Fe Doping in TiO2 via Anodic Dissolution of Iron: Synthesis, Characterization, and Electrophoretic Deposition on a Metal Substrate
Abstract
1. Introduction
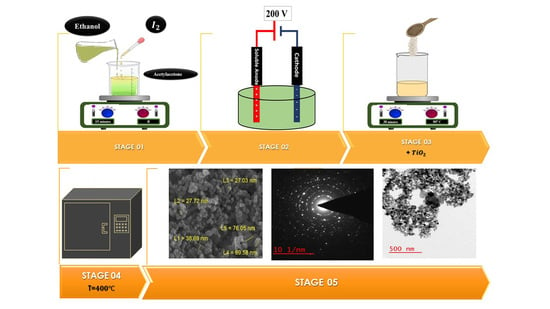
2. Materials and Methods
3. Results and Discussion
3.1. Dissolution of Fe in Different Conditions
3.1.1. Effect of Media on Anodic Dissolution
3.1.2. Effect of Voltage on Anodic Dissolution
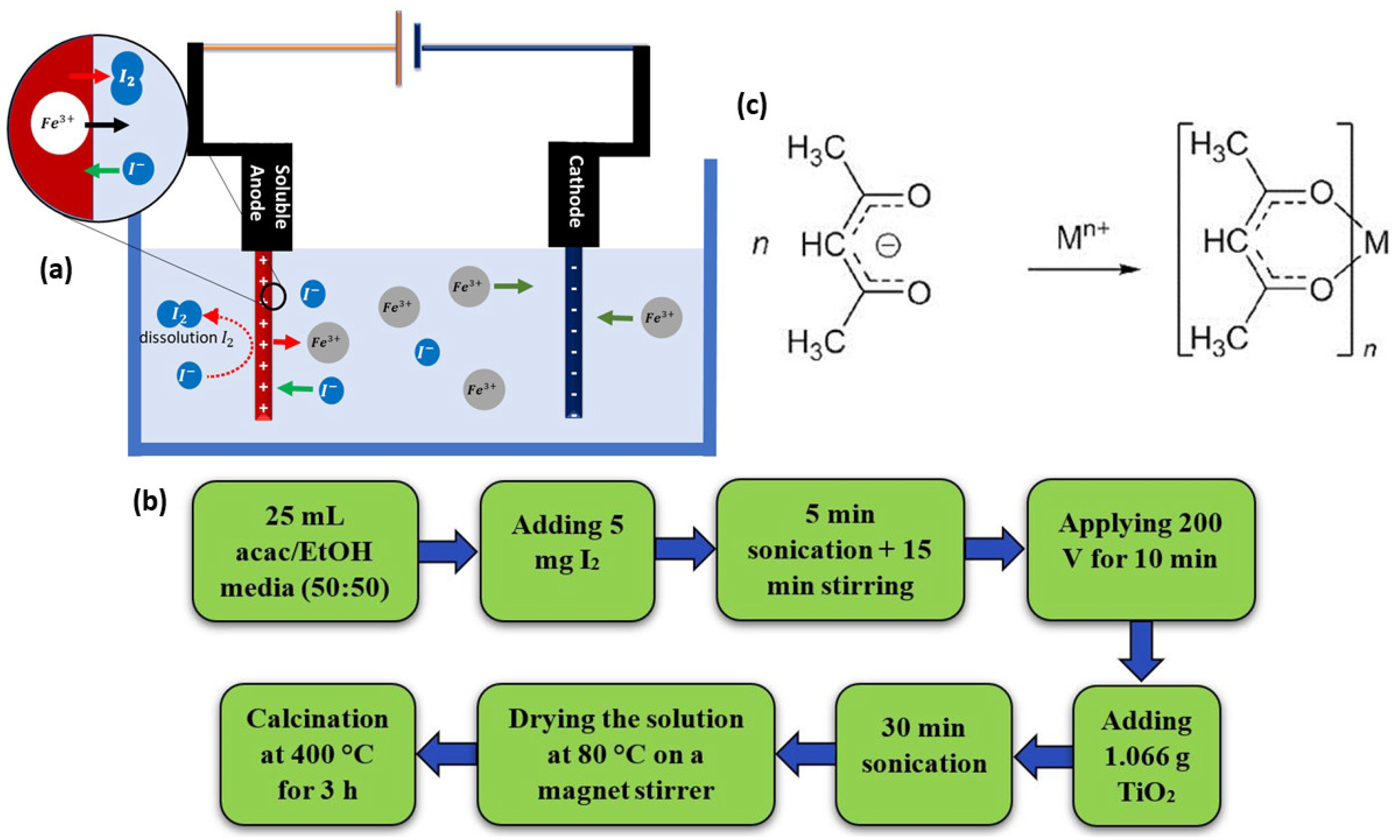
3.1.3. Anodic Dissolution in the Presence of Iodine
3.1.4. Effect of Time on Anodic Dissolution
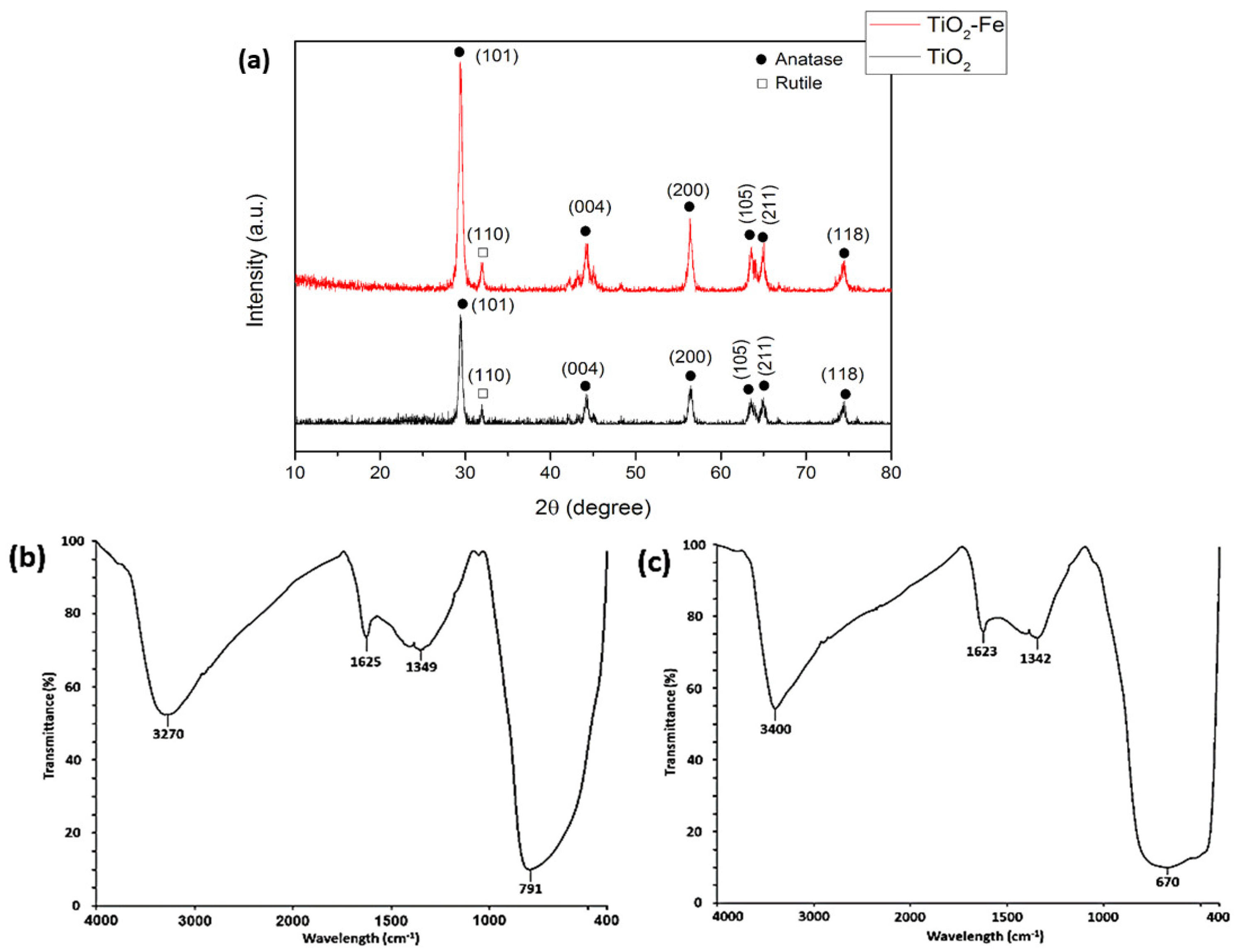
3.2. XRD Analysis
3.3. FTIR Analysis
3.4. TEM Analysis
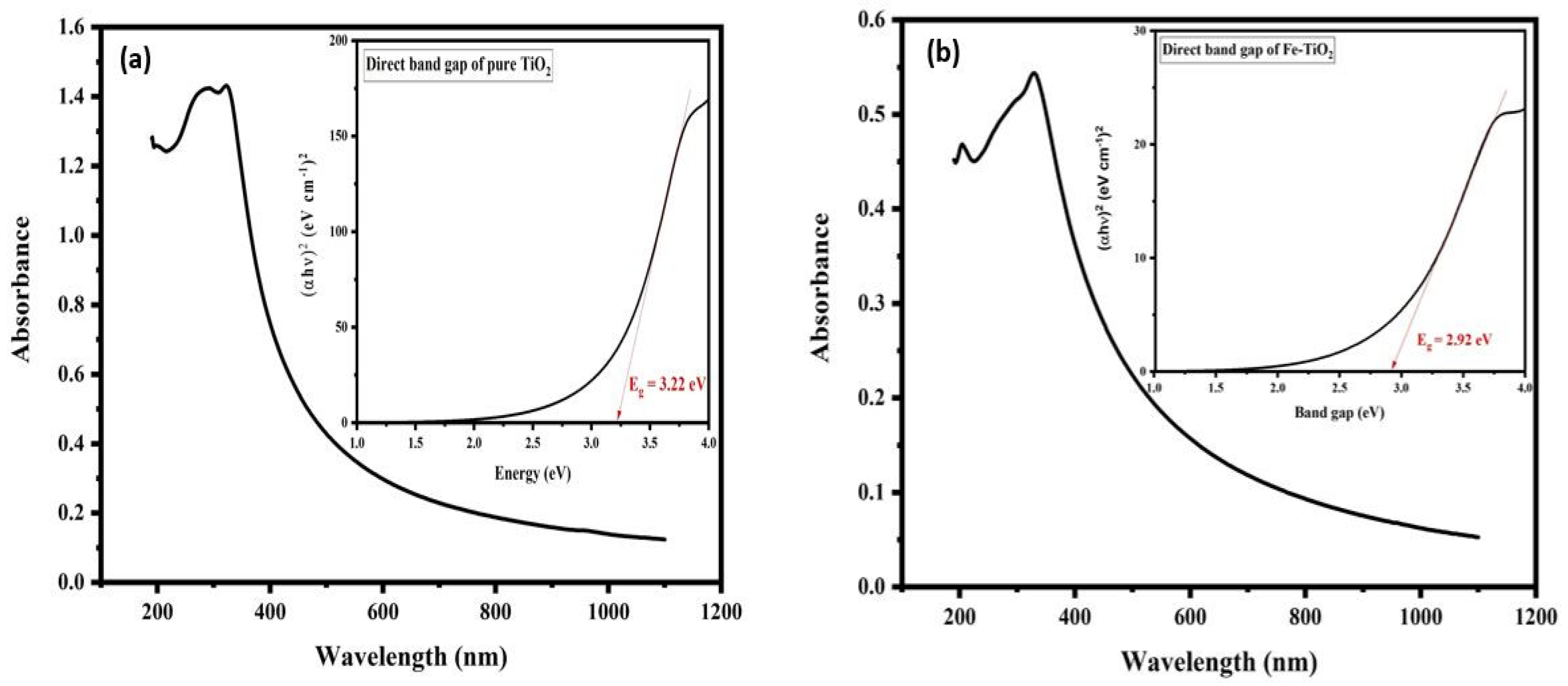
3.5. UV-Vis Analysis
3.6. Electrophoretic Deposition of Synthesized Fe-TiO2 Nanoparticle
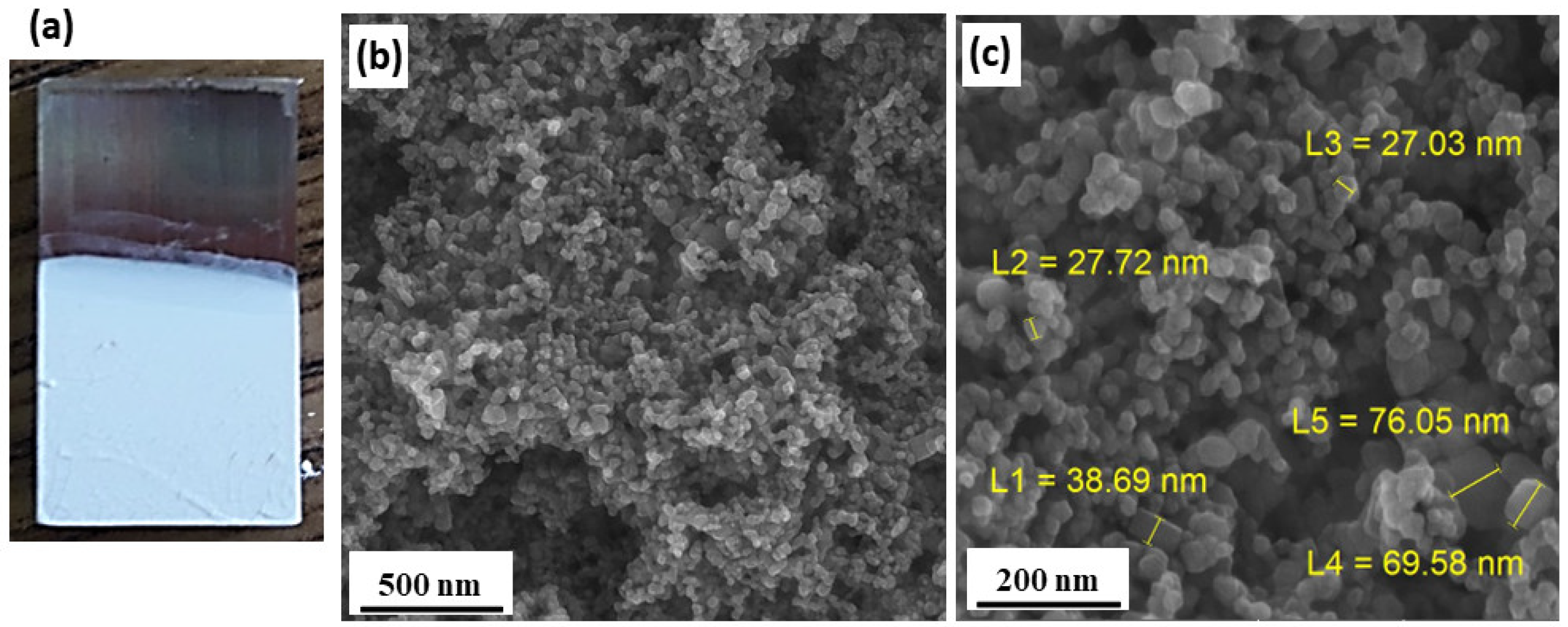
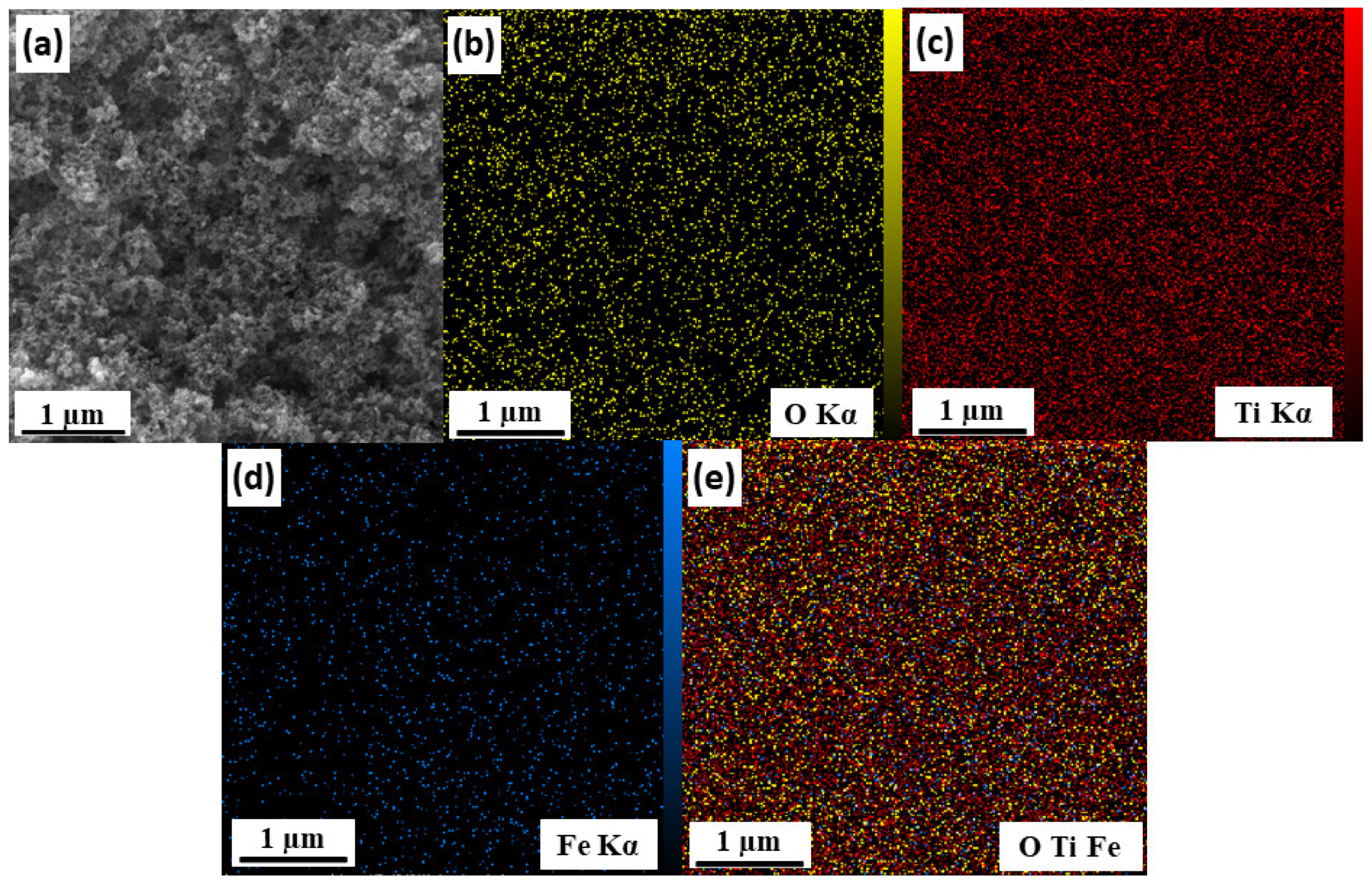
3.6.1. FESEM Analysis
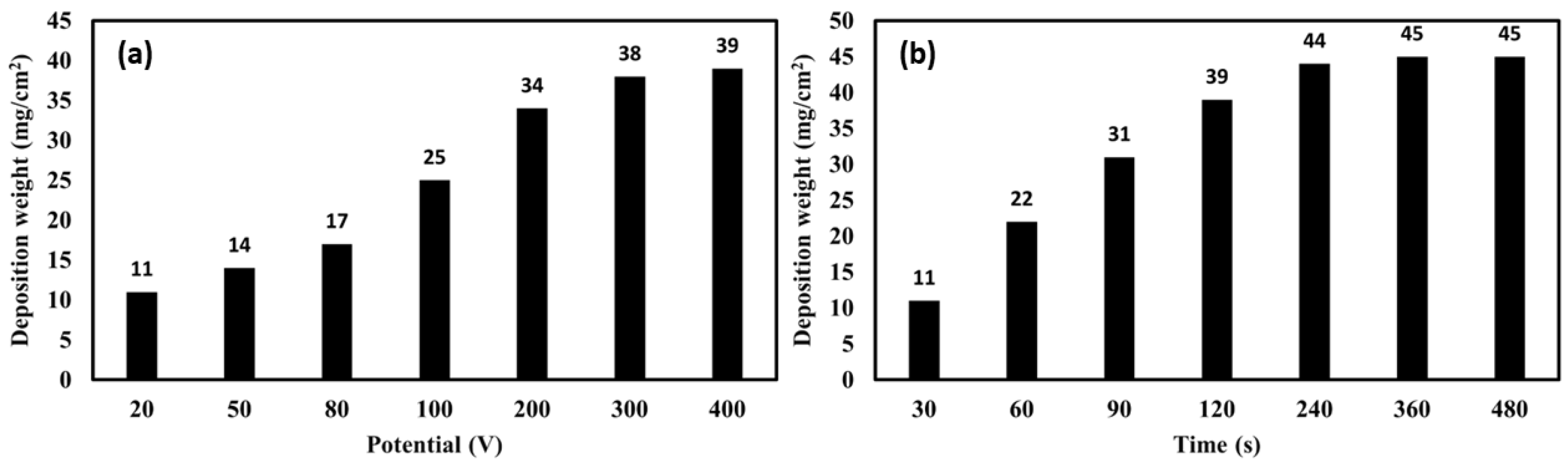
3.6.2. Deposition Weight of the Layer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castillo-Robles, J.A.; Rocha-Rangel, E.; Ramírez-de-León, J.A.; Caballero-Rico, F.C.; Armendáriz-Mireles, E.N. Armendáriz-Mireles, Advances on dye-sensitized solar cells (DSSCs) nanostructures and natural colorants: A review. J. Compos. Sci. 2021, 5, 288. [Google Scholar] [CrossRef]
- Negishi, N.; Sugasawa, M.; Miyazaki, Y.; Hirami, Y.; Koura, S. Effect of dissolved silica on photocatalytic water purification with a TiO2 ceramic catalyst. Water Res. 2019, 150, 40–46. [Google Scholar] [CrossRef]
- Silva, G.M.G.; Leão, V.N.S.; Pereira, M.F.G.; Faia, P.M.; Araújo, E.S. Zn2+-doped TiO2:WO3 films prepared by electrospinning and sintering: Microstructural characterization and electrical signature to moisture sensing. Ceramics 2021, 4, 576–591. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Ho, W.J.; Yeh, C.W. Fabrication of silicon solar cell with >18% efficiency using spin-on-film processing for phosphorus diffusion and SiO2/graded index TiO2 anti-reflective coating. Appl. Surf. Sci. 2015, 354, 20–24. [Google Scholar] [CrossRef]
- Nasir, A.; Khalid, S.; Yasin, T.; Mazare, A. A Review on the Progress and Future of TiO2/Graphene Photocatalysts. Energies 2022, 15, 6248. [Google Scholar] [CrossRef]
- Alem, S.A.A.; Latifi, R.; Angizi, S.; Hassanaghaei, F.; Aghaahmadi, M.; Ghasali, E.; Rajabi, M. Microwave sintering of ceramic reinforced metal matrix composites and their properties: A review. Mater. Manuf. Process. 2020, 35, 303–327. [Google Scholar] [CrossRef]
- Verchère, A.; Cottrino, S.; Fantozzi, G.; Mishra, S.; Gaudisson, T.; Blanchard, N.; Pailhès, S.; Daniele, S.; Le Floch, S. Effect of high pressure spark plasma sintering on the densification of a nb-doped TiO2 nanopowder. Ceramics 2020, 3, 507–520. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kato, H.; Miyazaki, T.; Kakihana, M. Hydrothermal synthesis of pseudocubic rutile-type titania particles. Ceramics 2019, 2, 56–63. [Google Scholar] [CrossRef]
- Tismanar, I.; Obreja, A.C.; Buiu, O.; Duta, A. TiO2–Graphene Oxide and TiO2–Reduced Graphene Oxide Composite Thin Films for Solar Photocatalytic Wastewater Treatment. Energies 2022, 15, 9416. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Buckeridge, Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef]
- Realpe, Á.; Núñez, D.P.; Herrera, A. Synthesis of Fe-Tio2 nanoparticles for photoelectrochemical generation of hydrogen. Int. J. ChemTech Res. 2016, 9, 453–464. [Google Scholar]
- Angizi, S.; Alem, S.A.A.; Azar, M.H.; Shayeganfar, F.; Manning, M.I.; Hatamie, A.; Pakdel, A.; Simchi, A. A comprehensive review on planar boron nitride nanomaterials: From 2D nanosheets towards 0D quantum dots. Prog. Mater. Sci. 2022, 124, 100884. [Google Scholar] [CrossRef]
- Angizi, S.; Alem, S.A.A.; Pakdel, A. Towards Integration of Two-Dimensional Hexagonal Boron Nitride (2D h-BN) in Energy Conversion and Storage Devices. Energies 2022, 15, 1162. [Google Scholar] [CrossRef]
- Sacco, O.; Sannino, D.; Matarangolo, M.; Vaiano, V. Room Temperature Synthesis of V-Doped TiO2 and Its Photocatalytic Activity in the Removal of Caffeine under UV Irradiation. Materials 2019, 12, 911. [Google Scholar] [CrossRef]
- Peng, Y.H.; Huang, G.F.; Huang, W.Q. Visible-light absorption and photocatalytic activity of Cr-doped TiO2 nanocrystal films. Adv. Powder Technol. 2012, 23, 8–12. [Google Scholar] [CrossRef]
- MMarami, B.; Farahmandjou, M.; Khoshnevisan, B. Sol–Gel Synthesis of Fe-Doped TiO2 Nanocrystals. J. Electron. Mater. 2018, 47, 3741–3748. [Google Scholar] [CrossRef]
- Xu, Z.; Li, C.; Fu, N.; Li, W.; Zhang, G. Facile synthesis of Mn-doped TiO2 nanotubes with enhanced visible light photocatalytic activity. J. Appl. Electrochem. 2018, 48, 1197–1203. [Google Scholar] [CrossRef]
- Jeon, M.S.; Yoon, W.S.; Joo, H.; Lee, T.K.; Lee, H. Preparation and characterization of a nano-sized Mo/Ti mixed photocatalyst. Appl. Surf. Sci. 2000, 165, 209–216. [Google Scholar] [CrossRef]
- Manzoor, M.; Rafiq, A.; Ikram, M.; Nafees, M.; Ali, S. Structural. optical, and magnetic study of Ni-doped TiO2 nanoparticles synthesized by sol–gel method. Int. Nano Lett. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Pankratova, V.; Popov, A.I.; Pankratov, V. Study of phase composition. photocatalytic activity, and photoluminescence of TiO2 with Eu additive produced by the extraction-pyrolytic method. J. Mater. Res. Technol. 2021, 13, 2350–2360. [Google Scholar] [CrossRef]
- Slimani, Y.; Almessiere, M.A.; Mohamed, M.J.; Hannachi, E.; Caliskan, S.; Akhtar, S.; Baykal, A.; Gondal, M.A. Synthesis of Ce and Sm Co-Doped TiO2 Nanoparticles with Enhanced Photocatalytic Activity for Rhodamine B Dye Degradation. Catalysts 2023, 13, 668. [Google Scholar] [CrossRef]
- Nair, P.B.; Justinvictor, V.B.; Daniel, G.P.; Joy, K.; Ramakrishnan, V.; Kumar, D.D.; Thomas, P.V. Structural. optical, photoluminescence and photocatalytic investigations on Fe doped TiO2 thin films. Thin Solid Films 2014, 550, 121–127. [Google Scholar] [CrossRef]
- Singh, A.P.; Kumari, S.; Shrivastav, R.; Dass, S.; Satsangi, V.R. Iron doped nanostructured TiO2 for photoelectrochemical generation of hydrogen. Int. J. Hydrog. Energy 2008, 33, 5363–5368. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Y.; Gu, F.; Wu, M.; Xie, Y.; Zhang, J. Influence of Fe ions in characteristics and optical properties of mesoporous titanium oxide thin films. Appl. Surf. Sci. 2009, 256, 85–89. [Google Scholar] [CrossRef]
- Ali, T.; Tripathi, P.; Azam, A.; Raza, W.; Ahmed, A.S.; Ahmed, A.; Muneer, M. Photocatalytic performance of Fe-doped TiO2 nanoparticles under visible-light irradiation. Mater. Res. Express 2017, 4, 015022. [Google Scholar] [CrossRef]
- Komaraiah, D.; Radha, E.; Kalarikkal, N.; Sivakumar, J.; Reddy, M.V.R.; Sayanna, R. Structural. optical and photoluminescence studies of sol-gel synthesized pure and iron doped TiO2 photocatalysts. Ceram. Int. 2019, 45, 25060–25068. [Google Scholar] [CrossRef]
- Ranjit, K.T.; Viswanathan, B. Synthesis. characterization and photocatalytic properties of iron-doped TiO2 catalysts. J. Photochem. Photobiol. A Chem. 1997, 108, 79–84. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Zakaria, R.; Ying, J.Y. Role of Particle Size in Nanocrystalline TiO2-Based Photocatalysts. J. Phys. Chem. B 1998, 102, 10871–10878. [Google Scholar] [CrossRef]
- Shi, J.; Chen, G.; Zeng, G.; Chen, A.; He, K.; Huang, Z.; Hu, L.; Zeng, J.; Wu, J.; Liu, W. Hydrothermal synthesis of graphene wrapped Fe-doped TiO2 nanospheres with high photocatalysis performance. Ceram. Int. 2018, 44, 7473–7480. [Google Scholar] [CrossRef]
- Aba-Guevara, C.G.; Medina-Ramírez, I.E.; Hernández-Ramírez, A.; Jáuregui-Rincón, J.; Lozano-Álvarez, J.A.; Rodríguez-López, J.L. Comparison of two synthesis methods on the preparation of Fe, N-Co-doped TiO2 materials for degradation of pharmaceutical compounds under visible light. Ceram. Int. 2017, 43, 5068–5079. [Google Scholar] [CrossRef]
- Dholam, R.; Patel, N.; Adami, M.; Miotello, A. Hydrogen production by photocatalytic water-splitting using Cr- or Fe-doped TiO2 composite thin films photocatalyst. Int. J. Hydrog. Energy 2009, 34, 5337–5346. [Google Scholar] [CrossRef]
- Sobczyk-Guzenda, A.; Owczarek, S.; Szymanowski, H.; Volesky, L.; Walkowiak, B.; Miszczak, S.; Gazicki-Lipman, M. Iron doped thin TiO2 films synthesized with the RF PECVD method. Ceram. Int. 2015, 41, 7496–7500. [Google Scholar] [CrossRef]
- Bally, A.R.; Korobeinikova, E.N.; Schmid, P.E.; Lévy, F.; Bussy, F. Structural and electrical properties of Fe-doped TiO2 thin films. J. Phys. D Appl. Phys. 1998, 31, 1149–1154. [Google Scholar] [CrossRef]
- Saadati, H.; Raissi, B.; Riahifar, R.; Yaghmaee, M.S. How preparation of suspensions affects the electrophoretic deposition phenomenon. J. Eur. Ceram. Soc. 2016, 36, 299–305. [Google Scholar] [CrossRef]
- Navidirad, M.; Raissi, B.; Riahifar, R.; Yaghmaee, M.S.; Kazemzadeh, A. Effect of polyethylenimine on electrophoretic deposition of TiO2 nanoparticles in alternating current electric field. J. Mater. Sci. Mater. Electron. 2014, 25, 5041–5050. [Google Scholar] [CrossRef]
- Raissi, B.; Riahifar, R.; Yaghmaee, M.S.; Taati-Asil, F.; Aghaei, A.; Chatrnoor, S.; Taghadossi, A.H.; Irankhah, R.; Karimi, M. DC-EPD of nanoceramic particles accelerated via anodic dissolution in organic media. Int. J. Mater. Res. 2018, 109, 356–361. [Google Scholar] [CrossRef]
- Ahmadi, S.S.S.; Raissi, B.; Riahifar, R.; Yaghmaee, M.S.; Saadati, H.R.; Ghashghaie, S.; Javaheri, M. Fabrication of counter electrode of electrochemical CO gas sensor by electrophoretic deposition of MWCNT. J. Electrochem. Soc. 2015, 162, D3101–D3108. [Google Scholar] [CrossRef]
- Narkevica, I.; Stradina, L.; Stipniece, L.; Jakobsons, E.; Ozolins, J. Electrophoretic deposition of nanocrystalline TiO2 particles on porous TiO2-x ceramic scaffolds for biomedical applications. J. Eur. Ceram. Soc. 2017, 37, 3185–3193. [Google Scholar] [CrossRef]
- Ishii, K.; Matsunaga, C.; Kobayashi, K.; Stevenson, A.J.; Tardivat, C.; Uchikoshi, T. Fabrication of BSCF-based mixed ionic-electronic conducting membrane by electrophoretic deposition for oxygen separation application. J. Eur. Ceram. Soc. 2019, 39, 5292–5297. [Google Scholar] [CrossRef]
- Mercier, H.; Malič, B.; Uršič, H.; Hreščak, J.; Levassort, F.; Kuscer, D. Electrophoretic deposition and properties of strontium-doped sodium potassium niobate thick films. J. Eur. Ceram. Soc. 2017, 37, 5305–5313. [Google Scholar] [CrossRef]
- Ahmadi, S.S.S.; Riahifar, R.; Raissi, B.; Yaghmaee, M.S.; Metselaar, H.S.C.; Javaheri, M. Electrophoretic deposition of MWCNT on a PTFE layer for making working electrode of oxygen sensor. J. Electrochem. Soc. 2017, 164, B506–B512. [Google Scholar] [CrossRef]
- Mishyn, V.; Aspermair, P.; Leroux, Y.; Happy, H.; Knoll, W.; Boukherroub, R.; Szunerits, S. “Click” Chemistry on Gold Electrodes Modified with Reduced Graphene Oxide by Electrophoretic Deposition. Surfaces 2019, 2, 193–204. [Google Scholar] [CrossRef]
- Van Dao, D.; Adilbish, G.; Le, T.D.; Lee, I.H.; Yu, Y.T. Triple phase boundary and power density enhancement in PEMFCs of a Pt/C electrode with double catalyst layers. RSC Adv. 2019, 9, 15635–15641. [Google Scholar] [CrossRef]
- Babadi, P.; Sadreddini, S.; Mohsenifar, F.; Roshani, M. The Influence of Electrophoretic Potential on Ni–Al2O3 Nano-Composite Coating. Prot. Met. Phys. Chem. Surfaces. 2016, 52, 249–253. [Google Scholar] [CrossRef]
- Kamada, K.; Maehara, K.; Mukai, M.; Ida, S.; Matsumoto, Y. Fabrication of metal oxide-diamond composite films by electrophoretic deposition and anodic dissolution. J. Mater. Res. 2003, 18, 2826–2831. [Google Scholar] [CrossRef]
- Moreno, C.H.A.; Cocke, D.L.; Gromes, J.A.; Morkovsky, P.; Parga, J.R.; Peterson, E.; Garcia, C. Electrochemical reactions for electrocoagulation using iron electrodes. Ind. Eng. Chem. Res. 2009, 48, 2275–2282. [Google Scholar] [CrossRef]
- Jackman, L.M.; Lange, B.C. Structure and Reactivity of Alkali Metal Enolates. Tetrahedron 1977, 22, 2737–2769. [Google Scholar] [CrossRef]
- Negishi, H.; Endo, A.; Ohraori, T.; Sakaki, K. Fabrication of mesoporous silica coating by electrophoretic deposition. Ind. Eng. Chem. Res. 2008, 47, 7236–7241. [Google Scholar] [CrossRef]
- Ishihara, T.; Shimose, K.; Shiomitsu, T.; Takita, Y. Electrophoretic Deposition of Y2O3-Stabilized ZrO2 on the Porous La0.8Sr0.2MnO3 Cathode Substrate for SOFC. ECS Proc. Vol. 1995, 1995, 334–343. [Google Scholar] [CrossRef]
- Karthik, T.; Rathinamoorthy, R.; Murugan, R. Enhancement of wrinkle recovery angle of cotton fabric using citric acid cross-linking agent with nano-TiO2 as a co-catalyst. J. Ind. Text. 2012, 42, 99–117. [Google Scholar] [CrossRef]
- Hajipour, F.; Asad, S.; Amoozegar, M.A.; Javidparvar, A.A.; Tang, J.; Zhong, H.; Khajeh, K. Developing a fluorescent hybrid nanobiosensor based on quantum dots and azoreductase enzyme formethyl red monitoring. Iran. Biomed. J. 2021, 25, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.M.; Badrinarayanan, S.; Sastry, M. Nanocrystalline TiO2 studied by optical. FTIR and X-ray photoelectron spectroscopy: Correlation to presence of surface states. Thin Solid Films. 2000, 358, 122–130. [Google Scholar] [CrossRef]
- Yu, J.; Yu, H.; Ao, C.H.; Lee, S.C.; Yu, J.C.; Ho, W. Preparation. characterization and photocatalytic activity of in situ Fe-doped TiO2 thin films. Thin Solid Films. 2006, 496, 273–280. [Google Scholar] [CrossRef]
- Bargui, M.; Messaoud, M.; Elleuch, K. Electrophoretic impregnation of porous anodizing layer by synthesized TiO2 nanoparticles. Surf. Eng. Appl. Electrochem. 2017, 53, 467–474. [Google Scholar] [CrossRef]
- Liu, M.; Piao, L.; Zhao, L.; Ju, S.; Yan, Z.; He, T.; Zhou, C.; Wang, W. Anatase TiO2 single crystals with exposed {001} and {110} facets: Facile synthesis and enhanced photocatalysis. Chem. Commun. 2010, 46, 1664–1666. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Gao, L. Enhancing the UV inducing hydrophilicity of TiO2 thin film by doping Fe ions. Mater. Chem. Phys. 2002, 77, 878–881. [Google Scholar] [CrossRef]
- Chin, M.; Jiuan, H.; Syh, T. Characteristics and optical properties of iron ion (Fe3+)-doped titanium oxide thin films prepared by a sol–gel spin coating. J. Alloys Compd. 2009, 473, 394–400. [Google Scholar] [CrossRef]
- Naceur, J.B.; Mechiakh, R.; Bousbih, F.; Chtourou, R. Applied Surface Science Influences of the iron ion (Fe3+)-doping on structural and optical properties of nanocrystalline TiO2 thin films prepared by sol–gel spin coating. Appl. Surf. Sci. 2011, 257, 10699–10703. [Google Scholar] [CrossRef]
- Lee, T.H.; Ryu, H.; Lee, W.J. Photoelectrochemical properties of iron (III)-doped TiO2 nanorods. Ceram. Int. 2015, 41, 7582–7589. [Google Scholar] [CrossRef]
- Zhu, J.; Zheng, W.; He, B.; Zhang, J.; Anpo, M. Characterization of Fe–TiO2 photocatalysts synthesized by hydrothermal method and their photocatalytic reactivity for photodegradation of XRG dye diluted in water. J. Mol. Catal. A Chem. 2004, 216, 35–43. [Google Scholar] [CrossRef]
- Hamaker, H.C.; Verwey, E.J. Part II.—(C) Colloid stability. The Role of The Forces Between The Particles in Electrodeposition and Other Phenomena. Trans. Faraday Soc. 1940, 35, 180–185. [Google Scholar] [CrossRef]
- Abudalazez, A.M.A.; Kasim, S.R.; Ariffin, A.B.; Ahmad, Z.A. Effect of the solid concentration in the suspension on electrophoretic deposition (EPD) coating parameters. Int. J. Eng. Res. Africa. 2012, 8, 47–54. [Google Scholar] [CrossRef]
- Ferrari, B.; Moreno, R. EPD kinetics: A review. J. Eur. Ceram. Soc. 2010, 30, 1069–1078. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]


| Applied Voltage (V) | Dissolved Fe Ion (ppm) in Different Medias | |||
|---|---|---|---|---|
| EtOH | Methanol | acac/EtOH (without I2) | acac/EtOH (with I2) | |
| 100 | 0 | 0 | 72 | 410 |
| 200 | 0 | 1 | 107 | 640 |
| 300 | 1.3 | 1.3 | 121 | 720 |
| 400 | 2.5 | 3.1 | 153 | 780 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatrnoor, S.; Taghaddosi, A.; Alem, S.A.A.; Taati-Asil, F.; Raissi, B.; Riahifar, R.; Sahba Yaghmaee, M. Fe Doping in TiO2 via Anodic Dissolution of Iron: Synthesis, Characterization, and Electrophoretic Deposition on a Metal Substrate. Ceramics 2023, 6, 1251-1262. https://doi.org/10.3390/ceramics6020076
Chatrnoor S, Taghaddosi A, Alem SAA, Taati-Asil F, Raissi B, Riahifar R, Sahba Yaghmaee M. Fe Doping in TiO2 via Anodic Dissolution of Iron: Synthesis, Characterization, and Electrophoretic Deposition on a Metal Substrate. Ceramics. 2023; 6(2):1251-1262. https://doi.org/10.3390/ceramics6020076
Chicago/Turabian StyleChatrnoor, Sara, Amirhossein Taghaddosi, Sayed Ali Ahmad Alem, Fatemeh Taati-Asil, Babak Raissi, Reza Riahifar, and Maziar Sahba Yaghmaee. 2023. "Fe Doping in TiO2 via Anodic Dissolution of Iron: Synthesis, Characterization, and Electrophoretic Deposition on a Metal Substrate" Ceramics 6, no. 2: 1251-1262. https://doi.org/10.3390/ceramics6020076
APA StyleChatrnoor, S., Taghaddosi, A., Alem, S. A. A., Taati-Asil, F., Raissi, B., Riahifar, R., & Sahba Yaghmaee, M. (2023). Fe Doping in TiO2 via Anodic Dissolution of Iron: Synthesis, Characterization, and Electrophoretic Deposition on a Metal Substrate. Ceramics, 6(2), 1251-1262. https://doi.org/10.3390/ceramics6020076