Combination of Physico-Chemical and Lead Isotope Analyses for the Provenance Study of the Archaeological Materials: Example of Saadien Ceramics (16th Century, Marrakech Morocco)
Abstract
1. Introduction
2. Materials and Methods
- -
- The clay–limestone of the Benjlikh site from Fez region (ARF).
- -
- The clay deposits used in the five most important potter villages of the Marrakech region, such as:
- -
- Quaternary clay deposits collected along rivers of the Marrakech Plain (TEN) and (ZAR);
- -
- The Pliocene clay deposits of Mzouda “MZD” (located 70 km from Marrakech city);
- -
- The Triassic clays of Ourika “OUR” and “ARK” (located 35 km from Marrakech city).
3. Results and Discussion
3.1. Chemical and Mineralogical Composition
3.2. Characterization by Lead Isotopes
3.3. Mineralogical Transformation During Firing
4. Conclusions
- The zellij of El Badi Palace and the Saadien Tombs were either imported by Saadien artisans directly from the Benjlikh site of Fez or manufactured in Marrakech using the raw clay materials brought from Fez clay deposits.
- Calcareous clay materials were used to manufacture the Saadien ceramics in traditional ovens with an oxidizing atmosphere.
- The firing temperature of the El Badi Palace ceramics ranges between 600 and 700 °C, while the Saadien Tomb zellij was manufactured at 800–900 °C.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mommsen, H.; Beier, T.; Hein, A. A complete chemical grouping of the Berkeley neutron activation analysis data on Mycenaean pottery. J. Archaeol. Sci. 2002, 29, 613–637. [Google Scholar] [CrossRef]
- Kennett, D.J.; Anderson, A.J.; Cruz, M.J.; Clarck, G.R.; Summerhayes, G.R. Geochemical characterization of Lapita pottery via inductively coupled plasma-mass spectrometry. Archaeometry 2004, 46, 35–46. [Google Scholar] [CrossRef]
- Klein, M.; Jesse, F.; Kasper, H.U.; Golden, A. Chemical characterization of ancient pottery from Sudan by X-ray fluorescence spectrometry (XRF), electron microprobe analyses (EMPA) and inductively coupled plasma mass spectrometry (ICP-MS). Archaeometry 2004, 46, 339–356. [Google Scholar] [CrossRef]
- Yellin, J. Instrumental neutron activation based provenance studies at the Hebrew University of Jerusalem, with a case study on Mycenaean pottery from Cyprus. Archaeometry 2007, 49, 271–288. [Google Scholar] [CrossRef]
- Ben-Shlomo, D.; Maier, A.M.; Mommsen, H. Neutron activation and petrographic analysis of selected Late Bronze Age and Iron Age pottery from Tell es-Safi/Gath, Israel. J. Archaeol. Sci. 2008, 35, 956–964. [Google Scholar] [CrossRef]
- Tschegg, C.; Ntaflos, T.; Hein, I. Integrated geological, petrologic and geochemical approach to establish source material and technology of Late Cypriot Bronze Age Plain White ware ceramics. J. Archaeol. Sci. 2009, 36, 1103–1114. [Google Scholar] [CrossRef]
- De Vleeschouwer, F.; Renson, V.; Claeys, P.; Nys, K.; Bindler, R. Quantitative WD-XRF calibration for small ceramic samples and their source material. Geoarchaeology 2011, 26, 440–450. [Google Scholar] [CrossRef]
- Renson, V.; Martiınez-Cortizas, A.; Mattielli, N.; Coenaerts, J.; Sauvage, C.; De Vleeschouwer, F.; Lorre, C.; Vanhaecke, F.; Bindler, R.; Rautman, M.; et al. Lead isotopic analysis within a multi-proxi approach to trace pottery sources. The example of White Slip II sherds from Late Bronze Age sites in Cyprus and Syria. Appl. Geochem. 2013, 28, 220–234. [Google Scholar] [CrossRef]
- Adan-Bayewitz, D.; Perlman, I. Local pottery provenience studies: A role for clay analysis. Archaeometry 1985, 27, 203–217. [Google Scholar] [CrossRef]
- Gomez, B.; Neff, H.; Rautman, M.L.; Vaughan, S.J.; Glascock, M.D. The source provenance of Bronze Age and Roman pottery from Cyprus. Archaeometry 2002, 44, 23–36. [Google Scholar] [CrossRef]
- Santacreu, A.; Vicens, G.M. Raw Materials and Pottery Production at the Late Bronze and Iron Age Site of Puig de Sa Morisca, Mallorca, Spain. Geoarchaeology 2012, 27, 285–299. [Google Scholar] [CrossRef]
- Segvic, B.; Seselj, L.; Slovenec, D.; Lugovi, B.; Ferreiro Mahlmann, R. Composition, technology of manufacture, and circulation of Hellenistic pottery from the Eastern Adriatic: A case study of three archaeological sites along the Dalmatian Coast, Croatia. Geoarchaeology 2012, 27, 63–87. [Google Scholar] [CrossRef]
- El Ouahabi, M.; El Boudour El Idrissi, H.; Daoudi, L.; El Halim, M.; Fagel, N. Moroccan clay deposits: Physico-chemical properties in view of provenance studies on ancient ceramics. Appl. Clay Sci. 2019, 172, 65–74. [Google Scholar] [CrossRef]
- Fowler, K.D.; Fayek, M.; Middleton, E. Clay acquisition and processing strategies during the first millennium A.D. in the Thukela River basin, South Africa: An ethnoarchaeological approach. Geoarchaeology 2011, 26, 762–785. [Google Scholar] [CrossRef]
- Nunes, K.P.; Toyota, R.G.; Oliveira, P.M.S.; Neves, E.G.; Soares, E.A.A.; Munita, C.S. Preliminary compositional evidence of provenance of ceramics from Hatahara archaeological site, central Amazonia. J. Chem. 2013, 2013, 701748. [Google Scholar] [CrossRef]
- Glascock, M.D. Characterization of archaeological ceramics at MURR by neutron activation analysis and multivariate statistics. In Chemical Characterization of Ceramic Pastes in Archaeology; Monographs in World Archaeology No. 7; Neff, H., Ed.; Prehistory Press: Madison, WI, USA, 1992; pp. 11–26. [Google Scholar]
- Reimann, C.; Filzmoser, P. Normal and lognormal data distribution in geochemistry: Death of a myth. Consequences for the statistical treatment of geochemical and environmental data. Environ. Geol. 2000, 39, 1001–1014. [Google Scholar] [CrossRef]
- Usman, A.A.; Speakman, R.J.; Glascock, M.D. An initial assessment of prehistoric ceramic production and exchange in northern Yoruba, north central Nigeria: Results of ceramic compositional analysis. Afr. Archaeol. Rev. 2005, 22, 141–168. [Google Scholar] [CrossRef]
- Tite, M.S. Ceramic production, provenance and use—A review. Archaeometry 2008, 50, 216–231. [Google Scholar] [CrossRef]
- Renson, V.; Coenaerts, J.; Nys, K.; Mattielli, N.; Vanhaecke, F.; Fagel, N.; Claeys, P. Lead isotopic analysis for the identification of Late Bronze Age pottery from Hala Sultan Tekke (Cyprus). Archaeometry 2011, 53, 37–57. [Google Scholar] [CrossRef]
- Medeghini, L.; Fayek, M.; Mignardi, S.; Coletti, F.; Contino, A.; De Vito, C. A provenance study of Roman lead-glazed ceramics using lead isotopes and secondary ion mass spectrometry (SIMS). Microchem. J. 2020, 154, 104519. [Google Scholar] [CrossRef]
- El Halim, M.; Daoudi, L.; El Ouahabi, M.; Fagel, N. Characterization of clays from Fez area (Northern Morocco) for potential uses in the ceramic industry. Clay Miner. 2023, 57, 139–149. [Google Scholar] [CrossRef]
- El Halim, M.; Daoudi, L.; El Ouahabi, M.; Rebbouh, L.; Rousseau, V.; Cools, C.; Fagel, N. Characterization of archaeological ceramics from Saadian Tombs (16th century) of Marrakech. Mater. Today Proc. 2022, 58, 1142–1148. [Google Scholar] [CrossRef]
- El Halim, M.; Daoudi, L.; El Ouahabi, M.; Rousseau, V.; Cools, C.; Fagel, N. Mineralogical and geochemical characterization of archaeological ceramics from the El Badi Palace (sixteenth century), Morocco. Clay Miner. 2018, 53, 459–470. [Google Scholar] [CrossRef]
- El Halim, M.; Daoudi, L.; El Alaoui El Fels, A.; Rebbou, L.; El Ouahabi, M.; Fagel, N. Non-destructive portable X-ray Fluorescence (pXRF) method for the characterization of Islamic architectural ceramic: Example of Saadien tombs and El Badi palace ceramics (Marrakech, Morocco). J. Archaeol. Sci. Rep. 2020, 32, 102422. [Google Scholar] [CrossRef]
- El Boudour El Idrissi, H. Caractérisation des Argiles Utilisées dans le Secteur de la Terre Cuite de la Région de Marrakech en vue D’améliorer la Qualité des Produits. Ph.D. Thesis, Université de Liège, Liège, Belgium, 2017; pp. 28–50. [Google Scholar]
- Duchesne, J.C.; Bologne, G. XRF major and trace element determination in Fe–Ti oxide minerals. Geol. Belg. 2009, 12, 205–212. [Google Scholar]
- Weis, D.; Kieffer, B.; Maerschalk, C.; Barling, J.; de Jong, J.; Williams, G.A.; Hanano, D.; Pretorius, W.; Mattielli, N.; Scoates, J.S.; et al. High-precision isotopic characterization of USGS reference materials by TIMS and MC-ICP-MS. Geochem. Geophys. Geosystems 2006, 7, Q08006. [Google Scholar] [CrossRef]
- Bühn, B.; Pimentel, M.M.; Matteini, M.; Dantas, E.L. High spatial resolution analysis of Pb and U isotopes for geochronology by laser ablation multi-collector inductively coupled plasma mass spectrometry (LA-MC-ICP-MS). An. Da Acad. Bras. De Ciências 2009, 81, 99–114. [Google Scholar] [CrossRef]
- Molera, J.; Pradell, T.; Vendrell-Saz, M. The colours of Ca-rich ceramic pastes: Origin and characterization. Appl. Clay Sci. 1998, 13, 187–202. [Google Scholar] [CrossRef]
- Ngun, B.K.; Mohamad, H.; Sulaiman, S.K.; Okada, K.; Ahmad, Z.A. Some ceramic properties of clays from central Cambodia. Appl. Clay Sci. 2011, 53, 33–41. [Google Scholar] [CrossRef]
- Milheiro, F.; Freire, M.; Silva, A.; Holanda, J. Densification behavior of a red firing Brazilian kaolinitic clay. Ceram. Int. 2005, 31, 757–763. [Google Scholar] [CrossRef]
- Baccour, H.; Medhioub, M.; Jamoussi, F.; Mhiri, T.; Daoud, A. Mineralogical evaluation and industrial applications of the Triassic clay deposits, Southern Tunisia. Mater. Charact. 2008, 59, 1613–1622. [Google Scholar] [CrossRef]
- El Boudour El Idrissi, H.; Daoudi, L.; El Ouahabi, M.; Balo Madi, A.; Collin, F.; Fagel, N. Suitability of soils and river deposits from Marrakech for the manufacturing of earthenware. Appl. Clay Sci. 2016, 129, 108–115. [Google Scholar] [CrossRef]
- De Ceuster, S.; Machaira, D.; Degryse, P. Lead isotope analysis for provenancing ancient materials: A comparison of approaches. RSC Adv. 2023, 13, 19595–19606. [Google Scholar] [CrossRef]
- Cultrone, G.; Rodriguez-Navarro, C.; Sebastian, E.; Cazalla, O.; De La Torre, M.J. Carbonate and silicate phase reactions during ceramic firing. Eur. J. Mineral. 2001, 13, 621–634. [Google Scholar] [CrossRef]
- Echallier, J.C.; Mery, S. Experimental Laboratory Approach of the Mineralogical and Physic-Chemical Evolution of Ceramics During Cooking; Document No. 74; GAL: Paris, France, 1989. [Google Scholar]
- Dondi, M.; Ercolani, G.; Fabri, B.; Marsigli, M. Chemical composition of melilite formed during the firing of carbonate-rich and iron-containing ceramic bodies. J. Am. Ceram. Soc. 1999, 82, 465–468. [Google Scholar] [CrossRef]
- Nagy, S.; Kuzmann, E.; Weiszburg, T.; Gyokeres-Toth, M.; Riedel, M. Oxide transformation during preparation of black pottery in Hungary. J. Radioanal. Nucl. Chem. 2000, 246, 91–96. [Google Scholar] [CrossRef]
- El Ouahabi, M.; Daoudi, L.; Hatert, F.; Fagel, N. Modified mineral phases during clay ceramic firing. Clay Clay Miner. 2015, 63, 404–413. [Google Scholar] [CrossRef]
- Fabri, B.; Gualtieri, S.; Shoval, S. The presence of calcite in archaeological ceramics. J. Eur. Ceram. Soc. 2014, 31, 1899–1911. [Google Scholar] [CrossRef]


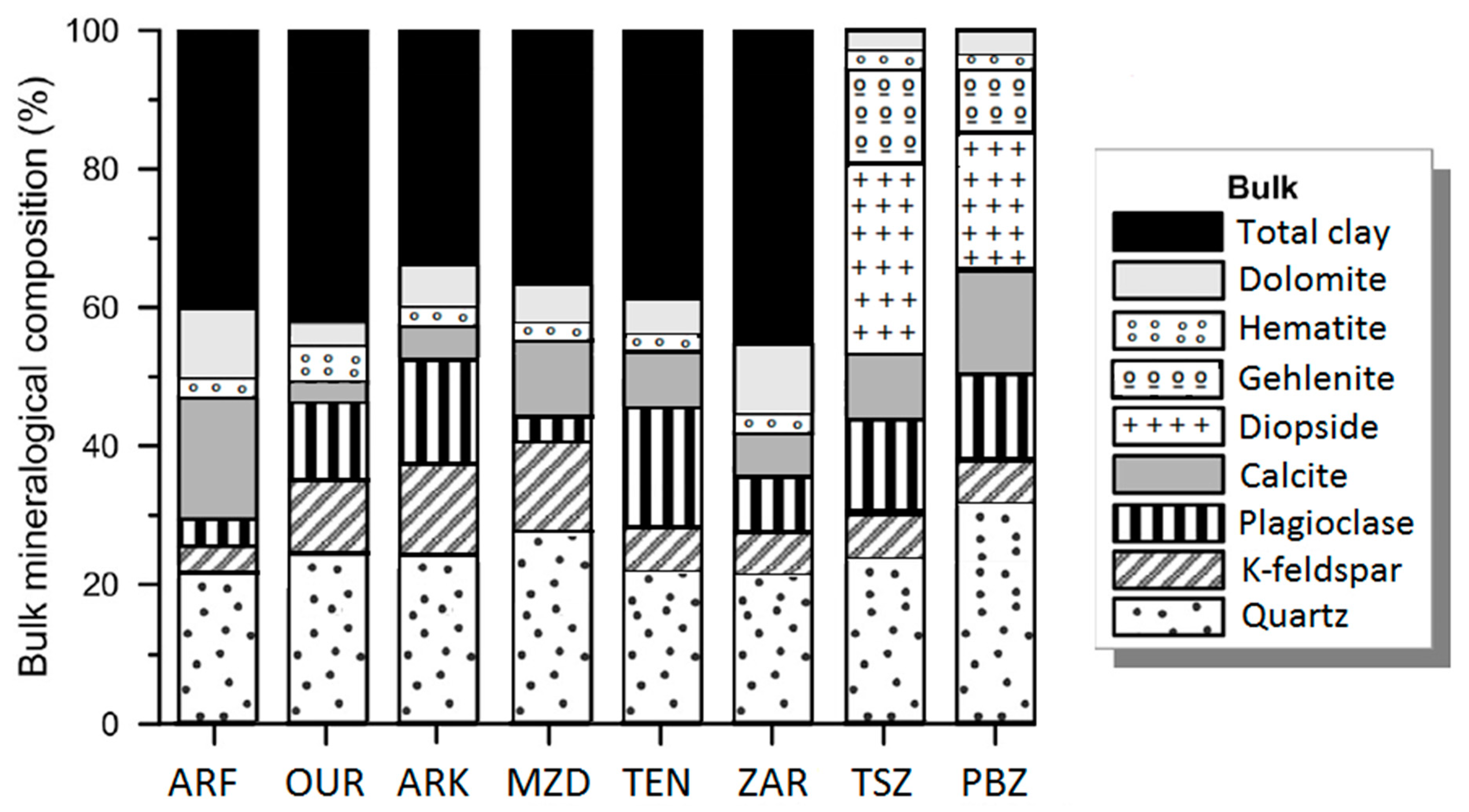
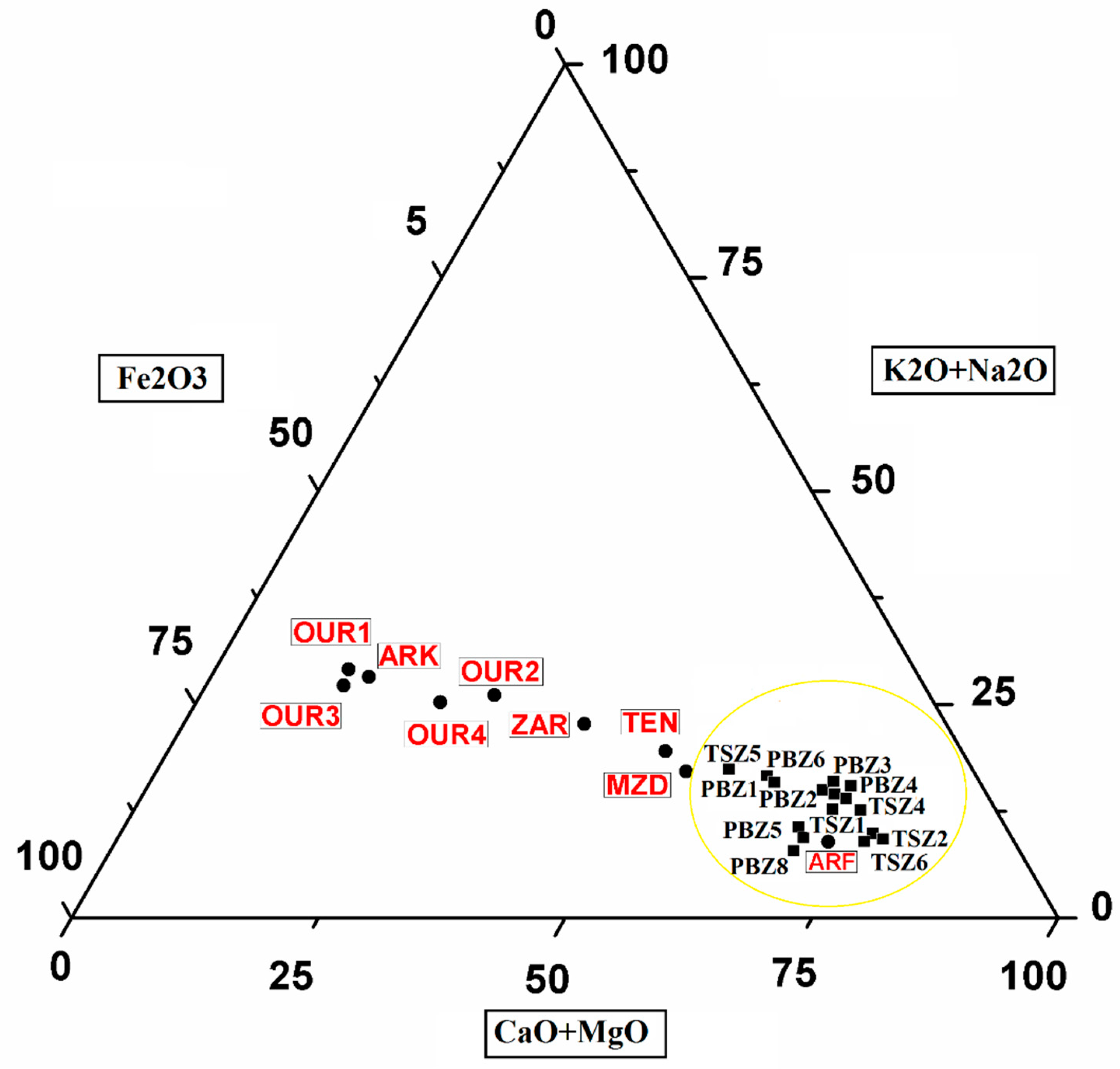
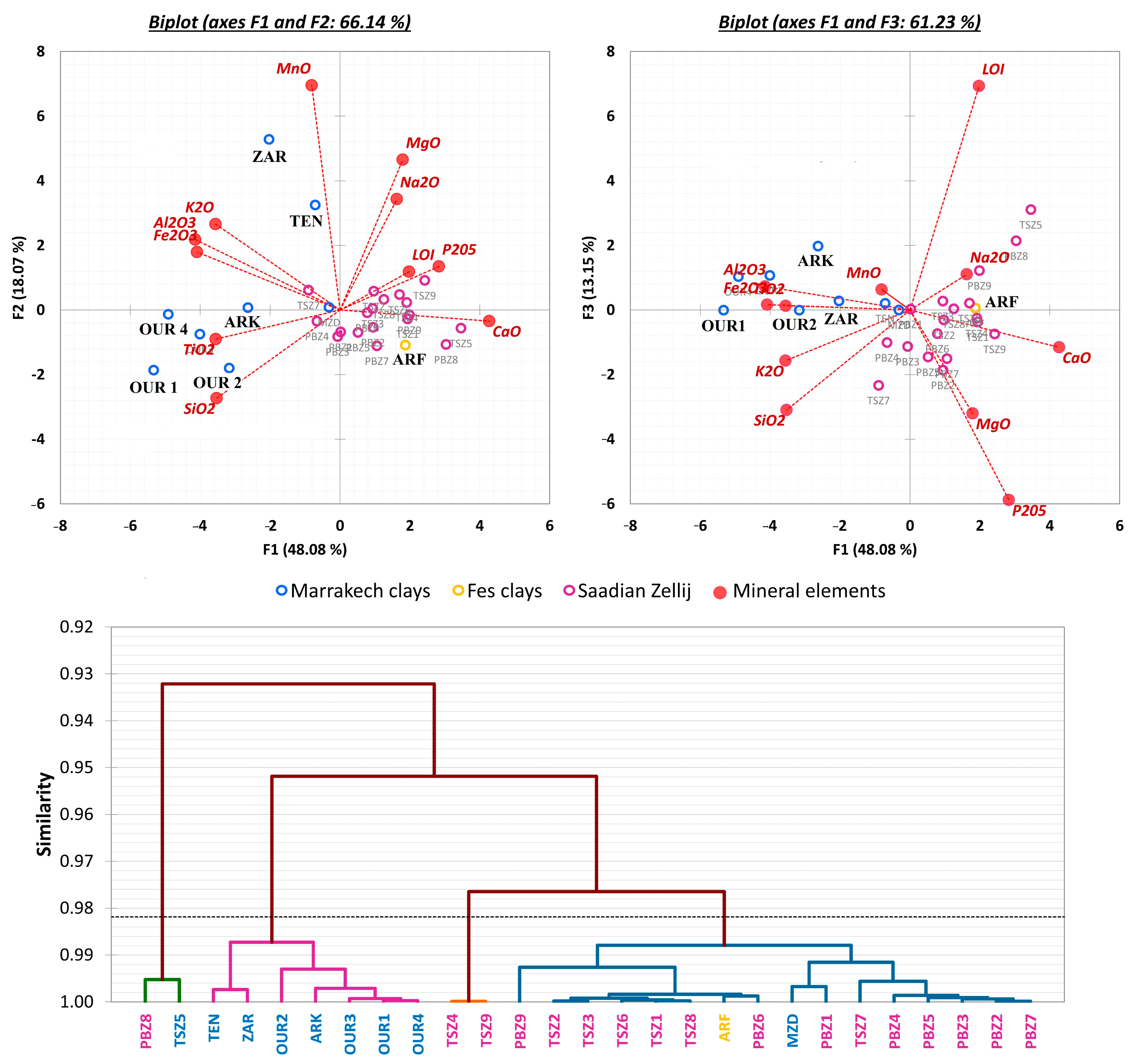

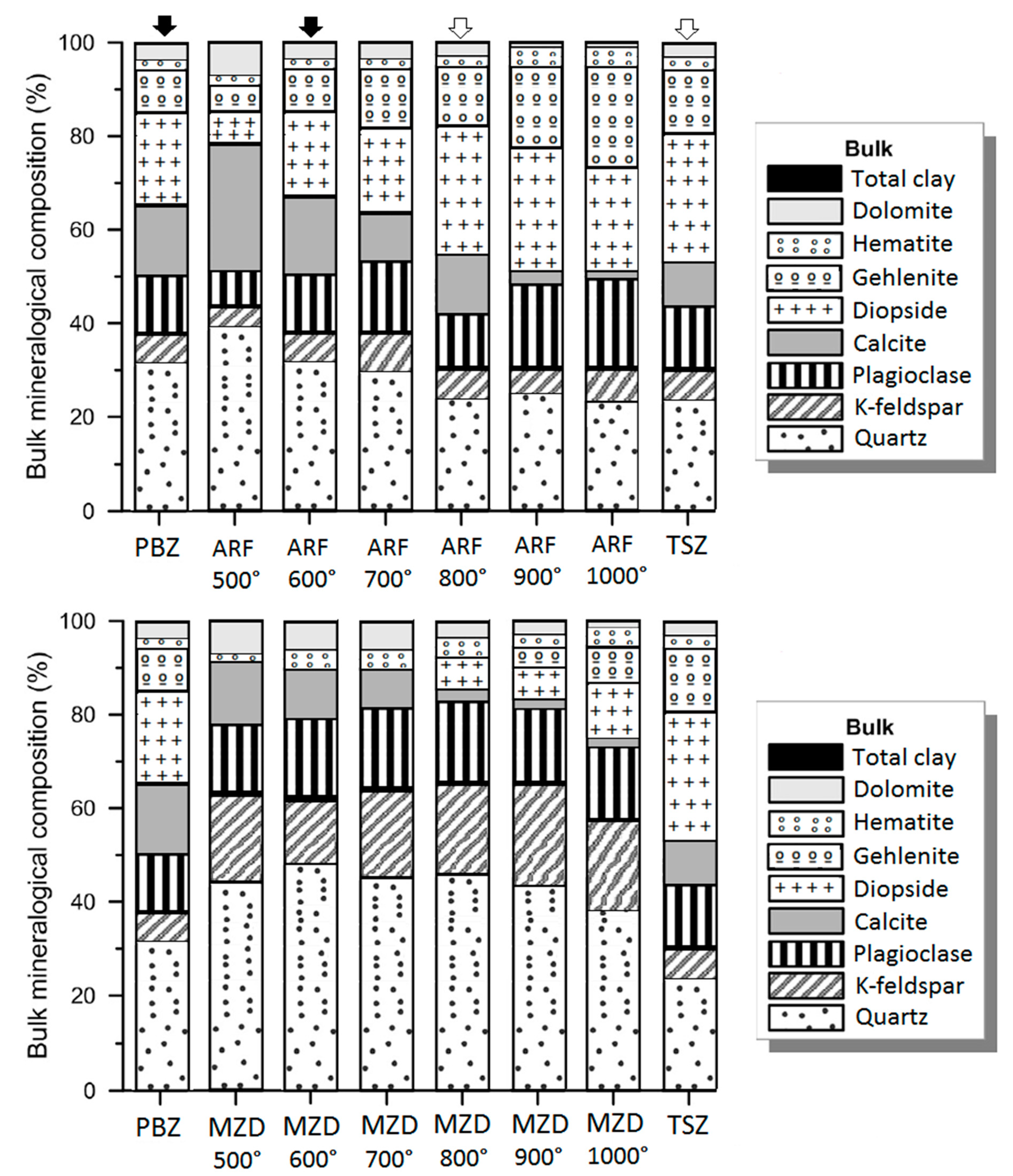
| Site | Sample | Lithology | Color | Type of Kiln | Firing Temperature | Firing Time | References |
|---|---|---|---|---|---|---|---|
| Fez (Benjlikh) | ARF | Marls | Grey | Traditional | 950 °C | 4 h (in summer), 8 h (in winter) | [22] |
| Ourika | OUR | Mudstone | Red | Gas kiln | 950 °C | 6 h | [26] |
| ARK | Mudstone | Red | |||||
| Saada | TEN | Silt/Clay Sediment | Red | Traditional and Gas kilns | 900 to 950 °C | 3 h | |
| ZAR | Silt/Clay Sediment | Grey | |||||
| Mzouda | MZD | Colluvial clays | Red | Gas kiln | 850 to 950 °C | 7 h (100°/h) + 4 h (50°/h) |
| SiO2 | Al2O3 | Fe2O3 | CaO | K2O | MgO | Na2O | TiO2 | MnO | P2O5 | LOI | |
| Clay minerals | |||||||||||
| OUR1 | 60.05 | 19.43 | 7.43 | 0.45 | 3.23 | 1.31 | 0.21 | 1.19 | 0.02 | 0.1 | 6.58 |
| OUR2 | 63.99 | 13.22 | 5.6 | 1.71 | 3.05 | 2.08 | 0.27 | 0.98 | 0.04 | 0.06 | 8.99 |
| OUR3 | 59.24 | 18.16 | 7.21 | 0.76 | 2.73 | 1.31 | 0.92 | 0.98 | 0.07 | 0.06 | 8.55 |
| OUR4 | 58.71 | 19.06 | 7.76 | 0.62 | 3.34 | 1.21 | 0.6 | 0.9 | 0.11 | 0.03 | 7.66 |
| ARK | 57.35 | 16.8 | 7.11 | 1.99 | 2.28 | 1.53 | 1.31 | 0.76 | 0.1 | 0.02 | 10.75 |
| MZD | 56.38 | 12.62 | 5.49 | 7.84 | 2.47 | 2.27 | 0.76 | 0.66 | 0.1 | 0.18 | 11.22 |
| TEN | 48.88 | 16.51 | 6.25 | 7.27 | 3.07 | 3.2 | 0.99 | 0.66 | 0.18 | 0.19 | 12.8 |
| ZAR | 48.57 | 17.61 | 7.22 | 4.42 | 3.5 | 3.58 | 0.97 | 0.77 | 0.28 | 0.17 | 12.9 |
| ARF | 47.43 | 10.29 | 5.26 | 13.63 | 1.55 | 2.55 | 0.34 | 0.55 | 0.04 | 0.19 | 10.73 |
| Archaeological ceramics | |||||||||||
| PBZ1 | 55.92 | 11.14 | 4.67 | 11.55 | 2.62 | 1.83 | 0.87 | 0.76 | 0.08 | 0.18 | 10.63 |
| PBZ2 | 55.46 | 11.72 | 5.34 | 14.09 | 2.09 | 2.82 | 0.57 | 0.62 | 0.05 | 0.27 | 7.09 |
| PBZ3 | 57.75 | 11.66 | 4.78 | 14 | 2.23 | 2.06 | 0.79 | 0.79 | 0.09 | 0.19 | 5.59 |
| PBZ4 | 56.63 | 12.52 | 5 | 12.26 | 2.76 | 2.04 | 0.87 | 0.82 | 0.09 | 0.19 | 6.27 |
| PBZ5 | 55.12 | 11.2 | 5.13 | 14.99 | 3.01 | 2.1 | 0.56 | 0.53 | 0.05 | 0.25 | 7.85 |
| PBZ6 | 52.6 | 11.7 | 4.75 | 14.64 | 2.54 | 1.94 | 0.92 | 0.75 | 0.09 | 0.28 | 10.11 |
| PBZ7 | 56.73 | 10.99 | 5.13 | 13.81 | 1.89 | 2.67 | 0.54 | 0.58 | 0.04 | 0.23 | 7.12 |
| PBZ8 | 45.94 | 8.82 | 3.65 | 17.88 | 1.78 | 1.78 | 0.74 | 0.58 | 0.06 | 0.17 | 18.76 |
| PBZ9 | 45.79 | 10.82 | 4.34 | 18.57 | 2.1 | 1.97 | 1.07 | 0.7 | 0.08 | 0.17 | 13.81 |
| TSZ1 | 49.42 | 10.96 | 5.07 | 16.4 | 1.84 | 3.05 | 0.57 | 0.58 | 0.05 | 0.2 | 11.11 |
| TSZ2 | 50.11 | 11.95 | 4.84 | 16.02 | 2.56 | 2.3 | 1.3 | 0.75 | 0.09 | 0.22 | 9.7 |
| TSZ3 | 49.72 | 11.58 | 4.66 | 16.21 | 2.42 | 2.27 | 1.1 | 0.75 | 0.08 | 0.16 | 10.37 |
| TSZ4 | 46.53 | 11.56 | 4.69 | 22.03 | 1.99 | 2.48 | 1.25 | 0.72 | 0.08 | 0.18 | 7.67 |
| TSZ5 | 41.45 | 9.13 | 3.67 | 19.41 | 1.68 | 1.84 | 1.08 | 0.61 | 0.06 | 0.13 | 20.55 |
| TSZ6 | 48.91 | 11.61 | 4.64 | 15.87 | 2.05 | 2.54 | 1.33 | 0.73 | 0.08 | 0.21 | 11.75 |
| TSZ7 | 57 | 14.07 | 5.65 | 11.7 | 2.93 | 2.28 | 1.16 | 0.84 | 0.1 | 0.29 | 3.59 |
| TSZ8 | 48.21 | 11.11 | 5.02 | 16.45 | 2.4 | 2.58 | 1.12 | 0.75 | 0.07 | 0.19 | 10.89 |
| TSZ9 | 44.96 | 11.17 | 4.52 | 21.45 | 2.11 | 2.19 | 1.84 | 0.78 | 0.09 | 0.29 | 7.55 |
| Name | Weigth (mg) | 208Pb/204Pb | 2se | 206Pb/204Pb | 2se | 208Pb/206Pb | 2se | 207Pb/206Pb | 2se | |
|---|---|---|---|---|---|---|---|---|---|---|
| Clays | MZD | 90.9 | 38.40 | 0.0019 | 18.41 | 0.00096 | 2.08 | 0.000040 | 0.84 | 0.000012 |
| Clays | OUR | 94.9 | 39.08 | 0.0020 | 18.93 | 0.00085 | 2.06 | 0.000037 | 0.82 | 0.000011 |
| Clays | ZAR | 100.5 | 38.96 | 0.0021 | 18.71 | 0.00083 | 2.08 | 0.000035 | 0.83 | 0.000012 |
| Clays | ARK | 105.6 | 38.64 | 0.0023 | 18.50 | 0.00095 | 2.08 | 0.000043 | 0.84 | 0.000012 |
| Clays | TEN | 99.5 | 38.58 | 0.0020 | 18.54 | 0.00077 | 2.08 | 0.000041 | 0.84 | 0.000013 |
| Clays | ARF | 106 | 38.21 | 0.0017 | 18.21 | 0.00070 | 2.09 | 0.000044 | 0.85 | 0.000011 |
| Zellige El Badi Palace | PBZ1 | 108.3 | 38.27 | 0.0022 | 18.43 | 0.00096 | 2.07 | 0.000038 | 0.84 | 0.000011 |
| Zellige El Badi Palace | PBZ2 | 93.7 | 38.83 | 0.0020 | 18.65 | 0.00088 | 2.08 | 0.000039 | 0.83 | 0.000011 |
| Zellige El Badi Palace | PBZ3 | 97.8 | 38.39 | 0.0022 | 18.36 | 0.00065 | 2.09 | 0.000043 | 0.85 | 0.000014 |
| Zellige El Badi Palace | PBZ4 | 109.6 | 38.36 | 0.0018 | 18.41 | 0.00074 | 2.08 | 0.000039 | 0.84 | 0.000011 |
| Zellige Saadien Tombs | TSZ1 | 100.8 | 38.46 | 0.0019 | 18.26 | 0.00076 | 2.10 | 0.000042 | 0.85 | 0.000013 |
| Zellige Saadien Tombs | TSZ2 | 95.1 | 38.31 | 0.0020 | 18.42 | 0.00077 | 2.07 | 0.000047 | 0.84 | 0.000012 |
| Zellige Saadien Tombs | TSZ3 | 100.2 | 38.35 | 0.0017 | 18.31 | 0.00080 | 2.09 | 0.000041 | 0.85 | 0.000013 |
| Zellige Saadien Tombs | TSZ4 | 102.5 | 38.38 | 0.0017 | 18.42 | 0.00077 | 2.08 | 0.000039 | 0.84 | 0.000011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Halim, M.; Daoudi, L.; El Idrissi, H.E.B.; El Ouahabi, M.; Omdi, F.E.; Gourfi, A.; Ait Hmeid, H.; Abdellah, H.I.; Fagel, N. Combination of Physico-Chemical and Lead Isotope Analyses for the Provenance Study of the Archaeological Materials: Example of Saadien Ceramics (16th Century, Marrakech Morocco). Ceramics 2025, 8, 13. https://doi.org/10.3390/ceramics8010013
El Halim M, Daoudi L, El Idrissi HEB, El Ouahabi M, Omdi FE, Gourfi A, Ait Hmeid H, Abdellah HI, Fagel N. Combination of Physico-Chemical and Lead Isotope Analyses for the Provenance Study of the Archaeological Materials: Example of Saadien Ceramics (16th Century, Marrakech Morocco). Ceramics. 2025; 8(1):13. https://doi.org/10.3390/ceramics8010013
Chicago/Turabian StyleEl Halim, Mouhssin, Lahcen Daoudi, Hicham El Boudour El Idrissi, Meriam El Ouahabi, Fatima Ezzahra Omdi, Abdelali Gourfi, Hanane Ait Hmeid, Hanane Id Abdellah, and Nathalie Fagel. 2025. "Combination of Physico-Chemical and Lead Isotope Analyses for the Provenance Study of the Archaeological Materials: Example of Saadien Ceramics (16th Century, Marrakech Morocco)" Ceramics 8, no. 1: 13. https://doi.org/10.3390/ceramics8010013
APA StyleEl Halim, M., Daoudi, L., El Idrissi, H. E. B., El Ouahabi, M., Omdi, F. E., Gourfi, A., Ait Hmeid, H., Abdellah, H. I., & Fagel, N. (2025). Combination of Physico-Chemical and Lead Isotope Analyses for the Provenance Study of the Archaeological Materials: Example of Saadien Ceramics (16th Century, Marrakech Morocco). Ceramics, 8(1), 13. https://doi.org/10.3390/ceramics8010013






