Features of Dielectric Properties of 0.20BiScO3·0.45PbTiO3·0.35PbMg1/3Nb2/3O3 Samples Obtained by the Melt-Hardening Method
Abstract
1. Introduction
2. Methods of Obtaining and Investigating the Samples
Obtaining of Samples
3. Results of the Samples and Their Discussion
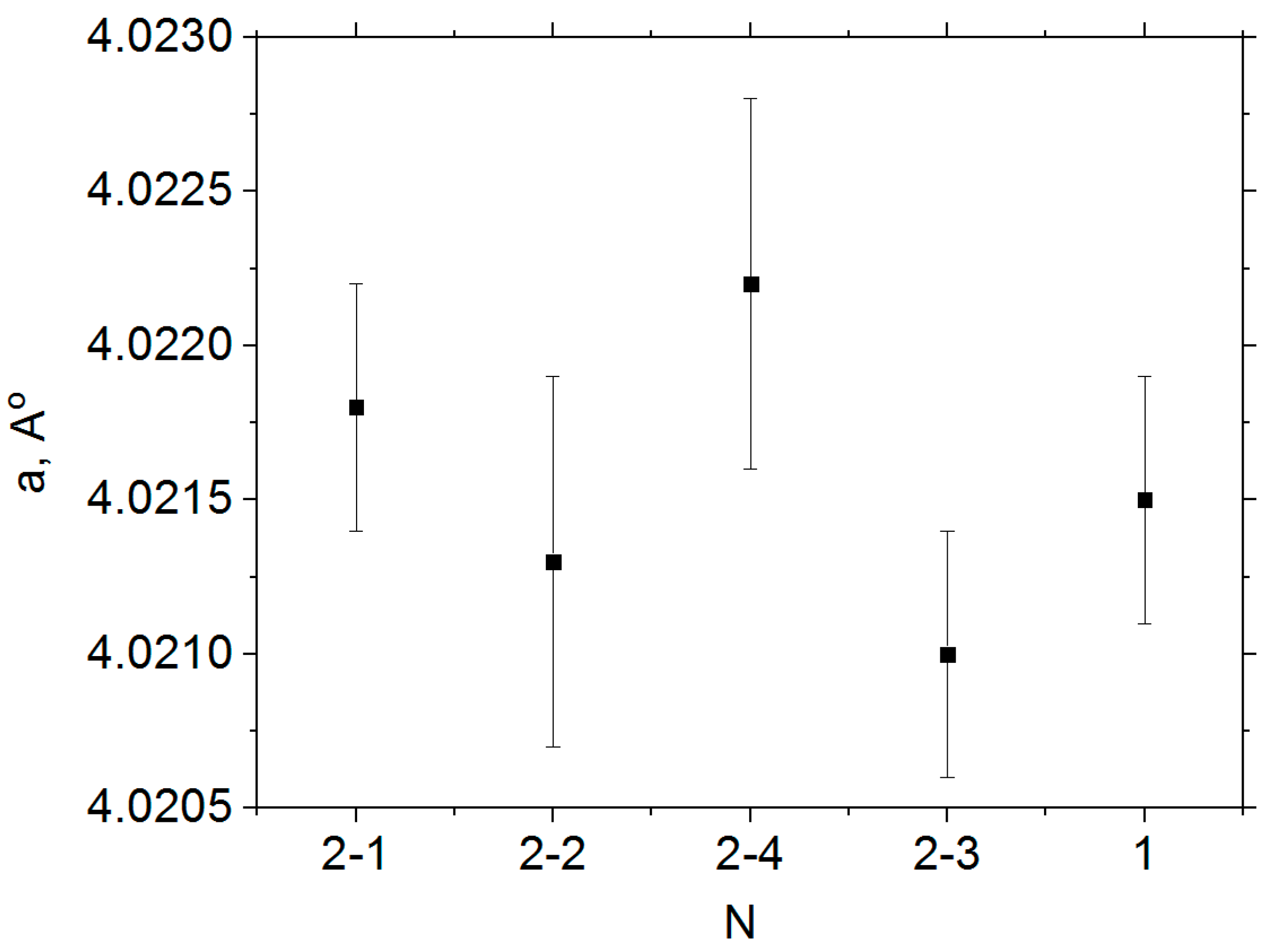
3.1. X-ray Phase Analysis
3.2. Microstructure of the Samples
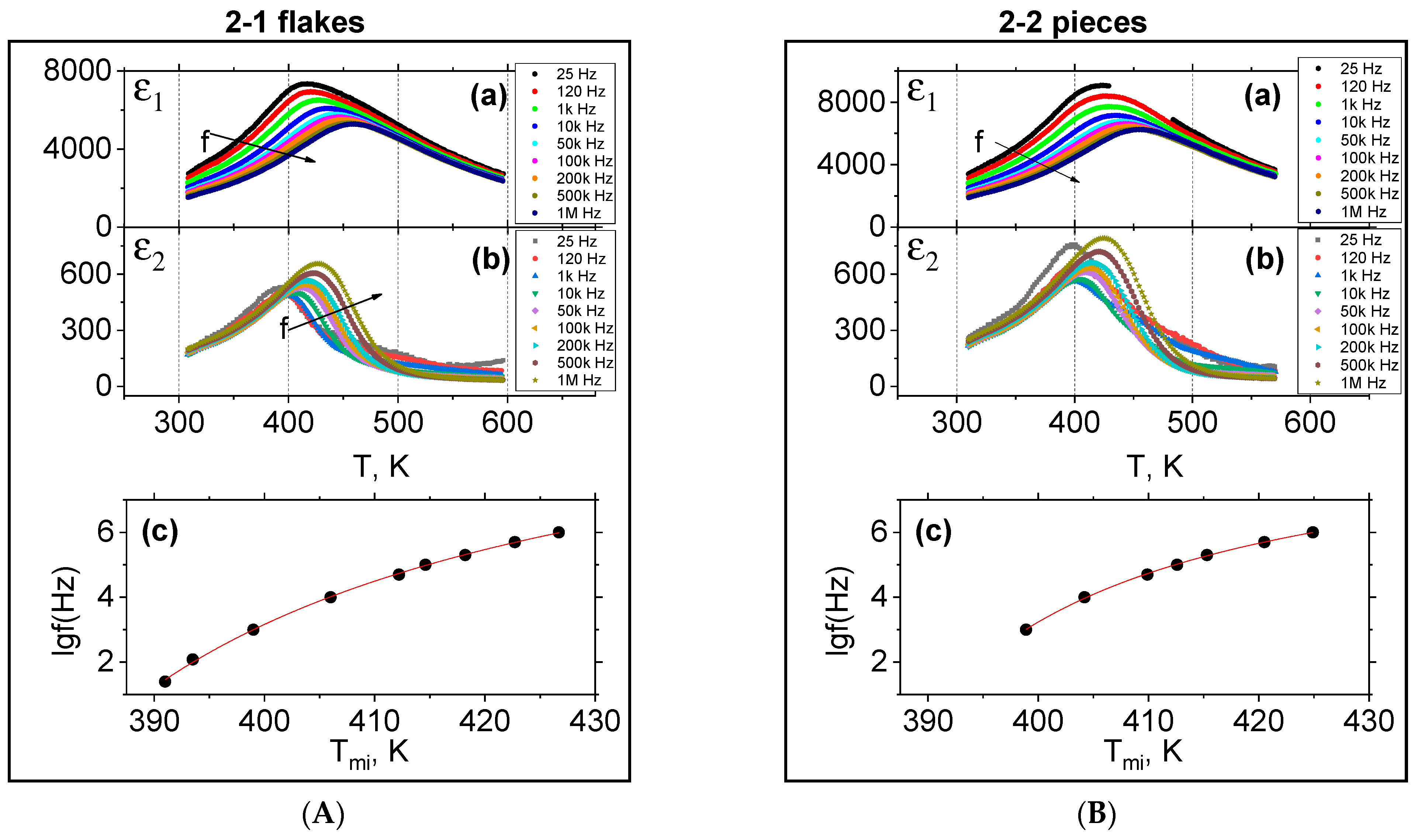
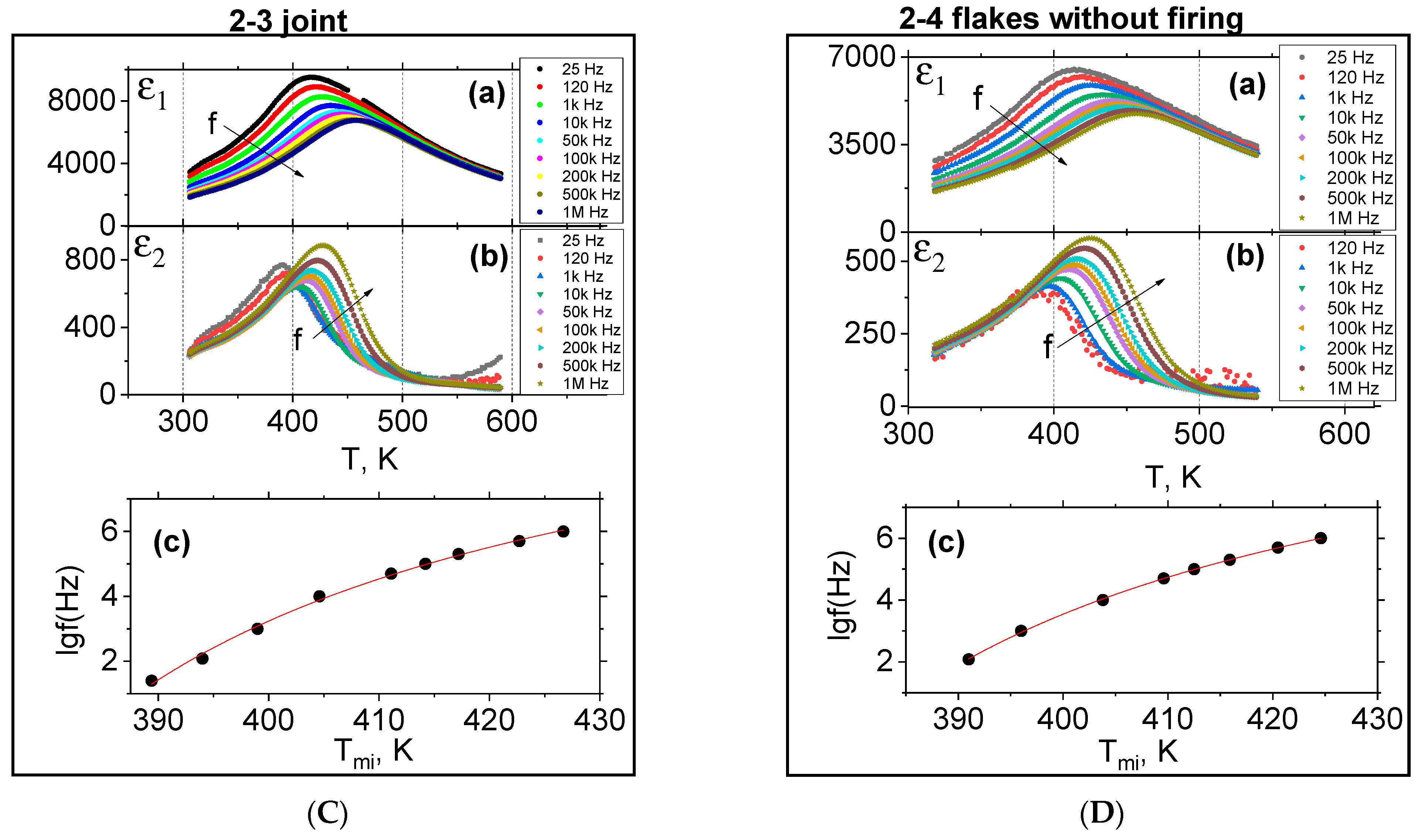
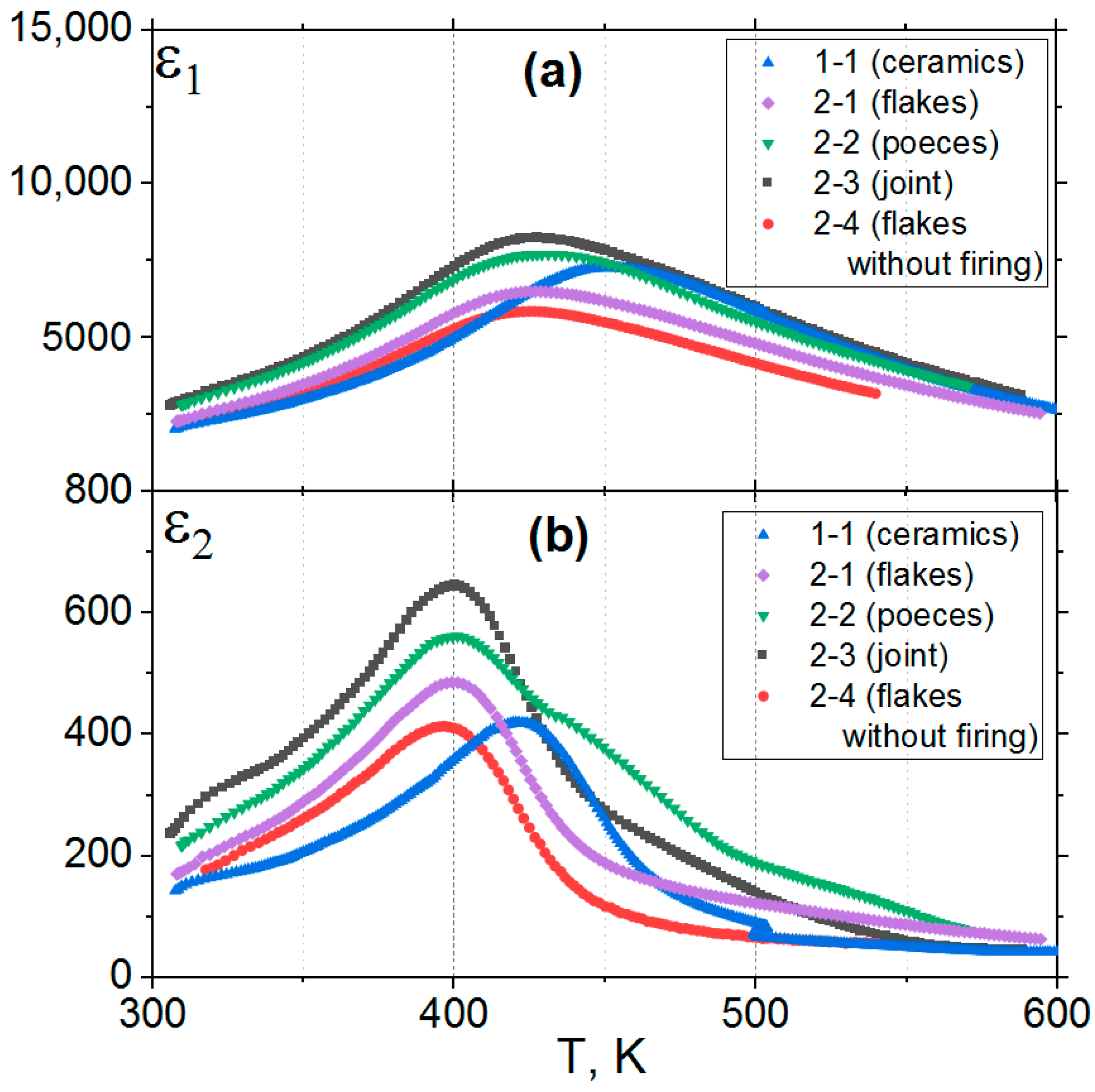
3.3. Dielectric Measurements
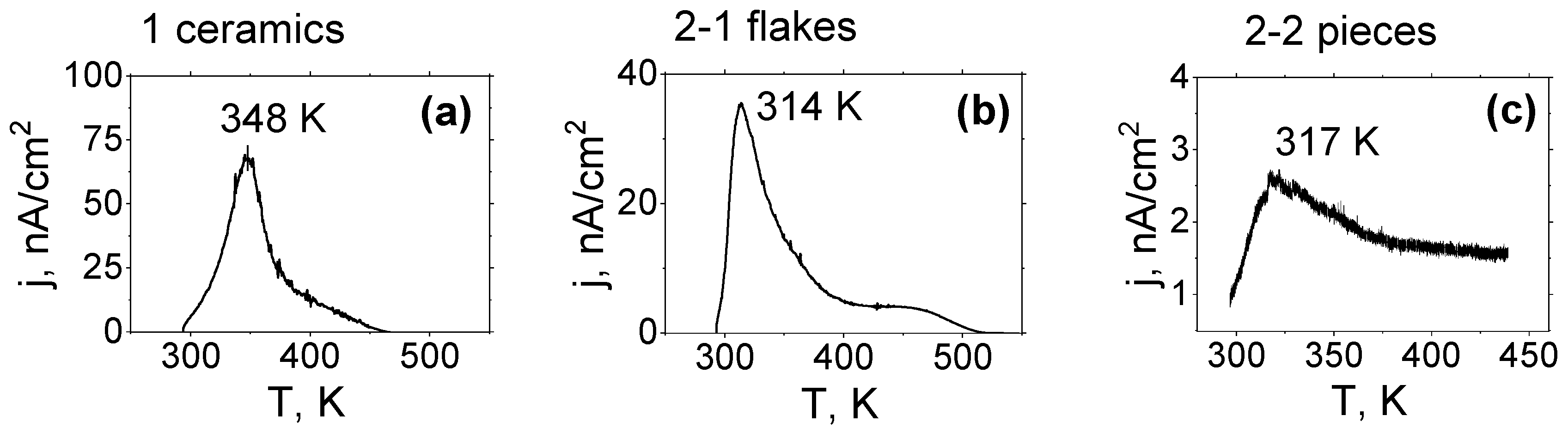
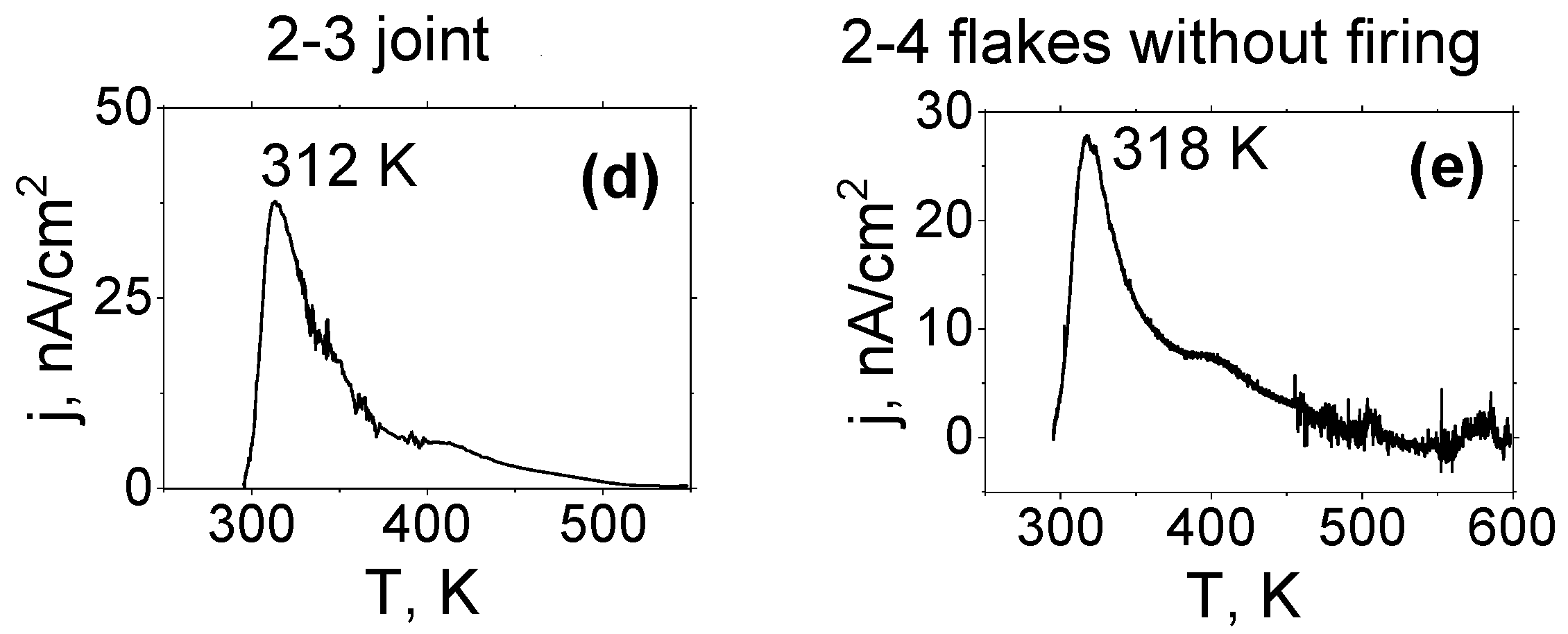
3.4. Study of Thermostimulated Depolarisation Currents (TSDC)
3.5. Discussion of Results
4. Conclusions
- Samples of solid solutions of composition 0.20BS·0.45PT·0.35PMN (with perovskite structure) can be obtained by ceramic and melt-quenching technology. Four kinds of samples (by melting and quenching of thermally treated and non-thermally treated initial oxide mixtures) were obtained using the melt-hardening method. The cubic unit cell sizes of 0.20BS·0.45PT·0.35PMN samples present in different samples are almost the same as each other. To obtain a higher density of the samples, they should be obtained by the melt-quenching method, but with a small, short pre-firing process.
- The synthesized 0.20BS·0.45PT·0.35PMN samples exhibit dielectric properties characteristic of relaxor ferroelectrics, and the polarized samples exhibit a pronounced piezo effect with a piezo modulus value of d33~200 pC/N. The ε1 value of type 2 samples is 13% higher than the ε1 value of type 1 samples. The mentioned differences between dielectric properties of samples of types 1 and 2 can relate to the difference in the technologies by which they were obtained. The differences between the samples of the second type (2-1, 2-2, 2-3, 2-4) can be related to the appearance of microstresses and deformations in the microstructures of the samples of type 2, manifested in different degrees in their structures depending on the forms of amorphous precursors, due to the difference in gradient-temperature conditions during their quenching.
- Application of the melt-quenching method has an advantage over the solid-phase method, as it allows to obtain practical single-phase samples of 0.20BS·0.45PT·0.35PMN solid solutions (four varieties), but the synthesis time is significantly reduced by 2–3 times. It is desirable to test this method for synthesis of other compositions of piezoelectric materials due to its efficiency.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, Z.-G. (Ed.) Handbook of Dielectric, Piezoelectric, and Ferroelectric materials: Synthesis, Properties, and Applications; Woodhead Publishing: Cambridge, UK, 2008; 1096p. [Google Scholar] [CrossRef]
- Uchino, K. (Ed.) Advanced Piezoelectric Materials. Science, and Technology, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2017; 848p. [Google Scholar]
- Reilly, E.K.; Burghardt, F.; Fain, R.; Wright, P. Powering a wireless sensor node with a vibration-driven piezoelectric energy harvester. Smart Mater. Struct. 2011, 20, 125006. [Google Scholar] [CrossRef]
- Murray, R.; Rastegar, J. Novel two-stage piezoelectric-based ocean wave energy harvesters for moored or unmoored buoys. In Active and Passive Smart Structures and Integrated Systems; SPIE: San Diego, CA, USA, 2009; Volume 7288, p. 72880E. [Google Scholar]
- Huang, C.; Lajnef, N.; Chakrabartty, S. Infrasonic energy harvesting for embedded structural health monitoring microsensors. In SPIE Smart Structures, and Materials Nondestructive Evaluation, and Health Monitoring; International Society for Optics, and Photonics: Bellingham, WA, USA, 2010; p. 764746. [Google Scholar]
- Cook-Chennault, K.A.; Thambi, N.; Sastry, A.M. Powering MEMS portable devices—A review of nonregenerative, and regenerative power supply systems with special emphasis on piezoelectric energy harvesting systems. Smart Mater. Struct. 2008, 17, 043001. [Google Scholar] [CrossRef]
- Fang, H.B.; Liu, J.Q.; Xu, Z.Y.; Dong, L.; Wang, L.; Chen, D.; Cai, B.-C.; Liu, Y. Fabrication, and performance of MEMS-based piezoelectric power generatorfor vibration energy harvesting. J. Microelectron. 2006, 37, 1280–1284. [Google Scholar] [CrossRef]
- Park, K.I.; Hassan, E.; Kouritem, S.A.; Amer, F.Z.; Mubarak, R.I. Acoustic energy harvesting using an array of piezoelectric cantilever plates for railways, and highways environmental noise. Ain Shams Eng. J. 2024, 15, 102461. [Google Scholar]
- Pradhan, L.K.; Kar, M. Relaxor Ferroelectric Oxides: Concept to Applications. In Multifunctional Ferroelectric Materials; Intech Open: London, UK, 2021; pp. 167–169. [Google Scholar] [CrossRef]
- Cross, L.E. Relaxor ferroelectrics. Ferroelectrics 1987, 76, 241–267. [Google Scholar] [CrossRef]
- Lalitha, K.V.; Hinterstein, M.; Lee, K.Y.; Yang, T.; Chen, L.Q.; Groszewicz, P.B.; Koruza, J.; Rödel, J. Spontaneous ferroelectric order in lead-free relaxor Na1/2Bi1/2TiO3-based composites. Phys. Rev. B 2020, 101, 174108. [Google Scholar]
- Hong, Z.; Tian, B.; Ke, X.; Yang, S.; Wang, Y. Origin of reentrant relaxor formation in ferroelectric solid solutions. Phys. Rev. B 2023, 107, 224105. [Google Scholar] [CrossRef]
- Jayakrishnan, A.R.; Silva, J.P.B.; Kamakshi, K.; Dastan, D.; Annapureddy, V.; Pereira, M.; Sekhar, K.C. Are lead-free relaxor ferroelectric materials the most promising candidates for energy storage capacitors? Prog. Mater. Sci. 2023, 132, 101046. [Google Scholar] [CrossRef]
- Al-Aaraji, M.N.; Hasan, W.N.; Al-Marzok, I.K. Progress in Lead Free- Relaxor Ferroelectrics for Energy Storage Applications. Conference, College of Material Engineering, University of Babylon, Iraq. J. Phys. Conf. Ser. 2021, 1973, 012117. [Google Scholar] [CrossRef]
- Veerapandiyan, V.; Benes, F.; Gindel, T.; Deluca, M. Strategies to Improve the Energy Storage Properties of Perovskite Lead-Free Relaxor Ferroelectrics. Materials 2020, 13, 5742. [Google Scholar] [CrossRef]
- Park, S.E.; Shrout, T.R. Ultrahigh strain, and piezoelectric behavior in relaxor based ferroelectric single crystals. J. Appl. Phys. 1997, 82, 1804. [Google Scholar] [CrossRef]
- Sahul, R. Effect of Manganese Doping on PIN-PMN-PT Single Crystals for High Power Applications. Ph.D. Thesis, Pennsylvania State University, State College, PA, USA, 2014. [Google Scholar]
- Stringer, C.J.; Donnelly, N.J.; Shrout, T.R.; Randall, C.A.; Alberta, E.F.; Hackenberger, W.S. Dielectric Characteristics of Perovskite-Structured High-Temperature Relaxor Ferroelectrics: The BiScO3–Pb(Mg1/3Nb2/3)O3–PbTiO3 Ternary System. J. Am. Ceram. Soc. 2008, 91, 1781–1787. [Google Scholar] [CrossRef]
- Bush, A.A.; Kamentsev, K.E.; Lavrentiev, A.M.; Segalla, A.G.; Fetisov, Y.K. Dielectric, and piezoelectric properties of ceramic samples of solid solutions (1-2x)BiScO3·xPbTiO3·xPbMg1/3Nb2/3O3 (0.30≤x≤0.46). Inorg. Mater. 2011, 47, 865–871. [Google Scholar] [CrossRef]
- Bush, A.A.; Kamentsev, K.E.; Bekhtin, M.A.; Segalla, A.G. Segnetoelectric-relaxor properties of samples of the system. (1-2x)BiScO3·xPbTiO3·xPbMg1/3Nb2/3O3 (0.30≤x≤0.46). FTT 2017, 59, 36–44. [Google Scholar]
- Xie, G. Structure, and electrical properties of PMN-BS-PT piezoelectric ceramics. In Proceedings of the Symposium on Piezoelectricity, Acoustic Waves, and Device Applications, Chengdu, China, 27–30 October 2017; pp. 537–540. [Google Scholar]
- Talanov, M.V.; Bush, A.A.; Kamentsev, K.E.; Sirotinkin, V.P.; Segalla, A.G. Structure-property relationships in BiScO3–PbTiO3–PbMg1/3Nb2/3O3 ceramics near the morphotropic phase boundary. J. Am. Ceram. Soc. 2018, 101, 683–693. [Google Scholar] [CrossRef]
- Spitsin, A.I.; Bush, A.A.; Kamentsev, K.E.; Sirotinkin, V.P.; Talanov, M.V. Preparation, structural, and electrophysical studies of segmented ceramic samples of the system (1–2x)BiScO3·xPbTiO3·xPbMg1/3Nb2/3O3, 0 ≤ x ≤ 0.50. Fine Chem. Eng. 2019, 14, 78–89. [Google Scholar]
- Sysoev, M.A.; Bush, A.A.; Kamentsev, K.E.; Sirotinkin, V.P.; Nogai, A.A.; Nogai, A.S. Preparation, Structure, and Electrophysical Properties of Ceramic Samples of (1–2x)BiScO3∙(2–y)xPbTiO3∙yxPbMg1/3Nb2/3O3 Perovskite Solid Solutions. Inorg. Mater. 2023, 59, 1345–1355. [Google Scholar] [CrossRef]
- Nogai, A.A.; Sysoev, M.A.; Bush, A.A.; Nogai, A. Synthesis and study of structural and electrophysical characteristics of piezoceramic section (1–x)(0.8PbMg1/3Nb2/3O3·0.2BiScO3)·x(0.8PbTiO3·0.2BiScO3) of the ternary system BiScO3–PbTiO3–PbMg1/3Nb2/3O3. J. Adv. Dielectr. 2024, 2450011. [Google Scholar] [CrossRef]
- Nogai, A.S.; Nogai, A.A.; Uskenbaev, D.E.; Utegulov, A.B.; Nogai, E.A.; Toleugulov, D.D. Features of Structures, and Ionic Conductivity of Na3Fe2(PO4)3 Polycrystals Obtained by Solid Phase, and Mellte Methods. Ceramics 2023, 6, 2295–2306. [Google Scholar] [CrossRef]
- Uskenbaev, D.; Zhetpisbayev, K.; Nogai, A.S.; Beissenov, R.; Zhetpisbayeva, A.; Baigisova, K.; Salmenov, Y.; Nogai, A.; Tursyntay, S. Synthesis of high temperature superconducting ceramics in the Bi(Pb)-Sr-Ca-Cu-O system based on amorphous precursors. East.-Eur. J. Enterp. Technol. 2022, 118, 29–37. [Google Scholar] [CrossRef]
- Uskenbaev, D.; Nogai, A.; Uskenbayev, A.; Zhetpisbayev, K.; Nogai, E.; Dunayev, P.; Zhetpisbayeva, A.; Nogai, A. Synthesis, and Research of Critical Parameters of Bi-HTSC Ceramics Based on Glass Phase Obtained by IR Heating. Chem. Eng. 2023, 7, 95. [Google Scholar] [CrossRef]
- Bokov, A.; Ye, Z.-G. Recent progress in relaxor ferroelectrics with perovskite structure. J. Mater. Sci. 2006, 41, 31–52. [Google Scholar] [CrossRef]
- Maiti, T.; Guo, R.; Bhalla, A.S. Structure-property phase diagram of BaZrxTi1-xO3 System. J. Am. Ceram. Soc. 2008, 91, 1769–1780. [Google Scholar] [CrossRef]
- Zhao, X.; Qu, W.; He, H.; Vittayakorn, N.; Tan, X. Influence of Cation Order on the Electric Field-Induced Phase Transition in Pb(Mg1/3Nb2/3)O3-Based Relaxor Ferroelectrics. J. Am. Ceram. Soc. 2006, 89, 202–209. [Google Scholar] [CrossRef]




| First Type | Second Type | ||||||
|---|---|---|---|---|---|---|---|
| 2-1, 2-2, 2-3 | 2-4 | ||||||
| PleSolid Solution Composition: 0.20BS3·0.45PT·0.35PMN | First Annealing | Second Annealing | First Annealing | Melting and Hardening | Second Annealing | Melting and Hardening | Firing |
| Firing temperatures T, K | 1133 | 1473 | 1133 | 1623 | 1623 | 1623 | 1473 |
| Firing time t, h | 4 | 2 | 2 | 0.0083 | 2 | 0.083 | 2 |
| Cooling time t, h | 0.5 | 0.5 | 0.5 | 0.0041 | 0.5 | 0.0041 | 0.5 |
| Cooling rate V, K/h | 2266 | 2046 | 2266 | 395,853 | 3246 | 395,853 | 2046 |
| Characteristics | Type of Sample | ||||
|---|---|---|---|---|---|
| 1-1 | 2-1 | 2-2 | 2-3 | 2-4 | |
| a, Å | 4.0215(4) | 4.0218(4) | 4.0213(6) | 4.0210(4) | 4.0222(6) |
| Tm1(1 kHz), K | 450 | 428 | 428 | 423 | 424 |
| Tm1(1 MHz), K | 477 | 460 | 455 | 456 | 455 |
| Tmp, K | 348 | 314 | 320 | 314 | 319 |
| TVF, K | 383(9) | 346(4) | 368(3) | 340(9) | 336(4) |
| ε1m (1 kHz) | 7320 | 6505 | 7685 | 8275 | 5870 |
| ε1 (296 K, 1 kHz) | 1970 | 2030 | 2540 | 2465 | 2070 |
| ε1m (1 MHz) | 6224 | 5300 | 6245 | 6745 | 4720 |
| ε1 (296 K, 1 MHz) | 1360 | 1400 | 1720 | 1645 | 1420 |
| tgδ (296 K, 1 kHz) | 0.076 | 0.077 | 0.076 | 0.087 | 0.073 |
| tgδ (296 K, 1 MHz) | 0.123 | 0.111 | 0.112 | 0.130 | 0.106 |
| jm, nA/cm2 | 10 | 35 | 2.6 | 38 | 28 |
| d33, pC/N | 220 | 195 | 193 | 178 | 190 |
| fo, Hz | 1.7 × 1010 | 5.5 × 1011 | 3.5 × 109 | 2.5 × 1012 | 2.7 × 1012 |
| Ea, eV | 0.056(21) | 0.093(12) | 0.040(5) | 0.11(2) | 0.12(2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogai, A.A.; Nogai, A.S.; Uskenbaev, D.E.; Nogai, E.A. Features of Dielectric Properties of 0.20BiScO3·0.45PbTiO3·0.35PbMg1/3Nb2/3O3 Samples Obtained by the Melt-Hardening Method. Ceramics 2024, 7, 1401-1412. https://doi.org/10.3390/ceramics7040091
Nogai AA, Nogai AS, Uskenbaev DE, Nogai EA. Features of Dielectric Properties of 0.20BiScO3·0.45PbTiO3·0.35PbMg1/3Nb2/3O3 Samples Obtained by the Melt-Hardening Method. Ceramics. 2024; 7(4):1401-1412. https://doi.org/10.3390/ceramics7040091
Chicago/Turabian StyleNogai, A. A., A. S. Nogai, D. E. Uskenbaev, and E. A. Nogai. 2024. "Features of Dielectric Properties of 0.20BiScO3·0.45PbTiO3·0.35PbMg1/3Nb2/3O3 Samples Obtained by the Melt-Hardening Method" Ceramics 7, no. 4: 1401-1412. https://doi.org/10.3390/ceramics7040091
APA StyleNogai, A. A., Nogai, A. S., Uskenbaev, D. E., & Nogai, E. A. (2024). Features of Dielectric Properties of 0.20BiScO3·0.45PbTiO3·0.35PbMg1/3Nb2/3O3 Samples Obtained by the Melt-Hardening Method. Ceramics, 7(4), 1401-1412. https://doi.org/10.3390/ceramics7040091





