Carbonate-Hydroxyapatite Cement: The Effect of Composition on Solubility In Vitro and Resorption In Vivo
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Composition of Cement at Figurative Points
3.1.1. X-ray Diffraction (XRD)
3.1.2. Fourier-Transform Infrared Spectroscopy (FTIR)
3.1.3. Volumetric Study
3.1.4. Calorimetric Study
3.1.5. Stoichiometric Formulas
- -
- the neutrality of the molecule;
- -
- the amount of Ca2+ = 10 − (HPO4)2− − (CO3)2v/2 − Na+/2;
- -
- the amount of OH− = 2 − (HPO4)2−.
3.1.6. Scanning Electron Microscopy (SEM)
3.2. Solubility of Cement Formed in the Ca3(PO4)2/CaCO3/Ca(H2PO4)2·H2O/Na2HPO4·12H2O System at Figurative Points
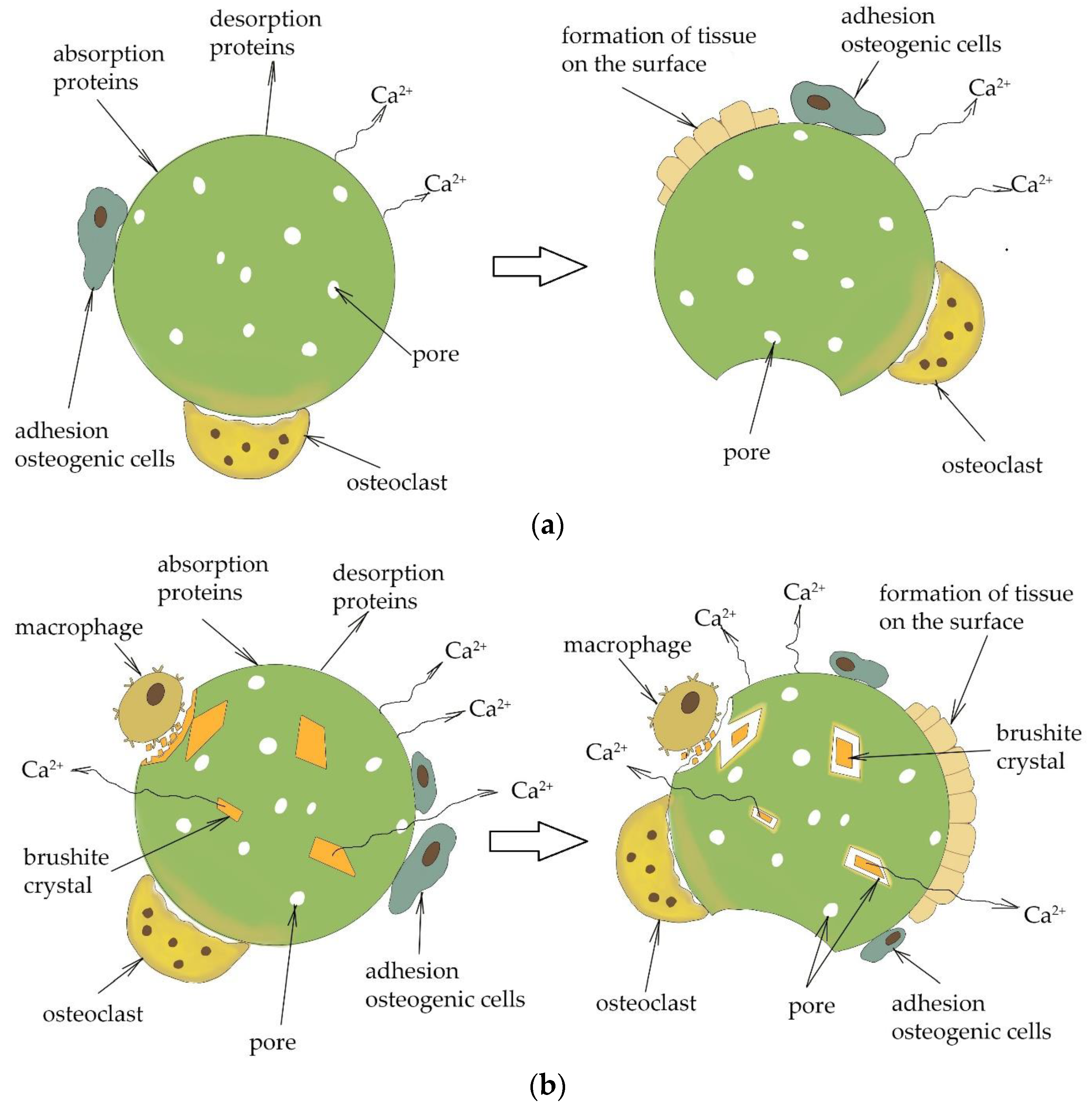
3.3. In Vivo Experiments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rau, J.V.; Cesaro, S.N.; Ferro, D.; Barinov, S.M.; Fadeeva, I.V. FTIR study of carbonate loss from carbonated apatites in the wide temperature range. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Landi, E.; Tampieri, A.; Celotti, G.; Vichi, L.; Sandri, M. Influence of synthesis and sintering parameters on the characteristics of carbonate apatite. Biomaterials 2004, 25, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K. Bone Substitute Fabrication Based on Dissolution-Precipitation Reactions. Materials 2010, 3, 1138–1155. [Google Scholar] [CrossRef]
- Ishikawa, K.; Matsuya, S.; Lin, X.; Lei, Z.; Yuasa, T.; Miyamoto, Y. Fabrication of low crystalline B-type carbonate apatite block from low crystalline calcite block. J. Ceram. Soc. Jpn. 2010, 118, 341–344. [Google Scholar] [CrossRef]
- Ishikawa, K.; Arifta, T.I.; Hayashi, K.; Tsuru, K. Fabrication and evaluation of interconnected porous carbonate apatite from alpha tricalcium phosphate spheres. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Mabroum, H.; Noukrati, H.; Benyoucef, H.; Lefeuvre, B.; Oudadesse, H.; Barroug, A. Physicochemical, setting, rheological, and mechanical properties of a novel bio-composite based on apatite cement, bioactive glass, and alginate hydrogel. Ceram. Int. 2021, 47, 23973–23983. [Google Scholar] [CrossRef]
- Ooms, E.M.; Wolke, J.G.C.; van de Heuvel, M.T.; Jeschel, B.; Jansen, J.A. Histological evalution of the bone response to calcium phosphate cement implanted in cortical bone. Biomaterials 2003, 24, 989–1000. [Google Scholar] [CrossRef]
- Khairoun, I.; Boltong, M.G.; Driessens, F.C.M.; Planell, J.A. Effect of calcium carbonate on the compliance of an apatitic calcium phosphate bone cement. Biomaterials 1997, 18, 1535–1539. [Google Scholar] [CrossRef] [PubMed]
- Boehm, A.V.; Meininger, S.; Tesch, A.; Gbureck, U.; Muller, F.A. The mechanical properties of biocompatible apatite bone cement reinforced with chemically activated carbon fibers. Materials 2018, 11, 192. [Google Scholar] [CrossRef]
- Lewis, G. Injectable bone cements for use in vertebroplasty and kyphoplasty: State-of-the-art review. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 76, 456–468. [Google Scholar] [CrossRef]
- Apelt, D.; Theiss, F.; El-Warrak, A.O.; Zlinszky, K.; Bettschart-Wolfisberger, R.; Bohner, M.; Matter, S.; Auer, J.; Von Rechenberg, B. In vivo behavior of three different injectable hydraulic calcium phosphate cements. Biomaterials 2004, 25, 1439–1451. [Google Scholar] [CrossRef]
- Bohner, M. Calcium orthophosphates in medicine: From ceramics to calcium phosphate cements. Injury 2000, 31, D37–D47. [Google Scholar] [CrossRef]
- Schröter, L.; Kaiser, F.; Stein, S.; Gbureck, U.; Ignatius, A. Biological and mechanical performance and degradation characteristics of calcium phosphate cements in large animals and humans. Acta Biomater. 2020, 117, 1–20. [Google Scholar] [CrossRef]
- Lukina, Y.; Panov, Y.; Panova, L.; Senyagin, A.; Bionyshev-Abramov, L.; Serejnikova, N.; Kireynov, A.; Sivkov, S.; Gavryushenko, N.; Smolentsev, D.; et al. Chemically Bound Resorbable Ceramics as an Antibiotic Delivery System in the Treatment of Purulent–Septic Inflammation of Bone Tissue. Ceramics 2022, 5, 330–350. [Google Scholar] [CrossRef]
- Spence, G.; Pate, N.; Brooks, R.; Bonfield, W.; Rushton, N. Osteoclastogenesis on hydroxyapatite ceramics: The effect of carbonate substitution. J. Biomed. Mater. Res. A 2010, 92, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Mano, T.; Akita, K.; Fukuda, N.; Kamada, K.; Kurio, N.; Ishikawa, K.; Miyamoto, Y. Histological comparison of three apatitic bone substitutes with different carbonate contents in alveolar bone defects in a beagle mandible with simultaneous implant installation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, K.; Akita, K.; Fukuda, N.; Kamada, K.; Kudoh, T.; Ohe, G.; Mano, T.; Tsuru, K.; Ishikawa, K.; Miyamoto, Y. Compositional and histological comparison of carbonate apatite fabricated by dissolution-precipitation reaction and Bio-Oss®. J. Mater. Sci. Mater. Med. 2018, 29, 121. [Google Scholar] [CrossRef]
- Hu, F.; Pan, L.; Zhang, K.; Xing, F.; Wang, X.; Lee, I.; Zhang, X.; Xu, J. Elevation of extracellular Ca2+ induces store-operated calcium entry via calcium-sensing receptors: A pathway contributes to the proliferation of osteoblasts. PLoS ONE 2014, 9, e107217. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Tsuru, K.; Nagai, H.; Fujisawa, K.; Kudoh, T.; Ohe, G.; Ishikawa, K.; Miyamoto, Y. Fabrication and evaluation of carbonate apatite-coated calcium carbonate bone substitutes for bone tissue engineering. J. Tissue Eng. Regen. Med. 2018, 12, 2077–2087. [Google Scholar] [CrossRef]
- Doi, Y.; Iwanaga, H.; Shibutani, T.; Moriwaki, Y.; Iwayama, Y. Osteoclastic responses to various calcium phosphates in cell cultures. J. Biomed. Mater. Res. 1999, 47, 424–433. [Google Scholar] [CrossRef]
- Ishikawa, K. Carbonate apatite bone replacement: Learn from the bone. J. Ceram. Soc. Jpn. 2019, 127, 595–601. [Google Scholar] [CrossRef]
- Hesaraki, S.; Nazarian, H.; Pourbaghi-Masouleh, M.; Borhan, S. Comparative study of mesenchymal stem cells osteogenic differentiation on low-temperature biomineralized nanocrystalline carbonated hydroxyapatite and sintered hydroxyapatite. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, W.; Lin, Y. The efficacy of parathyroid hormone analogues in combination with bisphosphonates for the treatment of osteoporosis: A meta-analysis of randomized controlled trials. Medicine 2015, 94, e1156. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Hu, Y.; Wang, B.; Zhu, J. Role of oral calcium supplementation alone or with vitamin D in preventing post-thyroidectomy hypocalcaemia: A meta-analysis. Medicine 2019, 98, e14455. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xu, L.; Jin, X.; Chen, D.; Jin, X.; Xu, G. Effect of calcium and magnesium on inflammatory cytokines in accidentally multiple fracture adults: A short-term follow-up. Medicine 2022, 101, e28538. [Google Scholar] [CrossRef]
- Notomi, T.; Kuno, M.; Hiyama, A.; Nozaki, T.; Ohura, K.; Ezura, Y.; Noda, M. Role of lysosomal channel protein TPC2 in osteoclast differentiation and bone remodeling under normal and low-magnesium conditions. J. Biol. Chem. 2017, 292, 20998–21010. [Google Scholar] [CrossRef]
- Nagai, H.; Kobayashi-Fujioka, M.; Fujisawa, K.; Ohe, G.; Takamaru, N.; Hara, K.; Uchida, D.; Tamatani, T.; Ishikawa, K.; Miyamoto, Y. Effects of low crystalline carbonate apatite on proliferation and osteoblastic differentiation of human bone marrow cells. J. Mater. Sci. Mater. Med. 2015, 26, 99. [Google Scholar] [CrossRef]
- Egashira, Y.; Atsuta, I.; Narimatsu, I.; Zhang, X.; Takahashi, R.; Koyano, K.; Ayukawa, Y. Effect of carbonate apatite as a bone substitute on oral mucosal healing in a rat extraction socket: In vitro and in vivo analyses using carbonate apatite. Int. J. Implant. Dent. 2022, 8, 11. [Google Scholar] [CrossRef]
- Kudoh, K.; Fukuda, N.; Kasugai, S.; Tachikawa, N.; Koyano, K.; Matsushita, Y.; Ogino, Y.; Ishikawa, K.; Miyamoto, Y. Maxillary sinus floor augmentation using low crystalline carbonate apatite granules with simultaneous implant installation: First-in-human clinical trial. J. Oral Maxillofac. Surg. 2019, 77, 985.E1–985.E11. [Google Scholar] [CrossRef]
- Nakagawa, T.; Kudoh, K.; Fukuda, N.; Kasugai, S.; Tachikawa, N.; Koyano, K.; Matsushita, Y.; Sasaki, M.; Ishikawa, K.; Miyamoto, Y. Application of low-crystalline carbonate apatite granules in 2-stage sinus floor augmentation: A prospective clinical trial and histomorphometric evaluation. J. Periodontal Implant. Sci. 2019, 49, 382–396. [Google Scholar] [CrossRef]
- Chow, L.C.; Markovic, M.; Takagi, S. A dual constant-composition titration system as an in vitro resorption model for comparing dissolution rates of calcium phosphate biomaterials. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 65, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Hurle, K.; Oliveira, J.; Reis, R.; Pina, S.; Goetz-Neunhoeffer, F. Ion-doped brushite cements for bone regeneration. Acta Biomater. 2021, 123, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Issa, K.; Alanazi, A.; Aldhafeeri, K.A.; Alamer, O.; Alshaaer, M. Brushite: Synthesis, Properties, and Biomedical Applications. In Crystallization and Applications; Smida, Y.B., Marzouki, R., Eds.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Morúa, O.C.; Cardoso, M.J.B.; da Silva, H.N.; Carrodeguas, R.G.; Rodríguez, M.A.; Fook, M.V.L. Synthesis of brushite/polyethylene glycol cement for filler in bone tissue injuries. Cerâmica 2021, 67, 289–294. [Google Scholar] [CrossRef]
- Tsuchiya, A.; Freitas, P.P.; Nagashima, N.; Ishikawa, K. Influence of pH and ion components in the liquid phase on the setting reaction of carbonate apatite granules. Dent. Mater. J. 2022, 41, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Shariff, K.A.; Tsuru, K.; Ishikawa, K. Fabrication of dicalcium phosphate dihydrate-coated β-TCP granules and evaluation of their osteoconductivity using experimental rats. Mater. Sci. Eng. C 2017, 75, 1411–1499. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, A.; Sato, M.; Takahashi, I.; Ishikawa, K. Fabrication of apatite-coated gypsum granules and histological evaluation using rabbits. Ceram. Int. 2018, 44, 20330–20336. [Google Scholar] [CrossRef]
- Fulmer, M.T.; Brown, P.W. Effects of Na2HPO4 and NaH2PO4 on hydroxyapatite formation. J. Biomed. Mater. Res. 1993, 27, 1095–1102. [Google Scholar] [CrossRef]
- Ishikawa, K.; Munar, M.L.; Tsuru, K.; Miyamoto, Y. Fabrication of carbonate apatite honeycomb and its tissue response. J. Biomed. Mater. Res. Part A 2019, 107, 1014–1020. [Google Scholar] [CrossRef]
- Cahyanto, A.; Maruta, M.; Tsuru, K.; Matsuya, S.; Ishikawa, K. Fabrication of bone cement that fully transforms to carbonate apatite. Dent. Mater. J. 2015, 34, 394–401. [Google Scholar] [CrossRef]
- Driessens, F.C.; Boltong, M.; de Maeyer, E.A.; Wenz, R.; Nies, B.; Planell, J. The Ca/P range of nanoapatitic calcium phosphate cements. Biomaterials 2002, 23, 4011–4017. [Google Scholar] [CrossRef]
- Lodoso-Torrecilla, I.; van den Beucken, J.J.; Jansen, J.A. Calcium phosphate cements: Optimization toward biodegradability. Acta Biomater. 2021, 119, 1–12. [Google Scholar] [CrossRef]
- Grossardt, C.; Ewald, A.; Grover, L.M.; Barralet, J.E.; Gbureck, U. Passive and active in vitro resorption of calcium and magnesium phosphate cements by osteclastic cells. Tissue Eng. Part A 2010, 16, 3687–3695. [Google Scholar] [CrossRef]
- Montazerolghaem, M.; Rasmusson, A.; Melhus, H.; Engqvist, H.; Karlsson Ott, M. Simvastatin-doped pre-mixed calcium phosphate cement inhibits osteoclast differentiation and resorption. J. Mater. Sci. Mater. Med. 2016, 27, 5. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Davison, N.L.; Gamblin, A.-L.; Layrolle, P.; Yuan, H.; de Bruijn, J.D.; Barrère-de Groot, F. Liposomal clodronate inhibition of osteoclastogenesis and osteoinduction by submicrostructured beta-tricalcium phosphate. Biomaterials 2014, 35, 5088–5097. [Google Scholar] [CrossRef] [PubMed]
- Odgren, P.R.; Witwicka, H.; Reyes-Gutierrez, P. The cast of clasts: Catabolism and vascular invasion during bone growth, repair, and disease by osteoclasts, chondroclasts, and septoclasts. Connect. Tissue Res. 2016, 57, 161–174. [Google Scholar] [CrossRef]
- Maeno, S.; Niki, Y.; Matsumoto, H.; Morioka, H.; Yatabe, T.; Funayama, A.; Toyama, Y.; Taguchi, T.; Tanaka, J. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials 2005, 26, 4847–4855. [Google Scholar] [CrossRef]
- Guo, H.; Su, J.; Wei, J.; Kong, H.; Liu, C. Biocompatibility and osteogenicity of degradable Ca-deficient hydroxyapatite scaffolds from calcium phosphate cement for bone tissue engineering. Acta Biomater. 2009, 5, 268–278. [Google Scholar] [CrossRef]

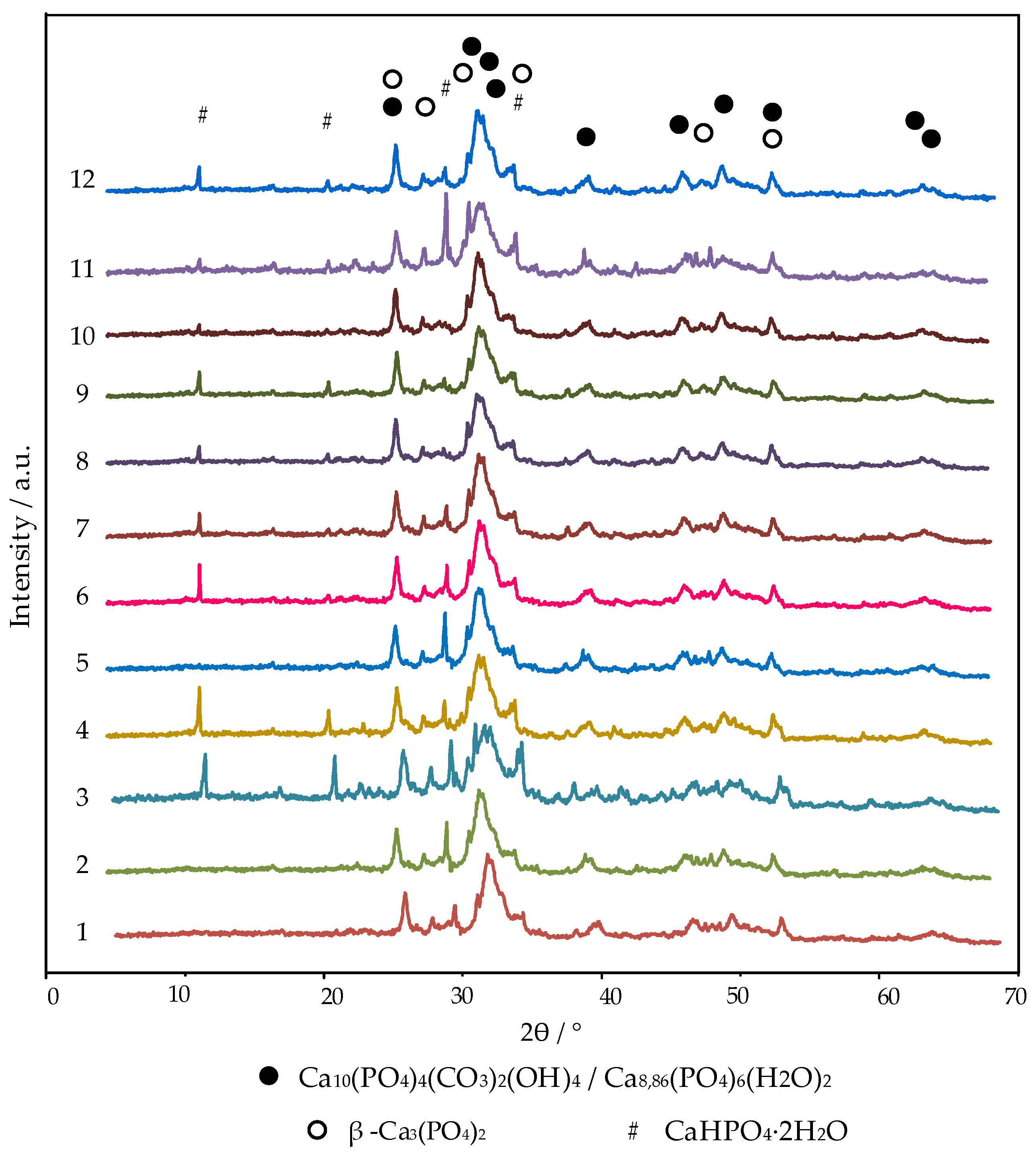
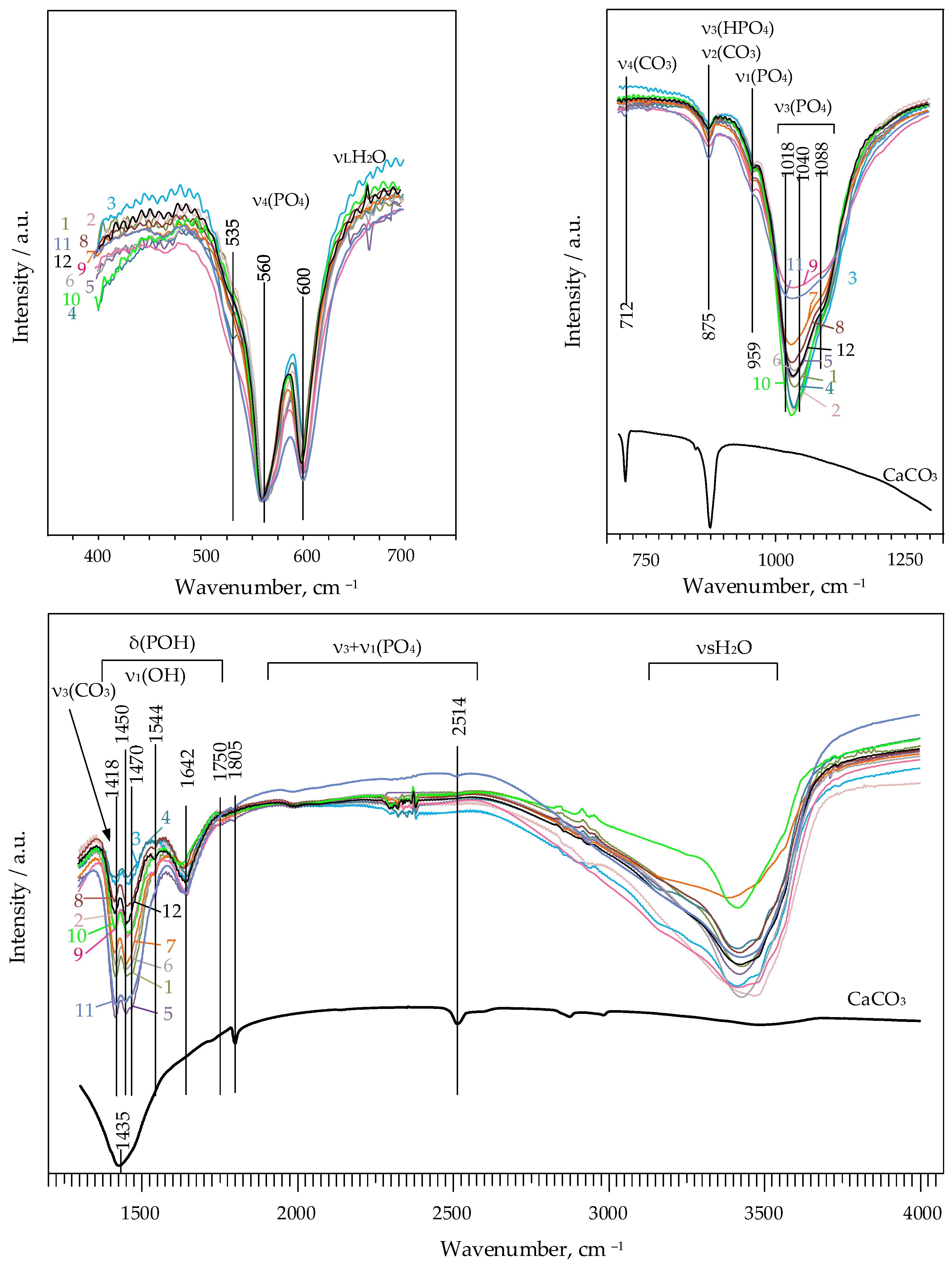
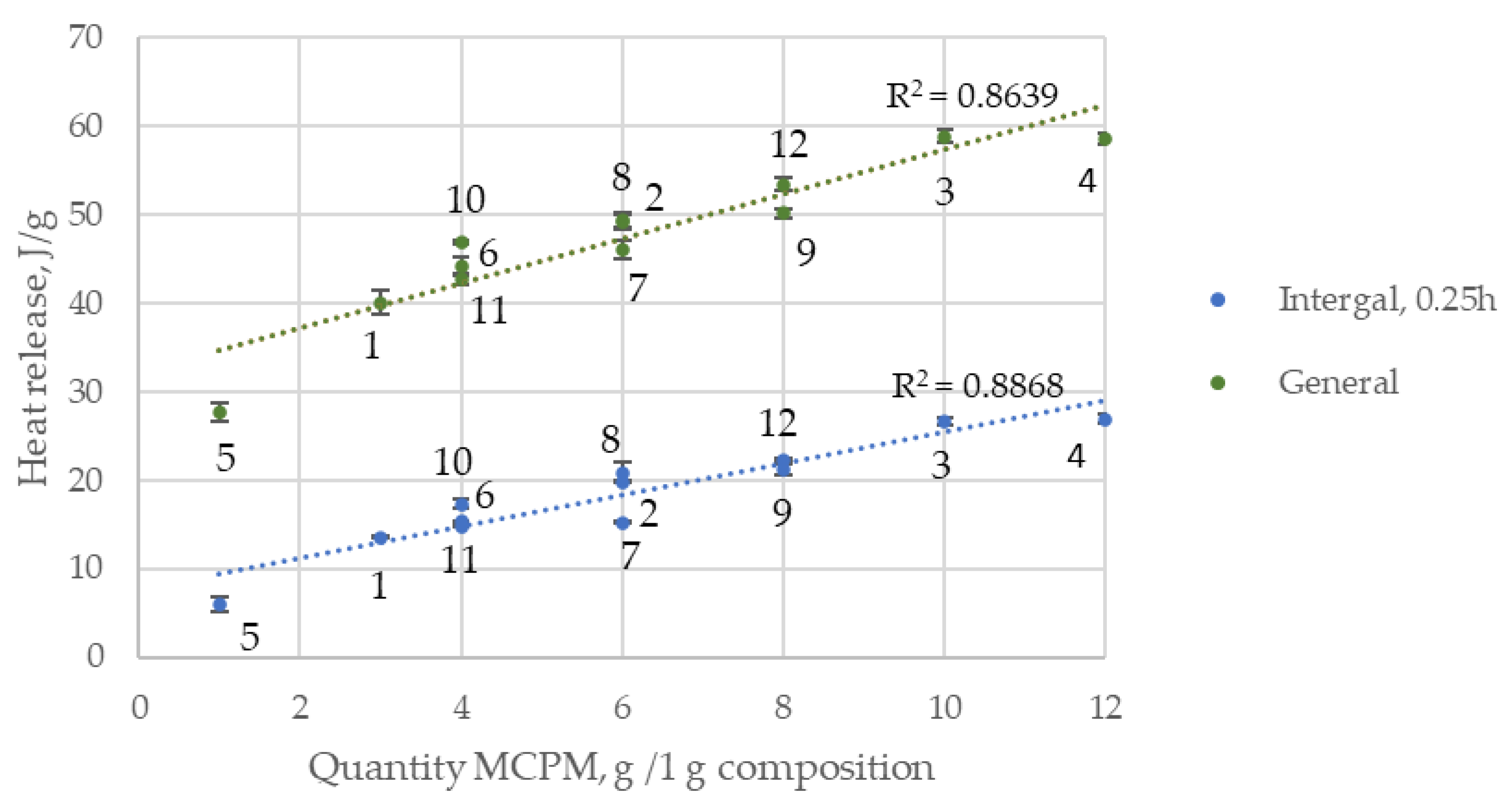
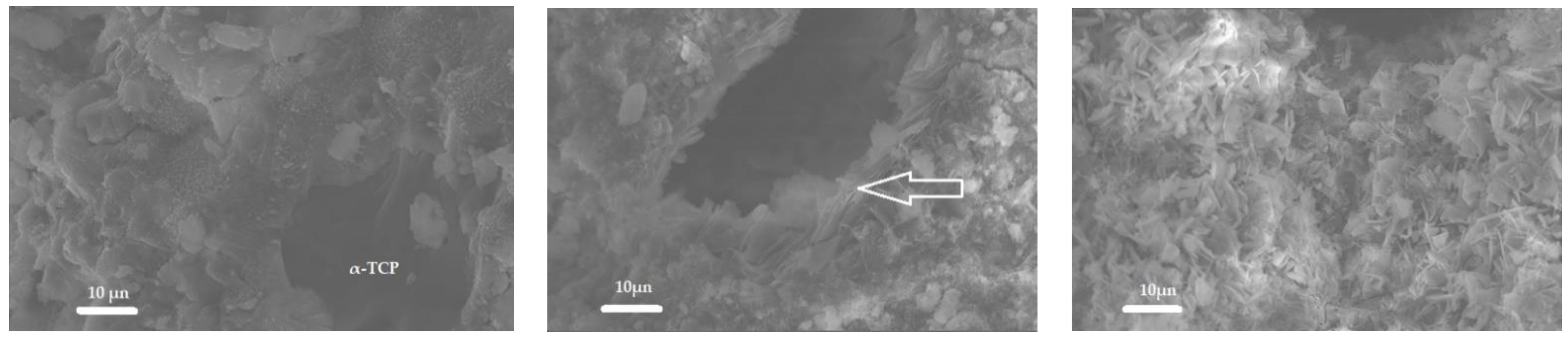

| Quantity, wt.% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Composition | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| CaCO3 | 10 | 8 | 4 | 5 | 13 | 10 | 10 | 6 | 6 | 8 | 12 | 8 |
| Ca(H2PO4)2∙H2O | 3 | 6 | 10 | 12 | 1 | 4 | 6 | 6 | 8 | 4 | 4 | 8 |
| Na2HPO4∙12H2O | 7 | 6 | 6 | 3 | 6 | 6 | 4 | 8 | 6 | 8 | 4 | 4 |
| Phase/Composition | Quantity, wt.% | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| CaHPO4·2H2O | 3.0 ± 0.3 | 3.4 ± 0.3 | 6.4 ± 0.5 | 5.7 ± 0.5 | 2.0 ± 0.3 | 3.7 ± 0.4 | 3.8 ± 0.3 | 4.2 ± 0.4 | 4.6 ± 0.4 | 3.3 ± 0.3 | 4.3 ± 0.4 | 4.4 ± 0.5 |
| Composition | Area, Conv. Unit2 | Composition | Area, Conv. Unit2 |
|---|---|---|---|
| 1 | 3745 | 7 | 3282 |
| 2 | 2388 | 8 | 1575 |
| 3 | 1139 | 9 | 2589 |
| 4 | 1000 | 10 | 2439 |
| 5 | 5158 | 11 | 4871 |
| 6 | 3505 | 12 | 1953 |
| Composition | CO32−, mg/1 g Cement | Composition | CO32−, mg/1 g Cement |
|---|---|---|---|
| 1 | 56.37 | 7 | 50.37 |
| 2 | 41.00 | 8 | 23.06 |
| 3 | 20.33 | 9 | 30.22 |
| 4 | 14.17 | 10 | 29.62 |
| 5 | 69.18 | 11 | 65.48 |
| 6 | 41.00 | 12 | 30.22 |
| Time, h Composition | Integral | General | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 1 | 3 | 6 | 12 | 24 | ||
| Point 1 | 13.5 ± 0.12 g | 4.3 ± 0.10 | 1.5 ± 0.15 | 1.9 ± 0.19 | 3.4 ± 0.10 | 4.1 ± 0.12 | 5.5 ± 0.09 | 40.1 ± 1.32 cde |
| Point 2 | 19.8 ± 0.05 d | 6.0 ± 0.32 | 2.2 ± 0.12 | 2.1 ± 0.09 | 3.6 ± 0.11 | 3.8 ± 0.18 | 4.3 ± 0.11 | 49.2 ± 0.85 cd |
| Point 3 | 26.7 ±0.42 b | 7.6 ± 0.15 | 2.4 ± 0.05 | 1.9 ± 0.12 | 3.5 ± 0.16 | 4.0 ± 0.22 | 4.7 ± 0.23 | 58.9 ± 0.79 a |
| Point 4 | 27.0 ± 0.43 b | 6.5 ± 0.48 | 2.0 ± 0.16 | 2.8 ± 0.43 | 4.7 ± 0.25 | 4.2 ± 0.09 | 3.7 ± 0.12 | 58.6 ± 0.70 a |
| Point 5 | 6.0 ± 0.78 h | 3.9 ± 0.11 | 1.6 ± 0.09 | 1.5 ± 0.22 | 2.4 ± 0.12 | 2.8 ± 0.24 | 4.1 ± 0.19 | 27.7 ± 1.00 g |
| Point 6 | 15.4 ± 0.08 ef | 5.1 ± 0.09 | 1.9 ± 0.20 | 2.1 ± 0.08 | 3.4 ± 0.27 | 3.5 ± 0.70 | 4.5 ± 0.27 | 42.7 ± 0.63 f |
| Point 7 | 15.2 ± 0.09 fg | 7.1 ± 0.20 | 2.2 ± 0.18 | 2.2 ± 0.10 | 3.6 ± 0.19 | 3.6 ± 0.17 | 4.2 ± 0.41 | 46.0 ± 1.03 e |
| Point 8 | 20.8 ± 1.17 d | 5.5 ± 0.37 | 1.9 ± 0.08 | 2.0 ± 0.56 | 3.5 ± 0.18 | 3.8 ± 0.08 | 4.9 ± 0.29 | 49.4 ± 0.77 cd |
| Point 9 | 21.2 ± 0.66 c | 7.1 ± 0.23 | 2.4 ± 0.37 | 2.2 ± 0.16 | 3.7 ± 0.09 | 3.9 ± 0.19 | 4.6 ± 0.23 | 53.4 ± 0.78 b |
| Point 10 | 17.3 ± 0.47 e | 4.7 ± 0.08 | 1.7 ± 0.21 | 2.0 ± 0.12 | 3.4 ± 0.20 | 3.8 ± 0.35 | 5.0 ± 0.65 | 44.2 ± 1.01 f |
| Point 11 | 14.8 ± 0.08 a | 9.2 ± 0.28 | 2.2 ± 0.18 | 2.0 ± 0.14 | 3.3 ± 0.18 | 3.3 ± 0.12 | 4.2 ± 0.15 | 46.9 ± 0.18 de |
| Point 12 | 22.3 ± 0.15 c | 6.9 ± 0.17 | 1.8 ± 0.07 | 2.0 ± 0.12 | 3.3 ± 0.19 | 3.4 ± 0.16 | 4.0 ± 0.12 | 50.2 ± 0.50 c |
| Composition | Stoichiometric Formula |
|---|---|
| 1 | Ca7.9Na0.3(HPO4)1.4(PO4)3.5(CO3)(OH)0.6 |
| 2 | Ca8Na0.3(HPO4)1.6(PO4)3.6(CO3)0.8(OH)0.4 |
| 3 | Ca7.6Na0.3(HPO4)2(PO4)3.4(CO3)0.6 |
| 4 | Ca8Na0.1(HPO4)1.7(PO4)4.0(CO3)0.3(OH)0.3 |
| 5 | Ca8Na0.3(HPO4)1.2(PO4)3.6(CO3)1.2(OH)0.8 |
| 6 | Ca8.2Na0.3(HPO4)1.2(PO4)4(CO3)0.8(OH)0.7 |
| 7 | Ca8Na0.2(HPO4)1.5(PO4)3.7(CO3)0.8(OH)0.5 |
| 8 | Ca8.2Na0.4(HPO4)1.4(PO4)4.1(CO3)0.5(OH)0.6 |
| 9 | Ca7.9Na0.3(HPO4)1.6(PO4)3.8(CO3)0.6(OH)0.3 |
| 10 | Ca8.3Na0.4(HPO4)1.2(PO4)4.2(CO3)0.5(OH)0.8 |
| 11 | Ca7.9Na0.2(HPO4)1.5(PO4)3.4(CO3)1.1(OH)0.5 |
| 12 | Ca8.1Na0.2(HPO4)1.5(PO4)4(CO3)0.5(OH)0.5 |
| Figurative Point | |||
|---|---|---|---|
| 3 | 5 | 8 | |
| 3D model and orthogonal projections Initial |  |  | 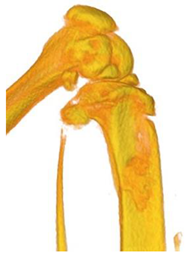 |
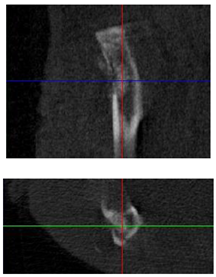 | 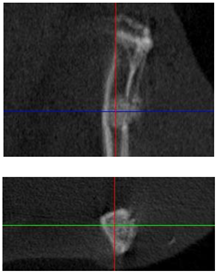 | 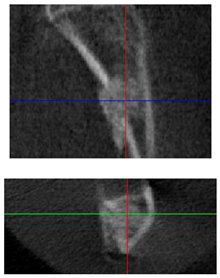 | |
| 3D model and orthogonal projectionsAfter implantation period of 3 months | 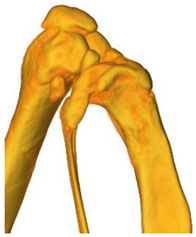 |  |  |
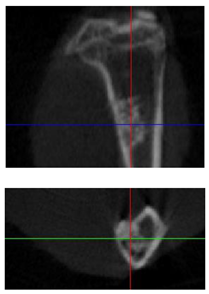 |  |  | |
| Point | Type of Microscopy | ||||
|---|---|---|---|---|---|
| Standard Light | Standard Light | Polarization | Standard Light | Phase Contrast | |
| Staining with Hematoxylin–Eosin | Staining with Picrosirius Red | Staining with Picrosirius Red | Staining with Hematoxylin–Eosin | Staining with Hematoxylin–Eosin | |
| 3 | 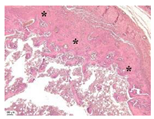 | 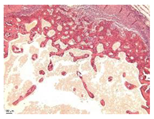 |  | 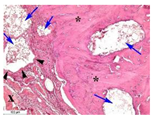 | 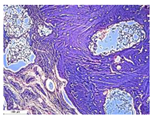 |
| 5 | 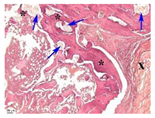 | 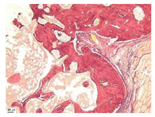 |  | 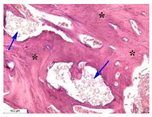 | 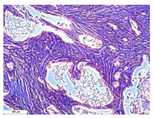 |
| 8 | 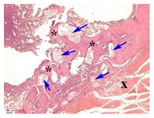 | 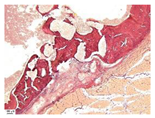 | 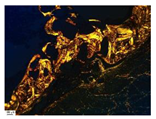 | 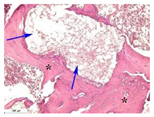 | 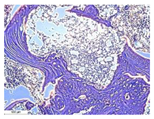 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukina, Y.; Bionyshev-Abramov, L.; Kotov, S.; Serejnikova, N.; Smolentsev, D.; Sivkov, S. Carbonate-Hydroxyapatite Cement: The Effect of Composition on Solubility In Vitro and Resorption In Vivo. Ceramics 2023, 6, 1397-1414. https://doi.org/10.3390/ceramics6030086
Lukina Y, Bionyshev-Abramov L, Kotov S, Serejnikova N, Smolentsev D, Sivkov S. Carbonate-Hydroxyapatite Cement: The Effect of Composition on Solubility In Vitro and Resorption In Vivo. Ceramics. 2023; 6(3):1397-1414. https://doi.org/10.3390/ceramics6030086
Chicago/Turabian StyleLukina, Yulia, Leonid Bionyshev-Abramov, Sergey Kotov, Natalya Serejnikova, Dmitriiy Smolentsev, and Sergey Sivkov. 2023. "Carbonate-Hydroxyapatite Cement: The Effect of Composition on Solubility In Vitro and Resorption In Vivo" Ceramics 6, no. 3: 1397-1414. https://doi.org/10.3390/ceramics6030086
APA StyleLukina, Y., Bionyshev-Abramov, L., Kotov, S., Serejnikova, N., Smolentsev, D., & Sivkov, S. (2023). Carbonate-Hydroxyapatite Cement: The Effect of Composition on Solubility In Vitro and Resorption In Vivo. Ceramics, 6(3), 1397-1414. https://doi.org/10.3390/ceramics6030086






