Insights for Precursors Influence on the Solar-Assisted Photocatalysis of Greenly Synthesizing Zinc Oxide NPs towards Fast and Durable Wastewater Detoxification
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Biogenesis-Mediated Synthesis of Zinc Oxide Nanoparticles (ZnO NPs) from Different Precursors
2.2.2. Spectrofluorimetric Monitoring of Solar-Driven Productivity of •OH Radicals Due to Greenly Synthesized ZnO NPs
2.2.3. Photocatalytic Experiments
2.3. Instruments
3. Results and Discussion
3.1. XRD Characteristics

3.2. Raman Spectroscopic Analysis
3.3. FTIR Analysis
3.4. BET Surface Analysis
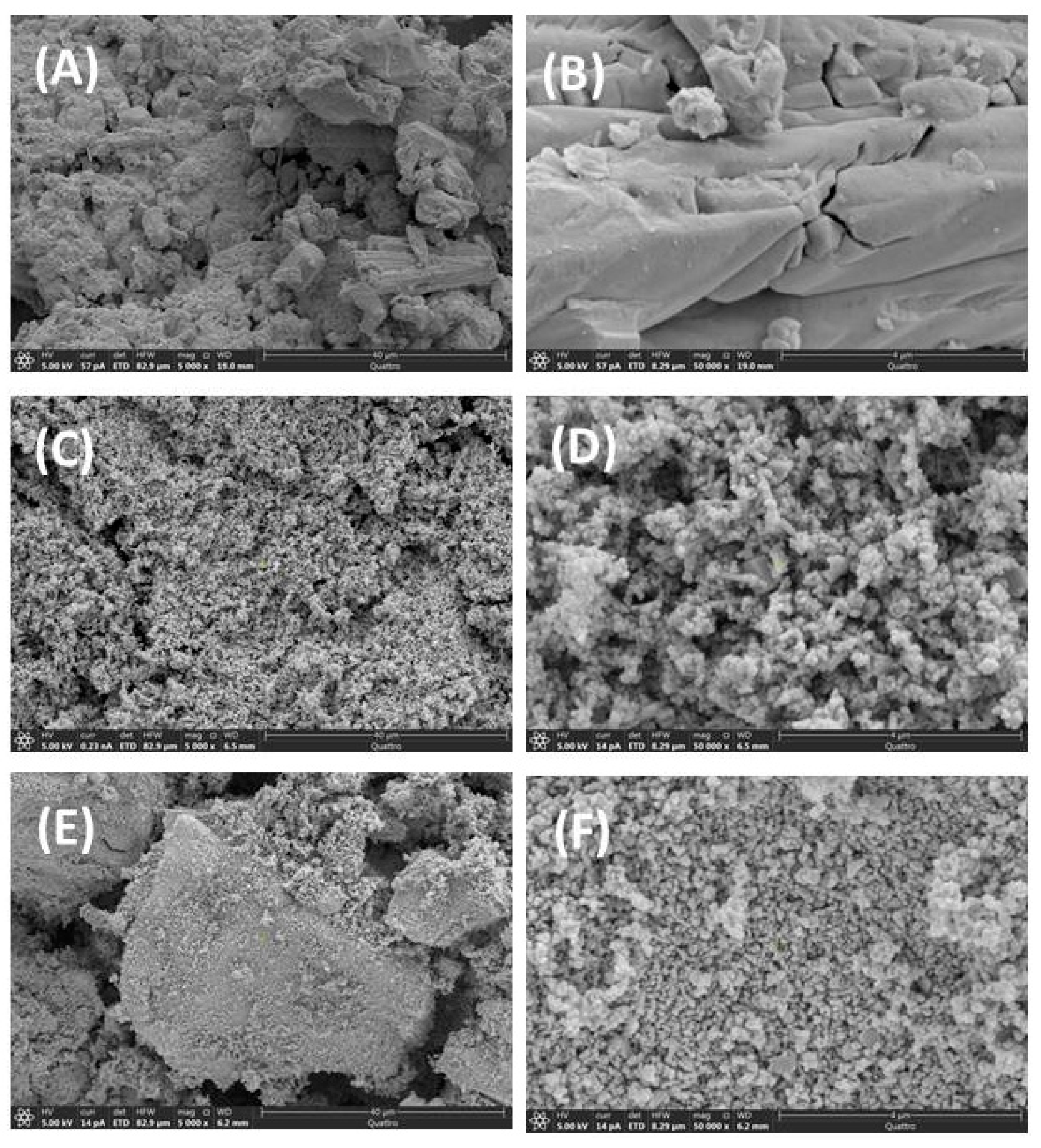
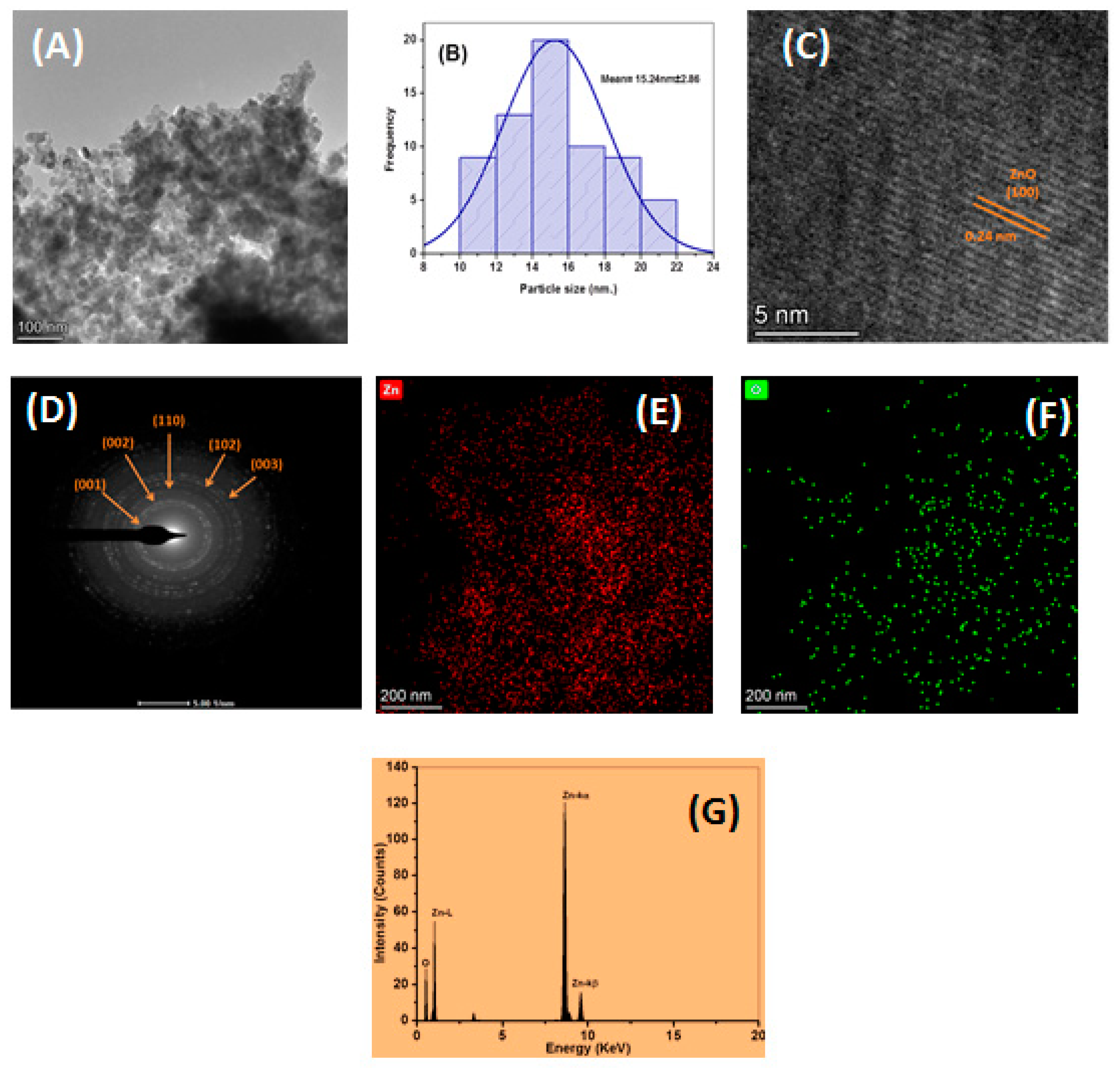
3.5. SEM and TEM Morphological Features
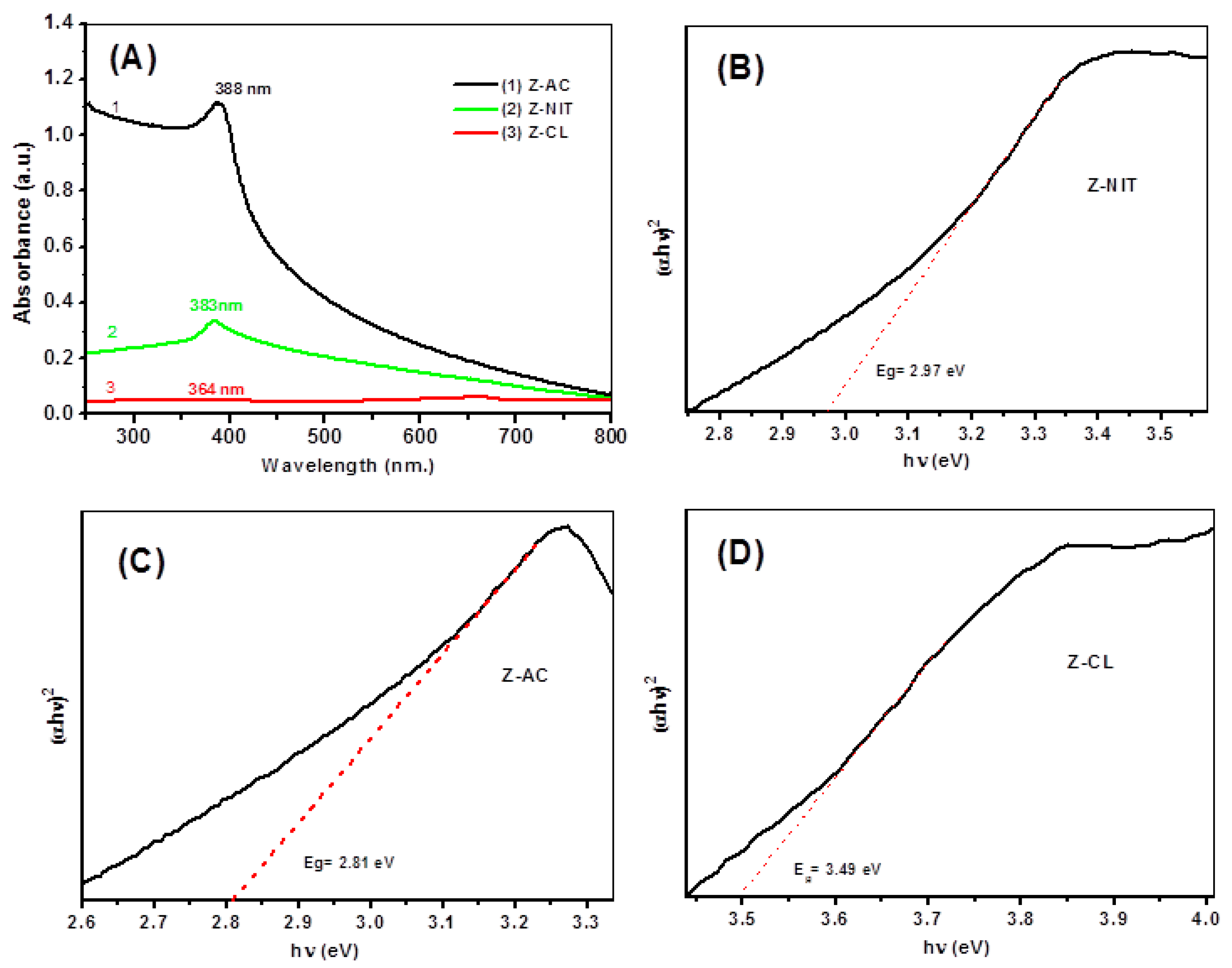
3.6. UV-Vis Absorption Spectroscopy and Band Gap Estimation
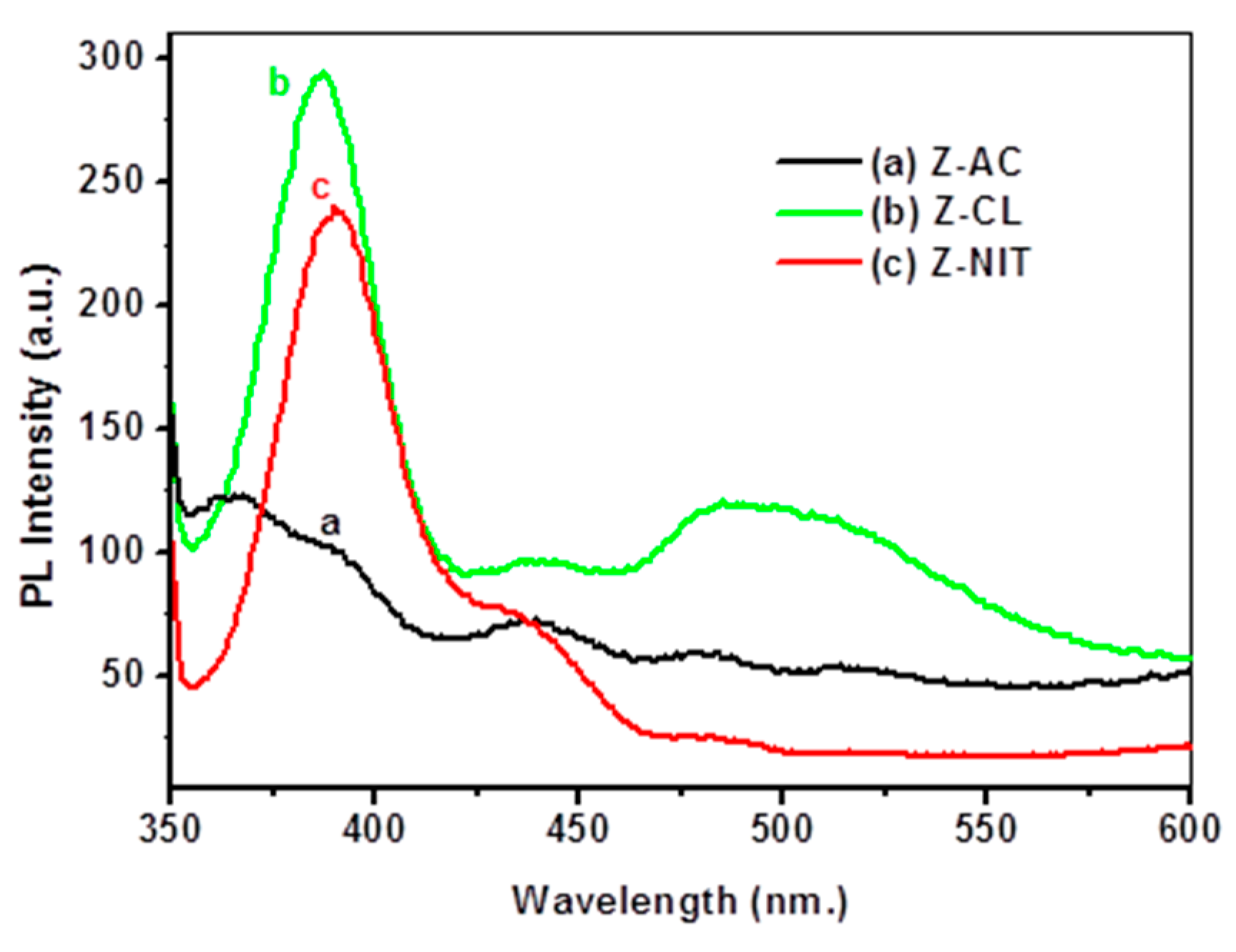
3.7. Photoluminescence Characteristics
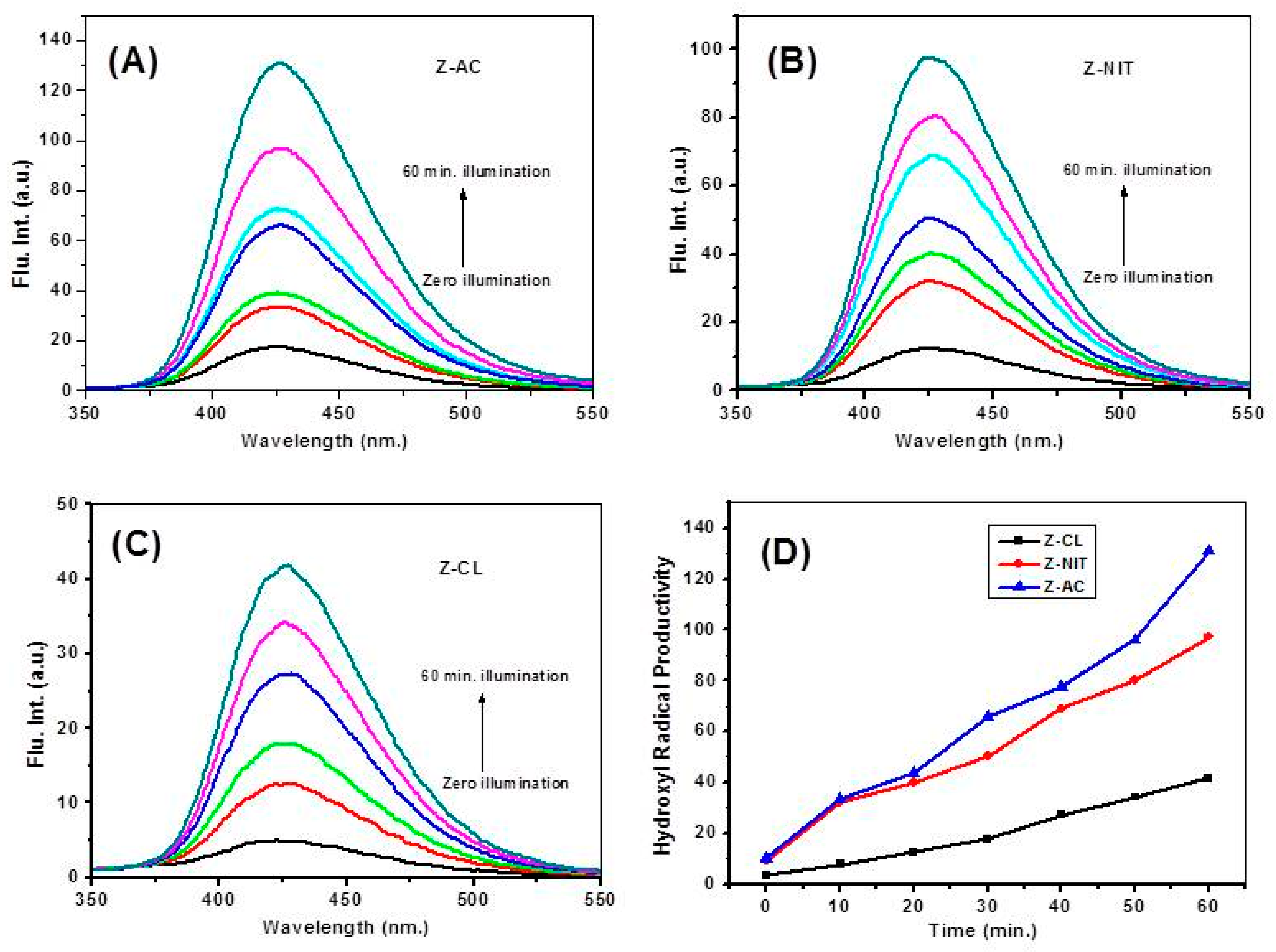
3.8. Comparison of Hydroxyl Radicals (•OH) Productivity Using Photoluminescence
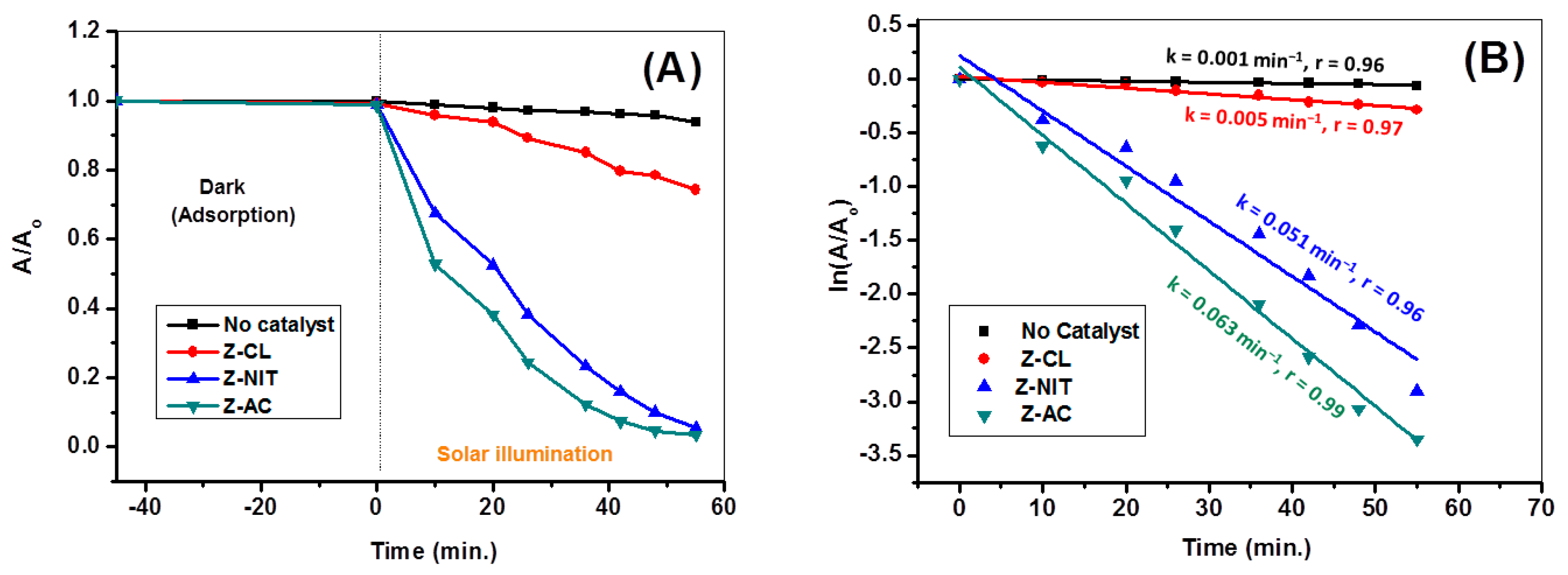
3.9. Evaluation of the Photocatalytic Activity
3.9.1. Comparison of Photocatalytic Activities of ZnO Particles
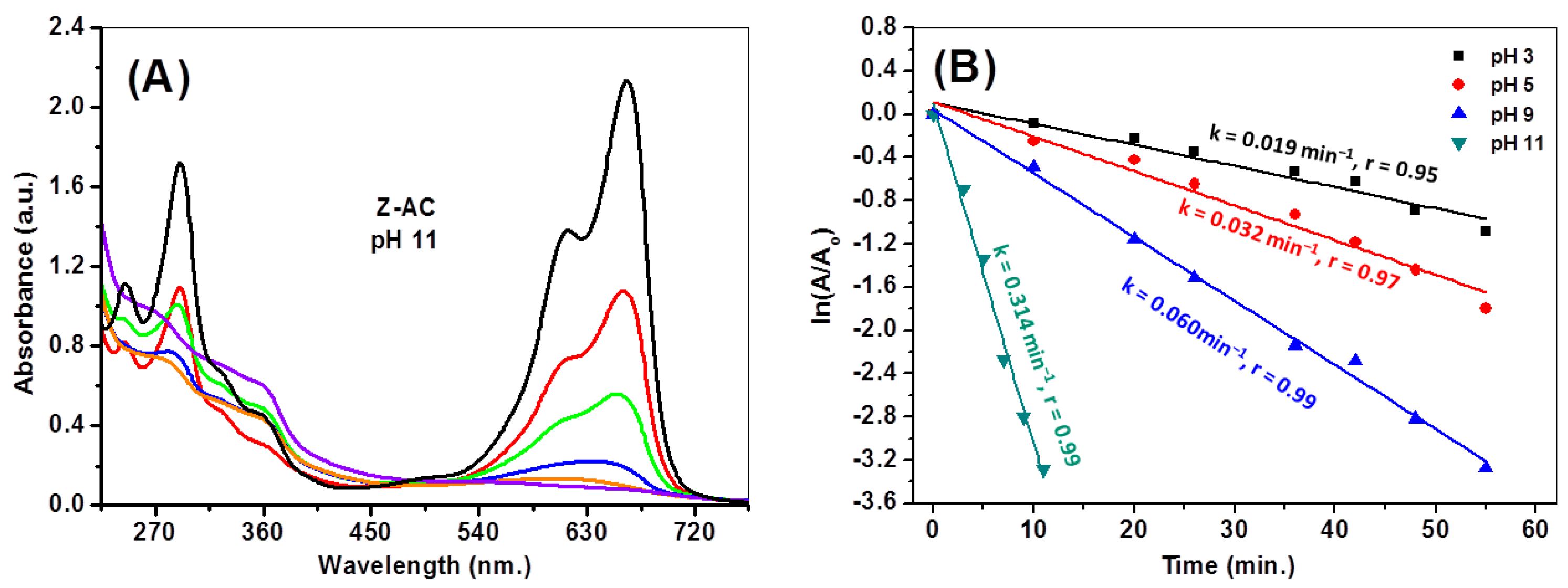
3.9.2. pH Influence on Photocatalytic Degradation Performance
3.10. Study for Trapping of Charge Carriers and Oxidizing Species
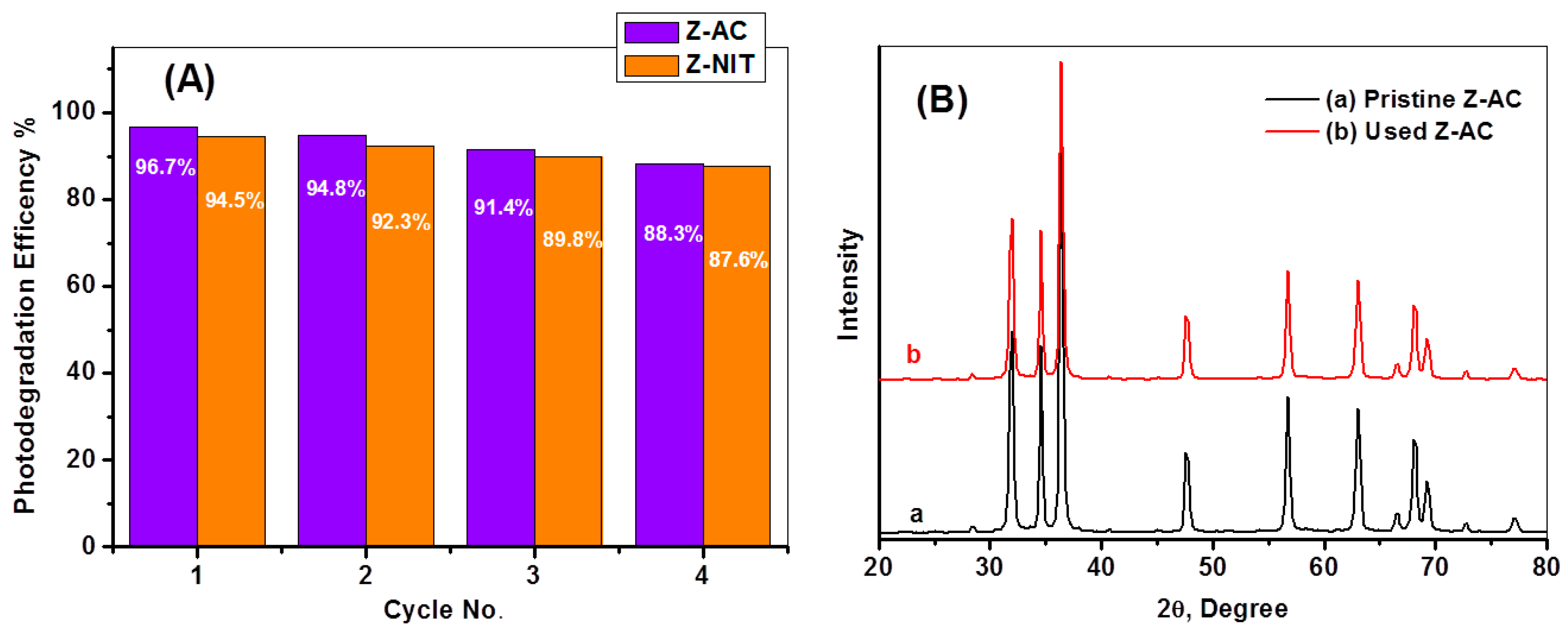
3.11. Stability and Life Span of Z-NIT and Z-AC Photocatalysts
3.12. Application in Photodegrading Dye Enriched Lake Water
3.13. Plausible Mechanism for the Photocatalytic Degradation Process
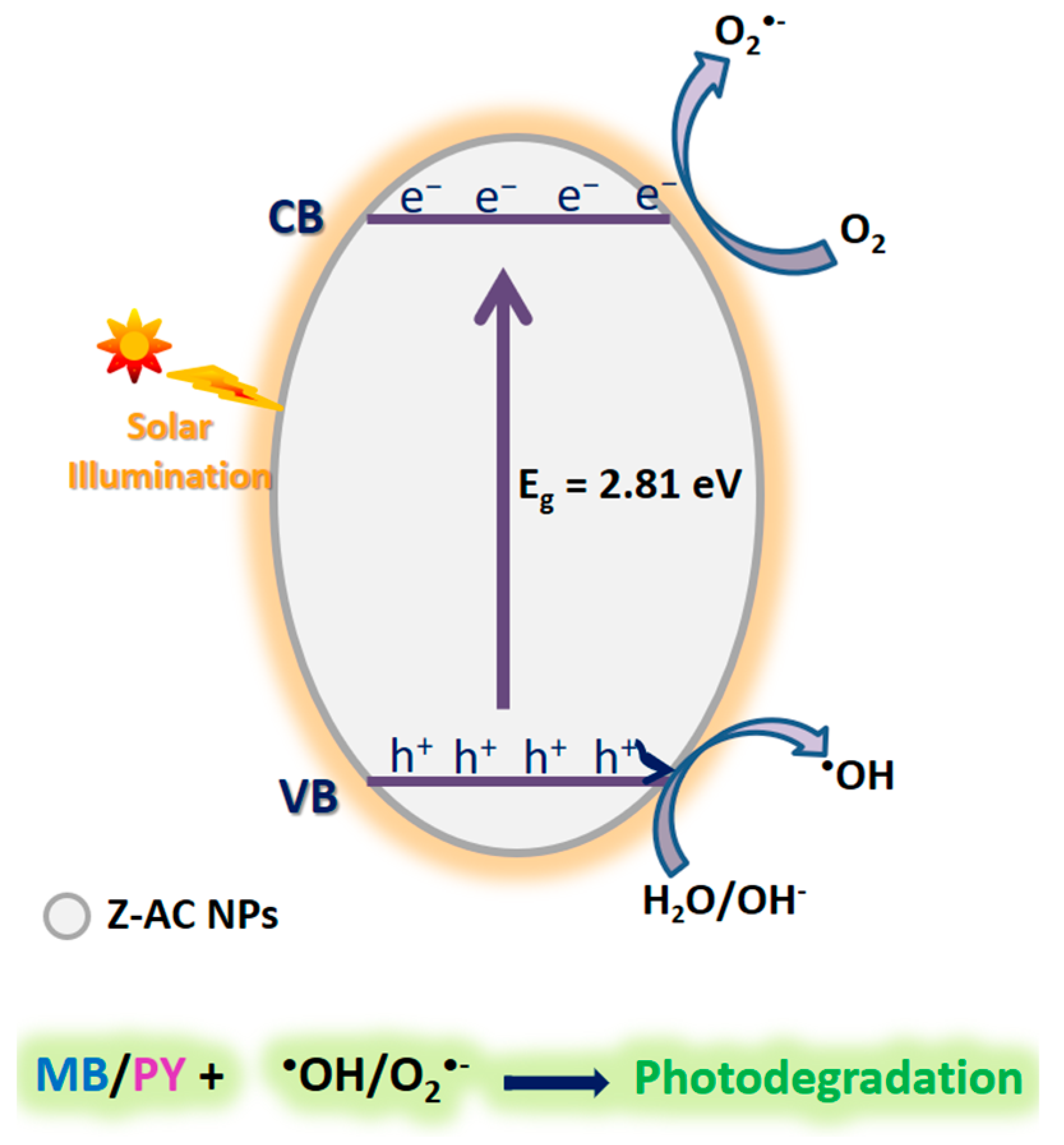
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, N.; Gusain, R.; Pandey, S.; Ray, S.S. Hydrogel Nanocomposite Adsorbents and Photocatalysts for Sustainable Water Purification. Adv. Mater. Interfaces 2023, 10, 2201375. [Google Scholar] [CrossRef]
- Pandey, S.; Son, N.; Kang, M. Synergistic Sorption Performance of Karaya Gum Crosslink Poly(Acrylamide-Co-Acrylonitrile)@Metal Nanoparticle for Organic Pollutants. Int. J. Biol. Macromol. 2022, 210, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Chankhanittha, T.; Nanan, S. Visible-light-driven photocatalytic degradation of ofloxacin (OFL) antibiotic and Rhodamine B (RhB) dye by solvothermally grown ZnO/Bi2MoO6 heterojunction. J. Colloid Interface Sci. 2020, 582, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Senasu, T.; Hemavibool, K.; Nanan, S. Hydrothermally grown CdS nanoparticles for photodegradation of anionic azo dyes under UV-visible light irradiation. RSC Adv. 2018, 8, 22592–22605. [Google Scholar] [CrossRef] [PubMed]
- Elfeky, A.S.; Salem, S.S.; Elzaref, A.S.; Owda, M.E.; Eladawy, H.A.; Saeed, A.M.; Awad, M.A.; Abou-Zeid, R.E.; Fouda, A. Multifunctional Cellulose Nanocrystal/Metal Oxide Hybrid, Photo-Degradation, Antibacterial and Larvicidal Activities. Carbohydr. Polym. 2020, 230, 115711. [Google Scholar] [CrossRef] [PubMed]
- Essawy, A.A. Silver imprinted zinc oxide nanoparticles: Green synthetic approach, characterization and efficient sunlight-induced photocatalytic water detoxification. J. Clean. Prod. 2018, 183, 1011–1020. [Google Scholar] [CrossRef]
- Pandit, V.U.; Arbuj, S.S.; Pandit, Y.B.; Naik, S.D.; Rane, S.B.; Mulik, U.P.; Gosavi, S.W.; Kale, B.B. Solar Light Driven Dye Degradation Using Novel Organo-Inorganic (6,13-Pentacenequinone/TiO2) Nanocomposite. RSC Adv. 2015, 5, 10326–10331. [Google Scholar] [CrossRef]
- Essawy, A.A.; Alsohaimi, I.H.; Alhumaimess, M.S.; Hassan, H.M.A.; Kamel, M.M. Green synthesis of spongy Nano-ZnO productive of hydroxyl radicals for unconventional solar-driven photocatalytic remediation of antibiotic enriched wastewater. J. Environ. Manag. 2020, 271, 110961. [Google Scholar] [CrossRef] [PubMed]
- Mdlovu, N.V.; Yang, N.-C.; Lin, K.-S.; Chang, C.-J.; Dinh, K.T.; Lin, Y.-G. Formulation and characterization of W-doped titania nanotubes for adsorption/photodegradation of methylene blue and basic violet 3 dyes. Catal. Today 2022, 388–389, 36–46. [Google Scholar] [CrossRef]
- Xu, J.; Cui, Z.; Liu, Z.; Xu, F.; Chen, Y. Organic-inorganic nanohybrid electrochemical sensors from multi-walled carbon nanotubes decorated with zinc oxide nanoparticles and insitu wrapped with poly(2-methacryloyloxyethyl-ferrocenecarboxylate) for detection of food additives. Nanomaterials 2019, 9, 1388. [Google Scholar] [CrossRef]
- Lam, S.M.; Sin, J.C.; Abdullah, A.Z.; Mohamed, A.R. Investigation on visible-light photocatalytic degradation of 2,4- dichlorophenoxyacetic acid in the presence of MoO3/ZnO nanorod composites. J. Mol. Catal. A Chem. 2013, 370, 123–131. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Gao, Y.; Zhang, Q. Microstructral evolution of well-aligned ZnO nanorods array films in aqueous solution. J. Wuhan Univ. Technol. 2007, 22, 603–606. [Google Scholar] [CrossRef]
- Gupta, M.; Sharma, V.; Shrivastava, J.; Solanki, A. Preparation and characterization of nanostructured ZnO thin films for photoelectrochemical splitting of water. Bull. Mater. Sci. 2009, 32, 23–30. [Google Scholar] [CrossRef]
- Singh, O.; Kohli, N.; Singh, R.C. Precursor controlled morphology of zinc oxide and its sensing behaviour. Sens. Actuators B Chem. 2013, 178, 149–154. [Google Scholar] [CrossRef]
- Essawy, A.A.; Abdel-Farid, I.B. Hybrid solvothermal/sonochemical-mediated synthesis of ZnO NPs generative of •OH radicals: Photoluminescent approach to evaluate •OH scavenging activity of Egyptian and Yemeni Punica granatum arils extract. Ultrason. Sonochem. 2022, 89, 106152. [Google Scholar] [CrossRef] [PubMed]
- Koutu, V.; Shastri, L.; Malik, M.M. Effect of NaOH concentration on optical properties of zinc oxide nanoparticles. Mater. Sci. Pol. 2016, 34, 819–827. [Google Scholar] [CrossRef]
- Varadavenkatesan, T.; Lyubchik, E.; Pai, S.; Pugazhendhi, A.; Vinayagam, R.; Selvaraj, R. Photocatalytic degradation of Rhodamine B by zinc oxide nanoparticles synthesized using the leaf extract of Cyanometra ramiflora. J. Photochem. Photobiol. B Biol. 2019, 199, 111621. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Watcharakitti, J.; Nanan, S. PVP-assisted synthesis of rod-like ZnO photocatalyst for photodegradation of reactive red (RR141) and Congo red (CR) azo dyes. J. Mater. Sci. Mater. Electron. 2019, 30, 17804–17819. [Google Scholar] [CrossRef]
- Kakarndee, S.; Nanan, S. SDS capped and PVA capped ZnO nanostructures with high photocatalytic performance toward photodegradation of reactive red (RR141) azo dye. J. Environ. Chem. Eng. 2018, 6, 74–94. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu; Chaudhry, S.A.; Ikram, S. A review on biogenic synthesis of ZnO nanoparticles using plant extracts and microbes: A prospect towards green chemistry. J. Photochem. Photobiol. B Biol. 2017, 166, 272–284. [Google Scholar] [CrossRef]
- Mydeen, S.S.; Kumar, R.R.; Kottaisamy, M.; Vasantha, V.S. Biosynthesis of ZnO nanoparticles through extract from Prosopis juliflora plant leaf: Antibacterial activities and a new approach by rust-induced photocatalysis. J. Saudi Chem. Soc. 2020, 24, 393–406. [Google Scholar] [CrossRef]
- Begum, J.P.S.; Manjunath, K.; Pratibha, S.; Dhananjaya, N.; Sahu, P.; Kashaw, S. Bioreduction synthesis of zinc oxide nanoparticles using Delonix regia leaf extract (Gul Mohar) and its agromedicinal applications. J. Sci. Adv. Mater. Devices 2020, 5, 468–475. [Google Scholar] [CrossRef]
- Kaliraj, L.; Ahn, J.C.; Rupa, E.J.; Abid, S.; Lu, J.; Yang, D.C. Synthesis of panos extract mediated ZnO nano-flowers as photocatalyst for industrial dye degradation by UV illumination. J. Photochem. Photobiol. B Biol. 2019, 199, 111588. [Google Scholar] [CrossRef]
- Essawy, A.A.; Sayyah, S.M.; El-Nggar, A.M. Ultrasonic-mediated synthesis and characterization of TiO2-loaded chitosan-grafted-polymethylaniline nanoparticles of potent efficiency in dye uptake and sunlight driven self-cleaning applications. RSC Adv. 2016, 6, 2279–2294. [Google Scholar] [CrossRef]
- Payra, S.; Reddy, K.L.; Sharma, R.S.; Singh, S.; Roy, S. A trade-off between adsorption and photocatalysis over ZIF-derived Composite. J. Hazard. Mater. 2020, 393, 122491. [Google Scholar] [CrossRef]
- JCPDS. Powder Diffraction File, Alphabetical Index, Inorganic Compounds; International Centre for Diffraction Data: Newtown Square, PA, USA, 1977. [Google Scholar]
- Al-Ariki, S.; Yahya, N.A.A.; Al-A’nsi, S.A.; Jumali, M.H.H.; Jannah, A.N.; Abd Shukor, R. Synthesis and comparative study on the structural and optical properties of ZnO doped with Ni and Ag nanopowders fabricated by sol gel technique. Sci. Rep. 2021, 11, 11948. [Google Scholar] [CrossRef]
- LBVS, G.; Dhiman, T.K.; Kumar, R.; Singh, A.K.; Solanki, P.R. One-Step Synthesized ZnO np-Based Optical Sensors for Detection of Aldicarb via a Photoinduced Electron Transfer Route. ACS Omega 2020, 5, 2552–2560. [Google Scholar]
- Aljabali, A.A.A.; Obeid, M.A.; Bakshi, H.A.; Alshaer, W.; Ennab, R.M.; Al-Trad, B.; Al Khateeb, W.; Al-Batayneh, K.M.; Al-Kadash, A.; Alsotari, S.; et al. Synthesis, Characterization, and Assessment of Anti-Cancer Potential of ZnO Nanoparticles in an In Vitro Model of Breast Cancer. Molecules 2022, 27, 1827. [Google Scholar] [CrossRef]
- Rafaja, D.; Wüstefeld, C.; Dopita, M.; Motylenko, M.; Baehtz, C. Capability of X ray diffraction for the study of microstructure of metastable thin films. IUCrJ 2014, 1 Pt 6, 446–456. [Google Scholar] [CrossRef]
- Uribe-López, M.C.; Hidalgo-López, M.C.; López-González, R.; Frías-Márquez, D.M.; Núñez-Nogueira, G.; Hernández-Castillo, D.; Alvarez-Lemus, M.A. Photocatalytic activity of ZnO nanoparticles and the role of the synthesis method on their physical and chemical properties. J. Photochem. Photobiol. A Chem. 2021, 404, 112866. [Google Scholar] [CrossRef]
- Payra, S.; Challagulla, S.; Bobde, Y.; Chakraborty, C.; Ghosh, B.; Roy, S. Probing the photo- and electro-catalytic degradation mechanism of methylene blue dye over ZIF-derived ZnO. J. Hazard. Mater. 2019, 373, 377–388. [Google Scholar] [CrossRef]
- Cusco, R.; Alarcón-Lladó, E.; Ib’añez, J.; Artús, L.; Jiménez, J.; Wang, B.; Callahan, M.J. Temperature Dependence of Raman Scattering in ZnO. Phys. Rev. B 2007, 75, 165202. [Google Scholar] [CrossRef]
- Kahouli, M.; Tounsi, N.; Mzabi, N.; Guermazi, H.; Guermazi, S. Enhanced structural and optical properties of ZnO nano-powder with tailored visible luminescence as a function of sodium hydroxide to zinc sulfate mass ratio. Adv. Powder Technol. 2018, 29, 325–332. [Google Scholar] [CrossRef]
- Moungsrijun, S.; Wongrerkdee, S. Investigation of structural, optical, and electrical properties of ZnO thin films for electrooptical devices. Suranaree J. Sci. Technol. 2022, 29, 030085. [Google Scholar]
- Gatou, M.-A.; Lagopati, N.; Vagena, I.-A.; Gazouli, M.; Pavlatou, E.A. ZnO Nanoparticles from Different Precursors and Their Photocatalytic Potential for Biomedical Use. Nanomaterials 2023, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Wongrerkdee, S.; Wongrerkdee, S.; Boonruang, C.; Sujinnapram, S. Enhanced Photocatalytic Degradation of Methylene Blue Using Ti-Doped ZnO Nanoparticles Synthesized by Rapid Combustion. Toxics 2023, 11, 33. [Google Scholar] [CrossRef]
- Krobthong, S.; Rungsawang, T.; Wongrerkdee, S. Comparison of ZnO Nanoparticles Prepared by Precipitation and Combustion for UV and Sunlight-Driven Photocatalytic Degradation of Methylene Blue. Toxics 2023, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Q.; Tian, S.; Zhao, X. Exposed facet dependent stability of ZnO micro/nano crystals as a photocatalyst. Appl. Surf. Sci. 2019, 470, 807–816. [Google Scholar] [CrossRef]
- De Corrado, J.M.; Fernando, J.F.S.; Shortell, M.P.; Poad, B.L.J.; Blanksby, S.J.; Waclawik, E.R. ZnO colloid crystal facet-type determines both Au photodeposition and photocatalytic activity. ACS Appl. Nano Mater. 2019, 2, 7856–7869. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Yenjai, C.; Nanan, S. Utilization of formononetin and pinocembrin from stem extract of Dalbergia parviflora as capping agents for preparation of ZnO photocatalysts for degradation of RR141 azo dye and ofloxacin antibiotic. Catal. Today 2022, 384–386, 279–293. [Google Scholar] [CrossRef]
- Zeng, B.H.; Duan, G.; Li, Y.; Yang, S.; Xu, X. Blue Luminescence of ZnO nanoparticles based on non-equilibrium processes: Defect origins and emission controls. J. Adv. Funct. Mater. 2010, 20, 561–572. [Google Scholar] [CrossRef]
- Alwan, R.M.; Kadhim, Q.A.; Sahan, K.M.; Ali, R.A.; Roaa, J.; Mahdi, N.A.K.; Jassim, A.N. Synthesis of Zinc oxide nanoparticles via Sol—Gel route and their characterization. Nanosci. Nanotechnol. 2015, 5, 1–6. [Google Scholar]
- Peter, I.J.; Praveen, E.; Vignesh, G.; Nithiananthi, P. ZnO nanostructures with different morphology for enhanced photocatalytic activity. Mater. Res. Express 2017, 4, 124003. [Google Scholar] [CrossRef]
- Bhatia, S.; Verma, N. Photocatalytic Activity of ZnO Nanoparticles with Optimization of Defects. Mater. Res. Bull. 2017, 95, 468–476. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Nanan, S. Hydrothermal synthesis, characterization and enhanced photocatalytic performance of ZnO toward degradation of organic azo dye. Mater. Lett. 2018, 226, 79–82. [Google Scholar] [CrossRef]
- Ozgur, U.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Dogan, S.; Avrutin, V.; Cho, S.-J.; Morkoc, H. A Comprehensive Review of ZnO Materials and Devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Studenikin, S.A.; Golego, N.; Cocivera, M. Fabrication of Green and Orange Photoluminescent, Undoped ZnO Films Using Spray Pyrolysis. J. Appl. Phys. 1998, 84, 2287–2294. [Google Scholar] [CrossRef]
- Wagh, S.S.; Kadam, V.S.; Jagtap, C.V.; Salunkhe, D.B.; Patil, R.S.; Pathan, H.M.; Patole, S.P. Comparative Studies on Synthesis, Characterization and Photocatalytic Activity of Ag Doped ZnO Nanoparticles. ACS Omega 2023, 8, 7779–7790. [Google Scholar] [CrossRef]
- Payra, S.; Ganeshan, S.K.; Challagulla, S.; Roy, S. A correlation story of syntheses of ZnO and their influence on photocatalysis. Adv. Powder Technol. 2020, 31, 510–520. [Google Scholar] [CrossRef]
- Chen, L.-L.; Zhai, B.-g.; Huang, Y.M. Rendering Visible-Light Photocatalytic Activity to Undoped ZnO via Intrinsic Defects Engineering. Catalysts 2020, 10, 1163. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Essawy, A.A.; Diab, A.K.; Mohamed, W.S. Facile synthesis and characterization of novel Gd2O3–CdO binary mixed oxide nanocomposites of highly photocatalytic activity for wastewater remediation under solar illumination. J. Phys. Chem. Solids 2021, 148, 109666. [Google Scholar] [CrossRef]
- Degen, A.; Kosec, M. Effect of pH and impurities on the surface charge of zinc oxide in aqueous solution. J. Eur. Ceram. Soc. 2000, 20, 667–673. [Google Scholar] [CrossRef]
- Basit, R.A.; Abbasi, Z.; Hafeez, M.; Ahmad, P.; Khan, J.; Khandaker, M.U.; Al-Mugren, K.S.; Khalid, A. Successive Photocatalytic Degradation of Methylene Blue by ZnO, CuO and ZnO/CuO Synthesized from Coriandrum sativum Plant Extract via Green Synthesis Technique. Crystals 2023, 13, 281. [Google Scholar] [CrossRef]
- Dhananjay, P.; Abhilash, M.R.; Shilpa, N.; NK, H.K.; Gowtham, H.G.; Aiyaz, M.; Singh, B.; Malik, A.; Akhtar, S.; Murali, M. Solar irradiation driven catalytic dye degradation by novel biosynthesized zinc oxide nanoparticles (ZnO–NPs) from Barleria mysorensis: Kinetics, reusability and mineralization studies. J. Mol. Struct. 2024, 1303, 137549. [Google Scholar]
- Rajalakshmi, P.; Vijayan, S.; Amirtharaj, M. A study on microwave synthesis of unique Zn1-3xCuxCexCrxO nanoparticles with efficient photocatalytic reactivity under visible sunlight irradiation. J. Mol. Struct. 2024, 1307, 137992. [Google Scholar] [CrossRef]
- Al-Zahrani, S.A.; Khedr, A.M.; Alturki, A.M.; El-Yazeed, W.S.A. Integration of 2D graphene oxide/zinc oxide nanohybrid for enhancement adsorption and photodegradation of organic pollutants. J. Mol. Liq. 2024, 395, 123956. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chang, T.F.M.; Chen, C.Y.; Sone, M.; Hsu, Y.J. Mechanistic insights into photodegradation of organic dyes using heterostructure photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef]
- Li, Z.; Jin, D.; Wang, Z. ZnO/CdSe-diethylenetriamine nanocomposite as a stepscheme photocatalyst for photocatalytic hydrogen evolution. Appl. Surf. Sci. 2020, 529, 147071. [Google Scholar] [CrossRef]
- Motelica, L.; Oprea, O.-C.; Vasile, B.-S.; Ficai, A.; Ficai, D.; Andronescu, E.; Holban, A.M. Antibacterial Activity of Solvothermal Obtained ZnO Nanoparticles with Different Morphology and Photocatalytic Activity against a Dye Mixture: Methylene Blue, Rhodamine B and Methyl Orange. Int. J. Mol. Sci. 2023, 24, 5677. [Google Scholar] [CrossRef] [PubMed]
- Popa, M.L.; Preda, M.D.; Neacşu, I.A.; Grumezescu, A.M.; Ginghina, O. Traditional vs. Microfluidic Synthesis of ZnO Nanoparticles. Int. J. Mol. Sci. 2023, 24, 1875. [Google Scholar] [CrossRef]
- Limón-Rocha, I.; Guzmán-González, C.A.; Anaya-Esparza, L.M.; Romero-Toledo, R.; Rico, J.L.; González-Vargas, O.A.; Pérez-Larios, A. Effect of the Precursor on the Synthesis of ZnO and Its Photocatalytic Activity. Inorganics 2022, 10, 16. [Google Scholar] [CrossRef]
- Flores, N.M.; Pal, U.; Galeazzi, R.; Sandoval, A. Effects of morphology, surface area, and defect content on the photocatalytic dye degradation performance of ZnO nanostructures. RSC Adv. 2014, 4, 41099. [Google Scholar] [CrossRef]
- Pawar, R.C.; Kim, H.; Lee, C.S. Defect-controlled growth of ZnO nanostructures using its different zinc precursors and their application for effective photodegradation. Curr. Appl. Phys. 2014, 14, 621–629. [Google Scholar] [CrossRef]
- El Golli, A.; Fendrich, M.; Bazzanella, N.; Dridi, C.; Miotello, A.; Orlandi, M. Wastewater remediation with ZnO photocatalysts: Green synthesis and solar concentration as an economically and environmentally viable route to application. J. Environ. Manag. 2021, 286, 112226. [Google Scholar] [CrossRef] [PubMed]
- Aliannezhadi, M.; Mirsanaee, S.Z.; Jamali, M.; Tehrani, F.S. The physical properties and photocatalytic activities of green synthesized ZnO nanostructures using different ginger extract concentrations. Sci. Rep. 2024, 14, 2035. [Google Scholar] [CrossRef]
- El Golli, A.; Contreras, S.; Dridi, C. Bio-synthesized ZnO nanoparticles and sunlight-driven photocatalysis for environmentally-friendly and sustainable route of synthetic petroleum refinery wastewater treatment. Sci. Rep. 2023, 13, 20809. [Google Scholar] [CrossRef]
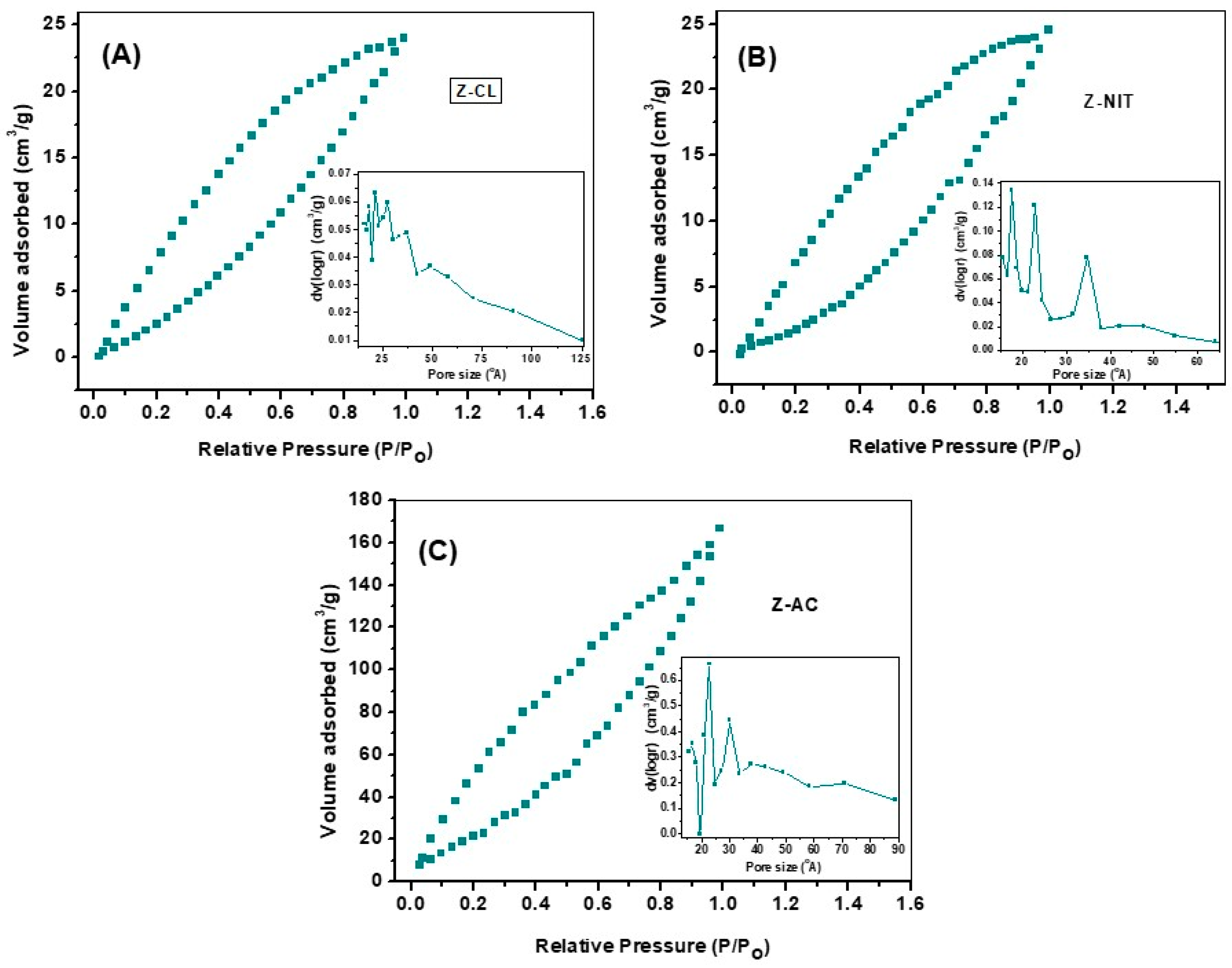



| Sample | Crystallite Particle Size, (nm.) | Surface Area (m2/g) | Pore Size (Å) | Pore Volume (m3/g) |
|---|---|---|---|---|
| Z-CL | 17.55 | 4.965 | 1.541 × 101 | 1.076 × 10−3 |
| Z-NIT | 15.97 | 31.81 | 1.838 × 101 | 1.136 × 10−2 |
| Z-AC | 15.54 | 43.04 | 2.276 × 101 | 1.67 × 10−2 |
| Photocatalyst | kapp, (min−1) | t1/2 (min.) (min) | R2 | Degradation (%) |
|---|---|---|---|---|
| Z-CL | 0.005 | 138.6 | 0.97 | 25.12 |
| Z-AC | 0.063 | 11.00 | 0.99 | 96.46 |
| Z-NIT | 0.051 | 13.58 | 0.96 | 94.47 |
| Catalyst | [MB] | Irradiation Source | Rate Constant (min−1) | Degradation Efficiency % | Reference |
|---|---|---|---|---|---|
| ZnO (combustion method) | 5 mg/L | Sunlight | 0.00856 | -- | [38] |
| ZnO (Precipitation method) | 0.03531 | -- | |||
| Reflux chemical method | 10 mg/L | Natural sunlight | [49] | ||
| ZnO (chloride precursor) | 0.02 | 51.0 | |||
| Ag (7 wt.%)/ZnO | 0.08 | 88.0 | |||
| ZnO (nitrate precursor) | 0.03 | 54.0 | |||
| Ag (10 wt.%)/ZnO | 0.13 | 84.0 | |||
| ZnO (Acetate precursor) | 0.03 | 64.0 | |||
| Ag (7 wt.%)/ZnO | 0.13 | 96.0 | |||
| ZnO | 4.8 mg/L | Natural sunlight | 0.0036 | -- | [54] |
| ZnO/CuO | 0.0148 | -- | |||
| Biosynthesizing ZnO | 100 mg/L | Natural sunlight | 0.01834 | 91.3 | [55] |
| ZnO, | 50 mg/L | Natural sunlight | -- | 56.65% | [56] |
| Zn0.91Cu0.03Ce0.03Cr0.03O | -- | 63.22% | |||
| Zn0.85Cu0.05Ce0.05Cr0.05O | -- | 67.28 | |||
| ZnO | 5 mg/L | Philips 100 W Mercury Vapor Lamp (420 nm) | -- | 76.2% | [57] |
| ZnO@GO | 0.1343 | 98.4% | |||
| ZnO | 50 mg/L | Natural sunlight | 0.0075 | 53% | [58] |
| Zn0.95Co0.05O | -- | 87% | |||
| Zn0.99Co0.01O | 0.037 | 97% | |||
| Greenly synthesized ZnO “Z-AC” | 20 mg/L | Natural sunlight | 0.314 at pH 11 | 96.4 | This work |
| Preparation Route | Application | Characterization Findings | Efficiency | Reference |
|---|---|---|---|---|
| Precipitation method using either NaOH or KOH and the sulfate, chloride, nitrates and acetates of Zn2+ followed by separation, drying and finally annealing | Photocatalytic degradation of Rhodamine B (10 ppm) under UV or visible light | Eg: 3.37–3.4 eV; BET: 6–11 m2 g−1; wurtzite structure with flak, spherical and hexagonal morphology; mean size distribution (25–41.3 nm) on DLS basis. | Rate constant: 0.006–0.017 min−1; t1/2 = 115.5–40.7 min. | [36] |
| Two steps sol-gel method using zinc acetate and zinc nitrate in presence of polyethylene glycol and NH4OH | Photocatalytic degradation of 2,4-Dichlorophenoxyacetic acid and 2,4-Dichlorophenol under UV light irradiation | Wurtzite hexagonal phase of near-spherical (99.28 nm) and elongated rod-like morphology (40.94 nm); BET: 0.85–2.24 m2 g−1 | Rate constant: 0.0004–0.002 min−1; t1/2 = 18.2–181.6 min. | [62] |
| Ultrasound-assisted hydrolysis of zinc acetate in presence of EDTA and NH4OH at different pHs. | Photocatalytic degradation of methylene blue (10 ppm) under UV light | Eg: 3.24–3.3 eV; BET: 2.20–5.16 m2 g−1; wurtzite structure with hexagonal disk morphology | Rate constant: 0.0159 min−1; t1/2 = 43.58 min.; 91.4% | [63] |
| Refluxing zinc chloride, nitrate, acetate, or sulfate with hexamethylenetetramine at 95 °C for 5 h | Photocatalytic degradation of methylene blue (3.2 ppm) under UV light | Eg: No data; BET: 4.83 m2 g−1; hexagonal wurtzite structure with flakes, nanowalls, nanopetals and nanodisks morphology | Rate constant: 0.027 min−1; t1/2 = 25.6 min.; 94% | [64] |
| Multistep synthetic route starting with heating zinc nitrate with garlic extract followed by drying, washing, calcination at 550 °C | Photocatalytic degradation of methylene blue (10 ppm) under solar light | Eg: 2.9–3.0 eV; BET: No data; wurtzite structure with alterations in diffraction positions with rod-like hexagonal facets diameter ~30–40 nm and length of ~100–200 nm | Rate constant: 0.009–0.0183 min−1; t1/2 = 77–37.8 min.; 94% | [65] |
| Multistep sol-gel green synthetic route starting with precipitation of mixture from zinc acetate ethanolic solution and ginger aqueous extract using NaOH followed by steps of long sonication, overnight heating, washing and finally drying. | Photocatalytic degradation of methylene blue (10 ppm) under UV light | Eg: 3.09–3.2eV; BET: 6.1–27.7 m2 g−1; Either pure hexagonal or orthorhombic phases; Nanoflakes and flower-like structure with average particle size of ~66–120 nm | Rate constant: No data; t1/2 = No data; Efficiency: 44–83% after 150 min of UV illumination | [66] |
| Multistep green synthetic route starting with mixing zinc nitrate with hot solution of Moringa oleifera leaves extract, then boiling until paste formation followed by calcination at 500 °C for 2 h. | Photocatalytic degradation of phenol, Toluene, xylene (10 ppm), cresol (20 ppm) under illumination of Xenon lamp (1500 W) | Eg: 3.16 eV; BET: 19.7 m2 g−1; hexagonal wurtzite phase phases; nearly spherical shaped particles with average size of ~9–18 nm | Rate constant: No data; t1/2 = No data; Efficiency: 51–93% after 180 min of Xenon lamp illumination | [67] |
| One-pot biogenic combustion route (450 °C, 2 h) using zinc nitrate mixed with different concentrations from bio-capping agent. | Photocatalytic degradation of flumequine antibiotic (15 ppm) under solar light | Eg: 3.07 eV; BET: 31.81 m2 g−1; Defective hexagonal wurtzite structure; Morphology is mostly spherical/hemispherical, some hexagonal and square shapes with particle sizes (6.3 to 26.4 nm) | Rate constant: 0.0397 min−1; t1/2 = 17.45 min.; 97.6% | [8] |
| One-pot biogenic combustion route using chloride, acetate and nitrate zinc precursors | Photocatalytic degradation of methylene blue (MB, 20 ppm) at pH 11 and pyronine Y (PY, 18 ppm) under solar light (Photodegradation also examined on dyes spiked in natural lake water) | Eg: 2.81–3.49 eV; BET: 4.96–43.04 m2 g−1; monocrystalline wurtzite structure with alterations in diffraction positions with microrod-like hexagonal morphology, particle size 15.4 nm. | (MB) Rate constant: 0.314 min−1; t1/2 = 2.2 min.; 96.46% (PY) Rate constant: 0.116 min−1; t1/2 = 5.9 min.; 95.4% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Essawy, A.A.; Hussein, M.F.; Hasanin, T.H.A.; El Agammy, E.F.; Alsaykhan, H.S.; Alanazyi, R.F.; Essawy, A.E.-N.I. Insights for Precursors Influence on the Solar-Assisted Photocatalysis of Greenly Synthesizing Zinc Oxide NPs towards Fast and Durable Wastewater Detoxification. Ceramics 2024, 7, 1100-1121. https://doi.org/10.3390/ceramics7030072
Essawy AA, Hussein MF, Hasanin THA, El Agammy EF, Alsaykhan HS, Alanazyi RF, Essawy AE-NI. Insights for Precursors Influence on the Solar-Assisted Photocatalysis of Greenly Synthesizing Zinc Oxide NPs towards Fast and Durable Wastewater Detoxification. Ceramics. 2024; 7(3):1100-1121. https://doi.org/10.3390/ceramics7030072
Chicago/Turabian StyleEssawy, Amr A., Modather F. Hussein, Tamer H. A. Hasanin, Emam F. El Agammy, Hissah S. Alsaykhan, Rakan F. Alanazyi, and Abd El-Naby I. Essawy. 2024. "Insights for Precursors Influence on the Solar-Assisted Photocatalysis of Greenly Synthesizing Zinc Oxide NPs towards Fast and Durable Wastewater Detoxification" Ceramics 7, no. 3: 1100-1121. https://doi.org/10.3390/ceramics7030072
APA StyleEssawy, A. A., Hussein, M. F., Hasanin, T. H. A., El Agammy, E. F., Alsaykhan, H. S., Alanazyi, R. F., & Essawy, A. E.-N. I. (2024). Insights for Precursors Influence on the Solar-Assisted Photocatalysis of Greenly Synthesizing Zinc Oxide NPs towards Fast and Durable Wastewater Detoxification. Ceramics, 7(3), 1100-1121. https://doi.org/10.3390/ceramics7030072





