Reactive Spark Plasma Sintering and Oxidation of ZrB2-SiC and ZrB2-HfB2-SiC Ceramic Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Sample Preparation
2.2. Instrumentation
3. Results and Discussion
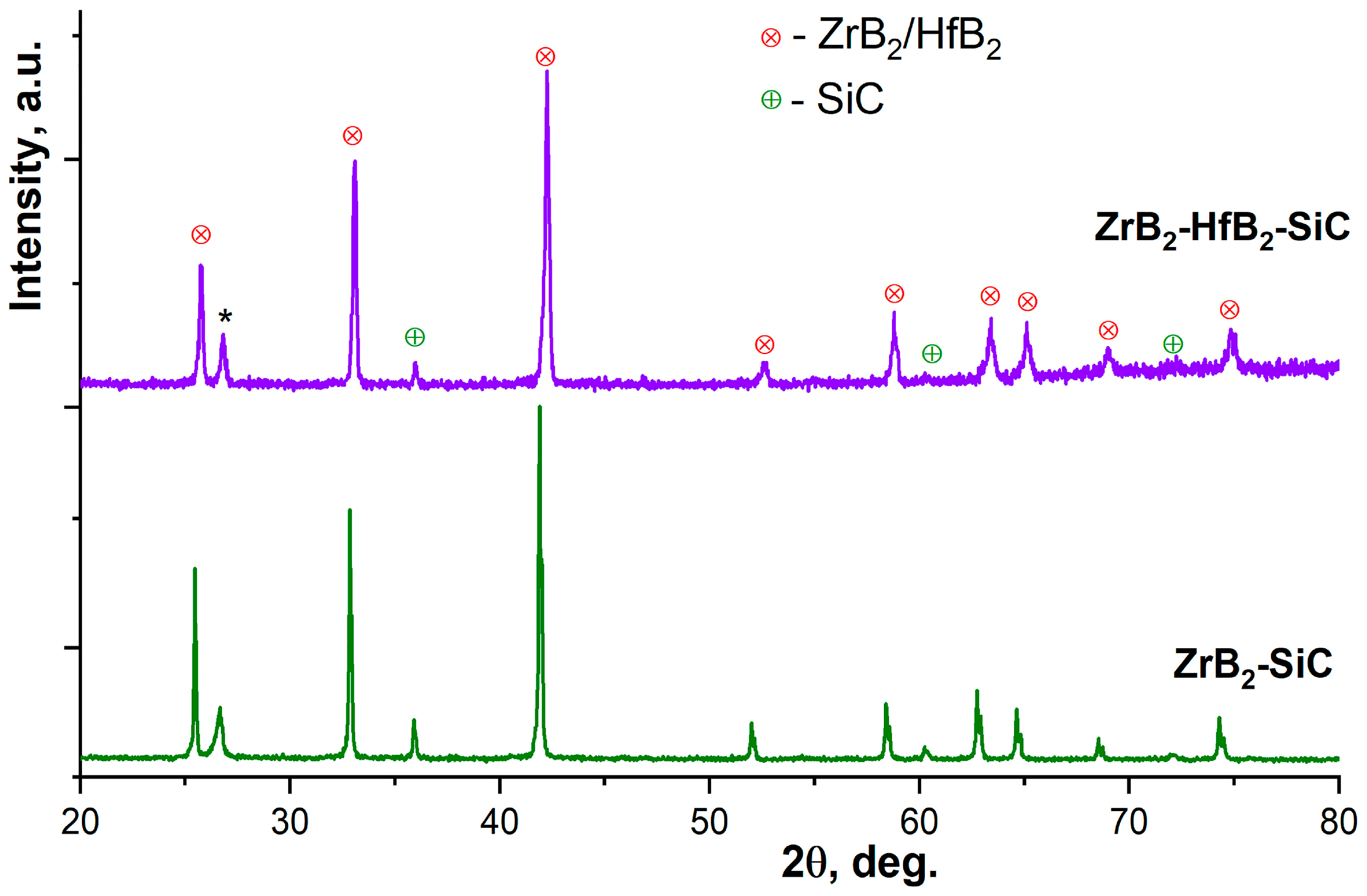
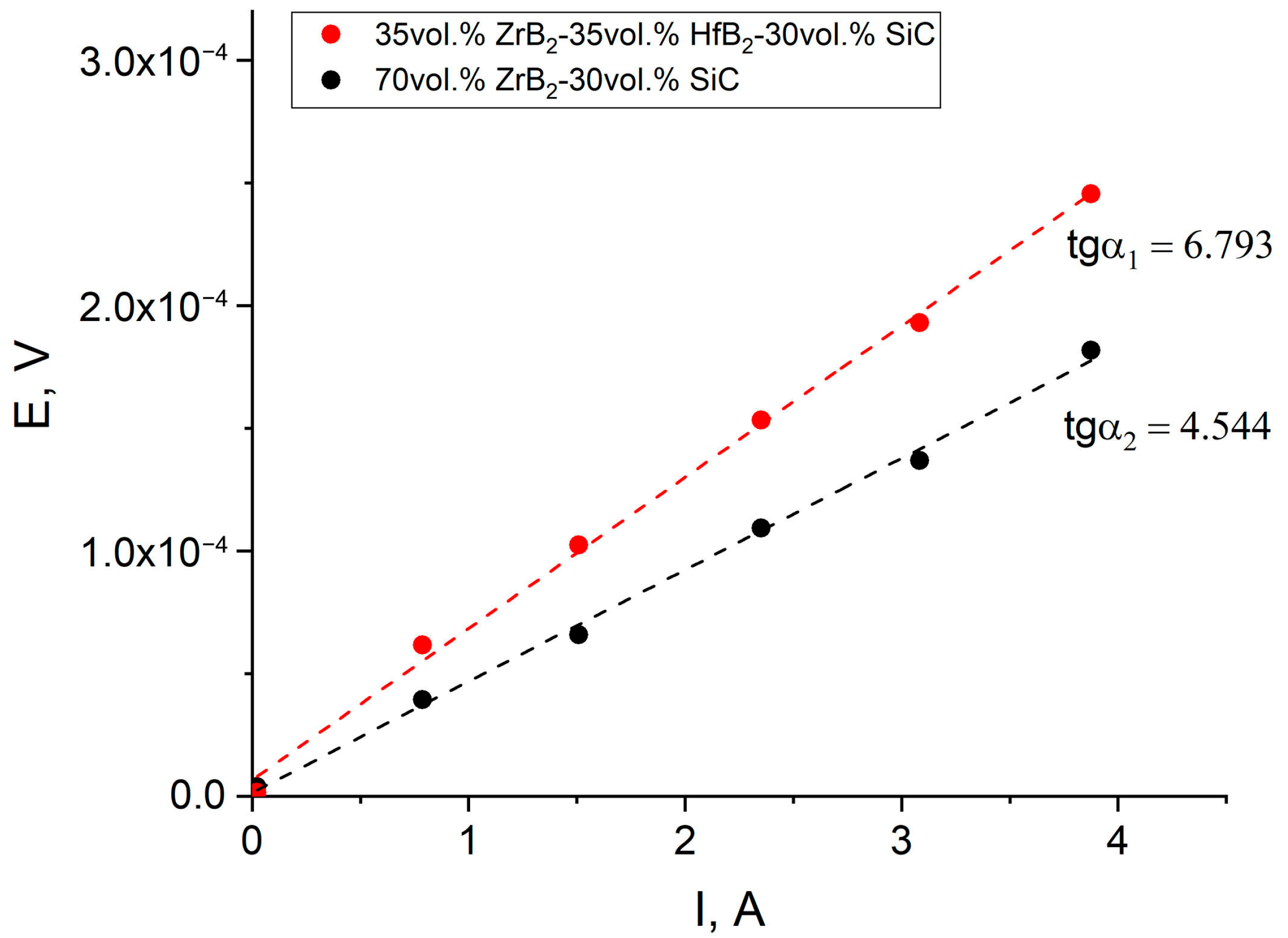
3.1. An Investigation of ZrB2-SiC and ZrB2-HfB2-SiC Ceramic Samples Obtained Using the SPS Method of Reaction
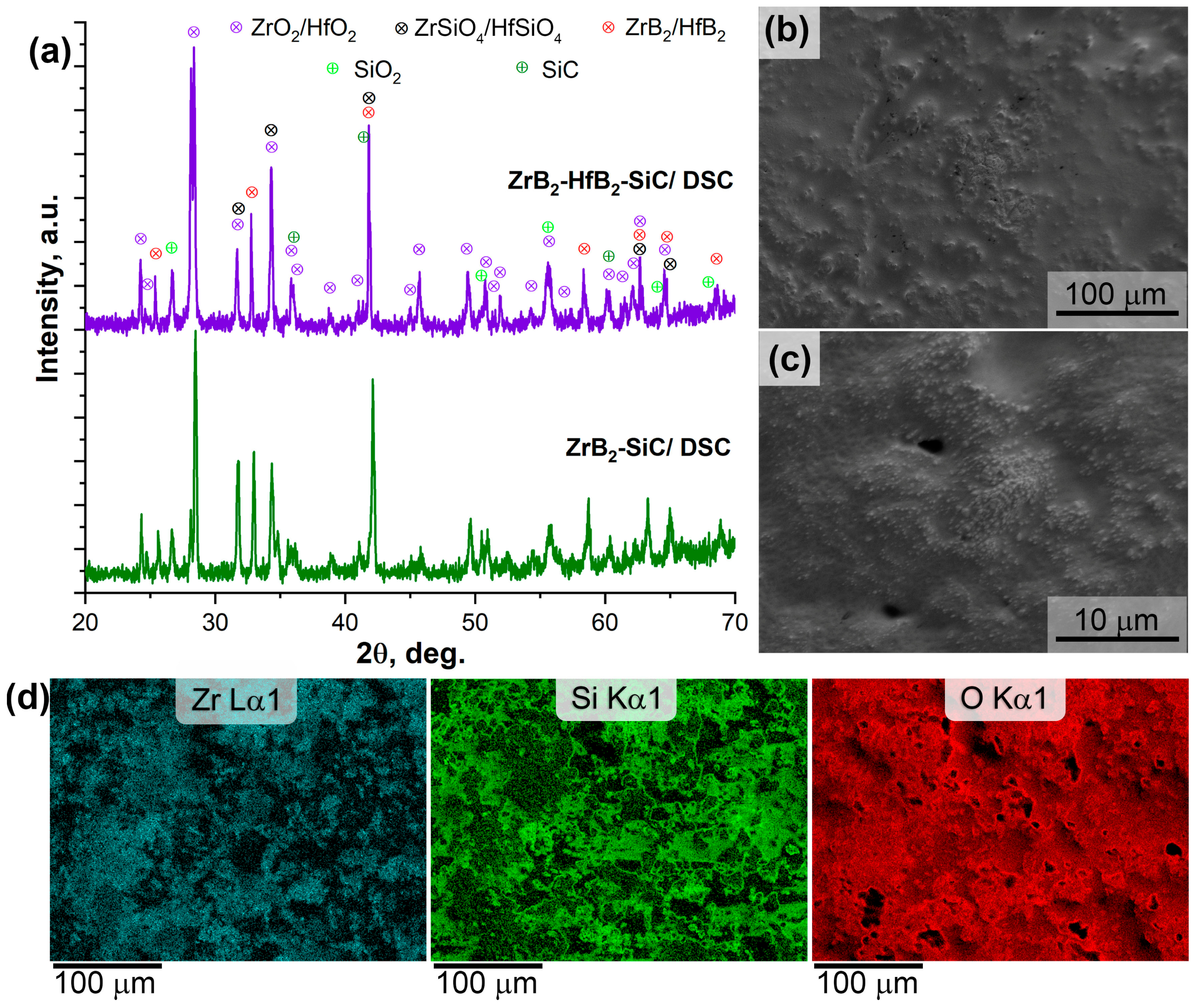
3.2. Heating Behavior of ZrB2-SiC and ZrB2-HfB2-SiC Materials during Heating in an Air Current (DSC/TG)
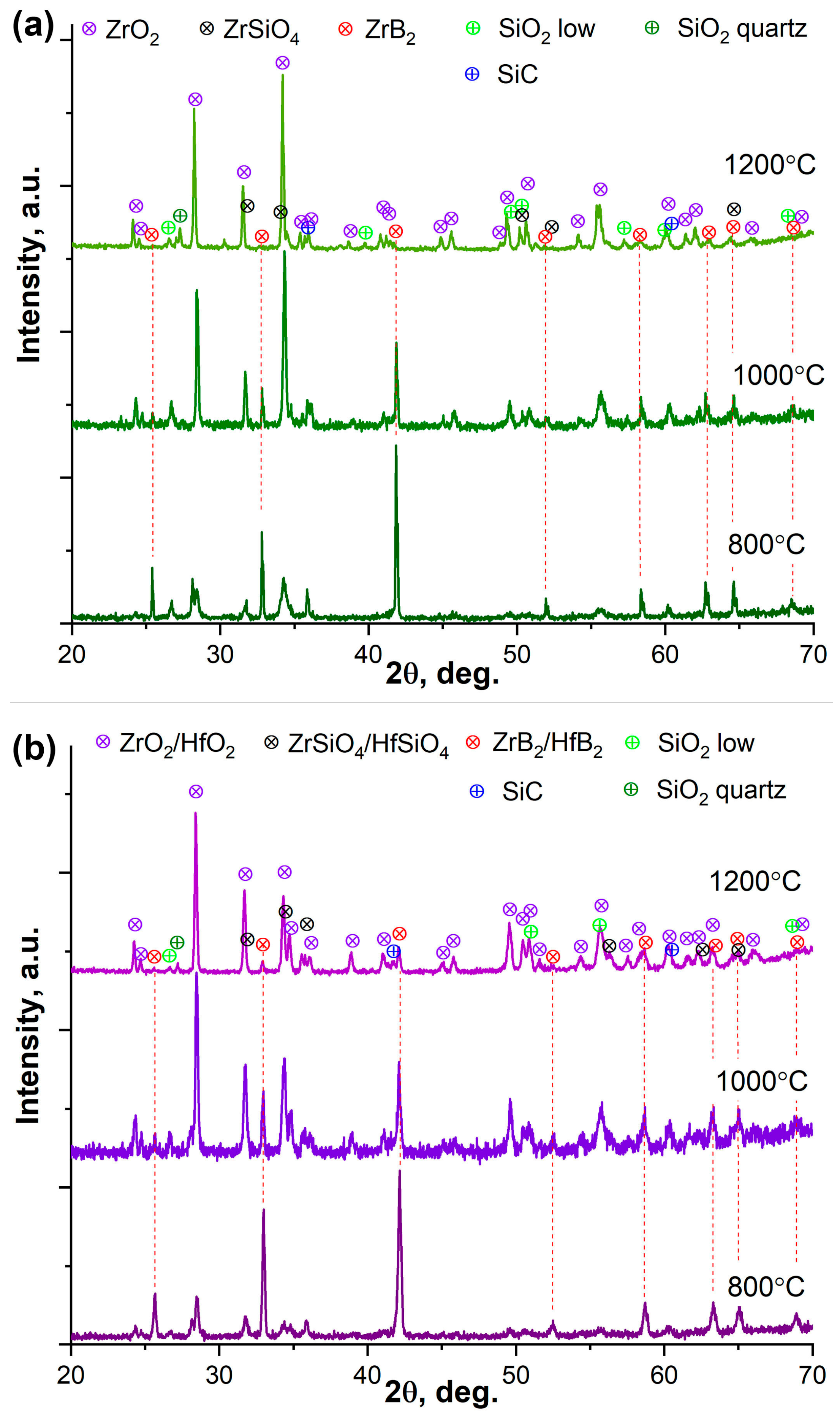
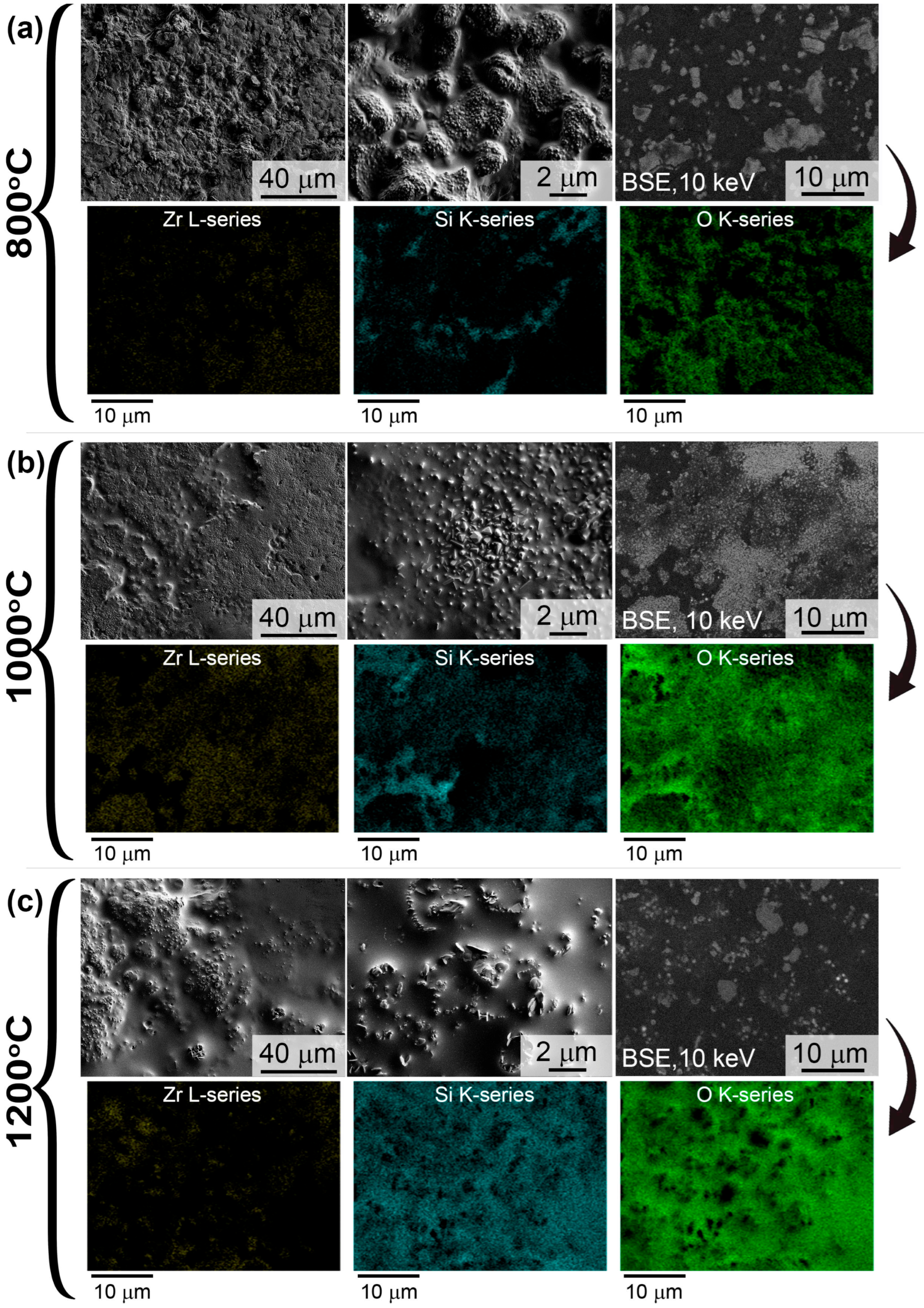
3.3. Oxidation Resistance of ZrB2-SiC and ZrB2-HfB2-SiC Materials Under Static Heating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simonenko, E.P.; Sevast’yanov, D.V.; Simonenko, N.P.; Sevast’yanov, V.G.; Kuznetsov, N.T. Promising ultra-high-temperature ceramic materials for aerospace applications. Russ. J. Inorg. Chem. 2013, 58, 1669–1693. [Google Scholar] [CrossRef]
- Opeka, M.M.; Talmy, I.G.; Zaykoski, J.A. Oxidation-based materials selection for 2000 °C + hypersonic aerosurfaces: Theoretical considerations and historical experience. J. Mater. Sci. 2004, 39, 5887–5904. [Google Scholar] [CrossRef]
- Wuchina, E.; Opila, E.; Opeka, M.; Fahrenholtz, B.; Talmy, I. UHTCs: Ultra-high temperature ceramic materials for extreme environment applications. Electrochem. Soc. Interface 2007, 16, 30. [Google Scholar] [CrossRef]
- Ni, D.; Cheng, Y.; Zhang, J.; Liu, J.-X.; Zou, J.; Chen, B.; Wu, H.; Li, H.; Dong, S.; Han, J.; et al. Advances in ultra-high temperature ceramics, composites, and coatings. J. Adv. Ceram. 2022, 11, 1–56. [Google Scholar] [CrossRef]
- Golla, B.R.; Mukhopadhyay, A.; Basu, B.; Thimmappa, S.K. Review on ultra-high temperature boride ceramics. Prog. Mater. Sci. 2020, 111, 100651. [Google Scholar] [CrossRef]
- Mor, M.; Vinci, A.; Sciti, D. Processing and oxidation resistance at 1650 °C of ZrB2-based UHTCMCs with short fibre gradients. J. Eur. Ceram. Soc. 2024, 44, 4536–4548. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, P.; Xun, L.; Zhou, Y.; Han, J.; Zhang, X. A universal strategy towards the fabrication of ultra-high temperature ceramic matrix composites with outstanding mechanical properties and ablation resistance. Compos. Part B Eng. 2024, 280, 111485. [Google Scholar] [CrossRef]
- Silvestroni, L.; Pavan, D.; Melandri, C.; Sciti, D.; Gilli, N.; Ortiz-Membrado, L.; Jiménez-Piqué, E.; Grande, A.M. Functionally graded ultra-high temperature ceramics: From thermo-elastic numerical analysis to damage tolerant composites. Mater. Des. 2022, 224, 111379. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, D.; Fu, Q. Ablation resistant behavior of silicide modified HfB2-SiC coating on graphite by spark plasma sintering: Role of MeSi2 addition. J. Eur. Ceram. Soc. 2024, 44, 6286–6297. [Google Scholar] [CrossRef]
- Han, J.; Hu, P.; Zhang, X.; Meng, S.; Han, W. Oxidation-resistant ZrB2–SiC composites at 2200 °C. Compos. Sci. Technol. 2008, 68, 799–806. [Google Scholar] [CrossRef]
- Tang, S.; Hu, C. Design, Preparation and Properties of Carbon Fiber Reinforced Ultra-High Temperature Ceramic Composites for Aerospace Applications: A Review. J. Mater. Sci. Technol. 2017, 33, 117–130. [Google Scholar] [CrossRef]
- Squire, T.H.; Marschall, J. Material property requirements for analysis and design of UHTC components in hypersonic applications. J. Eur. Ceram. Soc. 2010, 30, 2239–2251. [Google Scholar] [CrossRef]
- Nisar, A.; Hassan, R.; Agarwal, A.; Balani, K. Ultra-high temperature ceramics: Aspiration to overcome challenges in thermal protection systems. Ceram. Int. 2022, 48, 8852–8881. [Google Scholar] [CrossRef]
- Mungiguerra, S.; Cecere, A.; Savino, R.; Saraga, F.; Monteverde, F.; Sciti, D. Improved aero-thermal resistance capabilities of ZrB2-based ceramics in hypersonic environment for increasing SiC content. Corros. Sci. 2021, 178, 109067. [Google Scholar] [CrossRef]
- Mungiguerra, S.; Di Martino, G.D.; Cecere, A.; Savino, R.; Zoli, L.; Silvestroni, L.; Sciti, D. Ultra-high-temperature testing of sintered ZrB2-based ceramic composites in atmospheric re-entry environment. Int. J. Heat Mass Transf. 2020, 156, 119910. [Google Scholar] [CrossRef]
- Rita, C.C.P.; Miranda, F.d.S.; Caliari, F.R.; Rocha, R.M.; Essiptchouk, A.; Charakhovski, L.; Filho, G.P. Hypersonic Plasma Setup for Oxidation Testing of Ultrahigh Temperature Ceramic Composites. J. Heat Transf. 2020, 142, 082103. [Google Scholar] [CrossRef]
- Mor, M.; Meiser, M.; Langhof, N.; Vinci, A.; Zoli, L.; Alber-Laukant, B.; Tremmel, S.; Schafföner, S.; Sciti, D. Dry tribological behavior of 0/90° continuous carbon fiber reinforced ZrB2 based UHTC-material. J. Eur. Ceram. Soc. 2024, 44, 116664. [Google Scholar] [CrossRef]
- López-Arenal, J.; Moshtaghioun, B.M.; Gómez-García, D.; Ortiz, A.L. Processing of ZrB2 tribo-ceramics by reactive spark plasma sintering of ZrH2+2B subjected to high-energy pre-ball-milling. J. Eur. Ceram. Soc. 2023, 43, 5195–5206. [Google Scholar] [CrossRef]
- Hassan, R.; Patro, D.; Josyula, S.K.; Omar, S.; Balani, K. Incessant tribo-layer formation supresses high temperature wear damage in SiC reinforced equi-volume ZrB2-HfB2 composite. Surf. Coat. Technol. 2023, 465, 129586. [Google Scholar] [CrossRef]
- Sciti, D.; Guicciardi, S.; Zoli, L.; Failla, S.; Melandri, C. Dry sliding wear behaviour of ZrB2-based ceramics: Self-mated and cross coupling with alumina. J. Eur. Ceram. Soc. 2022, 42, 6335–6346. [Google Scholar] [CrossRef]
- Gupta, Y.; Kumar, B.V.M. ZrB2–SiC composites for sliding wear contacts: Influence of SiC content and counterbody. Ceram. Int. 2022, 48, 14560–14567. [Google Scholar] [CrossRef]
- Silvestroni, L.; Mungiguerra, S.; Sciti, D.; Di Martino, G.D.; Savino, R. Effect of hypersonic flow chemical composition on the oxidation behavior of a super-strong UHTC. Corros. Sci. 2019, 159, 108125. [Google Scholar] [CrossRef]
- Ma, H.-B.; Zou, J.; Zhu, J.-T.; Liu, L.-F.; Zhang, G.-J. Segregation of tungsten atoms at ZrB2 grain boundaries in strong ZrB2-SiC-WC ceramics. Scr. Mater. 2018, 157, 76–80. [Google Scholar] [CrossRef]
- Ma, H.-B.; Zou, J.; Zhu, J.-T.; Lu, P.; Xu, F.-F.; Zhang, G.-J. Thermal and electrical transport in ZrB2-SiC-WC ceramics up to 1800 °C. Acta Mater. 2017, 129, 159–169. [Google Scholar] [CrossRef]
- Ifti, H.S.; Hermann, T.; McGilvray, M.; Larrimbe, L.; Hedgecock, R.; Vandeperre, L. Flow Characterization of Porous Ultra-High-Temperature Ceramics for Transpiration Cooling. AIAA J. 2022, 60, 3286–3297. [Google Scholar] [CrossRef]
- Liu, D.; Jin, X.; Li, N.; Li, J.; Zhang, S.; Yi, T.; Li, X.; Li, X.; Zhang, B.; Dong, L.; et al. In-situ synthesis of hierarchically high porosity ZrB2 ceramics from carbon aerogel template with excellent performance in thermal insulation and light absorption. J. Eur. Ceram. Soc. 2024, 44, 738–747. [Google Scholar] [CrossRef]
- Zhao, K.; Ye, F.; Cheng, L.; Yang, J.; Chen, X. An overview of ultra-high temperature ceramic for thermal insulation: Structure and composition design with thermal conductivity regulation. J. Eur. Ceram. Soc. 2023, 43, 7241–7262. [Google Scholar] [CrossRef]
- Zhang, X.; He, J.; Han, L.; Huang, Z.; Xu, K.; Cai, W.; Wu, S.; Jia, Q.; Zhang, H.; Zhang, S. Foam gel-casting preparation of SiC bonded ZrB2 porous ceramics for high-performance thermal insulation. J. Eur. Ceram. Soc. 2023, 43, 37–46. [Google Scholar] [CrossRef]
- Zoli, L.; Failla, S.; Sani, E.; Sciti, D. Novel ceramic fibre-Zirconium diboride composites for solar receivers in concentrating solar power systems. Compos. Part B Eng. 2022, 242, 110081. [Google Scholar] [CrossRef]
- Barbarossa, S.; Orrù, R.; Cao, G.; Balbo, A.; Zanotto, F.; Sani, E. Optical properties of bulk high-entropy diborides for solar energy applications. J. Alloys Compd. 2023, 935, 167965. [Google Scholar] [CrossRef]
- Qiu, X.-L.; He, C.-Y.; Zhao, P.; Liu, B.-H.; Guo, H.-X.; Liu, G.; Gao, X.-H. Reinforcement optical performance and thermal tolerance in a TiB2-HfB2-based double-layer spectral selective absorber via a pre-annealing strategy. Mater. Today Phys. 2022, 24, 100690. [Google Scholar] [CrossRef]
- Chaplygin, A.; Simonenko, E.; Simonenko, N.; Kotov, M.; Yakimov, M.; Lukomskii, I.; Galkin, S.; Kolesnikov, A.; Vasil’evskii, S.; Shemyakin, A.; et al. Heat transfer and behavior of ultra high temperature ceramic materials under exposure to supersonic carbon dioxide plasma with additional laser irradiation. Int. J. Therm. Sci. 2024, 201, 109005. [Google Scholar] [CrossRef]
- Marschall, J.; Pejaković, D.A.; Fahrenholtz, W.G.; Hilmas, G.E.; Panerai, F.; Chazot, O. Temperature Jump Phenomenon During Plasmatron Testing of ZrB2-SiC Ultrahigh-Temperature Ceramics. J. Thermophys. Heat Transf. 2012, 26, 559–572. [Google Scholar] [CrossRef]
- Sevastyanov, V.G.; Simonenko, E.P.; Gordeev, A.N.; Simonenko, N.P.; Kolesnikov, A.F.; Papynov, E.K.; Shichalin, O.O.; Avramenko, V.A.; Kuznetsov, N.T. HfB2-SiC (45 vol %) ceramic material: Manufacture and behavior under long-term exposure to dissociated air jet flow. Russ. J. Inorg. Chem. 2014, 59, 1298–1311. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Kolesnikov, A.F.; Chaplygin, A.V.; Kotov, M.A.; Yakimov, M.Y.; Lukomskii, I.V.; Galkin, S.S.; Shemyakin, A.N.; Solovyov, N.G.; Lysenkov, A.S.; et al. Oxidation of Ceramic Materials Based on HfB2-SiC under the Influence of Supersonic CO2 Jets and Additional Laser Heating. Int. J. Mol. Sci. 2023, 24, 13634. [Google Scholar] [CrossRef]
- Chaplygin, A.V.; Simonenko, E.P.; Kotov, M.A.; Sakharov, V.I.; Lukomskii, I.V.; Galkin, S.S.; Kolesnikov, A.F.; Lysenkov, A.S.; Nagornov, I.A.; Mokrushin, A.S.; et al. Short-Term Oxidation of HfB2-SiC Based UHTC in Supersonic Flow of Carbon Dioxide Plasma. Plasma 2024, 7, 300–315. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Kolesnikov, A.F.; Chaplygin, A.V.; Papynov, E.K.; Shichalin, O.O.; Belov, A.A.; Nagornov, I.A.; Mokrushin, A.S.; Kuznetsov, N.T. Effect of Supersonic Nitrogen Flow on Ceramic Material Ta4HfC5–SiC. Russ. J. Inorg. Chem. 2023, 68, 479–486. [Google Scholar] [CrossRef]
- Monteverde, F.; Savino, R. Stability of ultra-high-temperature ZrB2–SiC ceramics under simulated atmospheric re-entry conditions. J. Eur. Ceram. Soc. 2007, 27, 4797–4805. [Google Scholar] [CrossRef]
- Savino, R.; Fumo, M.D.S.; Silvestroni, L.; Sciti, D. Arc-jet testing on HfB2 and HfC-based ultra-high temperature ceramic materials. J. Eur. Ceram. Soc. 2008, 28, 1899–1907. [Google Scholar] [CrossRef]
- Kolesnikov, A.F.; Kuznetsov, N.T.; Muravyeva, T.I.; Nagornov, I.A.; Sakharov, V.I.; Sevastyanov, V.G.; Simonenko, E.P.; Simonenko, N.P.; Chaplygin, A.V.; Shcherbakova, O.O. Investigation of Heat Transfer to HfB2-SiC-Based Ceramics in Underexpanded Dissociated-Nitrogen Flows and Analysis of the Surface. Fluid Dyn. 2022, 57, 513–523. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Kolesnikov, A.F.; Chaplygin, A.V.; Lysenkov, A.S.; Nagornov, I.A.; Mokrushin, A.S.; Kuznetsov, N.T. Investigation of the Effect of Supersonic Flow of Dissociated Nitrogen on ZrB2–HfB2–SiC Ceramics Doped with 10 vol.% Carbon Nanotubes. Materials 2022, 15, 8507. [Google Scholar] [CrossRef] [PubMed]
- Simonenko, E.P.; Kolesnikov, A.F.; Chaplygin, A.V.; Lysenkov, A.S.; Nagornov, I.A.; Lukomskii, I.V.; Galkin, S.S.; Mokrushin, A.S.; Simonenko, N.P.; Kuznetsov, N.T. Transformation of the Surface of HfB2–SiC–C(graphene) Ultrahigh-Temperature Ceramics in a High-Velocity Flow of Dissociated Nitrogen. Russ. J. Inorg. Chem. 2024, 69, 517. [Google Scholar] [CrossRef]
- Yang, M.; Tian, X.; Gao, C.; Guo, D.; Liu, J.; Xie, L.; Zhao, S.; Liu, H.; Sun, X. A 4.25-Gbps serializer ASIC in 180 nm for the monolithic active pixel sensor of the NICA-MPD Inner Tracker System. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2024, 1064, 169357. [Google Scholar] [CrossRef]
- Baryshnikov, V.; Babkin, V.; Buzin, S.; Burdyko, A.; Buryakov, M.; Golovatyuk, V.; Dmitriev, A.; Dulov, P.; Rumyantsev, M.; Romakhov, S.; et al. Status of the Time-of-Flight System of the MPD Experiment at the NICA Collider. Phys. At. Nucl. 2023, 86, 788–795. [Google Scholar] [CrossRef]
- Avdeev, S.P.; Busin, S.G.; Buryakov, M.G.; Golovatyuk, V.M.; Malakhov, A.I.; Milnov, G.D.; Kurepin, A.B.; Litvinenko, A.G.; Litvinenko, E.I. Detector for Setting Up Beam Convergence and Determining Luminosity at the Interaction Point on the MPD NICA. Phys. At. Nucl. 2023, 86, 680–691. [Google Scholar] [CrossRef]
- Guo, S.-Q. Densification of ZrB2-based composites and their mechanical and physical properties: A review. J. Eur. Ceram. Soc. 2009, 29, 995–1011. [Google Scholar] [CrossRef]
- Chamberlain, A.L.; Fahrenholtz, W.G.; Hilmas, G.E. Pressureless Sintering of Zirconium Diboride. J. Am. Ceram. Soc. 2006, 89, 450–456. [Google Scholar] [CrossRef]
- Zhu, S.; Fahrenholtz, W.G.; Hilmas, G.E. Influence of silicon carbide particle size on the microstructure and mechanical properties of zirconium diboride–silicon carbide ceramics. J. Eur. Ceram. Soc. 2007, 27, 2077–2083. [Google Scholar] [CrossRef]
- Rezaie, A.; Fahrenholtz, W.G.; Hilmas, G.E. Effect of hot pressing time and temperature on the microstructure and mechanical properties of ZrB2–SiC. J. Mater. Sci. 2007, 42, 2735–2744. [Google Scholar] [CrossRef]
- Mao, C.; Ren, X.; Ji, X.; Xu, L.; Wang, X.; Zhu, N.; Zhang, P.; Feng, P. High-temperature oxidation resistance of spent MoSi2 modified ZrB2–SiC–MoSi2 coatings prepared by spark plasma sintering. Ceram. Int. 2023, 49, 32913–32922. [Google Scholar] [CrossRef]
- Pellegrini, C.; Balat-Pichelin, M.; Rapaud, O.; Bêche, E.; Thébault, Y. Oxidation resistance up to 2600 K under air plasma of (Zr/Hf)B2 composites containing 20 vol% AlN for hypersonic applications. Ceram. Int. 2023, 49, 31634–31648. [Google Scholar] [CrossRef]
- Pellegrini, C.; Balat-Pichelin, M.; Rapaud, O.; Bêche, E. Oxidation resistance and emissivity of diboride-based composites containing tantalum disilicide in air plasma up to 2600 K for space applications. Ceram. Int. 2022, 48, 27878–27890. [Google Scholar] [CrossRef]
- Lu, J.; Zhu, S.; Liu, Y.; Xie, M. Effects of Gd2O3 Content on the Infrared Emissivity and Ablation Resistance of HfB2/SiC/TaSi2 Coating at 4400 kW/m. Coatings 2023, 13, 1397. [Google Scholar] [CrossRef]
- Sarkar, S.; Mondal, M.K.; Mallik, M. Correlation Between Pressureless Sintering, Microstructure, and Properties of ZrB2-SiC-Y2O3 Composites. J. Mater. Eng. Perform. 2024, 33, 5487–5500. [Google Scholar] [CrossRef]
- Chen, B.; Ni, D.; Bao, W.; Liao, C.; Luo, W.; Song, E.; Dong, S. Engineering Cf/ZrB2-SiC-Y2O3 for Thermal Structures of Hypersonic Vehicles with Excellent Long-Term Ultrahigh Temperature Ablation Resistance. Adv. Sci. 2023, 10, e2304254. [Google Scholar] [CrossRef]
- Monteverde, F.; Saraga, F.; Reimer, T.; Sciti, D. Thermally stimulated self-healing capabilities of ZrB2-SiC ceramics. J. Eur. Ceram. Soc. 2021, 41, 7423–7433. [Google Scholar] [CrossRef]
- Mor, M.; Vinci, A.; Failla, S.; Galizia, P.; Zoli, L.; Sciti, D. A novel approach for manufacturing of layered, ultra-refractory composites using pliable, short fibre-reinforced ceramic sheets. J. Adv. Ceram. 2023, 12, 155–168. [Google Scholar] [CrossRef]
- Galizia, P.; Failla, S.; Melandri, C.; Sciti, D. Local indentation response of carbon fibers embedded in a harsh environment: The sintered ultra-high temperature ceramic matrix. J. Eur. Ceram. Soc. 2024, 44, 5347–5357. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, J.; Ni, D.; Chen, B.; Cai, F.; Xuegang, Z.; Zhang, X.; Ding, Y.; Dong, S. Microstructure evolution and ablation mechanisms of Csf/ZrB2-SiC composites at different heat fluxes under air plasma flame. J. Eur. Ceram. Soc. 2024, 44, 3514–3524. [Google Scholar] [CrossRef]
- Zoli, L.; Servadei, F.; Failla, S.; Mor, M.; Vinci, A.; Galizia, P.; Sciti, D. ZrB 2–SiC ceramics toughened with oriented paper-derived graphite for a sustainable approach. J. Adv. Ceram. 2024, 13, 207–219. [Google Scholar] [CrossRef]
- Chen, Y. Ultra-high-temperature ceramic materials modified by graphene: An overview. Ceram. Silik. 2023, 67, 260–269. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Sevastyanov, V.G.; Kuznetsov, N.T. ZrB2/HfB2–SiC Ultra-High-Temperature Ceramic Materials Modified by Carbon Components: The Review. Russ. J. Inorg. Chem. 2018, 63, 1772–1795. [Google Scholar] [CrossRef]
- Opila, E.; Levine, S.; Lorincz, J. Oxidation of ZrB2- and HfB2-based ultra-high temperature ceramics: Effect of Ta additions. J. Mater. Sci. 2004, 39, 5969–5977. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, B.; Li, L.; An, L. Oxidation Behavior of ZrB2–SiC–TaC Ceramics. J. Am. Ceram. Soc. 2012, 95, 374–378. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Papynov, E.K.; Gridasova, E.A.; Sevastyanov, V.G.; Kuznetsov, N.T. Production of HfB2–SiC (10–65 vol % SiC) Ultra-High-Temperature Ceramics by Hot Pressing of HfB2–(SiO2–C) Composite Powder Synthesized by the Sol–Gel Method. Russ. J. Inorg. Chem. 2018, 63, 1–15. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Simonenko, N.P.; Gordeev, A.N.; Kolesnikov, A.F.; Sevastyanov, V.G.; Kuznetsov, N.T. Behavior of HfB2–30 vol% SiC UHTC obtained by sol–gel approach in the supersonic airflow. J. Sol-Gel Sci. Technol. 2019, 92, 386–397. [Google Scholar] [CrossRef]
- Zamora, V.; Ojalvo, C.; Guiberteau, F.; Ortiz, A.L. Fabricating ultrahard boron carbide–silicon carbide–zirconium diboride composites by spark plasma sintering from B4C with ZrSi2 aids. J. Eur. Ceram. Soc. 2024, 44, 4323–4329. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Jiao, Y.; Wei, B.; Ding, X.; Jin, X.; Ran, S. Reactive spark plasma sintering of an electrically conductive B4C-SiC-ZrB2 composite with enhanced mechanical properties. J. Eur. Ceram. Soc. 2024, 44, 2720–2729. [Google Scholar] [CrossRef]
- Tao, C.; Liao, H.; Chen, B.; Mao, W.; Wang, J.; Dai, C.; Fan, Z.; Zhou, Z.; Zuo, J. Ablation behavior and mechanism of (PyC-Csf)/ZrB2-SiC-ZrC ceramics sintered by reactive spark plasma sintering at low temperature. Corros. Sci. 2024, 229, 111873. [Google Scholar] [CrossRef]
- Shapkin, N.P.; Papynov, E.K.; Shichalin, O.O.; Buravlev, I.Y.; Simonenko, E.P.; Simonenko, N.P.; Zavjalov, A.P.; Belov, A.A.; Portnyagin, A.S.; Gerasimenko, A.V.; et al. Spark Plasma Sintering-Reactive Synthesis of SiC and SiC–HfB2 Ceramics Based on Natural Renewable Raw Materials. Russ. J. Inorg. Chem. 2021, 66, 629–637. [Google Scholar] [CrossRef]
- Papynov, E.K.; Shichalin, O.O.; Buravlev, I.Y.; Portnyagin, A.S.; Belov, A.A.; Maiorov, V.Y.; Skurikhina, Y.E.; Merkulov, E.B.; Glavinskaya, V.O.; Nomerovskii, A.D.; et al. Reactive Spark Plasma Synthesis of Porous Bioceramic Wollastonite. Russ. J. Inorg. Chem. 2020, 65, 263–270. [Google Scholar] [CrossRef]
- Papynov, E.; Belov, A.; Shichalin, O.; Buravlev, I.Y.; Azon, S.; Gerasimenko, A.; Parotkina, Y.; Zavjalov, A.; Tananaev, I.; Sergienko, V. SrAl2Si2O8 ceramic matrices for 90Sr immobilization obtained via spark plasma sintering-reactive synthesis. Nucl. Eng. Technol. 2021, 53, 2289–2294. [Google Scholar] [CrossRef]
- Papynov, E.K.; Belov, A.A.; Shichalin, O.O.; Buravlev, I.Y.; Azon, S.A.; Gridasova, E.A.; Parotkina, Y.A.; Yagofarov, V.Y.; Drankov, A.N.; Golub, A.V.; et al. Synthesis of Perovskite-Like SrTiO3 Ceramics for Radioactive Strontium Immobilization by Spark Plasma Sintering-Reactive Synthesis. Russ. J. Inorg. Chem. 2021, 66, 645–653. [Google Scholar] [CrossRef]
- Papynov, E.; Shichalin, O.; Buravlev, I.; Belov, A.; Portnyagin, A.; Fedorets, A.; Azarova, Y.; Tananaev, I.; Sergienko, V. Spark plasma sintering-reactive synthesis of SrWO4 ceramic matrices for 90Sr immobilization. Vacuum 2020, 180, 109628. [Google Scholar] [CrossRef]
- Bind, J.-M. Phase transformation during hot-pressing of cubic SiC. Mater. Res. Bull. 1978, 13, 91–96. [Google Scholar] [CrossRef]
- Ordan’yan, S.S.; Dmitriev, A.I.; Moroshkina, F.S. Carbon (Graphene/Graphite). Inorg. Mater. 1989, 25, 1487. [Google Scholar]
- Medvedeva, O.A. ChemInform Abstract: Legierungen von HfB2 mit ZrN. Chem. Informationsd. 1972, 3, 15. [Google Scholar] [CrossRef]
- Wyckoff, R.W.G. Interscience publishers, new york, new york rocksalt structure. Cryst. Struct. 1963, 1, 85. [Google Scholar]
- Kugai, L.N. IChemical stability of borides of transition metals of groups IV-VI in alkaline solutions. Izv. Akad. Nauk Neorg. Mater. 1972, 8, 669. (In Russian) [Google Scholar]
- Holleck, H. Legierungsverhalten von HfB2 mit uran- und übergangsmetalldiboriden. J. Nucl. Mater. 1967, 21, 14–20. [Google Scholar] [CrossRef]
- He, R.; Fang, D.; Wang, P.; Zhang, X.; Zhang, R. Electrical properties of ZrB2–SiC ceramics with potential for heating element applications. Ceram. Int. 2014, 40, 9549–9553. [Google Scholar] [CrossRef]
- Ghadami, S.; Taheri-Nassaj, E.; Baharvandi, H.R.; Ghadami, F. Improvement of mechanical properties of HfB2-based composites by incorporating in situ SiC reinforcement through pressureless sintering. Sci. Rep. 2021, 11, 9835. [Google Scholar] [CrossRef] [PubMed]
- Mallik, M.; Kailath, A.J.; Ray, K.; Mitra, R. Electrical and thermophysical properties of ZrB2 and HfB2 based composites. J. Eur. Ceram. Soc. 2012, 32, 2545–2555. [Google Scholar] [CrossRef]
- Winterer, M.; Delaplane, R.; McGreevy, R. X-ray diffraction, neutron scattering and EXAFS spectroscopy of monoclinic zirconia: Analysis by Rietveld refinement and reverse Monte Carlo simulations. J. Appl. Crystallogr. 2002, 35, 434–442. [Google Scholar] [CrossRef]
- Henderson, S.J.; Shebanova, O.; Hector, A.L.; McMillan, P.F.; Weller, M.T. Structural Variations in Pyrochlore-Structured Bi2Hf2O7, Bi2Ti2O7 and Bi2Hf2-xTixO7 Solid Solutions as a Function of Composition and Temperature by Neutron and X-ray Diffraction and Raman Spectroscopy. Chem. Mater. 2007, 19, 1712–1722. [Google Scholar] [CrossRef]
- Kusaba, K.; Yagi, T.; Kikuchi, M.; Syono, Y. Structural considerations on the mechanism of the shock-induced zircon-scheelite transition in ZrSiO. J. Phys. Chem. Solids 1986, 47, 675–679. [Google Scholar] [CrossRef]
- Speer, J.A.; Cooper, B.J. Crystal structure of synthetic hafnon, HfSiO4, Comparison with zircon and the actinide orthosilicates. Am. Mineral. 1982, 67, 804. [Google Scholar]
- Machatschki, F. Die Kristallstruktur von Tiefquarz SiO2 und Aluminiumorthoarsenat AlAsO4. Z. Krist.-Cryst. Mater. 1936, 94, 222–230. [Google Scholar] [CrossRef]
- Norton, J.T.; Blumenthal, H.; Sindeband, S.J. Structure of diborides of titanium, zirconium, columbium, tantalum and vanadium. JOM 1949, 1, 749–751. [Google Scholar] [CrossRef]
- Braekken, H. Zur Kristallstruktur des kubischen Karborunds. Z. Krist. 1930, 75, 572. [Google Scholar]
- Levien, L.; Prewitt, C.T.; Weidner, D.J. Structure and elastic properties of quartz at pressure. Am. Mineral. 1980, 65, 920. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonenko, E.P.; Papynov, E.K.; Shichalin, O.O.; Belov, A.A.; Nagornov, I.A.; Simonenko, T.L.; Gorobtsov, P.Y.; Teplonogova, M.A.; Mokrushin, A.S.; Simonenko, N.P.; et al. Reactive Spark Plasma Sintering and Oxidation of ZrB2-SiC and ZrB2-HfB2-SiC Ceramic Materials. Ceramics 2024, 7, 1566-1583. https://doi.org/10.3390/ceramics7040101
Simonenko EP, Papynov EK, Shichalin OO, Belov AA, Nagornov IA, Simonenko TL, Gorobtsov PY, Teplonogova MA, Mokrushin AS, Simonenko NP, et al. Reactive Spark Plasma Sintering and Oxidation of ZrB2-SiC and ZrB2-HfB2-SiC Ceramic Materials. Ceramics. 2024; 7(4):1566-1583. https://doi.org/10.3390/ceramics7040101
Chicago/Turabian StyleSimonenko, Elizaveta P., Eugeniy K. Papynov, Oleg O. Shichalin, Anton A. Belov, Ilya A. Nagornov, Tatiana L. Simonenko, Philipp Yu. Gorobtsov, Maria A. Teplonogova, Artem S. Mokrushin, Nikolay P. Simonenko, and et al. 2024. "Reactive Spark Plasma Sintering and Oxidation of ZrB2-SiC and ZrB2-HfB2-SiC Ceramic Materials" Ceramics 7, no. 4: 1566-1583. https://doi.org/10.3390/ceramics7040101
APA StyleSimonenko, E. P., Papynov, E. K., Shichalin, O. O., Belov, A. A., Nagornov, I. A., Simonenko, T. L., Gorobtsov, P. Y., Teplonogova, M. A., Mokrushin, A. S., Simonenko, N. P., & Kuznetsov, N. T. (2024). Reactive Spark Plasma Sintering and Oxidation of ZrB2-SiC and ZrB2-HfB2-SiC Ceramic Materials. Ceramics, 7(4), 1566-1583. https://doi.org/10.3390/ceramics7040101









