Compositional Analysis of SiOC(H) Powders: A Comparison of X-ray Photoelectron Spectroscopy (XPS) and Combustion Analysis
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Characterization
3. Results and Discussion
3.1. Comparison of Chemical Analysis by XPS and Combustion
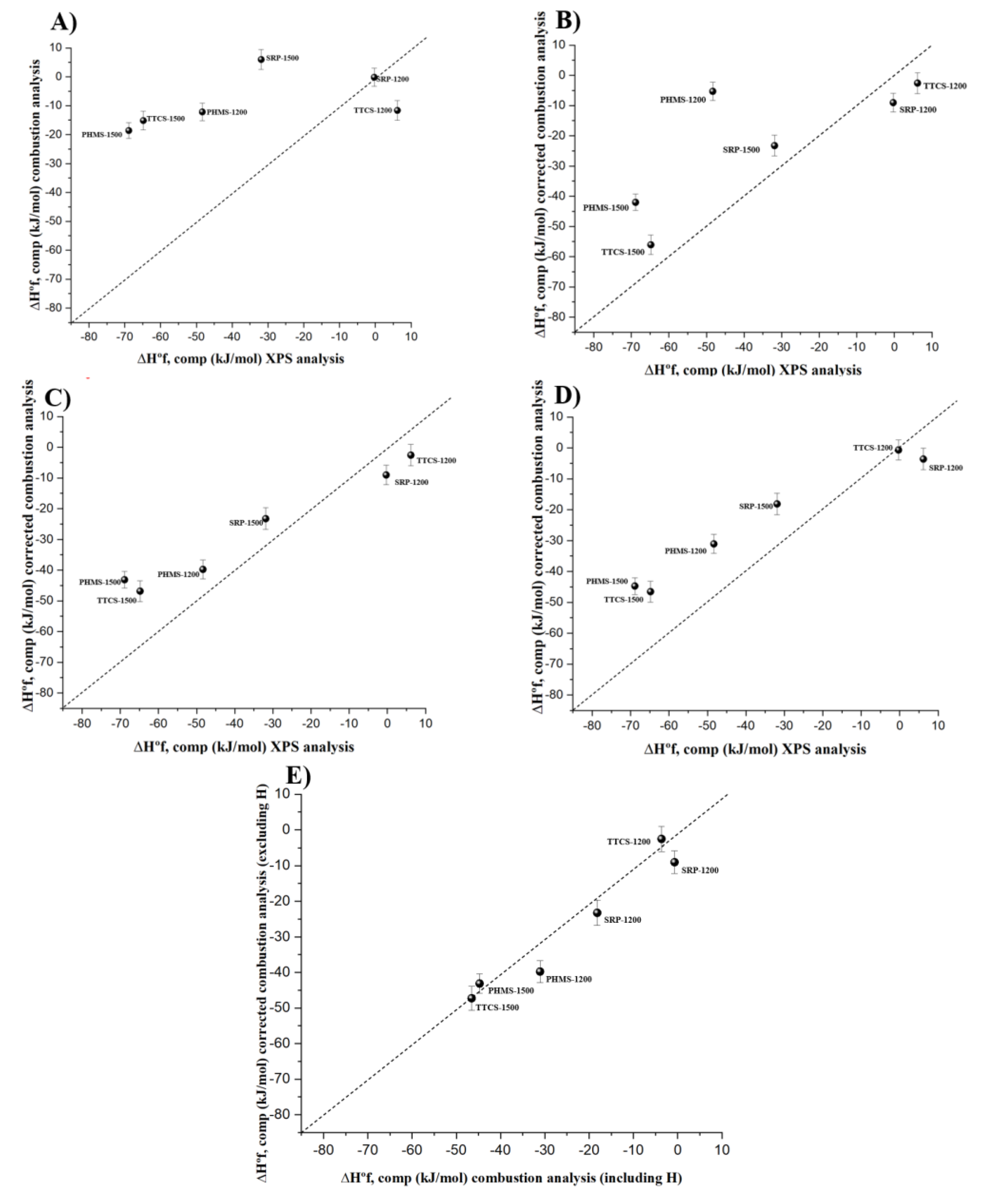
3.2. Thermochemical Calculations Using Differently Analyzed Chemical Compositions
a SiO2 (s, 800 °C) + c CO2 (g, 800 °C) + (d/2) H2O (g, 800 °C) ∆Hdis, SiOC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bozeman, T.B.; Lemon, R.R.; Eleazer, P.D. Elemental analysis of crystal precipitate from gray and white MTA. J. Endod. 2006, 32, 425–428. [Google Scholar] [CrossRef]
- Ninomiya, K.; Kubo, M.K.; Nagatomo, T.; Higemoto, W.; Ito, T.U.; Kawamura, N.; Strasser, P.; Shimomura, K.; Miyake, Y.; Suzuki, T.; et al. Nondestructive elemental depth-profiling analysis by muonic X-Ray measurement. Anal. Chem. 2015, 87, 4597–4600. [Google Scholar] [CrossRef] [PubMed]
- Nowak, S.; Winter, M. Elemental analysis of lithium ion batteries. J. Anal. At. Spectrom. 2017, 32, 1833–1847. [Google Scholar] [CrossRef]
- Atzei, D.; Fantauzzi, M.; Rossi, A.; Fermo, P.; Piazzalunga, A.; Valli, G.; Vecchi, R. Surface chemical characterization of PM10 samples by XPS. Appl. Surf. Sci. 2014, 307, 120–128. [Google Scholar] [CrossRef]
- Aquisman, A.E.; Assim, Z.B.; Wahi, R.B.; Kwabena, D.E.; Festus, W. Validation of the atomic absorption spectroscopy (AAS) for heavy metal analysis and geochemical exploration of sediment samples from the Sebangan river. Adv. Anal. Chem. 2019, 9, 23–33. [Google Scholar]
- Faubel, W.; Staub, S.; Simon, R.; Heissler, S.; Pataki, A.; Banik, G. Non-destructive analysis for the investigation of decomposition phenomena of historical manuscripts and prints. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 669–676. [Google Scholar] [CrossRef]
- Millett, E.J. Progress in the Analysis of Crystalline Solids. J. Cryst. Growth 1980, 48, 666–682. [Google Scholar] [CrossRef]
- Sujith, R.; Jothi, S.; Zimmermann, A.; Aldinger, F.; Kumar, R. Mechanical behaviour of polymer derived ceramics—A Review. Int. Mater. Rev. 2021, 66, 426–449. [Google Scholar] [CrossRef]
- Ma, B.; Cao, Y.; Gao, Y.; Wang, Y. Fabrication of a thin double-layer thermistor based on DVB-modified polymer-derived SiCN ceramics. J. Alloys Compd. 2018, 732, 491–497. [Google Scholar] [CrossRef]
- Ionescu, E.; Mera, G.; Riedel, R. Polymer-derived ceramics (PDCs): Materials design towards applications at ultrahigh-temperatures and in extreme environments. In Nanotechnology: Concepts, Methodologies, Tools, and Applications; Information Resources Management Association; Technische Universität Darmstadt: Darmstadt, Germany, 2014; pp. 1108–1139. [Google Scholar]
- Colombo, P.; Mera, G.; Riedel, R.; Sorarù, G.D. Polymer-derived ceramics: 40 years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Terauds, K.; Sanchez-Jimenez, P.E.; Raj, R.; Vakifahmetoglu, C.; Colombo, P. Giant Piezoresistivity of polymer-derived ceramics at high temperatures. J. Eur. Ceram. Soc. 2010, 30, 2203–2207. [Google Scholar] [CrossRef]
- Riedel, R.; Mera, G.; Hauser, R.; Klonczynski, A. Silicon-Based Ceramics Derived from Polymers-Review on Synthesis, Properties and Applications. J. Ceram. Soc. Jpn. 2006, 114, 425–444. [Google Scholar] [CrossRef]
- Duan, W.; Yin, X.; Li, Q.; Schlier, L.; Greil, P.; Travitzky, N. A Review of absorption properties in silicon-based polymer derived ceramics. J. Eur. Ceram. Soc. 2016, 36, 3681–3689. [Google Scholar] [CrossRef]
- Wen, Q.; Yu, Z.; Riedel, R. The fate and role of in situ formed carbon in polymer-derived ceramics. Prog. Mater. Sci. 2020, 109, 100623. [Google Scholar] [CrossRef]
- Ionescu, E.; Kleebe, H.-J.; Riedel, R. Silicon-containing polymer-derived ceramic nanocomposites (PDC-NCs): Preparative approaches and properties. Chem. Soc. Rev. 2012, 41, 5032–5052. [Google Scholar] [CrossRef]
- Ushakov, S.V.; Maram, P.S.; Kapush, D.; Pavlik, A.J.; Fyhrie, M.; Gallington, L.C.; Benmore, C.J.; Weber, R.; Neuefeind, J.C.; McMurray, J.W.; et al. Phase transformations in oxides above 2000 °C: Experimental technique development. Adv. Appl. Ceram. 2018, 117, s82–s89. [Google Scholar] [CrossRef]
- Ionescu, E.; Bernard, S.; Lucas, R.; Kroll, P.; Ushakov, S.; Navrotsky, A.; Riedel, R. Polymer-derived ultra-high temperature ceramics (UHTCs) and related materials. In Ceramics, Glass and Glass-Ceramics: From Early Manufacturing Steps Towards Modern Frontiers; Baino, F., Tomalino, M., Tulyaganov, D., Eds.; PoliTO Springer Series; Springer International Publishing: Cham, Switzerland, 2021; pp. 281–323. [Google Scholar]
- David, L.; Bhandavat, R.; Barrera, U.; Singh, G. Silicon oxycarbide glass-graphene composite paper electrode for long-cycle lithium-ion batteries. Nat. Commun. 2016, 7, 10998. [Google Scholar] [CrossRef]
- Bhandavat, R.; Singh, G. Stable and efficient Li-ion battery anodes prepared from polymer-derived silicon oxycarbide–carbon nanotube shell/core composites. J. Phys. Chem. C 2013, 117, 11899–11905. [Google Scholar] [CrossRef]
- Vakifahmetoglu, C.; Zeydanli, D.; Colombo, P. Porous polymer derived ceramics. Mater. Sci. Eng. R. Rep. 2016, 106, 1–30. [Google Scholar] [CrossRef]
- Leonel, G.J.; Mujib, S.B.; Singh, G.; Navrotsky, A. Thermodynamic stabilization of crystalline silicon carbide polymer-derived ceramic fibers. Int. J. Ceram. Eng. Sci. 2022, 4, 315–326. [Google Scholar] [CrossRef]
- Sugie, C.; Navrotsky, A.; Lauterbach, S.; Kleebe, H.-J.; Mera, G. Structure and thermodynamics of silicon oxycarbide polymer-derived ceramics with and without mixed-bonding. Materials 2021, 14, 4075. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Yu, Z.; Riedel, R.; Ionescu, E. Single-source-precursor synthesis and high-temperature evolution of a boron-containing SiC/HfC ceramic nano/micro composite. J. Eur. Ceram. Soc. 2021, 41, 3002–3012. [Google Scholar] [CrossRef]
- Reinold, L.M.; Graczyk-Zajac, M.; Gao, Y.; Mera, G.; Riedel, R. Carbon-rich SiCN ceramics as high capacity/high stability anode material for lithium-ion batteries. J. Power Sources 2013, 236, 224–229. [Google Scholar] [CrossRef]
- Widgeon, S.; Mera, G.; Gao, Y.; Sen, S.; Navrotsky, A.; Riedel, R. Effect of precursor on speciation and nanostructure of SiBCN polymer-derived ceramics. J. Am. Ceram. Soc. 2013, 96, 1651–1659. [Google Scholar] [CrossRef]
- Bhandavat, R.; Singh, G. Synthesis, Characterization, and high temperature stability of Si(B)CN-coated carbon nanotubes using a boron-modified poly(ureamethylvinyl)silazane chemistry. J. Am. Ceram. Soc. 2012, 95, 1536–1543. [Google Scholar] [CrossRef]
- Poerschke, D.L.; Braithwaite, A.; Park, D.; Lauten, F. Crystallization behavior of polymer-derived Si-O-C for ceramic matrix composite processing. Acta Mater. 2018, 147, 329–341. [Google Scholar] [CrossRef]
- Daccà, A.; Gemme, G.; Mattera, L.; Parodi, R. XPS Analysis of the Surface composition of niobium for superconducting RF cavities. Appl. Surf. Sci. 1998, 126, 219–230. [Google Scholar] [CrossRef]
- Hooshmand, T.; Daw, R.; van Noort, R.; Short, R.D. XPS Analysis of the surface of leucite-reinforced feldspathic ceramics. Dent. Mater. 2001, 17, 1–6. [Google Scholar] [CrossRef]
- Sarkar, S.; Chunder, A.; Fei, W.; An, L.; Zhai, L. Superhydrophobic mats of polymer-derived ceramic fibers. J. Am. Ceram. Soc. 2008, 91, 2751–2755. [Google Scholar] [CrossRef]
- Stojilovic, N. Why can’t we see hydrogen in X-ray photoelectron spectroscopy? J. Chem. Educ. 2012, 89, 1331–1332. [Google Scholar] [CrossRef]
- Guo, X.; Szenknect, S.; Mesbah, A.; Labs, S.; Clavier, N.; Poinssot, C.; Ushakov, S.V.; Curtius, H.; Bosbach, D.; Ewing, R.C.; et al. Thermodynamics of formation of coffinite, USiO4. Proc. Natl. Acad. Sci. USA 2015, 112, 6551–6555. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, A.H.; Armentrout, M.M.; Narisawa, M.; Sen, S.; Navrotsky, A. White Si–O–C ceramic: Structure and thermodynamic stability. J. Am. Ceram. Soc. 2015, 98, 242–246. [Google Scholar] [CrossRef]
- Tavakoli, A.H.; Golczewski, J.A.; Bill, J.; Navrotsky, A. Effect of boron on the thermodynamic stability of amorphous polymer-derived Si(B)CN ceramics. Acta Mater. 2012, 60, 4514–4522. [Google Scholar] [CrossRef]
- Niu, M.; Wang, H.; Chen, J.; Su, L.; Wu, D.; Navrotsky, A. Structure and energetics of SiOC and SiOC-modified carbon-bonded carbon fiber composites. J. Am. Ceram. Soc. 2017, 100, 3693–3702. [Google Scholar] [CrossRef]
- Tavakoli, A.H.; Campostrini, R.; Gervais, C.; Babonneau, F.; Bill, J.; Sorarù, G.D.; Navrotsky, A. Energetics and structure of polymer-derived Si–(B–)O–C glasses: Effect of the boron content and pyrolysis temperature. J. Am. Ceram. Soc. 2014, 97, 303–309. [Google Scholar] [CrossRef]
- Navrotsky, A. Progress and new directions in calorimetry: A 2014 perspective. J. Am. Ceram. Soc. 2014, 97, 3349–3359. [Google Scholar] [CrossRef]
- Widgeon, S.; Mera, G.; Gao, Y.; Stoyanov, E.; Sen, S.; Navrotsky, A.; Riedel, R. Nanostructure and energetics of carbon-rich SiCN ceramics derived from polysilylcarbodiimides: Role of the nanodomain interfaces. Chem. Mater. 2012, 24, 1181–1191. [Google Scholar] [CrossRef]
- Shen, C.; Barrios, E.; Zhai, L. Bulk polymer-derived ceramic composites of graphene oxide. ACS Omega 2018, 3, 4006–4016. [Google Scholar] [CrossRef]
- Sreeja, R.; Jayalatha, T.; Devapal, D. Silicon oxycarbide (SiOC) foam from methylphenylpoly(silsesquioxane)(PS) by direct foaming technique. J. Porous Mater. 2022. [Google Scholar] [CrossRef]
- Sun, X.; Yang, G.; Tian, Z.; Zhu, W.; Su, D. In-Situ Formation of titanium carbide in carbon-rich silicon oxycarbide ceramic for enhanced thermal stability. J. Eur. Ceram. Soc. 2022, 42, 6935–6941. [Google Scholar] [CrossRef]
- Seo, W.-S.; Koumoto, K. Stacking Faults in β-SiC Formed during Carbothermal Reduction of SiO2. J. Am. Ceram. Soc. 1996, 79, 1777–1782. [Google Scholar] [CrossRef]
- Berger, L.-M.; Gruner, W.; Langholf, E.; Stolle, S. On the mechanism of carbothermal reduction processes of TiO2 and ZrO2. Int. J. Refract. Met. Hard Mater. 1999, 17, 235–243. [Google Scholar] [CrossRef]

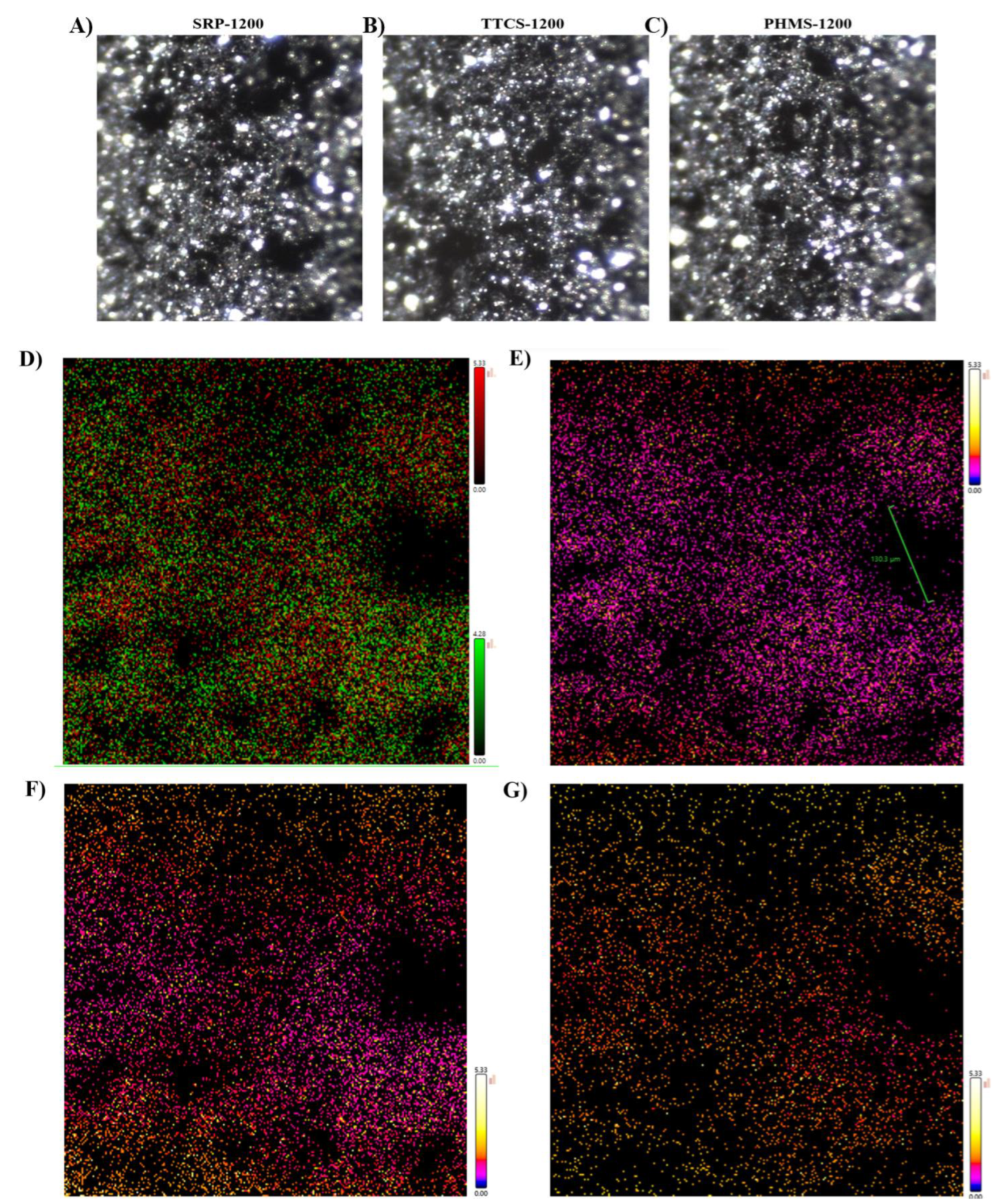
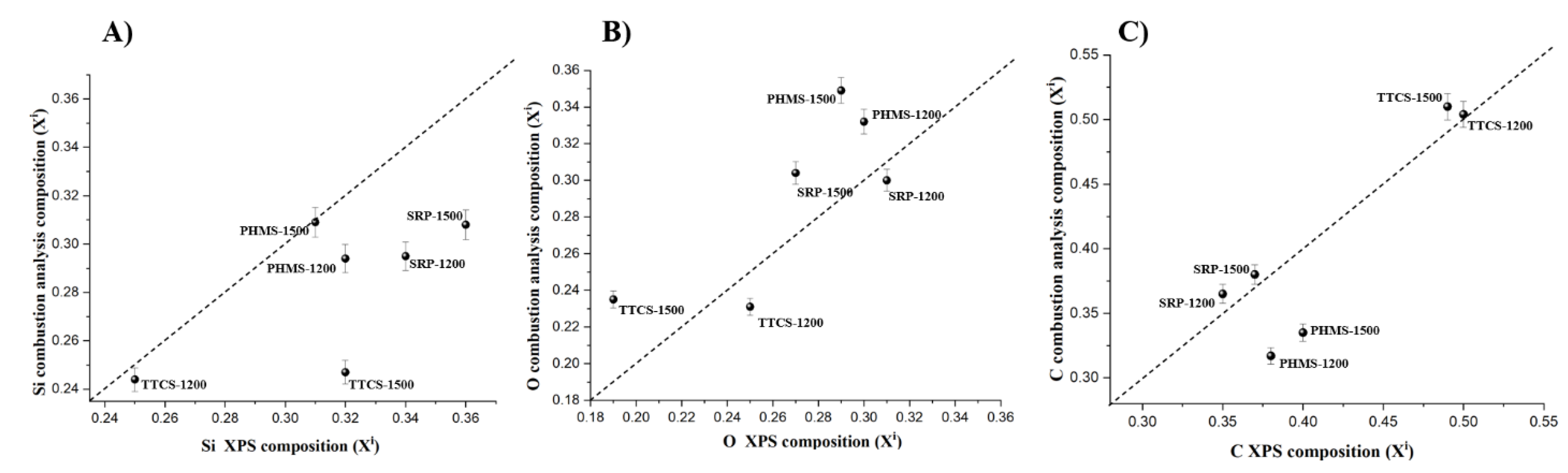
| Elemental Composition by Combustion Analysis | ||||
|---|---|---|---|---|
| Elements (at.%) | ||||
| SiOC Sample | C | O | Si | H |
| SRP-1200 | 36.54 ± 0.35 | 30.0 ± 0.17 | 29.52 | 3.94 ± 0.02 |
| SRP-1500 | 37.96 ± 0.34 | 30.40 ± 0.30 | 30.79 | 0.85 ± 0.01 |
| TTCS-1200 | 50.45 ± 0.48 | 23.09 ± 0.155 | 24.45 | 2.01 ± 0.02 |
| TTCS-1500 | 50.97 ± 0.49 | 23.47 ± 0.18 | 24.75 | 0.81 ± 0.08 |
| PHMS-1200 | 31.75 ± 0.30 | 33.24 ± 0.30 | 29.37 | 5.68 + 0.05 |
| PHMS-1500 | 33.54 ± 0.34 | 34.88 ± 0.34 | 30.94 | 0.67 ± 0.07 |
| Elemental Composition by XPS | |||
|---|---|---|---|
| Elements (at.%) | |||
| SiOC Sample | C1s | O1s | Si2p |
| SRP-1200 | 35.47 ± 0.55 | 30.56 ± 0.60 | 33.97 ± 0.05 |
| SRP-1500 | 37.12 ± 0.02 | 27.32 ± 0.74 | 35.56 ± 0.71 |
| TTCS-1200 | 50.28 ± 0.22 | 25.29 ± 0.62 | 24.43 ± 0.39 |
| TTCS-1500 | 48.76 ± 0.52 | 19.37 ± 0.05 | 31.87 ± 0.47 |
| PHMS-1200 | 38.16 ± 0.30 | 30.25 ± 1.00 | 31.62 ± 0.70 |
| PHMS-1500 | 40.35 ± 1.09 | 28.57 ± 0.03 | 31.15 ± 0.09 |
| Sample | Composition SixOyCz (x + y + z = 1) | ∆Hdis (kJ.mol−1) | ∆H°f, elem (kJ.mol−1) | ∆H°f, comp (kJ.mol−1) |
|---|---|---|---|---|
| SRP-1200 | Si0.34O0.31C0.35 | −275.41 ± 2.40 | −154.40 ± 3.19 | −0.31 ± 3.23 |
| TTCS-1200 | Si0.25O0.25C0.50 | −291.94 ± 2.77 | −116.35 ± 3.48 | +6.16 ± 3.51 |
| PHMS-1200 | Si0.32O0.30C0.38 | −226.92 ± 2.21 | −197.55 ± 3.05 | −48.34 ± 3.09 |
| SRP-1500 | Si0.36O0.27C0.37 | −284.91 ± 2.78 | −170.12 ± 3.50 | −31.88 ± 3.52 |
| TTCS-1500 | Si0.32O0.19C0.49 | −299.76 ± 2.60 | −167.29 ± 3.35 | −64.80 ± 3.38 |
| PHMS-1500 | Si0.31O0.29C0.40 | −210.21 ± 1.70 | −212.43 ± 2.71 | −68.87 ± 2.74 |
| Sample | Composition SiwOxCyHz (w + x + y + z = 1) | ∆Hdis (kJ.mol−1) | ∆H°f, elem (kJ.mol−1) | ∆H°f, comp (kJ.mol−1) |
|---|---|---|---|---|
| SRP-1200 | Si0.295O0.300C0.365H0.04 | −256.23 ± 2.23 | −144.18 ± 3.07 | −0.173 ± 3.10 |
| TTCS-1200 | Si0.244O0.231C0.504H0.021 | −283.70 ± 2.70 | −123.43 ± 3.42 | −11.65 ± 3.45 |
| PHMS-1200 | Si0.294O0.332C0.317H0.057 | −214.20 ± 2.09 | −168.54 ± 2.97 | −12.16 ± 3.0 |
| SRP-1500 | Si0.308O0.304C0.380H0.008 | −271.47 ± 2.65 | −142.50 ± 3.50 | +5.9828 ± 3.42 |
| TTCS-1500 | Si0.247O0.235C0.510H0.008 | −280.33 ± 2.43 | −130.23 ± 2.62 | −15.14 ± 3.24 |
| PHMS-1500 | Si0.309O0.349C0.335H0.007 | −210.51 ± 1.70 | −186.51 ± 2.70 | −18.61 ± 2.74 |
| Sample | Composition SixOyCz (x + y + z = 1) | ∆Hdis (kJ.mol−1) | ∆H°f, elem (kJ.mol−1) | ∆H°f, comp (kJ.mol−1) |
|---|---|---|---|---|
| SRP-1200 | Si0.34O0.30C0.36 | −274.82 ± 2.39 | −158.93 ± 3.18 | −9.03 ± 3.22 |
| TTCS-1200 | Si0.25O0.24C0.51 | −291.31 ± 2.76 | −120.95 ± 3.47 | −2.59 ± 3.50 |
| PHMS-1200 | Si0.32O0.35C0.33 | −229.37 ± 2.23 | −174.64 ± 3.07 | −5.29 ± 3.1 |
| SRP-1500 | Si0.36O0.28C0.36 | −285.43 ± 2.78 | −167.31 ± 3.49 | −23.25 ± 3.51 |
| TTCS-1500 | Si0.32O0.20C0.48 | −300.37 ± 2.60 | −162.74 ± 3.35 | −56.07 ± 3.37 |
| PHMS-1500 | Si0.31O0.31C0.34 | −205.57 ± 1.66 | −193.93 ± 2.68 | −42.02 ± 2.72 |
| Sample | Composition SixOyCz (x + y + z = 1) | ∆Hdis (kJ.mol−1) | ∆H°f, elem (kJ.mol−1) | ∆H°f, comp (kJ.mol−1) |
|---|---|---|---|---|
| SRP-1200 | Si0.34O0.30C0.36 | −274.82 ± 2.39 | −158.93 ± 3.18 | −9.03 ± 3.22 |
| TTCS-1200 | Si0.25O0.24C0.51 | −291.31 ± 2.76 | −120.95 ± 3.47 | −2.59 ± 3.50 |
| PHMS-1200 | Si0.32O0.31C0.37 | −227.39 ± 2.22 | −192.38 ± 3.06 | −39.78 ± 3.07 |
| SRP-1500 | Si0.36O0.28C0.36 | −285.43 ± 2.78 | −166.23 ± 3.49 | −23.25 ± 3.51 |
| TTCS-1500 | Si0.32O0.21C0.47 | −301.05 ± 2.61 | −158.12 ± 3.35 | −46.82 ± 3.36 |
| PHMS-1500 | Si0.31O0.32C0.37 | −211.59 ± 1.71 | −247.58 ± 2.71 | −43.13 ± 2.73 |
| Sample | Composition SixOyCz (x + y + z = 1) | ∆Hdis (kJ.mol−1) | ∆H°f, elem (kJ.mol−1) | ∆H°f, comp (kJ.mol−1) |
|---|---|---|---|---|
| SRP-1200 | Si0.34O0.305C0.315H0.04 | −270.97 ± 4.06 | −149.61 ± 4.57 | −0.70 ± 4.60 |
| TTCS-1200 | Si0.25O0.240C0.489H0.021 | −286.0 ± 2.76 | −120.59 ± 3.47 | −3.64 ± 3.50 |
| PHMS-1200 | Si0.32O0.316C0.307H0.057 | −219.61 ± 2.13 | −182.78 ± 3.0 | −31.09 ± 3.03 |
| SRP-1500 | Si0.36O0.287C0.345H0.008 | −283.17 ± 2.76 | −163.59 ± 3.47 | −18.16 ± 3.50 |
| TTCS-1500 | Si0.32O0.212C0.46H0.008 | −298.54 ± 2.59 | −157.72 ± 3.34 | −46.54 ± 3.37 |
| PHMS-1500 | Si0.31O0.319C0.364H0.007 | −209.388 ± 1.67 | −182.78 ± 2.69 | −44.73 ± 2.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonel, G.J.; Guo, X.; Singh, G.; Navrotsky, A. Compositional Analysis of SiOC(H) Powders: A Comparison of X-ray Photoelectron Spectroscopy (XPS) and Combustion Analysis. Ceramics 2023, 6, 74-85. https://doi.org/10.3390/ceramics6010006
Leonel GJ, Guo X, Singh G, Navrotsky A. Compositional Analysis of SiOC(H) Powders: A Comparison of X-ray Photoelectron Spectroscopy (XPS) and Combustion Analysis. Ceramics. 2023; 6(1):74-85. https://doi.org/10.3390/ceramics6010006
Chicago/Turabian StyleLeonel, Gerson J., Xin Guo, Gurpreet Singh, and Alexandra Navrotsky. 2023. "Compositional Analysis of SiOC(H) Powders: A Comparison of X-ray Photoelectron Spectroscopy (XPS) and Combustion Analysis" Ceramics 6, no. 1: 74-85. https://doi.org/10.3390/ceramics6010006
APA StyleLeonel, G. J., Guo, X., Singh, G., & Navrotsky, A. (2023). Compositional Analysis of SiOC(H) Powders: A Comparison of X-ray Photoelectron Spectroscopy (XPS) and Combustion Analysis. Ceramics, 6(1), 74-85. https://doi.org/10.3390/ceramics6010006







