Barium-Impregnated Ag3PO4 for Enhanced Visible Light Photocatalytic Degradation of Methyl Orange
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Characterization of Materials
2.4. Photocatalytic Activity Measurements
3. Results
3.1. Characterization
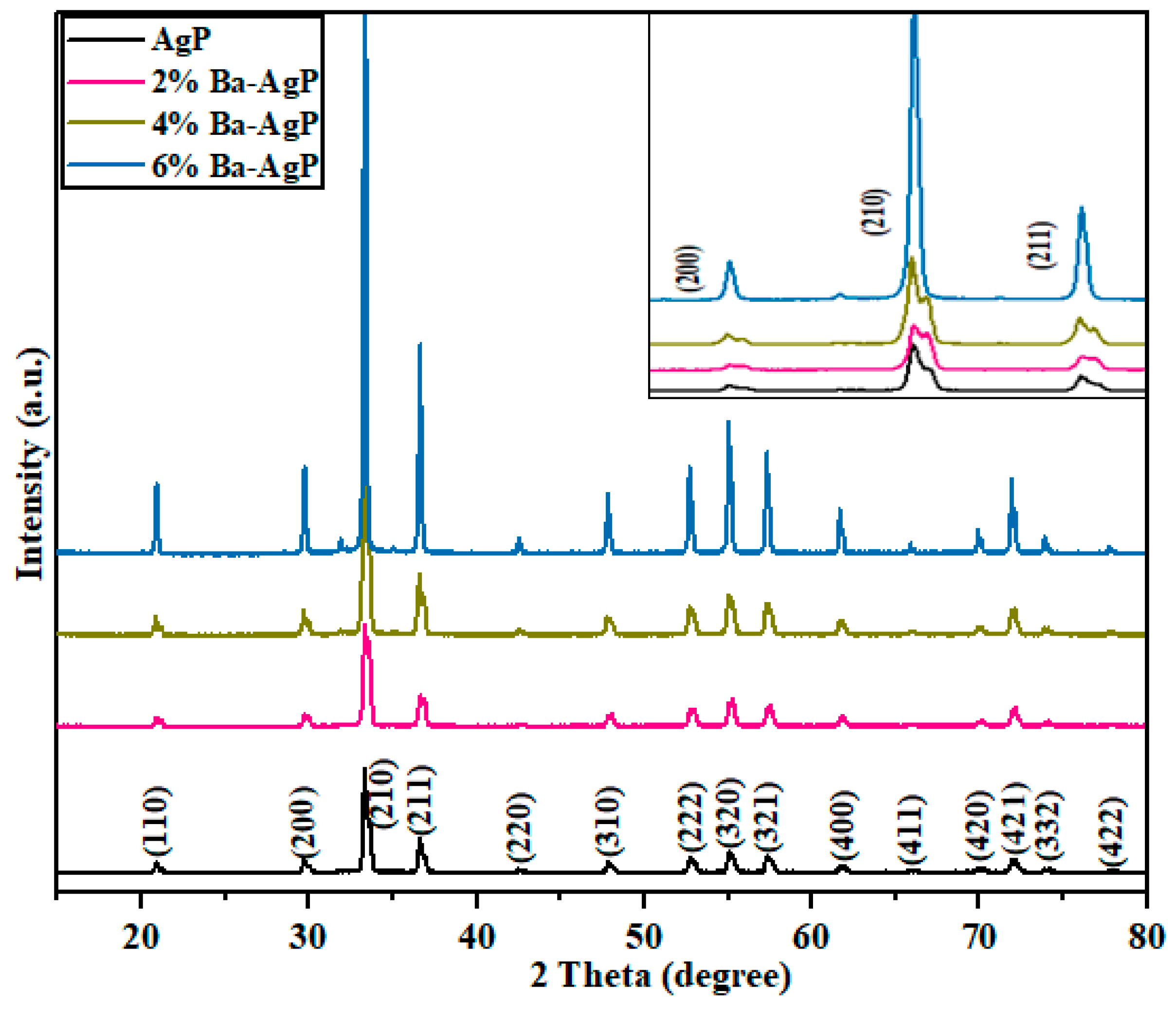
3.1.1. XRD Analysis
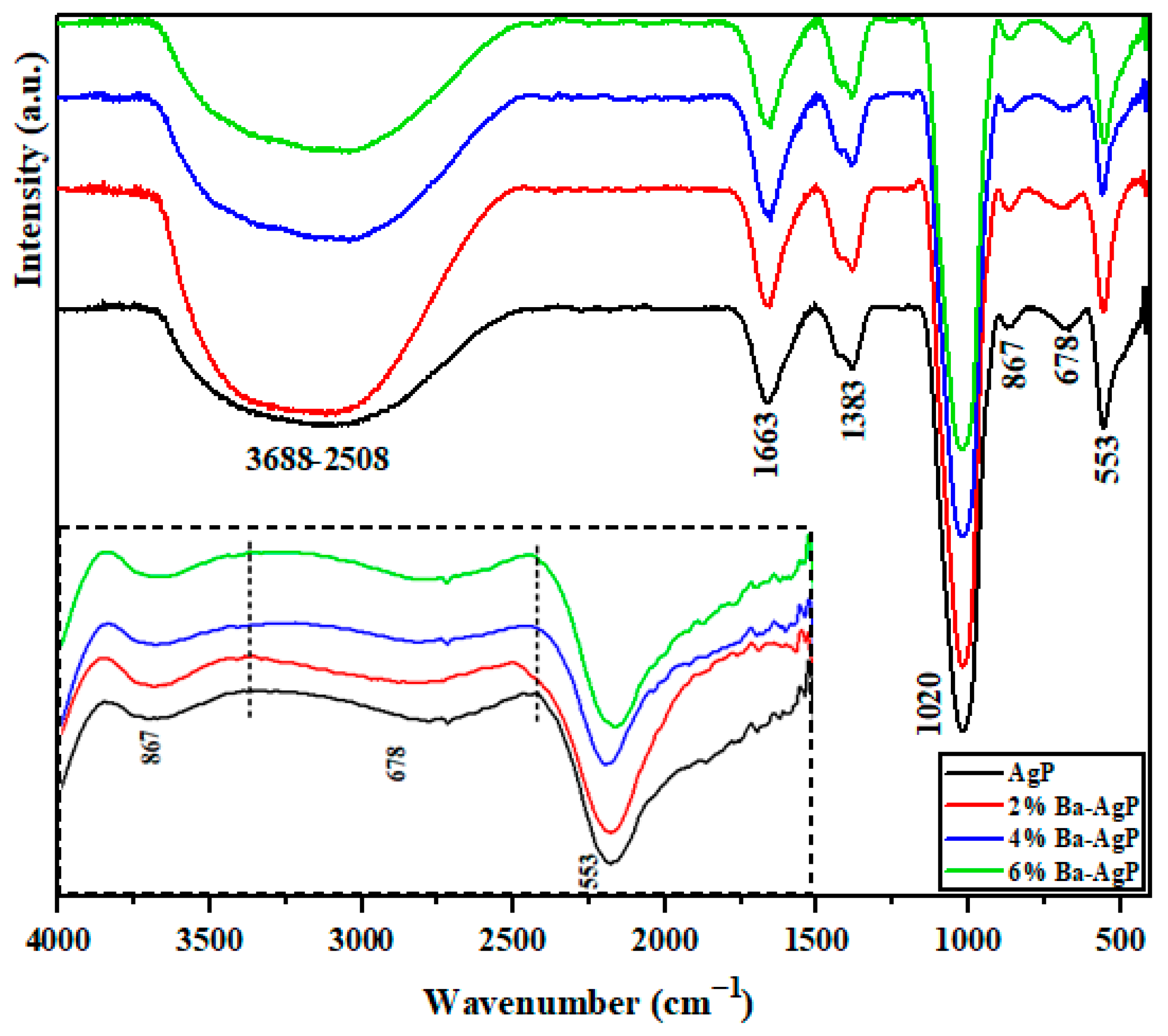
3.1.2. FTIR Analysis
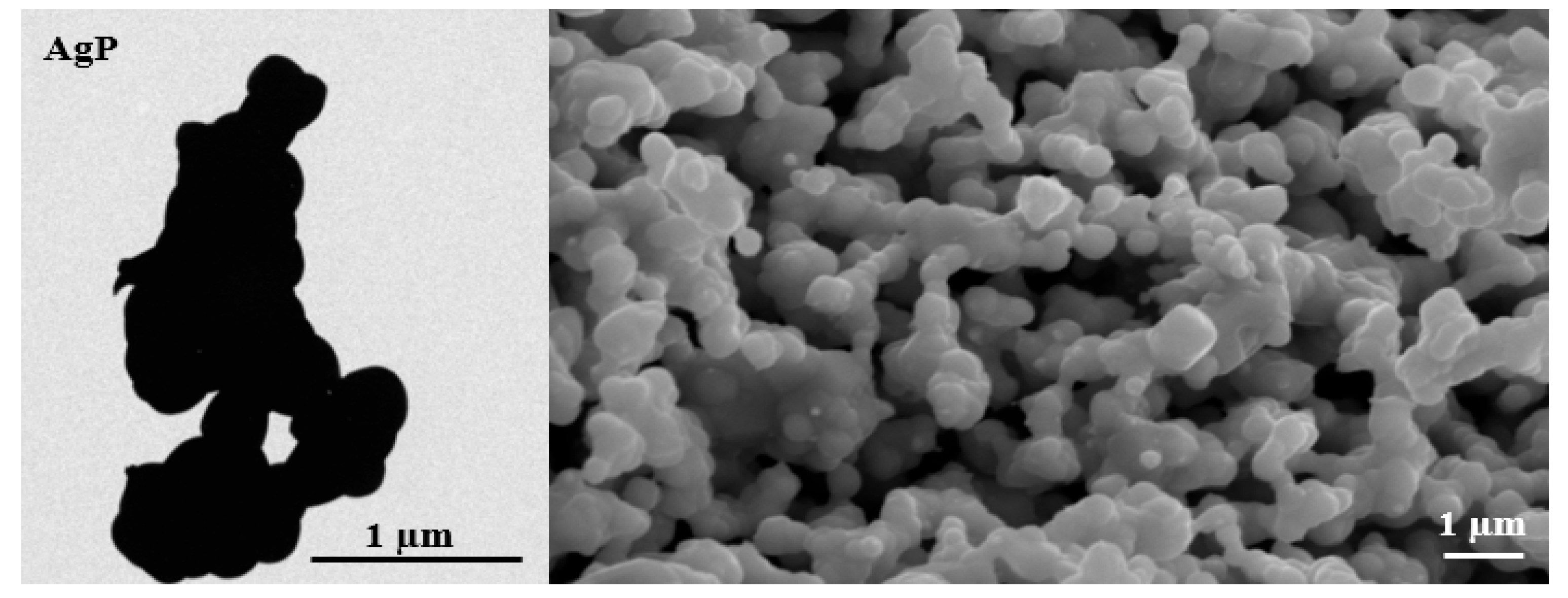
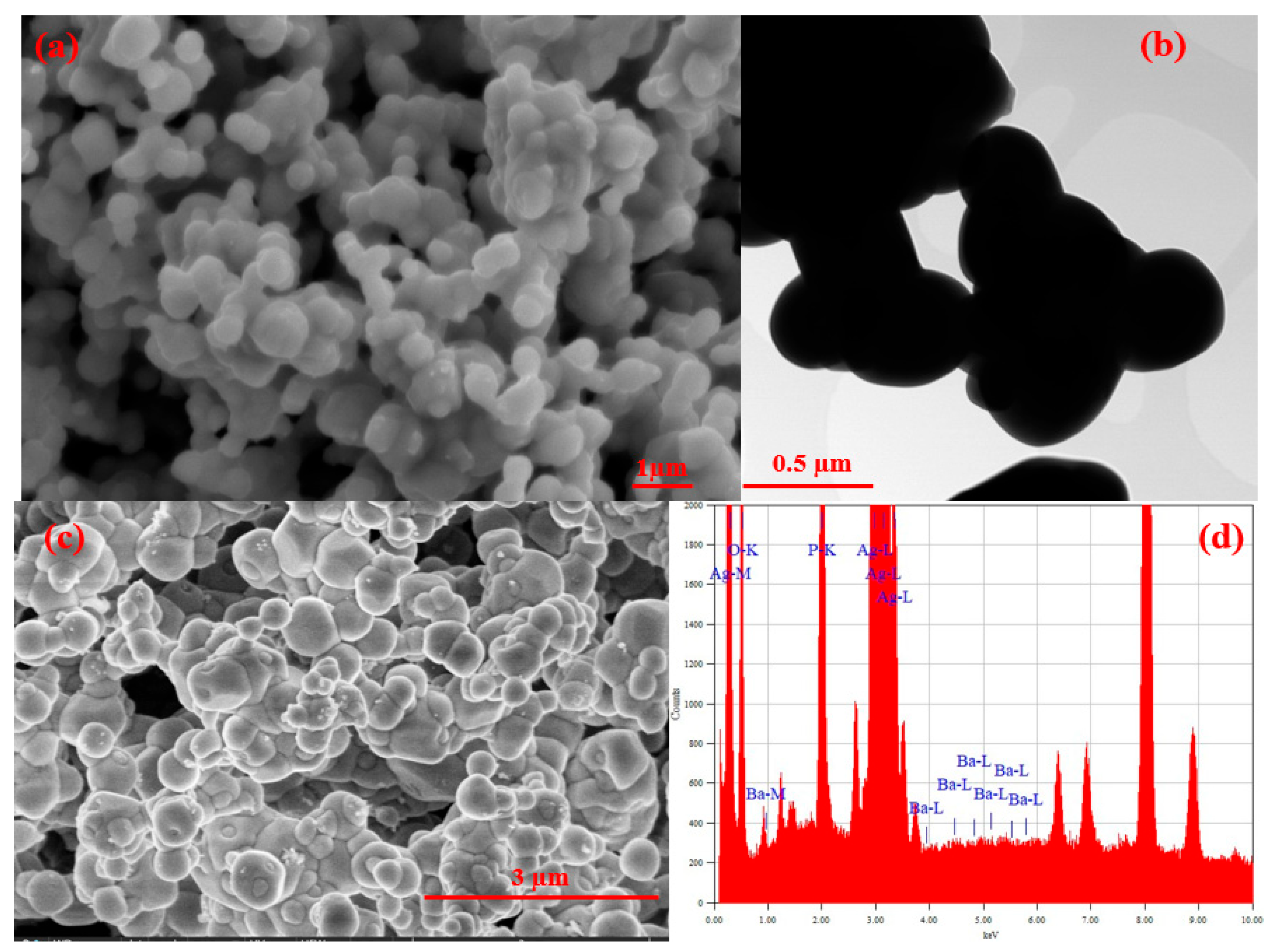
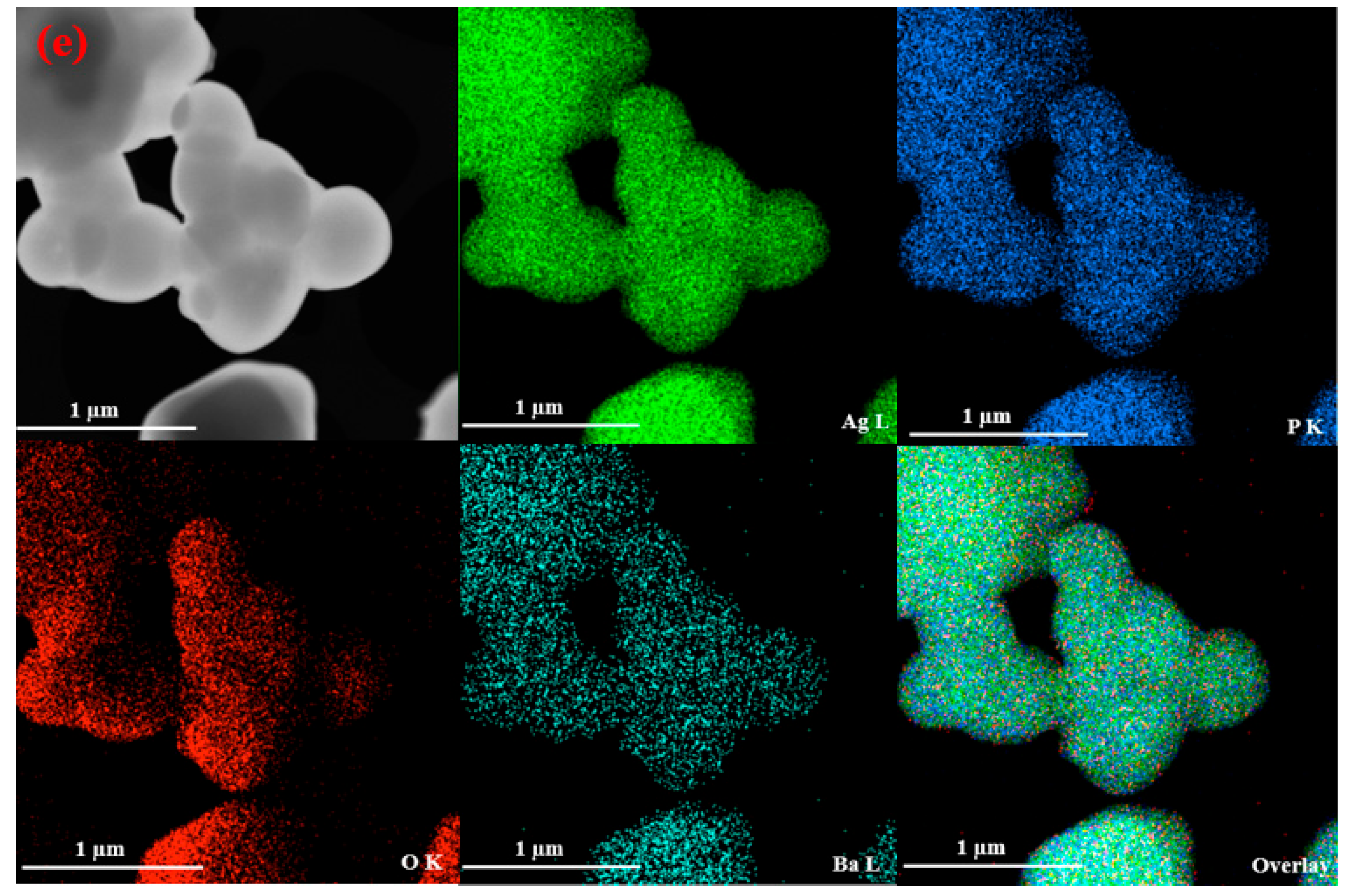
3.1.3. SEM, TEM, FESEM, and HRTEM/EDX Analysis
3.1.4. UV–Vis DRS Analysis
3.2. Photocatalytic Activity Evaluation
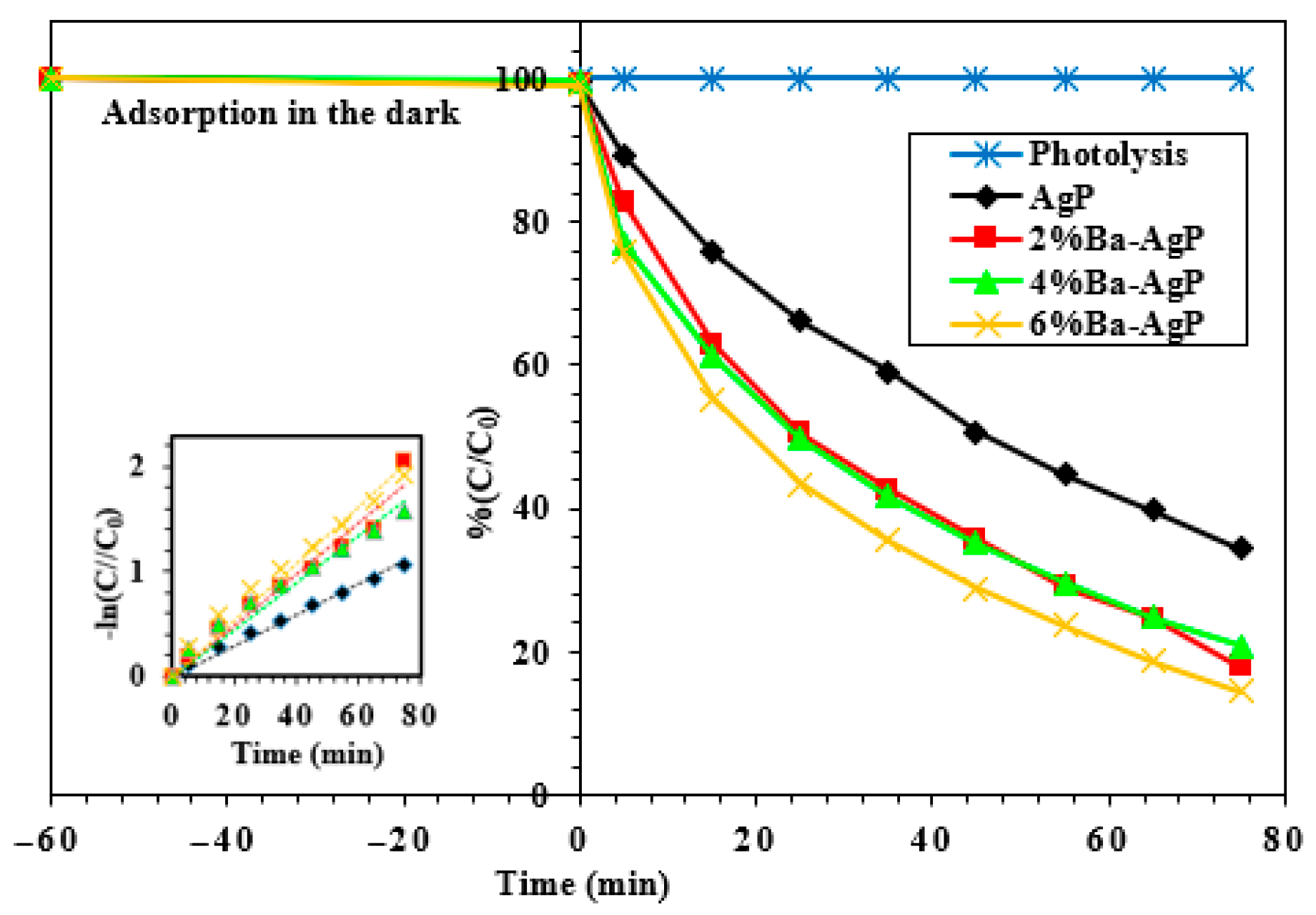
3.2.1. Effect of Ba on the Surface of AgP
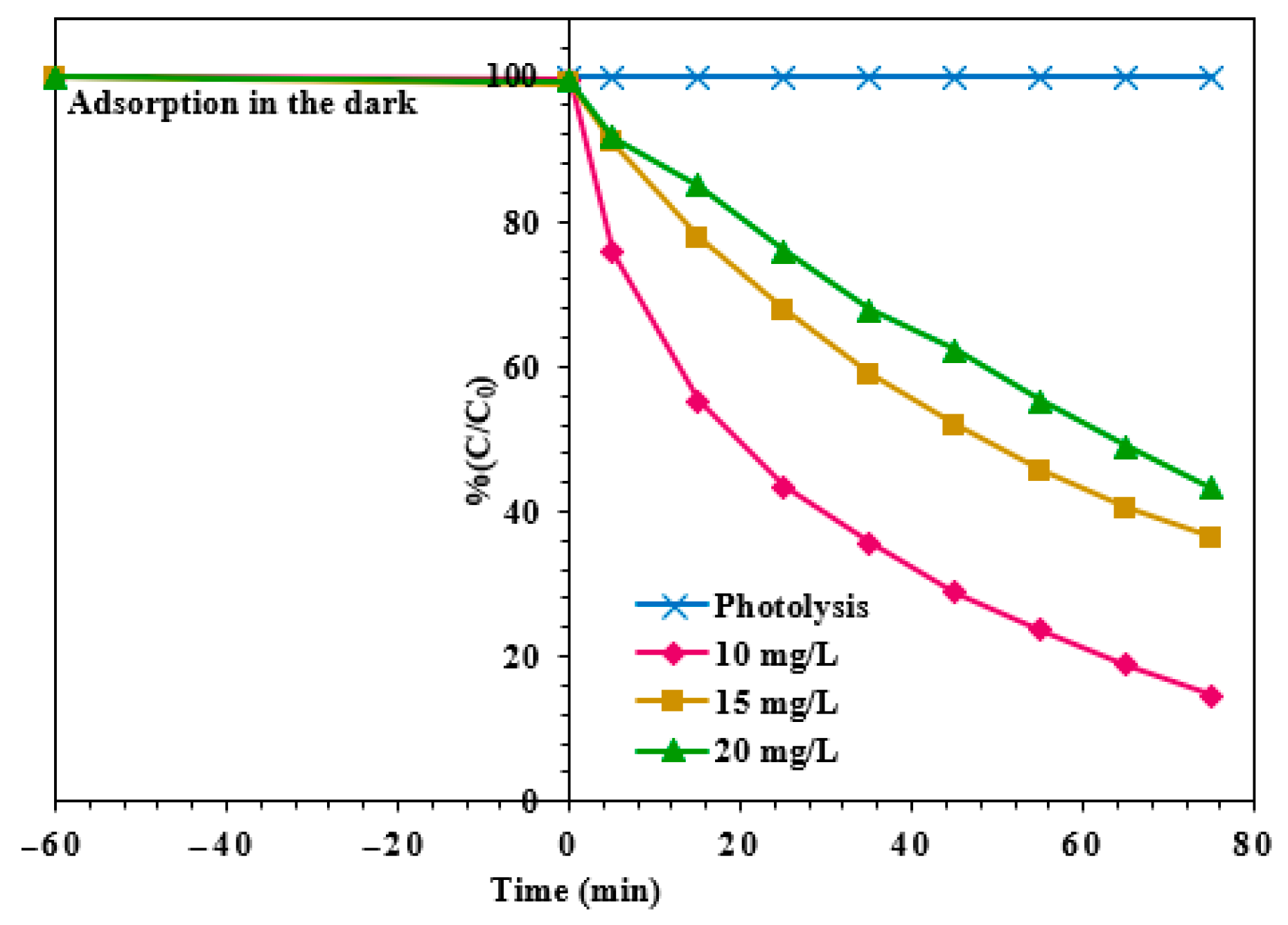
3.2.2. Effect of MO Concentration
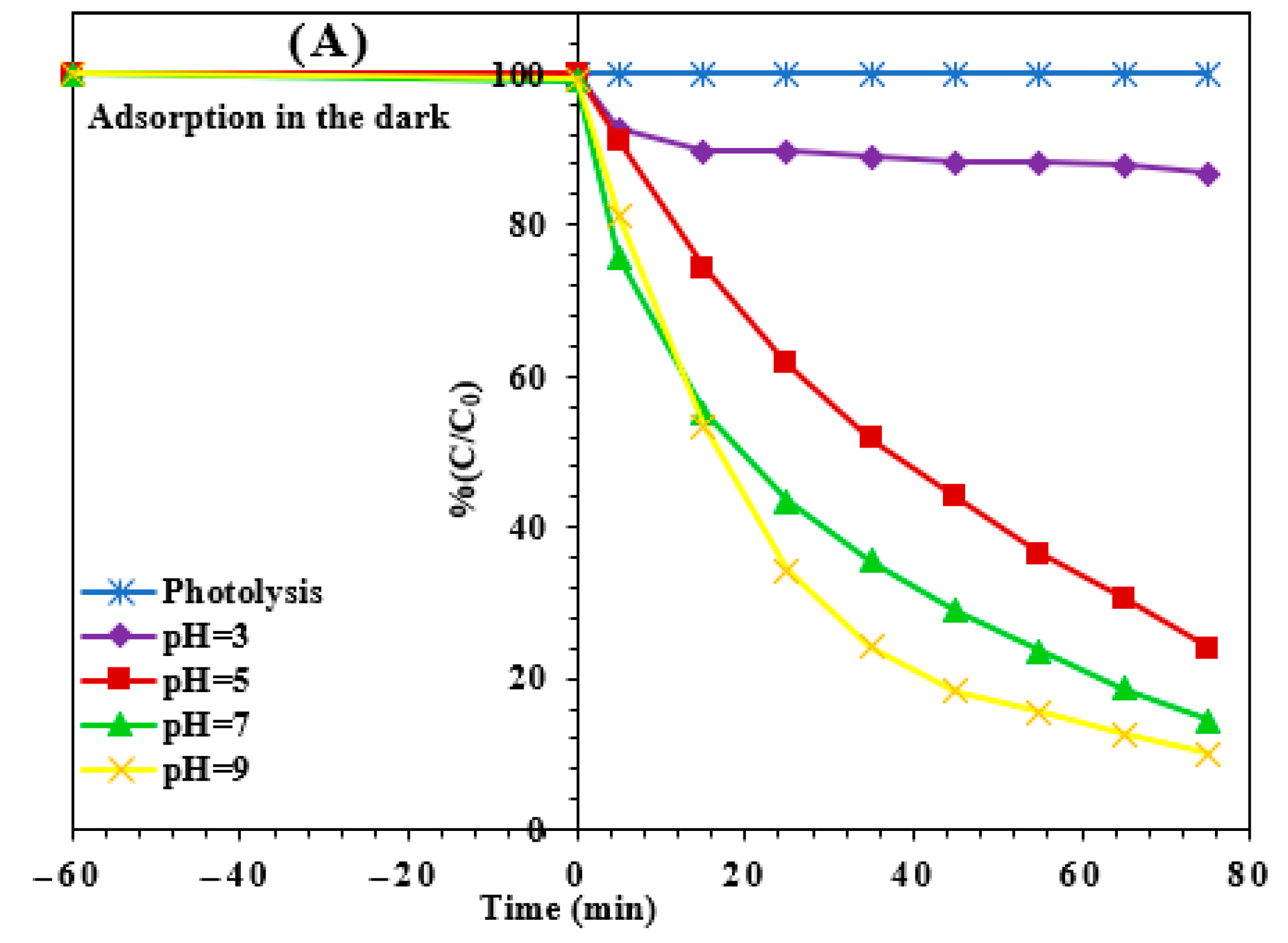
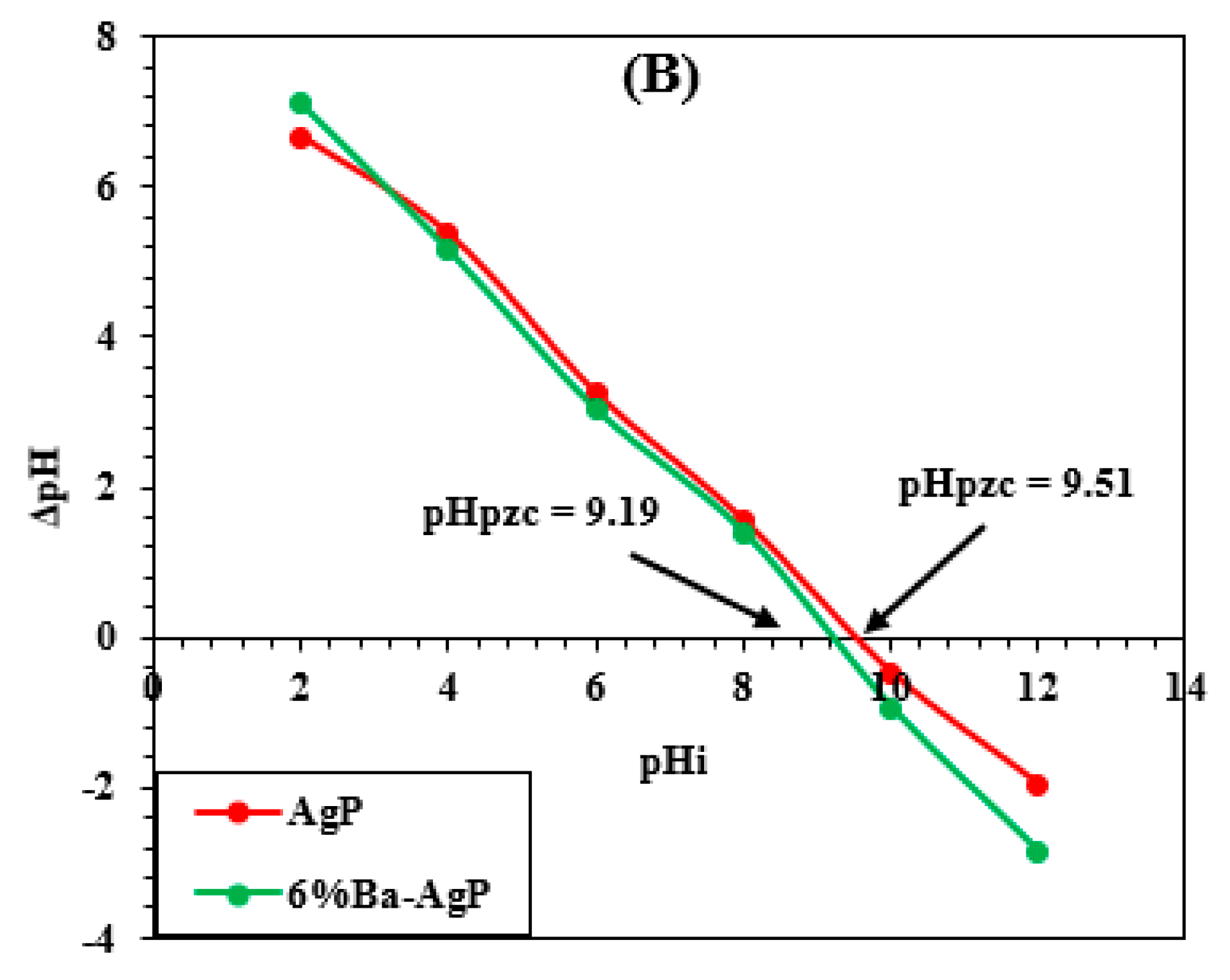
3.2.3. Effect of pH
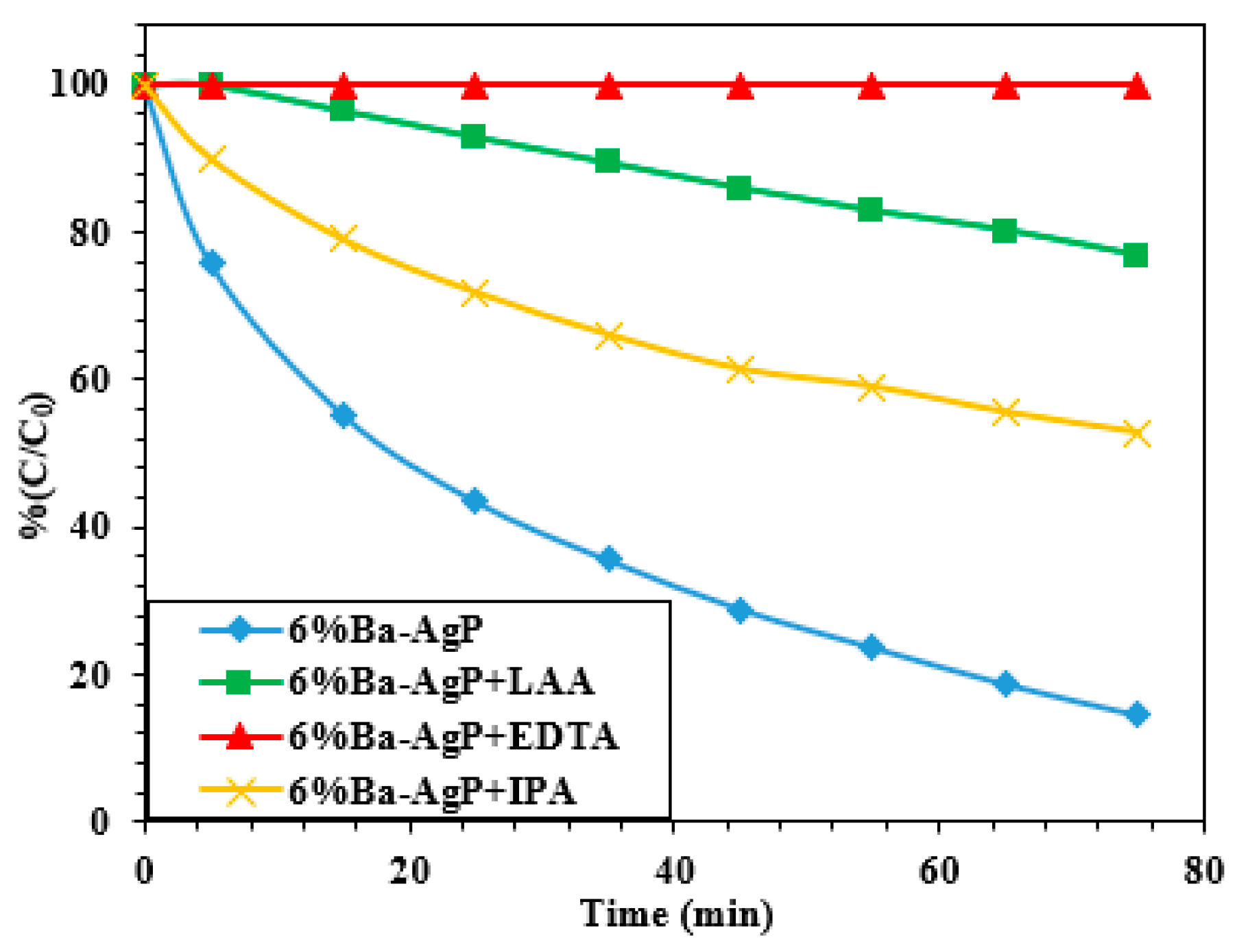
3.2.4. Photocatalytic Mechanism
3.2.5. Recyclability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amirulsyafiee, A.; Khan, M.M.; Harunsani, M.H. Ag3PO4 and Ag3PO4—based visible light active photocatalysts: Recent progress, synthesis, and photocatalytic applications. Catal. Commun. 2022, 172, 106556. [Google Scholar] [CrossRef]
- Luo, L.; Li, Y.; Hou, J.; Yang, Y. Visible potocatalysis and photostability of Ag3PO4 photocatalyst. Appl. Surf. Sci. 2014, 319, 332–338. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, C.; Yuan, J.; Wang, Z.; Wang, Y.; Zhou, S.; Gu, P.; Li, Y. Extended construction strategies of Ag3PO4-based heterojunction photocatalysts for robust environmental applications. J. Environ. Chem. Eng. 2023, 11, 110705. [Google Scholar] [CrossRef]
- Fechete, I.; Wang, Y.; Védrine, J.C. The past, present and future of heterogeneous catalysis. Catal. Today 2012, 189, 2–27. [Google Scholar] [CrossRef]
- Trench, A.B.; Alvarez, R.; Teodoro, V.; da Trindade, L.G.; Machado, T.R.; Teixeira, M.M.; de Souza, D.; Pinatti, I.M.; Simões, A.Z.; Gobato, Y.G.; et al. Interface matters: Design of an efficient α-Ag2WO4/Ag3PO4 photocatalyst. J. Mater. Chem. Phys. 2022, 280, 125710. [Google Scholar] [CrossRef]
- Kotp, M.G.; Kuo, S.-W. Harnessing solar energy with porous organic polymers: Advancements, challenges, economic, environmental impacts and future prospects in sustainable photocatalysis. Mater. Today Chem. 2024, 41, 102299. [Google Scholar] [CrossRef]
- Khiar, H.; Valencia-Valero, L.C.; Puga, A.; Barka, N. α-Ag2WO4/g-C3N4: Investigation of the synthesis medium on composite properties and H2 evolution under simulated sunlight. Environ. Res. 2025, 276, 121514. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, H.; Li, M.; Zhang, H.; Wei, L.; Yang, X. Synthesis and application of Ag3PO4 photocatalyst modified by g-C3N4 for tetracycline hydrochloride degradation. Solid State Sci. 2024, 155, 107654. [Google Scholar] [CrossRef]
- Sari, F.N.I.; Syue, M.T.; Purba, Y.; Ting, J.M. Fabrication of binary Ag3PO4 photocatalysts for enhanced photocatalytic degradation: Effect of PEDOT hole conductor and hybridized 1 T-containing MoS2 electron conductor. Sep. Purif. Technol. 2022, 278, 119650. [Google Scholar] [CrossRef]
- Chu, Y.; Miao, B.; Zheng, X.; Su, H. Fabrication of flower-globular Bi2WO6/BiOI@Ag3PO4 photocatalyst for the degradation of bisphenol A and cefepime under sunlight: Photoelectric properties, degradation performance, mechanism and biodegradability enhancement. Sep. Purif. Technol. 2021, 272, 118866. [Google Scholar] [CrossRef]
- Chen, R.; Ding, S.; Fu, N.; Ren, X. Preparation of a g-C3N4/Ag3PO4 composite Z-type photocatalyst and photocatalytic degradation of Ofloxacin: Degradation performance, reaction mechanism, degradation pathway and toxicity evaluation. J. Environ. Chem. Eng. 2023, 11, 109440. [Google Scholar] [CrossRef]
- Khiar, H.; Janani, F.Z.; Elhalil, A.; Sadiq, M.; Barka, N. Ni2+ grafted Ag3PO4: Enhanced photocatalytic performance under visible light. Appl. Surf. Sci. Adv. 2022, 11, 100288. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, L.; Liu, Y.; Hengchao, E.; Zhao, Z.; Chu, H.; Zhou, X.; Zhang, Y.; Zou, G. Novel synthesis of sulfur-doped Ag3PO4 photocatalyst for efficient degradation of cylindrospermopsin. Chem. Eng. J. 2025, 504, 158462. [Google Scholar] [CrossRef]
- Amirulsyafiee, A.; Khan, M.M.; Khan, M.Y.; Khan, A.; Harunsani, M.H. Visible light active La-doped Ag3PO4 for photocatalytic degradation of dyes and reduction of Cr(VI). Solid State Sci. 2022, 131, 106950. [Google Scholar] [CrossRef]
- Lu, R.; Luo, J.; Wang, X.; Chen, A.; Xie, Y.; Xu, Y.; Shang, T.; Li, R.; Jiang, D.; Zhan, Q. A Co-doped Ag3PO4 photocatalyst: Efficient degradation of organic pollutants and enhancement mechanism. Mol. Catal. 2024, 558, 114033. [Google Scholar] [CrossRef]
- Amirulsyafiee, A.; Khan, M.M.; Khan, A.; Khan, M.Y.; Harunsani, M.H. Influence of Zr doping on Ag3PO4 for photocatalytic degradation of dyes and Cr(VI) reduction under visible light irradiation. Mater. Chem. Phys. 2022, 291, 126673. [Google Scholar] [CrossRef]
- Khiar, H.; Janani, F.Z.; Sadiq, M.; Mansouri, S.; Puga, A.; Barka, N. Effect of indium (III) doping on Ag3PO4 catalyst stabilization and its visible light photocatalytic activity toward toxic dyes in water. Environ. Sci. Pollut. Res. 2023, 30, 100785–100798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Z.; Li, B.; Dai, W.; Si, Y.; Yang, L.; Luo, S. Hierarchical Ag3PO4@ZnIn2S4 nanoscoparium: An innovative Z-scheme photocatalyst for highly efficient and predictable tetracycline degradation. J. Colloid Interface Sci. 2021, 586, 708–718. [Google Scholar] [CrossRef]
- Yin, H.; Cao, Y.; Fan, T.; Zhang, M.; Yao, J.; Li, P.; Chen, S.; Liu, X. In situ synthesis of Ag3PO4/C3N5 Z-scheme heterojunctions with enhanced visible-light-responsive photocatalytic performance for antibiotics removal. Sci. Total Environ. 2021, 754, 141926. [Google Scholar] [CrossRef]
- Promnopas, S.; Promnopas, W.; Maisang, W.; Wannapop, S.; Thongtem, T.; Thongtem, S.; Wiranwetchayan, O. Synthesis of Ag3PO4/Ag4P2O7 by microwave-hydrothermal method for enhanced UV–visible photocatalytic performance. Sci. Rep. 2023, 13, 4742. [Google Scholar] [CrossRef]
- Khiar, H.; Janani, F.Z.; Sadiq, M.; Qourzal, S.; Puga, A.; Barka, N. MoO3 decorated Ag3PO4: n-n heterojunction for high photocatalytic performance and improved stability. Mater. Res. Bull. 2025, 184, 113253. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, S.; Li, S.; Zhou, X.; Yin, J.; Liu, P.; Shi, W.; Wu, M.; Shen, L. Electrospinning visible light response Bi2MoO6/Ag3PO4 composite photocatalytic nanofibers with enhanced photocatalytic and antibacterial activity. Appl. Surf. Sci. 2021, 569, 150955. [Google Scholar] [CrossRef]
- Fulzele, N.N.; Bhanvase, B.A.; Pandharipande, S.L. Sonochemically prepared rGO/Ag3PO4/CeO2 nanocomposite photocatalyst for effective visible light photocatalytic degradation of methylene dye and its prediction with ANN modeling. Mater. Chem. Phys. 2022, 292, 126809. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, Z.; Guo, L. Alkaline earth metal as a novel dopant for chalcogenide solid solution: Improvement of photocatalytic efficiency of Cd1−xZnxS by barium surface doping. Int. J. Hydrogen Energy 2011, 36, 9469–9478. [Google Scholar] [CrossRef]
- Lu, J.; Dai, Y.; Zhu, Y.; Huang, B. Density Functional Characterization of Pure and Alkaline Earth Metal-Doped Bi12GeO20, Bi12SiO20, and Bi12TiO20 Photocatalysts. ChemCatChem 2010, 3, 378–385. [Google Scholar] [CrossRef]
- Asaithambi, S.; Sakthivel, P.; Karuppaiah, M.; Hayakawa, Y.; Loganathan, A.; Ravi, G. Improved photocatalytic performance of nanostructured SnO2 via addition of alkaline earth metals (Ba2+, Ca2+ and Mg2+) under visible light irradiation. Appl. Phys. A 2020, 126, 265. [Google Scholar] [CrossRef]
- Iwase, A.; Kato, H.; Okutomi, H.; Kudo, A. Formation of Surface Nano-step Structures and Improvement of Photocatalytic Activities of NaTaO3 by Doping of Alkaline Earth Metal Ions. Chem. Lett. 2004, 33, 1260–1261. [Google Scholar] [CrossRef]
- Zhang, K.; Jing, D.; Chen, Q.; Guo, L. Influence of Sr-doping on the photocatalytic activities of CdS–ZnS solid solution photocatalysts. Int. J. Hydrogen Energy 2010, 35, 2048–2057. [Google Scholar] [CrossRef]
- Lv, Y.; Shi, K.; Zhang, Y.; He, S.; Guo, X.; Du, Z.; Chen, H.; Zhang, C.; Zhang, B. The characterization of Sr-doped nanocrystal grain microspheres and photodegradation of KN-R dye. Catal. Commun. 2008, 9, 557–562. [Google Scholar] [CrossRef]
- Li, Y.; Peng, S.; Jiang, F.; Lu, G.; Li, S. Effect of doping TiO2 with alkaline-earth metal ions on its photocatalytic activity. J. Serbian Chem. Soc. 2007, 72, 393–402. [Google Scholar] [CrossRef]
- Venkatachalam, N.; Palanichamy, M.; Murugesan, V. Sol–gel preparation and characterization of alkaline earth metal doped nano TiO2: Efficient photocatalytic degradation of 4-chlorophenol. J. Mol. Catal. A Chem. 2007, 273, 177–185. [Google Scholar] [CrossRef]
- Lv, C.; Lan, X.; Wang, L.; Yu, Q.; Zhang, M.; Sun, H.; Shi, J. Alkaline-earth-metal-doped TiO2 for enhanced photodegradation and H2 evolution: Insights into the mechanisms. Catal. Sci. Technol. 2019, 9, 6124–6135. [Google Scholar] [CrossRef]
- Iwase, A.; Kato, H.; Kudo, A. The Effect of Alkaline Earth Metal Ion Dopants on Photocatalytic Water Splitting by NaTaO3 Powder. ChemSusChem 2009, 2, 873–877. [Google Scholar] [CrossRef]
- Khiar, H.; Janani, F.Z.; Sadiq, M.; Elmoubarki, R.; Elhalil, A.; Barka, N. Bi3+/Ag3PO4 photocatalyst with higher absorption of visible light and improved stability. Results Surf. Interfaces 2025, 19, 100502. [Google Scholar] [CrossRef]
- Khan, M.M.A.; Siddique, S.; Liu, B.-T.; Latif, A. Barium-doped ZnO nanorods fabricated via Piper nigrum leaf extract: A green route for enhanced photocatalytic efficiency. React. Kinet. Mech. Catal. 2024, 138, 413–431. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J.; Wang, G.; Liu, D.; Liu, S. Construction of Cu-Fe bimetallic oxide/biochar/Ag3PO4 heterojunction for improving photocorrosion resistance and photocatalytic performance achieves efficient removal of phenol. Appl. Surf. Sci. 2022, 592, 153307. [Google Scholar] [CrossRef]
- Wei, X.; Yu, F.; Ji, J.; Cai, Y.; Zou, W.; Zheng, Y.; Huang, J.; Zhang, Y.; Yang, Y.; Naushad, M.; et al. Porous biochar supported Ag3PO4 photocatalyst for “two-in-one” synergistic adsorptive-photocatalytic removal of methylene blue under visible light irradiation. J. Environ. Chem. Eng. 2021, 9, 106753. [Google Scholar] [CrossRef]



| Concentration Effect | pH Effect | ||||||
|---|---|---|---|---|---|---|---|
| 10 mg/L | 15 mg/L | 20 mg/L | pH = 3 | pH = 5 | pH = 7 | pH = 9 | |
| AgP | 0.0146 | 0.0036 | 0.0078 | 0.0021 | 0.019 | 0.0146 | 0.0111 |
| 2%Ba-AgP | 0.0243 | 0.0126 | 0.0119 | 0.0023 | 0.0218 | 0.0243 | 0.0275 |
| 4%Ba-AgP | 0.0223 | 0.0119 | 0.009 | 0.0012 | 0.0184 | 0.0223 | 0.0311 |
| 6%Ba-AgP | 0.0268 | 0.0141 | 0.0112 | 0.0024 | 0.0186 | 0.0268 | 0.0336 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khiar, H.; Janani, F.Z.; Sadiq, M.; Al-Senani, G.M.; Al-Qahtani, S.D.; Barka, N. Barium-Impregnated Ag3PO4 for Enhanced Visible Light Photocatalytic Degradation of Methyl Orange. Ceramics 2025, 8, 44. https://doi.org/10.3390/ceramics8020044
Khiar H, Janani FZ, Sadiq M, Al-Senani GM, Al-Qahtani SD, Barka N. Barium-Impregnated Ag3PO4 for Enhanced Visible Light Photocatalytic Degradation of Methyl Orange. Ceramics. 2025; 8(2):44. https://doi.org/10.3390/ceramics8020044
Chicago/Turabian StyleKhiar, Habiba, Fatima Zahra Janani, M’hamed Sadiq, Ghadah M. Al-Senani, Salhah D. Al-Qahtani, and Noureddine Barka. 2025. "Barium-Impregnated Ag3PO4 for Enhanced Visible Light Photocatalytic Degradation of Methyl Orange" Ceramics 8, no. 2: 44. https://doi.org/10.3390/ceramics8020044
APA StyleKhiar, H., Janani, F. Z., Sadiq, M., Al-Senani, G. M., Al-Qahtani, S. D., & Barka, N. (2025). Barium-Impregnated Ag3PO4 for Enhanced Visible Light Photocatalytic Degradation of Methyl Orange. Ceramics, 8(2), 44. https://doi.org/10.3390/ceramics8020044







