Room Temperature Surfactant-Free Synthesis of Cobalt-Doped CaMoO4 Nanoparticles: Structural and Microstructural Insights
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanoparticles Preparation
2.2. Nanoparticles Characterization
3. Results and Discussion
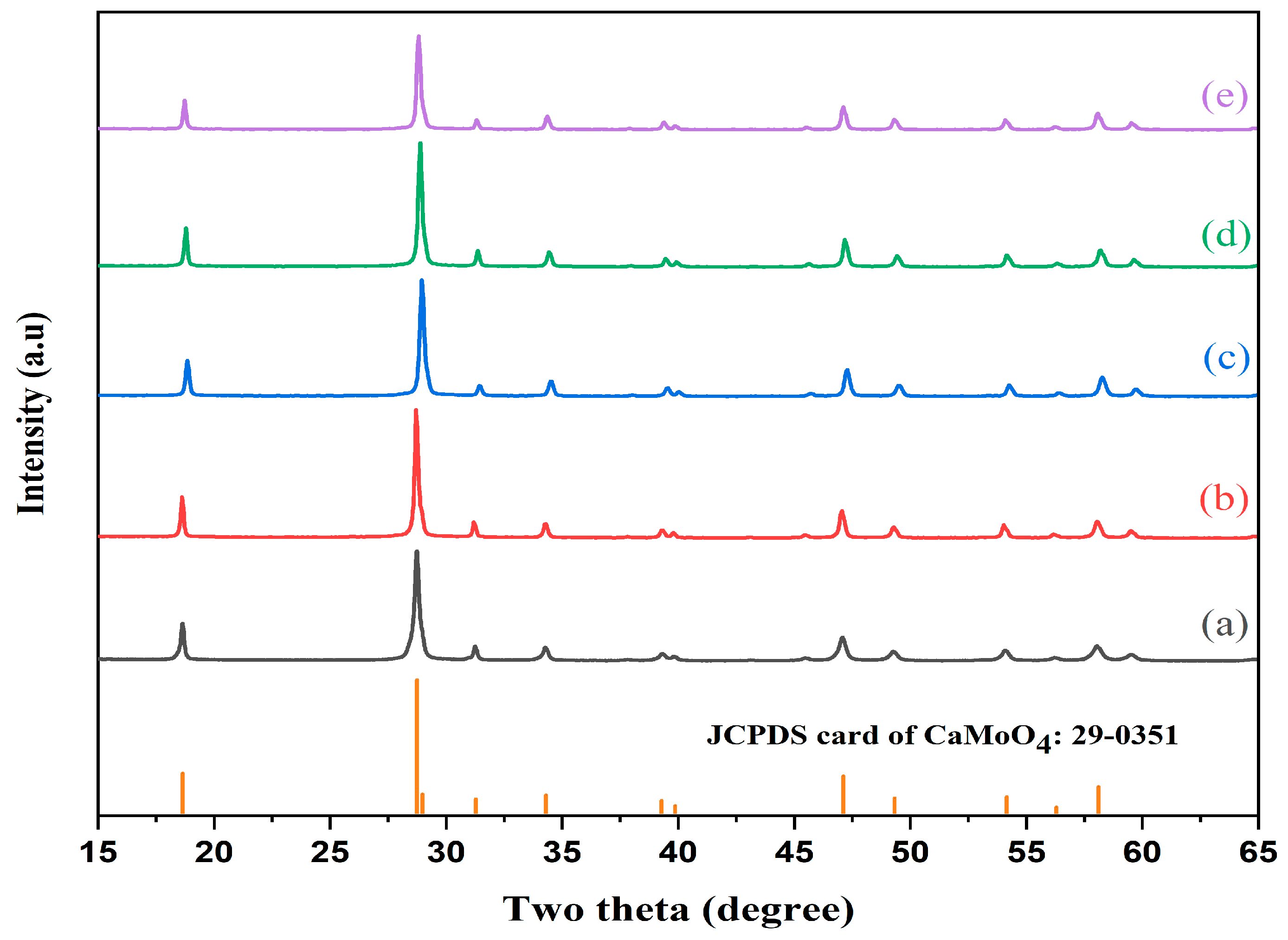
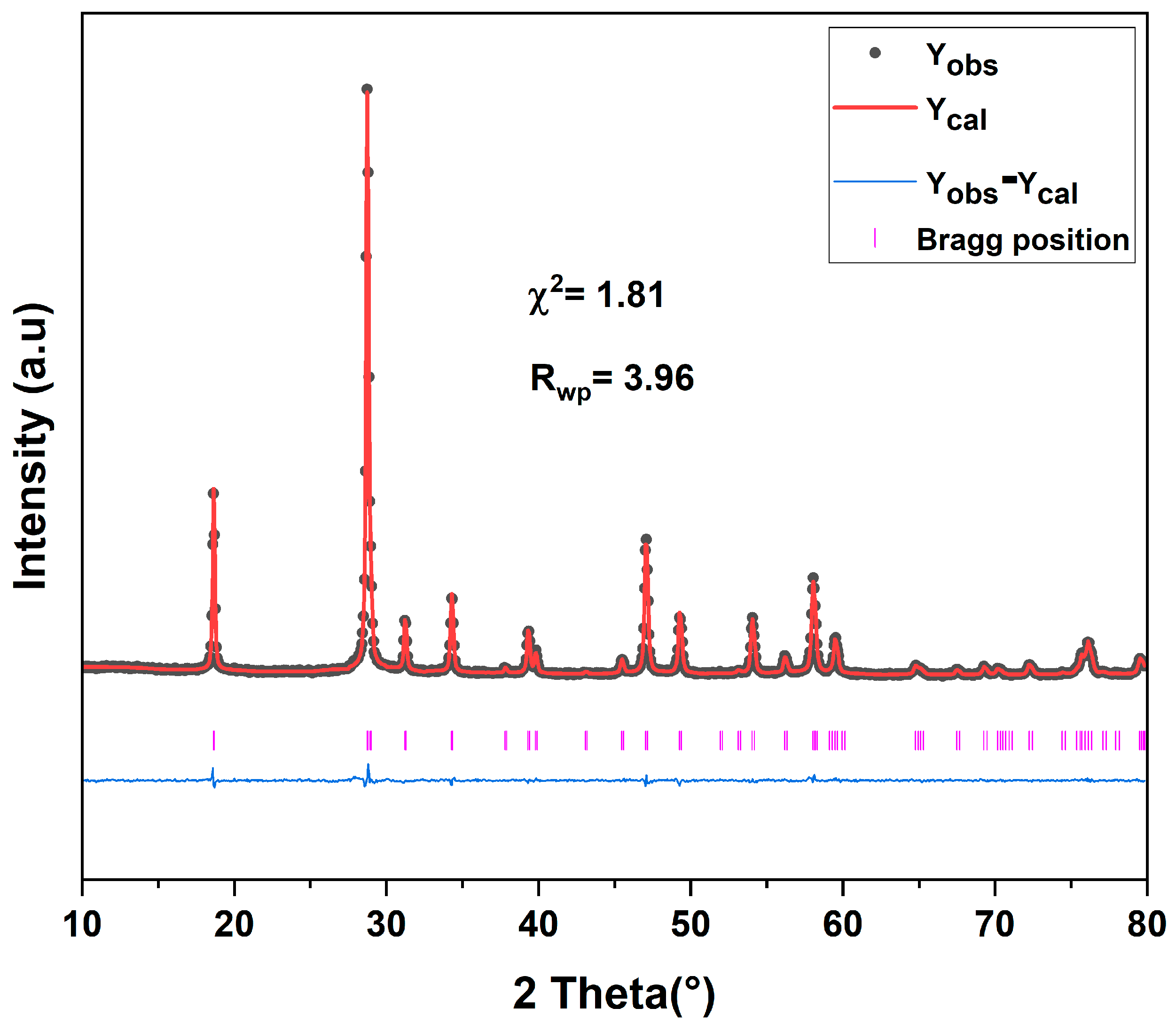
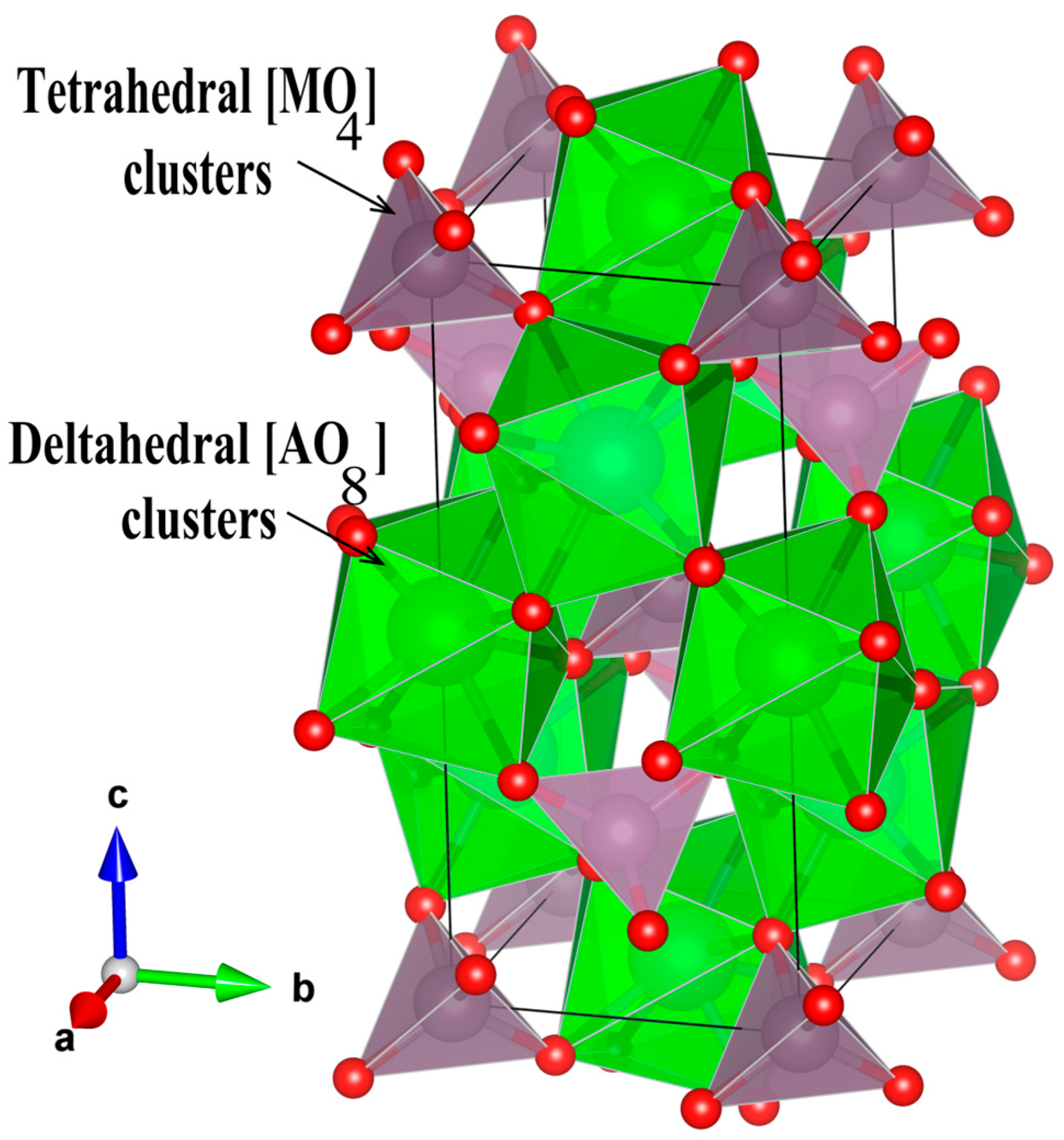
3.1. X-Ray Diffraction and Rietveld Refinement
3.2. Raman Spectroscopy
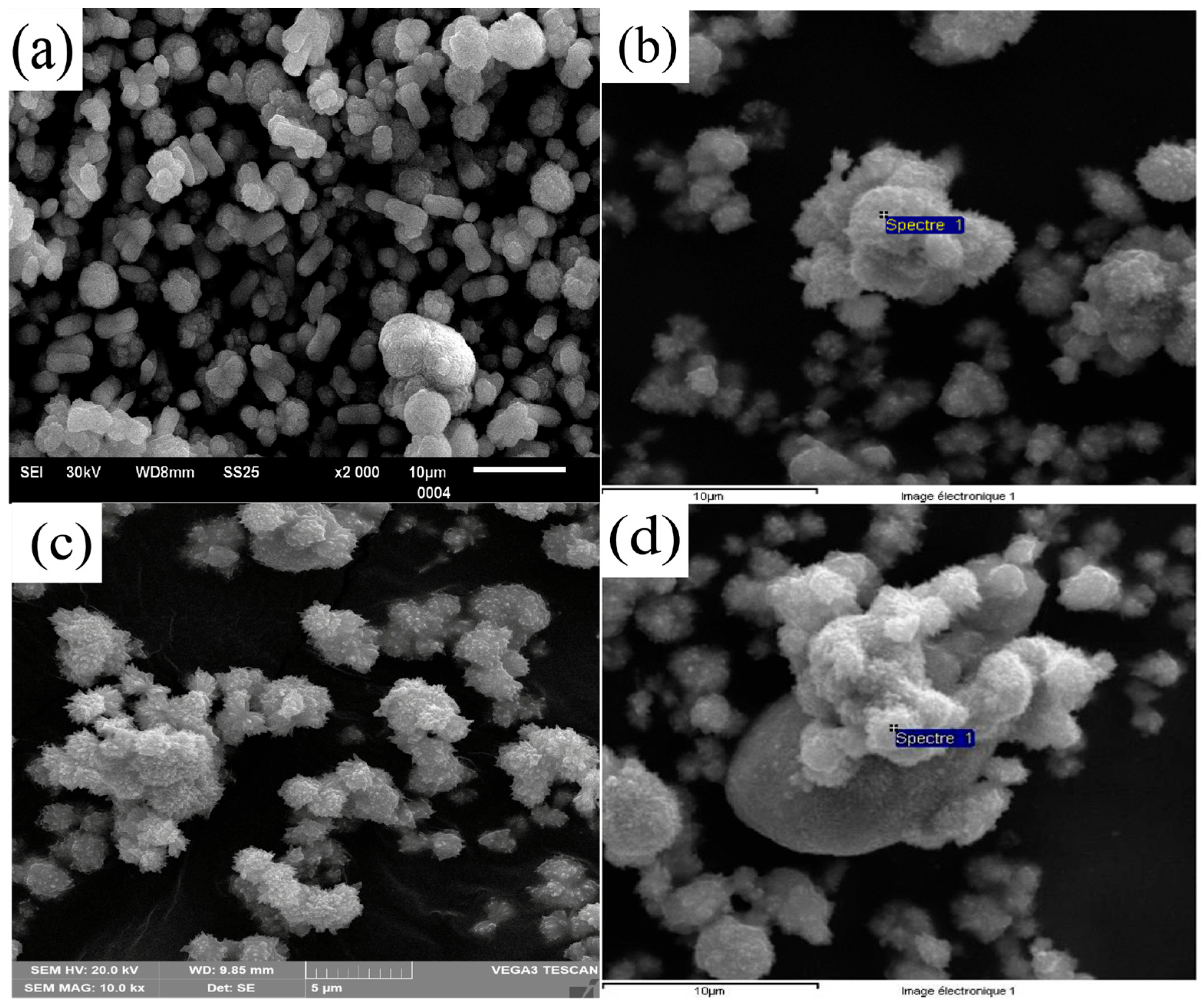
3.3. Scanning Electron Microscopy
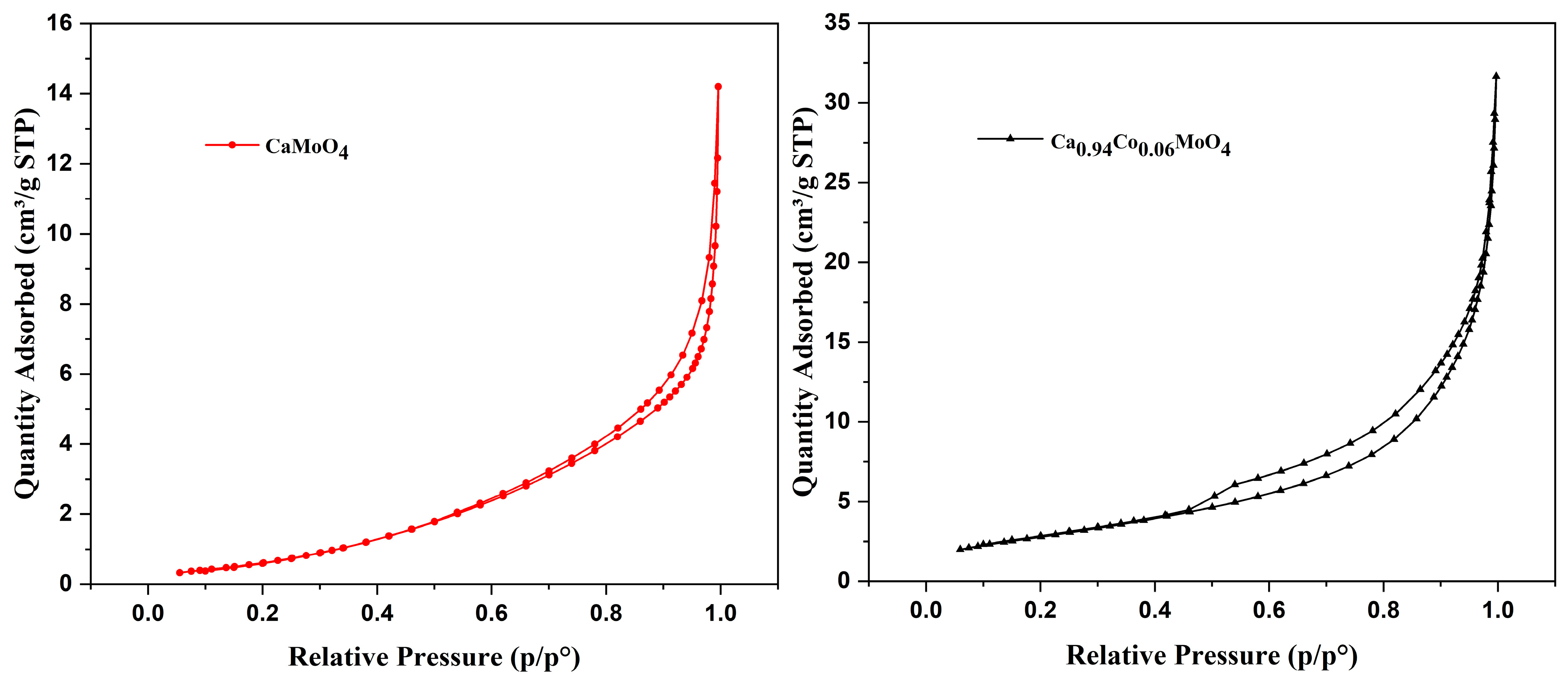
3.4. N2 Adsorption–Desorption Isotherm
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sawant, D.S.; Kulkarni, S.B.; Dubal, D.P.; Lohar, G.M. Transition Metal Molybdates Emerging Materials for High-Performance Supercapacitors: A Machine Learning Analysis. Battery Energy 2025, 4, e20240073. [Google Scholar] [CrossRef]
- Ge, X.; Zhu, Y.; Cao, Z.; Jia, J.; Zhao, Q.; Chang, S.; Liu, S.; Yang, X.; Feng, K. High-Specific-Capacity Molybdate Anode Materials for Lithium-Ion Batteries with Good Low-Temperature Performance. J. Alloys Compd. 2022, 903, 163914. [Google Scholar] [CrossRef]
- Kaminskii, A.A.; Bagaev, S.N.; Ueda, K.; Takaichi, K.; Eichler, H.J. High-Order Picosecond SRS and Self-SRS Generation in Nd3+-Doped CaMoO4, SrMoO4, and SrWO4 Laser Crystals. Crystallogr. Rep. 2002, 47, 653–657. [Google Scholar] [CrossRef]
- Yi, S.-S.; Jung, J.-Y. Barium Molybdate White Emitting Phosphor Synthesized at Room Temperature by Co-Precipitation. RSC Adv. 2022, 12, 21827–21835. [Google Scholar] [CrossRef]
- Mikhailik, V.B.; Elyashevskyi, Y.; Kraus, H.; Kim, H.J.; Kapustianyk, V.; Panasyuk, M. Temperature Dependence of Scintillation Properties of SrMoO4. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrometers Detect. Assoc. Equip. 2015, 792, 1–5. [Google Scholar] [CrossRef]
- Edwin Suresh Raj, A.M.; Mallika, C.; Swaminathan, K.; Sreedharan, O.M.; Nagaraja, K.S. Zinc(II) Oxide-Zinc(II) Molybdate Composite Humidity Sensor. Sens. Actuators B Chem. 2002, 81, 229–236. [Google Scholar] [CrossRef]
- Choi, G.-K.; Kim, J.-R.; Yoon, S.H.; Hong, K.S. Microwave Dielectric Properties of Scheelite (A=Ca, Sr, Ba) and Wolframite (A=Mg, Zn, Mn) AMoO4 Compounds. J. Eur. Ceram. Soc. 2007, 27, 3063–3067. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Rodriguez, J.A.; Hanson, J.C.; Albornoz, A.; Brito, J.L. Properties of Pure and Sulfided NiMoO4 and CoMoO4 Catalysts: Tpr, Xanes and Time-Resolved XRD Studdzs. MRS Online Proc. Libr. 1997, 497, 41–46. [Google Scholar] [CrossRef]
- Huerta-Flores, A.M.; Juárez-Ramírez, I.; Torres-Martínez, L.M.; Carrera-Crespo, J.E.; Gómez-Bustamante, T.; Sarabia-Ramos, O. Synthesis of AMoO4 (A = Ca, Sr, Ba) Photocatalysts and Their Potential Application for Hydrogen Evolution and the Degradation of Tetracycline in Water. J. Photochem. Photobiol. Chem. 2018, 356, 29–37. [Google Scholar] [CrossRef]
- Sleight, A.W. Accurate Cell Dimensions for ABO4 Molybdates and Tungstates. Acta Crystallogr. B 1972, 28, 2899–2902. [Google Scholar] [CrossRef]
- Errandonea, D.; Manjón, F.J. Pressure Effects on the Structural and Electronic Properties of ABX4 Scintillating Crystals. Prog. Mater. Sci. 2008, 53, 711–773. [Google Scholar] [CrossRef]
- Wu, W.-T.; Tiong, K.-K.; Lee, Y.-W.; Hu, S.-Y.; Lee, Y.-C.; Huang, W. Tunable Luminescence of Sm3+/Tb3+ Co-Doped CaMoO4 Phosphors Synthesized by Microwave-Assisted Heating. Appl. Sci. 2022, 12, 7883. [Google Scholar] [CrossRef]
- Dixit, P.; Chauhan, V.; Kumar, P.; Pandey, P.C. Enhanced Photoluminescence in CaMoO4:Eu3+ by Mn2+ Co-Doping. J. Lumin. 2020, 223, 117240. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Thongtem, S.; Thongtem, T. Effect of Ce Dopant on Photocatalytic Properties of CaMoO4 Nanoparticles Prepared by Microwave-Assisted Method. Mater. Res. Innov. 2022, 26, 84–90. [Google Scholar] [CrossRef]
- Jeseentharani, V.; Jeyaraj, B.; Dayalan, A.; Nagaraja, K.S. Fabrication and Characterisation of Nanocrystalline Composites of Ca1−xCoxMoO4 (x = 0, 0.3, 0.5, 0.7, 1) Prepared by Co-Precipitation Method as a Humidity Sensor. J. Mater. Sci. Mater. Electron. 2017, 28, 3548–3559. [Google Scholar] [CrossRef]
- Gancheva, M.; Iordanova, R.; Koseva, I.; Avdeev, G.; Burdina, G.; Ivanov, P. Synthesis and Luminescent Properties of Barium Molybdate Nanoparticles. Materials 2023, 16, 7025. [Google Scholar] [CrossRef] [PubMed]
- Millner, T.; Neugebauer, J. Volatility of the Oxides of Tungsten and Molybdenum in the Presence of Water Vapour. Nature 1949, 163, 601–602. [Google Scholar] [CrossRef]
- Senguttuvan, N.; Babu, S.M.; Subramanian, C. Synthesis, Crystal Growth and Mechanical Properties of Lead Molybdate. Mater. Sci. Eng. B 1997, 47, 269–273. [Google Scholar] [CrossRef]
- Chen, H.; Ge, C.; Li, R.; Wang, J.; Wu, C.; Zeng, X. Growth of Lead Molybdate Crystals by Vertical Bridgman Method. Bull. Mater. Sci. 2005, 28, 555–560. [Google Scholar] [CrossRef]
- Arora, S.K.; Trivikrama Rao, G.S. Crystal Growth of Barium Molybdate by Flux Evaporation. Bull. Mater. Sci. 1983, 5, 381–387. [Google Scholar] [CrossRef]
- Benchikhi, M.; El Ouatib, R.; Guillemet-Fritsch, S.; Yves Chane-Ching, J.; Er-Rakho, L.; Durand, B. Sol–Gel Synthesis and Sintering of Submicronic Copper Molybdate (α-CuMoO4) Powders. Ceram. Int. 2014, 40, 5371–5377. [Google Scholar] [CrossRef][Green Version]
- Ding, Y.; Wan, Y.; Min, Y.-L.; Zhang, W.; Yu, S.-H. General Synthesis and Phase Control of Metal Molybdate Hydrates MMoO4·nH2O (M = Co, Ni, Mn, n = 0, 3/4, 1) Nano/Microcrystals by a Hydrothermal Approach: Magnetic, Photocatalytic, and Electrochemical Properties. Inorg. Chem. 2008, 47, 7813–7823. [Google Scholar] [CrossRef]
- Rani, B.J.; Swathi, S.; Yuvakkumar, R.; Ravi, G.; Kumar, P.; Babu, E.S.; Alfarraj, S.; Alharbi, S.A.; Velauthapillai, D. Solvothermal Synthesis of CoMoO4 Nanostructures for Electrochemical Applications. J. Mater. Sci. Mater. Electron. 2021, 32, 5989–6000. [Google Scholar] [CrossRef]
- Sakthivel, S.; Shobika, S.; Dinesh, A.; Yogalakshmi, K.; Suriyaprakash, R.; Kabilan, B.; Sathyajith, P.; Elumalai, S.; Gnanasekaran, L.; Ayyar, M.; et al. Comparative Study of Synthesis Methods and Electrochemical Performance of Nickel Molybdate (NiMoO4) Nanostructures for Supercapacitor Applications. Results Chem. 2025, 13, 101956. [Google Scholar] [CrossRef]
- Afanasiev, P. Molten Salt Synthesis of Barium Molybdate and Tungstate Microcrystals. Mater. Lett. 2007, 61, 4622–4626. [Google Scholar] [CrossRef]
- Masteri-Farahani, M.; Mahdavi, S.; Rafizadeh, M. Microemulsion-Mediated Synthesis and Characterization of Monodispersed Nickel Molybdate Nanocrystals. Ceram. Int. 2013, 39, 4619–4625. [Google Scholar] [CrossRef]
- Rullens, F.; Deligne, N.; Laschewsky, A.; Devillers, M. A Facile Precursor Route to Transition Metal Molybdates Using a Polyzwitterionic Matrix Bearing Simultaneously Charged Moieties and Complexing Groups. J. Mater. Chem. 2005, 15, 1668. [Google Scholar] [CrossRef]
- Yoshimura, M.; Ohmura, M.; Cho, W.; Yashima, M.; Kakihana, M. Preparation of Crystallized Sr1−XCaXMoO4 Solid-Solution Films by an Electrochemical Method at Room Temperature and Their Luminescence. J. Am. Ceram. Soc. 1997, 80, 2464–2466. [Google Scholar] [CrossRef]
- Kianpour, G.; Salavati-Niasari, M.; Emadi, H. Sonochemical Synthesis and Characterization of NiMoO4 Nanorods. Ultrason. Sonochem. 2013, 20, 418–424. [Google Scholar] [CrossRef]
- Barmi, M.J.; Sundaram, M.M. Role of Polymeric Surfactant in the Synthesis of Cobalt Molybdate Nanospheres for Hybrid Capacitor Applications. RSC Adv. 2016, 6, 36152–36162. [Google Scholar] [CrossRef]
- Harichandran, G.; Radha, S.; Yesuraj, J.; Muthuraaman, B. Synthesis and Characterization of Cobalt Molybdate Dihydrate Nanorods Arrays for Supercapacitor Electrode Application. Appl. Phys. A 2021, 127, 627. [Google Scholar] [CrossRef]
- Longo, V.M.; Cavalcante, L.S.; Paris, E.C.; Sczancoski, J.C.; Pizani, P.S.; Li, M.S.; Andrés, J.; Longo, E.; Varela, J.A. Hierarchical Assembly of CaMoO4 Nano-Octahedrons and Their Photoluminescence Properties. J. Phys. Chem. C 2011, 115, 5207–5219. [Google Scholar] [CrossRef]
- Pourbaix, M. Electrochemical Equilibria of Molybdenum, 1st ed.; Pergamon Press: Oxford, UK, 1966; pp. 273–279. [Google Scholar]
- Rietveld, H.M. The Crystal Structure of Some Alkaline Earth Metal Uranates of the Type M3 UO6. Acta Crystallogr. 1966, 20, 508–513. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Abstracts of the Satellite Meeting on Powder Diffraction. In Proceedings of the XV Congress of the IUCr, Toulouse, France, 19–28 July 1990; p. 127. [Google Scholar]
- Regmi, C.; Kshetri, Y.K.; Kim, T.-H.; Pandey, R.P.; Lee, S.W. Visible-Light-Induced Fe-Doped BiVO4 Photocatalyst for Contaminated Water Treatment. Mol. Catal. 2017, 432, 220–231. [Google Scholar] [CrossRef]
- Neto, N.F.A.; Dias, B.P.; Tranquilin, R.L.; Longo, E.; Li, M.; Bomio, M.R.D.; Motta, F.V. Synthesis and Characterization of Ag+ and Zn2+ Co-Doped CaWO4 Nanoparticles by a Fast and Facile Sonochemical Method. J. Alloys Compd. 2020, 823, 153617. [Google Scholar] [CrossRef]
- Singh, N.; Kumar, U.; Jatav, N.; Sinha, I. Photocatalytic Degradation of Crystal Violet on Cu, Zn Doped BiVO4 Particles. Langmuir ACS J. Surf. Colloids 2024, 40, 8450–8462. [Google Scholar] [CrossRef]
- Hume-Rothery, W.; Raynor, G.V., XI. Atomic and Ionic Radii.—II. Application to the Theory of Solid Solubility in Alloys. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1938, 26, 143–152. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Skibiński, T.; Kaczmarek, S.M.; Leniec, G.; Tsuboi, T.; Nakai, Y.; Berkowski, M.; Kowalski, Z.; Huang, W. Propriétés Magnétiques et Optiques Des Monocristaux de PbMoO4 Co-Dopés. J. Cryst. Growth 2014, 401, 802–806. [Google Scholar] [CrossRef]
- Roy, K.S.; Subramaniam, C.; Panchakarla, L.S. Non-Stoichiometry Induced Exsolution of Metal Oxide Nanoparticles via Formation of Wavy Surfaces and Their Enhanced Electrocatalytic Activity: Case of Misfit Calcium Cobalt Oxide. ACS Appl. Mater. Interfaces 2021, 13, 9897–9907. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-Ray Diffraction Procedures for Polycrystalline and Amorphous Materials, 1st ed.; Wiley: New York, NY, USA, 1954; pp. 245–255. [Google Scholar]
- Basiev, T.T.; Sobol, A.A.; Voronko, Y.K.; Zverev, P.G. Spontaneous Raman Spectroscopy of Tungstate and Molybdate Crystals for Raman Lasers. Opt. Mater. 2000, 15, 205–216. [Google Scholar] [CrossRef]
- Porto, S.P.S.; Scott, J.F. Raman Spectra of CaWO4, SrWO4, CaMoO4, and SrMoO4. Phys. Rev. 1967, 157, 716–719. [Google Scholar] [CrossRef]
- Khanna, R.K.; Brower, W.S.; Guscott, B.R.; Lippincott, E.R. Laser Induced Raman Spectra of Some Tungstates and Molybdates. J. Res. Natl. Bur. Stand. Sect. Phys. Chem. 1968, 72A, 81. [Google Scholar] [CrossRef]
- Zhao, Z.; Sui, Z.; Wei, X.; Zuo, J.; Zhang, X.; Dai, R.; Zhang, Z.; Ding, Z. Structure Transformation and Remarkable Site-Distribution Modulation of Eu3+ Ions in CaMoO4:Eu3+ Nanocrystals under High Pressure. CrystEngComm 2015, 17, 7905–7914. [Google Scholar] [CrossRef]
- Guan, X.; Ren, Y.; Chen, S.; Yan, J.; Wang, G.; Zhao, H.; Zhao, W.; Zhang, Z.; Deng, Z.; Zhang, Y.; et al. Charge Separation and Strong Adsorption-Enhanced MoO3 Visible Light Photocatalytic Performance. J. Mater. Sci. 2020, 55, 5808–5822. [Google Scholar] [CrossRef]
- Schmid, T.; Dariz, P. Shedding Light onto the Spectra of Lime: Raman and Luminescence Bands of CaO, Ca(OH)2 and CaCO3. J. Raman Spectrosc. 2015, 46, 141–146. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Thongtem, T.; Thongtem, S. Barium Molybdate and Barium Tungstate Nanocrystals Synthesized by a Cyclic Microwave Irradiation. J. Phys. Chem. Solids 2009, 70, 955–959. [Google Scholar] [CrossRef]
- Elorika, P.; Anwar, S.; Anwar, S. Impact of Synthesis-Induced Disorder on the Structural, Electrical, and Optical Properties of BaTi1−xHfxO3, 0 ≤ x ≤ 0.08. Mater. Res. Bull. 2023, 167, 112424. [Google Scholar] [CrossRef]
- Aafiya; Abushad, M.; Arshad, M.; Naseem, S.; Ahmed, H.; Ansari, A.; Chakradhary, V.K.; Husain, S.; Khan, W. Synthesis and Role of Structural Disorder on the Optical, Magnetic and Dielectric Properties of Zn Doped NiFe2O4 Nanoferrites. J. Mol. Struct. 2022, 1253, 132205. [Google Scholar] [CrossRef]
- Bersani, D.; Lottici, P.P.; Ding, X.-Z. Phonon Confinement Effects in the Raman Scattering by TiO2 Nanocrystals. Appl. Phys. Lett. 1998, 72, 73–75. [Google Scholar] [CrossRef]
- Korepanov, V.I.; Chan, S.-Y.; Hsu, H.-C.; Hamaguchi, H. Phonon Confinement and Size Effect in Raman Spectra of ZnO Nanoparticles. Heliyon 2019, 5, e01222. [Google Scholar] [CrossRef]
- Kumar, V.; Saxena, S.K.; Kaushik, V.; Saxena, K.; Shukla, A.K.; Kumar, R. Silicon Nanowires Prepared by Metal Induced Etching (MIE): Good Field Emitters. RSC Adv. 2014, 4, 57799–57803. [Google Scholar] [CrossRef]
- Im, H.S.; Park, K.; Kim, J.; Kim, D.; Lee, J.; Lee, J.A.; Park, J.; Ahn, J.-P. Strain Mapping and Raman Spectroscopy of Bent GaP and GaAs Nanowires. ACS Omega 2018, 3, 3129–3135. [Google Scholar] [CrossRef]
- Xingchen, Y.; Zhitian, F.; Rui, N.; Long, Z.; Haiyan, L. A Facile Synthesis of Flower-like NiCo-LDH for High Specific Capacitance Pseudosupercapacitor Positive Materials | Request PDF. J. Mater. Sci. 2024, 59, 4225–4235. [Google Scholar] [CrossRef]
- Gao, X.; Zu, W.; Wu, B.; Dong, H.; Shi, W.; Wang, Y. Effects of Transition Metal Doping on Surface Properties and Resistance to Cl− Adsorption of α-Al2O3. Surf. Sci. 2021, 713, 121909. [Google Scholar] [CrossRef]
- Raizada, P.; Soni, V.; Kumar, A.; Singh, P.; Parwaz Khan, A.A.; Asiri, A.M.; Thakur, V.K.; Nguyen, V.-H. Surface Defect Engineering of Metal Oxides Photocatalyst for Energy Application and Water Treatment. J. Mater. 2021, 7, 388–418. [Google Scholar] [CrossRef]
- Tu, G.; Wang, H.; Tu, B.; Xu, P.; Ren, L.; Huang, Q.; Yang, R.; Wang, W.; Fu, Z. Achieving Near-Theoretical Transmittance in MgAl2O4 Ceramic at Reduced Sintering Temperature via Solution Substitution. J. Eur. Ceram. Soc. 2025, 45, 117382. [Google Scholar] [CrossRef]
- Perro, A.; Reculusa, S.; Ravaine, S.; Bourgeat-Lami, E.; Duguet, E. Design and synthesis of Janus micro- and nanoparticles. J. Mater. Chem. 2005, 15, 3745–3760. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, X.; Wu, P.; Zhang, Y.; Liu, B.; Hu, H.; Xue, G. Divanadium-Substituted Phosphotungstate Supported on Magnetic Mesoporous Silica Nanoparticles as Effective and Recyclable Catalysts for the Selective Oxidation of Alcohols. ChemCatChem 2016, 8, 3680–3687. [Google Scholar] [CrossRef]

| Parameters | Compositions | ||||
|---|---|---|---|---|---|
| x = 0.0 | x = 0.02 | x = 0.04 | x = 0.06 | x = 0.08 | |
| Atomic positions | |||||
| Cation 4a (Mo) | |||||
| x | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| y | 0.2500 | 0.2500 | 0.2500 | 0.2500 | 0.2500 |
| z | 0.1250 | 0.1250 | 0.1250 | 0.1250 | 0.1250 |
| Cation 4b (Ca/Co) | |||||
| x | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| y | 0.2500 | 0.2500 | 0.2500 | 0.2500 | 0.2500 |
| z | 0.6250 | 0.6250 | 0.6250 | 0.6250 | 0.6250 |
| Anion 16f (O) | |||||
| x | 0.15236 | 0.15384 | 0.14910 | 0.14759 | 0.14850 |
| y | 0.49495 | 0.49670 | 0.49619 | 0.49583 | 0.50044 |
| z | 0.20830 | 0.20880 | 0.21084 | 0.20736 | 0.21109 |
| Lattice parameters | |||||
| a (Å) | 5.22166 | 5.22285 | 5.22714 | 5.22567 | 5.23307 |
| c (Å) | 11.42460 | 11.44340 | 11.45060 | 11.45220 | 11.45686 |
| c/a | 2.18792 | 2.19103 | 2.19060 | 2.19153 | 2.18932 |
| Unit cell volume (Å3) | 311.500 | 312.154 | 312.864 | 312.732 | 313.746 |
| Bonds lengths (Å) | |||||
| (Ca/Co)-O | 2.45635(5) | 2.45214(6) | 2.46791(3) | 2.45869(4) | 2.46208(3) |
| 2.44428(5) | 2.43689(7) | 2.42918(3) | 2.45861(4) | 2.41577(3) | |
| Mo-O | 1.78173(3) | 1.79593(5) | 1.79710(2) | 1.77052(3) | 1.81503(2) |
| Crystallite size (nm) | 32± 4 | 43± 4 | 37± 2 | 41± 3 | 43 ± 3 |
| R factors | |||||
| Rp | 3.47 | 3.38 | 3.08 | 3.07 | 2.88 |
| Rwp | 4.52 | 4.41 | 3.96 | 4.06 | 3.71 |
| χ2 | 2.05 | 2.13 | 1.81 | 2.10 | 1.59 |
| x | υ1 (Ag) Mode | T(Bg) Mode | ||
|---|---|---|---|---|
| Raman Shift (cm−1) | FWHM (cm−1) | Raman Shift (cm−1) | FWHM (cm−1) | |
| 0.00 | 879.99 ± 0.10 | 11.54 ± 0.30 | 111.62 ± 0.23 | 10.11 ± 0.64 |
| 0.02 | 880.05 ± 0.10 | 11.60 ± 0.29 | 111.67 ± 0.23 | 10.09 ± 0.70 |
| 0.04 | 879.95 ± 0.11 | 11.48 ± 0.31 | 111.62 ± 0.23 | 10.10 ± 0.60 |
| 0.06 | 880.17 ± 0.10 | 10.86 ± 0.26 | 111.87 ± 0.24 | 9.84 ± 0.61 |
| 0.08 | 880.13 ± 0.09 | 10.83 ± 0.25 | 112.48 ± 0.24 | 9.30 ± 0.66 |
| Samples | Ca(at.%) | Co(at.%) | Mo(at.%) | O(at.%) | Co/(Ca + Co) | Mo/(Ca + Co) |
|---|---|---|---|---|---|---|
| CaMoO4 | 15.51 | - | 17.69 | 66.79 | 0 | 1.14 |
| 2% Co-CaMoO4 | 10.45 | 0.22 | 10.57 | 78.76 | 0.02 | 0.99 |
| 4% Co-CaMoO4 | 17.2 | 0.59 | 17.24 | 64.96 | 0.03 | 0.97 |
| 6% Co-CaMoO4 | 10.59 | 0.54 | 10.70 | 78.16 | 0.05 | 0.96 |
| 8% Co-CaMoO4 | 10.02 | 0.86 | 10.83 | 78.29 | 0.08 | 0.99 |
| Surface Area (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) | |
|---|---|---|---|
| CaMoO4 | 3.59 | 13.07 | 0.024 |
| 6% Co-CaMoO4 | 10.74 | 13.09 | 0.047 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abidi, S.; Benchikhi, M. Room Temperature Surfactant-Free Synthesis of Cobalt-Doped CaMoO4 Nanoparticles: Structural and Microstructural Insights. Ceramics 2025, 8, 110. https://doi.org/10.3390/ceramics8030110
Abidi S, Benchikhi M. Room Temperature Surfactant-Free Synthesis of Cobalt-Doped CaMoO4 Nanoparticles: Structural and Microstructural Insights. Ceramics. 2025; 8(3):110. https://doi.org/10.3390/ceramics8030110
Chicago/Turabian StyleAbidi, Said, and Mohamed Benchikhi. 2025. "Room Temperature Surfactant-Free Synthesis of Cobalt-Doped CaMoO4 Nanoparticles: Structural and Microstructural Insights" Ceramics 8, no. 3: 110. https://doi.org/10.3390/ceramics8030110
APA StyleAbidi, S., & Benchikhi, M. (2025). Room Temperature Surfactant-Free Synthesis of Cobalt-Doped CaMoO4 Nanoparticles: Structural and Microstructural Insights. Ceramics, 8(3), 110. https://doi.org/10.3390/ceramics8030110






