Reduction of Ceramic Wear by Concave Dimples on the Bearing Surface in CoC Hip Implants: A Finite Element Analysis
Abstract
1. Introduction
2. Materials and Methods
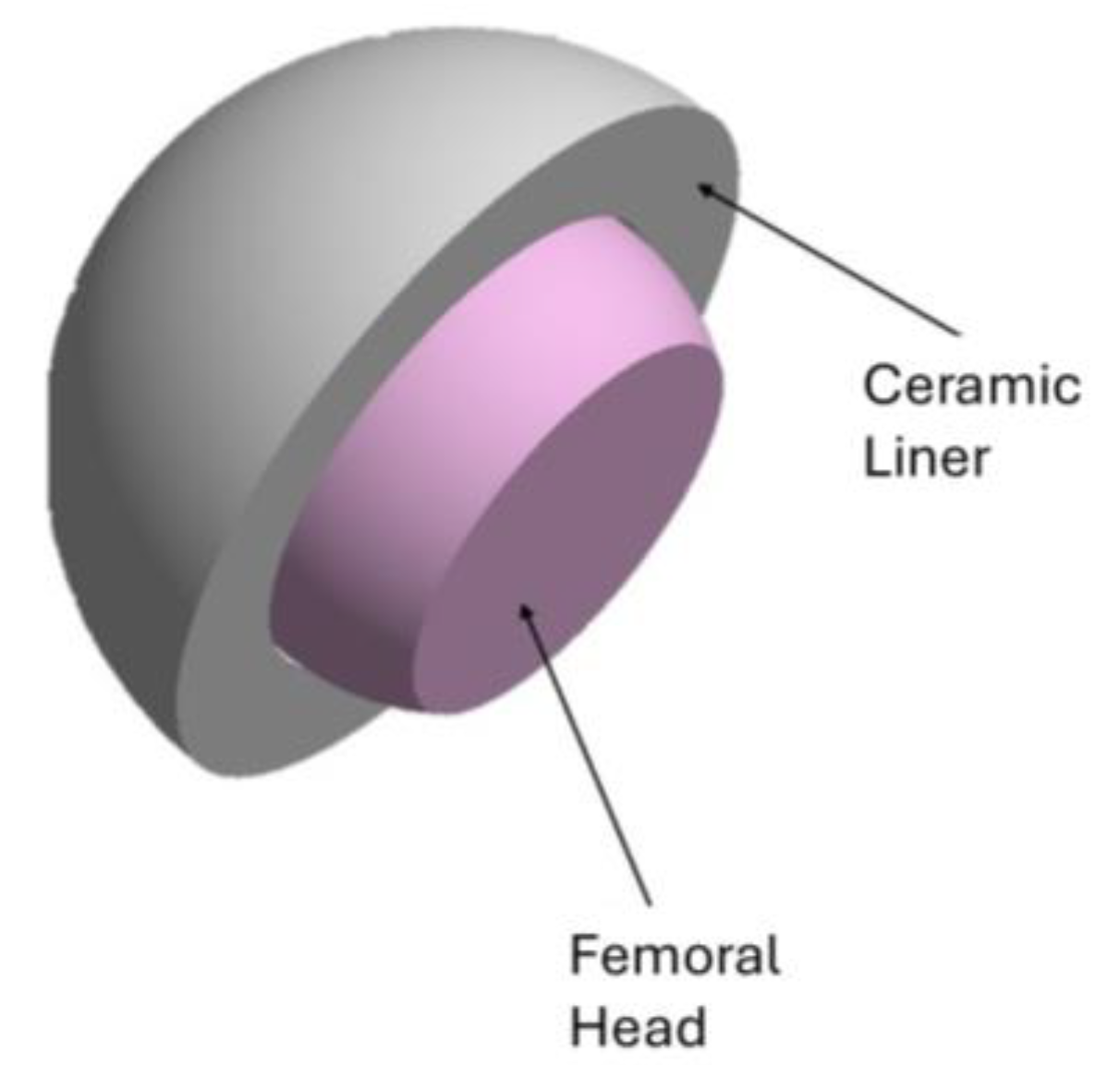
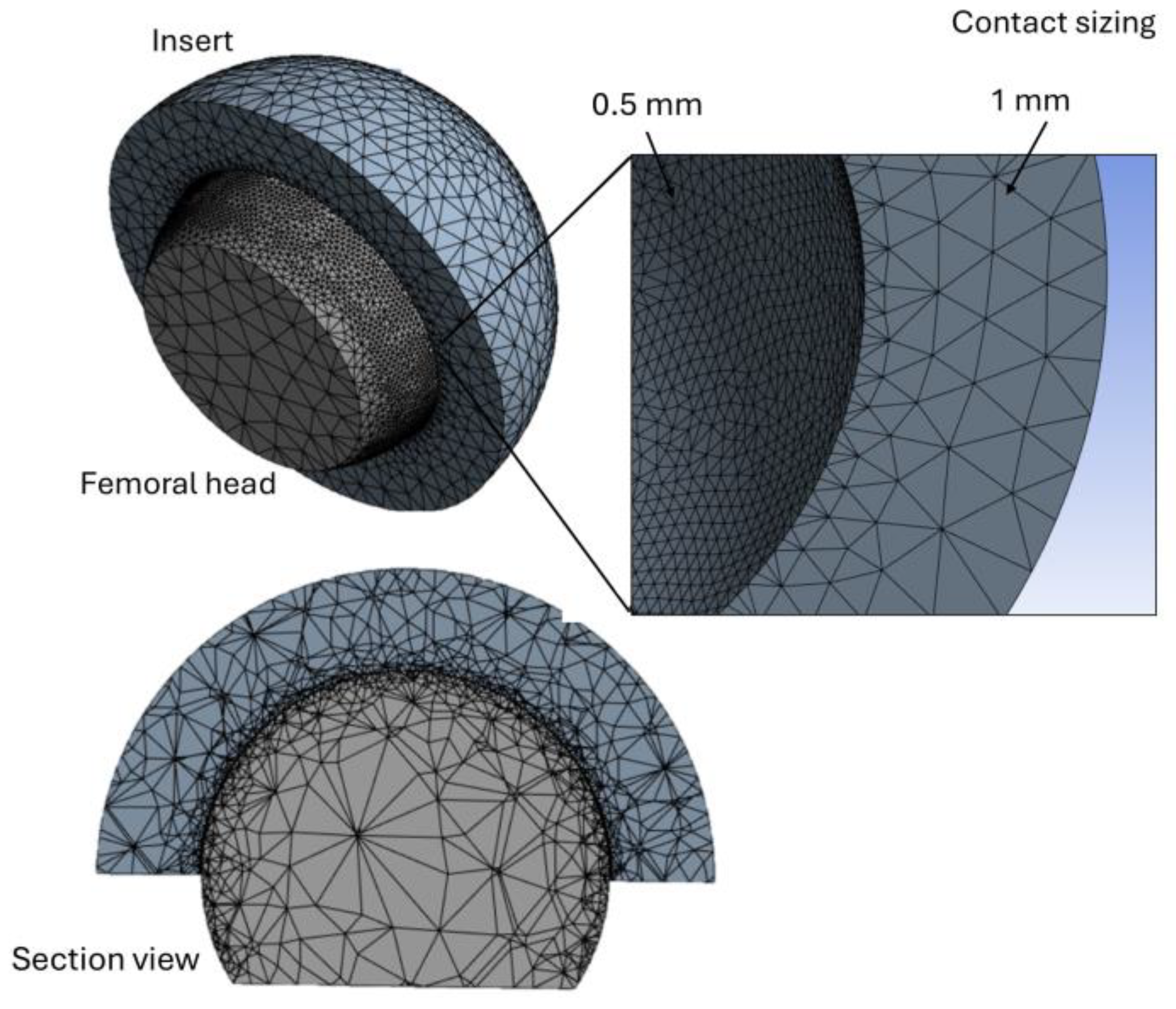
2.1. FEA Modeling
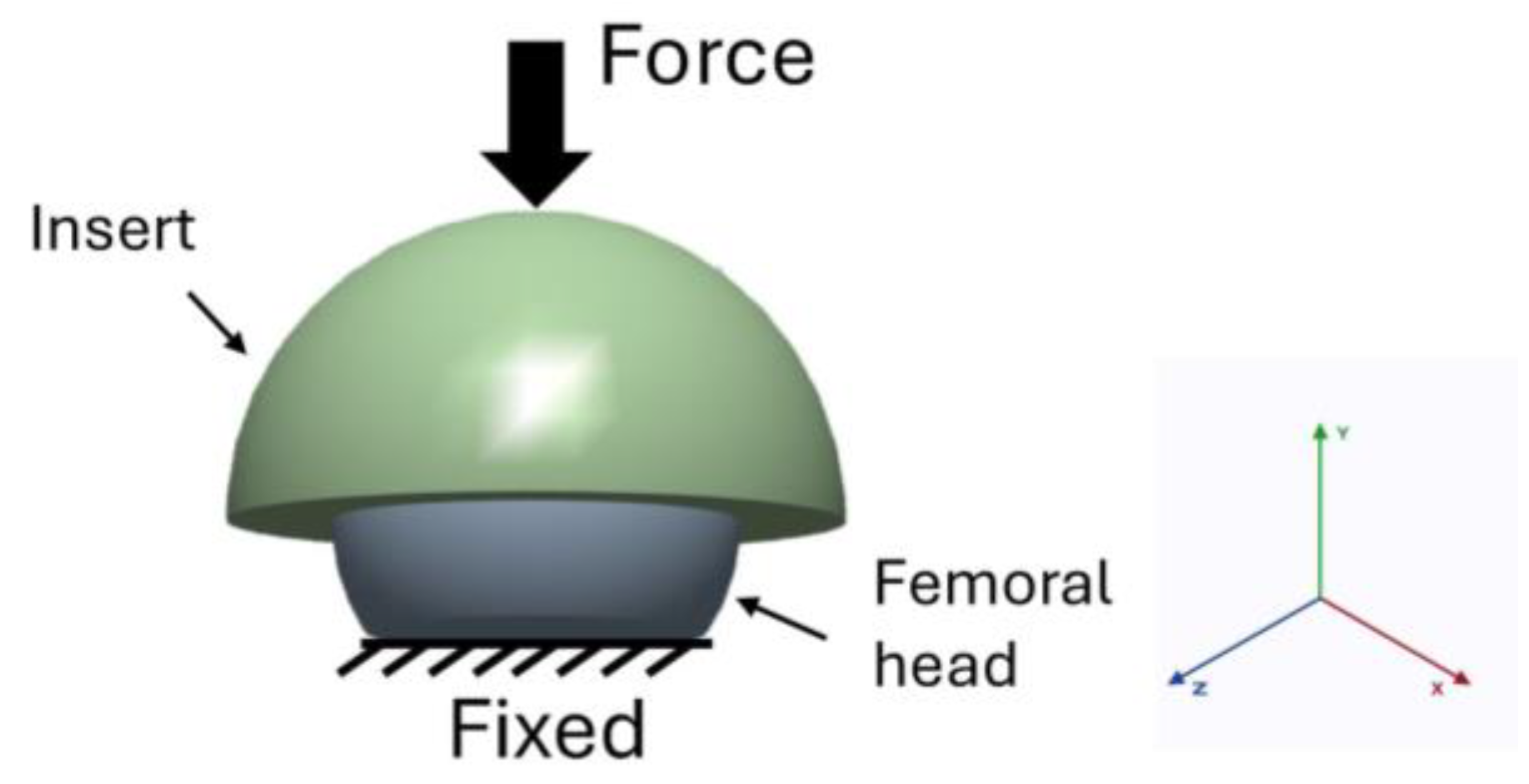
2.2. Boundary Conditions
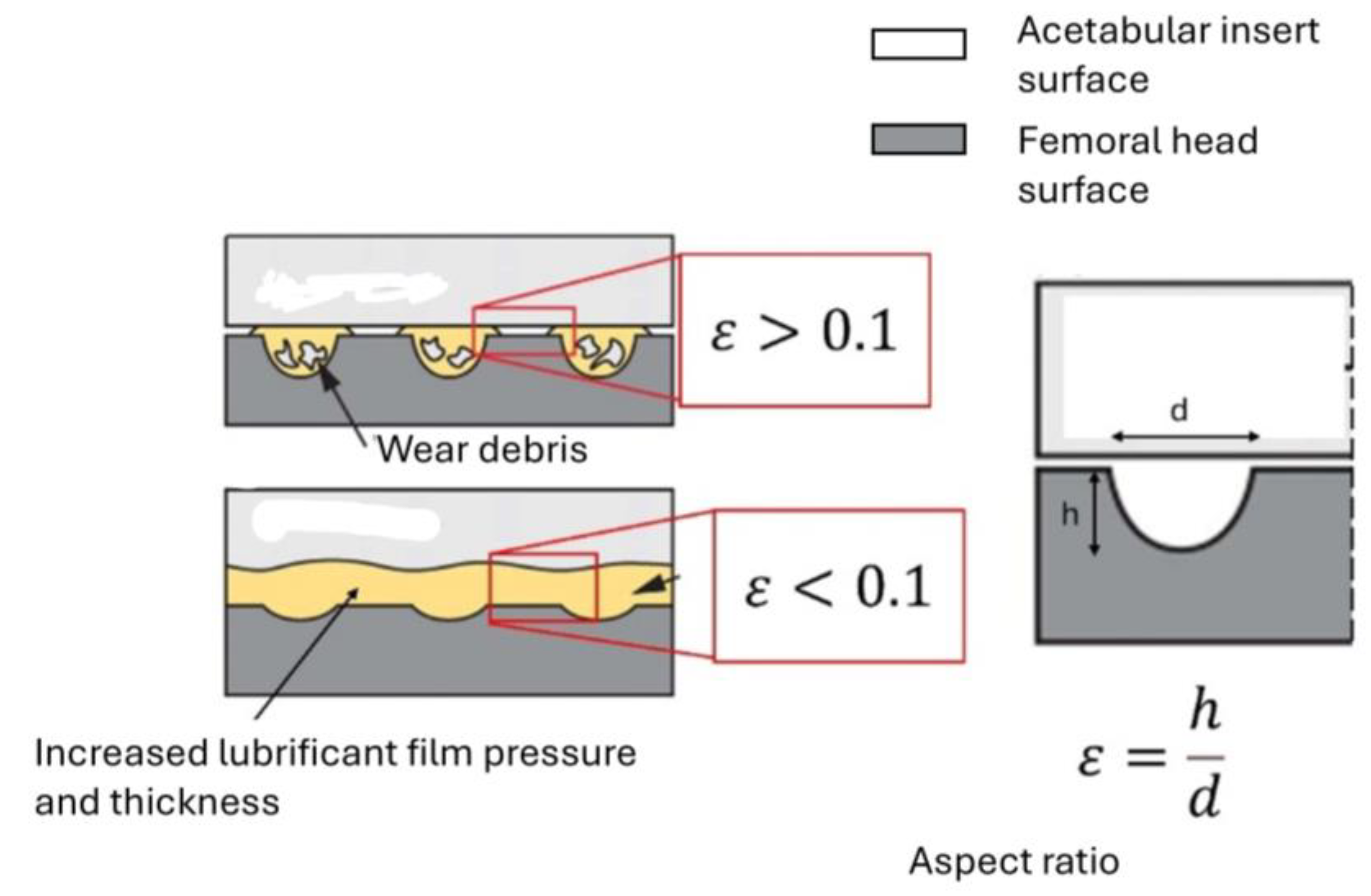
2.3. Calculation of the Wear Rate
- : wear coefficient in mm3/Nm.
- : contact pressure in MPa
- : contact area in mm2
- : sliding distance in mm.
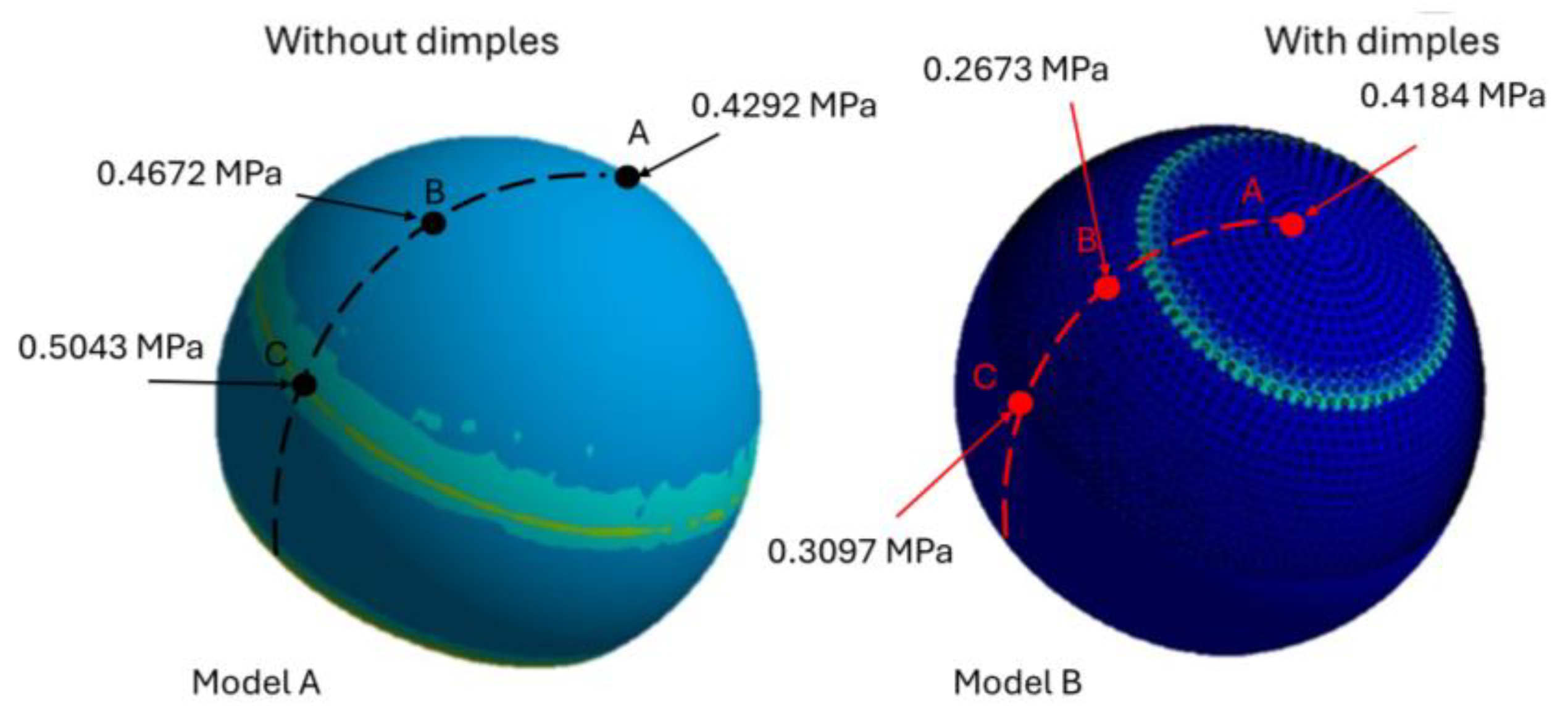
3. Results
Wear Volume
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kugelman, D.N.; Mahure, S.A.; Feng, J.E.; Rozell, J.C.; Schwarzkopf, R.; Long, W.J. Total knee arthroplasty is associated with greater immediate post-surgical pain and opioid use than total hip arthroplasty. Arch. Orthop. Trauma Surg. 2022, 142, 3575–3580. [Google Scholar] [CrossRef]
- Kim, J.S.; Moon, N.H.; Do, M.U.; Jung, S.W.; Suh, K.T.; Shin, W.C. The use of dual mobility acetabular cups in total hip replacement reduces dislocation rates in hip dysplasia patients. Sci. Rep. 2023, 13, 22404. [Google Scholar] [CrossRef] [PubMed]
- Merx, H.; Dreinhöfer, K.; Schräder, P.; Stürmer, T.; Puhl, W.; Günther, K.-P.; Brenner, H. International variation in hip replacement rates. Ann. Rheum. Dis. 2003, 62, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Svarnas, G.; Popa, V.; Patsiou, T.-S.; Schwab, J.M.; Tannast, M. Postoperative muscle atrophy and fatty degeneration with respect to surgical approaches in total hip arthroplasty. Arch. Orthop. Trauma Surg. 2025, 145, 177. [Google Scholar] [CrossRef] [PubMed]
- Hart, C.M.; Chen, C.; Hsiue, P.P.; Farshchi, R.; Silva, M.; Zeegen, E.; Thompson, R.; Stavrakis, A. National trends in total hip arthroplasty bearing surface usage in extremely young patients between 2006 and 2016. Arthroplast. Today 2021, 10, 51–56. [Google Scholar] [CrossRef]
- Kostova, I. The role of complexes of biogenic metals in living organisms. Inorganics 2023, 11, 56. [Google Scholar] [CrossRef]
- Singh, N.; Awasthi, A.; Pisulkar, G.; Taywade, S.; Samal, N. Adverse metal reaction in a case of metal-on-polyethylene hip arthroplasty. Cureus 2024, 16, e60810. [Google Scholar] [CrossRef] [PubMed]
- Almaawi, A.; Alabdullatif, F.S.; Alabdulkarim, N.; Benfaris, D.; Alamari, N.; Alzuhair, A. Different Types of Bearing Surfaces in Primary Total Hip Arthroplasty: A Systematic Review. Int. J. Med. Res. Health Sci. 2021, 10, 153–167. [Google Scholar]
- Crutsen, J.R.W.; Koper, M.C.; Jelsma, J.; Heymans, M.; Heyligers, I.C.; Grimm, B.; Mathijssen, N.M.C.; Schotanus, M.G.M. Prosthetic hip-associated cobalt toxicity: A systematic review of case series and case reports. EFORT Open Rev. 2022, 7, 188–199. [Google Scholar] [CrossRef]
- Meng, Y.; Xu, J.; Ma, L.; Jin, Z.; Prakash, B.; Ma, T.; Wang, W. A review of advances in tribology in 2020–2021. Friction 2022, 10, 1443–1595. [Google Scholar] [CrossRef]
- Pressato, D.; Battista, A.; Govoni, M.; Vivarelli, L.; Dallari, D.; Pellegrini, A. The intraoperative use of Defensive Antibacterial Coating (DAC®) in the form of a gel to prevent peri-implant infections in orthopaedic surgery: A clinical narrative review. Materials 2023, 16, 5304. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.K.; Abwini, L.Z.; Tang, A.; Yang, J.I.; Keller, D.M.; Menken, L.G.; Liporace, F.A.; Yoon, R.S. Comparative outcomes after treatment of peri-implant, periprosthetic, and interprosthetic femur fractures: Which factors increase mortality risk? OTA Int. Open Access J. Orthop. Trauma 2024, 7, e322. [Google Scholar] [CrossRef] [PubMed]
- Solarino, G.; Bizzoca, D.; Dramisino, P.; Vicenti, G.; Moretti, L.; Moretti, B.; Piazzolla, A. Total hip arthroplasty following the failure of intertrochanteric nailing: First implant or salvage surgery? World J. Orthop. 2023, 14, 763–770. [Google Scholar] [CrossRef]
- Polus, J.S.; Kaptein, B.L.; Lanting, B.A.; Teeter, M.G. Effect of head size, head material, and radiation dose on the repeatability of CT-RSA measurements of femoral head penetration. J. Mech. Behav. Biomed. Mater. 2025, 164, 106907. [Google Scholar] [CrossRef]
- Triantafyllou, A.; Papagiannis, G.; Stasi, S.; Georgios, P.; Koulouvaris, P.; Papagelopoulos, P.J.; Babis, G.C. Biomechanical assessment of wear in ceramic on ceramic and ceramic on xlpe THAs. J. Mech. Med. Biol. 2021, 21, 2150023. [Google Scholar] [CrossRef]
- Buttaro, M.A.; Zanotti, G.; Comba, F.M.; Piccaluga, F. Primary Total Hip Arthroplasty With Fourth-Generation Ceramic-on-Ceramic: Analysis of Complications in 939 Consecutive Cases Followed for 2-10 Years. J. Arthroplast. 2016, 32, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-C.; Kim, R.H. Incidence of Modern Alumina Ceramic and Alumina Matrix Composite Femoral Head Failures in Nearly 6 Million Hip Implants. J. Arthroplast. 2016, 32, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Solarino, G.; Spinarelli, A.; Virgilio, A.; Simone, F.; Baglioni, M.; Moretti, B. Outcomes of ceramic composite in total hip replacement bearings: A single-center series. J. Compos. Sci. 2021, 5, 320. [Google Scholar] [CrossRef]
- Matar, H.E.; van Duren, B.H.; Bloch, B.V.; Berber, R.; James, P.J.; Manktelow, A.R. Lower Risk of Revision With 32- and 36-Millimeter Femoral Heads Compared With 28-mm Heads in Primary Total Hip Arthroplasty: A Comparative Single-Center Study (10,104 Hips). J. Arthroplast. 2024, 39, 991–996. [Google Scholar] [CrossRef]
- Ho, K.W.K.; Chakravertty, R.; van Vliet, A.; Mahomed, N.N. Fracture of a 36-mm ceramic-on-ceramic total hip arthroplasty. Curr. Orthop. Pract. 2012, 23, 258–261. [Google Scholar] [CrossRef]
- Peker, G.; Bala, M.M.; Altun, I. Ceramic femoral head in highly cross-linked polyethylene cup. 5 year results of a randomized trial. Ann. Ital. Chirurgia 2022, 93, 202–209. [Google Scholar]
- Zampogna, B.; Papalia, G.F.; Ferrini, A.; Torre, G.; Vorini, F.; Balzani, L.A.D.; Farsetti, P.; Papalia, R. Dual-mobility total hip arthroplasty in patients younger than 55 years old: A systematic review. Arch. Orthop. Trauma Surg. 2023, 143, 6821–6828. [Google Scholar] [CrossRef]
- Deans, C.F.; Buckner, B.C.; Garvin, K.L. Wear, osteolysis, and aseptic loosening following total hip arthroplasty in young patients with highly cross-linked polyethylene: A review of studies with a follow-up of over 15 years. J. Clin. Med. 2023, 12, 6615. [Google Scholar] [CrossRef]
- Konopitski, A.; Okafor, C.; Smith, B.; Baldwin, K.; Sheth, N.P. Evolution of total hip arthroplasty in patients younger than 30 years of age: A systematic review and meta-analysis. Arch. Orthop. Trauma Surg. 2023, 143, 1081–1094. [Google Scholar] [CrossRef]
- Chapot, A.; Zambelli, P.-Y.; Merckaert, S.R. Functional and patient-related outcomes of total hip arthroplasty in patients younger than 20 years. Arthroplast. Today 2023, 20, 101100. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Liu, K.C.; Gettleman, B.S.; Chen, M.; Piple, A.S.; Yang, J.; Heckmann, N.D.; Christ, A.B. Characteristics of Very Young Patients Undergoing Total Hip Arthroplasty: A Contemporary Assessment. Arthroplast. Today 2024, 25, 101268. [Google Scholar] [CrossRef]
- Hidayat, T.; Ismail, R.; Tauviqirrahman, M.; Saputra, E.; Ammarullah, M.I.; Lamura, M.D.P.; Bayuseno, A.P.; Jamari. Running-in behavior of dual-mobility cup during the gait cycle: A finite element analysis. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2024, 238, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; van der Heide, E. In silico contact pressure of metal-on-metal total hip implant with different materials subjected to gait loading. Metals 2022, 12, 1241. [Google Scholar] [CrossRef]
- Ito, H.; Kaneda, K.; Yuhta, T.; Nishimura, I.; Yasuda, K.; Matsuno, T. Reduction of polyethylene wear by concave dimples on the frictional surface in artificial hip joints. J. Arthroplast. 2000, 15, 332–338. [Google Scholar] [CrossRef]
- Tewelde, F.B.; Allen, Q.; Yu, Q.; Guo, W.; Khan, M.; Zhou, T. Effect of Multiscale Texture Alignment on Friction in Prosthetic Hip Implants. SSRN Electron. J. 2025, in press. [Google Scholar] [CrossRef]
- Sirdeshmukh, N.; Dongre, G. Laser micro & nano surface texturing for enhancing osseointegration and antimicrobial effect of biomaterials: A review. Mater. Today Proc. 2021, 44, 2348–2355. [Google Scholar] [CrossRef]
- Arulkumar, M.; Rangan, R.P.; Ananth, M.P.; Srividhyasakthi, V.; Aaditya, R. Experimental verification on the influence of surface texturing on biomaterials and study of its tribological characteristics. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Rahman, M.; Biswas, A.S.; Hoque, K.N. Recent development on micro-texturing of UHMWPE surfaces for orthopedic bearings: A review. Biotribology 2022, 31, 100216. [Google Scholar] [CrossRef]
- Roy, N.A.P.; Rao, K.J.; Amrita, M. Influence of tool micro-texturing, cooling conditions and cutting conditions on workpiece surface integrity and tool condition. J. Micromanuf. 2025, in press. [Google Scholar] [CrossRef]
- Vaidyanathan, S. Tribological Performance of Ceramic on Textured Polyethylene Contact for Artificial Hip Implants; Rochester Institute of Technology: Rochester, NY, USA, 2024. [Google Scholar]
- Ammarullah, M.I.; Afif, I.Y.; Maula, M.I.; Winarni, T.I.; Tauviqirrahman, M.; Bayuseno, A.P.; Basri, H.; Syahrom, A.; Saad, A.P.M.; Jamari, J. Wear analysis of acetabular cup on metal-on-metal total hip arthroplasty with dimple addition using finite element method. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2022; Volume 2391. [Google Scholar]
- Ammarullah, M.I.; Saad, A.P.M.; Syahrom, A.; Basri, H. Contact pressure analysis of acetabular cup surface with dimple addition on total hip arthroplasty using finite element method. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021; Volume 1034, p. 012001. [Google Scholar]
- Qudeiri, J.E.A.; Abdudeen, A.; Sahadevan, M.R. Numerical investigation on the wear characteristics of hip implant under static loading. Heliyon 2024, 10, e26151. [Google Scholar] [CrossRef]
- Vignesh, G.; Barik, D.; Aravind, S.; Ragupathi, P.; Arun, M. Numerical investigation of dimple-texturing on the turning performance of hardened AISI H-13 steel. Int. J. Simul. Multidiscip. Des. Optim. 2022, 13, 10. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Saad, A.P.M.; Syahrom, A.; Uddin, M.; van der Heide, E.; Basri, H. The effect of bottom profile dimples on the femoral head on wear in metal-on-metal total hip arthroplasty. J. Funct. Biomater. 2021, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Marian, M.; Almqvist, A.; Rosenkranz, A.; Fillon, M. Numerical micro-texture optimization for lubricated contacts—A critical discussion. Friction 2022, 10, 1772–1809. [Google Scholar] [CrossRef]
- Döring, J.; Buchholz, A.; Herbster, M.; Gehring, J.; Betke, U.; Chodór, P.; Zabrzyński, J.; Bertrand, J.; Lohmann, C.H.; Łapaj, Ł. Damage analysis of retrieved Biolox® delta components used in hard and soft bearings. Acta Biomater. 2023, 158, 827–842. [Google Scholar] [CrossRef]
- Canossi, C.M. In Vivo Wear Performance of Total Hip Replacements with Oxinium Femoral Heads: A Retrieval Perspective. Master’s Thesis, University of Illinois at Chicago, Chicago, IL, USA, 2024. [Google Scholar]
- Paione, C.M.; Baino, F. Non-oxide ceramics for bone implant application: State-of-the-art overview with an emphasis on the acetabular cup of hip joint prosthesis. Ceramics 2023, 6, 994–1016. [Google Scholar] [CrossRef]
- Lee, H.H.; Kim, J.S.; Park, J.Y.; Lee, S. Investigation on the wear resistance of AISI 316L with micro dimples as a material for human implants’ structure. J. Mech. Sci. Technol. 2024, 38, 2507–2517. [Google Scholar] [CrossRef]
- Vogel, D.; Wehmeyer, M.; Kebbach, M.; Heyer, H.; Bader, R. Stress and strain distribution in femoral heads for hip resurfacing arthroplasty with different materials: A finite element analysis. J. Mech. Behav. Biomed. Mater. 2021, 113, 104115. [Google Scholar] [CrossRef]
- Sass, J.-O.; Kebbach, M.; Lork, C.; Johannsen, J.; Weinmann, M.; Stenzel, M.; Bader, R. Computational biomechanical study on hybrid implant materials for the femoral component of total knee replacements. J. Mech. Behav. Biomed. Mater. 2024, 158, 106681. [Google Scholar] [CrossRef]
- Pakhaliuk, V.; Poliakov, A.; Fedotov, I. The ceramic modular head improvement in the design of a total hip replacement. Facta Univ. Ser. Mech. Eng. 2021, 19, 067–078. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, X.; Feng, Z.; Cao, S.-J. Application of polyhedral meshing strategy in indoor environment simulation: Model accuracy and computing time. Indoor Built Environ. 2022, 31, 719–731. [Google Scholar] [CrossRef]
- Li, Y.; Okaze, T. Application of polyhedral meshes in large-eddy simulation of building array flow fields within neutral and unstable boundary layers. Build. Environ. 2024, 263, 111846. [Google Scholar] [CrossRef]
- Schneider, T.; Hu, Y.; Gao, X.; Dumas, J.; Zorin, D.; Panozzo, D. A large-scale comparison of tetrahedral and hexahedral elements for solving elliptic PDEs with the finite element method. ACM Trans. Graph. 2022, 41, 1–14. [Google Scholar] [CrossRef]
- Prasetiyo, A.B.; Sekarjati, K.A. Finite element simulation of power weeder machine frame. Indones. J. Comput. Eng. Des. 2022, 4, 25–34. [Google Scholar] [CrossRef]
- Bavil, A.Y.; Eghan-Acquah, E.; Diamond, L.E.; Barrett, R.; Carty, C.P.; Barzan, M.; Nasseri, A.; Lloyd, D.G.; Saxby, D.J.; Feih, S. Effect of different constraining boundary conditions on simulated femoral stresses and strains during gait. Sci. Rep. 2024, 14, 10808. [Google Scholar] [CrossRef]
- Gee, M.; Kamps, T.; Woolliams, P. A new paradigm in uniaxial wear testing for ceramics and ceramic coatings. Int. J. Refract. Met. Hard Mater. 2025, 130, 107127. [Google Scholar] [CrossRef]
- Bergmann, G.; Bender, A.; Dymke, J.; Duda, G.; Damm, P. Standardized Loads Acting in Hip Implants. PLoS ONE 2016, 11, e0155612. [Google Scholar] [CrossRef] [PubMed]
- Nagentrau, M.; Tobi, A.M.; Jamian, S.; Otsuka, Y.; Hussin, R. Delamination-fretting wear failure evaluation at HAp-Ti-6Al–4V interface of uncemented artificial hip implant. J. Mech. Behav. Biomed. Mater. 2021, 122, 104657. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Chen, F.; Ying, R.; Zhang, L. Ceramic–Titanium Alloy Artificial Hip Joint Wear Simulation and Experimental Study. Coatings 2023, 13, 1321. [Google Scholar] [CrossRef]
- Tang, K.; Deng, Z.; Wang, T.; Nie, M. Aseptic Loosening after total hip arthroplasty secondary to the disappearing coating? Asian J. Surg. 2022, 45, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Apostu, D.; Lucaciu, O.; Berce, C.; Lucaciu, D.; Cosma, D. Current methods of preventing aseptic loosening and improving osseointegration of titanium implants in cementless total hip arthroplasty: A review. J. Int. Med Res. 2018, 46, 2104–2119. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Gu, J.; Zhou, Y. Primary total hip arthroplasty failure: Aseptic loosening remains the most common cause of revision. Am. J. Transl. Res. 2022, 14, 7080–7089. [Google Scholar]
- Lee, C.R.; Kim, C.W.; Park, D.H.; Kwon, Y.U.; Yoon, J.M. Risk of infection after septic and aseptic revision total knee arthroplasty: A systematic review. JBJS 2023, 105, 1630–1637. [Google Scholar] [CrossRef]
- Apostu, D.; Piciu, D.; Oltean-Dan, D.; Cosma, D.; Lucaciu, O.; Popa, C.; Mester, A.; Benea, H. How to prevent aseptic loosening in cementless arthroplasty: A review. Appl. Sci. 2022, 12, 1571. [Google Scholar] [CrossRef]
- Liu, F.; He, Y.; Gao, Z.; Jiao, D. Enhanced computational modelling of UHMWPE wear in total hip joint replacements: The role of frictional work and contact pressure. Wear 2021, 482–483, 203985. [Google Scholar] [CrossRef]
- Raj, R.; Singh, G. A review on process prerequisites and biomedical applications of additively manufactured zirconia. Eng. Sci. Technol. Int. J. 2024, 59, 101876. [Google Scholar] [CrossRef]
- Nikpour, S. Corrosion and Mechanical Wear of Biomedical Metallic Implants and Corresponding Health Aspects. Ph.D. Thesis, The University of Western Ontario, London, ON, Canada, 2025. [Google Scholar]
- Sutter, L.; Hall, D.J.; Bischoff, L.; Dommann-Scherrer, C.; Schläppi, M.; Pourzal, R.; Hallab, N.; Meier, C.; Wahl, P. How In Vivo Alteration of Hip Replacement Wear Mode Can Cause a Voluminous Inflammatory Reaction and an Excessive Titanium Exposure. J. Clin. Med. 2025, 14, 210. [Google Scholar] [CrossRef] [PubMed]
- Ude, C.C.; Esdaille, C.J.; Ogueri, K.S.; Kan, H.-M.; Laurencin, S.J.; Nair, L.S.; Laurencin, C.T. The mechanism of metallosis after total hip arthroplasty. Regen. Eng. Transl. Med. 2021, 7, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Cannella, A.; Greco, T.; Polichetti, C.; De Martino, I.; Mascio, A.; Maccauro, G.; Perisano, C. A rare case of adverse reaction to metal debris in a ceramic-on-ceramic total hip replacement. J. Funct. Biomater. 2022, 13, 145. [Google Scholar] [CrossRef]
- Sathishkumar, S.; Paulraj, J.; Chakraborti, P.; Muthuraj, M. Comprehensive review on biomaterials and their inherent behaviors for hip repair applications. ACS Appl. Bio Mater. 2023, 6, 4439–4464. [Google Scholar] [CrossRef] [PubMed]
- Saracco, M.; Ciriello, V.; Spinarelli, A.; Solarino, G.; Goderecci, R.; Logroscino, G. Ceramic-on-Metal Bearing in Total Hip Arthroplasty—Was It So Bad? A Narrative Review and a Critical Analysis of the Literature. J. Compos. Sci. 2023, 8, 9. [Google Scholar] [CrossRef]
- Goswami, C.; Patnaik, A.; Bhat, I.; Singh, T. Mechanical physical and wear properties of some oxide ceramics for hip joint application: A short review. Mater. Today Proc. 2021, 44, 4913–4918. [Google Scholar] [CrossRef]
- Nikam, N.; Shenoy, B.S.; KN, C.; Keni, L.G.; Shetty, S.; Bhat, N.S. Advancements in Surface Coatings for Enhancing Longevity in Hip Implants: A Review. Prosthesis 2025, 7, 21. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, J.W.; Ha, J.H.; Lee, Y.K.; Ha, Y.C.; Koo, K.H. Third-generation ceramic-on-ceramic total hip arthroplasty in patients with osteonecrosis of the femoral head: A 10-to 16-year follow-up study. J. Bone Jt. Surg. 2022, 104 (Suppl. S2), 68–75. [Google Scholar] [CrossRef]
- Yin, C.; Eberhardt, L.; Cederman, M.; Haley, H.; Steffensmeier, A.; Karadsheh, M. Fourth-generation Ceramic Head Fracture in Total Hip Arthroplasty: A Case Report and Literature Review. Arthroplast. Today 2025, 32, 101614. [Google Scholar] [CrossRef]
- Blumenfeld, T.J.; Politi, J.; Croker, S.; O’dell, T.; Hamilton, W. Long-term results of delta ceramic-on-ceramic total hip arthroplasty. Arthroplast. Today 2022, 13, 130–135. [Google Scholar] [CrossRef]
- Prasad, K.N.; Ramkumar, P. Effect of geometrical, operational and material parameters in the lubrication regime of hard-on-hard hip implants. Mater. Chem. Phys. 2024, 317, 129171. [Google Scholar] [CrossRef]
- Fraval, A.; Parvizi, J. Bearing Surfaces in Patients Undergoing Total Hip Arthroplasty with Osteonecrosis of the Femoral Head. In Osteonecrosis; Springer Nature: Singapore, 2025; pp. 469–478. [Google Scholar]
- Shaikh, N.; Shenoy, B.S.; Bhat, N.S.; Shetty, S.; KN, C. Wear estimation at the contact surfaces of oval shaped hip implants using finite element analysis. Cogent Eng. 2023, 10, 2222985. [Google Scholar] [CrossRef]
- Choudhari, A.; Gupta, A.K.; Kumar, A.; Kumar, A.; Gupta, A.; Chowdhury, N.; Kumar, A. Wear and friction mechanism study in knee and hip rehabilitation: A comprehensive review. In Applications of Biotribology in Biomedical Systems; Springer: Cham, Switzerland, 2024; pp. 345–432. [Google Scholar]
- Joshi, B.; Tripathi, K.; Gyawali, G.; Lee, S.W. The effect of laser surface texturing on the tribological performance of different sialon ceramic phases. Prog. Nat. Sci. 2016, 26, 415–421. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, B.; He, J.; Lu, X. The optimization design for the journal-thrust couple bearing surface texture based on particle swarm algorithm. Tribol. Int. 2024, 198, 109874. [Google Scholar] [CrossRef]
- Muchammad, M.; Tauviqirrahman, M.; Ammarullah, M.I.; Iqbal, M.; Setiyana, B.; Jamari, J. Performance of textured dual mobility total hip prosthesis with a concave dimple during Muslim prayer movements. Sci. Rep. 2024, 14, 916. [Google Scholar] [CrossRef] [PubMed]
- Tauviqirrahman, M.; Husein, H.R.; Muchammad. Effect of surface texturing on the performance of artificial hip joint for Muslim prayer (Salat) activity. Biotribology 2021, 26, 100177. [Google Scholar] [CrossRef]
- Popov, V.L.; Poliakov, A.M.; Pakhaliuk, V.I. Is it possible to create an “ideal endoprosthesis” for an “ideal total hip replacement”? Prosthesis 2023, 5, 1020–1036. [Google Scholar] [CrossRef]
- Roy, T.; Choudhury, D.; Ghosh, S.; Bin Mamat, A.; Pingguan-Murphy, B. Improved friction and wear performance of micro dimpled ceramic-on-ceramic interface for hip joint arthroplasty. Ceram. Int. 2015, 41, 681–690. [Google Scholar] [CrossRef]
- Huang, W.; Wang, X. Biomimetic design of elastomer surface pattern for friction control under wet conditions. Bioinspiration Biomimetics 2013, 8, 046001. [Google Scholar] [CrossRef]
- Shen, C.; Khonsari, M. Numerical optimization of texture shape for parallel surfaces under unidirectional and bidirectional sliding. Tribol. Int. 2015, 82, 1–11. [Google Scholar] [CrossRef]
- Callea, C.; Ceddia, M.; Piattelli, A.; Specchiulli, A.; Trentadue, B. Finite element analysis (FEA) for a different type of cono-in dental implant. Appl. Sci. 2023, 13, 5313. [Google Scholar] [CrossRef]
- Comuzzi, L.; Ceddia, M.; Di Pietro, N.; Inchingolo, F.; Inchingolo, A.M.; Romasco, T.; Tumedei, M.; Specchiulli, A.; Piattelli, A.; Trentadue, B. Crestal and subcrestal placement of morse cone implant–abutment connection implants: An in vitro finite element analysis (FEA) Study. Biomedicines 2023, 11, 3077. [Google Scholar] [CrossRef] [PubMed]
- Sciammarella, C.A.; Singh, B.; Trentadue, B.; Sciammarella, F.M. Stress analysis of weldments by holographic moiré and the finite element method. Exp. Mech. 2000, 40, 15–21. [Google Scholar] [CrossRef]
- Ceddia, M.; Lamberti, L.; Trentadue, B. FEA comparison of the mechanical behavior of three dental crown materials: Enamel, ceramic, and zirconia. Materials 2024, 17, 673. [Google Scholar] [CrossRef] [PubMed]
- Ceddia, M.; Romasco, T.; Comuzzi, L.; Cipollina, A.; Piattelli, A.; Dipalma, G.; Inchingolo, A.M.; Inchingolo, F.; Di Pietro, N.; Trentadue, B. The influence of cement thickness within the cap on stress distribution for dental implants. J. Funct. Biomater. 2024, 15, 199. [Google Scholar] [CrossRef] [PubMed]
- Ceddia, M.; Trentadue, B. Evaluation of rotational stability and stress shielding of a stem optimized for hip replacements—A finite element study. Prosthesis 2023, 5, 678–693. [Google Scholar] [CrossRef]
- Ceddia, M.; Solarino, G.; Cassano, G.D.; Trentadue, B. Finite Element Study on Stability in the Femoral Neck and Head Connection to Varying Geometric Parameters with the Relates Implications on the Effect of Wear. J. Compos. Sci. 2023, 7, 387. [Google Scholar] [CrossRef]
- Elkins, J.M.; Callaghan, J.J.; Brown, T.D. Stability and trunnion wear potential in large-diameter metal-on-metal total hips: A finite element analysis. Clin. Orthop. Relat. Res. 2014, 472, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Tewelde, F.B.; Allen, Q.; Zhou, T. Multiscale Texture Features to Enhance Lubricant Film Thickness for Prosthetic Hip Implant Bearing Surfaces. Lubricants 2024, 12, 187. [Google Scholar] [CrossRef]
- Sagbas, B.; Durakbasa, M.N.; Sagbas, M.; Koyun, A. Measurement and theoretical determination of frictional temperature rise between sliding surfaces of artificial hip joints. Measurement 2014, 51, 411–419. [Google Scholar] [CrossRef]
- Ji, M.; Zhang, H.; Xu, J.; Li, C.; Yu, D.; Chen, M.; El Mansori, M. Toward the mechanisms of surface texturing on the wear behavior of dental zirconia ceramics under dry and saliva lubricated conditions. Wear 2021, 484–485, 203845. [Google Scholar] [CrossRef]





| Components | Young’s Modulus [GPa] | Poisson’s Ratio |
|---|---|---|
| Femoral head | 350 | 0.26 |
| Liner | 210 | 0.26 |
| Activity | Maximum Contact Force (% of Body Weight) or Activity Factor |
|---|---|
| Going upstairs | 2.6 |
| Going downstairs | 2.51 |
| Fast walking | 2.5 |
| Slow walking | 2.42 |
| Sitting down | 1.56 |
| Sliding Distance (mm) | |
|---|---|
| Without dimples | 0.01832 |
| With dimples | 0.00874 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceddia, M.; Morizio, A.; Solarino, G.; Trentadue, B. Reduction of Ceramic Wear by Concave Dimples on the Bearing Surface in CoC Hip Implants: A Finite Element Analysis. Ceramics 2025, 8, 51. https://doi.org/10.3390/ceramics8020051
Ceddia M, Morizio A, Solarino G, Trentadue B. Reduction of Ceramic Wear by Concave Dimples on the Bearing Surface in CoC Hip Implants: A Finite Element Analysis. Ceramics. 2025; 8(2):51. https://doi.org/10.3390/ceramics8020051
Chicago/Turabian StyleCeddia, Mario, Arcangelo Morizio, Giuseppe Solarino, and Bartolomeo Trentadue. 2025. "Reduction of Ceramic Wear by Concave Dimples on the Bearing Surface in CoC Hip Implants: A Finite Element Analysis" Ceramics 8, no. 2: 51. https://doi.org/10.3390/ceramics8020051
APA StyleCeddia, M., Morizio, A., Solarino, G., & Trentadue, B. (2025). Reduction of Ceramic Wear by Concave Dimples on the Bearing Surface in CoC Hip Implants: A Finite Element Analysis. Ceramics, 8(2), 51. https://doi.org/10.3390/ceramics8020051







