Application of Ceramic Membranes Derived from Waste and Natural Materials for the Removal of Organic Dyes from Wastewater: A Review
Abstract
1. Introduction
2. Organic Dyes as Emerging Contaminants
2.1. Dye Classification
2.2. Chemistry and Toxicity of Selected Synthetic Dyes
2.2.1. Congo Red
2.2.2. Malachite Green
2.2.3. Methyl Orange
2.2.4. Methylene Blue
2.2.5. Rhodamine
3. Ceramic Membrane Technology Overview
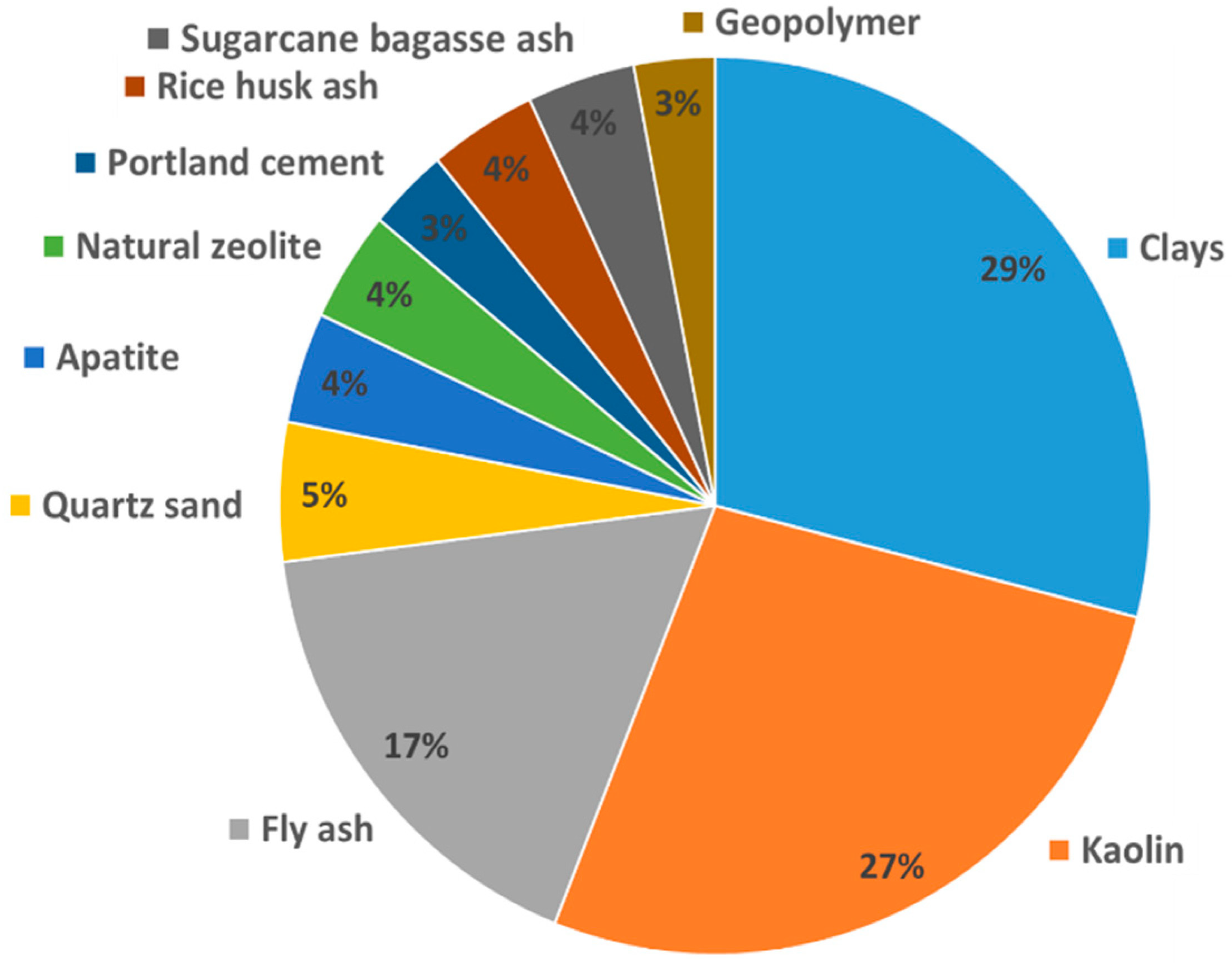
4. Application of Ceramic Membranes Derived from Waste and Natural Materials in the Removal of Dye Removal from Wastewater
| Membrane Type | Organic Dye Removed | Country of Study/Scale | Process Parameters | Efficiency | Ref. |
|---|---|---|---|---|---|
| Chocobofe clay-based ceramic membrane | Methylene blue dye | Brazil/Bench scale | Initial dye concentration: 50 mg/L, Pressure: 2 bar, Temperature: 25 °C, Porosity: 53%, Average pore size: 0.48 µm | 100% dye rejection; Good performance after 15 reuse cycles | [129] |
| Cordierite and an abundant clay-based ceramic membrane | Methylene blue dye | Morocco/Bench scale | Porosity: 18.65–29.63% | 99.8% dye filtration efficiency | [130] |
| Purified clay-based ceramic membrane | Methyl orange (MO), Rhodamine B (RhB) | Morocco/Bench scale | Average pore size: 5.4 nm Pressure: 4 bar, Dye concentration: 50 ppm, Water permeability: 26 L/h·m2·bar | Rejection rates: 84.5% (MO), 85.7% (RhB) | [144] |
| Clay-based ceramic membrane | Methylene blue dye | Egypt/Lab scale | Synthetic dye wastewater (100 ppm) | 42% dye rejection | [146] |
| COOH-carbon nanotube-functionalized clay-based ceramic membrane | Acid fuchsin dye | Pore size: 0.076 µm, Dye concentration: 100 ppm | 95% rejection | ||
| Sugar scum and fly ash-based ceramic membrane | Methylene blue dye | Morocco/Lab scale | Pore size: 0–4.5 μm, Pressure: 1 bar, Filtration time: 2 h, Water permeability: 2356.68 L/h·m2·bar | 99.9% retention | [147] |
| Natural clay-based ceramic membrane | Acid Yellow 49, Basic Violet 16, Disperse Red 167 | Iran/Lab scale | Porosity: 27%, Pore size: 3.3 µm, Pressure: 0.1 bar, Dye concentration: 50 ppm, Water permeability: 2491.4 L/m2·h·bar | 98% retention (anionic dyes), 93% retention (non-ionic dyes) | [148] |
| Bentonite-based ceramic membrane | Direct Red 80, Rhodamine B | Morocco/Lab scale | Pore size: 13 nm, Pressure: 4 bar, Dye concentration: 50 ppm, Water permeability: 30 L/h·m2·bar | 97% rejection (Direct Red 80), 80.1% rejection (Rhodamine B) | [56] |
| Clay-based ceramic membrane | Congo Red dye | Morocco/Lab scale | Average pore size: 1.21–1.76 µm | 99% dye removal | [149] |
| Zeolite-clay-based ceramic membrane | Indigo Blue dye | Tunisia/Lab scale | Pore size: 0.75 µm, Pressure: 1.0 bar, Permeability: 371 L/h·m2·bar | 95% dye removal | [150] |
| Potter’s clay-based ceramic membrane | Methylene blue, Congo Red | India/Lab scale | Porosity: 52.51%, Average pore size: 0.49 µm | High removal efficiency and reusability | [133] |
| Purified natural clay-based ceramic membrane | Direct Red 80 | Morocco/Lab scale | Pressure: 3 bar, Pore size: 75–90 nm, Permeability: 14.7–16.4 L/h·m2·bar, Dye concentration: 50 ppm | 97–99% dye removal | [151] |
| TiO2 functionalized low cost clay/alumina based ceramic membrane | Alizarin Red dye | Tunisia/Lab scale | Pressure of 5 bars, pH = 9, Permeability of 117 L h−1 m−2 bar−1. Dye concentration of 150 ppm | 99% dye removal | [152] |
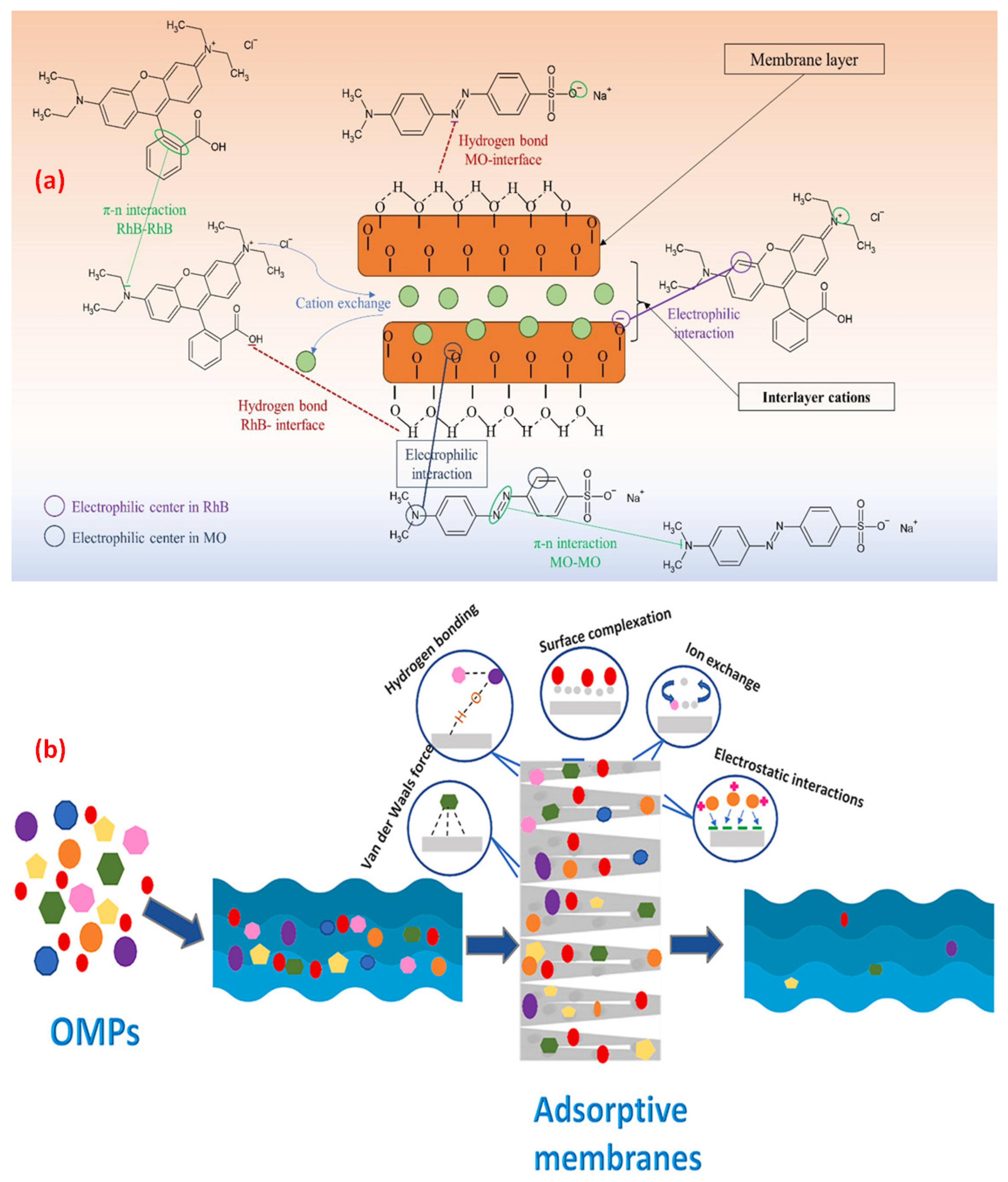
Factors That Affect the Removal Efficiency of Organic Dyes by Waste and Natural Material Derived Ceramic Membranes
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SO | Safranin O |
| MG | Malachite green |
| RO | Reverse osmosis |
| CR | Congo Red (CR) |
| RhB | Rhodamine B (RhB) |
| BG | Brilliant green (BG) |
| EBT | Eriochrome black T (EBT) |
| MV | Methyl violet (MV) |
| MB | Methylene blue (MB) |
| MO | Methyl orange (MO) |
References
- Nachiyar, C.V.; Rakshi, A.; Sandhya, S.; Jebasta, N.B.D.; Nellore, J. Developments in treatment technologies of dye-containing effluent: A review. Case Stud. Chem. Environ. Eng. 2023, 7, 100339. [Google Scholar] [CrossRef]
- Randhawa, K.S. Synthesis, Properties, and Environmental Impact of Hybrid Pigments. Sci. World J. 2024, 2024, 2773950. [Google Scholar] [CrossRef]
- Yusuf, M. Synthetic dyes: A threat to the environment and water ecosystem. Text. Cloth. 2019, 11–26. [Google Scholar] [CrossRef]
- Ismail, M.; Akhtar, K.; Khan, M.; Kamal, T.; Khan, M.A.; Asiri, A.M.; Seo, J.; Khan, S.B. Pollution, toxicity and carcinogenicity of organic dyes and their catalytic bio-remediation. Curr. Pharm. Des. 2019, 25, 3645–3663. [Google Scholar] [CrossRef]
- Singh, K.; Arora, S. Removal of synthetic textile dyes from wastewaters: A critical review on present treatment technologies. Crit. Rev. Environ. Sci. Technol. 2011, 41, 807–878. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, S.; Soni, V. Classification and impact of synthetic textile dyes on Aquatic Flora: A review. Reg. Stud. Mar. Sci. 2021, 45, 101802. [Google Scholar] [CrossRef]
- Suresh, R.; Subramaniyan, R.; Senthil Kumar, K.; Kumar, N.; Chenniappan, M. Sustainable technologies for treatment of industrial wastewater and its potential for reuse. In Microbial Technologies in Industrial Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2023; pp. 143–168. [Google Scholar]
- Ahmed, S.; Khan, M.; DHAKA, B. Study of Wastewater Treatment Process of a Synthetic Fabric Dyeing Plant; Department of Chemical Engineerig, Bangladesh University of Engineering and Technology: Dhaka, Bangladesh, 2004; pp. 1–97. [Google Scholar]
- Pereira, P.C.G.; Reimão, R.V.; Pavesi, T.; Saggioro, E.M.; Moreira, J.C.; Correia, F.V. Lethal and sub-lethal evaluation of Indigo Carmine dye and byproducts after TiO2 photocatalysis in the immune system of Eisenia andrei earthworms. Ecotoxicol. Environ. Saf. 2017, 143, 275–282. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Sharma, P.; Haq, N.; Ahmad, M.; Siddiqui, K.A. Mo@ Zinc-CP composite for dual applications: Luminescent detection of pesticides and photodegradation of organic dyes. J. Mol. Struct. 2025, 1327, 141249. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Bamigboye, M.O.; Ogunbiyi, O.D.; Akano, M.T. Toxicity and decontamination strategies of Congo red dye. Groundw. Sustain. Dev. 2022, 19, 100844. [Google Scholar] [CrossRef]
- Khan, R.R.M.; Qamar, H.; Hameed, A.; Rehman, A.u.; Pervaiz, M.; Saeed, Z.; Adnan, A.; Ch, A.R. Biological and photocatalytic degradation of congo red, a diazo sulfonated substituted dye: A review. Water Air Soil. Pollut. 2022, 233, 468. [Google Scholar] [CrossRef]
- Dube, D. Degradation of a Xanthene Recalcitrant Dye in Aqueous Phase by the Fenton Process, UV Irradiation and Photo-Fenton Process. Ph.D. Thesis, Universite Badji Mokhtar-Annaba, Annaba, Algeria, 2018. [Google Scholar]
- Irwin, J.A.; Erisir, A.; Kwon, I. Oral triphenylmethane food dye analog, brilliant blue G, prevents neuronal loss in APPSwDI/NOS2-/-mouse model. Curr. Alzheimer Res. 2016, 13, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Balabanova, M.; Popova, L.; Tchipeva, R. Dyes in dermatology. Clin. Dermatol. 2003, 21, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Bharagava, R.N. Exposure to Crystal Violet, Its Toxic, Genotoxic and Carcinogenic Effects on Environment and Its Degradation and Detoxification for Environmental Safety. Rev. Environ. Contam. Toxicol. 2016, 237, 71–104. [Google Scholar] [CrossRef]
- Kaur, Y.; Jasrotia, T.; Kumar, R.; Chaudhary, G.R.; Chaudhary, S. Adsorptive removal of eriochrome black T (EBT) dye by using surface active low cost zinc oxide nanoparticles: A comparative overview. Chemosphere 2021, 278, 130366. [Google Scholar] [CrossRef]
- Bansal, M.; Patnala, P.K.; Dugmore, T. Adsorption of Eriochrome Black-T (EBT) using tea waste as a low cost adsorbent by batch studies: A green approach for dye effluent treatments. Curr. Res. Green Sustain. Chem. 2020, 3, 100036. [Google Scholar] [CrossRef]
- Khan, M.I.; Shanableh, A.; Javid, A.; Manzoor, S. Removal of Eriochrome black-T from wastewater by utilizing the commercial DF-120 anion exchange membrane. Next Energy 2024, 3, 100096. [Google Scholar] [CrossRef]
- Cooksey, C.J. Quirks of dye nomenclature. 11. Safranine and its relatives. Biotech. Histochem. 2019, 94, 225–233. [Google Scholar] [CrossRef]
- Pinto da Costa, J.; Girão, A.V.; Monteiro, O.C.; Trindade, T.; Costa, M.C. Biotechnologically obtained nanocomposites: A practical application for photodegradation of Safranin-T under UV-Vis and solar light. J. Environ. Sci. Health Part A 2015, 50, 996–1010. [Google Scholar] [CrossRef]
- Umar, A.; Claudia, C.; Khalil, M.; Bakri, R.; Bae, H.B.; Tae-Hwan, K.; Apriandanu, D.O.B. Photocatalytic Activity of CuBi2O4/CuO Heterojunction for Methylene Blue Degradation under Visible Light Irradiation. Vacuum 2025, 238, 114246. [Google Scholar] [CrossRef]
- Yildirim, O.A.; Bahadir, M.; Pehlivan, E. Detrimental effects of commonly used textile dyes on the aquatic environment and human health—A review. Fresenius Environ. Bull. 2022, 31, 9329–9345. [Google Scholar]
- Hanafi, M.F.; Sapawe, N. A review on the water problem associate with organic pollutants derived from phenol, methyl orange, and remazol brilliant blue dyes. Mater. Today Proc. 2020, 31, A141–A150. [Google Scholar] [CrossRef]
- Hashemi, S.H.; Kaykhaii, M. Azo dyes: Sources, occurrence, toxicity, sampling, analysis, and their removal methods. In Emerging Freshwater Pollutants; Elsevier: Amsterdam, The Netherlands, 2022; pp. 267–287. [Google Scholar]
- Zhao, J.; Dang, Z.; Muddassir, M.; Raza, S.; Zhong, A.; Wang, X.; Jin, J. A New Cd(II)-Based Coordination Polymer for Efficient Photocatalytic Removal of Organic Dyes. Molecules 2023, 28, 6848. [Google Scholar] [CrossRef] [PubMed]
- Adenan, N.H.; Lim, Y.Y.; Ting, A.S.Y. Removal of triphenylmethane dyes by Streptomyces bacillaris: A study on decolorization, enzymatic reactions and toxicity of treated dye solutions. J. Environ. Manag. 2022, 318, 115520. [Google Scholar] [CrossRef]
- Gbajabiaimila, A.T.; Elemike, E.E.; Akpeji, B.H.; Bawa, H.A.; Hossain, I.; Pere, C.; Anekwe-Nwekeaku, O.J. Enhanced Solar-Assisted Degradation of Sunset Yellow Dye Using Plant-Mediated Ag-TiO2/Na-BNT Nanocomposite: Synthesis, Characterization, and Performance Optimization. J. Hazard. Mater. Adv. 2025, 18, 100682. [Google Scholar] [CrossRef]
- Sultana, S.; Rahman, M.M.; Aovi, F.I.; Jahan, F.I.; Hossain, M.S.; Brishti, S.A.; Yamin, M.; Ahmed, M.; Rauf, A.; Sharma, R. Food Color Additives in Hazardous Consequences of Human Health: An Overview. Curr. Top. Med. Chem. 2023, 23, 1380–1393. [Google Scholar] [CrossRef]
- Chung, K.T. Azo dyes and human health: A review. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2016, 34, 233–261. [Google Scholar] [CrossRef]
- Ambroziewicz, Z.M.; Siemiątkowski, R.; Łata, M.; Dowgiert, S.; Sikorska, M.; Kamiński, J.; Więcław, K.; Grabowska, H.; Chruściel, J.; Mąsior, G. Long-Term Health Effects of Artificially Colored Foods in Adults and Children: A Review of Scientific Literature on Attention Deficits, Carcinogenicity, and Allergy Risks. J. Educ. Health Sport 2024, 76, 56522. [Google Scholar] [CrossRef]
- Poopal, R.-K.; Ashwini, R.; Ramesh, M.; Li, B.; Ren, Z. Triphenylmethane dye (C52H54N4O12) is potentially a hazardous substance in edible freshwater fish at trace level: Toxicity, hematology, biochemistry, antioxidants, and molecular docking evaluation study. Environ. Sci. Pollut. Res. 2023, 30, 28759–28779. [Google Scholar] [CrossRef]
- Bora, R.; Rasid, S.S.; Hussain, M.A. Toxicological Impact of Malachite Green on Freshwater Fish: A Comprehensive Review. Uttar Pradesh J. Zool. 2024, 45, 659–668. [Google Scholar] [CrossRef]
- Rehman, R.; Hussain, M.S.; Abidin, A.; Ghfar, A.A.; Hossain, N.; Akram, M.; Dar, A. Exploring feasibility of citric acid infused lignocellulosic waste derived from chestnut and water melon peels for phytofiltration of Eosin yellow dye from water. Int. J. Biol. Macromol. 2024, 276, 133878. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Jhare, D.; Mittal, J. Adsorption of hazardous dye Eosin Yellow from aqueous solution onto waste material De-oiled Soya: Isotherm, kinetics and bulk removal. J. Mol. Liq. 2013, 179, 133–140. [Google Scholar] [CrossRef]
- Mittal, J.; Jhare, D.; Vardhan, H.; Mittal, A. Utilization of bottom ash as a low-cost sorbent for the removal and recovery of a toxic halogen containing dye eosin yellow. Desalination Water Treat. 2014, 52, 4508–4519. [Google Scholar] [CrossRef]
- Mittal, A.; Mittal, J.; Kurup, L. Batch and bulk removal of hazardous dye, indigo carmine from wastewater through adsorption. J. Hazard. Mater. 2006, 137, 591–602. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, G.; Pan, J. Spectroscopic studies of DNA interactions with food colorant indigo carmine with the use of ethidium bromide as a fluorescence probe. J. Agric. Food Chem. 2012, 60, 10867–10875. [Google Scholar] [CrossRef]
- Zaouak, A.; Noomen, A.; Jelassi, H. Gamma-radiation induced decolorization and degradation on aqueous solutions of Indigo Carmine dye. J. Radioanal. Nucl. Chem. 2018, 317, 37–44. [Google Scholar] [CrossRef]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological methods for textile dye removal from wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Paz, A.; Carballo, J.; Perez, M.J.; Dominguez, J.M. Biological treatment of model dyes and textile wastewaters. Chemosphere 2017, 181, 168–177. [Google Scholar] [CrossRef]
- Kim, T.-H.; Park, C.; Kim, S. Water recycling from desalination and purification process of reactive dye manufacturing industry by combined membrane filtration. J. Clean. Prod. 2005, 13, 779–786. [Google Scholar] [CrossRef]
- Moradihamedani, P. Recent advances in dye removal from wastewater by membrane technology: A review. Polym. Bull. 2022, 79, 2603–2631. [Google Scholar] [CrossRef]
- Ghanbari, S.; Fatehizadeh, A.; Khiadani, M.; Taheri, E.; Iqbal, H.M.N. Treatment of synthetic dye containing textile raw wastewater effluent using UV/Chlorine/Br photolysis process followed by activated carbon adsorption. Environ. Sci. Pollut. Res. Int. 2022, 29, 39400–39409. [Google Scholar] [CrossRef] [PubMed]
- Ledakowicz, S.; Pazdzior, K. Recent Achievements in Dyes Removal Focused on Advanced Oxidation Processes Integrated with Biological Methods. Molecules 2021, 26, 870. [Google Scholar] [CrossRef] [PubMed]
- Beluci, N.d.C.L.; Mateus, G.A.P.; Miyashiro, C.S.; Homem, N.C.; Gomes, R.G.; Fagundes-Klen, M.R.; Bergamasco, R.; Vieira, A.M.S. Hybrid treatment of coagulation/flocculation process followed by ultrafiltration in TiO2-modified membranes to improve the removal of reactive black 5 dye. Sci. Total Environ. 2019, 664, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Fouda-Mbanga, B.G.; Onotu, O.; Tywabi-Ngeva, Z. Advantages of the reuse of spent adsorbents and potential applications in environmental remediation: A review. Green Anal. Chem. 2024, 11, 100156. [Google Scholar] [CrossRef]
- Sawunyama, L.; Oyewo, O.A.; Makgato, S.S.; Bopape, M.F.; Onwudiwe, D.C. TiO2–ZnO functionalized low-cost ceramic membranes from coal fly ash for the removal of tetracycline from water under visible light. Discov. Nano 2025, 20, 1. [Google Scholar] [CrossRef]
- Osman, A.I.; Chen, Z.; Elgarahy, A.M.; Farghali, M.; Mohamed, I.M.; Priya, A.; Hawash, H.B.; Yap, P.S. Membrane technology for energy saving: Principles, techniques, applications, challenges, and prospects. Adv. Energy Sustain. Res. 2024, 5, 2400011. [Google Scholar] [CrossRef]
- Senthil Rathi, B.; Senthil Kumar, P.; Parthasarathy, V.; Dinesh Aravind, V.; Sanjay, S.; Rangasamy, G.; Vo, D.-V.N. Recent progress in ceramic membrane technology for the removal of emerging contaminant from wastewater: A critical review. Chem. Eng. Commun. 2025, 212, 304–328. [Google Scholar] [CrossRef]
- Asif, M.B.; Zhang, Z. Ceramic membrane technology for water and wastewater treatment: A critical review of performance, full-scale applications, membrane fouling and prospects. Chem. Eng. J. 2021, 418, 129481. [Google Scholar] [CrossRef]
- Sawunyama, L.; Ajiboye, T.O.; Oyewo, O.; Onwudiwe, D.C. Ceramic-polymer composite membranes: Synthesis methods and environmental applications. Ceram. Int. 2024, 50, 5067–5079. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, H.; Yang, F.; Gray, S. Cost and efficiency perspectives of ceramic membranes for water treatment. Water Res. 2022, 220, 118629. [Google Scholar] [CrossRef]
- Silveira, C.; Mulinari, J.; Junior, A.D.N.; Ambrosi, A.; Hotza, D.; Di Luccio, M. Low-cost ceramic membranes prepared from kaolin and quartz via tape casting using different pore formers. Open Ceram. 2025, 22, 100765. [Google Scholar] [CrossRef]
- Saja, S.; Bouazizi, A.; Achiou, B.; Ouaddari, H.; Karim, A.; Ouammou, M.; Aaddane, A.; Bennazha, J.; Younssi, S.A. Fabrication of low-cost ceramic ultrafiltration membrane made from bentonite clay and its application for soluble dyes removal. J. Eur. Ceram. Soc. 2020, 40, 2453–2462. [Google Scholar] [CrossRef]
- Artun, G.; AŞKIN, A. Studies on Production of Low-Cost Ceramic Membranes and Their Uses in Wastewater Treatment Processes. Eur. J. Res. Dev. 2022, 2, 126–140. [Google Scholar] [CrossRef]
- Salleh, S.Z.; Kechik, A.A.; Yusoff, A.H.; Taib, M.A.A.; Nor, M.M.; Mohamad, M.; Tan, T.G.; Ali, A.; Masri, M.N.; Mohamed, J.J. Recycling food, agricultural, and industrial wastes as pore-forming agents for sustainable porous ceramic production: A review. J. Clean. Prod. 2021, 306, 127264. [Google Scholar] [CrossRef]
- Qin, H.; Guo, W.; Gao, P.; Xiao, H. Spheroidization of low-cost alumina powders for the preparation of high-flux flat-sheet ceramic membranes. Ceram. Int. 2020, 46, 13189–13197. [Google Scholar] [CrossRef]
- Sawunyama, L.; Oyewo, O.A.; Bopape, M.F.; Onwudiwe, D.C. Fabrication and characterization of low-cost ceramic membranes from coal fly ash and natural sand. Sustain. Chem. Environ. 2024, 8, 100165. [Google Scholar] [CrossRef]
- Akash, F.A.; Shovon, S.M.; Rahman, W.; Rahman, M.A.; Chakraborty, P.; Prasetya, T.A.E.; Monir, M.U. Advancements in ceramic membrane technology for water and wastewater treatment: A comprehensive exploration of current utilizations and prospective horizons. Desalination Water Treat. 2024, 319, 100569. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, D.; Soni, V. Copper and mercury induced oxidative stresses and antioxidant responses of Spirodela polyrhiza (L.) Schleid. Biochem. Biophys. Rep. 2020, 23, 100781. [Google Scholar] [CrossRef]
- Lin, J.; Ye, W.; Xie, M.; Seo, D.H.; Luo, J.; Wan, Y.; Van der Bruggen, B. Environmental impacts and remediation of dye-containing wastewater. Nat. Rev. Earth Environ. 2023, 4, 785–803. [Google Scholar] [CrossRef]
- Mahlaule-Glory, L.M.; Mapetla, S.; Makofane, A.; Mathipa, M.M.; Hintsho-Mbita, N.C. Biosynthesis of iron oxide nanoparticles for the degradation of methylene blue dye, sulfisoxazole antibiotic and removal of bacteria from real water. Heliyon 2022, 8, e10536. [Google Scholar] [CrossRef]
- Munyai, S.; Mahlaule-Glory, L.M.; Hintsho-Mbita, N.C. Green synthesis of Zinc sulphide (ZnS) nanostructures using S. frutescences plant extract for photocatalytic degradation of dyes and antibiotics. Mater. Res. Express 2022, 9, 015001. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Olisah, C.; Adams, J.B.; Rubidge, G. The state of persistent organic pollutants in South African estuaries: A review of environmental exposure and sources. Ecotoxicol. Environ. Saf. 2021, 219, 112316. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Mongkholrattanasit, R.; Kryštůfek, J.; Wiener, J.; Studničková, J. Natural dye from eucalyptus leaves and application for wool fabric dyeing by using padding techniques. Nat. Dye. 2011, 57–78. [Google Scholar] [CrossRef]
- Booth, G.; Zollinger, H.; McLaren, K.; Sharples, W.G.; Westwell, A. Dyes, general survey. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Firmansyah, M.L.; Ashraf, M.; Ullah, N. A critical review on the removal of anionic dyes by cross-linked resin: Recent progress, challenges and future perspective. Sep. Purif. Technol. 2025, 360, 131111. [Google Scholar] [CrossRef]
- Ihsanullah, I.; Jamal, A.; Ilyas, M.; Zubair, M.; Khan, G.; Atieh, M.A. Bioremediation of dyes: Current status and prospects. J. Water Process Eng. 2020, 38, 101680. [Google Scholar] [CrossRef]
- Pizzicato, B.; Pacifico, S.; Cayuela, D.; Mijas, G.; Riba-Moliner, M. Advancements in sustainable natural dyes for textile applications: A review. Molecules 2023, 28, 5954. [Google Scholar] [CrossRef]
- Jain, S.; Jain, P.K. Classification, chemistry, and applications of chemical substances that are harmful to the environment: Classification of dyes. In Impact of Textile Dyes on Public Health and the Environment; IGI Global Scientific Publishing: Hershey, PA, USA, 2020; pp. 20–49. [Google Scholar]
- Baldwinson, T.M. Classification of dyeing and printing auxiliaries by function. Color. Aux. 2002, 2, 497–759. [Google Scholar]
- Zollinger, H. Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
- Oladoye, P.; Ajiboye, T.; Omotola, E.; Oyewola, O. Methylene blue dye: Toxicity and potential technologies for elimination from (waste)water. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Krishna Moorthy, A.; Govindarajan Rathi, B.; Shukla, S.P.; Kumar, K.; Shree Bharti, V. Acute toxicity of textile dye Methylene blue on growth and metabolism of selected freshwater microalgae. Environ. Toxicol. Pharmacol. 2021, 82, 103552. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Sikarwar, S. Adsorption and desorption studies of Congo red using low-cost adsorbent: Activated de-oiled mustard. Desalination Water Treat. 2014, 52, 7400–7411. [Google Scholar] [CrossRef]
- Singh, H.; Chauhan, G.; Jain, A.K.; Sharma, S.K. Adsorptive potential of agricultural wastes for removal of dyes from aqueous solutions. J. Environ. Chem. Eng. 2017, 5, 122–135. [Google Scholar] [CrossRef]
- Iqbal, M.M.; Imran, M.; Hussain, T.; Naeem, M.A.; Al-Kahtani, A.A.; Shah, G.M.; Ahmad, S.; Farooq, A.; Rizwan, M.; Majeed, A.; et al. Effective sequestration of Congo red dye with ZnO/cotton stalks biochar nanocomposite: MODELING, reusability and stability. J. Saudi Chem. Soc. 2021, 25, 101176. [Google Scholar] [CrossRef]
- Naseem, K.; Farooqi, Z.; Begum, R.; Irfan, A. Removal of Congo red dye from aqueous medium by its catalytic reduction using sodium borohydride in the presence of various inorganic nano-catalysts: A review. J. Clean. Prod. 2018, 187, 296–307. [Google Scholar] [CrossRef]
- Shah, N.S.; Khan, J.A.; Sayed, M.; Khan, Z.U.H.; Iqbal, J.; Arshad, S.; Junaid, M.; Khan, H.M. Synergistic effects of H2O2 and S2O82− in the gamma radiation induced degradation of congo-red dye: Kinetics and toxicities evaluation. Sep. Purif. Technol. 2020, 233, 115966. [Google Scholar] [CrossRef]
- Ismail, G.A.; Sakai, H. Review on effect of different type of dyes on advanced oxidation processes (AOPs) for textile color removal. Chemosphere 2022, 291, 132906. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, S.; Soni, V. Toxicity of malachite green on plants and its phytoremediation: A review. Reg. Stud. Mar. Sci. 2023, 62, 102911. [Google Scholar] [CrossRef]
- Jasim, M.A.; Abbas, A.M. Adsorption of Malachite Green Dye by Bio- micro-adsorbent from Aqueous Solution at Different Temperatures. J. Phys. Conf. Ser. 2019, 1294, 052015. [Google Scholar] [CrossRef]
- Samuchiwal, S.; Gola, D.; Malik, A. Decolourization of textile effluent using native microbial consortium enriched from textile industry effluent. J. Hazard. Mater. 2021, 402, 123835. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, A.K.; Roy, D. Malachite green (triarylmethane dye) and pyceze (bronopol) induced histopathological and biochemical changes in the liver of stinging catfish Heteropneustes fossilis (Bloch, 1794). Indian J. Fish. 2016, 63, 135–139. [Google Scholar] [CrossRef]
- Nandhini, N.T.; Rajeshkumar, S.; Mythili, S. The possible mechanism of eco-friendly synthesized nanoparticles on hazardous dyes degradation. Biocatal. Agric. Biotechnol. 2019, 19, 101138. [Google Scholar] [CrossRef]
- Mittal, J. Permissible synthetic food dyes in India. Resonance 2020, 25, 567–577. [Google Scholar] [CrossRef]
- Bai, Y.-N.; Wang, X.-N.; Zhang, F.; Wu, J.; Zhang, W.; Lu, Y.-Z.; Fu, L.; Lau, T.-C.; Zeng, R.J. High-rate anaerobic decolorization of methyl orange from synthetic azo dye wastewater in a methane-based hollow fiber membrane bioreactor. J. Hazard. Mater. 2020, 388, 121753. [Google Scholar] [CrossRef]
- Haque, M.M.; Haque, M.A.; Mosharaf, M.K.; Marcus, P.K. Decolorization, degradation and detoxification of carcinogenic sulfonated azo dye methyl orange by newly developed biofilm consortia. Saudi J. Biol. Sci. 2021, 28, 793–804. [Google Scholar] [CrossRef]
- Dardouri, S.; Sghaier, J. Adsorptive removal of methylene blue from aqueous solution using different agricultural wastes as adsorbents. Korean J. Chem. Eng. 2017, 34, 1037–1043. [Google Scholar] [CrossRef]
- Saeed, M.; Usman, M.; Haq, A. Catalytic Degradation of Organic Dyes in Aqueous Medium. In Photochemistry and Photophysics-Fundamentals to Applications; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Khodaie, M.; Ghasemi, N.; Moradi, B.; Rahimi, M. Removal of methylene blue from wastewater by adsorption. J. Chem. 2013, 91, 1–6. [Google Scholar]
- Shakoor, S.; Nasar, A. Adsorptive treatment of hazardous methylene blue dye from artificially contaminated water using cucumis sativus peel waste as a low-cost adsorbent. Groundw. Sustain. Dev. 2017, 5, 152–159. [Google Scholar] [CrossRef]
- Waghchaure, R.H.; Adole, V.A.; Jagdale, B.S. Photocatalytic degradation of methylene blue, rhodamine B, methyl orange and Eriochrome black T dyes by modified ZnO nanocatalysts: A concise review. Inorg. Chem. Commun. 2022, 143, 109764. [Google Scholar] [CrossRef]
- Hermanson, G.T. Chapter 10—Fluorescent Probes. In Bioconjugate Techniques, 3rd ed.; Hermanson, G.T., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 395–463. [Google Scholar] [CrossRef]
- Sulistina, D.R.; Martini, S. The effect of Rhodamine B on the cerebellum and brainstem tissue of Rattus norvegicus. J. Public Health Res. 2020, 9, 1812. [Google Scholar] [CrossRef] [PubMed]
- Kotobuki, M.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-Polymer Composite Membranes for Water and Wastewater Treatment: Bridging the Big Gap between Ceramics and Polymers. Molecules 2021, 26, 3331. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Peng, Y.; Zheng, X.; Wang, W.; Zhang, J. Comparative analysis of pork tenderness prediction using different optical scattering parameters. J. Food Eng. 2019, 248, 1–8. [Google Scholar] [CrossRef]
- Sawunyama, L.; Olatunde, O.C.; Oyewo, O.A.; Bopape, M.F.; Onwudiwe, D.C. Application of coal fly ash based ceramic membranes in wastewater treatment: A sustainable alternative to commercial materials. Heliyon 2024, 10, e24344. [Google Scholar] [CrossRef]
- Dmitrieva, E.S.; Anokhina, T.S.; Novitsky, E.G.; Volkov, V.V.; Borisov, I.L.; Volkov, A.V. Polymeric Membranes for Oil-Water Separation: A Review. Polymers 2022, 14, 980. [Google Scholar] [CrossRef]
- Ghadhban, M.Y.; Rashid, K.T.; AbdulRazak, A.A.; Alsalhy, Q.F. Recent progress and future directions of membranes green polymers for oily wastewater treatment. Water Sci. Technol. 2022, 87, 57–82. [Google Scholar] [CrossRef]
- Aliyu, U.M.; Rathilal, S.; Isa, Y.M. Membrane desalination technologies in water treatment: A review. Water Pract. Technol. 2018, 13, 738–752. [Google Scholar] [CrossRef]
- Ismail, S.B.; de La Parra, C.J.; Temmink, H.; van Lier, J.B. Extracellular polymeric substances (EPS) in upflow anaerobic sludge blanket (UASB) reactors operated under high salinity conditions. Water Res. 2010, 44, 1909–1917. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.; Sun, R.; Zhang, W. Preparation of Ceramic Membranes and Their Application in Wastewater and Water Treatment. Water 2023, 15, 3344. [Google Scholar] [CrossRef]
- Salleh, W.N.W.; Ismail, A.F.; Matsuura, T.; Abdullah, M.S. Precursor Selection and Process Conditions in the Preparation of Carbon Membrane for Gas Separation: A Review. Sep. Purif. Rev. 2011, 40, 261–311. [Google Scholar] [CrossRef]
- Shehata, N.; Egirani, D.; Olabi, A.G.; Inayat, A.; Abdelkareem, M.A.; Chae, K.-J.; Sayed, E.T. Membrane-based water and wastewater treatment technologies: Issues, current trends, challenges, and role in achieving sustainable development goals, and circular economy. Chemosphere 2023, 320, 137993. [Google Scholar] [CrossRef] [PubMed]
- Lorente-Ayza, M.M.; Mestre, S.; Sanz, V.; Sánchez, E. On the underestimated effect of the starch ash on the characteristics of low cost ceramic membranes. Ceram. Int. 2016, 42, 18944–18954. [Google Scholar] [CrossRef]
- Li, L.; Chen, M.; Dong, Y.; Dong, X.; Cerneaux, S.; Hampshire, S.; Cao, J.; Zhu, L.; Zhu, Z.; Liu, J. A low-cost alumina-mullite composite hollow fiber ceramic membrane fabricated via phase-inversion and sintering method. J. Eur. Ceram. Soc. 2016, 36, 2057–2066. [Google Scholar] [CrossRef]
- Koros, W.J.; Mahajan, R. Pushing the limits on possibilities for large scale gas separation: Which strategies? J. Membr. Sci. 2000, 175, 181–196. [Google Scholar] [CrossRef]
- Li, C.; Sun, W.; Lu, Z.; Ao, X.; Li, S. Ceramic nanocomposite membranes and membrane fouling: A review. Water Res. 2020, 175, 115674. [Google Scholar] [CrossRef]
- He, Z.; Lyu, Z.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-based membranes for water and wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123513. [Google Scholar] [CrossRef]
- Abdullayev, A.; Bekheet, M.F.; Hanaor, D.A.; Gurlo, A. Materials and applications for low-cost ceramic membranes. Membranes 2019, 9, 105. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, H.; Zhang, Y.; Liang, D.; Chen, H. Recycle coal fly ash for preparing tubular ceramic membranes applied in transport membrane condenser. Sep. Purif. Technol. 2022, 282, 119972. [Google Scholar] [CrossRef]
- Saxena, A.K.S.; Soni, A.B.; Jayapal, A. Fabrication of Novel Ceramic Membrane Using Coal Fly Ash Blended with Fuller’s Clay for Separation of Technical Lignin. J. Hazard. Toxic. Radioact. Waste 2025, 29, 04025006. [Google Scholar] [CrossRef]
- Muhamad, A.I.; Zulkipli, F.F.; Mohamed, A.H.; Abdullah, N.; Dabwan, A.H. Fabrication of ceramic membrane from local raw materials for treatment different wastes. IOP Conf. Ser. Earth Environ. Sci. 2021, 646, 012053. [Google Scholar] [CrossRef]
- Taha, M.; Abdel-Ghafar, H.; Amin, S.K.; Ali, M.; Mohamed, E.; Mohamed, F. Development of low-cost ceramic membranes from industrial ceramic for enhanced wastewater treatment. Int. J. Environ. Sci. Technol. 2025, 22, 6005–6020. [Google Scholar] [CrossRef]
- Goswami, K.P.; Pugazhenthi, G. Treatment of starch-rich wastewater using fly ash-based low-cost tubular ceramic membrane. Environ. Prog. Sustain. Energy 2022, 41, e13906. [Google Scholar] [CrossRef]
- Lagdali, S.; Miyah, Y.; El-Habacha, M.; Mahmoudy, G.; Benjelloun, M.; Iaich, S.; Zerbet, M.; Chiban, M.; Sinan, F. Performance assessment of a phengite clay-based flat membrane for microfiltration of real-wastewater from clothes washing: Characterization, cost estimation, and regeneration. Case Stud. Chem. Environ. Eng. 2023, 8, 100388. [Google Scholar] [CrossRef]
- Haque, F.; Fan, C.; Lee, Y.-y. From waste to value: Addressing the relevance of waste recovery to agricultural sector in line with circular economy. J. Clean. Prod. 2023, 415, 137873. [Google Scholar] [CrossRef]
- Dalbanjan, N.P.; Eelager, M.P.; Korgaonkar, K.; Gonal, B.N.; Kadapure, A.J.; Arakera, S.B.; Kumar, S.P. Descriptive review on conversion of waste residues into valuable bionanocomposites for a circular bioeconomy. Nano-Struct. Nano-Objects 2024, 39, 101265. [Google Scholar] [CrossRef]
- Bandyopadhyay, S. An Overview on Recent Progress in Wastewater Treatment Using Low Cost Ceramic Membrane. In Application of Low Cost Ceramic Membranes in Wastewater Treatment: Issues on Reusability of Treated Water; Springer: Berlin/Heidelberg, Germany, 2025; pp. 1–40. [Google Scholar] [CrossRef]
- Wei, T.; Chen, X.; Guo, Z. Ceramic membrane composites for highly efficient oil–water separation: A review. J. Mater. Chem. A 2024, 12, 20803–20837. [Google Scholar] [CrossRef]
- Suresh, K.; Pugazhenthi, G.; Uppaluri, R. Fly ash based ceramic microfiltration membranes for oil-water emulsion treatment: Parametric optimization using response surface methodology. J. Water Process Eng. 2016, 13, 27–43. [Google Scholar] [CrossRef]
- do Carmo, E.S.; Silva, W.L.; dos Santos Barbosa, A.; Rodrigues, M.G.F. Low-cost ceramic membrane production for dye removal. Desalination Water Treat. 2024, 320, 100726. [Google Scholar] [CrossRef]
- Boutaleb, M.; Tabit, K.; Mansori, M.; Saadi, L.; Waqif, M. Facile and low-cost method for preparing multilayer ceramic membranes based on cordierite and abundant clay: Application to dye removal. Sep. Purif. Technol. 2025, 362, 131752. [Google Scholar] [CrossRef]
- Rani, S.L.S.; Kumar, R.V. Fabrication and characterization of ceramic membranes derived from inexpensive raw material fuller’s earth clay. Mater. Sci. Eng. B 2022, 284, 115877. [Google Scholar] [CrossRef]
- Vasanth, D.; Suresh, K.; Pugazhenthi, G. Fabrication of circular shaped ceramic membrane using mixed clays by uniaxial compaction method for the treatment of oily wastewater. Int. J. Nano Biomater. 2014, 5, 75–88. [Google Scholar] [CrossRef]
- Saikia, J.; Sarmah, S.; Bora, J.J.; Das, B.; Goswamee, R.L. Preparation and characterization of low cost flat ceramic membranes from easily available potters’ clay for dye separation. Bull. Mater. Sci. 2019, 42, 104. [Google Scholar] [CrossRef]
- Achiou, B.; Adlane, S.; Harrati, A.; Belgada, A.; Aaddane, A.; Chafiq Elidrissi, Z.; Ouammou, M.; Sadik, C.; Alami Younssi, S. Low-cost ceramic peridotite membrane developed with geomaterial-based sintering aids for textile wastewater treatment. J. Environ. Chem. Eng. 2025, 13, 116375. [Google Scholar] [CrossRef]
- Kyrii, S. Materials and methods for ceramic membrane synthesis. Short review. Water Water Purif. Technol. Sci. Tech. NEWS 2023, 35, 26–45. [Google Scholar]
- Intelligence, H.M. Global Alumina Ceramic Membrane Market Research Report 2020. Absolute Reports. 2020. Available online: https://360researchreports.com (accessed on 21 March 2025).
- Hotza, D.; Di Luccio, M.; Wilhelm, M.; Iwamoto, Y.; Bernard, S.; da Costa, J.C.D. Silicon carbide filters and porous membranes: A review of processing, properties, performance and application. J. Membr. Sci. 2020, 610, 118193. [Google Scholar] [CrossRef]
- Chen, M. SiC-Deposited Ceramic Membranes for Treatment of Oil-in-Water Emulsions. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2023. [Google Scholar]
- Zhu, Z.; Wei, Z.; Sun, W.; Hou, J.; He, B.; Dong, Y. Cost-effective utilization of mineral-based raw materials for preparation of porous mullite ceramic membranes via in-situ reaction method. Appl. Clay Sci. 2016, 120, 135–141. [Google Scholar] [CrossRef]
- Fu, Z.; Zhou, Z.; Liu, Z.; Yang, H.; Chen, Z. Feasibility and challenges of low-cost ceramic membranes in water treatment applications. Desalination Water Treat. 2024, 320, 100739. [Google Scholar] [CrossRef]
- Siah, T.; Foo, H.C.Y.; Tan, I.S.; Yeong, Y.F.; Lam, M.K.; Lee, K.T.; Wong, M.K. Surface charge-switchable polyacrylonitrile/cellulose acetate/soluble eggshell membrane protein (PAN/CA/SEP) mixed matrix membrane for methylene blue removal. Sep. Purif. Technol. 2025, 370, 133211. [Google Scholar] [CrossRef]
- Al-Jubouri, S.M.; Al-Batty, S.; Al-Hamd, R.K.S.; Sims, R.; Hakami, M.W.; SK, M.H. Sustainable environment through using porous materials: A review on wastewater treatment. Asia-Pac. J. Chem. Eng. 2023, 18, e2941. [Google Scholar] [CrossRef]
- Tabasum, A.; Siddique, A.; Razzaq, H.; Nawaz, H.H.; Razzaque, S.; Tahir, S.; Taimur, S.; Jabeen, N.; Shehzadi, S. Integrated synergy: PSF/PANI/GO membranes for dual-action textile dye detoxification. Mater. Adv. 2024, 5, 4736–4752. [Google Scholar] [CrossRef]
- Rakcho, Y.; Baidou, M.; Naboulsi, A.; Bouazizi, A.; Mouiya, M.; Sehaqui, H.; Abouliatim, Y.; Benhammou, A.; Ouammou, M.; Abourriche, A. Fabrication of low-cost ceramic nanofiltration membrane from natural resources for the removal of cationic and anionic dyes: Experimental and DFT investigations. Chem. Eng. J. 2025, 505, 159779. [Google Scholar] [CrossRef]
- Fonseca, F.C.; Borrell, A.; Moya, M.D.S.; Benavente, R.; Bernal, J.F.P. Technological Advances in Ceramic Membranes for Water Treatment. In Advanced Ceramic Materials-Emerging Technologies; IntechOpen: London, UK, 2025. [Google Scholar] [CrossRef]
- Hamad, K.H.; Abdallah, H.; Aly, S.T.; Abobeah, R.; Amin, S.K. Fabrication and assessment of performance of clay based ceramic membranes impregnated with CNTs in dye removal. Sci. Rep. 2024, 14, 26728. [Google Scholar] [CrossRef] [PubMed]
- Chikri, R.; Elataoui, K.; Said, H.A.; Benchanaa, M.; Elhadiri, N. Highly efficient ceramic membrane synthesized from sugar scum and fly ash as sustainable precursors for dyes removal. Heliyon 2024, 10, e27915. [Google Scholar]
- Foorginezhad, S.; Zerafat, M. Preparation of low-cost ceramic membranes using Persian natural clay and their application for dye clarification. Desalination Water Treat. 2019, 145, 378–392. [Google Scholar] [CrossRef]
- Iaich, S.; Miyah, Y.; Elazhar, F.; Lagdali, S.; El-Habacha, M. Low-cost ceramic microfiltration membranes made from Moroccan clay for domestic wastewater and Congo Red dye treatment. Desalination Water Treat. 2021, 235, 251–271. [Google Scholar] [CrossRef]
- Khmiri, Y.; Attia, A.; Aloulou, H.; Dammak, L.; Baklouti, L.; Ben Amar, R. Preparation and Characterization of New and Low-Cost Ceramic Flat Membranes Based on Zeolite-Clay for the Removal of Indigo Blue Dye Molecules. Membranes 2023, 13, 865. [Google Scholar] [CrossRef]
- Ouaddari, H.; Karim, A.; Achiou, B.; Saja, S.; Aaddane, A.; Bennazha, J.; El Hassani, I.E.A.; Ouammou, M.; Albizane, A. New low-cost ultrafiltration membrane made from purified natural clays for direct Red 80 dye removal. J. Environ. Chem. Eng. 2019, 7, 103268. [Google Scholar] [CrossRef]
- Oun, A.; Tahri, N.; Mahouche-Chergui, S.; Carbonnier, B.; Majumdar, S.; Sarkar, S.; Sahoo, G.C.; Amar, R.B. Tubular ultrafiltration ceramic membrane based on titania nanoparticles immobilized on macroporous clay-alumina support: Elaboration, characterization and application to dye removal. Sep. Purif. Technol. 2017, 188, 126–133. [Google Scholar] [CrossRef]
- Saad, E.M.; Wagdy, M.; Orabi, A.S. Advanced nano modification of ecofriendly glauconite clay for high efficiency methylene blue dye adsorption. Sci. Rep. 2024, 14, 23614. [Google Scholar] [CrossRef]
- Diachenko, A.; Hutsul, K.; Dontsova, T. Prospects for Using 3D Printing to Form Ceramic Membranes: A Brief Review. Water Water Purif. Technol. Sci. Tech. News 2024, 38, 14–26. [Google Scholar] [CrossRef]
- Roy Barman, S.; Gavit, P.; Chowdhury, S.; Chatterjee, K.; Nain, A. 3D-Printed Materials for Wastewater Treatment. JACS Au 2023, 3, 2930–2947. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, J.K.; Zou, M. 3D Printing of High-Porosity Membranes with Submicron Pores for Microfluidics. Nanomanufacturing 2024, 4, 120–137. [Google Scholar] [CrossRef]
- Argurio, P.; Fontananova, E.; Molinari, R.; Drioli, E. Photocatalytic membranes in photocatalytic membrane reactors. Processes 2018, 6, 162. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.K.; da Costa, J.C.D. Recent progresses on fabrication of photocatalytic membranes for water treatment. Catal. Today 2014, 230, 47–54. [Google Scholar] [CrossRef]
- Kim, H.J.; Pant, H.R.; Kim, J.H.; Choi, N.J.; Kim, C.S. Fabrication of multifunctional TiO2–fly ash/polyurethane nanocomposite membrane via electrospinning. Ceram. Int. 2014, 40, 3023–3029. [Google Scholar] [CrossRef]
- Ferrer-Nicomedes, S.; Mormeneo-Segarra, A.; Vicente-Agut, N.; Barba-Juan, A. Introducing an ionic conductive matrix to the cold-sintered Li1.3Al0.3Ti1.7(PO4)3-based composite solid electrolyte to enhance the electrical properties. J. Power Sources 2023, 581, 233494. [Google Scholar] [CrossRef]
- Ilyasoglu, G.; Kose-Mutlu, B.; Mutlu-Salmanli, O.; Koyuncu, I. Removal of organic micropollutans by adsorptive membrane. Chemosphere 2022, 302, 134775. [Google Scholar] [CrossRef]
- Priyadarshini, A. Design and Engineering of Dynamic and High Affinity Carbon-Based Hybrid Membranes for Nanofiltration. Ph.D. Thesis, National University of Singapore, Singapore, 2020. [Google Scholar]
- Braun, H. Particle size and solubility of disperse dyes. Rev. Prog. Color. Relat. Top. 1983, 13, 62–72. [Google Scholar] [CrossRef]
- Rashed, M.N. Adsorption technique for the removal of organic pollutants from water and wastewater. In Organic Pollutants-Monitoring, Risk and Treatment; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Cheng, X.; Li, N.; Zhu, M.; Zhang, L.; Deng, Y.; Deng, C. Positively charged microporous ceramic membrane for the removal of Titan Yellow through electrostatic adsorption. J. Environ. Sci. 2016, 44, 204–212. [Google Scholar] [CrossRef]
- Alias, N.H.; Norddin, M.N.A.M. Advanced ceramics for photocatalytic membranes: Application of photocatalytic membrane for dyes removal. In Advanced Ceramics for Photocatalytic Membranes; Elsevier: Amsterdam, The Netherlands, 2024; pp. 389–408. [Google Scholar] [CrossRef]




| Organic Dyes | Classification | Adverse Impacts of the Dye | Ref |
|---|---|---|---|
| Congo Red (CR) | Anionic azo dye | Exhibit carcinogenic and mutagenic properties. Toxicity to animals and humans. | [11,12,13] |
| Rhodamine B (RhB) | Cationic xanthene dye | Exhibit carcinogenic and mutagenic properties. Causes cancer in humans. | [11,14] |
| Brilliant green (BG) | Triphenylmethane dye | Highly toxic to aquatic organisms. Causes skin and eye irritation. Potentially carcinogenic. | [15,16,17] |
| Eriochrome black T (EBT) | Anionic azo dye | Causes undesirable anomalies such as astigmatism and skin allergies. Causes high pH, chemical oxygen demand, suspended solids, and salinity. Affects the re-oxygenation ability of water bodies. Forms carcinogenic byproducts (naphthoquinones) upon degradation. Affects the photosynthetic abilities of phytoplanktons and aquatic plants. | [18,19,20] |
| Safranin O (SO) | A phenazine dye | Toxicity to aquatic organisms Causes serious eye damage | [21,22] |
| Methylene blue (MB) | thiazine cationic and basic synthetic dyes | Induce fatal serotonin toxicity in humans. A threat to fauna in aquatic ecosystems. It causes cancer in humans. | [23,24] |
| Methyl orange (MO) | Anionic azo dye | Causes skin irritation Causes allergic dermatitis | [4,25,26] |
| Methyl violet (MV) | Cationic triphenylmethane dye class | It can cause toxicity in living organisms. Possible mutagenic effects. | [27,28] |
| Sunset Yellow dye | Synthetic azo dye | Causes allergic reactions, It can cause behavioral changes in children and has possible genotoxicity. | [29,30] |
| Yellow dye 5 (Tartrazine) | Synthetic azo dye | Associated with hyperactivity in children. It can cause skin rashes and asthma. Potential carcinogenic effects. | [31,32] |
| Malachite green | Triphenylmethane dye | Highly toxic to aquatic life. Carcinogenic and mutagenic. It can cause organ damage, particularly in the liver and kidneys. | [33,34] |
| Eosin Y | Xanthene dye | It can cause skin and eye irritation. Possible toxic effects on aquatic organisms. | [35,36,37] |
| Indigo Carmine (Indigo Blue) | Water-soluble indigoid dye | Possible adverse effects like hypertension and skin irritation. Highly toxic to humans and can cause tumors. When in contact with the skin, it irritates and causes permanent injury to the cornea and conjunctiva when in contact with the eyes. It can cause gastrointestinal irritation with nausea, vomiting, and diarrhea. It displays carcinogenic properties, which can cause acute toxicity of organs related to reproduction, development, and the neurological system. | [38,39,40] |
| Raw Materials for Membranes | Cost of Raw Materials (USD/Kg) | Total Cost of Membrane Production (USD/m2) | Ref. |
|---|---|---|---|
| Flay ash, quartz, calcium carbonate | - | 250 | [128] |
| Chocobofe clay, kaolin, magnesite concentrate, and starch | 27.80 | 233.55 | [129] |
| Phengite clay, distilled water | 0.313 | 3.398 | [123] |
| Abundant clay | 0.09 | 12.75 | [130] |
| Fuller’s earth clay | 45 | 99.03 | [131] |
| Clay (~70% clay) | 7 | [132] | |
| Potter’s clay | 1.083 | 42.46 | [133] |
| Peridotite, perlite | 2.96 | 9.91 | [134] |
| Fly ash-based low-cost tubular ceramic membrane | 0.43 | 250 | [127] |
| Commercial Starting Materials for Ceramic Membranes | Cost of Raw Materials (USD/Kg) | Total Cost of Membrane Production (USD/m2) | Ref. |
|---|---|---|---|
| Alumina α-Al2O3 | 760 | [135,136] | |
| Zirconia ZrO2 | 500–3000 | [117] | |
| Silicon carbide SiC | 100–1000 | [137] | |
| Silicon carbide SiC | 200 | [138] | |
| Commercial mullite | 20–30 | 150–200 | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malebadi, K.A.; Sawunyama, L.; Seheri, N.H.; Onwudiwe, D.C. Application of Ceramic Membranes Derived from Waste and Natural Materials for the Removal of Organic Dyes from Wastewater: A Review. Ceramics 2025, 8, 80. https://doi.org/10.3390/ceramics8030080
Malebadi KA, Sawunyama L, Seheri NH, Onwudiwe DC. Application of Ceramic Membranes Derived from Waste and Natural Materials for the Removal of Organic Dyes from Wastewater: A Review. Ceramics. 2025; 8(3):80. https://doi.org/10.3390/ceramics8030080
Chicago/Turabian StyleMalebadi, Keotshepile A., Lawrence Sawunyama, Naledi H. Seheri, and Damian C. Onwudiwe. 2025. "Application of Ceramic Membranes Derived from Waste and Natural Materials for the Removal of Organic Dyes from Wastewater: A Review" Ceramics 8, no. 3: 80. https://doi.org/10.3390/ceramics8030080
APA StyleMalebadi, K. A., Sawunyama, L., Seheri, N. H., & Onwudiwe, D. C. (2025). Application of Ceramic Membranes Derived from Waste and Natural Materials for the Removal of Organic Dyes from Wastewater: A Review. Ceramics, 8(3), 80. https://doi.org/10.3390/ceramics8030080








