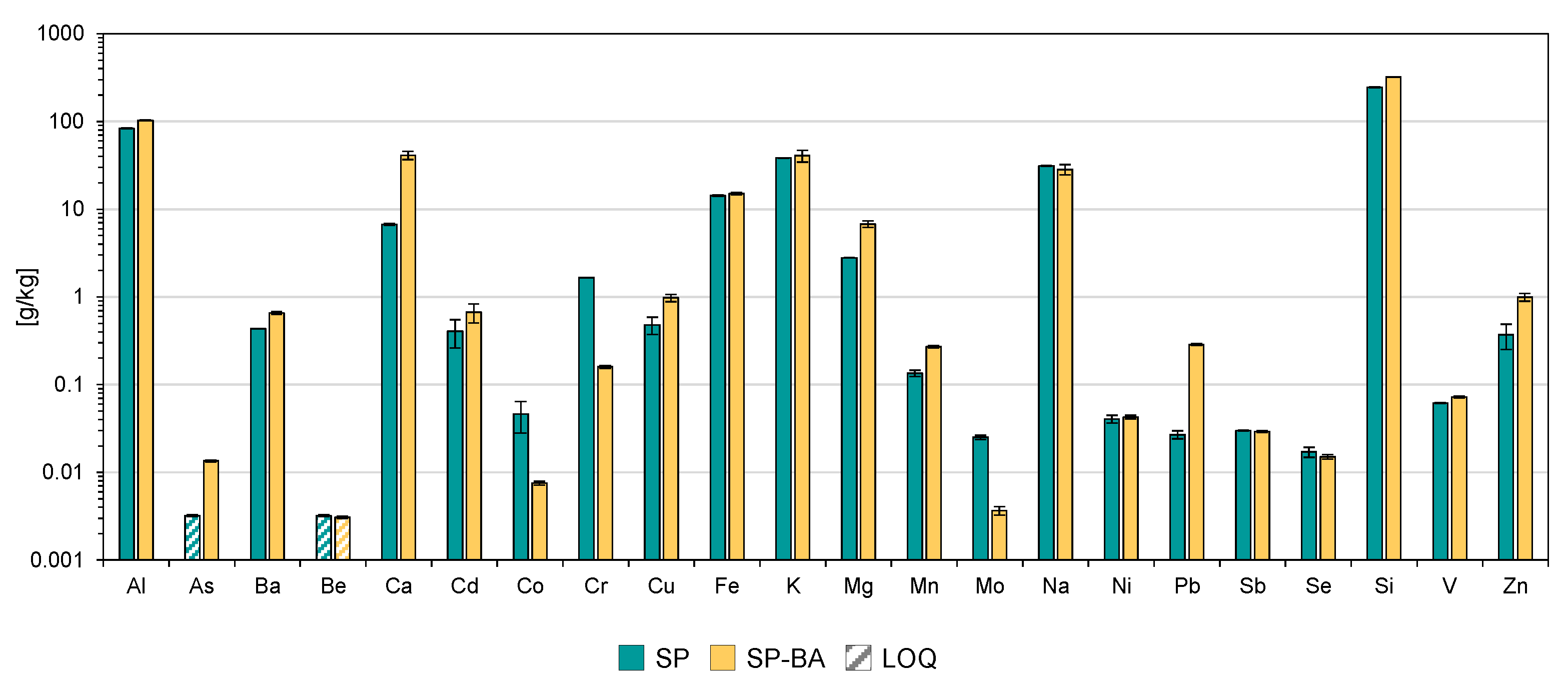1. Introduction
The increasing global production of municipal solid waste has led to a growing interest in waste-to-energy (WtE) technologies such as incineration, which significantly reduces the waste volume while recovering energy. However, this process generates various by-products, the most significant in terms of weight being bottom ash (BA) [
1].
Bottom ash accounts for roughly 20% of the total weight of waste incinerated in WtE plants [
2] and makes up around 80% of the solid residues produced by such facilities [
3]. Until recently, in Italy and other European countries, this by-product was primarily disposed of in landfills; however, over the past two decades, significant efforts have been made to find more sustainable and valuable uses for this residue [
4]. The mineral fraction of BA is widely used as a replacement for sand or aggregates in concrete and asphalt mixtures [
5,
6], as well as in unbound applications such as road sub-base layers [
4,
7,
8]. Beyond these uses, BA has also been explored as a raw material in cement production [
9] and, more recently, in ceramic manufacturing for products like tiles, bricks, refractories, glass, and sanitary ware [
10].
Ceramic materials present an excellent opportunity for the integration of alternative and more sustainable raw materials like bottom ash from incineration of municipal solid waste. This is mainly due to the high-temperature manufacturing process, which allows the stabilization and inertization of potential inorganic contaminants present in the BA. Additionally, ceramics manufacturing can incorporate industrial by-products without requiring significant modifications to processing methods. As a result, the use of BA in ceramics formulations represents a promising opportunity for sustainable material innovation and the practical implementation of circular economy strategies. However, it is fundamental to assess the effect of BA addition on product performance, durability, and environmental compatibility in terms of potential negative impacts on the environment and human health.
The preprocessing stage is essential for preparing bottom ash before its incorporation into ceramic formulations. This typically involves processes such as sieving to select particles of the optimal size, improving homogeneity, and removing ferrous and non-ferrous metals. Additionally, particle size reduction through milling or grinding is generally performed to enhance the reactivity and compatibility of the BA with other ceramic raw materials. These steps are largely consistent with traditional ceramic processing techniques [
11], which commonly include washing, sieving, ball milling, drying, and compaction/pelletizing. Through these treatments, BA, as well as the other raw materials, can be more uniformly and effectively integrated into the ceramic matrix, ensuring better control over the final properties of the product.
After preprocessing, thermal treatment is essential for manufacturing the final ceramic product. This process, generally named firing, can be classified into three main categories: melting, sintering, or vitrification [
1,
12,
13,
14]. Melting and vitrification involve the complete fusion of the material at very high temperatures (typically between 1000 and 1500 °C) [
1,
11], which makes them less suitable for traditional ceramic processes. In contrast, sintering, the most widely used thermal treatment process for ceramics manufacturing, is performed at lower temperatures, typically between 700 and 1200 °C [
1,
10], involving the partial fusion of the material and resulting in improved density, mechanical strength, and durability, while preserving the structural integrity of the final product. It is widely applied in the production of various types of ceramic materials, including those that incorporate bottom ash as an additive. Sintering allows the BA to bond with the other raw materials without fully melting, enhancing the final product’s performance [
11].
Several studies have analyzed key technical parameters to evaluate the feasibility of incorporating bottom ash in the formulation of ceramic materials, focusing on aspects such as sintering behavior, mechanical properties, and environmental behavior, particularly in relation to the release of potential contaminants, such as metals, metalloids, and salts, and the durability of the product [
10,
15].
Studies on the sintering behavior of bottom ash have demonstrated that the temperature employed significantly influences the product’s density, mineralogical transformations, and metal encapsulation. A study identified 1080 °C as the temperature at which the maximum density is achieved [
16]. Similar findings were reported by Cheeseman et al. [
17], who examined BA that was milled, pelletized, and sintered at temperatures between 900 °C and 1080 °C, confirming the improved densification and reduction in porosity of the treatment product. Other research highlighted that the use of pre-vitrified BA reduces the sintering temperatures by approximately 20 °C compared to non-vitrified BA, facilitating crystallization and improving material stability [
18,
19]. Regarding the mineralogical composition, it was observed that ceramics composed of 60% by weight of BA and 40% by weight of industrial clays exhibit a different sintering process. In these materials, the liquid phase forms through transformations involving CaO, SiO
2, and Al
2O
3, rather than feldspar melting as in traditional ceramic formulations [
20].
The influence of the variation in BA incorporation percentages on the properties of ceramic materials has been extensively investigated. Research on ceramic materials containing up to 20% BA has consistently shown that higher BA contents lead to increased water absorption, while reducing shrinkage, bending strength, and wear resistance [
21]. Dagnew [
22], who examined BA percentages ranging from 0 to 60%, identified 30% BA as the optimal content for maximizing flexural strength and water absorption, with the breaking strength reaching a plateau at this percentage. Another study indicated that incorporating up to 60% BA can still lead to a product with mechanical properties comparable to commercial products [
20]. Specifically, a mixture of 60% BA and 40% clay, sintered at temperatures between 1190 and 1240 °C, demonstrated high crystallinity, low water absorption, and good mechanical resistance. Experimental work on glass–ceramic materials has also confirmed pre-vitrified BA’s potential to enhance mechanical properties, including bending strength and Young’s modulus, also when combined with other industrial waste and sintered at high temperatures [
23].
The environmental impact of the utilization of the mineral fraction of BA for different applications has been widely studied, particularly through the analysis of the leaching behavior of the initial residues and of the obtained products, and also through life cycle assessment (LCA) studies comparing the manufacturing of the products with or without the use of BA. As for the latter, LCA has been employed to quantify the environmental footprint of BA utilization in ceramics manufacturing both as a replacement for feldspar sand in conventional materials [
24] and for the production of frit for ceramic glaze [
25], making use of data regarding energy consumption, raw material savings, emissions, and the management of the solid residues. The results of these studies indicate that BA incorporation can lead to a decrease in environmental impacts compared to traditional formulations, particularly related to the avoidance of the quarrying and transport of the substituted raw materials and to the avoidance of BA landfilling [
24].
Leaching refers to the process by which substances are released from a material into the environment when in contact with an eluent such as water. The alkalinity and potential leaching of inorganic contaminants from BA, such as metals, metalloids, and salts, are the critical issues that are considered when assessing the potential adverse environmental effects related to BA use, since the leached pollutants could affect the quality of water resources and hence human and ecosystem health. In materials with low permeability, such as ceramics, contaminants primarily diffuse through the material, which often limits the rate of leaching. This is particularly relevant for monolithic materials, which tend to exhibit slower leaching rates due to the lower specific surface area in contact with the extracting liquid compared to granular materials. Studies have shown that sintered BA in granular form exhibits a reduced acid neutralization capacity, especially at low pH values, and its neutralization capacity decreases when there is an increase in the sintering temperature [
7,
8]. Multiple investigations have reported a significant reduction in the leaching of metals and metalloids (Ni, Cr, Cd, Pb) and major elements (Ca, Mg, Na, K) as a result of their encapsulation in glassy and crystalline phases during sintering [
16,
17,
18,
26]. In particular, one study reported an up to 90% reduction in Ni release and up to 99% decrease in the leaching of other metals from sintered BA, even for acidic environments, under which these metals typically exhibit the highest release from untreated bottom ash [
16]. Only in one case the leaching of elements such as Cr, Zn, and Cd was reported to have been slightly higher from sintered products compared to untreated bottom ash [
17]. Regarding vitrified bottom ash, studies have shown a significant improvement in their leaching behavior, with metal and metalloid release reduced to nearly zero and a one-order-of-magnitude decrease in the leaching of macro-elements such as Ca and Mg [
18,
26].
Although the literature on the environmental behavior of bottom ash employed in granular form is extensive, studies on the leaching behavior of monolithic materials obtained employing bottom ash as one of the raw materials is instead quite limited. This is especially true for ceramic materials incorporating BA, despite the growing interest in sustainable construction materials and waste valorization options. Studies on monolithic applications have demonstrated that leaching from BA-containing monolithic products is significantly reduced, particularly when BA is incorporated into concrete, asphalt, or other construction materials [
27,
28,
29,
30]. Clavier et al. [
31] also observed acceptable leaching values for BA used in cement production as a kiln feed ingredient, replacing traditional kiln feed components, further supporting the potential of BA utilization for manufacturing sustainable construction materials.
In summary, the research on incorporating bottom ash into ceramic materials consistently shows that BA can be an effective alternative raw material, providing benefits such as reduced sintering temperature, enhanced mechanical properties, and reduced environmental impacts. However, further studies are necessary to assess the long-term environmental behavior of BA-containing monolithic materials, especially in ceramic products.
This study primarily aims to determine whether the partial replacement of traditional materials with bottom ash in porcelain stoneware tile formulations can be considered as an environmentally compatible solution, i.e., that does not lead to additional risks to the environment or human health. The main objective is to understand whether this substitution leads to significant variations in terms of chemical composition, leaching behavior, and compliance with regulatory requirements, when available. First, the total content of major elements and trace contaminants in tiles manufactured with BA and in those obtained with the traditional formulation was performed. This allowed to assess whether the use of BA could significantly alter the chemical composition of the material, potentially affecting its environmental compatibility. The focus then shifted to the end-of-life stage of the tiles. Here, the utilization potential of BA-containing ceramics as recycled aggregates in the construction sector at the end of their life (i.e., after demolition) was assessed. Compliance leaching tests were conducted on crushed tile samples manufactured with or without BA. The resulting concentrations were compared with the Italian End-of-Waste (EoW) criteria for the utilization of construction and demolition waste as recycled aggregates. This step was essential to verify that the BA-based tiles would meet environmental requirements not only during their use, but also when reintroduced into the material cycle after disposal. To evaluate the environmental behavior of the materials during their service life, monolith leaching tests were carried out on intact tile samples. This approach enables to perform a realistic evaluation of the materials’ environmental behavior under actual use conditions. In particular, depending on the utilization scenario, the amount of eluent and pH conditions may change, affecting in turn the release behavior of the material. The release of potential contaminants has been shown to vary depending on the characteristics of the element, but also on those of the solid matrix. It is hence important to identify the mechanisms controlling the release of the different constituents of the ceramic tile and how they may be modified if BA is employed in the formulation of the ceramics. The results of these tests were then integrated into a site-specific human health risk assessment study designed to simulate worst-case scenarios and assess the potential impact on groundwater quality and human health.
By examining variations in the chemical composition and the leaching mechanisms of the products manufactured with or without BA, the study aims to provide a clear and evidence-based framework for evaluating if the integration of BA into ceramic formulations could be a viable solution also from an environmental compatibility perspective, on the basis of the release behavior of the product during its use and at its end of life. This contributes to the broader goal of promoting resource efficiency and circularity in the ceramics industry, while ensuring long-term safety and environmental protection.
3. Results
3.1. Total Content of Major Elements and of Potential Contaminants
The results obtained from the total content analysis, performed after alkaline fusion of the samples, provide a comprehensive overview of the elemental composition of the two types of samples. This method allows for the complete dissolution of the ceramic matrix, ensuring the quantification of all of the non-volatile elements, including those bound within crystalline or amorphous phases. The results expressed in grams of constituent per kilogram of product are reported in
Figure 2, which shows the average concentrations obtained from three replicates, along with the corresponding standard deviations.
Silicon and aluminum were the most abundant elements retrieved in both samples, with silicon concentrations exceeding 300 g/kg in the SP-BA formulation and aluminum levels around 100 g/kg for both types of samples. These high levels are attributable to the use of feldspar sand and clay in the tiles’ formulation, but also to the presence of the BA in samples SP-BA, since all of these materials are rich in silicates and aluminosilicates [
52,
53]. Potassium, sodium, iron, calcium, and magnesium were also present in significant amounts. The addition of BA appeared to slightly increase the concentration of some elements, including Si, Ca, Mg, Ba, Cu, Mn, Pb, and Zn, suggesting a contribution of these elements from the BA source. Conversely, elements such as Co, Cr, and Mo showed to be present in lower amounts in the SP-BA sample, indicating a dilution effect or lack of contribution from the BA. Trace elements like As, Be, Mo, Ni, Sb, Se, and V were found at much lower concentrations, i.e., below 0.1 g/kg, for both materials.
Overall, from the perspective of the total content, the incorporation of bottom ash did not appear to cause significant variations in elemental composition compared to the traditional formulation, except for a one-order-of-magnitude-higher content of Pb in the SP-BA samples that, however, exhibited a one-order-of-magnitude-lower Cr and Mo content compared to the SP tiles. The concentrations of major and trace elements remained largely consistent, suggesting that the partial substitution of traditional materials with BA did not substantially alter the chemical composition of the final ceramic product.
3.2. Compliance Leaching Test
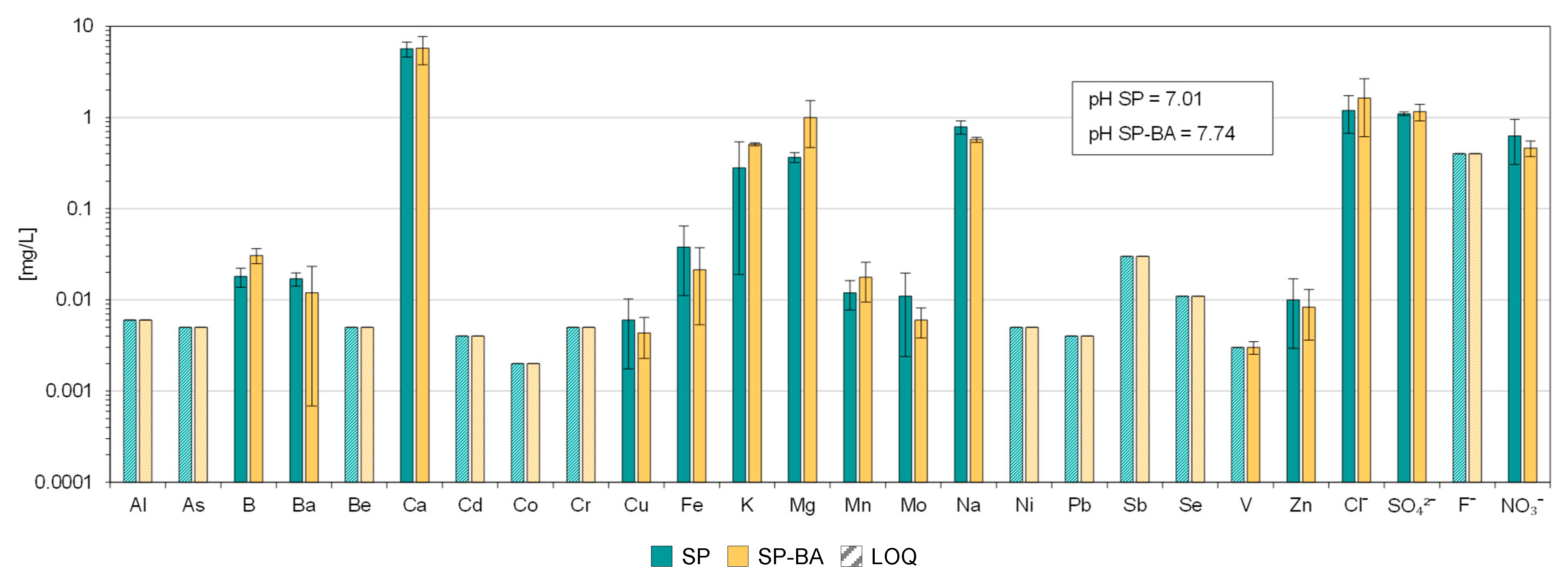
The compliance leaching test results and the comparison with the limits set by the Italian regulation for the utilization of construction and demolition materials as recycled aggregates are shown, respectively, in
Figure 3 and
Table 2. The results include all of the analyzed elements, while the comparison only considers the regulated elements.
As shown in
Figure 3, on average, the tiles containing bottom ash exhibited a pH increase of 0.7 units, which could influence the release of certain elements. The leaching of many of the constituents, however, remained below the quantification limit (LOQ) in both cases. The addition of BA appeared to slightly increase the eluate concentration of a few elements, including B, Mg, Mn, and chlorides, suggesting that the use of the BA as raw material increased their contents or that changes in pH may have affected their release. In contrast, for other elements such as Cu, Fe, Mo, and Zn, the addition of BA seemed to have reduced their release. Overall, it does not seem that the addition of BA negatively affected the leaching behavior of the material in its granular form. In fact, the results showed a similar release behavior for the two tile formulations, indicating that BA incorporation did not significantly affect the environmental behavior of the ground tile material.
The comparison of the results of the compliance leaching test with the Italian End-of-Waste (EoW) criteria for construction and demolition (C&D) waste use as recycled aggregates is shown in
Table 2, which reports the results obtained for the three replicates of the crushed samples, indicated as D, E, and F. Some of the analyzed elements (Al, B, Ca, Fe, K, Mg, Mn, Na, Sb) are not included in
Table 2, as the Italian End-of-Waste (EoW) Decree 152/22 does not report limit values for these constituents. Examining the concentrations resulting from each replicate, it can be noted that for all constituents, apart from Ba, chlorides, and sulfates, at least one of the replicates was below the quantification limit. In some cases, e.g., Zn, Cu, and nitrates, one of the replicates presented a higher concentration than the others; this result may be related to possible heterogeneity within the samples that in this case were only crushed and not finely milled as in the case of the total composition analysis. Nonetheless, the absence of any exceedances, even for the single replicates, demonstrates that both materials may be suitable for use as recycled aggregates at the end of their application as pavement tiles.
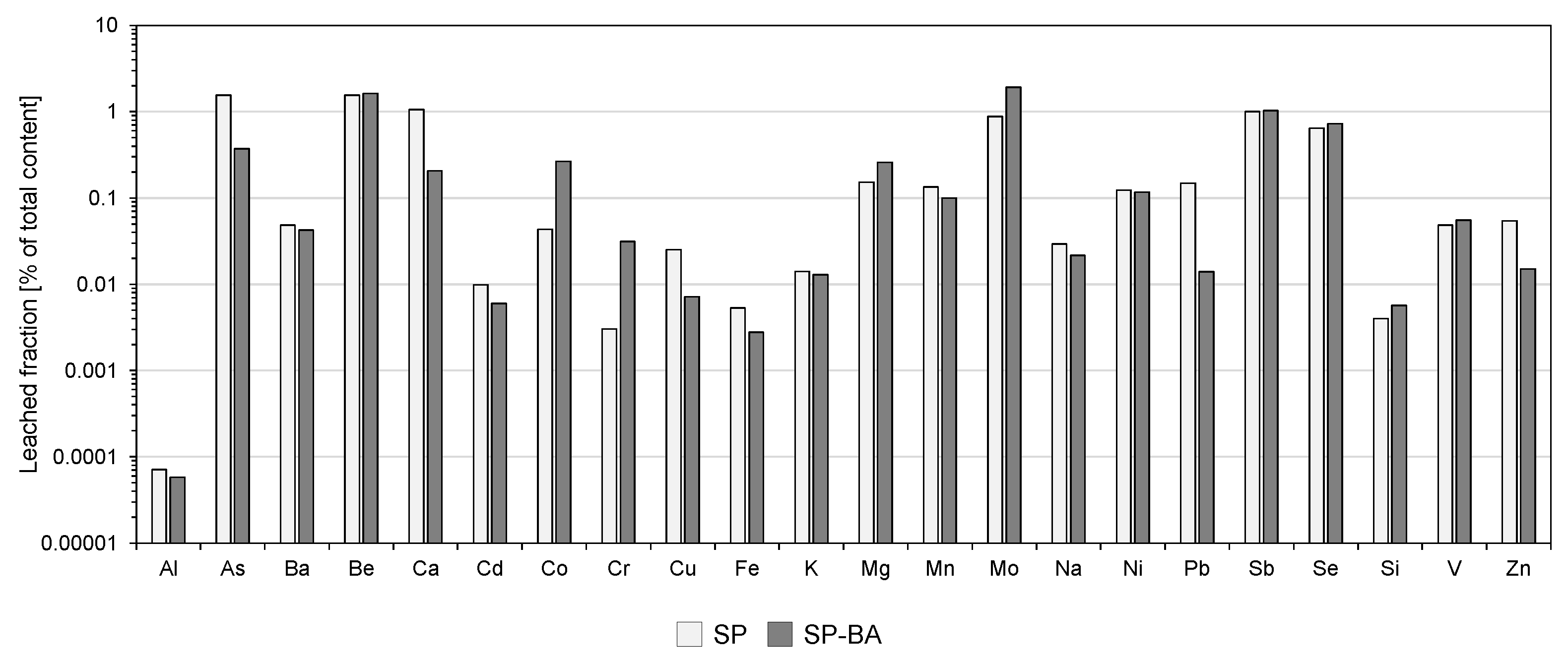
To further assess the leaching behavior of each type of material, for each element, the released percentage was calculated as the ratio between the maximum concentration leached during the compliance leaching test and the corresponding total content in the material determined by the alkaline fusion procedure. The results are presented in
Figure 4. It should be noted that this evaluation was performed only for constituents for which the total content was determined, i.e., for all constituents apart from boron, since lithium tetraborate was used in the alkaline fusion procedure, and the anions analyzed in the leachate solutions, since their total contents cannot be determined through the employed method. In cases where the maximum concentration detected in the leachate corresponded to the instrumental quantification limit (LOQ) (i.e., Al, As, Be, Cd, Co, Cr, Ni, Pb, Sb, Se, and V), this value was used as a conservative estimate for the calculation.
As shown, the majority of the elements exhibited leaching percentages well below 0.1%, indicating a very low release. A few elements displayed slightly higher percentages around 1–2%. Among these, elements such as As, Be, Sb, and Se were detected at concentrations lower than the respective LOQs in the compliance leaching test. In these cases, the release percentages may have been hence overestimated. This conservative assumption was intentionally adopted to ensure that even potentially critical elements were evaluated under worst-case conditions. Overall, these results clearly confirm the effective immobilization of the constituents within the ceramic matrix, demonstrating that the incorporation of bottom ash did not compromise the material’s leaching behavior even after the material was ground.
Such limited release is most likely attributable to the high temperature used in porcelain stoneware tile production, which promotes the formation of a dense structure and the inertization of the bottom ash. High temperatures in fact promote the incorporation of elements, including those introduced via bottom ash, into both the crystalline and the amorphous phases of the ceramic matrix. This process significantly reduces the mobility of the elements, effectively stabilizing them within the structure of the product. The encapsulation of potentially hazardous elements limits their solubility under the leaching test conditions [
26].
Even for elements present in relatively high concentrations in terms of total content (e.g., Si, Al, Ca, K, and Fe), the leached percentages were negligible. This confirms the high chemical stability of the products. Importantly, the inclusion of bottom ash in the SP-BA formulation did not lead to an increase in the release of constituents of potential environmental concern, even after the material was ground, reinforcing the conclusion that 30% by weight of bottom ash could be incorporated into the ceramic matrix without compromising its leaching performance at the end of life of the product.
3.3. Monolith Leaching Test
The leaching behavior of monolithic samples of traditional porcelain stoneware tiles (SP) and tiles containing bottom ash (SP-BA) was analyzed through three replicate tests. The results obtained for pH and conductivity in each leaching interval are presented in
Table 3 as averages of the replicates; the correlated standard deviations are also shown. For both tile formulations, the pH remained relatively stable, with pH values generally falling in the slightly acidic range (values around 5–6), showing only minor fluctuations and a slight increase over time, especially for the last sampling interval.
Conductivity also fluctuated, without a clear trend in this case, indicating small variations in ion concentrations. Notably, the conductivity values remained very low, typically in the range of 2–8 µS/cm, reflecting minimal ion dissolution from the samples. No significant differences were observed between the two types of tiles, suggesting that the addition of this secondary raw material did not significantly affect pH or conductivity.
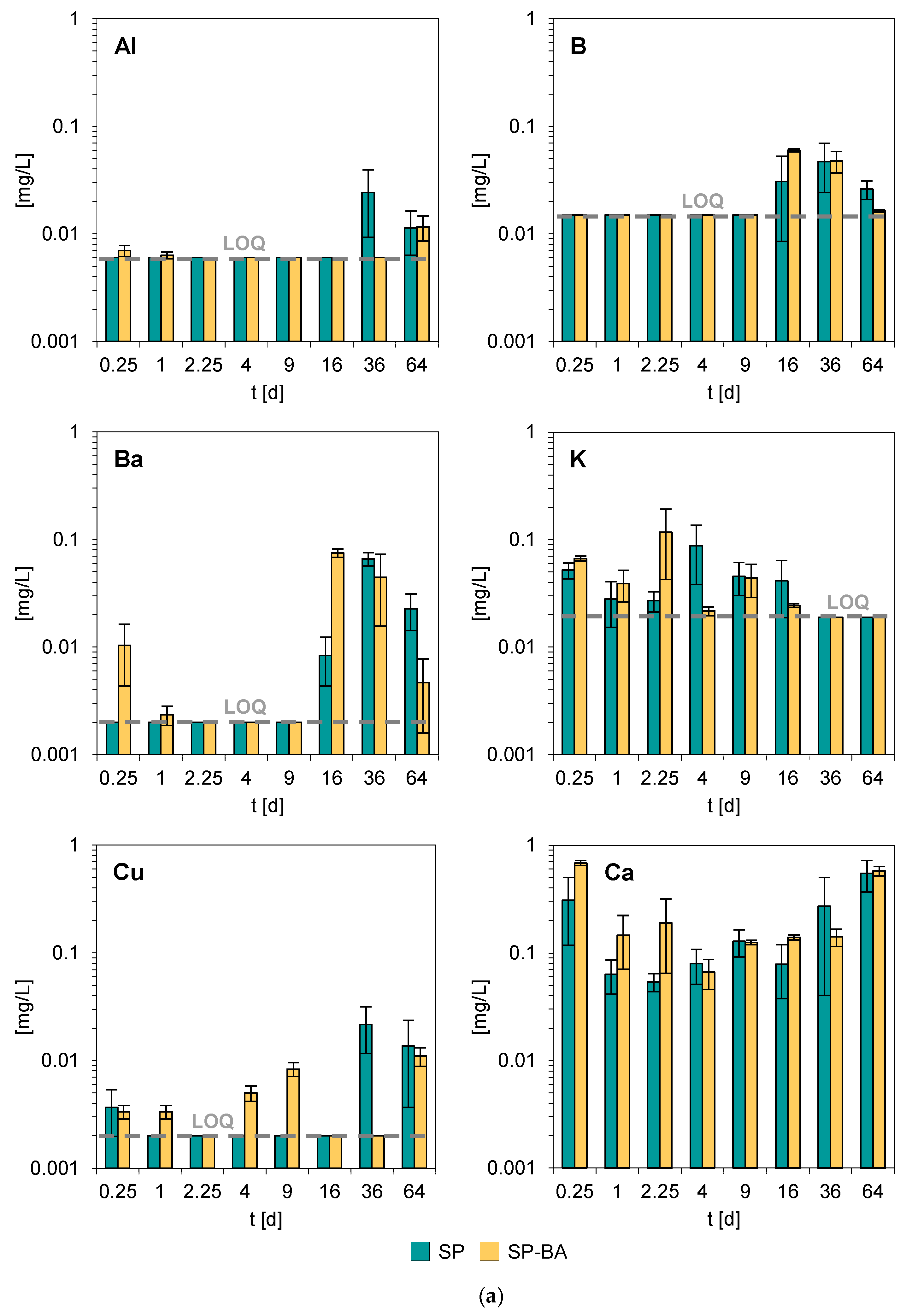
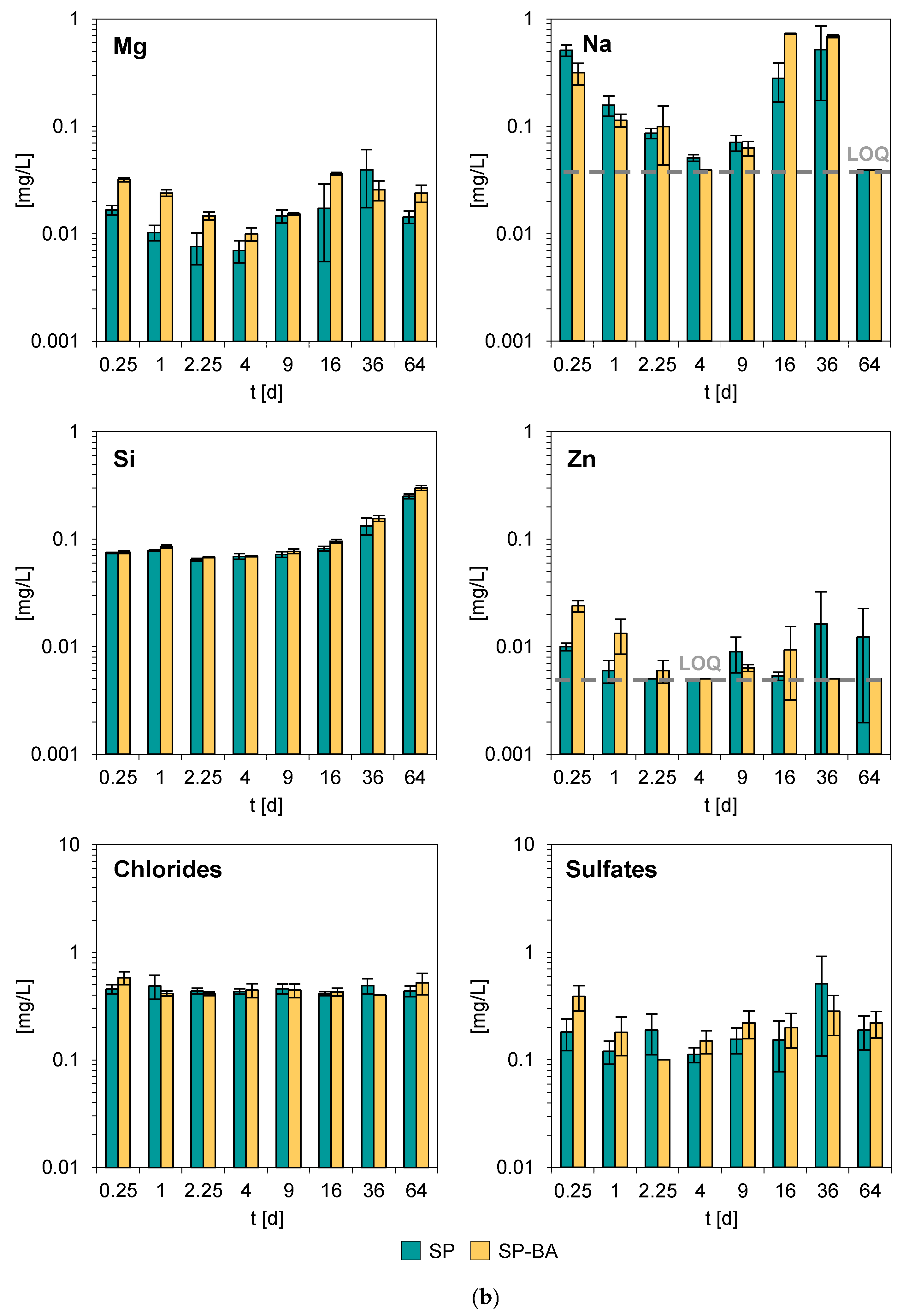
Figure 5a,b reports the average concentrations of the investigated elements released during the monolith leaching tests, along with the corresponding standard deviations (SD), based on three replicates. The constituents resulting consistently below the respective limits of quantification (LOQ) across all replicates and time points were omitted from the figure; the LOQ values are reported in
Table S2 in the
Supporting Information section.
Also in this case, the constituents that presented the highest concentrations in the collected leachates were major elements such as calcium, potassium, magnesium, sodium, and silicon. For most of the other analyzed elements, the concentrations in the eluates remained consistently close or below the respective LOQs throughout the entire testing period. Notably, the samples containing bottom ash (SP-BA) did not display significant differences in their dynamic leaching behavior compared to the traditional SP formulation. A slightly higher release was found in some leaching intervals for Ba, Ca, Mg, and Zn, which is consistent with the results of the total content analysis, which indicated that these elements were more abundant in the SP-BA samples. This minor increase, however, did not affect the overall environmental performance of the material. As already observed for pH and conductivity, the elemental release trends remained comparable for both formulations. This suggests that the incorporation of bottom ash did not significantly alter the leaching characteristics of the tiles. The overall stability of both formulations during all tested intervals, combined with the absence of substantial variations in the release of potentially hazardous elements, supports the conclusion that the addition of bottom ash did not negatively impact the environmental performance of the porcelain stoneware tiles. Additionally, the tests showed a good reproducibility between the three replicates, with consistent leaching trends observed throughout the experiment.
The very limited release exhibited by both types of products suggests a high stability of these elements within the ceramic matrix, also considering that some of them, e.g., Fe, Mn, and Pb, were present in significant concentrations, as shown by the results of the total content analysis (see
Figure 2). The differences between the total contents and the leached concentrations from the monolith test highlight again the strong binding of these elements within the tile structure. In fact, as for the compliance leaching test (see
Section 3.2), also for the monolith tests, the maximum concentrations obtained for each constituent, considering all eight leaching intervals and three replicates, were divided by the respective total contents to obtain the release percentages. Since the maximum concentrations observed in the monolith tests were slightly lower than those resulting from the compliance test, the calculated leachate percentages, shown in
Figure S2 of the
Supporting Information section, were even lower in this case.
3.4. Assessment of the Results of the Monolith Leaching Tests
3.4.1. Identification of the Main Release Mechanisms
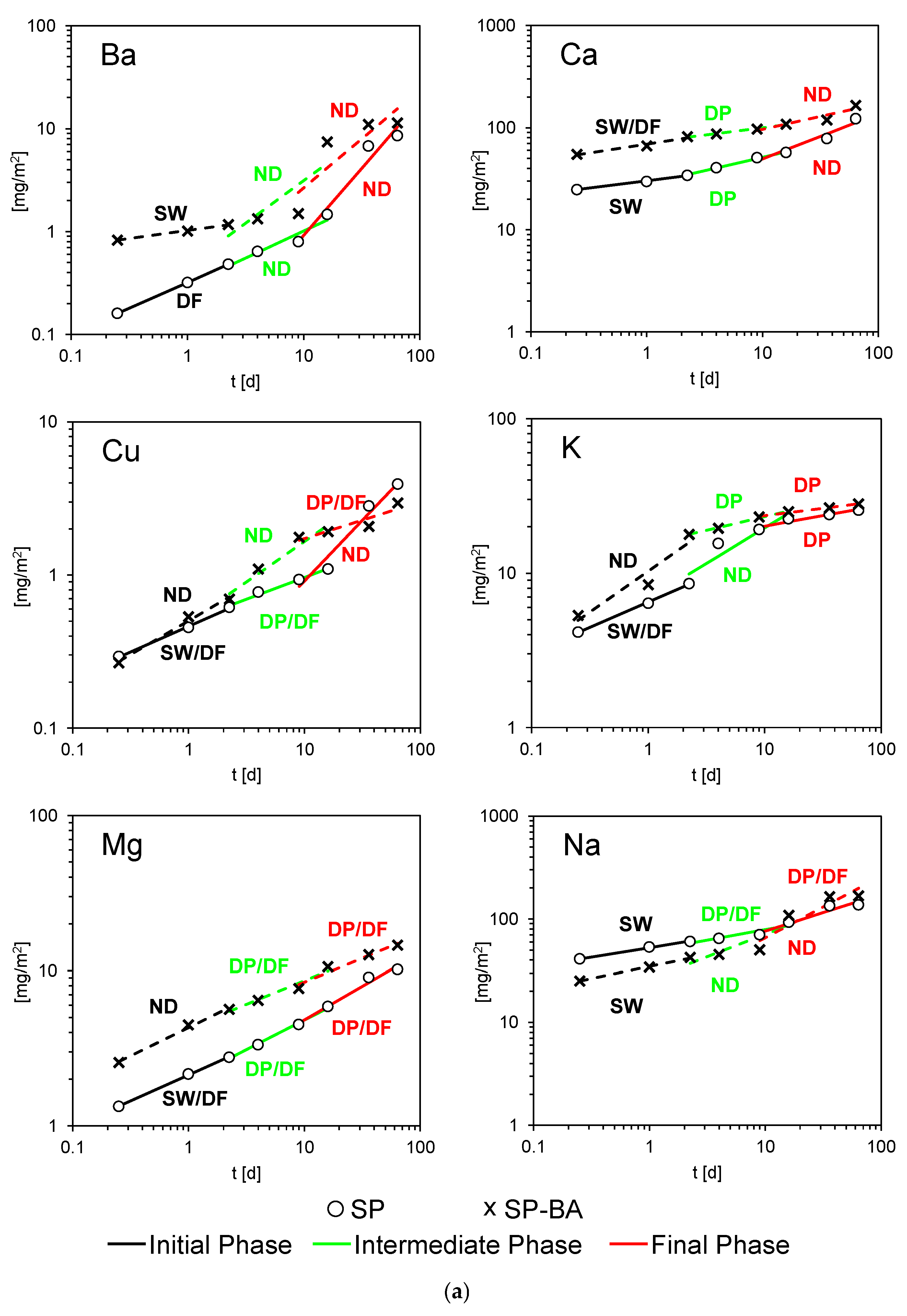
Figure 6a,b reports the release mechanisms resulting for 10 constituents for both the traditional and BA-containing tiles, following the methodology described in
Section 2.5.1. Constituents that consistently exhibited concentrations below the respective LOQs, or those for which detectable concentrations were only found in one or two leaching intervals, were excluded from the analysis.
The graphs reported in the previously mentioned figures focus on the three temporal phases of leaching (initial, intermediate, and final) and show the evolution over time in a log–log scale of the average cumulative release resulting from the three replicate samples. When considering the cumulative release values, a trend can be noted: tiles incorporating bottom ash tended to release higher amounts over time of several elements, such as Ba, Ca, Cu, K, Mg, Zn, and . For other elements like chlorides and Si, the cumulative release appeared very similar between the two materials. Only Na showed a lower release from BA-containing tiles, possibly indicating a stronger retention or incorporation within less soluble phases. As for the release mechanisms, as can be seen, for all constituents, more than one type of mechanism prevailed depending on the leaching interval considered. Furthermore, differences in the main mechanisms governing release were highlighted for the tiles containing BA compared to those with the traditional formulation.
The release of constituents from a solid material typically occurs through four distinct mechanisms: surface wash-off, dissolution, diffusion, and depletion, depending on the characteristics of the constituent and of the material and on other parameters such as the liquid-to-solid ratio and pH.
Considering the 95% confidence interval (CI) analysis, which allows a more rigorous assessment of the uncertainty in mechanism assignment, clearly defined single mechanisms could be identified in only a few cases. Surface wash-off (SW), occurring exclusively in the initial phase, was clearly identified for Ca and Na in SP tiles, and for Ba and Na in BA-containing tiles, reflecting the immediate availability of these elements at the surface. Diffusion (DF) was observed as a single mechanism only for Ba in the initial phase for SP tiles. Depletion (DP), which occurs when the source of an element within the tile matrix becomes progressively exhausted, leading to a decrease in the release rate over time, was observed more frequently than other single mechanisms. In SP tiles, DP was clearly identified for Ca (intermediate), K (final), and Cl− (final). In BA-containing tiles, it occurred for Ca (intermediate), K (intermediate and final), Zn (intermediate and final), Cl− (final), and SO42− (intermediate and final). Overall, the DP mechanism was more pronounced for SP-BA tiles, particularly during the final phases of leaching, highlighting a stronger tendency for progressive exhaustion of certain elements in the tile matrix.
In many cases, the release behavior could not be clearly attributed to a single mechanism. When the CI overlapped two mechanisms (e.g., DP/DF), the dominant process was considered to be potentially combined. This situation often arose when the slope of the linear regression was very close to the thresholds defining mechanism boundaries (e.g., 0.35 or 0.65), so that even minor fluctuations in the leaching data could shift the apparent mechanism.
In other cases, the CI spanned three mechanisms, and the release mechanism was classified as not defined (ND). These ND cases indicate that the observed release may have been influenced by more than one process without a clearly dominant mechanism.
A particularly distinct behavior was observed for copper. In BA-containing tiles, copper was released more quickly during the early and intermediate stages, resulting in higher cumulative release concentrations than the SP tiles at corresponding time intervals. During these phases, the release could not be clearly attributed to a single mechanism, likely reflecting contributions from different mechanisms. In contrast, SP tiles showed a lower initial release of Cu, with surface wash-off and combined diffusion–depletion observed in the intermediate phase. However, in the final stage, the release from SP tiles increased, ultimately exceeding that of the SP-BA tiles. Overall, this pattern indicates that BA-containing tiles released more copper at the beginning of the leaching process, but over the long term, the total release became greater for traditional SP tiles.
A similar behavior, though less pronounced, was also observed for Ba, Ca, K, Na, Zn, and sulfates: SP-BA tiles showed a higher initial release and a slope that decreased over time, whereas SP tiles presented lower initial values but a higher release in the final stage. As time progressed, the release curves of the two tile types tended to converge, and in the case of copper, as already discussed, the release from traditional SP tiles eventually exceeded that of the SP-BA tiles.
In these cases, the influence on the release mechanisms of the presence of bottom ash in the tile matrix may be related to microstructural features (e.g., porosity and specific surface area), which were not investigated here and, to our knowledge, have not been reported in the literature, warranting focused attention in future research. Concerning the comparison of the results of this study with those reported in previous works, it should be considered that the approach that was employed has been widely applied in the literature to investigate the leaching behavior of various monolithic matrices incorporating waste materials. These include hazardous waste stabilized through cement-based solidification processes, such as municipal solid waste incineration fly ash, filter ashes, metal sludge, and filter cakes derived from wastewater treatment [
41]. Other examples involve bottom ash stabilized with Portland cement concrete and hot mix asphalt [
29], as well as concrete made with cement from cement kiln co-processing of hazardous waste [
40]. In these systems, diffusion is commonly identified as the predominant release mechanism throughout all leaching phases for many metals. In some cases, it has been observed that deviations from diffusion-controlled behavior may occur in the later stages of leaching, often as a result of changes in environmental conditions. In particular, shifts in pH can significantly affect the release of certain elements, indicating a transition in the mechanisms controlling leaching [
41]. In this study, the incorporation of bottom ash showed to reduce early surface availability and shift the leaching mechanism towards depletion. At the same time, the tiles containing BA showed to be characterized by a higher early cumulative release for certain elements such as Cu, due to more sustained diffusion in the early phases, contrasted by the delayed release behavior observed in the latter stages of leaching. For a comprehensive summary of all the release mechanisms, including the total cumulative release, the reader is referred to the table in the
Supporting Information section (Table S6), which includes a detailed overview of the data.
3.4.2. Human Health Risk Assessment
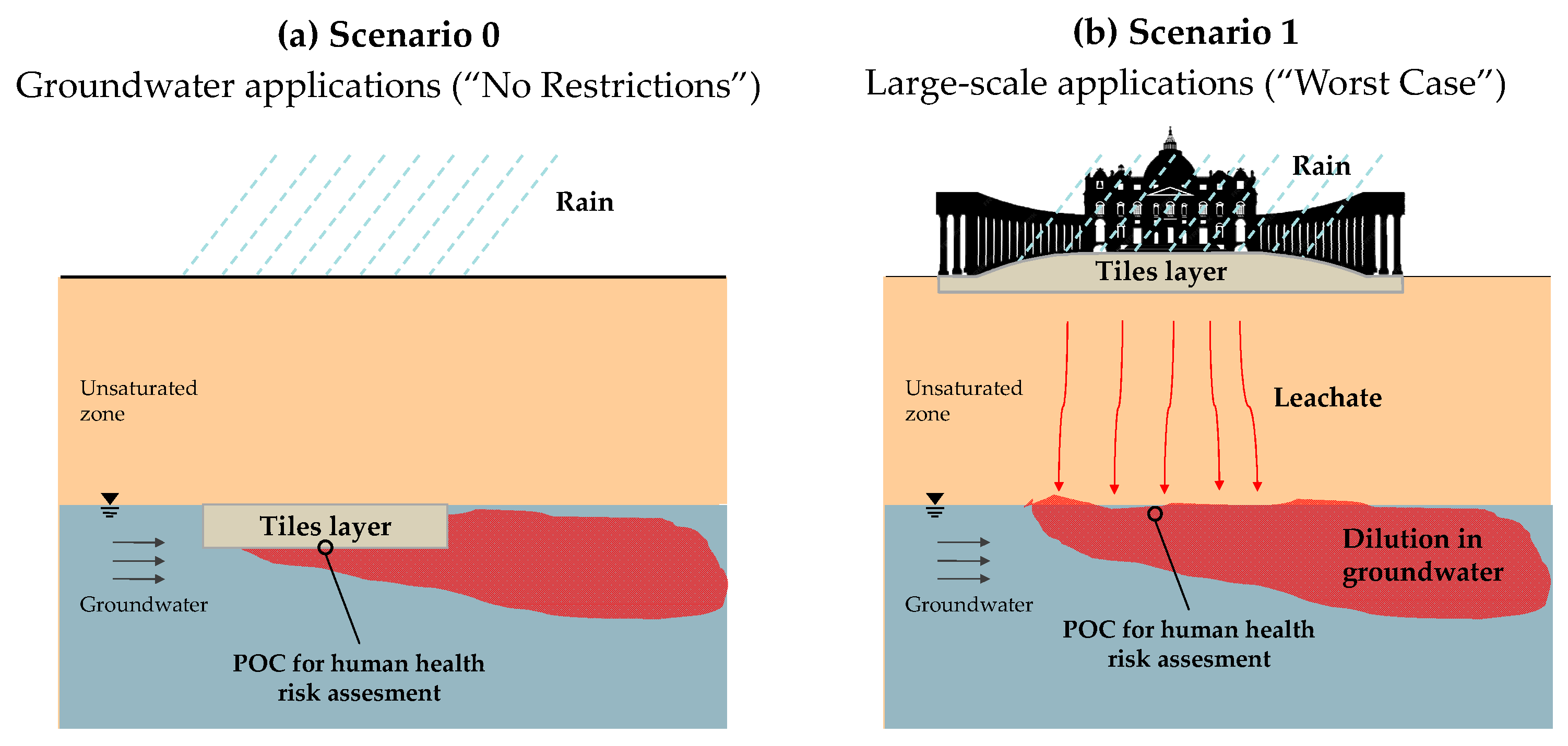
The results obtained from the monolith leaching tests were employed to assess the potential impact on water resources associated with the use of the tiles. This evaluation was carried out through a risk assessment analysis, considering the two utilization scenarios described in section “Utilization Scenarios Considered for the Risk Assessment”. The first scenario (Scenario 0) involves the use of the porcelain stoneware tiles in direct contact with the groundwater, while the second one (Scenario 1) involves using the material for paving a large outdoor area. As mentioned, the size of St. Peter’s Square in Vatican City was considered. To perform the risk assessment, the maximum concentrations obtained from the eight leaching intervals from the monolith tests for each target contaminant (
Supporting Information, Table S4) were used as the starting point. This approach ensures a precautionary evaluation by considering the highest concentrations of leached substances measured, which serve as the basis for estimating the potential impact on groundwater quality. By considering the leaching factor (see section “Human Health Risk Calculation”) resulting for each scenario, the Predicted Environmental Concentrations (PEC) in the groundwater were determined.
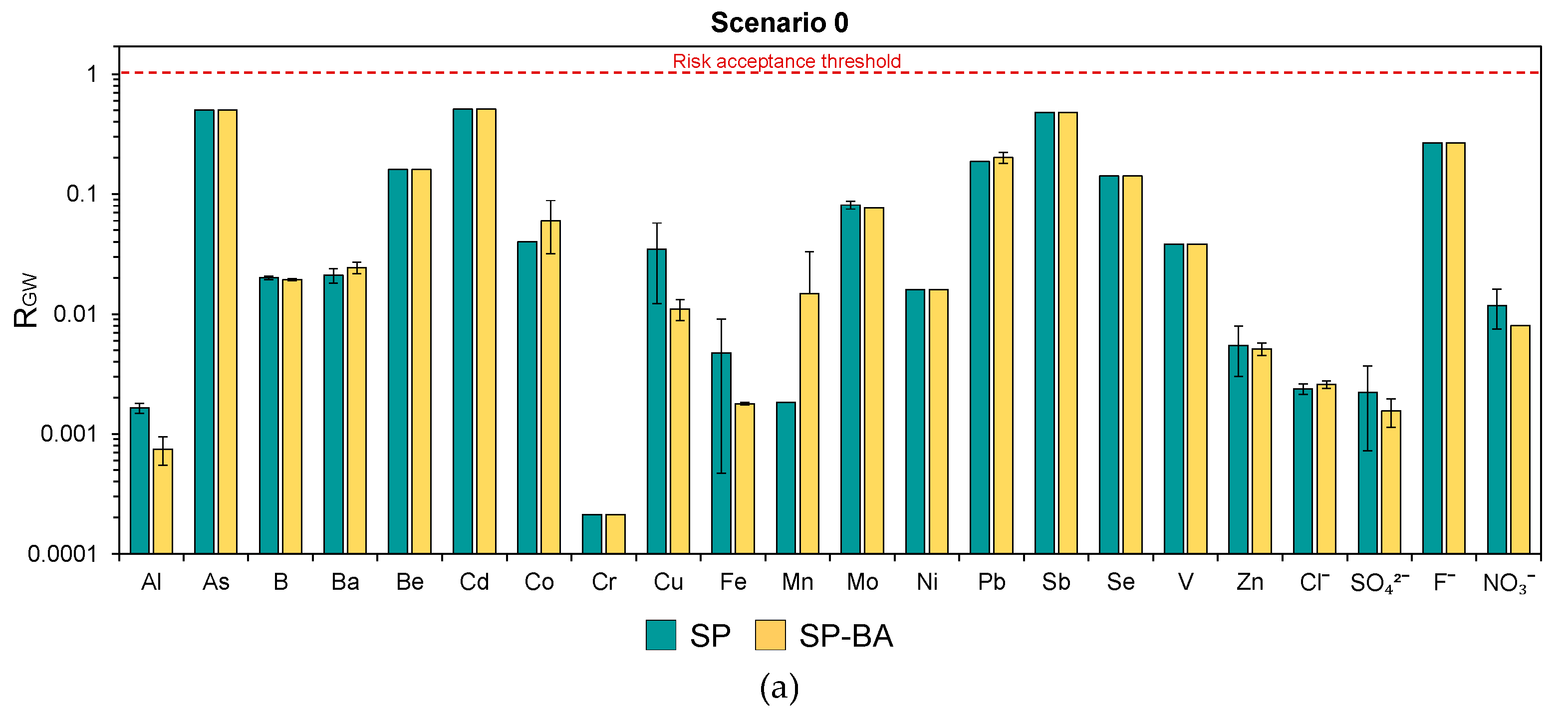
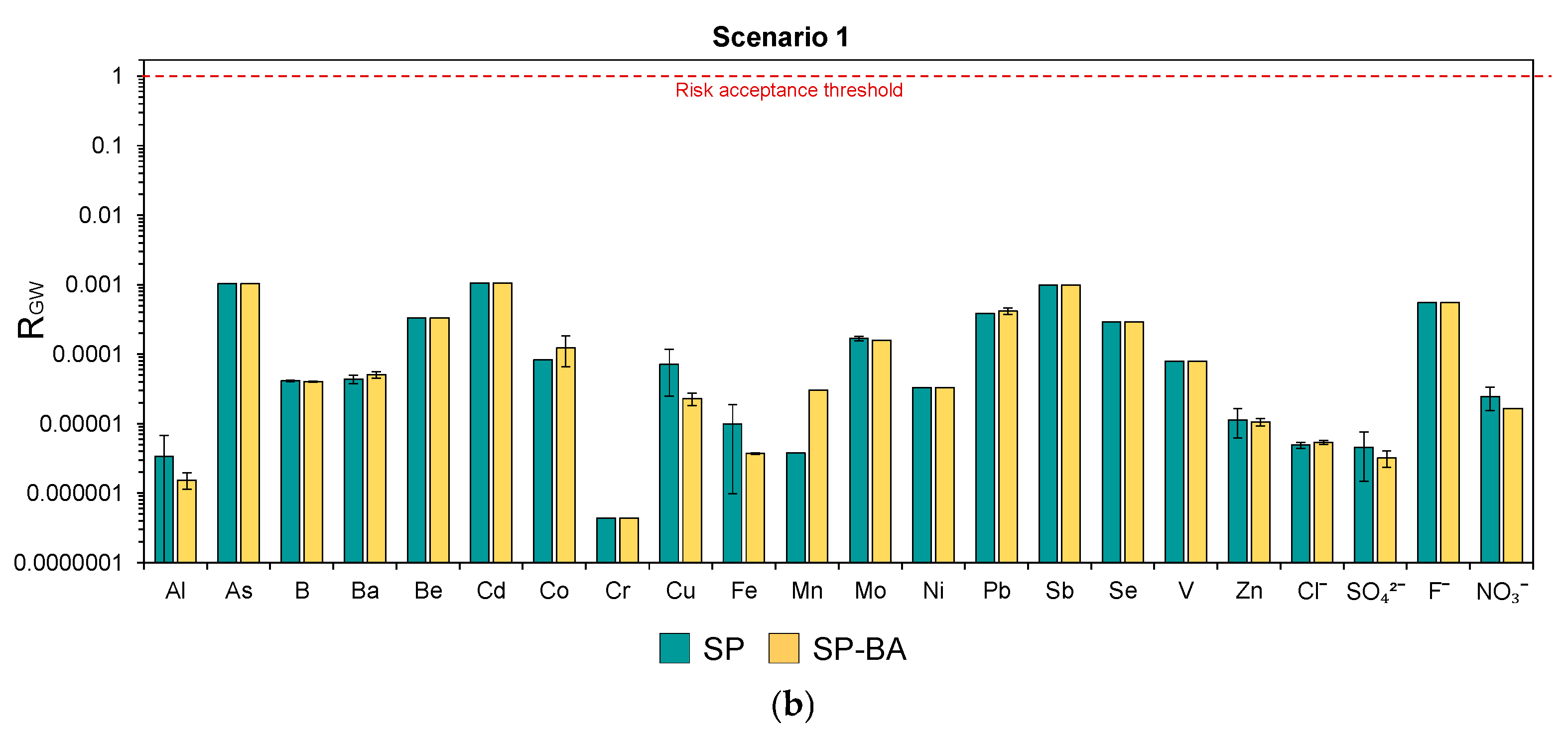
In
Figure 7a,b, the risks for the groundwater (R
GW) resulting from each constituent are reported for the two scenarios. These were calculated as the ratios between the PEC and the PNEC values for each relevant constituent. For more details on these parameters and their determination, refer to section “Human Health Risk Calculation”. For the constituents whose maximum leaching concentration from the monolith test was below the respective LOQ, the risk was estimated by assuming the concentration to be equal to the LOQ value.
The results show that, in both scenarios and for both types of tiles, the calculated risks remained consistently below the acceptability threshold of 1 (i.e., PEC < PNEC). This suggests that the leached concentrations would not pose a significant concern to groundwater quality under any of the considered conditions. In addition, the SP-BA formulation showed risk levels comparable to those resulting for the traditional SP tiles. This confirms that the addition of bottom ash would not increase the environmental risk and can be considered safe from the perspective of its leaching behavior during use and also in terms of its long-term impacts on groundwater.
Notably, even in Scenario 0 (an extreme, unrealistic case for which higher risks are expected due to direct contact with the groundwater and the absence of natural attenuation processes), the risk for all elements remained below the acceptable limit. In some cases, the difference was of several orders of magnitude (e.g., Al, Cr, Ni, Zn, chlorides, and sulfates). This result is particularly relevant, as the absence of a significant risk even under these unrealistic assumptions demonstrates a strong safety margin, supporting the feasibility of the use of the BA-based tiles without restrictions. Hence, in this case, the product could be considered as a viable End-of-Waste product suitable for free use from an environmental perspective.
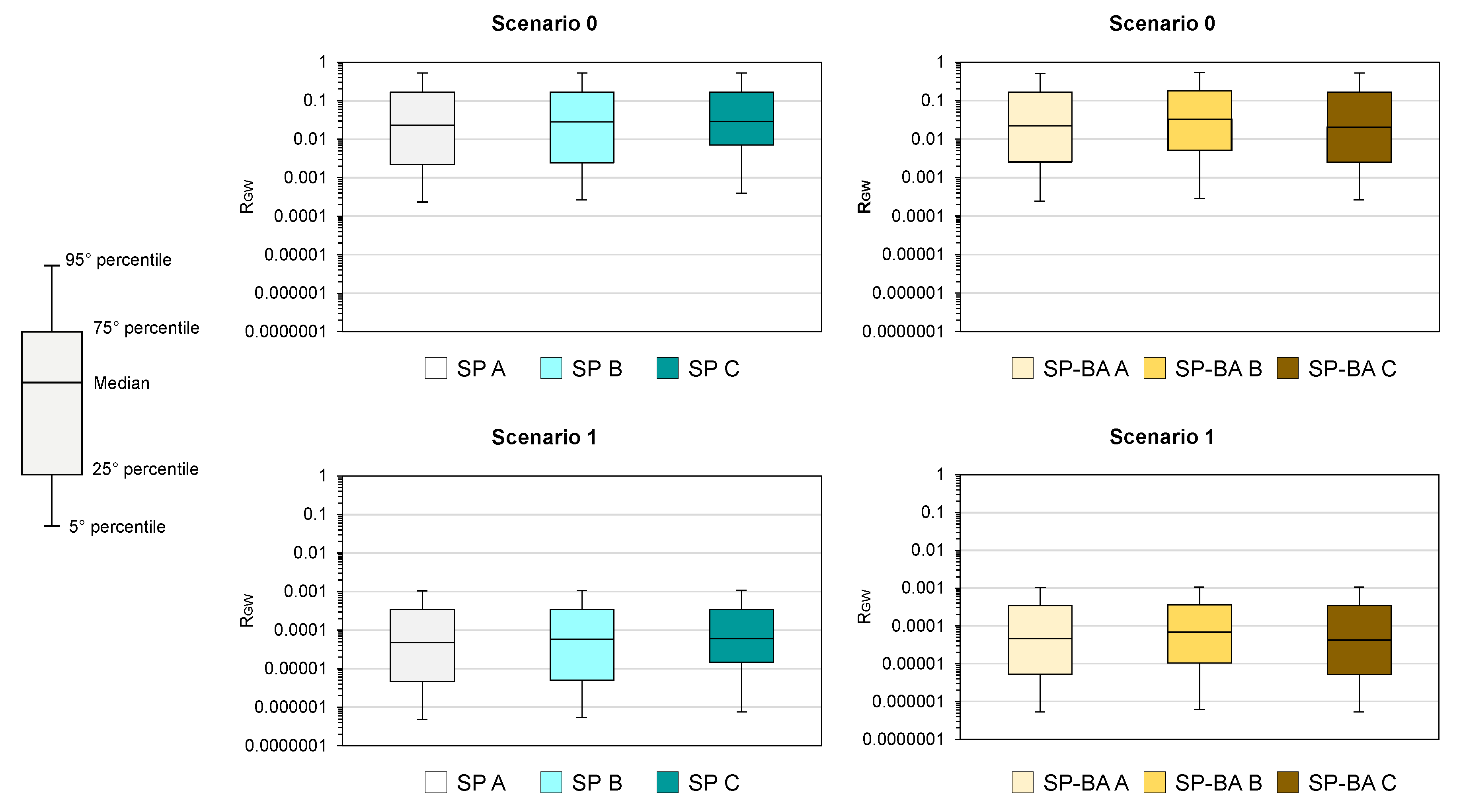
Additionally, a statistical analysis was conducted to globally compare the risk levels obtained for the SP and SP-BA samples, considering all of the involved analytes. This comparison was made by determining the 5th, 25th, 50th, 75th, and 95th percentiles of all the calculated risks for the water resource. The results are illustrated in
Figure 8. To carry out this analysis, a box plot was created, offering an immediate visual summary of the dispersion of the values in the dataset.
The statistical analysis showed that the calculated risks were very similar among the three replicates in both scenarios. Specifically, referring to Scenario 0, it can be seen that 50% of the data showed a risk lower by one to two orders of magnitude than the acceptance threshold, both for the traditional samples (SP) and for the samples containing incineration residues (SP-BA). The results suggest that there would be no significant differences in terms of risks posed by both types of porcelain stoneware tiles, indicating that the tested materials were comparable in terms of their environmental impact and safety for use. For Scenario 1, 50% of the data resulted within four to five orders of magnitude below the limit for both materials. Furthermore, the 95th percentile consistently yielded values at least three orders of magnitude below the risk threshold of 1.
The overall distribution of the risk values indicates a low level of risk, with only a very small fraction of the data approaching the threshold for both types of samples. This suggests that the addition of BA in the tile samples did not significantly affect the leaching behavior of the material and hence did not lead to an increased human health risk related to its use in outdoor pavements.
4. Conclusions
This study aimed to evaluate the environmental viability of incorporating bottom ash (BA) as a raw material for porcelain stoneware tile manufacturing. The primary objective was to assess whether the addition of BA would negatively impact the chemical properties or the environmental behavior of the tiles. By comparing the properties of BA-based tiles to the traditional porcelain formulation, we sought to determine whether this waste material could serve as a sustainable alternative material in tile production while maintaining compliance with environmental standards. Furthermore, this work aimed to fill a gap in the existing literature by conducting monolith leaching tests on ceramic materials containing BA, an area that has been scarcely explored to date.
In the initial phase, we focused on characterizing both materials through elemental composition analysis to determine whether the addition of 30% BA by weight in the tile formulation would significantly alter their chemical properties of the product. This analysis demonstrated that the incorporation of BA did not result in substantial changes in the elemental content of the tiles.
Subsequently, a standardized batch compliance leaching test was performed on crushed tile samples to assess the material’s compliance with the Italian End-of-Waste (EoW) criteria for construction and demolition waste (Legislative Decree 27 September 2022). This test was performed to assess if at the end-of-life stage of the tiles they could be used as recycled aggregates in construction from an environmental compliance point of view. The results showed that the BA-containing ceramics met the EoW thresholds, indicating their suitability for use also in granular form.
Following these assessments, more detailed evaluations were carried out to assess potential risks during the use phase of the product. In particular, the monolith leaching test on intact tiles was conducted to evaluate their behavior under direct exposure conditions in scenarios such as the use for outdoor paving. The results were then integrated into a human health risk assessment methodology to quantify the potential impacts resulting under assumed exposure scenarios. The comparison between the two tile types also allowed us to identify differences in release mechanisms. Although a single mechanism was not always clearly identifiable, BA-containing tiles showed a reduced early surface availability, leading to a shift toward depletion-controlled leaching. These tiles also presented a higher early release of some elements, likely due to increased diffusion at the beginning of the leaching process. Despite these variations in release behavior, the contaminant concentrations measured in the leachate led to no significant differences in risk between the two types of tile formulations, which, under all the conditions assumed, remained below the risk acceptance threshold of 1. These findings provide robust evidence supporting the environmental compatibility and safety of the free use of the analyzed BA-based tiles.
In conclusion, the results of this study demonstrate that the integration of bottom ash from Waste-to-Energy processes into the porcelain stoneware tiles examined in this study represents an environmentally viable solution. The addition of BA showed not to negatively impact the chemical stability nor the environmental performance of the tiles. The results highlight the potential of BA to be employed as a secondary raw material in ceramics manufacturing, contributing to circular economy strategies and reducing the need for waste disposal.