Ceramics—The Forgotten but Essential Ingredients for a Circular Economy on the Moon
Abstract
1. Introduction
2. Lunar Resources
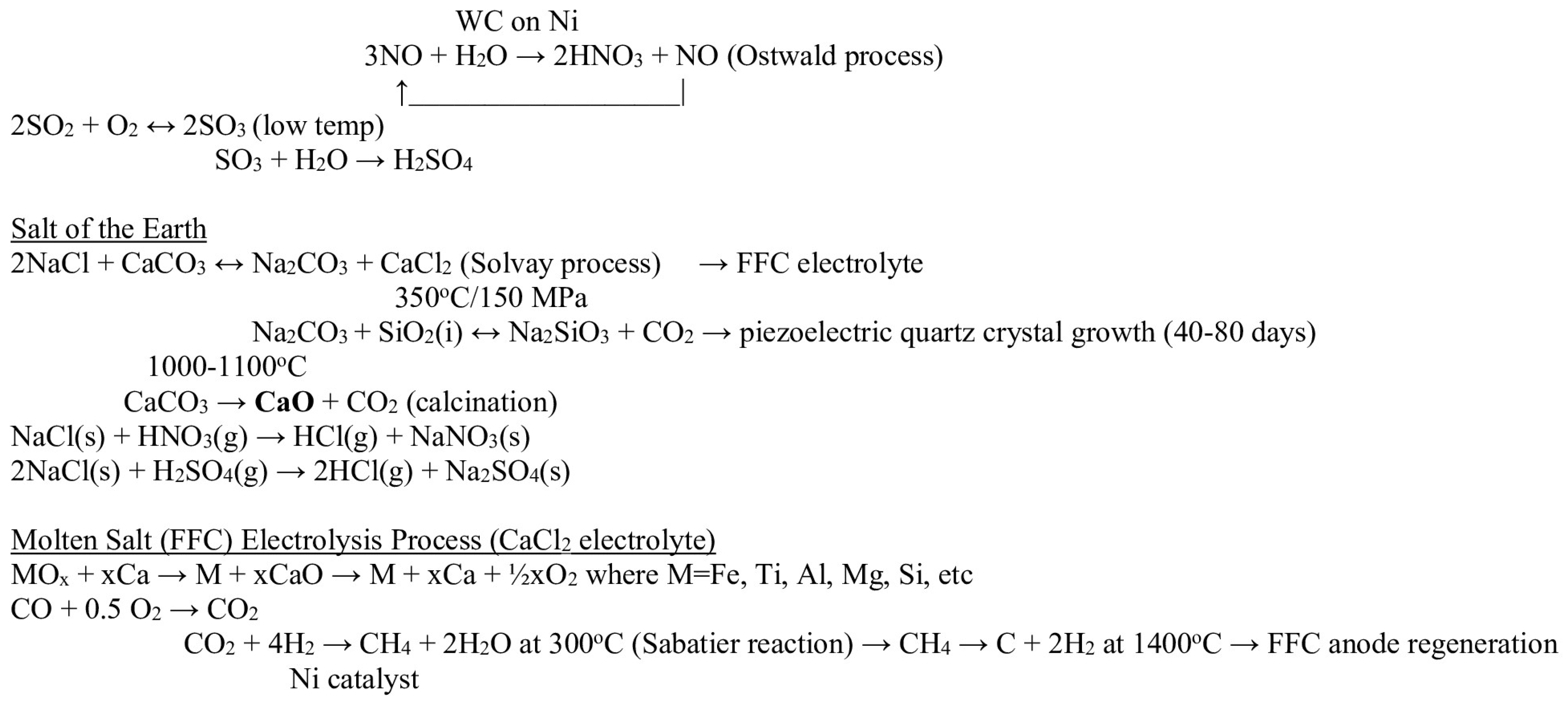
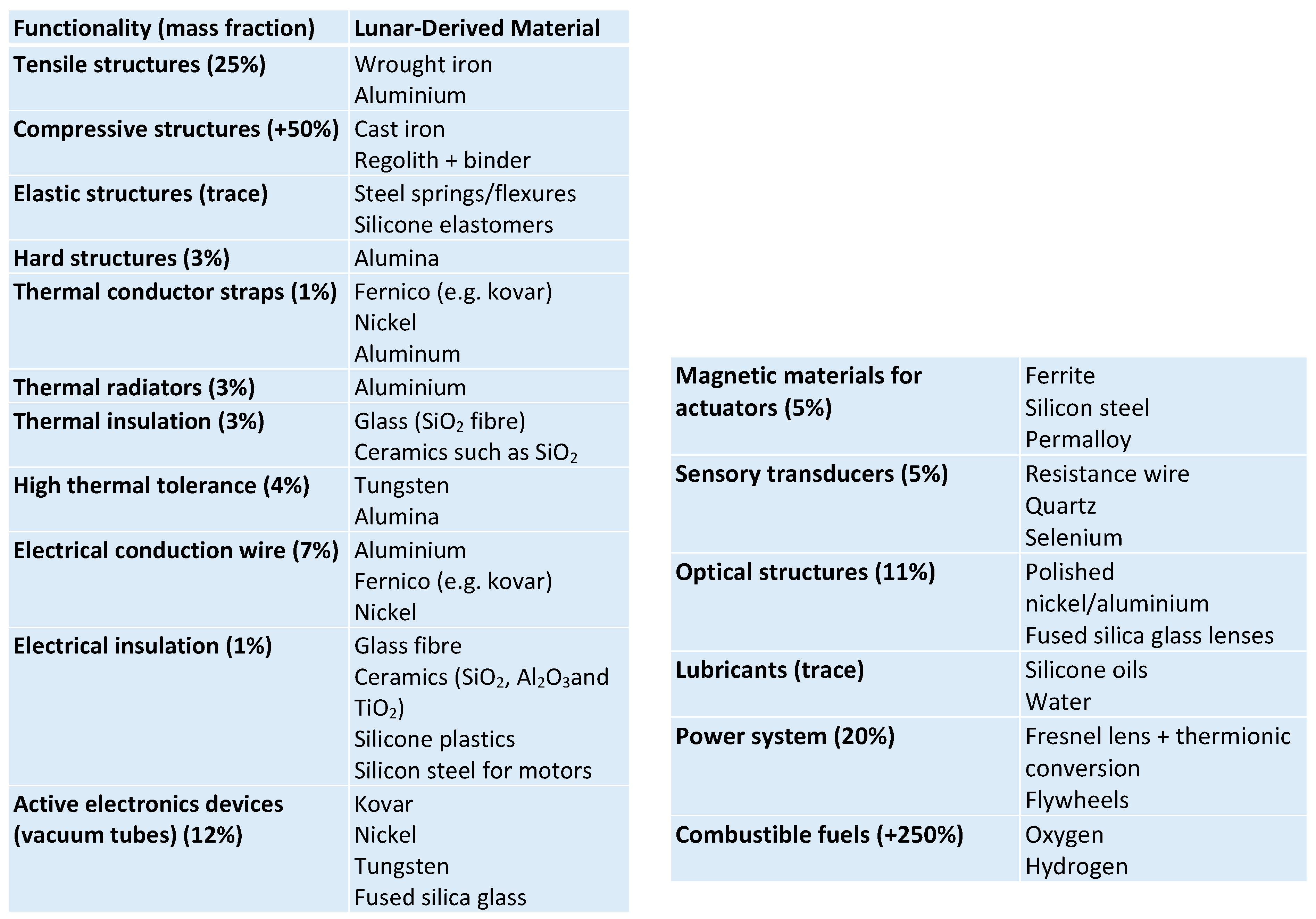
3. Circular Lunar Industrial Ecology
4. Thermal Sintering of Lunar-Derived Ceramics
5. Three-Dimensional Printing of Lunar-Derived Ceramics
6. Problem of Polymers
7. Lunar-Derived Clays
8. Non-Clay Minerals and Their Uses
9. Direct Solar Sintering/Melting
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Hao, L.; Li, Y.; Sun, Q.; Sun, M.; Huang, Y.; Li, Z.; Tang, D.; Wang, Y.; Xiao, L. In-situ utilisation of regolith resource and future exploration of additive manufacturing for lunar/martian habitats: A review. Appl. Clay Sci. 2022, 229, 106673. [Google Scholar] [CrossRef]
- Mueller, R.; Howe, S.; Kochmann, D.; Ali, H.; Anderson, C.; Burgoyne, H.; Chambers, W.; Clinton, R.; De Kestellier, X.; Ebelt, K.; et al. Automated additive construction (AAC) for Earth and space using in-situ resources. In Proceedings of the Biennial ASCE Conference on Engineering, Science, Construction & Operations in Challenging Environments (Earth & Space 2016), Reston, VA, USA, 11–15 April 2016. [Google Scholar]
- Fiske, M.; Ellery, A. On the Development of an ISRU-Based Calcium Sulphoaluminate Concrete for 3D Printed and Cast Lunar Surface Infrastructure Applications; AIAA SciTech Forum: Orlando, FL, USA, 2025; p. 1863. [Google Scholar]
- Ellery, A. Leveraging in-situ resources for lunar base construction. Can. J. Civ. Eng. 2021, 49, 657–674. [Google Scholar] [CrossRef]
- Ellery, A. Generating and storing power on the Moon using in-situ resources. Proc. Inst. Mech. Eng. J. Aerosp. Eng. 2021, 236, 1045–1063. [Google Scholar] [CrossRef]
- Ellery, A. Can we build nuclear-electric propulsion systems from lunar resources? In Space Resources Roundtable; Colorado School of Mines: Golden, CO, USA, 2025; Available online: https://isruinfo.com/public/index.php?page=srr_25 (accessed on 12 August 2025).
- Ellery, A. Supplementing closed ecological life support systems with in-situ resources on the Moon. Life 2021, 11, 770. [Google Scholar] [CrossRef]
- Azami, M.; Kazemi, Z.; Moazen, S.; Dube, M.; Potvin, M.-J.; Skonieczny, K. Comprehensive review of lunar-based manufacturing and construction. Prog. Aerosp. Sci. 2024, 150, 101045. [Google Scholar] [CrossRef]
- Zocca, A.; Wilbig, J.; Gunster, J.; Widjaja, P.; Neumann, C.; Clozel, M.; Meyer, A.; Ding, J.; Zhou, Z.; Tian, X. Challenges in the technology development for additive manufacturing in space. Chin. J. Mech. Eng. Addit. Manuf. Front. 2022, 1, 100018. [Google Scholar] [CrossRef]
- Ellery, A. Challenges of robotic milli-g operations for asteroid mining. In Proceedings of the Future Technologies Conference 2024, London, UK, 14–15 November 2024; Arai, K., Ed.; Lecture Notes on Networks & Systems. Springer Nature Switzerland AG: Cham, Switzerland, 2024; Volume 1157, pp. 45–64. [Google Scholar]
- McKay, D.; Hiken, G.; Basu, A.; Blanford, G.; Simon, S.; Reedy, R.; French, B.; Papike, J. Lunar regolith. In Lunar Sourcebook; Heiken, G., Vaniman, D., French, B., Eds.; Cambridge University Press: Cambridge, UK, 1991; pp. 287–356. [Google Scholar]
- Klein, C. Lunar materials: Their mineralogy, petrology and chemistry. Earth Sci. Rev. 1972, 8, 169–204. [Google Scholar] [CrossRef]
- Ellery, A. Sustainable in-situ resource utilisation on the Moon. Planet. Space Sci. 2020, 184, 104870. [Google Scholar] [CrossRef]
- Sodha, G.; Dhingra, D. Novel geological framework to understand the origin and diversity of orthopyroxene, olivine, spinel (OOS) lithologies on the Moon. Sci. Rep. 2025, 15, 2426. [Google Scholar] [CrossRef]
- Papike, J.; Taylor, L.; Simon, S. Lunar minerals. In Lunar Sourcebook; Heiken, G., Vaniman, D., French, B., Eds.; Cambridge University Press: Cambridge, UK, 1991; pp. 121–181. [Google Scholar]
- Crawford, I.; Anand, M.; Barber, S.; Cowley, A.; Crites, S.; Fa, W.; Flahaut, J.; Gaddis, L.; Greenhagen, B.; Haruyama, J.; et al. Lunar resources. Rev. Mineral. Geochem. 2023, 89, 829–868. [Google Scholar] [CrossRef]
- Reveron, H.; Gutierrez-Campos, D.; Rodriguez, R.; Bonassin, J. Chemical synthesis and thermal evolution of MgAl2O4 spinel precursor prepared from industrial gibbsite and magnesia powder. Mater. Lett. 2002, 56, 97–101. [Google Scholar] [CrossRef]
- Ewais, E.; Besisa, D.; El-Amir, A.; El-Sheikh, S.; Rayan, D. Optical properties of nanocrystalline magnesium aluminate spinel synthesised from industrial waste. J. Alloys Compd. 2015, 649, 159–166. [Google Scholar] [CrossRef]
- Lemeshev, D.; Senina, M.; Pedchenko, M.; Boyko, A. Transparent ceramic based on magnesium aluminate spinel for armour. IOP Conf. Ser. Mater. Sci. Eng. 2019, 525, 012081. [Google Scholar] [CrossRef]
- Shi, Z.; Zhao, Q.; Guo, B.; Ji, T.; Wang, H. Review on processing polycrystalline magnesium aluminate spinel (MgAl2O4): Sintering techniques, material properties and machinability. Mater. Des. 2020, 193, 108858. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, T.; Chang, X.; Wei, N.; Qi, J. Unique mechanical properties of nanostructured transparent MgAl2O4 ceramics. Nanoscale Res. Lett. 2013, 8, 261. [Google Scholar] [CrossRef]
- Mroz, T.; Goldman, L.; Gledhill, A.; Li, D.; Padture, N. Nanostructured infrared-transparent magnesium aluminate spinel with superior mechanical properties. Int. J. Appl. Ceram. Technol. 2012, 9, 83–90. [Google Scholar] [CrossRef]
- Campos-Quiros, A.; Zughbi, M.; Kundu, A.; Watanabe, M. Correlation between grain boundary segregation behaviours of calcium and yttrium and enhanced fracture toughness in magnesium aluminate spinel. J. Mater. Sci. 2025, 60, 1826–1852. [Google Scholar] [CrossRef]
- Voytovych, R.; MacLaren, I.; Gulgun, M.; Cannon, R.; Ruhle, M. Effect of yttrium on densification and grain growth in α-alumina. Acta Mater. 2002, 50, 3453–3463. [Google Scholar] [CrossRef]
- Xu, P.; Wang, H.; Tu, B.; Gu, H.; Wang, W.; Liu, S.; Ma, C.; Fu, Z. Effect of yttrium-doped grain boundary on sintering behaviour and properties of transparent ZnAl2O4 ceramics. J. Eur. Ceram. Soc. 2024, 44, 6597–6606. [Google Scholar] [CrossRef]
- Hou, Z.; Gong, Q.; Liu, N.; Jiang, B.; Li, J.; Wu, Y.; Huang, J.; Gu, W. Elemental abundances of Moon samples based on statistical distributions of analytical data. Appl. Sci. 2023, 13, 360. [Google Scholar] [CrossRef]
- Heiken, G.; Vaniman, D.; French, B. Lunar Sourcebook: A Users Guide to the Moon; Cambridge University Press: Cambridge, UK, 1991; Available online: https://www.lpi.usra.edu/publications/books/lunar_sourcebook/pdf/LunarSourceBook.pdf (accessed on 12 August 2025).
- Ellery, A. Mining asteroid versus mining the Moon—Can you have your cake and eat it? In Space Resources Roundtable; Colorado School of Mines: Golden, CO, USA, 2025. [Google Scholar]
- Unterclass, M. Geomimetics and extreme biomimetics inspired by hydrothermal systems—What can we learn from nature for materials synthesis? Biomimetics 2017, 2, 8. [Google Scholar] [CrossRef]
- Lasaga, A.; Soler, J.; Ganor, J.; Burch, T.; Nagy, K. Chemical weathering rate laws and global geochemical cycles. Geochim. Cosmochim. Acta 1994, 58, 2361–2386. [Google Scholar] [CrossRef]
- Thibodeau, B.; Walls, X.; Ellery, A.; Cousens, B.; Marczenko, K. Extraction of silica and alumina from lunar highland simulant. In Proceedings of the ASCE Earth & Space Conference 2024, Miami, FL, USA, 15–18 April 2024; p. 6962. [Google Scholar]
- Ellery, A.; Mellor, I.; Wanjara, P.; Conti, M. Metalysis FFC process as a strategic lunar in-situ resource utilisation technology. New Space J. 2022, 10, 224–238. [Google Scholar] [CrossRef]
- Walls, X.; Ellery, A.; Marczenko, K.; Wanjara, P. Aluminium metal extraction from lunar highland simulant using electrochemistry. In Proceedings of the ASCE Earth & Space Conference 2024, Miami, FL, USA, 15–18 April 2024. Paper No. 7061. [Google Scholar]
- Ellery, A. Is electronics fabrication feasible on the Moon? In Proceedings of the ASCE Earth & Space Conference 2022, Denver, CO, USA, 25–28 April 2022; pp. 759–772. [Google Scholar]
- Dorcheh, S.; Abbasi, M. Silica aerogel: Synthesis, properties and characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Hogue, M.; Mueller, R.; Sibille, L.; Hintze, P.; Rasky, D. Extraterrestrial regolith derived atmospheric entry heat shields. In Proceedings of the 15th Biennial ASCE International Conference on Engineering, Science, Construction, and Operations in Challenging Environments, Orlando, FL, USA, 11–15 April 2016. [Google Scholar]
- Chen, Y.; Wang, X.; Yu, C.; Ding, J.; Deng, C.; Zhu, H. Properties of inorganic high temperature adhesive for high temperature furnace connection. Ceram. Int. 2019, 45, 8684–8689. [Google Scholar] [CrossRef]
- McAdam, A.; Zolotiv, M.; Sharp, T.; Leshkin, L. Preferential low pH dissolution of pyroxene in plagioclase-pyroxene mixtures: Implications for martian surface materials. Icarus 2008, 196, 90–96. [Google Scholar] [CrossRef]
- Baasch, J.; Windisch, L.; Koch, F.; Linke, S.; Stoll, E.; Schilde, C. Regolith as substitute mould material for aluminium casting on the Moon. Acta Astronaut. 2021, 182, 1–12. [Google Scholar] [CrossRef]
- Marques, A.; Miglietta, D.; Gaspar, G.; Baptista, A.; Gaspar, A.; Perdigao, P.; Soares, I.; Bianchi, C.; Sousa, D.; Faustino, M.; et al. Synthesis of thermoelectric magnesium-silicide pastes for 3D printing, electrospinning and low-pressure spray. Mater. Renew. Sustain. Energy 2019, 8, 21. [Google Scholar] [CrossRef]
- Frankberg, E. Ceramic that bends instead of shattering. Science 2022, 378, 359–360. [Google Scholar] [CrossRef]
- Kuang, X.; Carotenuto, G.; Nicolais, L. Review of ceramic sintering and suggestions on reducing sintering temperatures. Adv. Perform. Mater. 1997, 4, 257–274. [Google Scholar] [CrossRef]
- Vakifahmetoglu, C.; Karacasulu, L. Cold sintering of ceramics and glasses: A review. Curr. Opin. Solid. State Mater. Sci. 2020, 24, 100807. [Google Scholar] [CrossRef]
- Yamasaki, N.; Yanagisawa, K.; Nishioka, M.; Kanahara, S. Hydrothermal hot-pressing method: Apparatus and application. J. Mater. Sci. Lett. 1986, 5, 355–356. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Nishioka, M.; Ioku, K.; Yamasaki, N. Densification of silica gels by hydrothermal hot-pressing. J. Mater. Sci. Lett. 1993, 12, 1073–1075. [Google Scholar] [CrossRef]
- Chen, W.; Shui, A.; Wang, C.; Li, J.; Ma, J.; Tian, W.; Ota, T.; Xi, X. Preparation of aluminium titanate flexible ceramic by solid phase sintering and its mechanical behaviour. J. Alloys Compd. 2019, 777, 119–126. [Google Scholar] [CrossRef]
- Kishimoto, A.; Obata, M.; Asaoka, H.; Hayashi, H. Fabrication of alumina-based ceramic foams utilising superplasticity. J. Eur. Ceram. Soc. 2007, 27, 41–45. [Google Scholar] [CrossRef]
- Wakai, F.; Kondo, N.; Shinoda, Y. Ceramics superplasticity. Curr. Opin. Solid. State Mater. Sci. 1999, 4, 461–465. [Google Scholar] [CrossRef]
- Pilling, J.; Payne, J. Superplasticity in Al2O3-ZrO2-Al2TiO5 ceramics. Scr. Metall. Mater. 1995, 32, 1091–1097. [Google Scholar] [CrossRef]
- Shen, Z.; Peng, H.; Nygren, M. Formidable increase in the superplasticity of ceramics in the presence of an electric field. Adv. Mater. 2003, 15, 1006–1009. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, J.; Li, Z.; Gao, Y.; Wang, M.; Huang, M.; Wang, J.; Chen, K. Borrowed dislocations for ductility in ceramics. Science 2024, 385, 422–428. [Google Scholar] [CrossRef]
- Peng, L.; Cao, J.; Noda, K.; Han, K. Mechanical properties of ceramic-metal composites by pressure infiltration of metal into porous ceramics. Mater. Sci. Eng. A 2004, 374, 1–9. [Google Scholar] [CrossRef]
- Wan, F.; Zuo, Y.; Pan, Y.; Yu, A.; Qi, J.; Lu, X. Iron-enhanced simulated lunar regolith sintered blocks: Preparation, sintering and mechanical properties. Constr. Build. Mater. 2024, 439, 137395. [Google Scholar] [CrossRef]
- Rahimian, M.; Ehsani, N.; Parvin, N.; Baharvandi, H. Effect of particle size, sintering temperature and sintering time on the properties of Al-Al2O3 composites made by powder metallurgy. J. Mater. Process. Technol. 2009, 209, 5387–5393. [Google Scholar] [CrossRef]
- Azami, M.; Siahsarani, A.; Hadian, A.; Kazemi, Z.; Rahmatabadi, D.; Kashani-Bozorg, F.; Abrinia, K. Laser powder bed fusion of alumina/Fe-Ni ceramic matrix particulate composites impregnated with a polymeric resin. J. Mater. Res. Technol. 2023, 24, 3133–3144. [Google Scholar] [CrossRef]
- Laot, M.; Rich, B.; Cheibas, I.; Fu, J.; Zhu, J.-N.; Popovich, V. Additive manufacturing and spark plasma sintering of lunar regolith for functionally graded materials. Spool 2021, 8, 7–23. [Google Scholar]
- Faes, M.; Valkenaers, H.; Vogeler, F.; Vleugels, J.; Ferraris, E. Extrusion-based 3D printing of ceramic components. Procedia CIRP 2015, 28, 76–81. [Google Scholar] [CrossRef]
- Ngo, T.; Kashani, A.; Imbalzano, G.; Nguyen, K.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Fang, W.; Mu, Z.; He, Y.; Kong, K.; Jiang, K.; Tang, R.; Liu, Z. Organic-inorganic covalent-ionic molecules for elastic ceramic plastic. Nature 2023, 619, 293–299. [Google Scholar] [CrossRef]
- Sun, S.; Mao, L.-B.; Lei, Z.; Yu, S.-H.; Colfen, H. Hydrogels from amorphous calcium carbonate and polyacrylic acid: Bio-inspired materials for ‘mineral plastic’. Angew. Chem. Int. Ed. 2016, 55, 11765–11769. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, T.; Huang, G. State-of-the-art research progress and challenge of the printing techniques, potential applications for advanced ceramic materials 3D printing. Mater. Today Commun. 2024, 40, 110001. [Google Scholar] [CrossRef]
- Clemens, F.; Sarraf, F.; Borzi, A.; Neels, A.; Hadian, A. Material extrusion additive manufacturing of advanced ceramics: Towards the production of large components. J. Eur. Ceram. Soc. 2023, 43, 2752–2760. [Google Scholar] [CrossRef]
- Yeh, T.-S.; Sacks, M. Effect of particle size distribution on the sintering of alumina. Commun. Am. Ceram. Soc. 1988, 71, C484–C487. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.; Dong, X.; Wu, J.; Zhou, Q.; Li, S.; Shen, C.; Liu, W.; Wang, G.; He, R. Additive manufacturing of fibre reinforced ceramic matrix composites: Advances, challenges and prospects. Ceram. Int. 2021, 48, 19542–19556. [Google Scholar] [CrossRef]
- Martin, J.; Fiore, B.; Erb, R. Designing bioinspired composite reinforcement architectures via 3D magnetic printing. Nat. Commun. 2015, 6, 8641. [Google Scholar] [CrossRef]
- Romanczuk-Ruszuk, E.; Sztorch, B.; Pakula, D.; Gabriel, E.; Nowak, K.; Przekop, R. 3D printing ceramics—Materials for direct extrusion process. Ceramics 2023, 6, 364–385. [Google Scholar] [CrossRef]
- Hao, L.; Tang, D.; Sun, T.; Xiong, W.; Feng, Z.; Evans, K.; Li, Y. Direct ink writing of mineral materials—A review. Int. J. Precis. Eng. Manuf. Green. Technol. 2021, 8, 665–685. [Google Scholar] [CrossRef]
- Lamnini, S.; Elsaed, H.; Lakhdar, Y.; Baino, F.; Smeacetto, F.; Bernado, E. Robocasting of advanced ceramics: Ink optimization and protocol to predict the printing parameters—A review. Helyon 2022, 8, e10651. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D printing of ceramics: A review. J. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Shahzad, A.; Lazoglu, I. Direct ink writing of structural and functional ceramics: Recent achievements and future challenges. Compos. B 2021, 225, 109249. [Google Scholar] [CrossRef]
- Jakus, A.; Koube, K.; Geisendorfer, N.; Shah, R. Robust and elastic lunar and martian structures from 3D printed regolith inks. Sci. Rep. 2017, 7, 44931. [Google Scholar] [CrossRef]
- Rocha, V.; Saiz, E.; Tirichenko, I.; Garcia-Tunon, E. Direct ink writing advances in multi-material structures for a sustainable future. J. Mater. Chem. A 2020, 8, 15646–15657. [Google Scholar] [CrossRef]
- Bland, P.; Cintala, M.; Horz, F.; Cressey, G. Survivability of meteorite projectiles—Results from impact experiments. In Proceedings of the 32nd Lunar & Planetary Science Conference, Houston, TX, USA, 12–16 March 2001; p. 1764. [Google Scholar]
- Bland, P.; Artemieva, N.; Collins, G.; Bottke, W.; Bussey, D.; Joy, K. Asteroids on the Moon: Projectile survival during low velocity impacts. In Proceedings of the 39th Lunar & Planetary Science Conference, League City, TX, USA, 10–14 March 2008; p. 2045. [Google Scholar]
- Halim, S.; Crawford, I.; Collins, G.; Joy, K.; Davison, T. Assessing the survival of carbonaceous chondrites impacting the lunar surface as a potential resource. Planet. Space Sci. 2024, 246, 105905. [Google Scholar] [CrossRef]
- Joy, K.; Crawford, I.; Curran, N.; Zolensky, M.; Fagan, A.; King, D. Moon: An archive of small body migration in the solar system. Earth Moon Planets 2016, 118, 133–158. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Zhu, M.-H.; Liu, Y.; Wu, B.; Du, J.; Fa, W.; Xu, R.; He, Z.; Wang, C.; et al. Impact remnants rich in carbonaceous chondrites detected on the Moon by the Chang’e 4 rover. Nat. Astron. 2022, 6, 207–213. [Google Scholar] [CrossRef]
- Thomas-Keprta, K.; Clemett, S.; Messenger, S.; Ross, D.; Le, L.; Rahman, Z.; McKay, D.; Gibson, E.; Gonzalez, C.; Peabody, W. Organic matter on the Earth’s Moon. Geochim. Cosmochim. Acta 2014, 134, 1–15. [Google Scholar] [CrossRef]
- Ellery, A. Trials and tribulations of asteroid mining. In Proceedings of the ASCE Earth & Space Conference 2024, Miami, FL, USA, 15–18 April 2024; p. 8087. [Google Scholar]
- O’Lenick, A. Basic silicone chemistry—A review. Silicone Spect. 2009. Available online: https://www.scientificspectator.com/documents/silicone%20spectator/Silicone_Spectator_January_2009.pdf (accessed on 12 August 2025).
- Chaudhary, R.; Parameswaran, C.; Idrees, M.; Rasaki, A.; Liu, C.; Chen, Z.; Colombo, P. Additive manufacturing of polymer-derived ceramics: Materials, technologies, properties and potential applications. Prog. Mater. Sci. 2022, 128, 100969. [Google Scholar] [CrossRef]
- Davis, P.; Murugavel, R. Recent developments in the chemistry of molecular titanosiloxanes and titanophosphonates. Synth. React. Inorg. Met. Org. Nano-Met. Chem. 2005, 35, 591–622. [Google Scholar] [CrossRef]
- Unny, R.; Gopinathan, S.; Gopinathan, C. Chelated aluminosiloxanes. Indian. J. Chem. 1980, 19A, 484–486. [Google Scholar]
- Puxviel, J.; Boilot, J.; Poncelet, O.; Hubert-Pfalzgraf, L.; Lecomte, A.; Dauger, A.; Beloeil, J. Aluminosiloxane as a ceramic precursor. J. Non-Cryst. Solids 1987, 93, 277–286. [Google Scholar] [CrossRef]
- Griffith, M.; Halloran, J. Freeform fabrication of ceramics vis stereolithography. J. Am. Ceram. Soc. 1996, 79, 2601–2608. [Google Scholar] [CrossRef]
- Dou, R.; Tang, W.; Hu, K.; Wang, L. Ceramic paste for space stereolithography 3D printing technology in microgravity environments. J. Eur. Ceram. Soc. 2022, 42, 3968–3975. [Google Scholar] [CrossRef]
- Gibson, M.; Mykulowycz, N.; Shim, J.; Fontana, R.; Schmitt, P.; Roberts, A.; Ketkaew, J.; Shao, L.; Chen, W.; Bordeenithikasem, P.; et al. 3D printing metals like thermoplastics: Fused filament fabrication of metallic glasses. Mater. Today 2018, 21, 697–702. [Google Scholar] [CrossRef]
- Liao, C.; Liu MZhang, Q.; Dong, W.; Zhao, R.; Li, B.; Jiao, Z.; Somg, J.; Yao, W.; Zhao, S.; Bai, H.; et al. Low temperature thermoplastic welding of metallic glass ribbons for in-space manufacturing. Sci. China Mater. 2020, 64, 979–986. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, J.; Jiang, P.; Liu, D.; Jia, X.; Wang, X.; Zhou, F. Direct ink writing of aluminium-phosphate-bonded Al2O3 ceramic with ultralow dimensional shrinkage. Ceram. Int. 2022, 48, 864–871. [Google Scholar] [CrossRef]
- Revelo, C.; Colorado, H. 3D printing of kaolinite clay ceramics using direct ink writing (DIW) technique. Ceram. Int. 2018, 44, 5673–5682. [Google Scholar] [CrossRef]
- Marquez, C.; Mata, J.; Renteria, A.; Gonzalez, D.; Gomez, G.; Lopez, A.; Baca, A.; Nunez, A.; Hassan, S.; Burke, V.; et al. Direct ink-write printing of ceramic clay with an embedded wireless temperature and relative humidity sensor. Sensors 2023, 23, 3352. [Google Scholar] [CrossRef] [PubMed]
- Karl, D.; Kamutzki, F.; Lima, P.; Gili, A.; Duminy, T.; Zocca, A.; Gunster, J.; Gurlo, A. Sintering of ceramics for clay in situ resource utilisation of Mars. Open Ceram. 2020, 3, 100008. [Google Scholar] [CrossRef]
- Tong, W.; Jaw, W.; Pothunuri, L.; Soh, E.; Le Ferrand, H. Easily applicable protocol to formulate inks for extrusion-based 3D printing. Ceramica 2022, 68, 152–159. [Google Scholar] [CrossRef]
- Lazorenko, G.; Kasprzhitskii, A. Geopolymer additive manufacturing: A review. Addit. Manuf. 2022, 55, 102782. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, Z.; Fu, S.; He, P.; Sun, C.; Duan, X.; Jia, D.; Colombo, P.; Zhou, Y. 3D printed lunar regolith simulant-based geopolymer composites with bio-inspired sandwich architectures. J. Adv. Ceram. 2023, 12, 510–525. [Google Scholar] [CrossRef]
- Abdulkareem, S.; Orkuma, G.; Apasi, A. Talc as a substitute for dry lubricant (an overview). AIP Proc. 2011, 1315, 1400. [Google Scholar]
- TM-2011-217114/AIAA-2011-704; Thermal Energy for Lunar In Situ Resource Utilisation: Technical Challenges and Technology Opportunities. NASA: Washington, DC, USA, 2011.
- Zhang, Y.; Shaw, M.; Brooks, G.; Rhamdhani, A.; Guo, C.; Han, Z.; Jackson, T.; Judkins, G. Investigation of heat transfer processes in multi-sized solar-sintered regolith for lunar ISRY program. Int. J. Heat. Mass. Transf. 2023, 214, 124387. [Google Scholar] [CrossRef]
- Meurisse, A.; Makaya, A.; Willsch, C.; Sperl, M. Solar 3D printing of lunar regolith. Acta Astronaut. 2018, 142, 800–810. [Google Scholar] [CrossRef]
- Imhof, B.; Urbina, D.; Weiss, P.; Sperl, M. Advancing solar sintering for building a base on the Moon. In Proceedings of the 67th International Astronautical Congress, Adelaide, Australia, 25–29 September 2017. IAC-17.C2.9.13.x37414. [Google Scholar]
- Imhof, B.; Sperl, M.; Urbina, D.; Weiss, P.; Preisinger, C.; Waclavicek, R.; Hoheneder, W.; Meurisse, A.; Fateri, M.; Gobert, T.; et al. Using solar sintering to build infrastructure on the Moon: Latest advancements in the Regolight project. In Proceedings of the 69th International Astronautical Congress, Bremen, Germany, 1–5 October 2018. IAC-18.E5.1.x47746. [Google Scholar]
- Fateri, M.; Meurisse, A.; Sperl, M.; Urbina, D.; Madakashira, K.; Govindaraj, S.; Gancet, J.; Imhof, B.; Hoheneder, W.; Waclavicek, R.; et al. Solar sintering for lunar additive manufacturing. ASCE J. Aerosp. Eng. 2019, 32, 04019101. [Google Scholar] [CrossRef]
- Biswas, D.; Cox, T.; Lapp, J. Sintering behaviour of lunar soil heated by indirect and direct concentrated sunlight. In Proceedings of the ASME 16th International Conference on Energy, Philadelphia, PA, USA, 11–13 July 2022. Paper No. ESS2022-81630. [Google Scholar]
- Gupta, N.; Dawara, V.; Kumar, A.; Viswanathan, K. Synthetic space bricks from lunar and martian regolith via sintering. Adv. Space Res. 2024, 74, 3902–3915. [Google Scholar] [CrossRef]


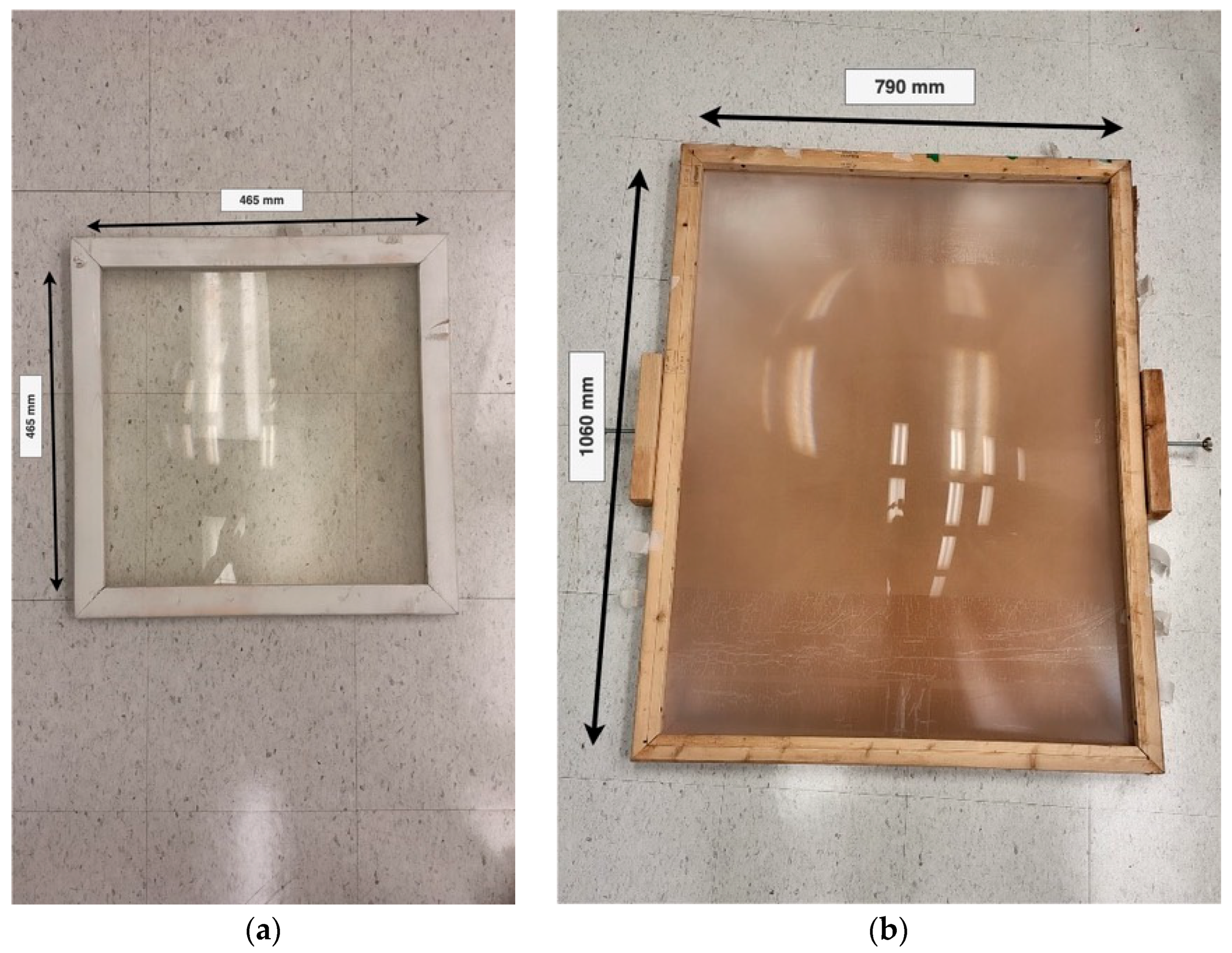



| Sample | Compressive Load Limit (N) |
|---|---|
| 2 | 239 |
| 3 | 267 |
| 4 | 136 (crack 1)/446 (crack 2)—291 (av) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellery, A. Ceramics—The Forgotten but Essential Ingredients for a Circular Economy on the Moon. Ceramics 2025, 8, 107. https://doi.org/10.3390/ceramics8030107
Ellery A. Ceramics—The Forgotten but Essential Ingredients for a Circular Economy on the Moon. Ceramics. 2025; 8(3):107. https://doi.org/10.3390/ceramics8030107
Chicago/Turabian StyleEllery, Alex. 2025. "Ceramics—The Forgotten but Essential Ingredients for a Circular Economy on the Moon" Ceramics 8, no. 3: 107. https://doi.org/10.3390/ceramics8030107
APA StyleEllery, A. (2025). Ceramics—The Forgotten but Essential Ingredients for a Circular Economy on the Moon. Ceramics, 8(3), 107. https://doi.org/10.3390/ceramics8030107











