Characterization of ZnWO4, MgWO4, and CaWO4 Ceramics Synthesized in the Field of a Powerful Radiation Flux
Abstract
1. Introduction
2. Materials
2.1. Morphology of Samples
2.2. Synthesis Efficiency
3. Results and Discussions
3.1. The Structure of Ceramic Samples
3.2. Cathodoluminescence Spectra
3.3. Kinetics of Cathodoluminescence Attenuation
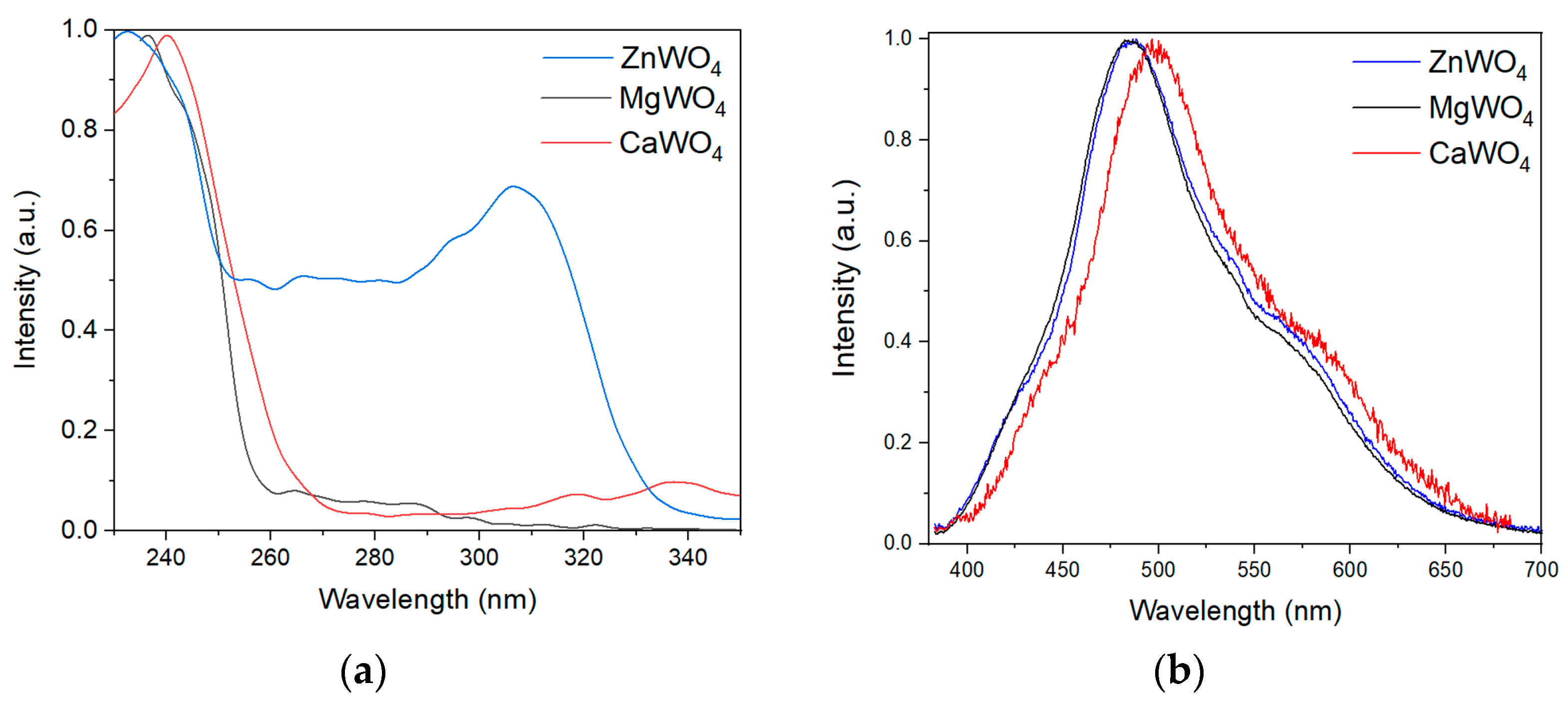
3.4. Photoluminescence Spectra
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mikhailik, V.B.; Kraus, H. Performance of Scintillation Materials at Cryogenic Temperatures. Phys. Status Solidi B 2010, 247, 1583–1599. [Google Scholar] [CrossRef]
- Dev Bhuyan, P.; Deobrat, S.; Shivam, K.; Sinha, E. Experimental and Theoretical Analysis of Electronic and Optical Properties of MgWO4. J. Mater. Sci. 2017, 52, 4934–4943. [Google Scholar] [CrossRef]
- Mikhailik, V.B.; Kraus, H.; Kapustyanyk, V.; Panasyuk, V.; Prots, Y.; Tsybulskyi, V.; Vasylechko, L. Structure, Luminescence and Scintillation Properties of the MgWO4-MgMoO4 System. J. Phys. Condens. Matter 2008, 20, 365219. [Google Scholar] [CrossRef]
- Chernov, S.; Grigorjeva, L.; Millers, D.; Watterich, A. Luminescence Spectra and Decay Kinetics in ZnWO4 and CdWO4 Crystals. Phys. Status Solidi B 2004, 241, 1945–1948. [Google Scholar] [CrossRef]
- Derraji, K.; Lucena, L.; Favotto, C.; Valmalette, J.-C.; Villain, S.; Nolibe, G.; Lyoussi, A.; Guinneton, F.; Gavarri, J.-R. Structural, vibrational and photoluminescence properties of samarium doped cobalt tungstates. J. Mol. Struct. 2022, 1254, 131983. [Google Scholar] [CrossRef]
- Sofronov, D.S.; Sofronova, E.M.; Starikov, V.V.; Voloshko, A.Y.; Baymer, V.N.; Kudin, K.A.; Matejchenko, P.V.; Mamalis, A.G.; Lavrynenko, S.N. Microwave Synthesis of Cadmium and Zinc Tungstates. J. Mater. Eng. Perform. 2012, 21, 2323–2327. [Google Scholar] [CrossRef]
- Mikhailik, V.B.; Kraus, H.; Miller, G.; Mykhaylyk, M.S.; Wahl, D. Luminescence of CaWO4, CdMoO4, and ZnWO4 scintillating crystals under different excitations. J. Appl. Phys. 2005, 97, 083523. [Google Scholar] [CrossRef]
- Itoh, M.; Fujita, N.; Inabe, Y. X-Ray Photoelectron Spectroscopy and Electronic Structures of Scheelite- and Wolframite-Type Tungstate Crystals. J. Phys. Soc. Jpn. 2006, 75, 084705. [Google Scholar] [CrossRef]
- Nagornyi, V.; Feldbach, E.; Jonsson, L.; Kirm, M.; Kotlov, A.; Lushchik, A.; Nefedov, V.; Zadneprovski, B. Energy transfer in ZnWO4 and CdWO4 scintillators. Nucl. Instrum. Methods Phys. Res. A 2002, 486, 395–398. [Google Scholar] [CrossRef]
- Pereira, P.F.S.; Gouveia, A.F.; Assis, M.; de Oliveira, R.C.; Pinatti, I.M.; Penha, M.; Goncalves, R.F.; Gracia, L.; Andres, J.; Longo, E. ZnWO4 nanocrystals: Synthesis, morphology, photoluminescence and photocatalytic properties. Phys. Chem. Chem. Phys. 2018, 20, 1923–1937. [Google Scholar] [CrossRef]
- Kraus, H.; Mikhailik, V.B.; Ramachers, Y.; Day, D.; Hutton, K.B.; Telfer, J. Feasibility study of a ZnWO4 scintillator for exploiting materials signature in cryogenic WIMP dark matter searches. Phys. Lett. B 2005, 610, 37–44. [Google Scholar] [CrossRef]
- Belli, P.; Bernabei, R.; Borovlev, Y.A.; Cappella, F.; Caracciolo, V.R.; Cerulli, F.A.; Danevich, V.Y.; Degoda, A.; Incicchitti, D.V.; Kasperovych, G.; et al. Optical, luminescence, and Scintillation Properties of Advanced ZnWO4 Crystal Scintillators. Creative Commons Attribution 4.0 International. 2022, pp. 1–14. Available online: https://arxiv.org/ftp/arxiv/papers/2202/2202.10111.pdf (accessed on 18 February 2024).
- Priyanka, K.P.; Sabu, N.A.; Sunny, A.T.; Sheena, P.A.; Varghese, T. Effect of Electron Beam Irradiation on Optical Properties of Manganese Tungstate Nanoparticles. J. Nanotechnol. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Ganiger, S.K.; Chaluvaraju, B.; Ananda, S.R.; Murugendrappa, M. A Feasibility Study of Polypyrrole/Zinc Tungstate (Ceramics) Nano Composites for D. C. Conductivity and as a Humidity Sensor. Mater. Today Proc. 2018, 5, 2803–2810. [Google Scholar] [CrossRef]
- de Macedo, O.B.; de Oliveira, A.L.M.; dos Santos, I.M.G. Zinc tungstate: A review on its application as heterogeneous photocatalyst. Cerâmica 2022, 68, 294–315. [Google Scholar] [CrossRef]
- Fang, J.; Hurley, N.; Chien, C.T.; Guo, A.; Khan, T.A.; Li, M.; Cotlet, M.; Moretti, F.; Bourret, E.; Shifman, S.; et al. Probing the optical properties and toxicological profile of zinc tungstate nanorods. J. Chem. Phys. 2024, 160, 234701. [Google Scholar] [CrossRef] [PubMed]
- Korzhik, M.; Brinkmann, K.-T.; Dormenev, V.; Follin, M.; Houzvicka, J.; Kazlou, D.; Kopal, J.; Mechinsky, V.; Nargelas, S.; Orsich, P.; et al. Ultrafast PWO scintillator for future high energy physics instrumentation. Nucl. Instrum. Methods Phys. Res. 2022, 1034, 166781. [Google Scholar] [CrossRef]
- Belli, P.; Bernabei, R.; Borovlev, Y.; Cappella, F.; Caracciolo, V.; Cerulli, R.; Danevich, F.; Degoda, V.; Incicchitti, A.; Kasperovych, D.; et al. Optical, luminescence, and scintillation properties of advanced ZnWO4 crystal scintillators. Nucl. Instrum. Methods Phys. Res. 2022, 1029, 166400. [Google Scholar] [CrossRef]
- Galashov, E.N.; Gusev, V.A.; Shlegel, V.N.; Vasiliev, Y.V. Growing of ZnWO4 Single Crystals from Melt by the Low Thermal Gradient Czochralski Technique (LTG Cz). Funct. Mater. 2009, 16, 63–66. [Google Scholar]
- Galashov, E.N.; Gusev, V.A.; Shlegel, V.N.; Vasiliev, Y.V. The growth of ZnWO4 and CdWO4 single crystals from melt by the low thermal gradient Czochralski technique. Cryst. Rep. 2009, 54, 689. [Google Scholar] [CrossRef]
- Pourmasoud, S.; Eghbali-Arani, M.; Ameri, V.; Rahimi-Nasrabadi, M.; Ahmadi, F.; Sobhani-Nasab, A. Synthesis of some transition MWO4 (M: Mn, Fe, Co, Ni, Cu, Zn, Cd) nanostructures by hydrothermal method. J. Mater. Sci. Mater. Electron. 2019, 30, 8105–8144. [Google Scholar] [CrossRef]
- Oliveira, Y.; Costa, M.; Jucá, A.; Silva, L.; Longo, E.; Arul, N.; Cavalcante, L. Structural characterization, morphology, optical and colorimetric properties of NiWO4 crystals synthesized by the co-precipitation and polymeric precursor methods. J. Mol. Struct. 2020, 1221, 128774. [Google Scholar] [CrossRef]
- Lisitsyn, V.; Tulegenova, A.; Golkovski, M.; Polisadova, E.; Lisitsyna, L.; Mussakhanov, D.; Alpyssova, G. Radiation Synthesis of High-Temperature Wide-Bandgap Ceramics. Micromachines 2023, 14, 2193. [Google Scholar] [CrossRef] [PubMed]
- Lisitsyn, V.; Tulegenova, A.; Kaneva, E.; Mussakhanov, D.; Gritsenko, B. Express Synthesis of YAG:Ce Ceramics in the High-Energy Electrons Flow Field. Materials 2023, 16, 1057. [Google Scholar] [CrossRef] [PubMed]
- Lisitsyn, V.; Polisadova, E.; Lisitsyna, L.; Tulegenova, A.; Denisov, I.; Golkovski, M. Efficiency Dependence of Radiation-Assisted Ceramic Synthesis Based on Metal Oxides and Fluorides on Initial Powder Particle Sizes. Photonics 2023, 10, 1084. [Google Scholar] [CrossRef]
- Itoh, M.; Katagiri, T.; Aoki, T.; Fujita, M. Photo-stimulated luminescence and photo-induced infrared absorption in ZnWO4. Radiat. Meas. 2007, 42, 545–548. [Google Scholar] [CrossRef]
- Kolobanov, V.; Kamenskikh, I.; Mikhailin, V.; Shpinkov, I.; Spassky, D.; Zadneprovsky, B.; Potkin, L.; Zimmerer, G. Optical and luminescent properties of anisotropic tungstate crystals. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2002, 486, 496–503. [Google Scholar] [CrossRef]
- Lisitsyn, V.M.; Valiev, D.T.; Tupitsyna, I.A.; Polisadova, E.F.; Oleshko, V.I.; Lisitsyna, L.A.; Andryuschenko, L.A.; Yakubovskaya, A.G.; Vovk, O.M. Effect of particle size and morphology on the properties of luminescence in ZnWO4. J. Lumin. 2014, 153, 130–135. [Google Scholar] [CrossRef]
- Mikhailik, V.B.; Kraus, H. Scintillators for Cryogenic Applications: State-of-Art. J. Phys. Stud. 2010, 14, 4201. [Google Scholar] [CrossRef]
- Yakovyna, V.; Zhydachevskii’, Y.; Mikhailik, V.B.; Solskii, I.; Sugak, D.; Vakiv, M. Effect of thermo-chemical treatments on the luminescence and scintillation properties of CaWO4. Opt. Mater. 2008, 30, 1630–1634. [Google Scholar] [CrossRef]
- Lisitsyn, V.; Lisitsyna, L.; Akilbekov, A.; Tupitsyna, I.; Dauletbekova, A.; Zdorovets, M. Photoluminescence of oxygen doped crystals of ZnWO4. In Proceedings of the 12th Europhysical Conference on Defects in Insulating Materials, University of Kent, Canterbury, UK, 13–19 July 2014. [Google Scholar]
- Zehani, F.; Sebais, M. UV-visible emission of (O2−–F+) centres in KBr. Cryst. Res. Technol. 2007, 42, 1123–1125. [Google Scholar] [CrossRef]
- Kotomin, E.A.; Popov, A.I. Radiation-induced point defects in simple oxides. Nucl. Instrum. Methods Phys. Res. 1998, 141, 1–15. [Google Scholar] [CrossRef]
- Zvonarev, S.V.; Kortov, V.S.; Shtang, T.V.; Ananchenko, D.V.; Petrovykh, K.A. Effect of structural changes on luminescent and dosimetric properties of nanoscale aluminum oxide. Appl. Radiat. Isot. 2015, 95, 44–47. [Google Scholar] [CrossRef]
- Garmysheva, T.Y.; Shendrik, R.Y.; Paklin, A.S.; Shalaev, A.A.; Kaneva, E.V.; Nepomnyashchikh, A.I. Luminescence of Oxygen-Deficient Centers in Quartz Glasses. Glass Phys. Chem. 2022, 48, 232–235. [Google Scholar] [CrossRef]
- Grassmann, H.; Moser, H.-G. Scintillation properties of ZnWO4. J. Lumin. 1985, 33, 109–113. [Google Scholar] [CrossRef]
- Lisitsyn, V.; Lisitsyna, L.; Tulegenova, A.; Ju, Y.; Polisadova, E.; Lipatov, E.; Vaganov, V. Nanodefects in YAG:Ce-Based Phosphor Microcrystals. Crystals 2019, 9, 476. [Google Scholar] [CrossRef]
- Krutyak, N.R.; Spassky, D.A.; Tupitsyna, I.A.; Dubovik, A.M. Influence of peculiarities of electronic excitation relaxation on luminescent properties of MgWO4. Opt. Spectrosc. 2016, 121, 45–51. [Google Scholar] [CrossRef]
- Alpyssova, G.K.; Afanasyev, D.A.; Bakieva, Z.K.; Lisitsyna, L.A.; Golkovski, M.G.; Tussupbekova, A.K.; Kissabekova, A.A.; Tuleuov, S.D. Optical properties of ZnWO4 ceramics obtained by radiation synthesis. Bull. Univ. Karaganda-Phys. 2024, 3. in press. [Google Scholar]
- Yi, S.-S.; Jung, J.-Y. Calcium Tungstate Doped with Rare Earth Ions Synthesized at Low Temperatures for Photoactive Composite and Anti-Counterfeiting Applications. Crystals 2021, 11, 1214. [Google Scholar] [CrossRef]


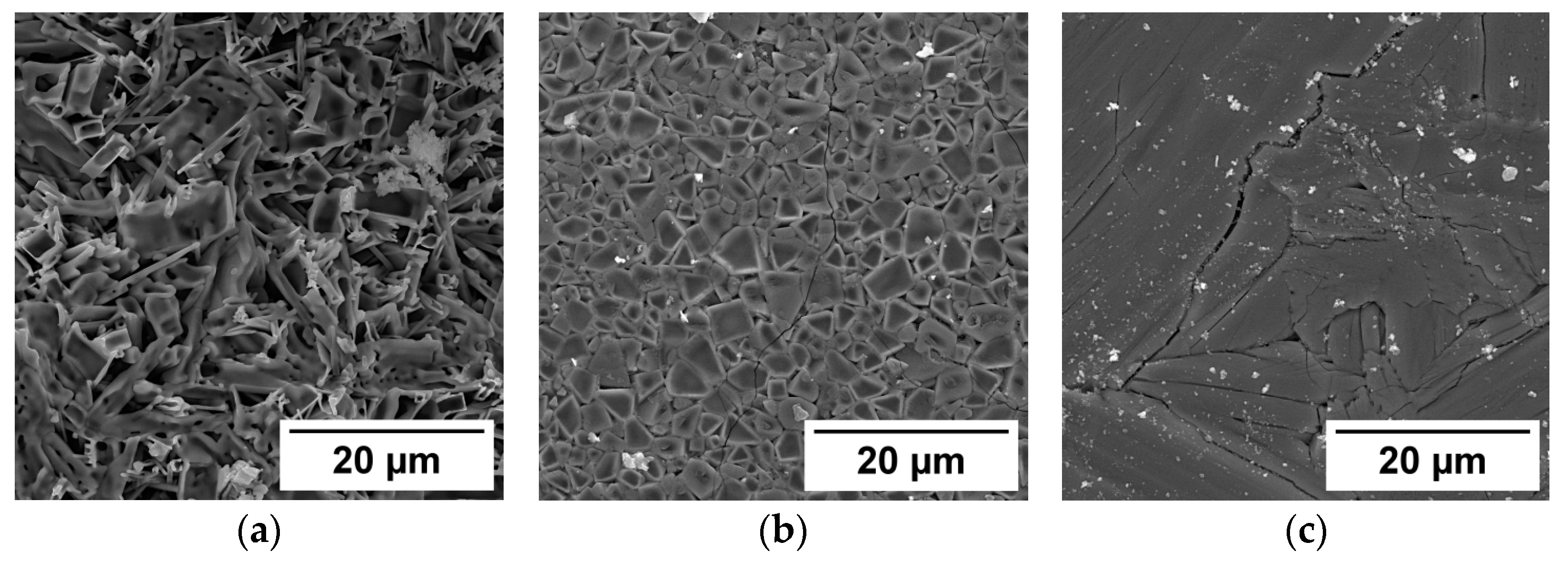
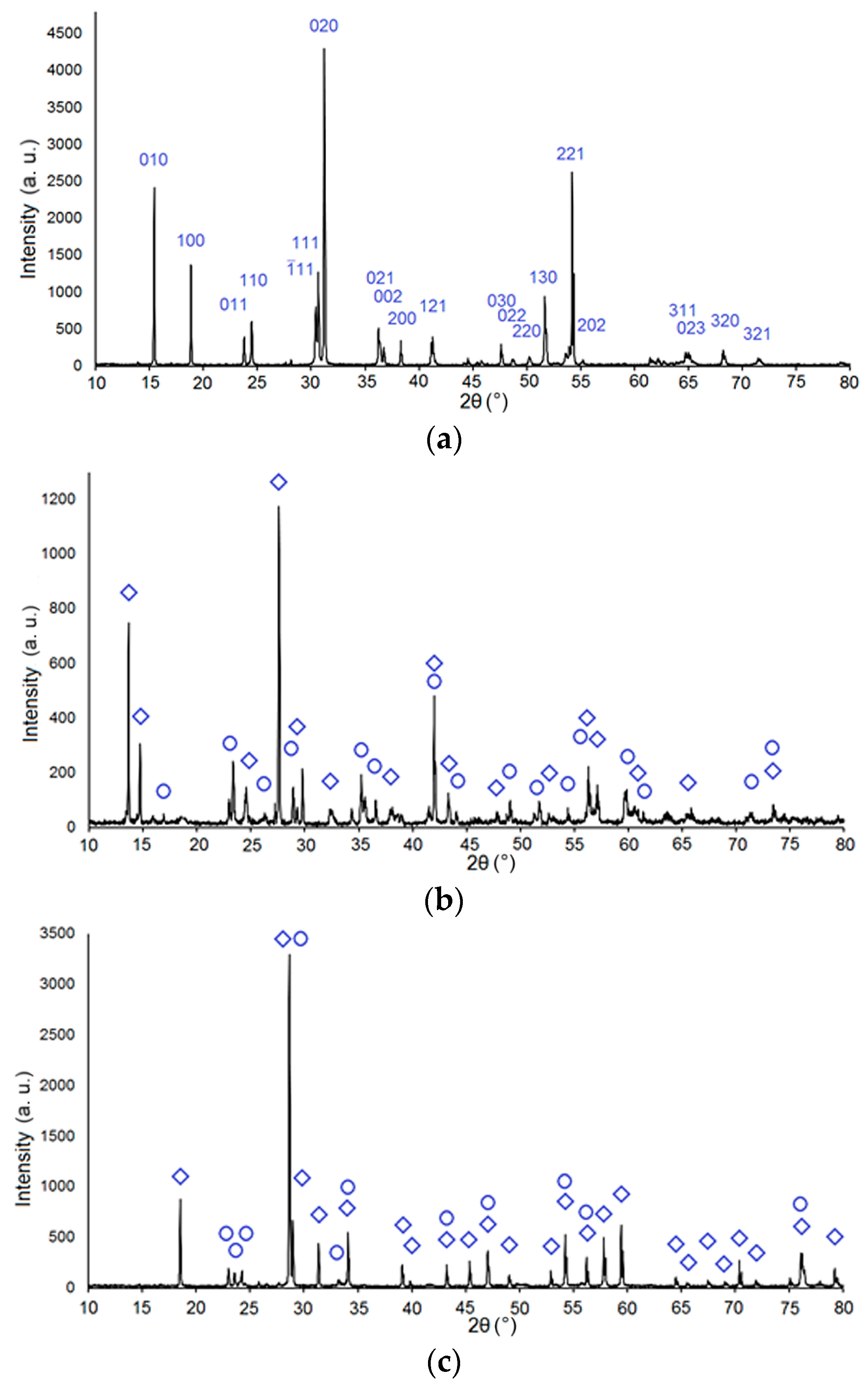
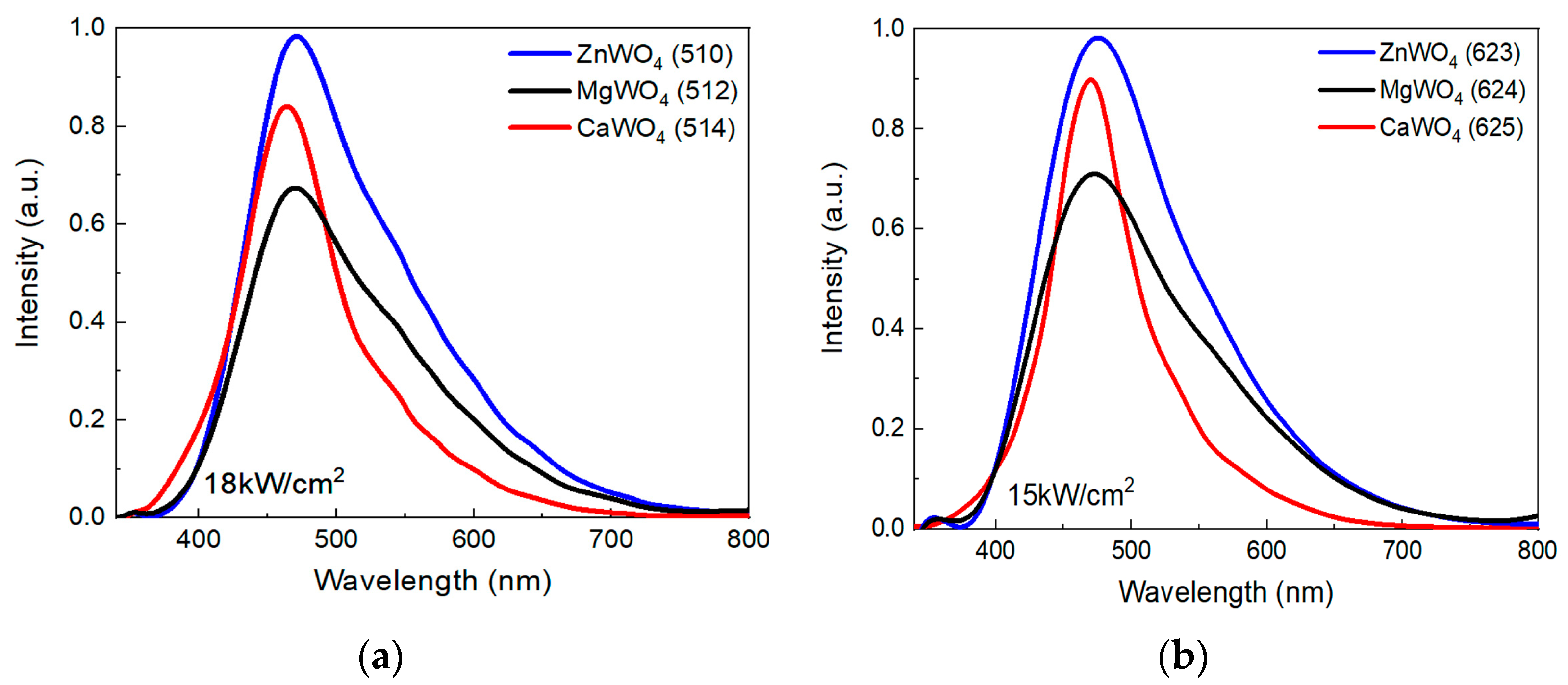
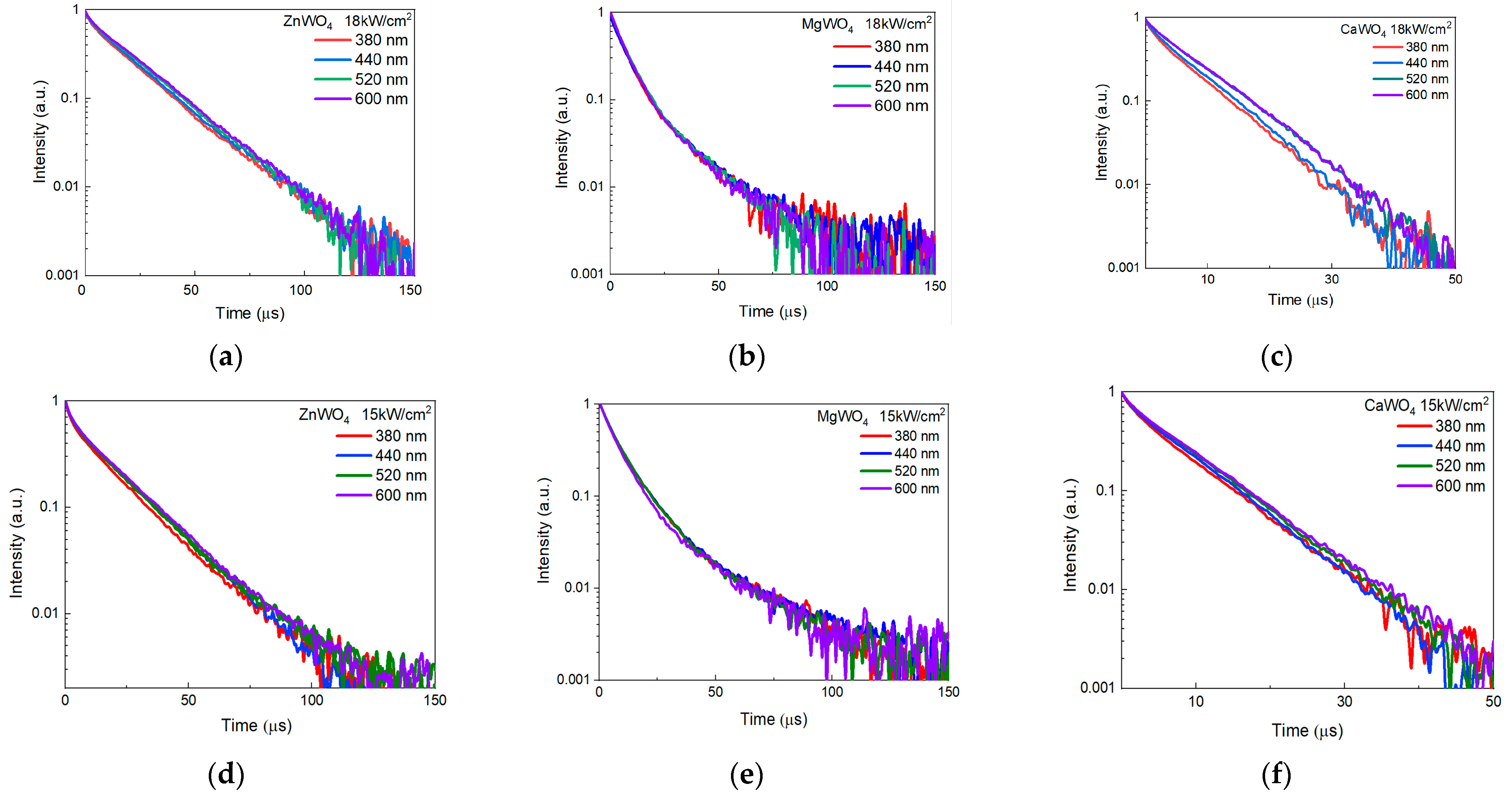
| Sample Number | Charge, Description | Power Density, kW/cm2 | Weight of Samples, g | The Output of the Synthesis Reaction * % | Mass Loss, % | Ceramic Density g/cm3 | Crystal Density g/cm3 |
| 510 | ZnWO4 (ZnO 26%, WO3 74%) | 18 | 86.9 | 98.7 | 1.3 | 5.9 | 7.79 |
| 512 | MgWO4 (MgO 14.8%, WO3 85.2%) | 18 | 71.6 | 99.3 | 0.7 | 3.75 | 6.89 |
| 514 | CaWO4 (CaO 19.5%, WO3 80.5%) | 18 | 50.7 | 75.7 | 24.3 | 3.9 | 6.06 |
| 623 | ZnWO4 (ZnO 26%,WO3 74%) | 15 | 64.6 | 91.3 | 8.9 | 6.38 | 7.79 |
| 624 | MgWO4 (MgO 14.8%, WO3 85.2%) | 15 | 47.3 | 97.2 | 2.8 | 4.26 | 6.89 |
| 625 | CaWO4 (CaO 19.5%, WO3 80.5%) | 15 | 39.5 | 73.3 | 26.7 | 4.1 | 6.06 |
| Sample Number * | Phase | Degree of Crystallinity | Crystallite Size | Refined Unit Cell Parameters |
|---|---|---|---|---|
| 509 | ZnWO4 | 99.9 (±5) % | 131 (±15) nm | P2/c, a = 4.689(4) Å, b = 5.716(7) Å, c = 4.925(3) Å, β = 90.6(1) °, V = 132.0(1) Å3 |
| 510 | ZnWO4 | 99.8 (±5) % | 113 (±11) nm | P2/c, a = 4.691(4) Å, b = 5.718(7) Å, c = 4.927(3) Å, β = 90.6(1) °, V = 132.1(1) Å3 |
| 511 | See below | |||
| 512 | See below | |||
| 513 | CaWO4 (~86%) | 99.9 (±5) % | 167 (±35) nm | I41/a, a = 5.243(2) Å, c = 11.371(4) Å, V = 312.5(2) Å3 |
| WO3 (~14%) | 114 (±28) nm | P21/n, a = 7.311(2) Å, b = 7.532(2) Å, c = 7.694(2) Å, β = 90.8(1) °, V = 423.6(1) Å3 | ||
| 514 | CaWO4 (~92%) | 99.7 (±5) % | 200 (±32) nm | I41/a, a = 5.242(1) Å, c = 11.372(4) Å, V = 312.5(1) Å3 |
| WO3 (~8%) | 127 (±24) nm | P21/n, a = 7.318(4) Å, b = 7.559(3) Å, c = 7.694(4) Å, β = 90.8(1) °, V = 425.6(3) Å3 |
| Sample | Sample Number | λm, nm | ΔW, eV | Sample Number | λm, nm | ΔW, eV |
|---|---|---|---|---|---|---|
| Cathodoluminescence | ||||||
| ZnWO4 | 510 | 467 | 0.58 | 623 | 472 | 0.60 |
| MgWO4 | 512 | 464 | 0.57 | 624 | 470 | 0.58 |
| CaWO4 | 514 | 468 | 0.44 | 625 | 465 | 0.44 |
| Photoluminescence | ||||||
| ZnWO4 | 510 | 482 | 0.54 | 623 | 489 | 0.54 |
| MgWO4 | 512 | 482 | 0.49 | 624 | 480 | 0.58 |
| CaWO4 | 514 | 491 | 0.48 | 625 | 475 | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alpyssova, G.; Lisitsyn, V.; Bakiyeva, Z.; Chakin, I.; Kaneva, E.; Afanasyev, D.; Tussupbekova, A.; Vaganov, V.; Tulegenova, A.T.; Tuleuov, S. Characterization of ZnWO4, MgWO4, and CaWO4 Ceramics Synthesized in the Field of a Powerful Radiation Flux. Ceramics 2024, 7, 1085-1099. https://doi.org/10.3390/ceramics7030071
Alpyssova G, Lisitsyn V, Bakiyeva Z, Chakin I, Kaneva E, Afanasyev D, Tussupbekova A, Vaganov V, Tulegenova AT, Tuleuov S. Characterization of ZnWO4, MgWO4, and CaWO4 Ceramics Synthesized in the Field of a Powerful Radiation Flux. Ceramics. 2024; 7(3):1085-1099. https://doi.org/10.3390/ceramics7030071
Chicago/Turabian StyleAlpyssova, Gulnur, Viktor Lisitsyn, Zhanara Bakiyeva, Ivan Chakin, Ekaterina Kaneva, Dmitriy Afanasyev, Ainura Tussupbekova, Vitalii Vaganov, Aida T. Tulegenova, and Serik Tuleuov. 2024. "Characterization of ZnWO4, MgWO4, and CaWO4 Ceramics Synthesized in the Field of a Powerful Radiation Flux" Ceramics 7, no. 3: 1085-1099. https://doi.org/10.3390/ceramics7030071
APA StyleAlpyssova, G., Lisitsyn, V., Bakiyeva, Z., Chakin, I., Kaneva, E., Afanasyev, D., Tussupbekova, A., Vaganov, V., Tulegenova, A. T., & Tuleuov, S. (2024). Characterization of ZnWO4, MgWO4, and CaWO4 Ceramics Synthesized in the Field of a Powerful Radiation Flux. Ceramics, 7(3), 1085-1099. https://doi.org/10.3390/ceramics7030071








