An Experimental Study of the Effects of Low-Calcium Fly Ash on Type II Concrete
Abstract
:1. Introduction
- The reaction between the sulphate ions and C3A compound in the cement content is based on Equation (6), which will be faced with the volume enhancement by 220%.
- The chemical reaction of sulphate ions and calcium hydroxide formed in the cement hydration will be exposed with a 120% volume increase.
2. Methodology and Materials
- The assessment of compressive strength of GPC and conventional concretes throughout time.
- The influence of environmental factors (high temperature) on the formation of GPC concretes with the inclusion of T2PC and FA.
- A comparison of various concrete characteristics for GPC and PPC (workability/setting time/elasticity modules/tensile strength/flexural strength).
- A comparison of GPC and PPC water absorption (permeability).
- Determination of the optimal mix design for concrete mixes through comparing the dosages of FA and PC between the current experimental program and previous studies.
2.1. Materials
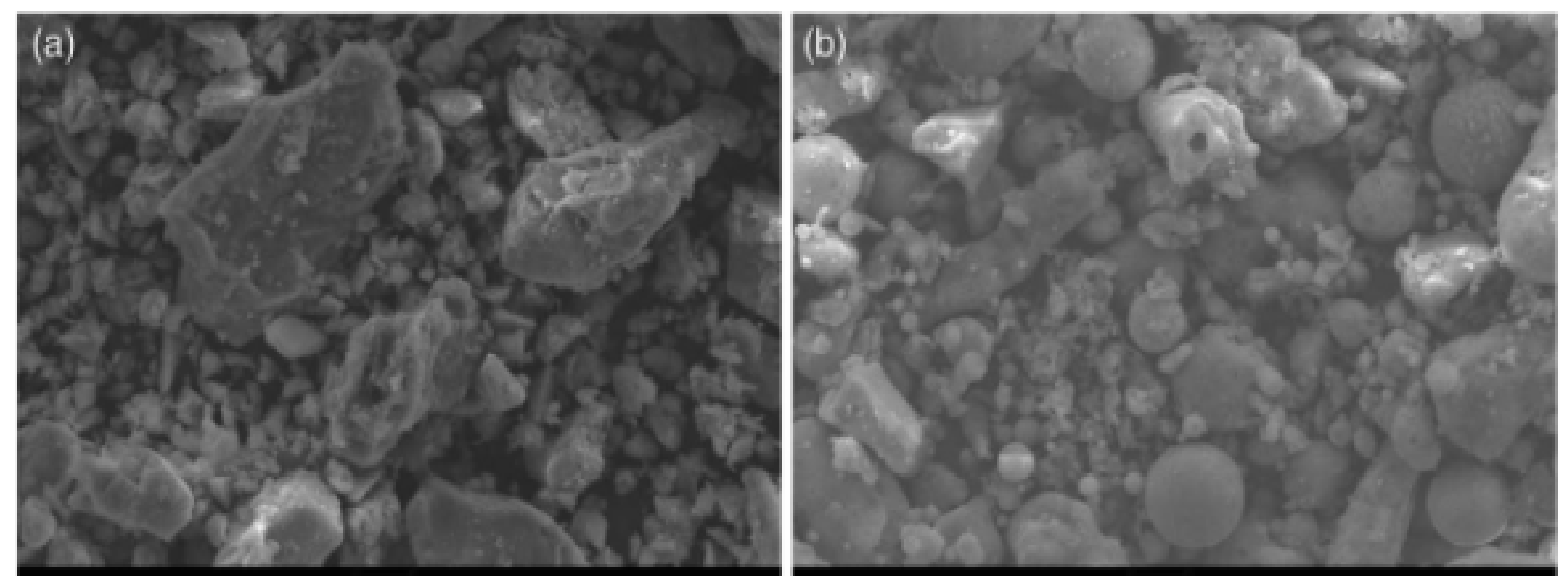
2.2. Scanning Electron Microscopy (SEM)
2.3. Experimental Design and Sieve Test
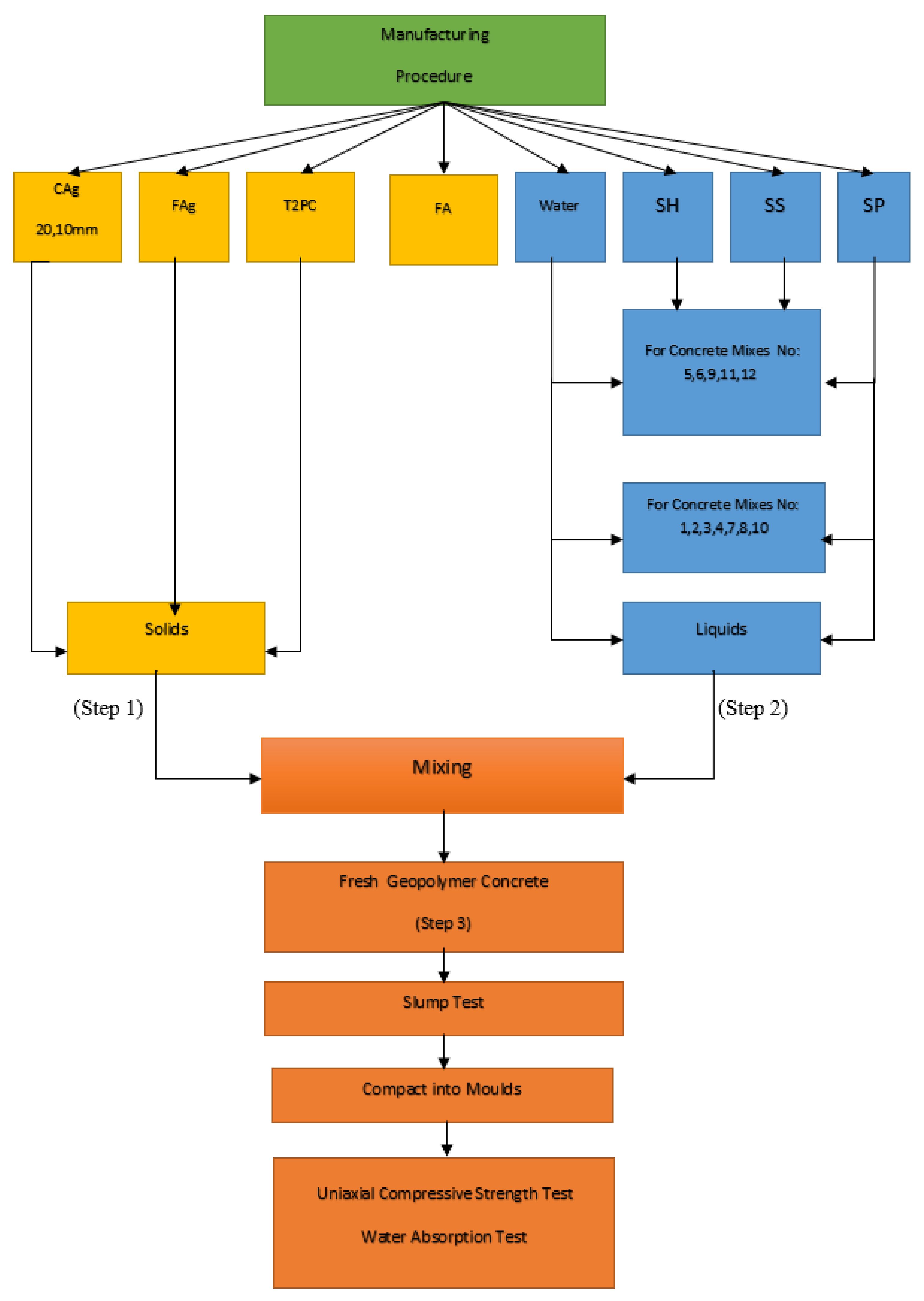
2.4. Specimen Preparation and Concrete Manufacturing Process
2.5. Test Procedure
2.6. Compressive Strength
2.7. Water Absorption
3. Results and Discussion
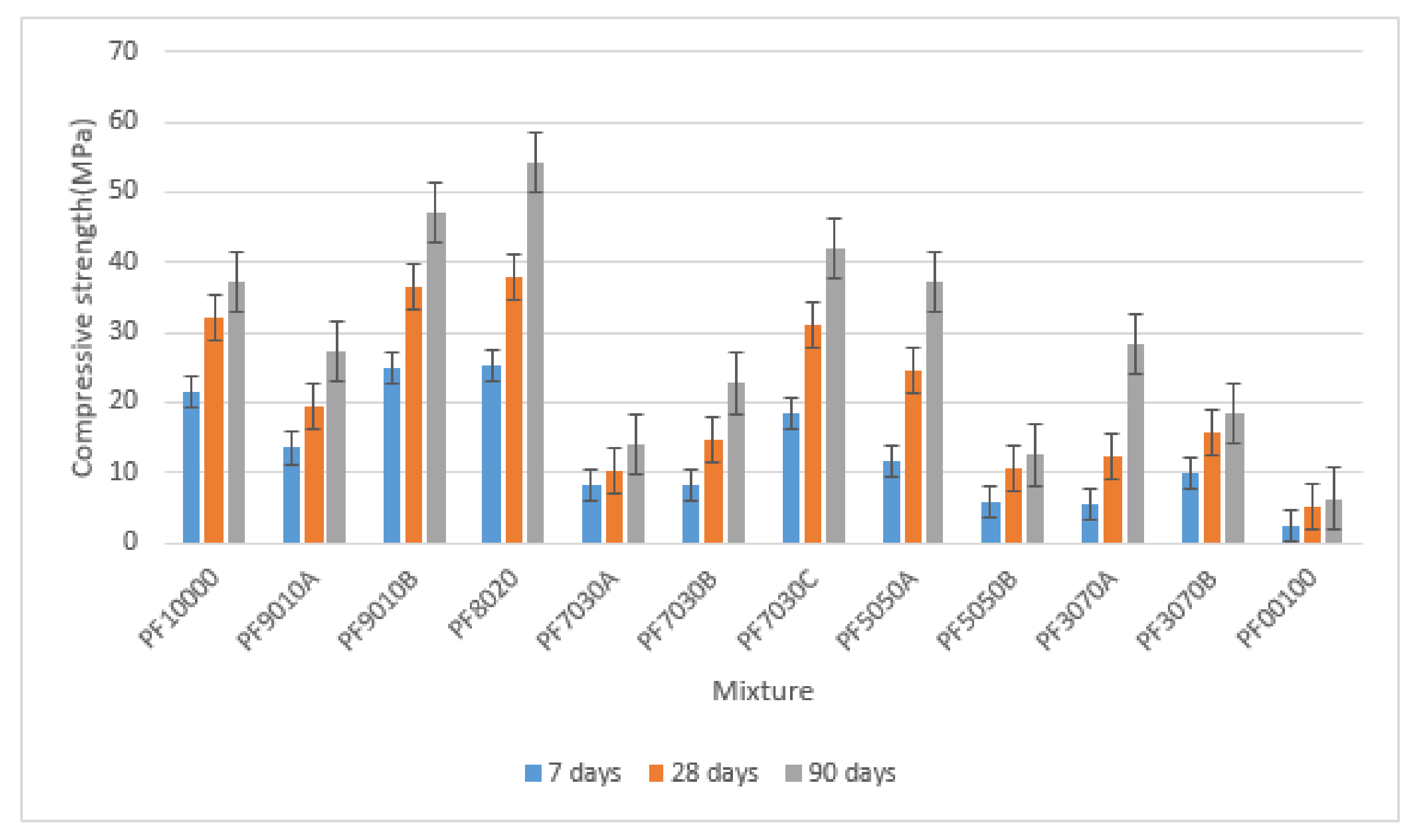
3.1. Compressive Strength
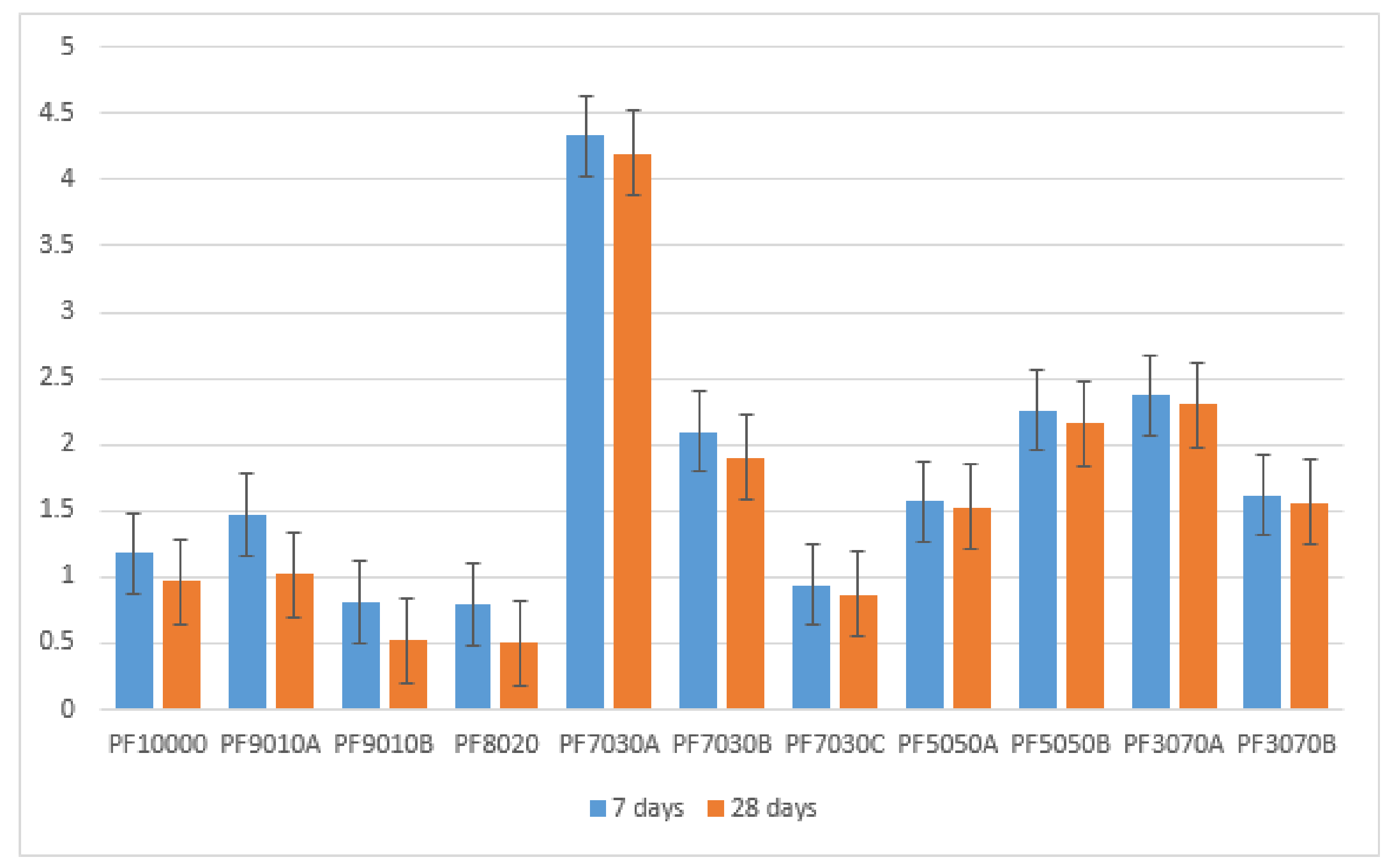
3.2. Water Absorption
4. Conclusions
- The compressive strength of low-calcium, FA-based geopolymer concrete was enhanced with the inclusion of FA as a replacement of T2PC up to 20% at all ages.
- The increment in concrete strength was observed due to additional calcium available in the chemical products formed by the hydration mechanism, which coexisted with the polymeric products of alumina-silicate.
- Although the use of fly ash with a percentage higher than 20% and alkaline solutions significantly enhanced the water absorption amount, the notable reduction of concrete strength was also observed, which was attributed to the relatively low balance in the value of ions in the chemical and hydration processes such as Na, Ca, Al2O3, and SiO2. The ions were associated with the chemical combinations of T2PC participating in the concrete mixtures of this investigation.
- Concrete specimens including 30% of FA and alkaline solutions kept under the heat of oven at 110 °C for the initial 24 h as a curing condition (PF7030A) were found to have the highest values for both water absorption and porosity as well as relatively low compressive strength among all concrete mixtures at all ages. These results were comparable with previous studies described earlier in this field. Thus, the typical curing condition based on BS 1881 [57] is strongly recommended in terms of the concrete binders consisting of T2PC and low-calcium FA up to 30% as cement replacement, and, beyond that, further considerations such as the appropriate ratio of alkaline solution and using superplasticizers in which their chemical combinations are compatible with polymeric products are suggested for this purpose.
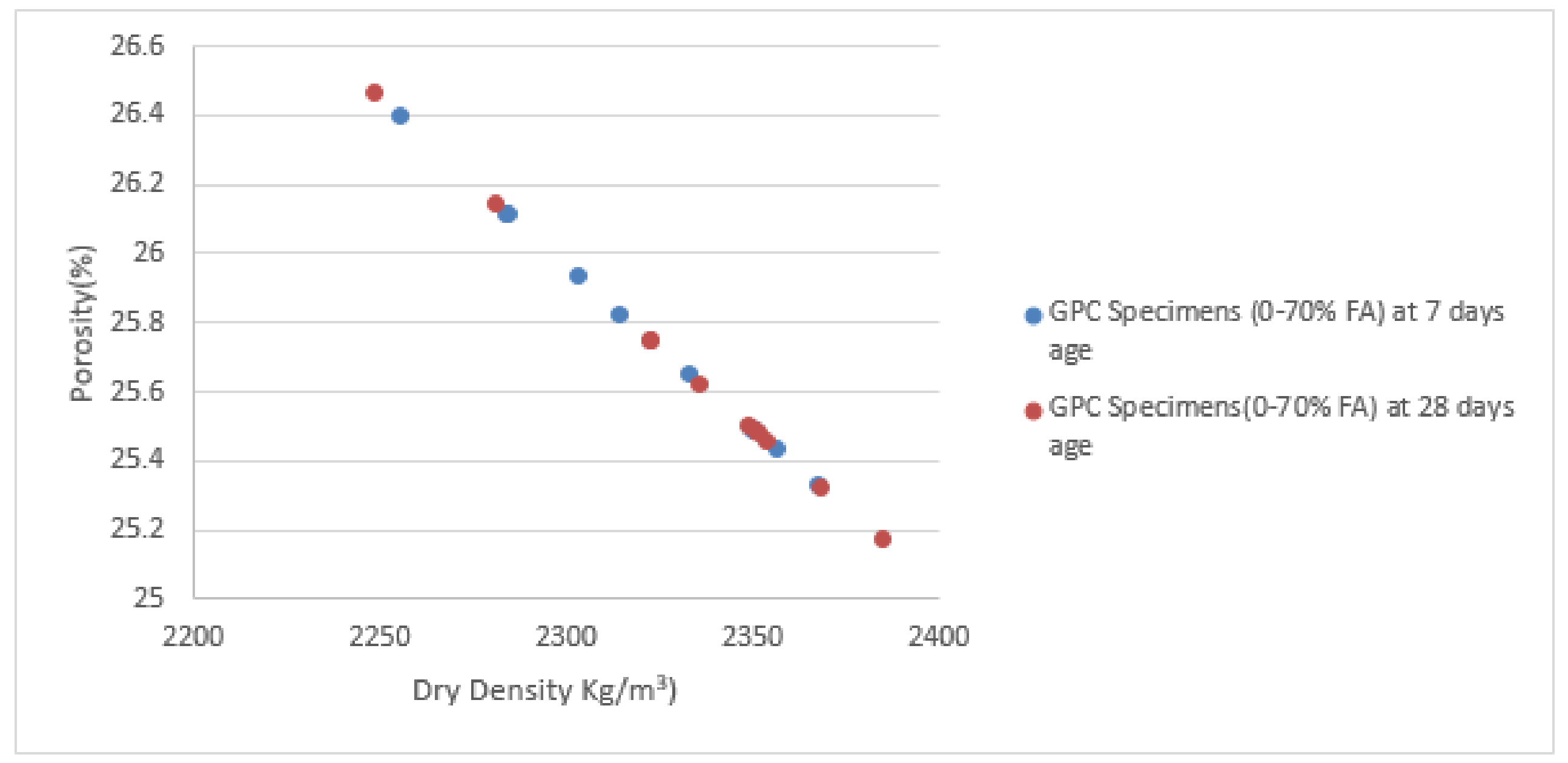
- Porosity is not the significant factor affecting the water absorption of Portland paste involving FA content. There is a relative relationship between water absorption and porosity; however, a little change was observed between 7 and 28 days for both water absorption and porosity in each concrete mixture. Porosity is significantly dependent on dry density regardless of fly ash class (low or high calcium) and FA percentage in concrete content.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samad, A.A.A.; Mohamad, N.; Ali, A.Z.M.; Ali, N.; Goh, W.I.; Hadipramana, J.; Tee, K.F. Trends and Development of Green Concrete Made from Agricultural and Construction Waste, in the Advanced Concrete Technology and Green Materials; Penerbit UTHM: Johor, Malaysia, 2017. [Google Scholar]
- Samad, A.A.A.; Hadipramana, J.; Mohamad, N.; Ali, A.Z.M.; Ali, N.; Goh, W.I.; Tee, K.F. Development of Green Concrete from Agricultural and Construction Waste. In Transition Towards 100% Renewable Energy; Springer: Cham, Switzerland, 2018; pp. 399–410. [Google Scholar]
- Kurtoğlu, A.; Alzeebaree, R.; Aljumaili, O.; Niş, A.; Gülşan, M.; Humur, G.; Çevik, A. Mechanical and durability properties of fly ash and slag based geopolymer concrete. Adv. Concr. Constr. 2018, 6, 345–362. [Google Scholar]
- Burris, L.E.; Alapati, P.; Moser, R.D.; Ley, M.T.; Berke, N.; Kurtis, K.E. Alternative cementitious materials: Challenges and opportunities, ACI SP-305-27. In Proceedings of the International Workshop on Durability and Sustainability of Concrete Structures, Bologna, Italy, 1–3 October 2015; pp. 27.1–27.10. [Google Scholar]
- Maria, C.G.J.; Rafat, S. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem. Concr. Res. 2015, 78, 71–80. [Google Scholar]
- Saricimen, H.; Maslehuddin, M.; Al-Tayyib, A.J.; Al-Mana, A.I. Permeability and durability of plain and blended cement concrete cured in field and laboratory conditions. ACI Mater. J. 1995, 9, 111–116. [Google Scholar]
- Sunil, B.; Manjunatha, L.; Ravi, L.; Yaragal, S.C. Potential use of mine tailings and fly ash in concrete. Adv. Concr. Constr. 2015, 3, 55–69. [Google Scholar] [CrossRef]
- Tee, K.F.; Agba, I.F.; Samad, A.A.A. Experimental and Numerical Study of Green Concrete. Int. J. Forensic Eng. 2019, 4, 238–254. [Google Scholar] [CrossRef]
- Ismail, A.H.; Kusbiantoro, A.; Chin, S.C.; Muthusamy, K.; Islam, M.; Tee, K.F. Pozzolanic reactivity and strength activity index of mortar containing palm oil clinker pretreated with hydrochloric acid. J. Clean. Prod. 2020, 242, 118565. [Google Scholar] [CrossRef]
- Gharehbaghi, K.; Tee, K.F.; Gharehbaghi, S. Review of geopolymer concrete: A structural integrity evaluation. Int. J. Forensic Eng. 2021, 5, 59. [Google Scholar] [CrossRef]
- Chore, H.; Joshi, M. Strength evaluation of concrete with fly ash and GGBFS as cement replacing materials. Adv. Concr. Constr. 2015, 3, 223–236. [Google Scholar] [CrossRef]
- Jenna, T.; Panda, K. Mechanical and Durability Properties of Marine Concrete Using Fly Ash and Silpozz. Adv. Concr. Constr. 2018, 6, 47–68. [Google Scholar]
- Łach, M.; Korniejenko, K.; Walter, J.; Stefanska, A. Decreasing of Leaching and Improvement of Geopolymer Properties by Addition of Aluminum Calcium Cements and Titanium Oxide. Materials 2020, 13, 495. [Google Scholar] [CrossRef] [Green Version]
- ASTM C150/C150M-15. Standard Specification for Portland Cement; American Standard for Testing and Materials, ASTM: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ASTM D3042-09. Standard Test Method for Insoluble Residue in Carbonate Aggregates; American Standard for Testing and Materials, ASTM: West Conshohocken, PA, USA, 2009. [Google Scholar]
- Kosmatka, S.H.; Kerkhoff, B.; Panarese, W.C. Design and Control of Concrete Mixtures, 14th ed.; Portland Cement Association: Skokie, IL, USA, 2002. [Google Scholar]
- Shi, C.; Roy, D.; Krivenko, P. Alkali-Activated Cements and Concretes; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Singhal, D. Development of mix design method for geopolymer concrete. Adv. Concr. Constr. 2017, 5, 377–390. [Google Scholar]
- BSI British Standards EN 450. Fly Ash for Concrete. Definitions, Requirements, and Quality Control; London BSI British Standards: London, UK, 1994. [Google Scholar]
- BSI British Standards EN450. Fly Ash for Concrete. Part 2, Conformity Evaluation; London BSI British Standards: London, UK, 2011. [Google Scholar]
- British Standards Institution. (BSI BS EN 450-1:2012. Fly Ash for Concrete. Part 1. Definition, Specifications, and Conformity Criteria; London BSI British Standards: London, UK, 2012. [Google Scholar]
- Ohenoja, K.; Wigren, V.; Österbacka, J.; Illikainen, M. Applicability of Fly Ash from Fluidized Bed Combustion of Peat, Wood, or Wastesi to Concrete. Waste Biomass Valorization 2019, 10, 3525–3534. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.J.; Shih, N.H.; Wu, C.H.; Lin, S.K. Effects of the Loss on Ignition of Fly Ash on the Properties of High-Volume Fly Ash Concrete. Sustainability 2019, 11, 2704. [Google Scholar] [CrossRef] [Green Version]
- Bilodeau, A.; Sivasundarm, V.; Painter, K.E.; Malhotra, V.M. Durability of concrete incorporating high volumes of fly ash from sources in U.S. ACI Mater. J. 1994, 91, 3–12. [Google Scholar]
- Tee, K.F.; Mostofizadeh, S. A Mini Review on Properties of Portland Cement Concrete with Geopolymer Materials as Partial or Entire Replacement. Infrastructures 2021, 6, 26. [Google Scholar] [CrossRef]
- Limbachiya, M.; Bostanci, S.C.; Kew, H. Suitability of BS EN 197-1 CEM II and CEM v Cement for Production of Low Carbon Concrete. Constr. Build. Mater. 2014, 71, 397–405. [Google Scholar] [CrossRef]
- IS EN 197-1: Cement—Composition, Specifications and Conformity Criteria for Common Cements. No. BS EN 197-1:2011; British Standards Institution (BSI): London, UK, 2011.
- Mostofizadeh, S.; Tee, K.F. Evaluation of Impact of Fly Ash on the Improvement on Type II Concrete Strength. In Proceedings of the 39th Cement and Concrete Science Conference, Bath, UK, 9–10 September 2019; pp. 190–193. [Google Scholar]
- Malhotra, V.M. Superplasticized fly ash concrete for structural applications. Concr. Int. 1986, 8, 28–31. [Google Scholar]
- Malhotra, V.M.; Mehta, P.K. High Performance, High-Volume Fly Ash Concrete: Materials, Mixture Proportioning, Properties, Construction Practice, and Case Histories, 3rd ed.; Supplementary Cementing Materials for Sustainable Development Inc.: Ottawa, BC, Canada, 2008. [Google Scholar]
- Vassilev, S.V.; Vassileva, C.G. Methods for characterization of composition of fly ashes from coal-fired power stations: A critical overview. Energy Fuels 2005, 19, 1084–1098. [Google Scholar] [CrossRef]
- Postek, M.T.; Howard, K.S.; Johnson, A.H.; McMichael, K.L. Scanning Electron Microscopy: A Student’s Handbook; Ladd Research Industries: Burlington, VT, USA, 1980. [Google Scholar]
- Kutchko, B.; Kim, A. Fly Ash Characterization by SEM–EDS. Fuel 2006, 85, 2537–2544. [Google Scholar] [CrossRef]
- Kumar, M.; Sinha, A.K.; Kujur, J. Mechanical and Durability Studies on High-Volume Fly-Ash Concrete. Struct. Concr. 2020, 22, E1036–E1049. [Google Scholar] [CrossRef]
- BS 812-103. Method for Determining Sieve Analysis; British Standards Institution: London, UK, 1985. [Google Scholar]
- Davidovits, J. Geopolymer: Chemistry & Applications; Institut Géopolymère, Dl: Saint-Quentin, France, 2015. [Google Scholar]
- Yip, C.K.; Lukey, G.C.; Van Deventer, J.S.J. The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Investigations of tungsten mine waste geopolymeric binder: Strength and microstructure. Constr. Build. Mater. 2008, 22, 2212–2219. [Google Scholar] [CrossRef] [Green Version]
- Nath, P.; Sarker, P.K. Geopolymer Concrete for Ambient Curing Condition. In Proceedings of the Australasian Structural Engineering Conference, Perth, Australia, 11–13 July 2012. [Google Scholar]
- Alonso, S.; Palomo, A. Alkaline activation of metakaolin and calcium hydroxide mixtures: Influence of temperature, activator concentration and solids ratio. Mater. Lett. 2001, 47, 55–62. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. Properties of low-calcium fly ash based geopolymer concrete incorporating OPC as partial replacement of fly ash. Constr. Build. Mater. 2017, 150, 792–807. [Google Scholar] [CrossRef]
- Temuujin, J.; Van Riessen, A.; Williams, R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J. Hazard. Mater. 2009, 167, 82–88. [Google Scholar] [CrossRef]
- Oh, J.E.; Monteiro, P.J.; Jun, S.S.; Choi, S.; Clark, S. The evolution of strength and crystalline phases for alkali-activated ground blast furnace slag and fly ash-based geopolymers. Cem. Concr. Res. 2010, 40, 189–196. [Google Scholar] [CrossRef]
- Buchwald, A.; Hilbig, H.; Kaps, C. Alkali-activated metakaolin-slag blends—Performance and structure in dependence of their composition. J. Mater. Sci. 2007, 42, 3024–3032. [Google Scholar] [CrossRef]
- Ghrici, M.; Kenai, S.; Mansour, M.S. Mechanical properties and durability of mortar and concrete containing natural pozzolana and limestone blended cements. Cem. Concr. Compos. 2007, 29, 542–549. [Google Scholar] [CrossRef]
- ASTM C1585. Standard Test Method for Measurement of Rate of Absorption of Water by Hydraulic-Cement Concretes; ASTM: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Andrade, C. Resistivity Test Criteria for Durability Design and Quality Control of Concrete in Chloride Exposures. Concr. Aust. 2014, 40, 57–64. [Google Scholar]
- Nadelman, E.I.; Kurtis, K.E. A Resistivity-Based Approach to Optimizing Concrete Performance. Concr. Int. 2014, 36, 50–54. [Google Scholar]
- Amin, N.; Castel, A. The Effect of Heat-Curing on Transport Properties of Low-Calcium Fly Ash-Based Geopolymer Concrete. Constr. Build. Mater. 2016, 112, 464–477. [Google Scholar]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; Deventer, J.S.J. Modification of Phase Evolution in Alkali-Activated Blast Furnace Slag by the Incorporation of Fly Ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary Cementitious Materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Provis, J.; Myers, R.; White, C.; Rose, V.; van Deventer, J.S. X-ray microtomography shows pore structure and tortuosity in alkali-activated binders. Cem. Concr. Res. 2012, 42, 855–864. [Google Scholar] [CrossRef]
- Naik, T.R.; Singh, S.S.; Hossain, M.M. Permeability of concrete containing large amounts of fly ash. Cem. Concr. Res. 1994, 24, 913–922. [Google Scholar] [CrossRef]
- Yu, Z.; Ni, C.; Tang, M.; Shen, X. Relationship between water permeability and pore structure of Portland cement paste blended with fly ash. Constr. Build. Mater. 2018, 175, 458–466. [Google Scholar] [CrossRef]
- Kearsley, E.; Wainwright, P. Porosity and permeability of foamed concrete. Cem. Concr. Res. 2001, 31, 805–812. [Google Scholar] [CrossRef]
- British Standard Institution. BS 1881: Part 116, Testing Concrete: Method for Determination of Compressive Strength of Concrete Cubes; British Standard Institution: London, UK, 1983. [Google Scholar]


| Property | Unit | Requirement Based on EN 450-1: 2012 |
|---|---|---|
| Loss on ignition for Category B | % By mass | 2.0–7.0 |
| Water Requirement | % | ≤95 |
| Fineness Fraction for Category N ≥ 45 µm | % By mass | ≤40 |
| Soluble Phosphate (P2O5) | % By mass | ≤100 |
| Total Phosphate | Mg/kg | --- |
| Initial Setting | Min | 2 |
| Sum SiO2 + Al2O3 + Fe2O3 | % By mass | ≥70 |
| Reactive SiO2 | % By mass | ≥25 |
| Reactive CaO | % By mass | ≤10 |
| Sulphate (SO3) | % By mass | ≤3 |
| Free Calcium Oxide | % By mass | 2.5 |
| Soundness | Mm | ≤10 |
| Magnesium Oxide (MgO) | % By mass | ≤4.0 |
| Chloride (Cl ion) | % By mass | ≤0.10 |
| Clinker [1] | Blast Furnace [2] | Silica Fume | Fly Ash | Limestone (CaCO3) [5] | Minor Additional Constituents | Strength Class | Initial Setting Time (min) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 32.5 R [8] | ||||||||||||
| Siliceous [3] | Calcareous [4] | L [6] | LL [7] | Compressive Strength (MPa) | ||||||||
| Early Strength | StandardStrength | |||||||||||
| 2 Days | 7 Days | 28 Days | ||||||||||
| 80–94 | 0 | 0 | 0 | 0 | 6–20 | 0 | 0–5 | ≥10 | 0 | ≥32.5 | ≤52.5 | ≥75 |
| Assay | Min. 98% |
|---|---|
| Heavy metals (as Pb) | Max. 5 ppm |
| Total N (Nitrogen) | Max. 5 ppm |
| CO3 (as Na2CO3) | Max. 1.0% |
| Cl (Chloride) | Max. 50 ppm |
| PO4 (Phosphate) | Max. 20 ppm |
| SiO4 (as SiO2) | Max. 100 ppm |
| SO4 (Sulphate) | Max. 50 ppm |
| Al (Aluminium) | Max. 10 ppm |
| Fe (Iron) | Max. 10 ppm |
| K (Potassium) | Max. 0.1% |
| Pb (Lead) | Max. 5 ppm |
| Mix | NaOH (gr) | Na2SiO3 (gr) | Water (mL) | Na2SiO3/NaOH | NaOH Molarity (M) | Alkaline Solution/FA (By Mass) |
|---|---|---|---|---|---|---|
| PF7030A | 250 | 625 | 1500 | 2.5 | 4.16 | 0.25 |
| PF7030B | 250 | 625 | 1500 | 2.5 | 4.16 | 0.25 |
| PF5050B | 428 | 1070 | 1500 | 2.5 | 7.13 | 0.25 |
| PF3070B | 600 | 1500 | 1500 | 2.5 | 10 | 0.25 |
| PF00100 | 830 | 1600 | 1500 | 2.5 | 12.96 | 0.25 |
| Mix | CAg 20 mm (Kg) | CAg 10 mm (Kg) | FAg (Kg) | FA (Kg) (%) | T2PC (Kg) | Water (L) | Superplasticizer (mL) (2.5%) | Curing Condition | w/cm | SS/SH | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PF10000 | 32.84 | 16.4 | 26.52 | 0 (0%) | 12 | 7.2 | 0 | Water Bath at 21 °C | 0.6 | ------ |
| 2 | PF9010A | 32.84 | 16.4 | 26.52 | 1.2 (10%) | 10.8 | 7.2 | 0 | Water Bath at 21° C | 0.6 | ------ |
| 3 | PF9010B | 32.84 | 16.4 | 26.52 | 1.2 (10%) | 10.8 | 6.6 | 30 | Water Bath at 21° C | 0.55 | ------ |
| 4 | PF8020 | 32.84 | 16.4 | 26.52 | 2.4 (20%) | 9.6 | 6 | 30 | Water Bath at 21 °C | 0.5 | ------ |
| 5 | PF7030A | 32.84 | 16.4 | 26.52 | 3.6 (30%) | 8.4 | 6 | 30 | 24 h heat (110° C) 4–5 h keep at room temperature Water bath at 21 °C | 0.5 | 2.5 |
| 6 | PF7030B | 32.84 | 16.4 | 26.52 | 3.6 (30%) | 8.4 | 6 | 30 | Water Bath at 21 °C | 0.5 | 2.5 |
| 7 | PF7030C | 32.84 | 16.4 | 26.52 | 3.6 (30%) | 8.4 | 6.6 | 30 | Water Bath at 21 °C | 0.55 | ------ |
| 8 | PF5050A | 32.84 | 16.4 | 26.52 | 6 (50%) | 6 | 6 | 30 | Water Bath at 21 °C | 0.5 | ------ |
| 9 | PF5050B | 32.84 | 16.4 | 26.52 | 6 (50%) | 6 | 5.5 | 30 | Water Bath at 21 °C | 0.45 | 2.5 |
| 10 | PF3070A | 32.84 | 16.4 | 26.52 | 8.4 (70%) | 3.6 | 5.7 | 30 | Water Bath at 21 °C | 0.48 | ------ |
| 11 | PF3070B | 32.84 | 16.4 | 26.52 | 8.4 (70%) | 3.6 | 4 | 30 | Water Bath at 21 °C | 0.33 | 2.5 |
| 12 | PF00100 | 32.84 | 16.4 | 26.52 | 12 (100%) | 0 | 3 | 30 | Room temperature (14–17 °C) | 0.25 | 2.5 |
| Sieve Size (mm) | Mass Retained | Mass Passing | Weight Total | % Passing | BS Sieve Test Standard |
|---|---|---|---|---|---|
| 8.0 | 0 | 166.63 | 166.63 | 100 | 100 |
| 6.3 | 0 | 166.63 | 100 | 95–100 | |
| 4.0 | 3.99 | 162.64 | 97.61 | 85–99 | |
| 1.0 | 35.76 | 126.88 | 76.14 | 57–97 | |
| 0.5 | 44.53 | 82.35 | 49.42 | 30–70 | |
| 0.25 | 62.33 | 20.02 | 12.01 | 0–40 | |
| 0.063 | 19.66 | 0.36 | 0.22 | 0–4 | |
| Pan | 0.36 |
| Sieve Size (mm) | Mass Retained | Mass Passing | Weight Total | % Passing | BS Sieve Test Standard |
|---|---|---|---|---|---|
| 20.0 | 0 | 677 | 677 | 100 | 100 |
| 14.0 | 0 | 677 | 100 | 98–100 | |
| 10.0 | 39 | 638 | 94.239 | 85–99 | |
| 4.0 | 580 | 58 | 8.567 | 0–20 | |
| 2.0 | 46 | 12 | 1.773 | 0–5 | |
| Pan | 12 |
| Sieve Size (mm) | Mass Retained | Mass Passing | Weight Total | % Passing | BS Sieve Test Standard |
|---|---|---|---|---|---|
| 40.0 | 0 | 1097 | 1097 | 100 | 100 |
| 31.5 | 0 | 1097 | 100 | 98–100 | |
| 20.0 | 39 | 1058 | 96.445 | 85–99 | |
| 10.0 | 1000 | 58 | 5.287 | 0–20 | |
| 4.0 | 46 | 12 | 1.094 | 0–5 | |
| Pan | 12 |
| Mix | % T2PC | % Fly Ash | Average Dry Density (Kg/m3) | Workability (mm) | Average Compressive Strength (MPa) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 7 Days | 28 Days | 90 Days | 7 Days | 28 Days | 90 Days | |||||
| 1 | PF10000 | 100 | 0 | 2332.8 | 2322.9 | 2322.46 | 70 | 21.56 | 31.93 | 37.13 |
| 2 | PF9010A | 90 | 10 | 2255.26 | 2248.8 | 2256.73 | 140 | 13.54 | 19.43 | 27.29 |
| 3 | PF9010B | 90 | 10 | 2367.86 | 2385.13 | 2385.2 | 22 | 24.99 | 36.63 | 47.10 |
| 4 | PF8020 | 80 | 20 | 2356.5 | 2349.1 | 2339.23 | 30 | 25.24 | 37.84 | 54.11 |
| 5 | PF7030A | 70 | 30 | 2284.4 | 2281.4 | 2266.13 | 22 | 8.28 | 10.43 | 13.93 |
| 6 | PF7030B | 70 | 30 | 2314.53 | 2322.9 | 2307.1 | 22 | 8.14 | 14.58 | 22.73 |
| 7 | PF7030C | 70 | 30 | 2350.6 | 2351.56 | 2353.03 | 40 | 18.44 | 31.03 | 41.83 |
| 8 | PF5050A | 50 | 50 | 2350.56 | 2353.56 | 2371.3 | 40 | 11.68 | 24.53 | 37.07 |
| 9 | PF5050B | 50 | 50 | 2303.16 | 2351.03 | 2293.26 | 120 | 5.97 | 10.55 | 12.52 |
| 10 | PF3070A | 70 | 30 | 2349.6 | 2368.36 | 2351 | 45 | 5.49 | 12.33 | 28.43 |
| 11 | PF3070B | 70 | 30 | 2283.93 | 2335.76 | 2329.86 | 45 | 10.02 | 15.63 | 18.52 |
| 12 | PF00100 | 0 | 100 | 2251.43 | 2173.8 | 2173.76 | 140 | 2.35 | 5.05 | 6.35 |
| Mix | NaOH (Kg) | Na2SiO3 (Kg) | Compressive Strength (MPa) | % Water Absorption | Alkaline Solution/Fly Ash | ||
|---|---|---|---|---|---|---|---|
| 7 Days | 28 Days | 7 Days | 28 Days | ||||
| PF10000 | 0 | 0 | 21.56 | 31.93 | 1.18% | 0.97% | N/A |
| PF9010A | 0 | 0 | 13.54 | 19.43 | 1.47% | 1.02 | N/A |
| PF9010B | 0 | 0 | 24.99 | 36.63 | 0.81% | 0.52% | 0.25 |
| PF8020 | 0 | 0 | 25.24 | 37.84 | 0.79% | 0.5% | 0.25 |
| PF7030A | 0.25 | 0.625 | 8.28 | 10.43 | 4.33% | 4.2% | 0.25 |
| PF7030B | 0.25 | 0.625 | 8.14 | 14.58 | 2.1% | 1.9% | 0.25 |
| PF7030C | 0 | 0 | 18.44 | 31.03 | 0.94% | 0.87% | N/A |
| PF5050A | 0 | 0 | 11.68 | 24.53 | 1.57% | 1.53% | N/A |
| PF5050B | 0.428 | 1.070 | 5.97 | 10.55 | 2.26% | 2.16% | 0.25 |
| PF3070A | 0 | 0 | 5.49 | 12.33 | 2.37% | 2.30% | N/A |
| PF3070B | 0.600 | 1.5 | 10.02 | 15.63 | 1.62% | 1.56% | 0.25 |
| Mix No | FA Content (%) | Dry Density (γd) (Kg/m3) | Porosity (ρ) (%) | ||
|---|---|---|---|---|---|
| 7 Days | 28 Days | 7 Days | 28 Days | ||
| PF10000 | 0% | 2332.8 | 2322.9 | 25.65345 | 25.74635 |
| PF9010A | 10% | 2255.26 | 2248.8 | 26.10125 | 26.0657 |
| PF9010B | 10% | 2367.86 | 2385.13 | 25.33022 | 25.17424 |
| PF8020 | 20% | 2356.5 | 2349.1 | 25.43398 | 25.40206 |
| PF7030A | 30% | 2284.4 | 2281.4 | 26.17471 | 26.1439 |
| PF7030B | 30% | 2314.53 | 2322.9 | 25.82547 | 25.74635 |
| PF7030C | 30% | 2350.6 | 2351.56 | 25.48823 | 25.47939 |
| PF5050A | 50% | 2350.56 | 2353.56 | 25.4886 | 25.46098 |
| PF5050B | 50% | 2303.16 | 2351.03 | 25.9338 | 25.48427 |
| PF3070A | 70% | 2349.6 | 2368.36 | 25.49745 | 25.32568 |
| PF3070B | 70% | 2283.93 | 2335.76 | 26.11928 | 25.62581 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tee, K.F.; Mostofizadeh, S. An Experimental Study of the Effects of Low-Calcium Fly Ash on Type II Concrete. Ceramics 2021, 4, 600-617. https://doi.org/10.3390/ceramics4040043
Tee KF, Mostofizadeh S. An Experimental Study of the Effects of Low-Calcium Fly Ash on Type II Concrete. Ceramics. 2021; 4(4):600-617. https://doi.org/10.3390/ceramics4040043
Chicago/Turabian StyleTee, Kong Fah, and Sayedali Mostofizadeh. 2021. "An Experimental Study of the Effects of Low-Calcium Fly Ash on Type II Concrete" Ceramics 4, no. 4: 600-617. https://doi.org/10.3390/ceramics4040043
APA StyleTee, K. F., & Mostofizadeh, S. (2021). An Experimental Study of the Effects of Low-Calcium Fly Ash on Type II Concrete. Ceramics, 4(4), 600-617. https://doi.org/10.3390/ceramics4040043






