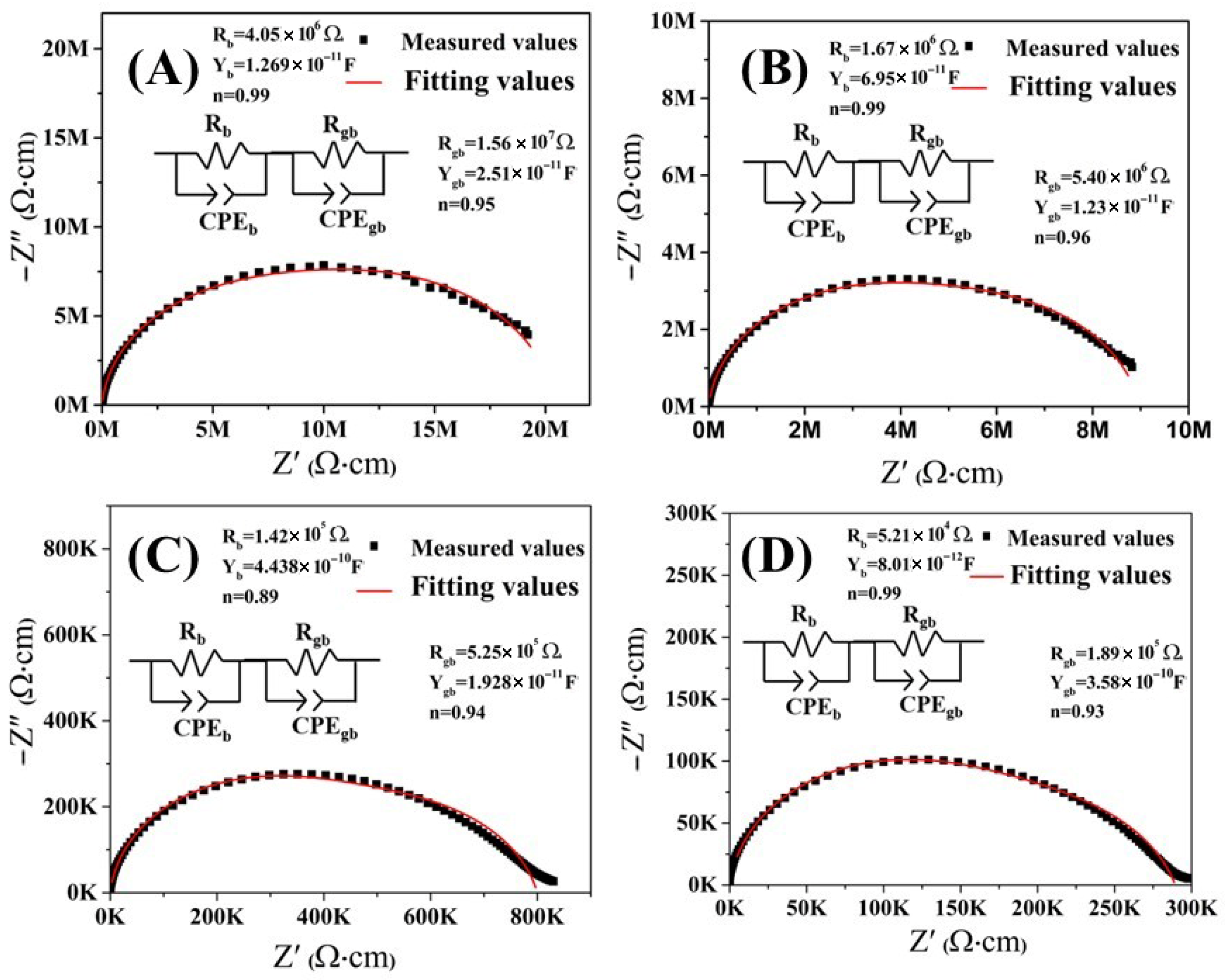
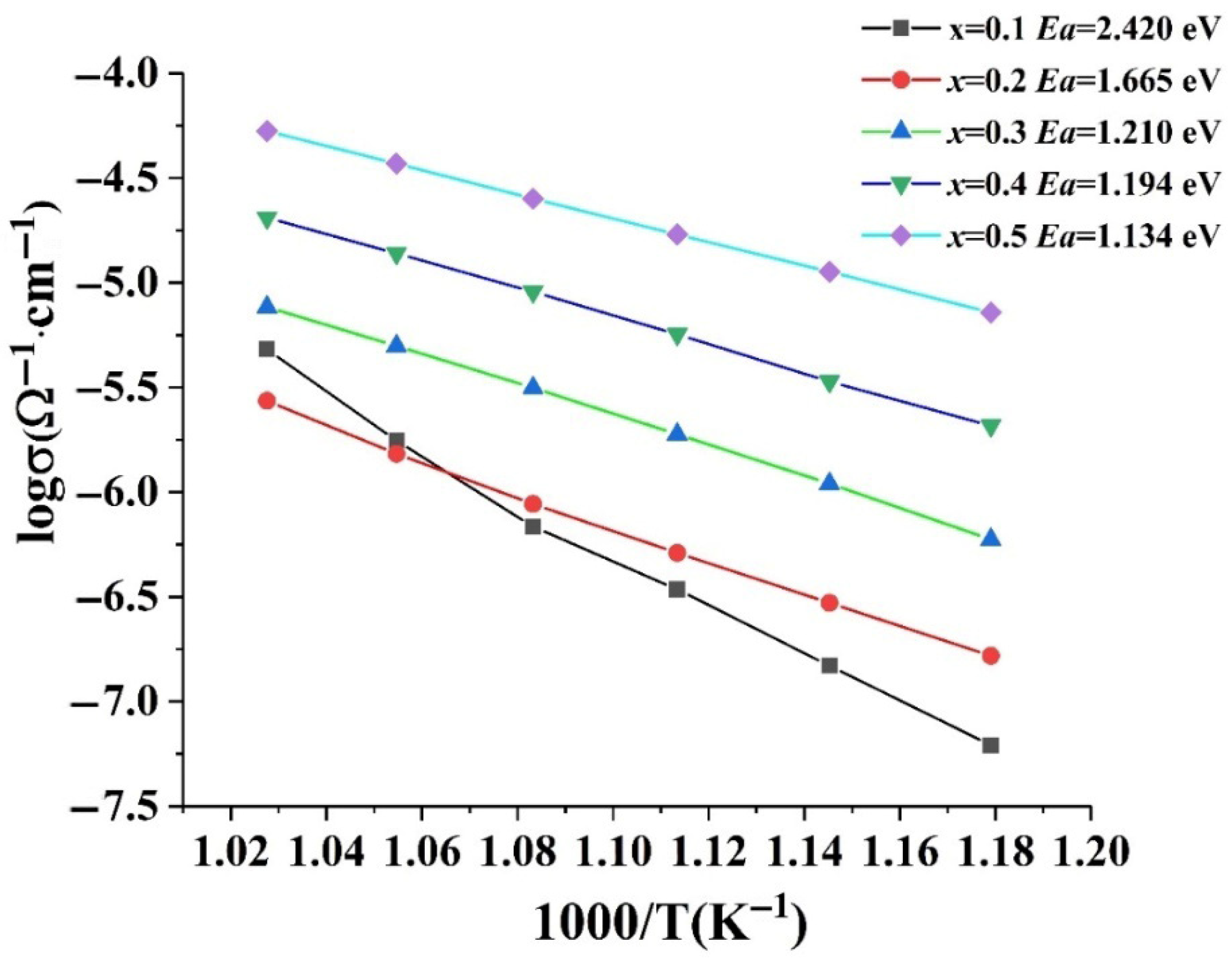
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; Xiang, H.C.; Tang, Y.; Li, J.; Fang, L. Constructing the cationic rattling effect to realize the adjustability of the temperature coefficient in Nd2-xSmxO3 microwave dielectric ceramics. J. Eur. Ceram. Soc. 2024, 44, 2859–2865. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, K.H.; Wang, W.; Liu, Z.Y.; Fang, Z.; Wang, X.; Wang, C.H.; Li, Y.C.; He, G.Q.; Zhou, T.; et al. A Series of Ultra-low Permittivity ALaP4O12 (A = Li, Na, K) Metaphosphate Microwave Dielectric Ceramics for Ultra-wideband Dielectric Resonant Antenna Application. ACS Appl. Mater. Interfaces 2024, 16, 58898–58911. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.W.; Yang, X.H.; Zhang, Y.C. Novel single-phase Li2SiO3 microwave dielectric ceramic with low permittivity. J. Eur. Ceram. Soc. 2025, 45, 116940. [Google Scholar] [CrossRef]
- Wang, H.Y.; Li, X.Y.; Xu, X.W.; Cheng, J.; Guo, B.; Chen, X.Q.; Wang, H. MgF2-based microwave dielectric ceramics with ultra-low sintering temperature and high thermal expansion coefficient. J. Eur. Ceram. Soc. 2024, 44, 2150–2156. [Google Scholar] [CrossRef]
- Mo, S.; Zhang, B.K.; Zhang, K.C.; Li, S.N.; Pan, F. LiAl5O8 as a potential coating material in lithium-ion batteries: A first principles study. Phys. Chem. Chem. Phys. 2019, 21, 13758–13765. [Google Scholar] [CrossRef]
- Miyakawa, S.; Matsuda, S.; Tanibata, N.; Takeda, H. Computational studies on defect chemistry and Li-ion conductivity of spinel-type LiAl5O8 as coating material for Li-metal electrode. Sci. Rep. 2022, 12, 16672. [Google Scholar] [CrossRef]
- Temeche, E.; Indris, S.; Laine, R. LiAlO2/LiAl5O8 membranes derived from flame-synthesized nanopowders as a potential electrolyte and coating material for all-solid-state batteries. ACS Appl. Mater. Interfaces 2020, 12, 46119–46131. [Google Scholar] [CrossRef]
- Roy, A.; Senso, D.; Casella, A.; Devanathan, R. Molecular dynamics simulations of radiation response of LiAlO2 and LiAl5O8. J. Nucl. Mater. 2023, 576, 154280. [Google Scholar] [CrossRef]
- Kniec, K.; Tikhomirov, M.; Tikhomirov, M.; Pozniak, B.; Pozniak, K.; Marciniak, L. LiAl5O8: Fe3+ and LiAl5O8: Fe3+, Nd3+ as a new luminescent nanothermometer operating in 1st biological optical window. Nanomaterials 2020, 10, 189. [Google Scholar] [CrossRef]
- Lan, X.K.; Li, J.; Li, J.P.; Wang, F.; Lu, W.Z.; Wang, X.C.; Lei, W. Phase evolution and microwave dielectric properties of novel LiAl5-xZnxO8-0.5x-based (0 ≤ x ≤ 0.5) ceramics. J. Am. Ceram. Soc. 2020, 103, 1105–1112. [Google Scholar] [CrossRef]
- Ao, L.Y.; Tang, Y.; Li, J.; Fang, W.S.; Duan, L.; Su, C.X.; Sun, Y.H.; Liu, L.J.; Fang, L. Structure characterization and microwave dielectric properties of LiGa5O8 ceramic with low-εr and low loss. J. Eur. Ceram. Soc. 2020, 40, 5498–5503. [Google Scholar] [CrossRef]
- Yang, J.; Ao, L.Y.; Luo, X.F.; Chen, C.Q.; Zhang, X.; Fang, L.; Tang, B.; Tang, X.Z. Influence of (Zn0.5Ti0.5)3+ substitution on structural evolution, bond characteristics, and microwave dielectric properties of spinel Li(Zn0.5Ti0.5)xGa5-xO8 solid solutions. Ceram. Int. 2023, 49, 27965–27974. [Google Scholar] [CrossRef]
- Donegan, J.F.; Bergin, F.J.; Glynn, T.J.; Imbusch, G.F.; Remeika, J.P. The optical spectroscopy of LiGa5O8: Ni2+. J. Lumin. 1986, 35, 57–63. [Google Scholar] [CrossRef]
- Izumi, K.; Miyazaki, S.; Yoshida, S.; Mizokawa, T.; Hanamura, E. Optical properties of 3d transition-metal-doped MgAl2O4 spinels. Phys. Rev. B 2007, 76, 075111. [Google Scholar] [CrossRef]
- Li, C.; Ding, S.H.; Zhang, Y.; Zhu, H.; Song, T.X. Effects of Ni2+ substitution on the crystal structure, bond valence, and microwave dielectric properties of BaAl2-2xNi2xSi2O8-x ceramics. J. Eur. Ceram. Soc. 2021, 41, 2610–2616. [Google Scholar] [CrossRef]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Hakki, B.W.; Coleman, P.A. Dielectric resonator method of measuring inductive capacities in the millimeter range. IEEE T. Microw. Theory 1960, 8, 402–410. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Zhang, G.; Guo, J.; Yuan, X.F.; Wang, H. Ultra-low temperature sintering and microwave dielectric properties of a novel temperature stable Na2Mo2O7-Na0.5Bi0.5MoO4 ceramic. J. Eur. Ceram. Soc. 2018, 38, 813–816. [Google Scholar] [CrossRef]
- Shannon, R.D. Dielectric polarizabilities of ions in oxides and fluorides. J. Appl. Phys. 1993, 73, 348–366. [Google Scholar] [CrossRef]
- Li, M.; Pietrowski, M.; Souza, R.; Zhang, H.R.; Reaney, I.M.; Cook, S.N.; Kilner, J.A.; Sinclair, D.C. A family of oxide ion conductors based on the ferroelectric perovskite Na0.5Bi0.5TiO3. Nat. Mater. 2014, 13, 31–35. [Google Scholar] [CrossRef]
- Zang, J.; Li, M.; Sinclair, D.C.; Jo, W.; Rodel, J. Impedance spectroscopy of (Bi1/2 Na1/2)TiO3–BaTiO3 ceramics modified with (K0.5Na0.5)NbO3. J. Am. Ceram. Soc. 2014, 97, 1523–1529. [Google Scholar] [CrossRef]
- Choi, G.K.; Kim, J.R.; Yoon, S.H.; Hong, K.S. Microwave dielectric properties of scheelite (A = Ca, Sr, Ba) and wolframite (A= Mg, Zn, Mn) AMoO4 compounds. J. Eur. Ceram. Soc. 2007, 27, 3063–3067. [Google Scholar] [CrossRef]
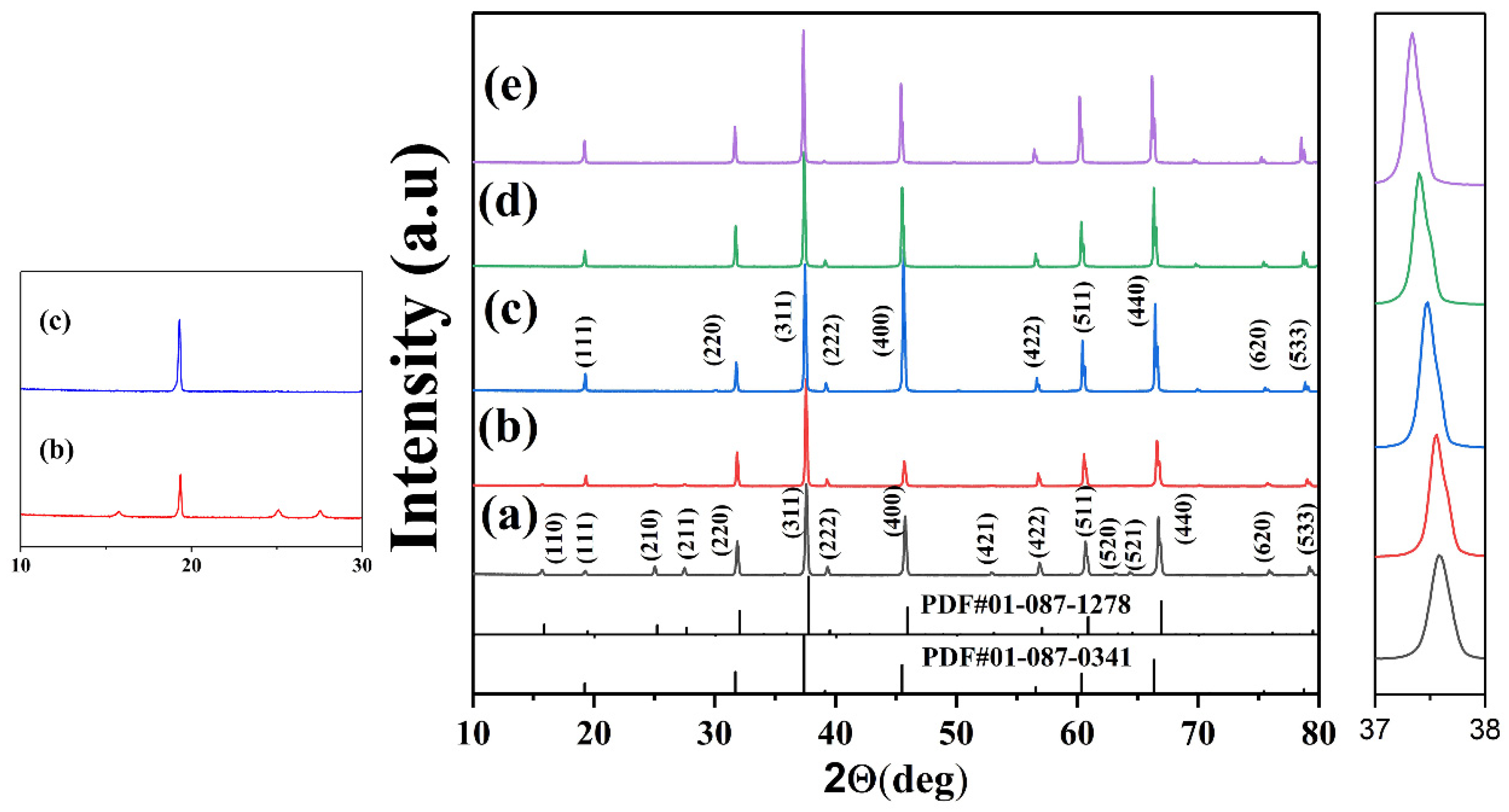
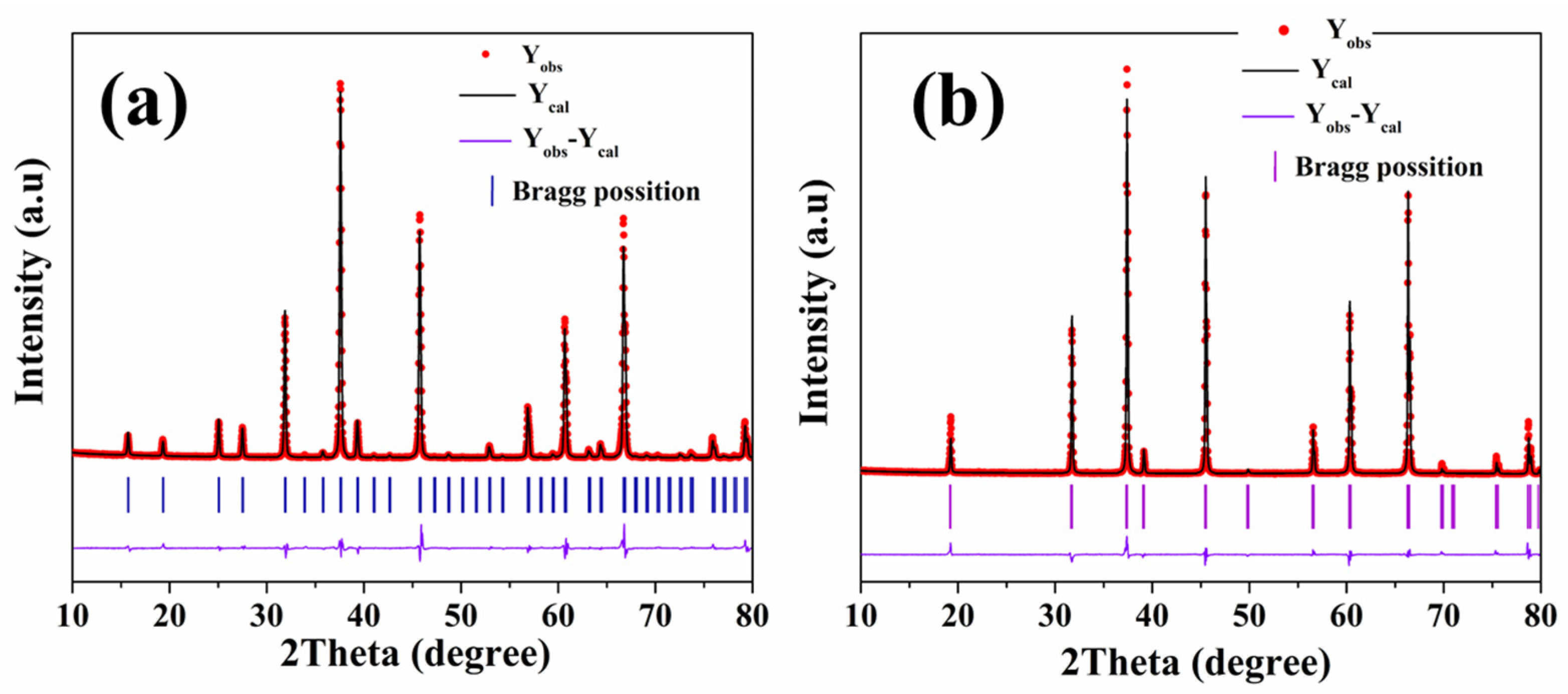
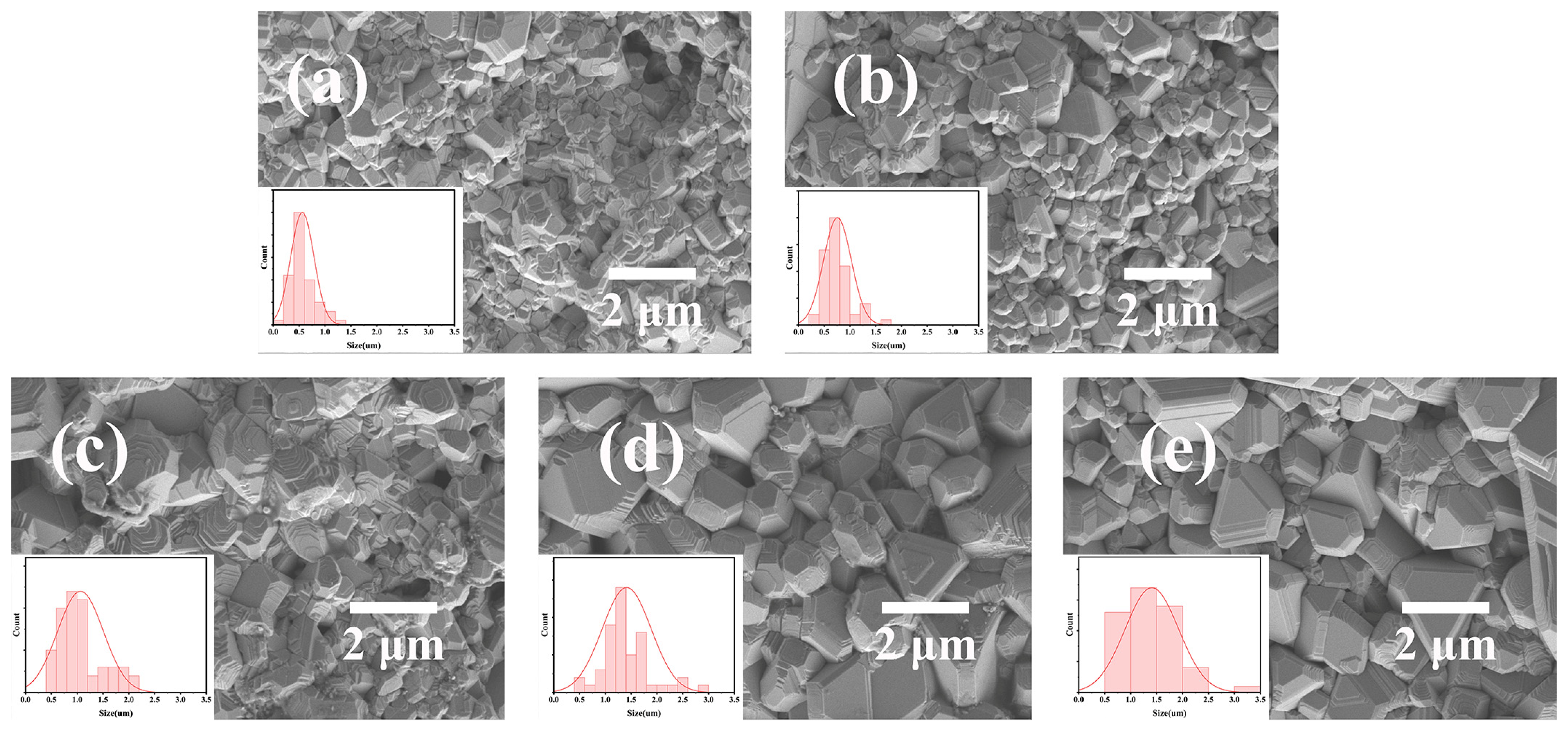
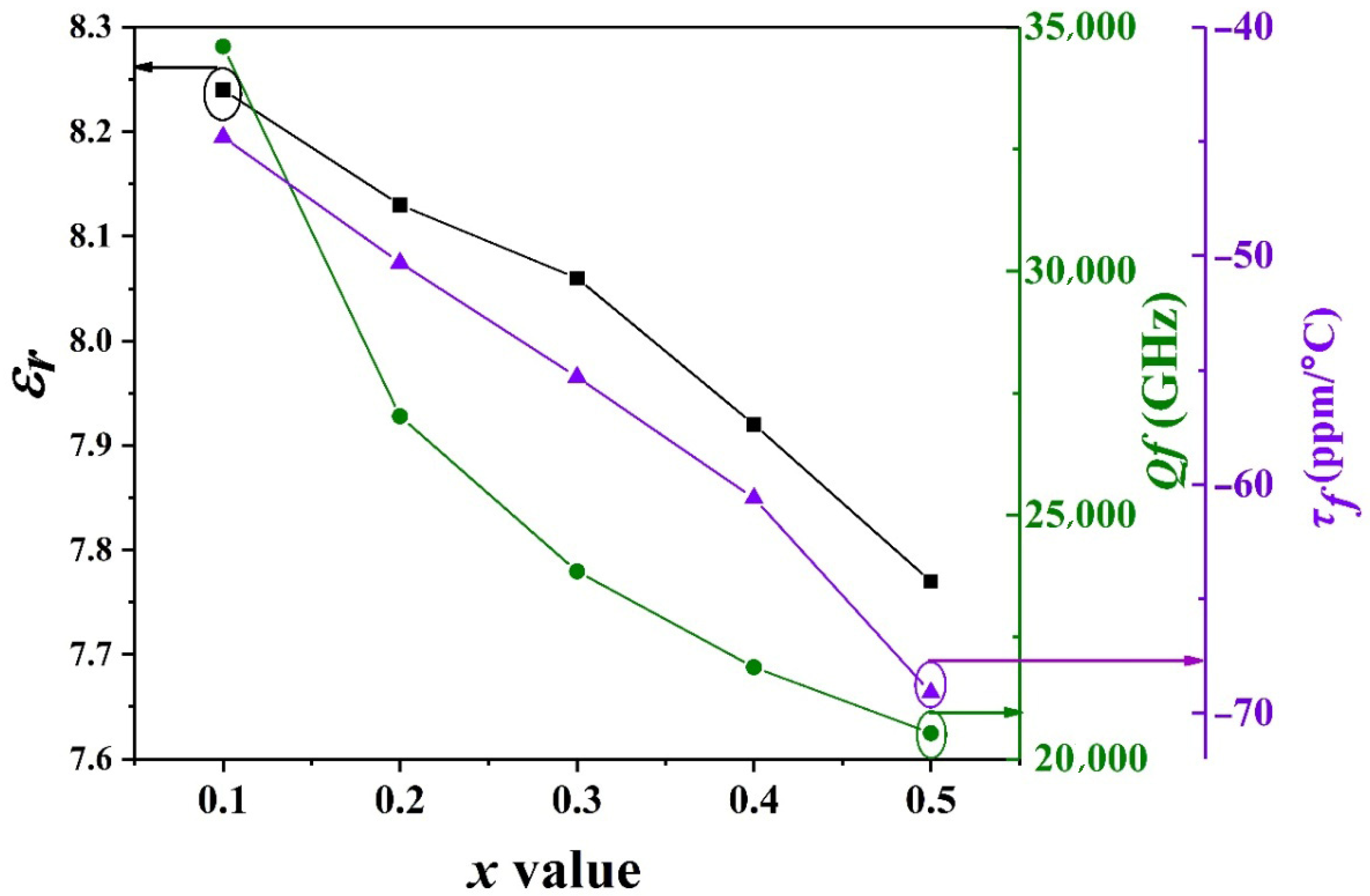

| Atom | x | y | z | Fraction | Rp | Rwp | χ2 |
|---|---|---|---|---|---|---|---|
| Li11 | 0.6250 | 0.6250 | 0.6250 | 1.00 | 0.053 | 0.081 | 4.869 |
| Li21 | −0.8756 | 1.1256 | 0.1250 | 1.00 | |||
| Al11 | 0.0024 | 0.0024 | 0.0024 | 1.00 | |||
| Al21 | 0.6250 | 0.6250 | 0.6250 | 1.00 | |||
| Al31 | 0.3725 | 0.1225 | 0.1250 | 1.00 | |||
| Ni11 | −0.0024 | −0.0024 | −0.0024 | 1.00 | |||
| Ni21 | 0.6250 | 0.6250 | 0.6250 | 1.00 | |||
| O11 | 0.1135 | 0.1231 | 0.3871 | 1.00 | |||
| O21 | 0.4023 | 0.4023 | 0.4023 | 1.00 | |||
| Li2 | 0.5000 | 0.5000 | 0.5000 | 1.00 | 0.048 | 0.071 | 4.235 |
| Al12 | 0.1250 | 0.1250 | 0.1250 | 0.94 | |||
| Al22 | 0.5000 | 0.5000 | 0.5000 | 0.94 | |||
| Ni12 | 0.1250 | 0.1250 | 0.1250 | 0.06 | |||
| Ni22 | 0.5000 | 0.5000 | 0.5000 | 0.06 | |||
| O2 | 0.2559 | 0.2559 | 0.2559 | 1.00 |
| x | Lattice Parameter a (Å) | Unit Cell Volume (Å3) | Measured Density (g/cm3) | Calculated Density (g/cm3) | Relative Density (%) |
|---|---|---|---|---|---|
| 0.1 | 7.909 | 494.726 | 3.528 | 3.656 | 96.5 |
| 0.2 | 7.944 | 501.323 | 3.552 | 3.639 | 97.6 |
| 0.3 | 7.952 | 502.839 | 3.565 | 3.660 | 97.4 |
| 0.4 | 7.963 | 504.929 | 3.536 | 3.676 | 96.2 |
| 0.5 | 7.975 | 507.215 | 3.582 | 3.690 | 97.1 |
| x | (Å3) | Vm (Å3) | εr | εc |
|---|---|---|---|---|
| 0.1 | 84.18 | 494.726 | 8.24 | 8.43 |
| 0.2 | 83.96 | 501.323 | 8.13 | 8.04 |
| 0.3 | 83.74 | 502.839 | 8.06 | 7.94 |
| 0.4 | 83.52 | 504.929 | 7.92 | 7.76 |
| 0.5 | 83.30 | 507.215 | 7.77 | 7.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, X.; Tang, H.; Chen, B.; Tian, B. Effect of Ni2+ Doping on the Crystal Structure and Properties of LiAl5O8 Low-Permittivity Microwave Dielectric Ceramics. Ceramics 2025, 8, 85. https://doi.org/10.3390/ceramics8030085
Lan X, Tang H, Chen B, Tian B. Effect of Ni2+ Doping on the Crystal Structure and Properties of LiAl5O8 Low-Permittivity Microwave Dielectric Ceramics. Ceramics. 2025; 8(3):85. https://doi.org/10.3390/ceramics8030085
Chicago/Turabian StyleLan, Xuekai, Huatao Tang, Bairui Chen, and Bin Tian. 2025. "Effect of Ni2+ Doping on the Crystal Structure and Properties of LiAl5O8 Low-Permittivity Microwave Dielectric Ceramics" Ceramics 8, no. 3: 85. https://doi.org/10.3390/ceramics8030085
APA StyleLan, X., Tang, H., Chen, B., & Tian, B. (2025). Effect of Ni2+ Doping on the Crystal Structure and Properties of LiAl5O8 Low-Permittivity Microwave Dielectric Ceramics. Ceramics, 8(3), 85. https://doi.org/10.3390/ceramics8030085




