Microstructure and Mechanical Properties of Diamond–Ceramic Composites Fabricated via Reactive Spark Plasma Sintering
Abstract
1. Introduction
2. Experimental Procedure
3. Results and Discussion
4. Conclusions
- (1)
- Through the absorption of graphite by Ti and Si, the liquid phase provided by Si, and spark plasma sintering, diamond–ceramic composites were successfully prepared under conventional conditions.
- (2)
- Characteristic of variable-temperature sintering, the chemical reaction was activated at a higher temperature, and then the sintering temperature was reduced to prevent excessive graphitisation. This method is particularly suitable for preparing diamond–ceramic composites and has certain research value.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shul’zhenko, A.A.; Sokolov, A.N.; Gargin, V.G. New Diamond-Based Superhard Materials. Production and Properties. Review. J. Superhard Mater. 2018, 40, 304–314. [Google Scholar] [CrossRef]
- Zhang, J.B.; Wang, J.P.; Zhang, G.Q.; Huo, Z.X.; Huang, Z.J.; Wu, L.J. A review of diamond synthesis, modification technology, and cutting tool application in ultra-precision machining. Mater. Des. 2024, 237, 112577. [Google Scholar] [CrossRef]
- Chen, F.; Yan, Z.Q.; Liu, Z.P.; Long, Y.; Fu, N.K.; Zhang, F.L.; Liu, B.; Liu, Y. Preparation and properties of Al2O3-reinforced Cu-Ni-Sn metallic matrix for applications in diamond-cutting tools. Diam. Relat. Mater. 2020, 109, 108025. [Google Scholar] [CrossRef]
- Xiang, J.F.; Pang, S.Q.; Xie, L.J.; Gao, F.N.; Hu, X.; Yi, J.; Hu, F. Mechanism-Based FE Simulation of Tool Wear in Diamond Drilling of SiCp/Al Composites. Materials 2018, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Jia, Q.J.; Sun, J.; Li, Y.Q.; He, Y.S.; Bi, W.S.; Zheng, W.Y. Improved Bending Strength and Thermal Conductivity of Diamond/Al Composites with Ti Coating Fabricated by Liquid-solid Separation Method. Materials 2024, 17, 1485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Bai, G.Z.; Zhu, X.Y.; Dai, J.J.; Wang, X.T.; Wang, J.G.; Kim, M.J.; Zhang, H.L. Manipulating in-situ discrete carbide interlayer to achieve high thermal conductivity in Cu–B/diamond composite. Mater. Today Commun. 2023, 34, 105357. [Google Scholar] [CrossRef]
- Li, M.; Sun, Y.H.; Meng, Q.N.; Wu, H.D.; Gao, K.; Liu, B.C. Fabrication of Fe-Based Diamond Composites by Pressureless Infiltration. Materials 2016, 9, 1006. [Google Scholar] [CrossRef]
- Aldwell, B.; Yin, S.; McDonnell, K.A.; Trimble, D.; Hussain, T.; Lupoi, R. A novel method for metal-diamond composite coating deposition with cold spray and formation mechanism. Scripta. Mater. 2016, 115, 10–13. [Google Scholar] [CrossRef]
- Wei, C.L.; Wang, X.X.; Tong, P.; Wang, P.; Wen, J. Effect of different titanium addition methods on the properties of diamond/Cu composites. J. Mater. Res. Technol. 2024, 31, 2014–2022. [Google Scholar] [CrossRef]
- Ekimov, E.A.; Borovikov, N.F.; Ivanov, A.S.; Pal, A.F.; Rusinkevich, A.A.; Serov, A.O.; Starostin, A.N.; Fortov, V.E.; Gromnitskaya, E.L. Application of the dusty plasma method for preparation of diamond ceramics. Diam. Relat. Mater. 2014, 41, 1–5. [Google Scholar] [CrossRef]
- Kailer, A.; Matthey, B.; Kunze, S.; Herrmann, M.; Tschirpke, C. SiC-bonded diamond ceramics for extreme conditions in subsea applications. Int. J. Appl. Ceram. Technol. 2024, 21, 2690–2701. [Google Scholar] [CrossRef]
- Qian, J. High-pressure, high-temperature sintering of diamond–SiC composites by ball-milled diamond-Si mixtures. J. Mater. Res. 2002, 17, 2153–2160. [Google Scholar] [CrossRef]
- Herrmann, M.; Matthey, B.; Höhn, S.; Kinski, I.; Rafaja, D. Diamond–ceramics composites—New materials for a wide range of challenging applications. J. Eur. Ceram. Soc. 2012, 32, 1915–1923. [Google Scholar] [CrossRef]
- Zhang, J.W.; Zhan, G.D.; He, D.W.; Li, D.; Li, Q.; Du, C.C.; Dai, Q.S.; Liu, F.M.; Yan, X.Z. Transparent diamond ceramics from diamond powder. J. Eur. Ceram. Soc. 2023, 43, 853–861. [Google Scholar] [CrossRef]
- Jaworska, K.; Gibas, T.; Wyczesany, A.; Królicka, B.; Rajchel, B. Study on interactions in diamond–ceramic material systems. J. Mater. Process. Technol. 2003, 133, 118–121. [Google Scholar] [CrossRef]
- Lai, S.L.; Zang, J.H.; Shen, W.X.; Huang, G.F.; Fang, C.; Zhang, Y.W.; Chen, L.C.; Wang, Q.Q.; Wan, B.; Jia, X.P.; et al. High hardness and high fracture toughness B4C–diamond ceramics obtained by high-pressure sintering. J. Eur. Ceram. Soc. 2023, 43, 3090–3095. [Google Scholar] [CrossRef]
- Beffort, O.; Vaucher, S.; Khalid, F.A. On the thermal and chemical stability of diamond during processing of Al/diamond composites by liquid metal infiltration. Diam. Relat. Mater. 2004, 13, 1834–1843. [Google Scholar] [CrossRef]
- Zhu, C.X.; Lang, J.; Ma, N.G. Preparation of Si–diamond–SiC composites by in-situ reactive sintering and their thermal properties. Ceram. Int. 2012, 38, 6131–6136. [Google Scholar] [CrossRef]
- Liu, G.; Wang, A.Y.; Tang, R.; Bai, W.H.; Song, Y.W.; Ji, W.; Wang, W.M. Fabrication and modeling of ultra-hard and high-strength B4C-based laminated ceramics by brazing joining. Ceram. Int. 2022, 48, 27982–27987. [Google Scholar] [CrossRef]
- Zhou, L.; Li, Y.Y.; Kou, Z.L.; Zheng, L.P.; Li, Q.; Ma, G.L.; Zhang, Y.J.; He, D.W. Heterogeneous diamond–TiC composites with high fracture toughness and electrical conductivity. J. Eur. Ceram. Soc. 2024, 44, 4887–4894. [Google Scholar] [CrossRef]
- Zhou, X.L.; Wang, Y.H.; Li, T.H.; Li, X.H.; Cheng, X.Z.; Dong, L.; Yuan, Y.G.; Zang, J.B.; Lu, J.; Yu, Y.Q.; et al. Fabrication of diamond–SiC–TiC composite by a spark plasma sintering-reactive synthesis method. J. Eur. Ceram. Soc. 2015, 35, 69–76. [Google Scholar] [CrossRef]
- Yoshida, K.; Nishiyama, N.; Shinoda, Y.; Akarsu, T.; Wakai, F. Evaluation of effects of crack deflection and grain bridging on toughening of nanocrystalline SiO2 stishovite. J. Eur. Ceram. Soc. 2017, 37, 5113–5117. [Google Scholar] [CrossRef]
- Wang, A.Y.; He, Q.L.; Guo, W.C.; Liu, C.; Tian, T.; Hu, L.X.; Liu, L.S.; Wang, W.M.; Fu, Z.Y. Microstructure and properties of hot pressed TiB2 and SiC reinforced B4C-based composites. Mater. Today Commun. 2021, 26, 102082. [Google Scholar] [CrossRef]
- Tian, S.; Guo, W.C.; He, Q.L.; Wang, A.Y.; Tian, T.; Liu, C.; Hu, L.X.; Zhang, Z.X.; Wang, H.; Wang, W.M.; et al. Synthesis of TiN–TiB2–hBN composites powders with a core-shell structure and preparation of bulk samples. Mater. Today Commun. 2021, 29, 102783. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Y.S.; Wang, C.H.; Nan, B.Y.; Zhao, Z.F.; Cheng, L.F.; Zhang, L.T. Microstructure and Properties of Diamond/SiC Composites Via Hot Molding Forming and CVI Densifying. Adv. Eng. Mater. 2019, 21, 1800640. [Google Scholar] [CrossRef]
- Matthey, B.; Kunze, S. SiC-bonded diamond materials produced by pressureless silicon infiltration. J. Mater. Res. 2017, 32, 3362–3371. [Google Scholar] [CrossRef]
- Zhao, Z.F.; Liu, Y.S.; Feng, W.; Zhang, Q.; Cheng, L.F.; Zhang, L.T. Improvement on the thermal conductivity of Diamond/CVI-SiC composites using diamond particles. Diam. Relat. Mater. 2017, 74, 1–8. [Google Scholar] [CrossRef]
- Hong, T.X.; Chen, Y.H.; Liu, R.B.; Hai, W.X.; Zhang, X.L.; Liu, M.L.; Yan, S. Effect of infiltration temperature on graphitization and properties of diamond/SiC composites with SiC matrix. Int. J. Appl. Ceram. Technol. 2024, 21, 1829–1838. [Google Scholar] [CrossRef]
- Liu, Y.S.; Hu, C.H.; Men, J.; Feng, W.; Cheng, L.F.; Zhang, L.T. Effect of diamond content on microstructure and properties of diamond/SiC composites prepared by tape-casting and CVI process. J. Eur. Ceram. Soc. 2015, 35, 2233–2242. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
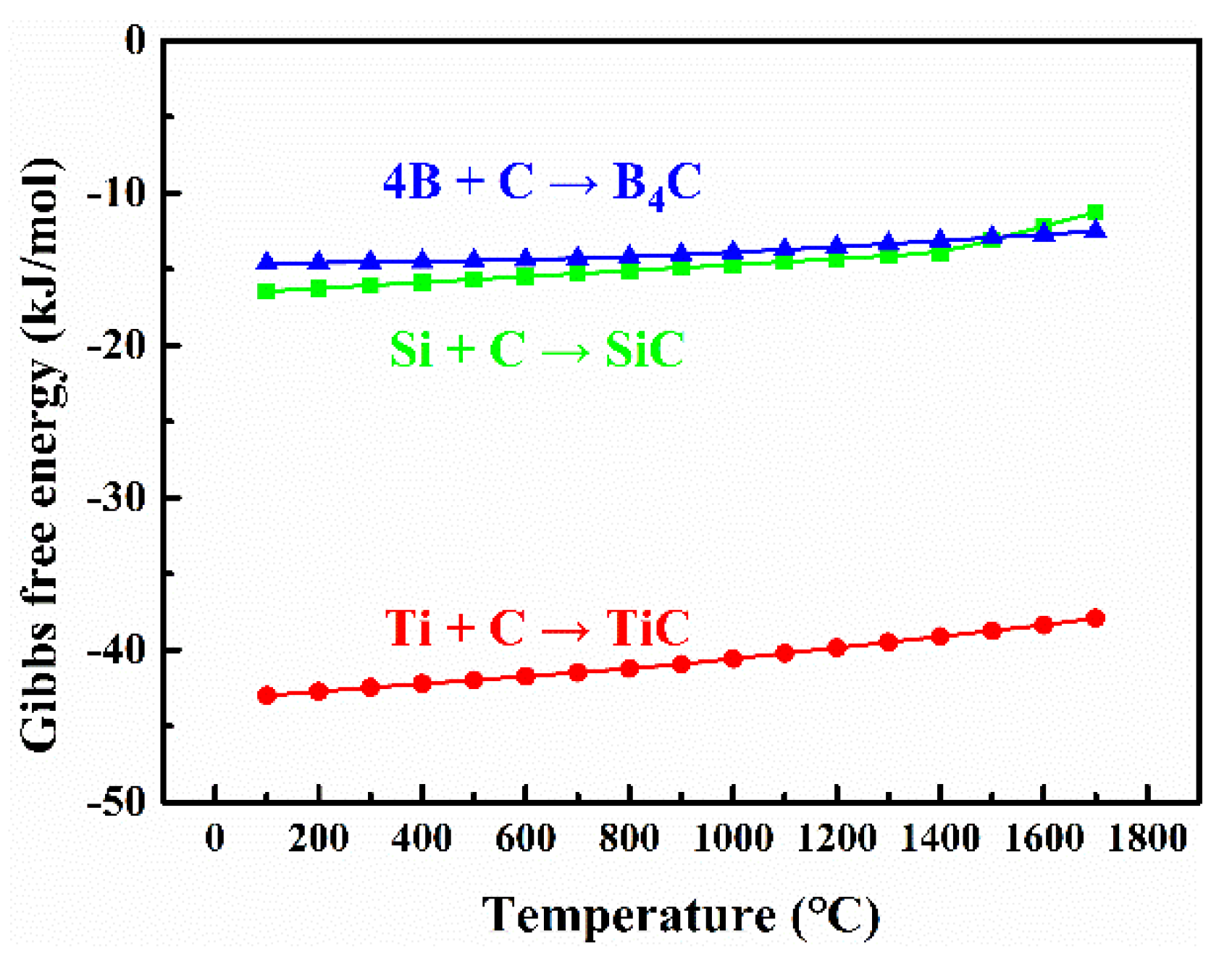
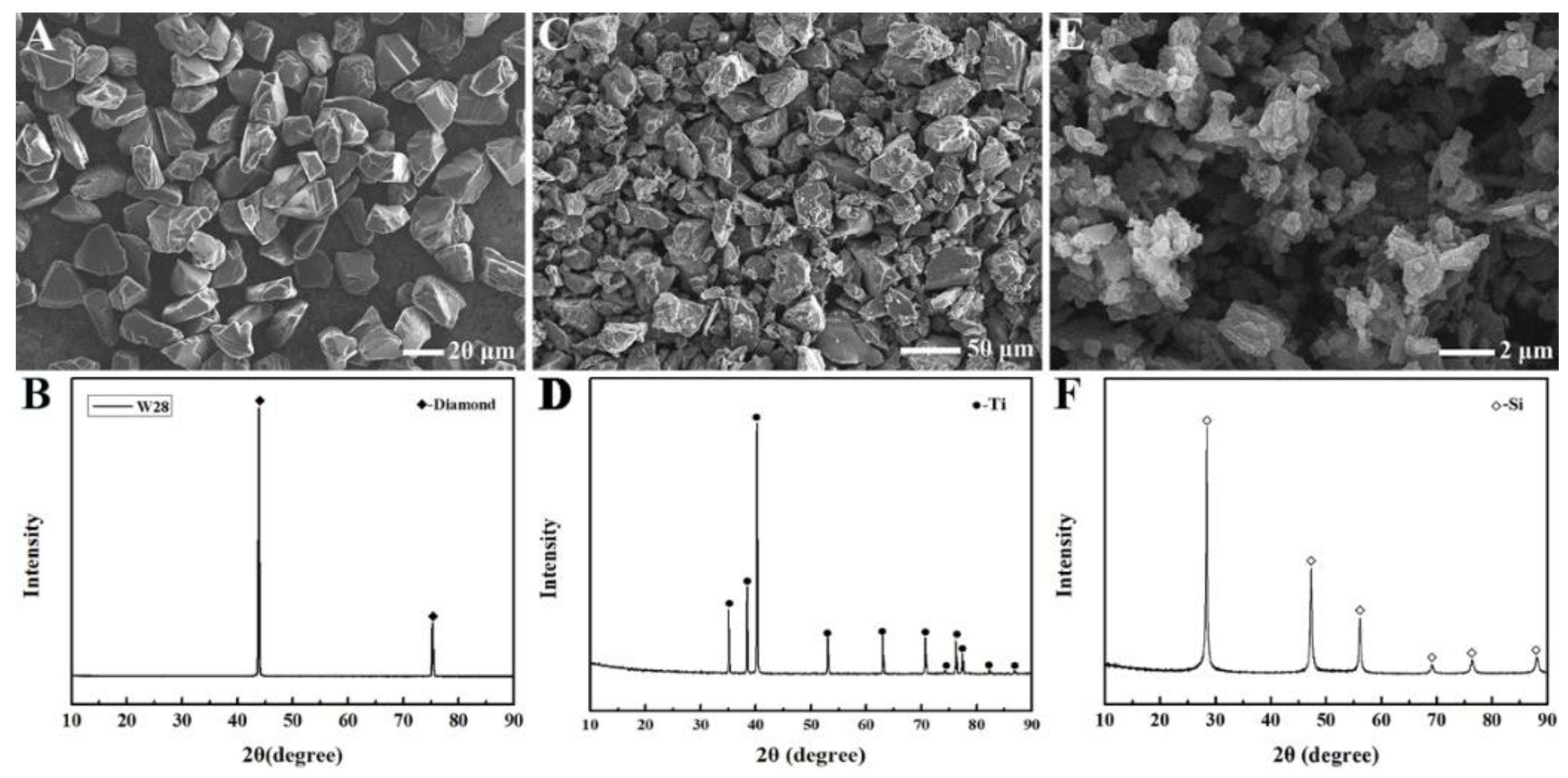

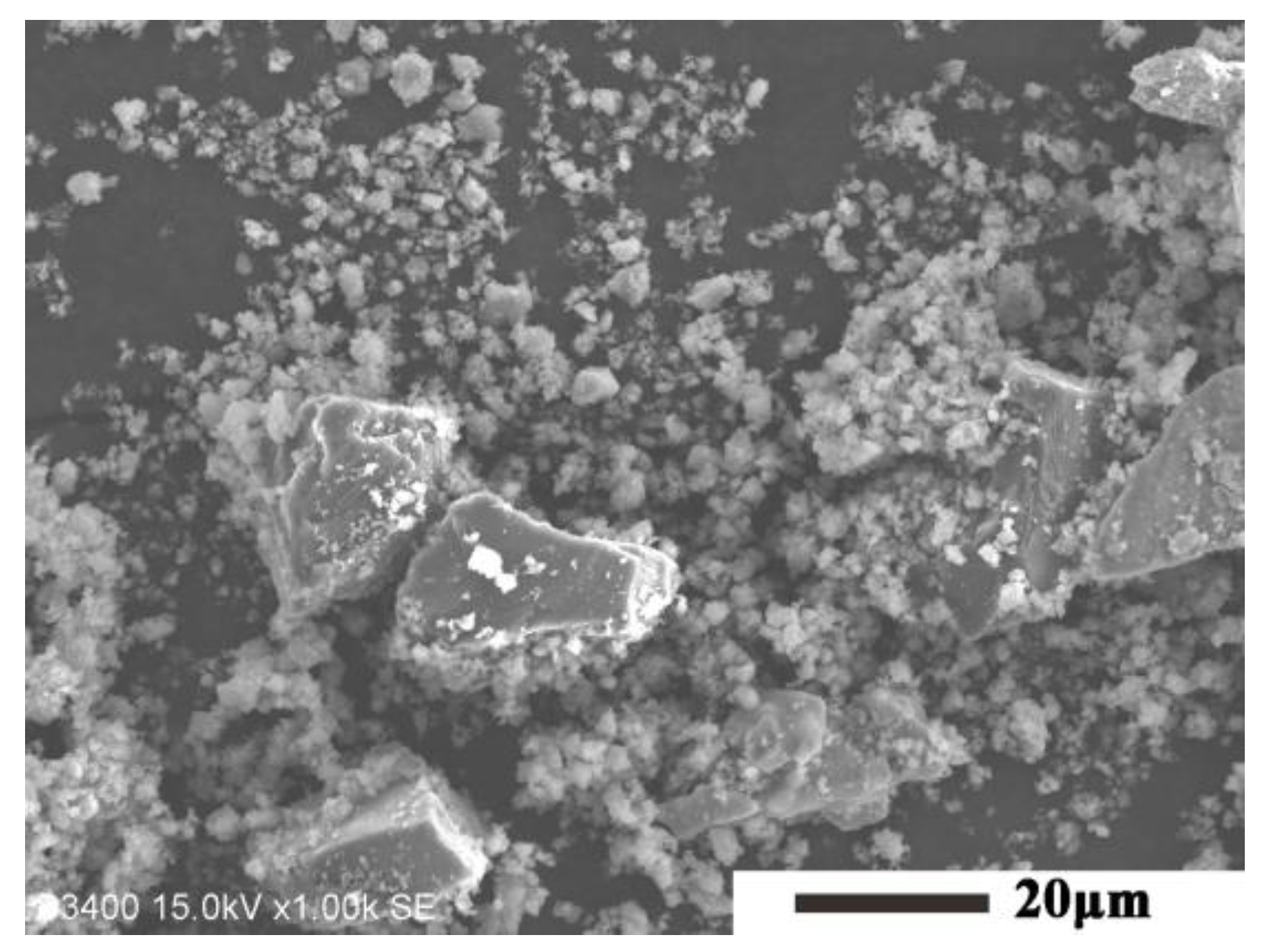
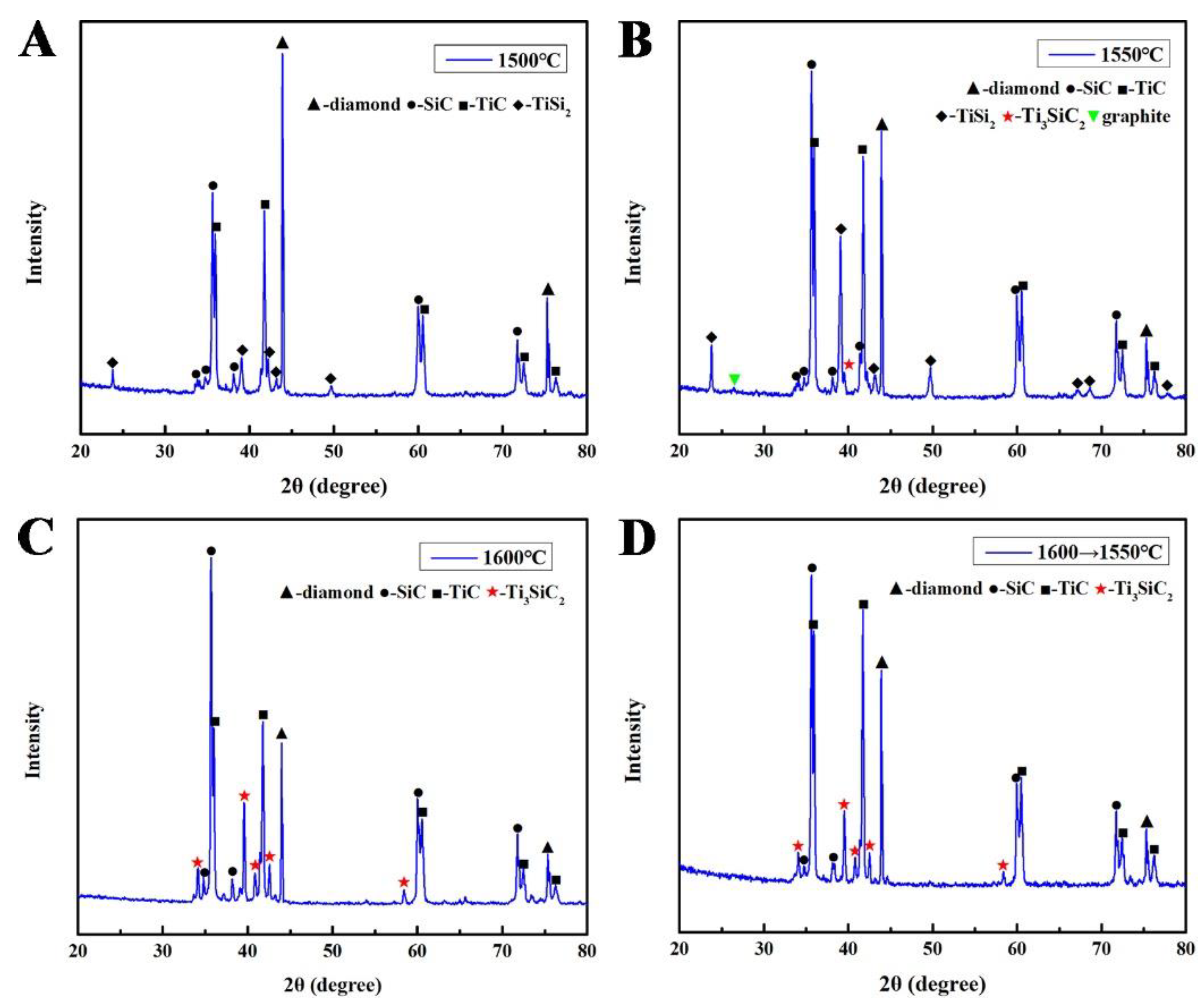
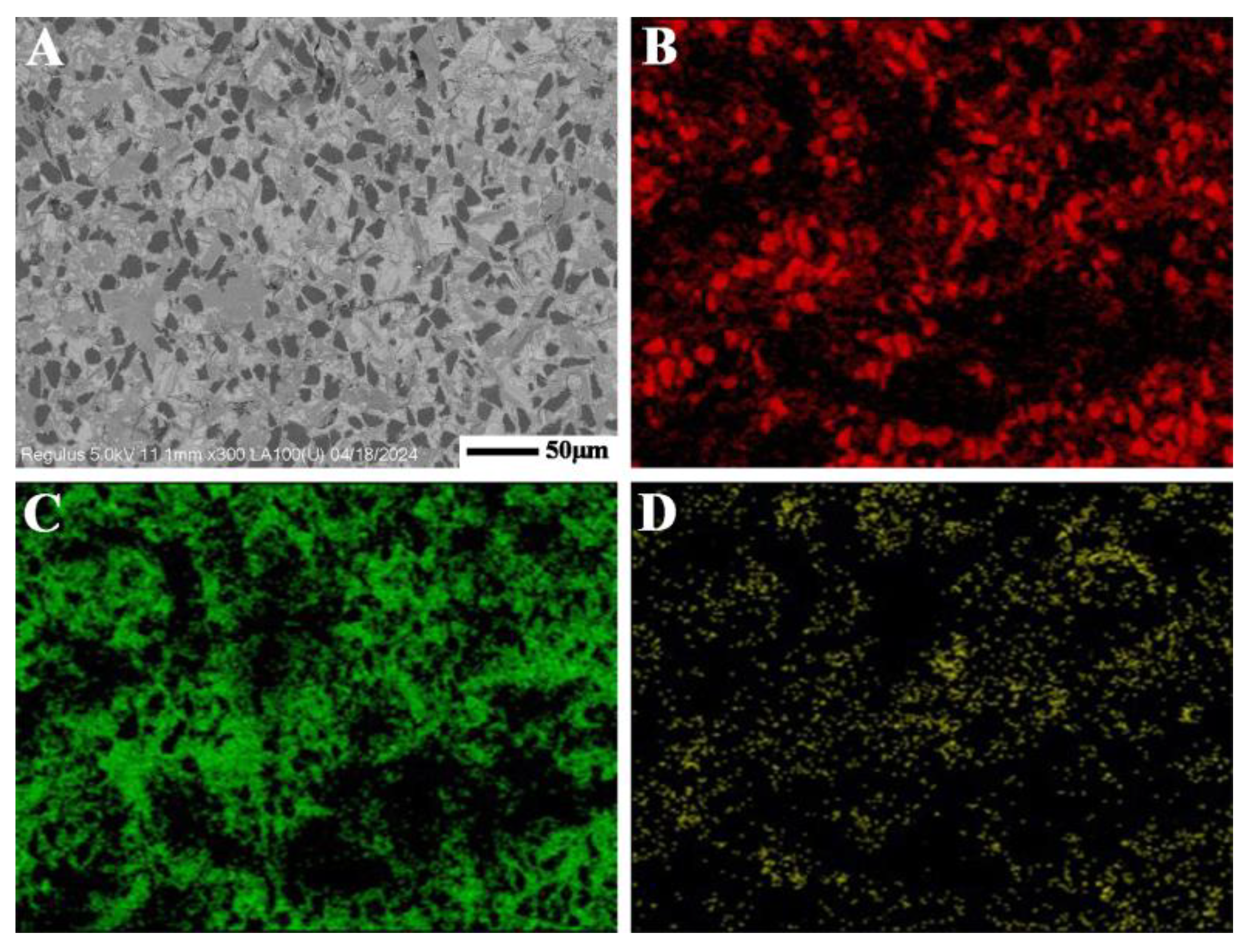

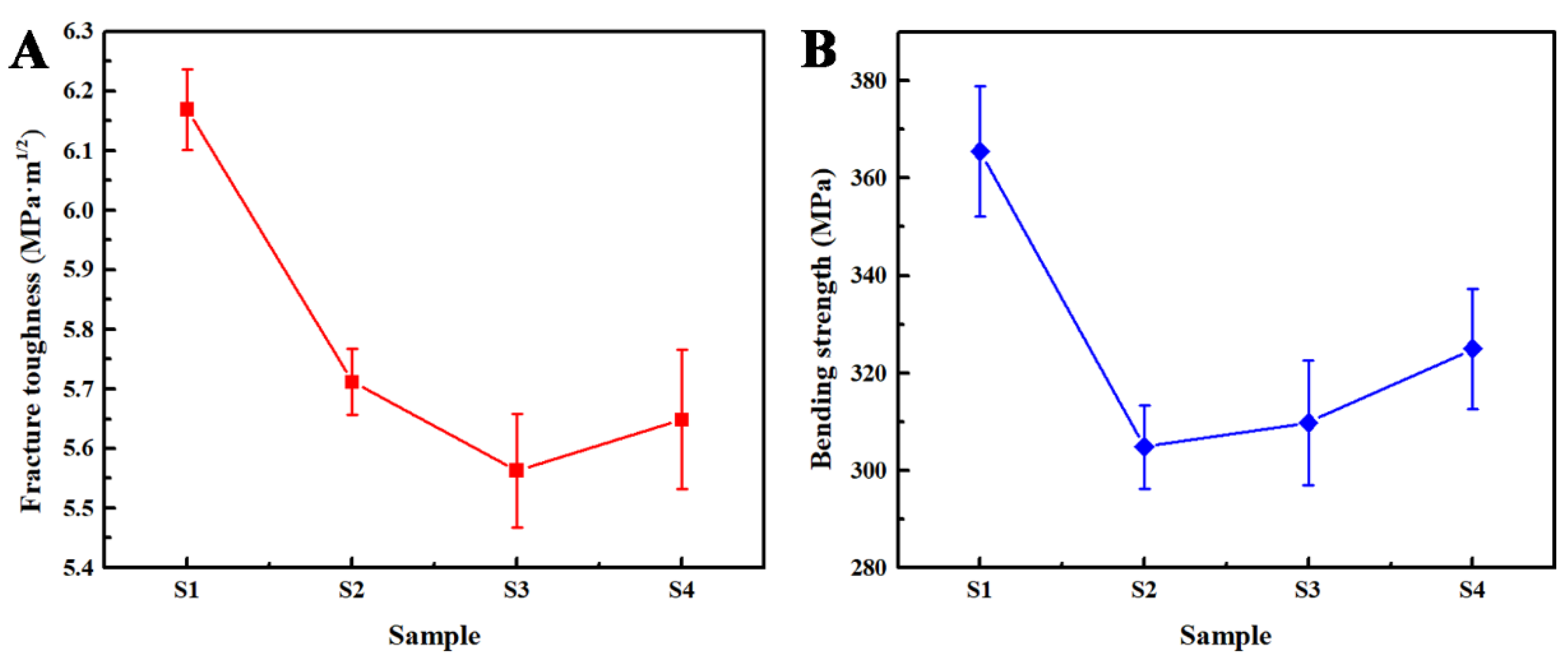
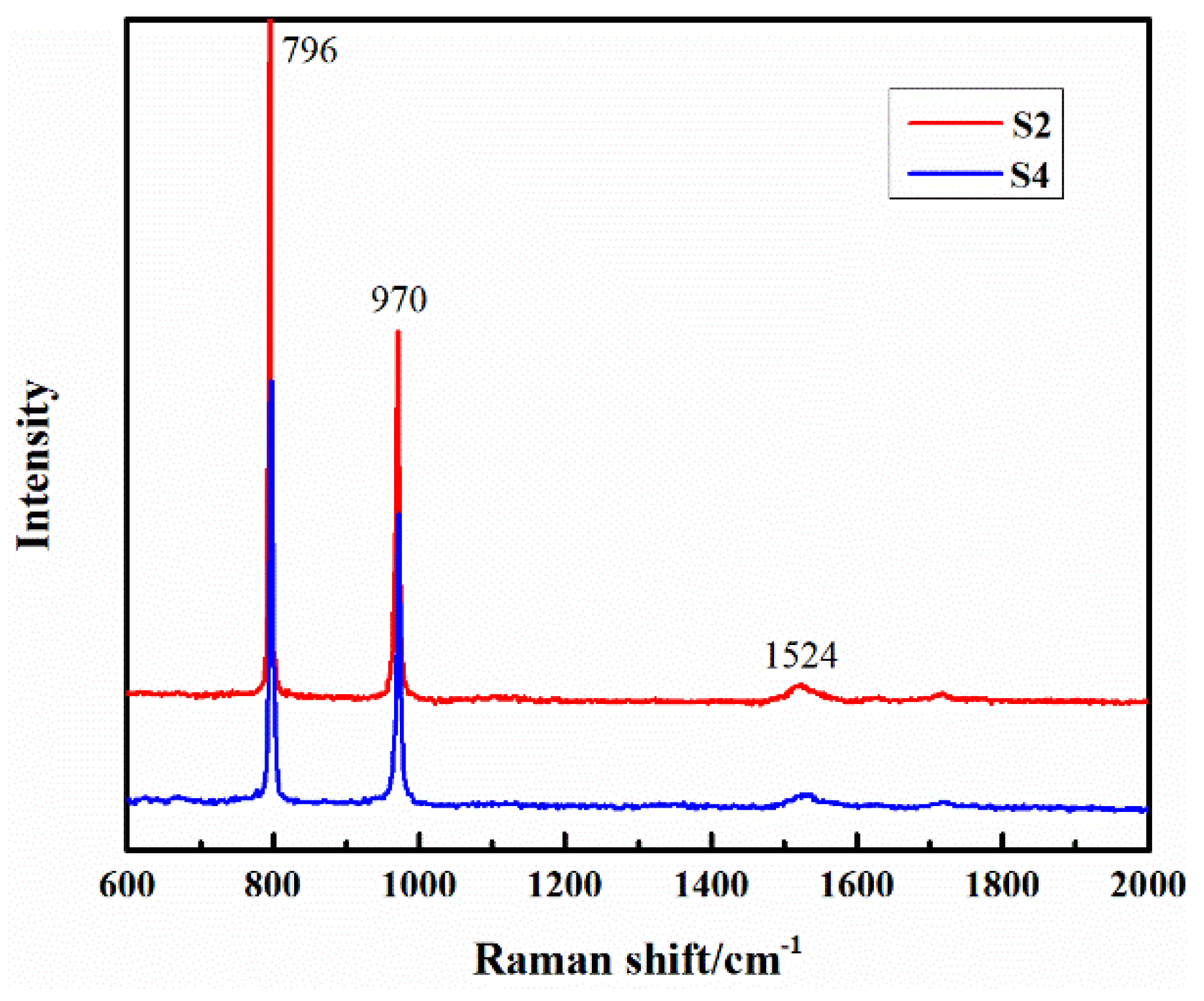
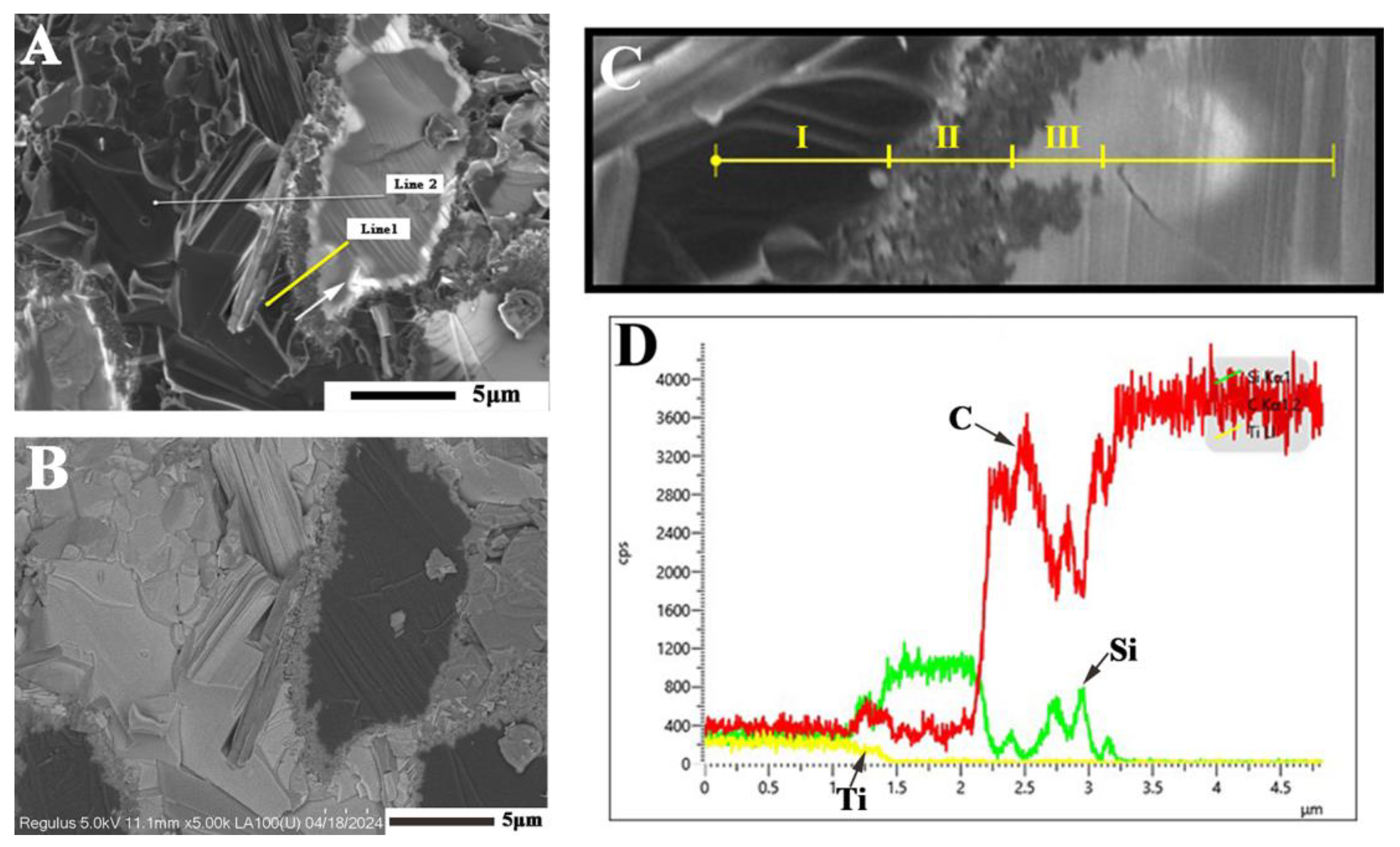
| Samples | S1 (1500 °C) | S2 (1550 °C) | S3 (1600 °C) | S4 (1600→1550 °C) |
|---|---|---|---|---|
| Volume density (g/cm3) | 3.647 | 3.604 | 3.555 | 3.596 |
| Reactants | Products | ||||
|---|---|---|---|---|---|
| Matter | 2TiC | TiSi2 | C | Ti3SiC2 | SiC |
| Relative atomic mass | 119.76 | 104.05 | 12.01 | 195.72 | 41 |
| Density/(g/cm3) | 4.93 | 4.39 | 3.52 | 4.53 | 3.2 |
| 4.60 | 4.21 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Hu, L.; Wang, A.; Liu, C.; He, Q.; Wang, W. Microstructure and Mechanical Properties of Diamond–Ceramic Composites Fabricated via Reactive Spark Plasma Sintering. Ceramics 2024, 7, 1390-1400. https://doi.org/10.3390/ceramics7040090
Shi Y, Hu L, Wang A, Liu C, He Q, Wang W. Microstructure and Mechanical Properties of Diamond–Ceramic Composites Fabricated via Reactive Spark Plasma Sintering. Ceramics. 2024; 7(4):1390-1400. https://doi.org/10.3390/ceramics7040090
Chicago/Turabian StyleShi, Yunwei, Lanxin Hu, Aiyang Wang, Chun Liu, Qianglong He, and Weimin Wang. 2024. "Microstructure and Mechanical Properties of Diamond–Ceramic Composites Fabricated via Reactive Spark Plasma Sintering" Ceramics 7, no. 4: 1390-1400. https://doi.org/10.3390/ceramics7040090
APA StyleShi, Y., Hu, L., Wang, A., Liu, C., He, Q., & Wang, W. (2024). Microstructure and Mechanical Properties of Diamond–Ceramic Composites Fabricated via Reactive Spark Plasma Sintering. Ceramics, 7(4), 1390-1400. https://doi.org/10.3390/ceramics7040090








