Hybrid Carbon Nano-Fibers with Improved Oxidation Resistance
Abstract
1. Introduction
2. Experimental Setup
- Heating from 25 °C to 230 °C and holding for 1 h.
- Heating from 230 °C to 250 °C and holding for 2 h.
- Heating from 250 °C to 280 °C and holding for 2 h.
3. Results and Discussion
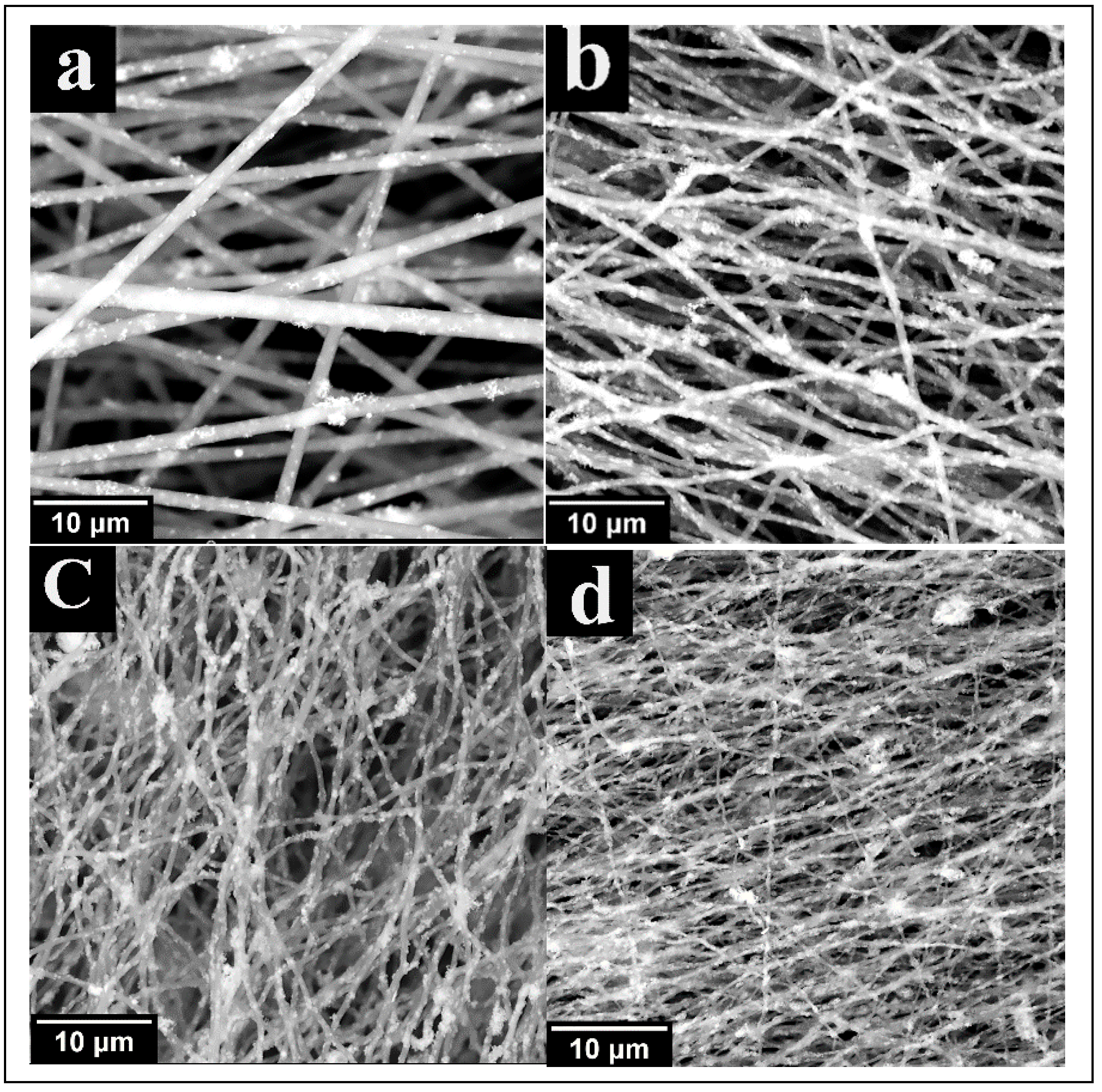
3.1. Scanning Electron Microscopy (SEM)
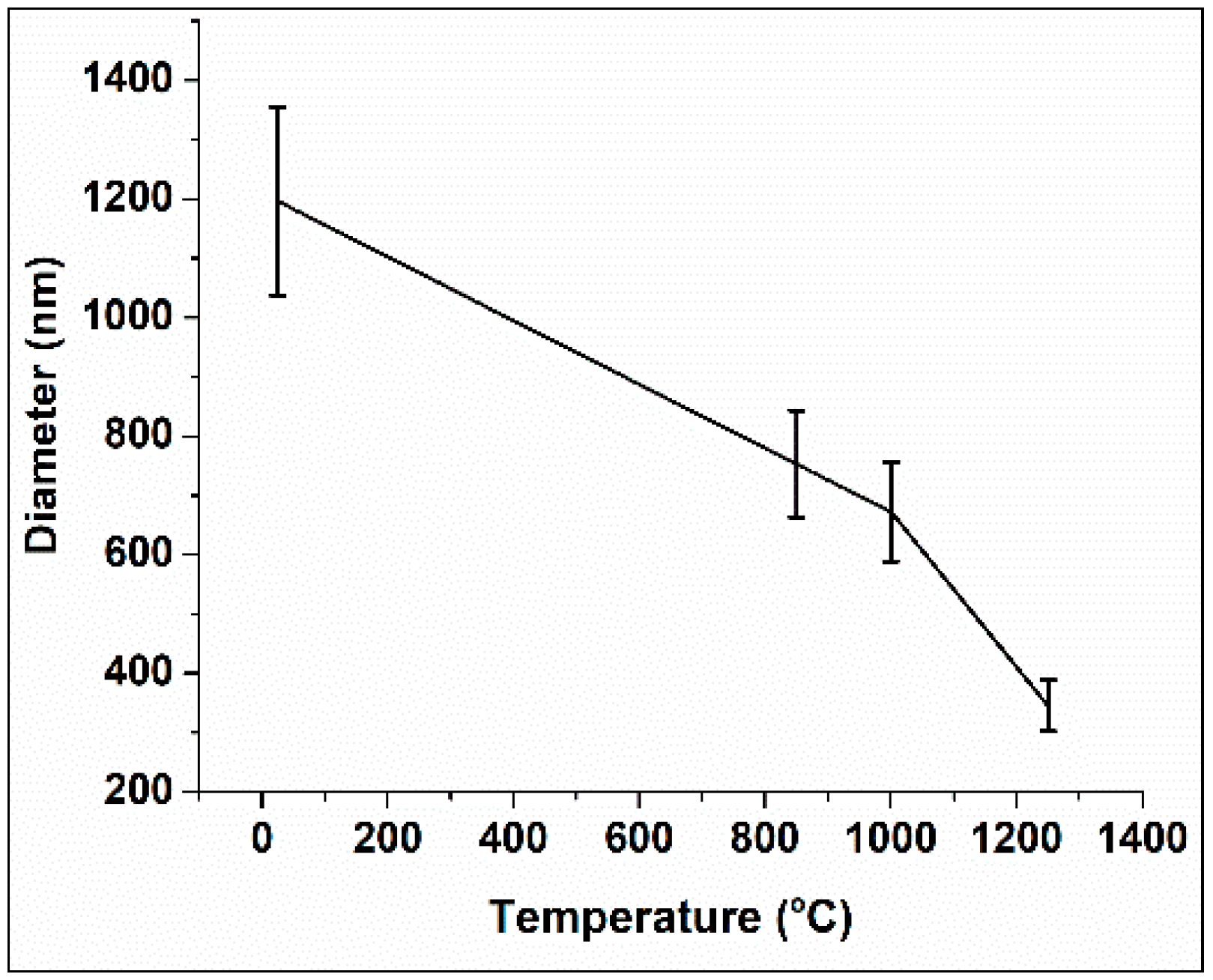
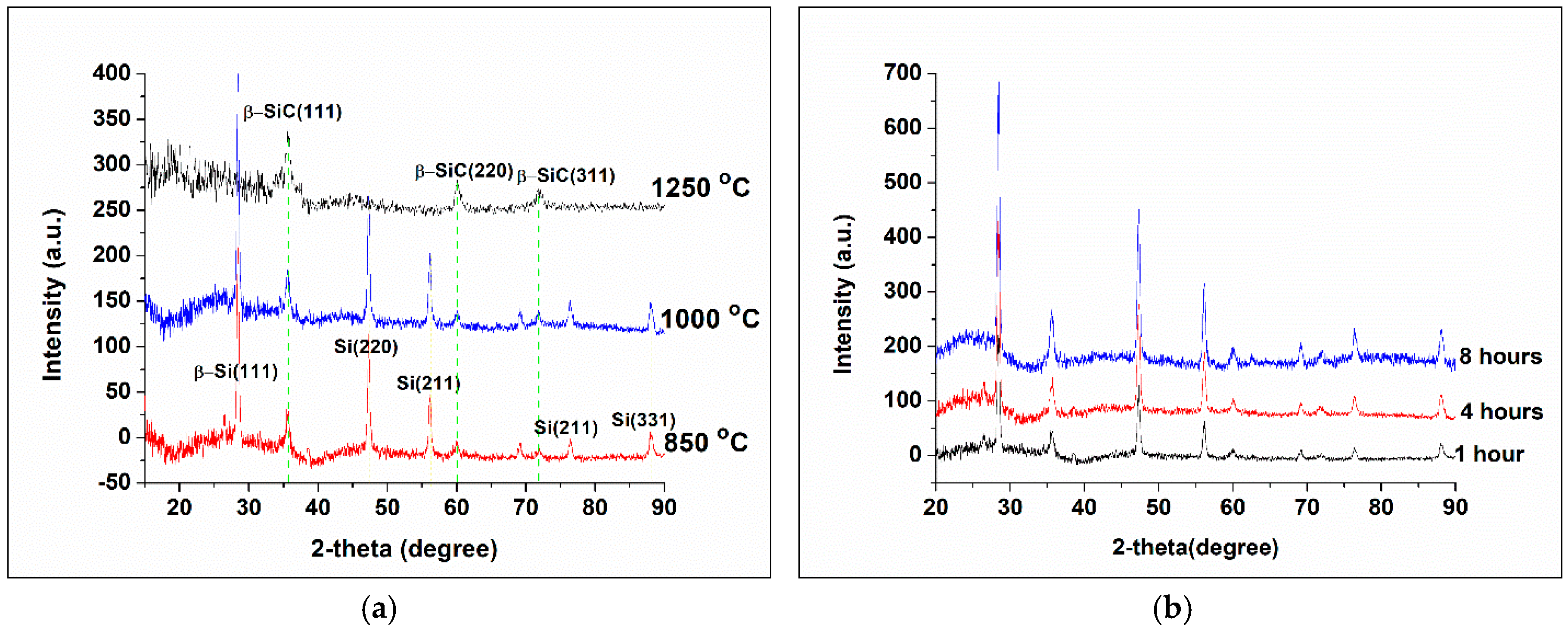
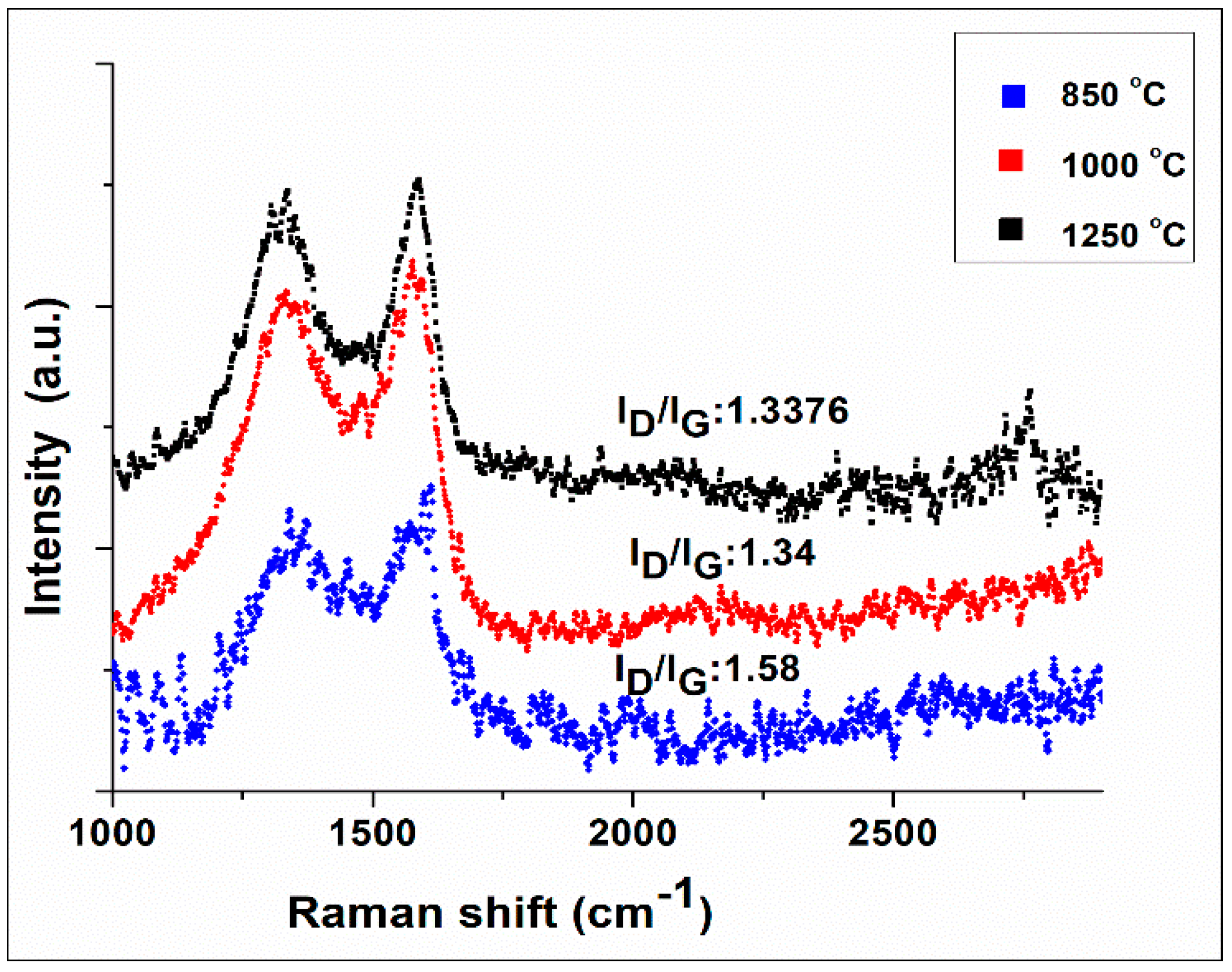
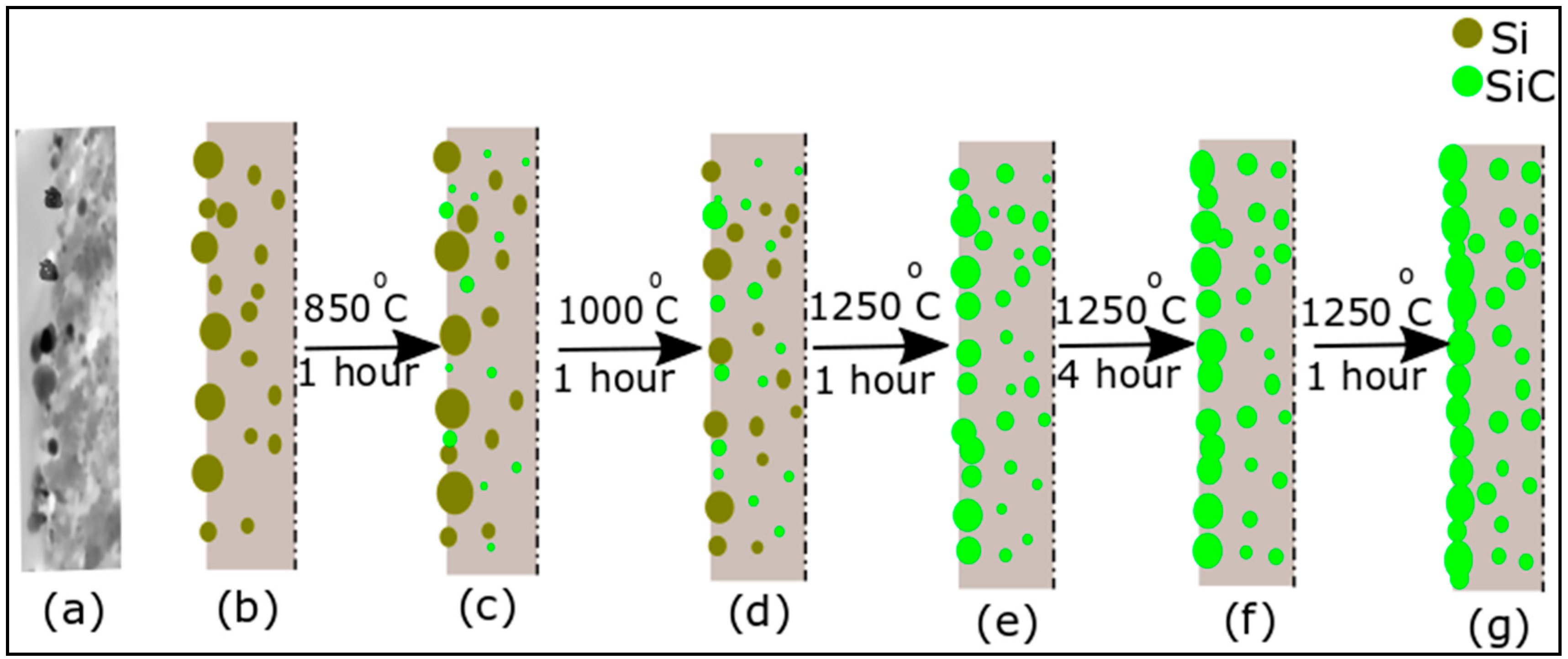
3.2. Nano-Fibers Structural Examinations
3.3. Thermogravimetric Analysis (TGA)
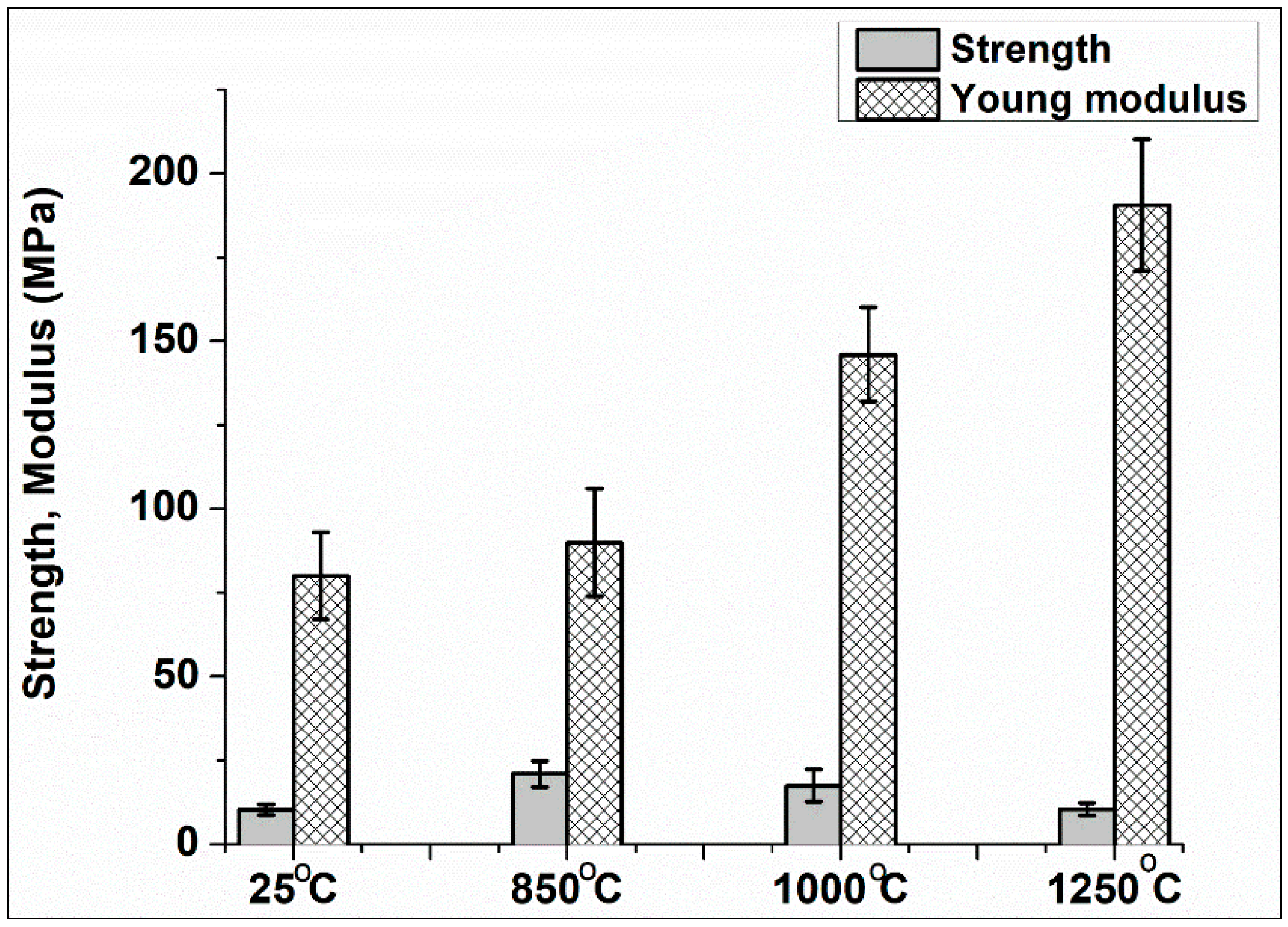
3.4. Mechanical Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tong, Y.; Wang, X.; Su, H.; Xu, L. Oxidation kinetics of polyacrylonitrile-based carbon fibers in air and the effect on their tensile properties. Corros. Sci. 2011, 53, 2484–2488. [Google Scholar] [CrossRef]
- Park, S.-J.; Heo, G.-Y. Precursors and Manufacturing of Carbon Fibers BT—Carbon Fibers; Park, S.-J., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 31–66. ISBN 978-94-017-9478-7. [Google Scholar]
- Huang, X. Fabrication and Properties of Carbon Fibers. Materials 2009, 2, 2369–2403. [Google Scholar] [CrossRef]
- Lu, P.; Huang, Q.; Mukherjee, A.; Hsieh, Y.-L. SiCO-doped Carbon Fibers with Unique Dual Superhydrophilicity/Superoleophilicity and Ductile and Capacitance Properties. ACS Appl. Mater. Interfaces 2010, 2, 3738–3744. [Google Scholar] [CrossRef]
- Fukuoka, H.; Ueno, S.; Aramata, M.; Okada, T.; Hamaya, N.; Maeda, T. Graphite-Silicon Carbide Composite and Making Method. U.S. Patent 12/055985, 2 October 2008. [Google Scholar] [CrossRef]
- Feng, L.; Xie, N.; Zhong, J. Carbon Nanofibers and Their Composites: A Review of Synthesizing, Properties and Applications. Materials 2014, 7, 3919–3945. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, I.M.; Sarasini, F.; Sarto, M.S.; Tamburrano, A. EMC Impact of Advanced Carbon Fiber/Carbon Nanotube Reinforced Composites for Next-Generation Aerospace Applications. IEEE Trans. Electromagn. Compat. 2008, 50, 556–563. [Google Scholar] [CrossRef]
- Al-Qureshi, H.A. Automobile leaf springs from composite materials. J. Mater. Process. Technol. 2001, 118, 58–61. [Google Scholar] [CrossRef]
- Tarfaoui, M.; El Moumen, A.; Lafdi, K. Progressive damage modeling in carbon fibers/carbon nanotubes reinforced polymer composites. Compos. Part B Eng. 2017, 112, 185–195. [Google Scholar] [CrossRef]
- Tual, N.; Carrere, N.; Davies, P.; Bonnemains, T.; Lolive, E. Characterization of sea water ageing effects on mechanical properties of carbon/epoxy composites for tidal turbine blades. Compos. Part A Appl. Sci. Manuf. 2015, 78, 380–389. [Google Scholar] [CrossRef]
- Ali, A.H.; Mohamed, H.M.; Benmokrane, B.; ElSafty, A. Theory-based approaches and microstructural analysis to evaluate the service life-retention of stressed carbon fiber composite strands for concrete bridge applications. Compos. Part B Eng. 2019, 165, 279–292. [Google Scholar] [CrossRef]
- Pervaiz, M.; Sain, M.M. Carbon storage potential in natural fiber composites. Resour. Conserv. Recycl. 2003, 39, 325–340. [Google Scholar] [CrossRef]
- Luan, C.; Yao, X.; Liu, C.; Lan, L.; Fu, J. Self-monitoring continuous carbon fiber reinforced thermoplastic based on dual-material three-dimensional printing integration process. Carbon N. Y. 2018, 140, 100–111. [Google Scholar] [CrossRef]
- Liu, C.; Lafdi, K. Fabrication and characterization of carbon nanofibers from polyacrylonitrile/pitch blends. J. Appl. Polym. Sci. 2017, 134, 45388. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, C. Graphene fiber: A new trend in carbon fibers. Mater. Today 2015, 18, 480–492. [Google Scholar] [CrossRef]
- Morgan, B.A.B.; Matters, M.; Issue, V. Polymer-Clay Nanocomposites : Design and Application of Multi-Functional Materials. Mater. Matters 2011, 2, 20–25. [Google Scholar]
- Xu, F.; Wu, D.; Fu, R.; Wei, B. Design and preparation of porous carbons from conjugated polymer precursors. Mater. Today 2017, 20, 629–656. [Google Scholar] [CrossRef]
- Al-Ajrah, S.; Lafdi, K.; Liu, Y.; Le Coustumer, P. Fabrication of ceramic nanofibers using polydimethylsiloxane and polyacrylonitrile polymer blends. J. Appl. Polym. Sci. 2017, 135, 45967. [Google Scholar] [CrossRef]
- Schöppl, O.; Ruess, G.; Lengauer, W.; Liersch, A.; Köttritsch, H. Polymer Precursor Ceramics and Their Applications; SKF Science Days: Steyr, Austria, 2004. [Google Scholar]
- Ji, L.; Jung, K.-H.; Medford, A.J.; Zhang, X. Electrospun polyacrylonitrile fibers with dispersed Si nanoparticles and their electrochemical behaviors after carbonization. J. Mater. Chem. 2009, 19, 4992–4997. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Shao, C.; Kim, H.-Y.; Gong, J.; Ding, B.; Lee, D.-R.; Park, S.-J. Fiber mats of poly(vinyl alcohol)/silica composite via electrospinning. Mater. Lett. 2003, 57, 1579–1584. [Google Scholar] [CrossRef]
- Han, P.; Yuan, T.; Yao, L.; Han, Z.; Yang, J.; Zheng, S. Copper Nanoparticle-Incorporated Carbon Fibers as Free-Standing Anodes for Lithium-Ion Batteries. Nanoscale Res. Lett. 2016, 11, 172. [Google Scholar] [CrossRef]
- Dadvar, S.; Tavanai, H.; Morshed, M. Fabrication of nanocomposite PAN nanofibers containing MgO and Al2O3 nanoparticles. Polym. Sci. Ser. A 2014, 56, 358–365. [Google Scholar] [CrossRef]
- Lee, S.-H.; Yun, S.-M.; Kim, S.J.; Park, S.-J.; Lee, Y.-S. Characterization of nanoporous β-SiC fiber complex prepared by electrospinning and carbothermal reduction. Res. Chem. Intermed. 2010, 36, 731–742. [Google Scholar] [CrossRef]
- Kancheva, M.; Toncheva, A.; Manolova, N.; Rashkov, I. Enhancing the mechanical properties of electrospun polyester mats by heat treatment. Express Polym. Lett. 2015, 9, 49–65. [Google Scholar] [CrossRef]
- Rošic, R.; Pelipenko, J.; Kocbek, P.; Baumgartner, S.; Bešter-Rogač, M.; Kristl, J. The role of rheology of polymer solutions in predicting nanofiber formation by electrospinning. Eur. Polym. J. 2012, 48, 1374–1384. [Google Scholar] [CrossRef]
- Chikvaidze, G.; Mironova-Ulmane, N.; Plaude, A.; Sergeev, O. Investigation of silicon carbide polytypes by Raman spectroscopy. Latv. J. Phys. Tech. Sci. 2014, 51, 51–57. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, B.-H.; Yang, K.S.; Kim, K.-Y. The formation of silica nanoparticles on the polyacrylonitrile-based carbon nanofibers by graphene via electrospinning. Mater. Lett. 2012, 71, 74–77. [Google Scholar] [CrossRef]
- Yue, Y. Synthesis of Silicon Carbide Fibers From Polycarbosilane by Electrospinning Method. Master’s Thesis, Clemson University, Clemson, SC, USA, 2014. [Google Scholar]
- Kaniyoor, A.; Ramaprabhu, S. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012, 2, 32183. [Google Scholar] [CrossRef]
- Lu, P.; Huang, Q.; Mukherjee, A.; Hsieh, Y.-L. Effects of polymer matrices to the formation of silicon carbide (SiC) nanoporous fibers and nanowires under carbothermal reduction. J. Mater. Chem. 2011, 21, 1005–1012. [Google Scholar] [CrossRef]
- Al-Ajrash, S.M.N.; Lafdi, K.; Vasquez, E.S.; Chinesta, F.; Le Coustumer, P. Experimental and Numerical Investigation of the Silicon Particle Distribution in Electrospun Nanofibers. Langmuir 2018, 34, 7147–7152. [Google Scholar] [CrossRef]
- Prakash, J.; Dasgupta, K.; Kumar, B.; Ghosh, S.K.; Chakravartty, J.K. Role of SiC nanowire coating on oxidation behavior of carbon fibers: Kinetic and thermodynamic study. Surf. Coat. Technol. 2014, 259, 637–646. [Google Scholar] [CrossRef]
- Zhou, W.; Long, L.; Xiao, P.; Li, Y.; Luo, H.; Hu, W.; Yin, R. Silicon carbide nano-fibers in-situ grown on carbon fibers for enhanced microwave absorption properties. Ceram. Int. 2017, 43, 5628–5634. [Google Scholar] [CrossRef]
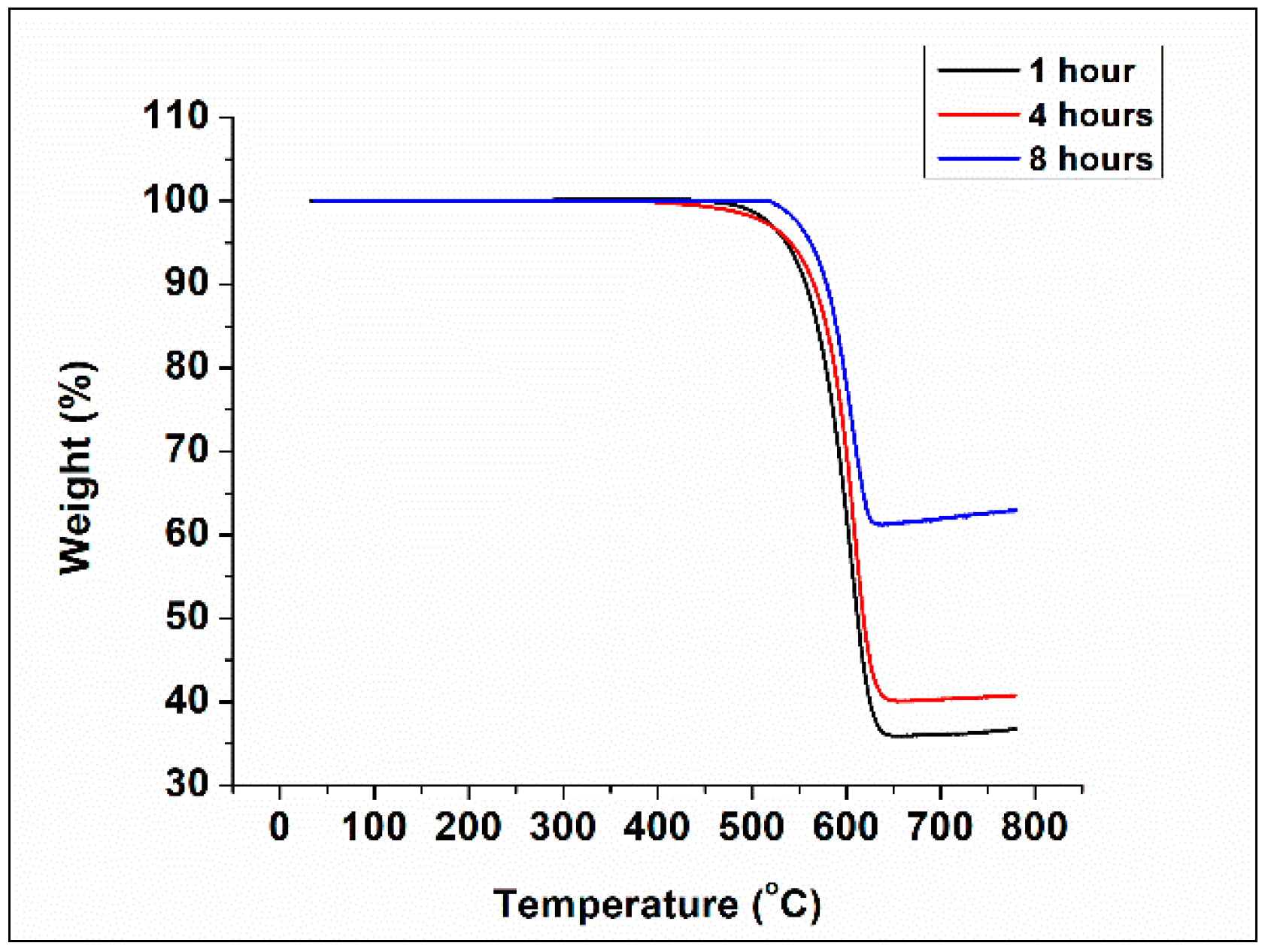
| Heat Treatment Temperature (°C) | Holding Time (Hours) | Char Yield (%) |
|---|---|---|
| 850 | 1 | 29 |
| 1000 | 1 | 33 |
| 1250 | 1 | 37 |
| 4 | 41 | |
| 8 | 62 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ajrash, S.M.N.; Lafdi, K. Hybrid Carbon Nano-Fibers with Improved Oxidation Resistance. Ceramics 2019, 2, 25-33. https://doi.org/10.3390/ceramics2010003
Al-Ajrash SMN, Lafdi K. Hybrid Carbon Nano-Fibers with Improved Oxidation Resistance. Ceramics. 2019; 2(1):25-33. https://doi.org/10.3390/ceramics2010003
Chicago/Turabian StyleAl-Ajrash, Saja M. Nabat, and Khalid Lafdi. 2019. "Hybrid Carbon Nano-Fibers with Improved Oxidation Resistance" Ceramics 2, no. 1: 25-33. https://doi.org/10.3390/ceramics2010003
APA StyleAl-Ajrash, S. M. N., & Lafdi, K. (2019). Hybrid Carbon Nano-Fibers with Improved Oxidation Resistance. Ceramics, 2(1), 25-33. https://doi.org/10.3390/ceramics2010003





