Sustainable Use of Gypsum Waste for Applications in Soil–Cement Bricks: Mechanical, Environmental, and Durability Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Specimen Preparation
2.3. Mineralogical and Chemical Characterization
2.4. Thermal Analysis
2.5. Scanning Electron Microscopy (SEM) Procedures
2.6. Mechanical and Physical Testing
2.6.1. Compressive Strength Test
2.6.2. Water Absorption and Apparent Density
2.6.3. Durability Test Under Wet–Dry Cycles
2.7. Statistical Analysis
3. Results
3.1. Physical and Chemical Characterization of Raw Materials
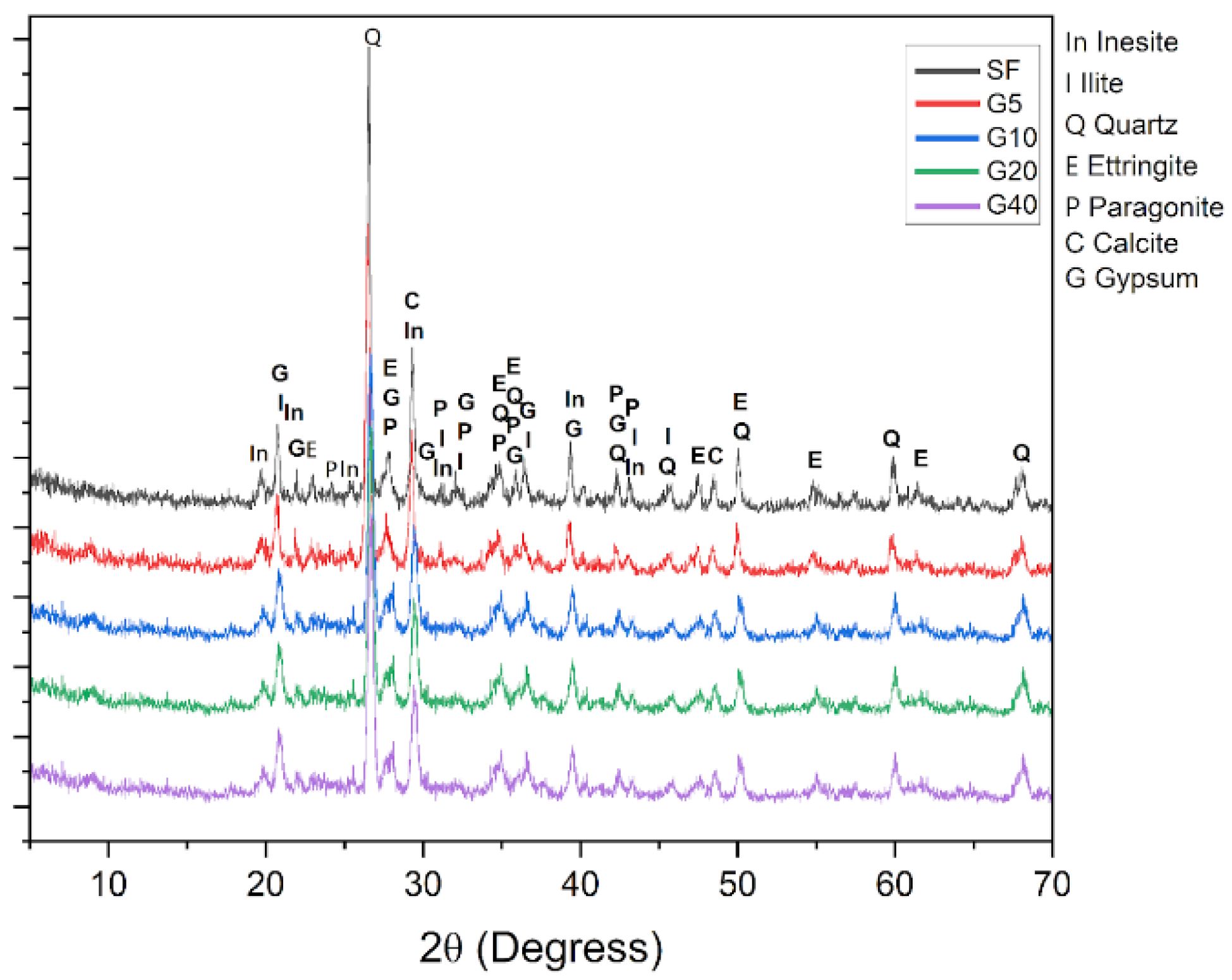
3.2. Mineralogical and Phase Analysis
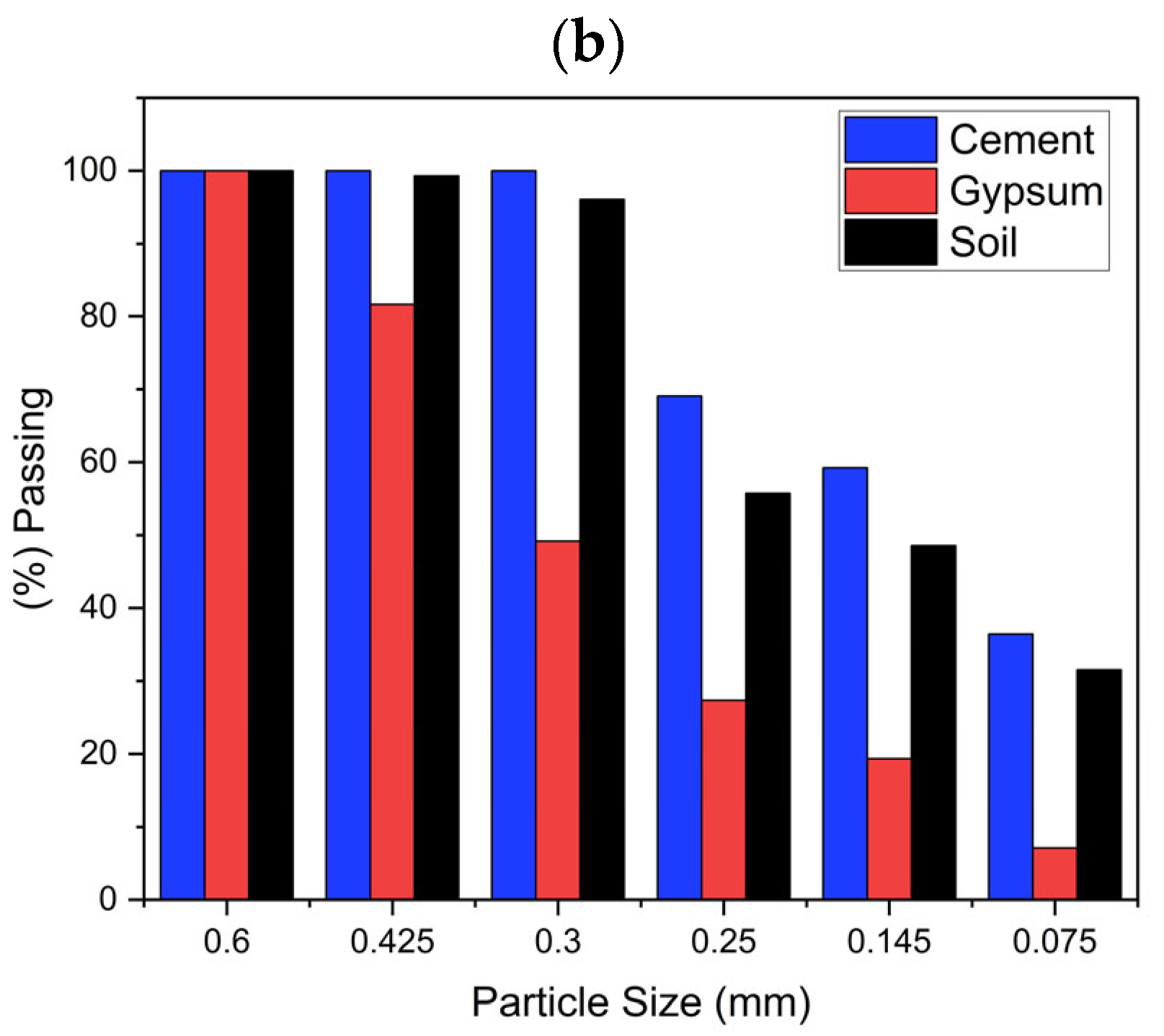
3.3. Particle Size Distribution and Plasticity
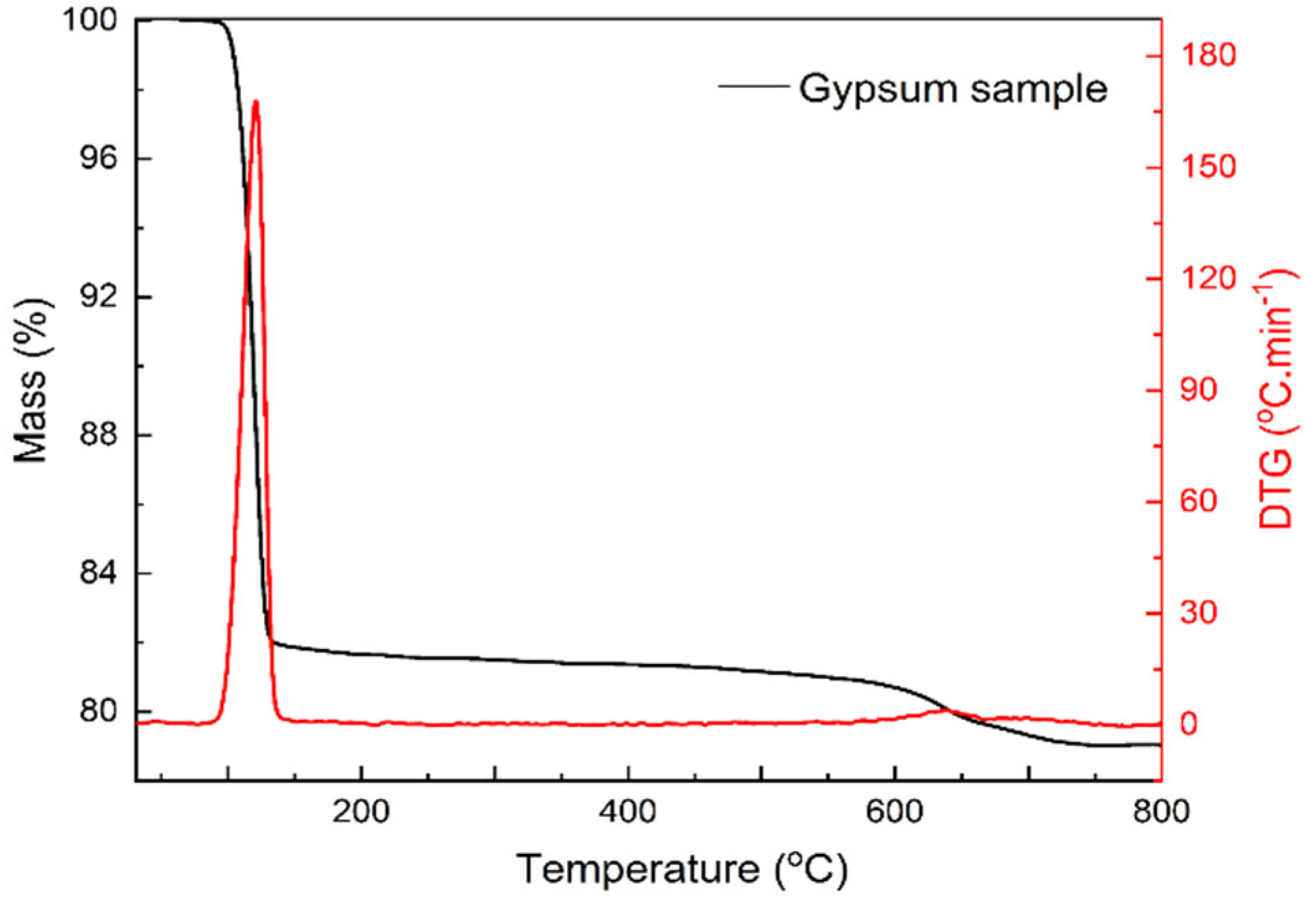
3.4. Thermal Analysis of Gypsum and Soil
3.5. Mechanical Performance
3.5.1. Compressive Strength
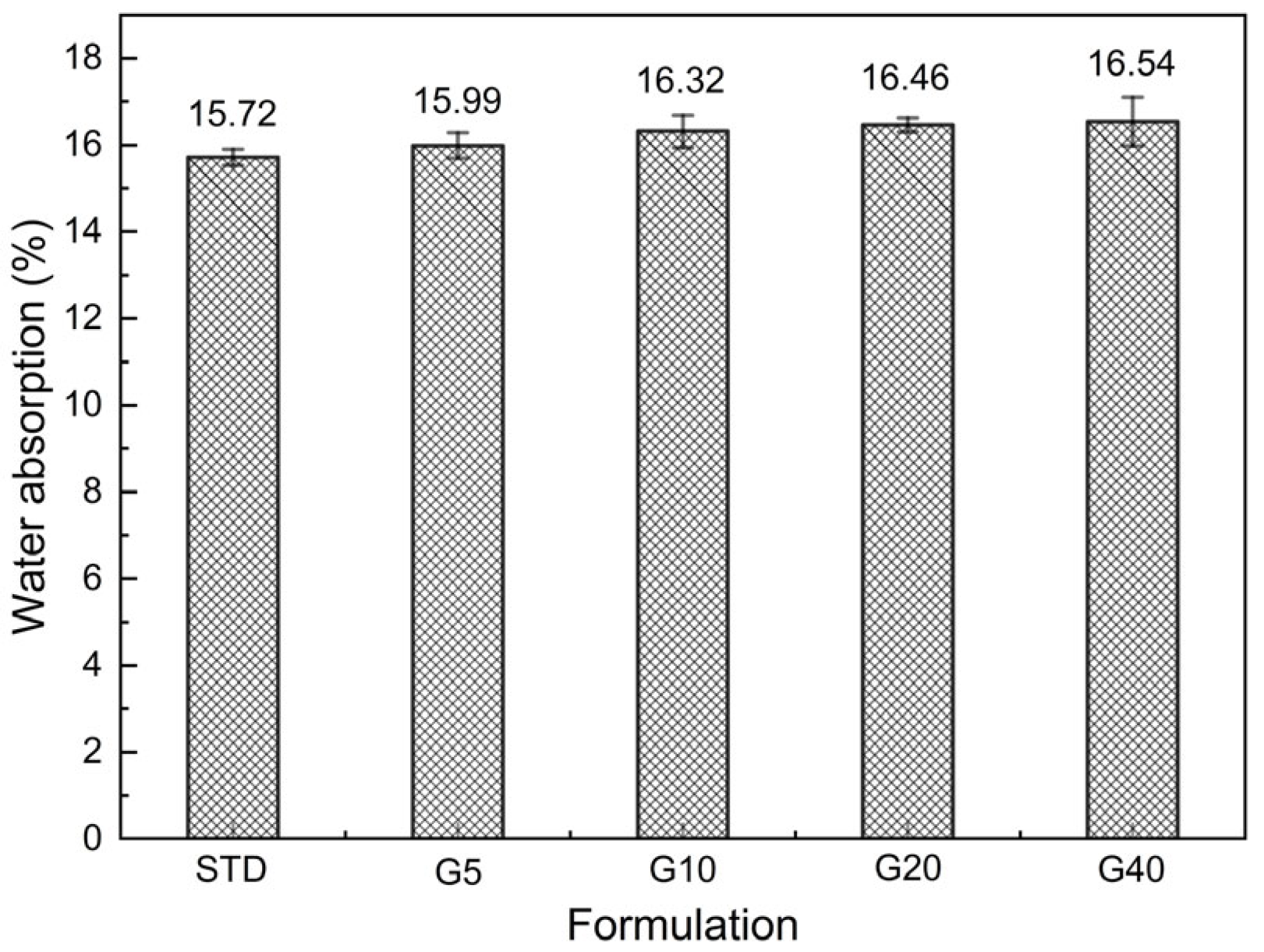
3.5.2. Water Absorption
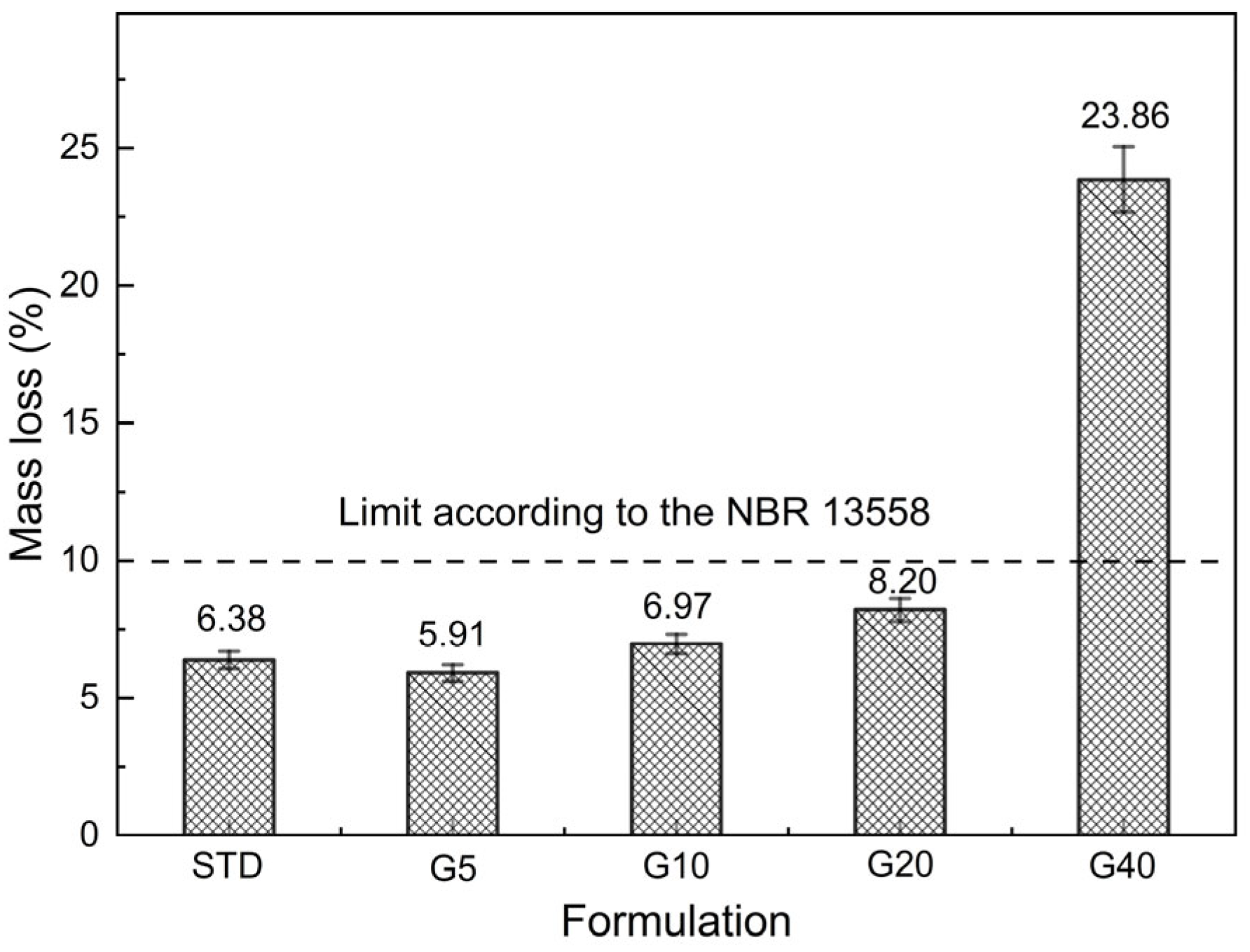
3.5.3. Durability
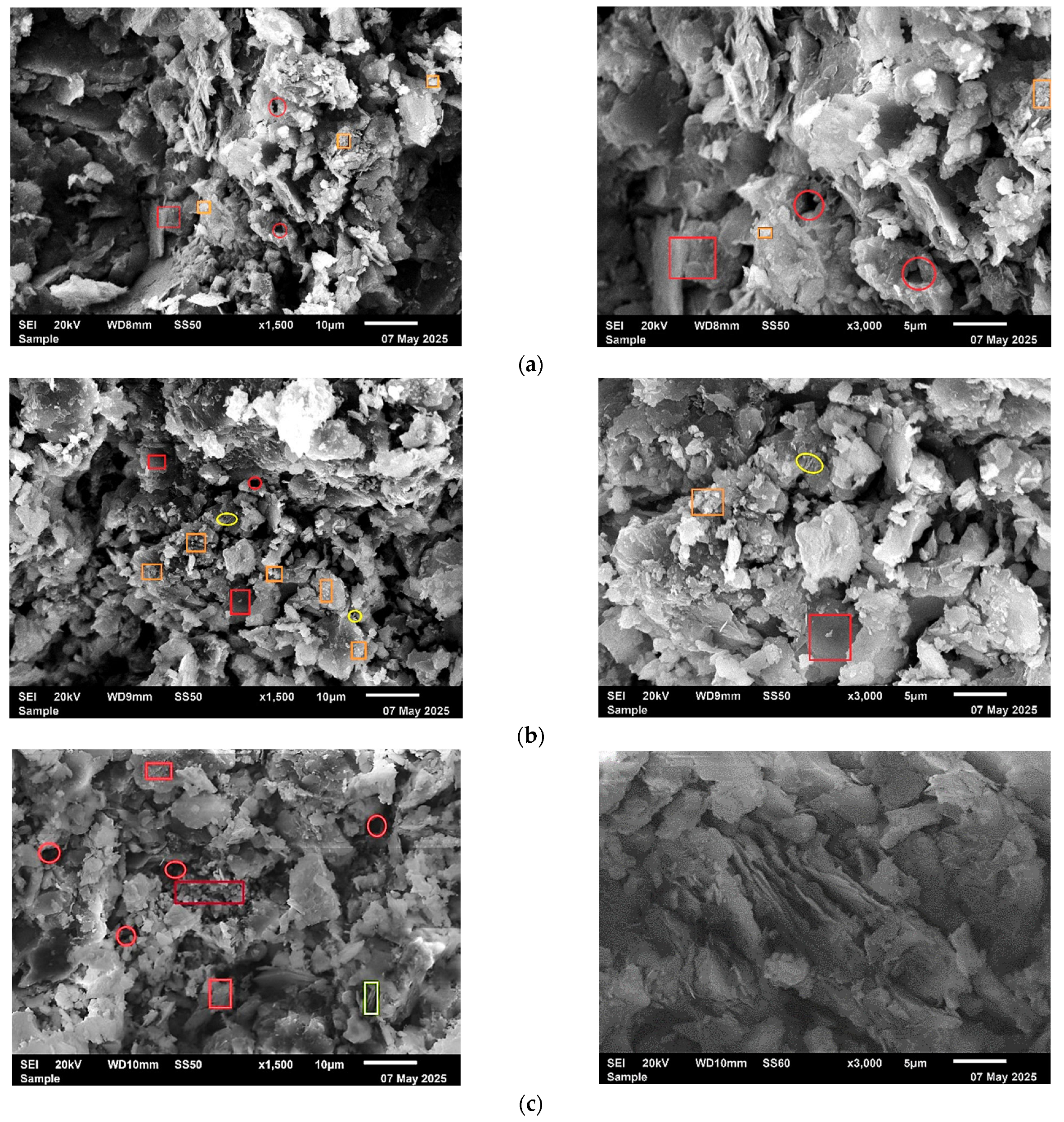
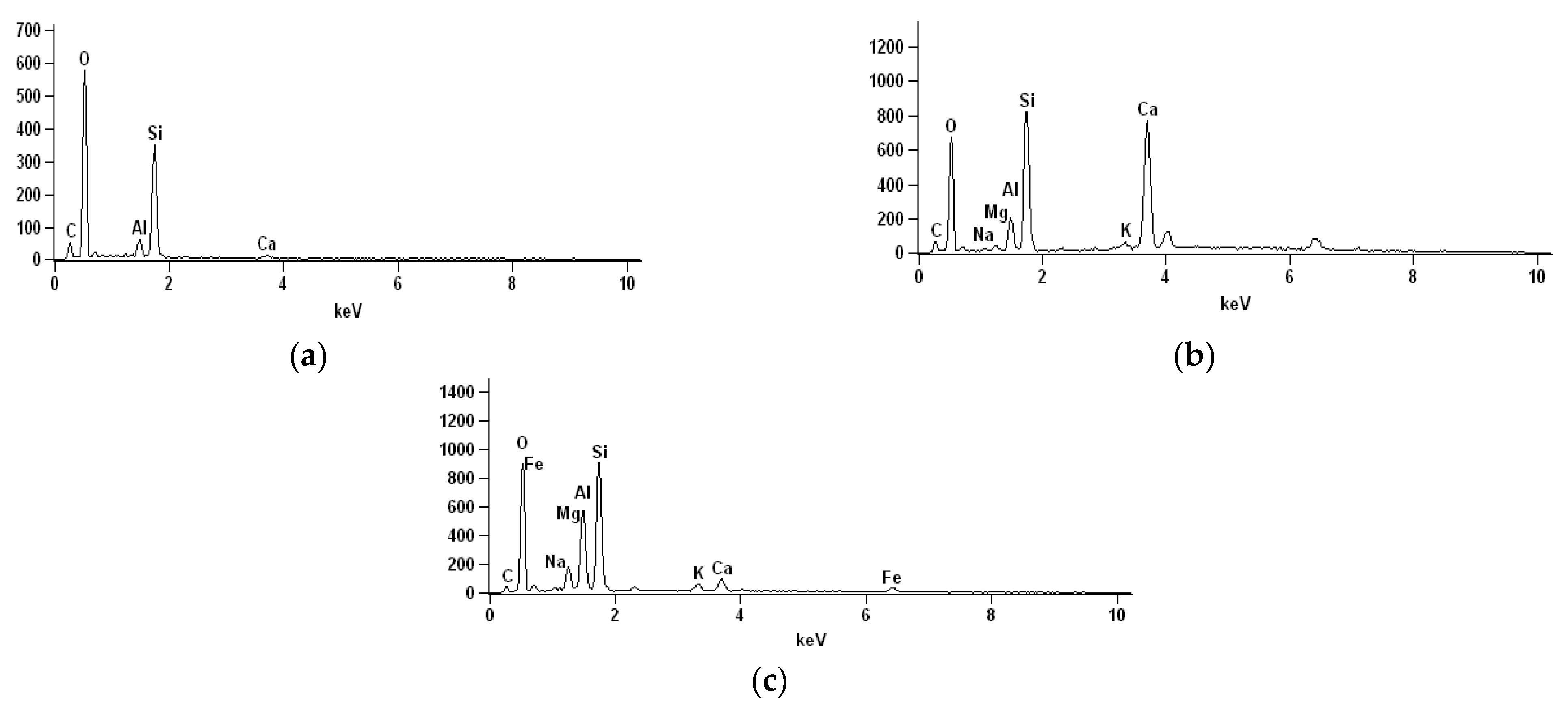
3.6. Microstructural Analysis
3.7. FTIR Analysis
3.8. Environmental Implications and Limitations Related to Gypsum Impurities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mariyam, S.; Cochrane, L.; Al-Ansari, T.; McKay, G. A framework to support localized solid waste management decision making: Evidence from Qatar. Environ. Dev. 2024, 50, 100986. [Google Scholar] [CrossRef]
- Beccarello, M.; Di Foggia, G. Sustainable Development Goals data-driven local policy: Focus on SDG 11 and SDG 12. Adm. Sci. 2022, 12, 167. [Google Scholar] [CrossRef]
- Vilela, A.P.; Eugênio, T.M.C.; de Oliveira, F.F.; Mendes, J.F.; Ribeiro, A.G.C.; Vaz, L.E.V.d.S.B.; Mendes, R.F. Technological properties of soil-cement bricks produced with iron ore mining waste. Constr. Build. Mater. 2020, 262, 120883. [Google Scholar] [CrossRef]
- da Silva, M.V.V.; Garcia, R.L.M.; de Siqueira, R.A.; Júnior, G.A.d.S. Técnicas de estabilização e reforço sustentável do solo. Rev. FT 2024, 29, 29–30. [Google Scholar] [CrossRef]
- Xiong, W.; Chen, Y.; Xu, J.; Zhang, Z.; Liang, C. Reuse of engineering waste soil and recycled fine aggregate to manufacture eco-friendly unfired clay bricks: Experimental assessment, data-driven modeling and environmental friendliness evaluation. Case Stud. Constr. Mater. 2023, 19, e02608. [Google Scholar] [CrossRef]
- Hashim, A.A.; Anaee, R.A.; Nasr, M.S. Improving the mechanical, corrosion resistance, microstructural and environmental performance of recycled aggregate concrete using ceramic waste powder as an alternative to cement. Ceramics 2025, 8, 11. [Google Scholar] [CrossRef]
- Rocha Silva, B.; Junior, H.S.M.; Filho, A.J.d.S.; Santos, A.M.S.; de Melo, F.M.C.; Almeira, V.G.d.O.; de Oliveira, H.A. Soil cement brick production process: Literature review. MOJ Civ. Eng. 2023, 7, 19–26. [Google Scholar] [CrossRef]
- Ângelo, F.A.; Simões, G.F. Tijolos ecoeficientes de barro cru com resíduos sólidos e efluente industrial utilizando tecnologias não convencionais. Ambiente Construído 2023, 23, 131–148. [Google Scholar] [CrossRef]
- de Castro, E.D.; Villella, L.S.; Mendes, L.M.; Mendes, R.F.; Ribeiro, A.G.C.; Júnior, J.B.G.; Rabelo, G.F. Analysis of the coffee peel application over the soil-cement bricks properties. Coffee Sci. 2019, 14, 12. [Google Scholar] [CrossRef]
- Azevedo, A.R.G.; Marvila, T.; Fernandes, W.J.; Alexandre, J.; Xavier, G.; Zanelato, E.; Cerqueira, N.; Pedroti, L.; Mendes, B. Assessing the potential of sludge generated by the pulp and paper industry in assembling locking blocks. J. Build. Eng. 2019, 23, 334–340. [Google Scholar] [CrossRef]
- Araújo, V.D.; Martins, V.A.; Cavalheri, P.S.; Honório, L.; Lima, P.D.M.; Filho, F.J.C.M. Incorporation of tannery waste and sugarcane bagasse ash in soil–cement bricks. Rev. AIDIS De Ing. Y Cienc. Ambient. 2022, 15, 154. [Google Scholar] [CrossRef]
- Ahmed, A.; Ugai, K.; Kamei, T. Laboratory and field evaluations of recycled gypsum as a stabilizer agent in embankment construction. Soils Found. 2011, 51, 975–990. [Google Scholar] [CrossRef]
- Azevedo, A.C.; Delgado, J.M.P.Q.; Neves, T.H.C.; Costa e Silva, A.J. Adhesion of gypsum plaster coatings: Experimental evaluation. In Building Pathology and Rehabilitation; Springer: Berlin/Heidelberg, Germany, 2021; pp. 41–66. [Google Scholar] [CrossRef]
- Camarini, G.; Pimentel, L.L.; De Sá, N.H.R. Assessment of the material loss in walls renderings with β-hemihydrate paste. Appl. Mech. Mater. 2011, 71–78, 1242–1245. [Google Scholar] [CrossRef]
- Jiménez-Rivero, A.; García-Navarro, J. Exploring factors influencing post-consumer gypsum recycling and landfilling in the European Union. Resour. Conserv. Recycl. 2017, 116, 116–123. [Google Scholar] [CrossRef]
- Antunes, M.L.P.; de Sá, A.B.; Oliveira, P.S.; Rangel, E.C. Utilization of gypsum from construction and demolition waste in Portland cement mortar. Cerâmics 2019, 65 (Suppl. S1), 1–6. [Google Scholar] [CrossRef]
- Torres-Ortega, R.; Torres-Sanchez, D.L.; López-Lara, T. Mechanical properties of hydraulic concretes with partial replacement of Portland cement by pozzolans obtained from agro-industrial residues: A review. Heliyon 2024, 11, e41004. [Google Scholar] [CrossRef] [PubMed]
- Puerto, J.D.; Uribe, S.; Ayala, L.; Padilla, A.; Rodriguez, A. Reliability of reusing gypsum flat board grinded waste as a conventional plaster replacement for buildings. Sustainability 2024, 16, 7889. [Google Scholar] [CrossRef]
- ASTM D6913; Standard Test Methods for Particle-Size Distribution (Gradation) of Soils Using Sieve Analysis. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D4318-18; Standard Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils. ASTM International: West Conshohocken, PA, USA, 2018.
- DNER-ME 093/94; Solos—Determinação da Densidade Real. DNER: Rio de Janeiro, Brazil, 1994.
- Kang, H.; Kang, S.-H.; Moon, J. A comparative investigation of hydration reaction of Portland cement with the use of alkanolamine as a chemical admixture or grinding agent under laboratory condition. J. Build. Eng. 2024, 88, 109214. [Google Scholar] [CrossRef]
- Axthammer, D.; Lange, T.; Dengler, J.; Gädt, T. Early hydration and viscoelastic properties of tricalcium aluminate pastes influenced by soluble sodium salts. Cem. Concr. Res. 2025, 190, 107788. [Google Scholar] [CrossRef]
- NBR 10833; Fabricação de Tijolo e Bloco de Solo-Cimento com Utilização de Prensa Manual ou Hidráulica—Procedimento. ABNT: Rio de Janeiro, Brazil, 2012.
- ASTM C191; Standard Test Methods for Time of Setting of Hydraulic Cement by Vicat Needle. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM C188; Standard Test Method for Density of Hydraulic Cement. ASTM International: West Conshohocken, PA, USA, 2022.
- NBR 16372; 2015—Cimento Portland e Outros Materiais em pó—Determinação da Finura pelo Método de Permeabilidade ao ar (Método de Blaine). ABNT: Rio de Janeiro, Brazil, 2015.
- ASTM C109/C109M; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars. ASTM International: West Conshohocken, PA, USA, 2023.
- Santa Rosa, J.M.S.; Silva, R.G.P.; Lafayette, K.P.V. Sustainable Alternative for the Production of Soil Cement Bricks. J. Manag. Sustain. 2023, 13, 45. [Google Scholar] [CrossRef]
- Ashour, T.; Korjenic, A.; Korjenic, S. Equilibrium Moisture Content of Earth Bricks Biocomposites Stabilized with Cement and Gypsum. Cem. Concr. Compos. 2015, 59, 18–25. [Google Scholar] [CrossRef]
- Metzker, S.L.O.; Sabino, T.P.F.; Mendes, J.F.; Ribeiro, A.G.C.; Mendes, R.F. Soil-cement bricks development using polymeric waste. Environ. Sci. Pollut. Res. 2022, 29, 21034–21048. [Google Scholar] [CrossRef] [PubMed]
- Kongkajun, N.; Laitila, E.A.; Ineure, P.; Prakaypan, W.; Cherdhirunkorn, B.; Chakartnarodom, P. Soil-Cement Bricks Produced from Local Clay Brick Waste and Soft Sludge from Fiber Cement Production. Case Stud. Constr. Mater. 2020, 13, e00448. [Google Scholar] [CrossRef]
- Leonel, R.F.; Folgueras, M.V.; Dalla Valentina, L.V.O.; Prim, S.R.; Prates, G.A.; Caraschi, J.C. Characterization of Soil-Cement Bricks with Incorporation of Used Foundry Sand. Cerâmica 2017, 63, 329–335. [Google Scholar] [CrossRef]
- NBR 8492; Tijolo de Solo-Cimento—Análise Dimensional, Determinação da Resistência à Compressão e da Absorção de Água—Método de Ensaio. ABNT: Rio de Janeiro, Brazil, 2012.
- NBR 5739; Cimento Portland—Determinação da Resistência à Compressão. ABNT: Rio de Janeiro, Brazil, 2007.
- NBR 10836; Bloco de Solo-Cimento Sem Função Estrutural—Análise Dimensional, Determinação da Resistência à Compressão e da Absorção de Água—Método de Ensaio. ABNT: Rio de Janeiro, Brazil, 2012.
- NBR 13554; 2012—Solo-Cimento—Ensaio de Durabilidade por Molhagem e Secagem—Método de Ensaio. ABNT: Rio de Janeiro, Brazil, 2012.
- Hammer, Ø. Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics software package for education and data analysis. Palaeontol Electron. 2001, 4, 1–9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 27 June 2025).
- Chernyshova, I.V.; Lesovik, V.S.; Fediuk, R.S.; Amran, M.; Vatin, N.I. Enhancement of Fresh Properties and Performances of the Eco-Friendly Gypsum-Cement Composite (EGCC). Constr. Build. Mater. 2020, 260, 120462. [Google Scholar] [CrossRef]
- Alves, M.L.X.F.N.; Barros, S.V.A.; de Oliveira, F.N. Physical and Chemical Characterization of Soil for Use in Ecological Bricks. In Uniting Knowledge: Integrated Scientific Research for Global Development; Seven Publicações: São José dos Pinhais, Brazil, 2024; Volume 2, pp. 532–539. [Google Scholar] [CrossRef]
- Qi, J.; Ma, W.; Song, C. Influence of Freeze—Thaw on Engineering Properties of a Silty Soil. Cold Reg. Sci. Technol. 2008, 53, 397–404. [Google Scholar] [CrossRef]
- Melnick, V.; Pianaro, S.A.; Cava, S.; Tebcherani, S.M. Application of Oil Shale Mining By-Products as Raw Materials in the Determination of the Vitrification Curve of Red Porcelain Stoneware Tiles by Dilatometric Method. Appl. Clay Sci. 2010, 50, 311–314. [Google Scholar] [CrossRef]
- Peffers, K.; Tuunanen, T.; Rothenberger, M.A.; Chatterjee, S. A Design Science Research Methodology for Information Systems Research. In Design Science Research: A Method for Science and Engineering; Peffers, K., Rothenberger, M.A., Kuechler, B., Eds.; CRC Press: Boca Raton, FL, USA, 2023; pp. 11–28. [Google Scholar] [CrossRef]
- Harrison, T.; Jones, M.R.; Lawrence, D. The Production of Low Energy Cements. In Lea’s Chemistry of Cement and Concrete, 5th ed.; Hewlett, P.C., Liska, M., Eds.; Butterworth-Heinemann: Oxford, UK, 2019; pp. 341–361. [Google Scholar] [CrossRef]
- Reigl, S.; Van Driessche, A.E.S.; Wagner, E.; Montes-Hernandez, G.; Mehringer, J.; Koltzenburg, S.; Kunz, W.; Kellermeier, M. Toward More Sustainable Hydraulic Binders: Controlling Calcium Sulfate Phase Selection via Specific Additives. ACS Sustain. Chem. Eng. 2023, 11, 8450–8461. [Google Scholar] [CrossRef]
- Stawski, T.M.; Besselink, R.; Chatzipanagis, K.; Hövelmann, J.; Benning, L.G.; Van Driessche, A.E.S. Nucleation Pathway of Calcium Sulfate Hemihydrate (Bassanite) from Solution: Implications for Calcium Sulfates on Mars. J. Phys. Chem. C 2020, 124, 8411–8422. [Google Scholar] [CrossRef]
- Gitirana, G.F.N.; Bandeira, A.P.N.; Dourado, K.C.A.; Souza Neto, J.B. Solos Não Saturados no Contexto Geotécnico, 2nd ed.; Editora Cubo: São Carlos, Brazil, 2023. [Google Scholar] [CrossRef]
- Macioski, G.; Soto, N.T.A.; Medeiros, M.H.F.d.; Hoppe Filho, J.; Araújo, M.S.d.; Cerri, J.A. Portlandite consumption by red ceramic waste due to alkali activation reaction. Ambiente Construído 2021, 21, 7–21. [Google Scholar] [CrossRef]
- Kleib, J.; Amar, M.; Aouad, G.; Bourbon, X.; Benzerzour, M.; Abriak, N.-E. The use of Callovo-Oxfordian Argillite as a raw material for Portland cement clinker production. Buildings 2022, 12, 1421. [Google Scholar] [CrossRef]
- Bošnjak, J.; Ožbolt, J.; Hahn, R. Permeability Measurement on High Strength Concrete without and with Polypropylene Fibers at Elevated Temperatures Using a New Test Setup. Cem. Concr. Res. 2013, 53, 104–111. [Google Scholar] [CrossRef]
- Park, S.-S.; Suh, E.-H.; Chae, K.-H.; Jang, S.-K.; Kim, J.-H. A Study on Soil Cementation and Calcite Precipitation with Clay as a Medium. J. Korean Geotech. Soc. 2015, 31, 17–27. [Google Scholar] [CrossRef]
- Krause, F.; Renner, B.; Coppens, F.; Dewanckele, J.; Schwotzer, M. Reactivity of gypsum-based materials subjected to thermal load: Investigation of reaction mechanisms. Materials 2020, 13, 1427. [Google Scholar] [CrossRef]
- Azimi, G.; Papangelakis, V.G. Mechanism and kinetics of gypsum–anhydrite transformation in aqueous electrolyte solutions. Hydrometallurgy 2011, 108, 122–129. [Google Scholar] [CrossRef]
- Voigt, W.; Freyer, D. Solubility of anhydrite and gypsum at temperatures below 100 °C and the gypsum-anhydrite transition temperature in aqueous solutions: A re-assessment. Front. Nucl. Eng. 2023, 2, 1208582. [Google Scholar] [CrossRef]
- Al Disi, Z.A.; Sadooni, F.; Al-Kuwari, H.A.-S.; Bontognali, T.R. Microbially influenced formation of anhydrite at low temperature. Sci. Total Environ. 2023, 902, 165820. [Google Scholar] [CrossRef]
- Engbrecht, D.C.; Hirschfeld, D.A. Thermal analysis of calcium sulfate dihydrate sources used to manufacture gypsum wallboard. Thermochim. Acta 2016, 639, 173–185. [Google Scholar] [CrossRef]
- Lira, M.C.d.A.; Neiva, L.S. Investigative study of the potentialities of noble applications for the Brazilian calcium sulfate α-hemihydrate. Pesqui. E Ensino Em Ciências Exatas E Da Nat. 2020, 4, e1516. [Google Scholar] [CrossRef]
- Vipulanandan, C.; Mohammed, A. XRD and TGA, swelling and compacted properties of polymer treated sulfate contaminated CL soil. J. Test. Eval. 2016, 44, 2270–2284. [Google Scholar] [CrossRef]
- Cai, H.; Chen, C.; Wei, S.; Zhu, S. Strength development of cemented soil cured in water-air conditions at varied temperatures: Experimental investigation and model characterization. J. Mater. Civ. Eng. 2023, 35, 3. [Google Scholar] [CrossRef]
- Zak, P.; Ashour, T.; Korjenic, A.; Korjenic, S.; Wu, W. The influence of natural reinforcement fibers, gypsum and cement on compressive strength of earth bricks materials. Constr. Build. Mater. 2016, 106, 179–188. [Google Scholar] [CrossRef]
- Zhu, Y.; Jia, Y.; Xie, X.S.; Xu, J. Study on the Macroscopic Properties and Microstructure of High Fly Ash Content Alkali-Activated Fly Ash Slag Concrete Cured at Room Temperature. Materials 2025, 18, 547. [Google Scholar] [CrossRef] [PubMed]
- Rohman, M.N.; Simanihuruk, B.; Dewita, H. Karakteristik Beton Mutu FC 30 Dengan Menggunakan Limbah Peleburan Baja (Slag) Dan Pembakaran Batubara (Fly Ash). Pros. Semin. Nas. Rekayasa Dan Teknol. 2025, 5, 61–65. [Google Scholar] [CrossRef]
- Kirgiz, M.S.; Khatib, J.; Galdino, A.G.S.; Kenaï, S.; Ashteyat, A.M.; Campilho, R.D.S.G.; González-Lezcano, R.A.; Bamigboye, G.; Nagaprasad, N.; Ramaswamy, K. Waste Marble Sludge and Calcined Clay Brick Powders in Conventional Cement Farina Production for Cleaner Built Environment. Dent. Sci. Rep. 2025, 15, 3467. [Google Scholar] [CrossRef]
- Li, Y.; Yan, X.; Wan, M.P.; Zhou, J.; Liu, J. Research on Recycling and Utilization of Shredded Waste Mask Fibers to Prepare Sustainable Engineered Cementitious Composites. Buildings 2025, 15, 402. [Google Scholar] [CrossRef]
- Jafari, M.M.; Bagheripour, M.H.; Yaghoubi, E. Prediction of Strength Properties of Reinforced and Stabilized Sandy Soil as a Building Foundation Material. Buildings 2025, 15, 382. [Google Scholar] [CrossRef]
- NBR 10834; 2012—Bloco de Solo-Cimento Sem Função Estrutural—Requisitos. ABNT: Rio de Janeiro, Brazil, 2012.
- Dulal, P.; Maharjan, S.; Timalsina, M.P.; Maharjan, Y.; Giri, A.; Tamang, A. Engineering properties of cement-stabilized compressed earth bricks. J. Build. Eng. 2023, 77, 107453. [Google Scholar] [CrossRef]
- Thennarasan Latha, A.; Murugesan, B.; Thomas, B.S. Compressed stabilized earth block incorporating municipal solid waste incinerator bottom ash as a partial replacement for fine aggregates. Buildings 2023, 13, 1114. [Google Scholar] [CrossRef]
- Corrêa-Silva, M.; Cristelo, N.; Rouainia, M.; Araújo, N.; Miranda, T. Constitutive behaviour of a clay stabilised with alkali-activated cement based on blast furnace slag. Sustainability 2022, 14, 13736. [Google Scholar] [CrossRef]
- Kamran, F.; Basavarajappa, M.; Bala, N.; Hashemian, L. Laboratory Evaluation of Stabilized Base Course Using Asphalt Emulsion and Asphaltenes Derived from Alberta Oil Sands. Constr. Build. Mater. 2021, 283, 122735. [Google Scholar] [CrossRef]
- Dong, W.; Li, W.; Tao, Z.; Wang, K. Piezoresistive Properties of Cement-Based Sensors: Review and Perspective. Constr. Build. Mater. 2019, 203, 146–163. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, S.; Zhang, J.; Liu, X.; Wu, H. Effect of fly ash and gypsum on drying shrinkage and mechanical properties of one-part alkali-activated slag mortar. Struct. Concr. 2025, 26, 1275–1288. [Google Scholar] [CrossRef]
- NBR 13553; Materiais Para Emprego em Parede Monolítica de Solo-Cimento Sem Função estrutural—Requisitos. ABNT: Rio de Janeiro, Brasil, 2012.
- Höglund, L.O. Some notes of ettringite formation in cementitious materials; Influence of hydration and thermodynamic constraints for durability. Cem. Concr. Res. 1992, 22, 217–228. [Google Scholar] [CrossRef]
- Gu, Y.; Martin, R.-P.; Metalssi, O.O.; Fen-Chong, T.; Dangla, P. Pore size analyses of cement paste exposed to external sulfate attack and delayed ettringite formation. Cem. Concr. Res. 2019, 123, 105766. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Cai, J.; Zhou, F.; Cheng, Z.; Zhang, J. Experimental Study on the Mechanical Properties and Microstructural Mechanisms of Coal Gangue-Based Cementitious Materials Synergistically Activated by Desulfurization Gypsum and Lime. Polymers 2025, 17, 932. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, Z.; Yang, J.; Zhang, J. An Eco-Friendly Phosphogypsum-Based Cementitious Material. Front. Phys. 2022, 10, 892037. [Google Scholar] [CrossRef]
- Fu, Y.; Beaudoin, J.J. Mechanisms of Delayed Ettringite Formation in Portland Cement Systems. ACI Mater. J. 1996, 93, 327–333. [Google Scholar] [CrossRef]
- Li, L.; Darquennes, A.; Hannawi, K.; Che, C. Effects of Sulfates on the Hydration of Portland Cement—A Review. Constr. Build. Mater. 2021, 270, 121134. [Google Scholar] [CrossRef]
- Taman, M.; Abd Elaty, M.A.; Behiry, R.N. Codes Applicability of Estimating the FRC Compressive Strength by the Core-Drilling Method. Constr. Build. Mater. 2022, 330, 127227. [Google Scholar] [CrossRef]
- Wild, S.; Khatib, J.M. Portlandite consumption in metakaolin cement pastes and mortars. Cem. Concr. Res. 1997, 27, 137–146. [Google Scholar] [CrossRef]
- Habert, G.; d’Espinose de Lacaillerie, J.B.; Roussel, N. An environmental evaluation of geopolymer based concrete production: Reviewing current research trends. J. Clean. Prod. 2010, 19, 1229–1238. [Google Scholar] [CrossRef]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Habert, G. Environmental Impact of Portland Cement Production. In Eco-Efficient Concrete; Woodhead Publishing Series in Civil and Structural Engineering; Woodhead Publishing: Cambridge, UK, 2013; pp. 3–25. [Google Scholar] [CrossRef]
- Born, K. Exploring the Spatial Dynamics of Circular Economy Transitions: Insights and Lessons from Chile’s Mining Territories. Prog. Econ. Geogr. 2025, 3, 100041. [Google Scholar] [CrossRef]






| Compositions | Soil | Cement | Sand | Gypsum |
|---|---|---|---|---|
| SF | 80 | 10 | 10 | - |
| G5 | 80 | 9.5 | 10 | 0.5 |
| G10 | 80 | 9 | 10 | 1 |
| G20 | 80 | 8 | 10 | 2 |
| G40 | 80 | 6 | 10 | 4 |
| Specific Density (g/cm3) | Bulk Density (g/cm3) | Specific Surface Area (cm2/g) | |
|---|---|---|---|
| Soil | 2.71 ± 0.02 | 1.15 ± 0.01 | 4243 ± 100 |
| Gypsum | 2.25 ± 0.02 | 1.10 ± 0.02 | 5500 ± 150 |
| Cement | 3.10 ± 0.02 | 1.40 ± 0.02 | 4400 ± 120 |
| Sand | 2.63 ± 0.01 | 1.65 ± 0.01 | - |
| Component | Soil | Cement | Sand | Gypsum |
|---|---|---|---|---|
| CaO | 22.97 | 80.95 | 1.54 | 68.01 |
| SiO2 | 39.39 | 8.46 | 89.79 | 0.63 |
| Fe2O3 | 18.45 | 5.95 | 1.721 | - |
| SO3 | 0.11 | 2.06 | 0.32 | 29.97 |
| K2O | 6.95 | 1.60 | 0.66 | 0.30 |
| Al2O3 | 9.40 | - | 1.82 | - |
| TiO2 | 2.09 | 0.37 | 3.56 | - |
| Loss on ignition | 0.64 | 0.61 | 0.59 | 1.09 |
| Mineral Phase | Concentration Range (%) |
|---|---|
| Quartz | 24.7–38.0 |
| Paragonite | 18.4–22.8 |
| Inesite | 10.1–15.8 |
| Ettringite | 8.0–10.0 |
| Calcite | 12.5–16.2 |
| Gypsum | 0.9–2.5 |
| Ilite | 16.9–20.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, E.S.S.; Oliveira, H.A.d.; Santos, C.P.d.; Gomes, M.d.A.; Valerio, M.E.G.; Macedo, Z.S. Sustainable Use of Gypsum Waste for Applications in Soil–Cement Bricks: Mechanical, Environmental, and Durability Performance. Ceramics 2025, 8, 83. https://doi.org/10.3390/ceramics8030083
Nascimento ESS, Oliveira HAd, Santos CPd, Gomes MdA, Valerio MEG, Macedo ZS. Sustainable Use of Gypsum Waste for Applications in Soil–Cement Bricks: Mechanical, Environmental, and Durability Performance. Ceramics. 2025; 8(3):83. https://doi.org/10.3390/ceramics8030083
Chicago/Turabian StyleNascimento, Elvia Soraya Santos, Herbet Alves de Oliveira, Cochiran Pereira dos Santos, Maria de Andrade Gomes, Mário Ernesto Giroldo Valerio, and Zélia Soares Macedo. 2025. "Sustainable Use of Gypsum Waste for Applications in Soil–Cement Bricks: Mechanical, Environmental, and Durability Performance" Ceramics 8, no. 3: 83. https://doi.org/10.3390/ceramics8030083
APA StyleNascimento, E. S. S., Oliveira, H. A. d., Santos, C. P. d., Gomes, M. d. A., Valerio, M. E. G., & Macedo, Z. S. (2025). Sustainable Use of Gypsum Waste for Applications in Soil–Cement Bricks: Mechanical, Environmental, and Durability Performance. Ceramics, 8(3), 83. https://doi.org/10.3390/ceramics8030083











