Total Quality and Innovation Management in Healthcare (TQIM-H) for an Effective Innovation Development: A Conceptual Framework and Exploratory Study
Abstract
:1. Introduction
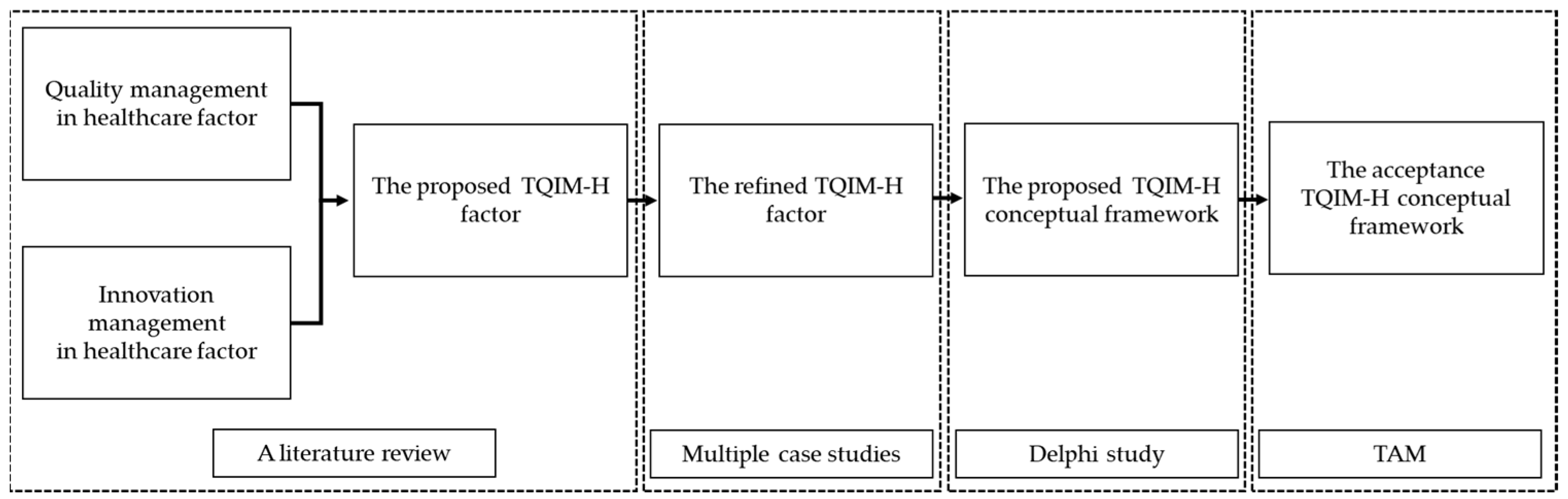
2. Research Methodology
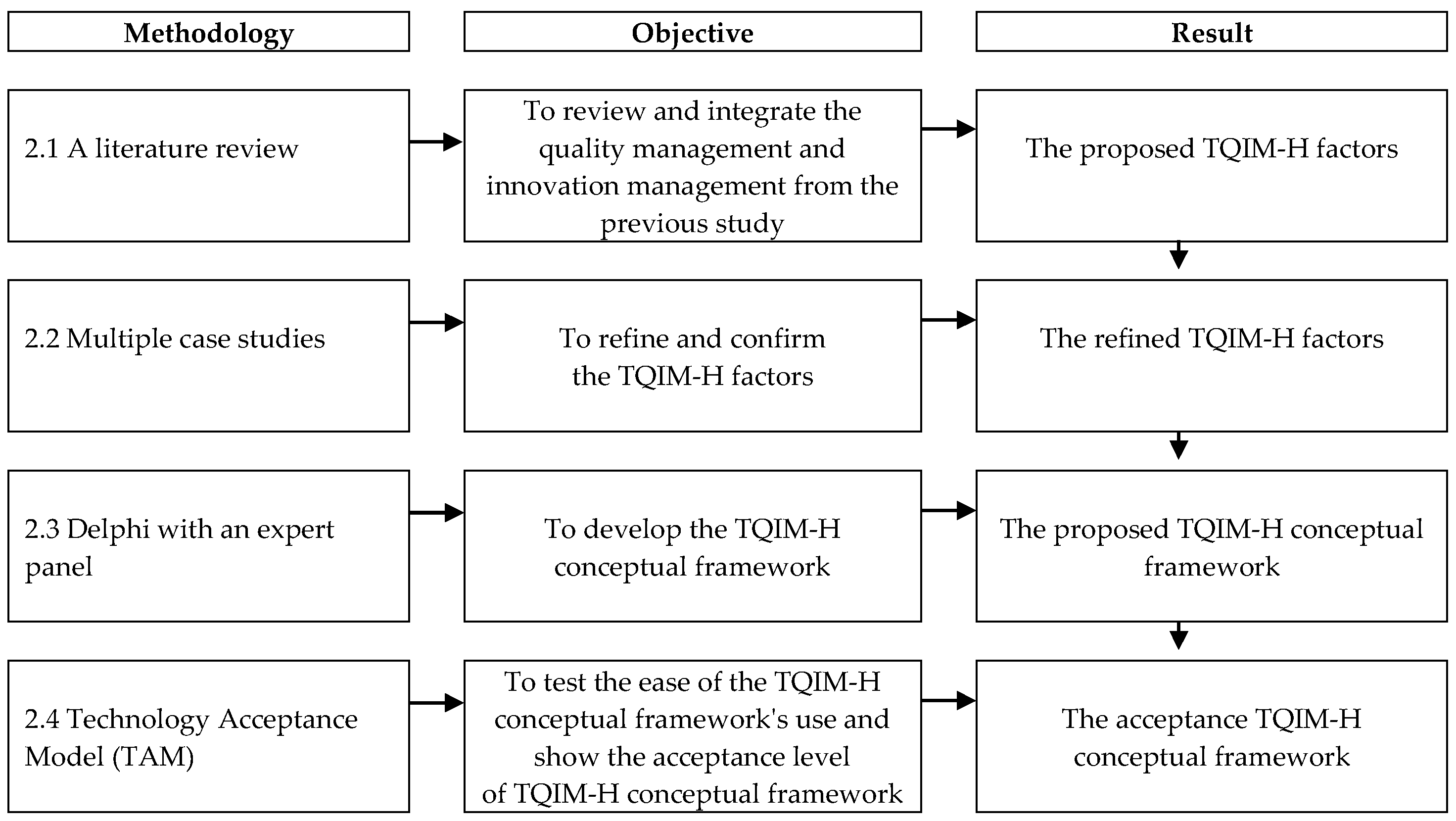
2.1. A Literature Review
2.2. Case Study Analysis
2.3. Delphi Study with Expert Panels
2.3.1. Selection of Experts
2.3.2. Delphi First Round
2.3.3. Delphi Second Round
2.3.4. Delphi Third Round
2.4. Technology Acceptance Model (TAM)
2.5. The Triangulation Technique with the Healthcare Innovation Development
3. Results
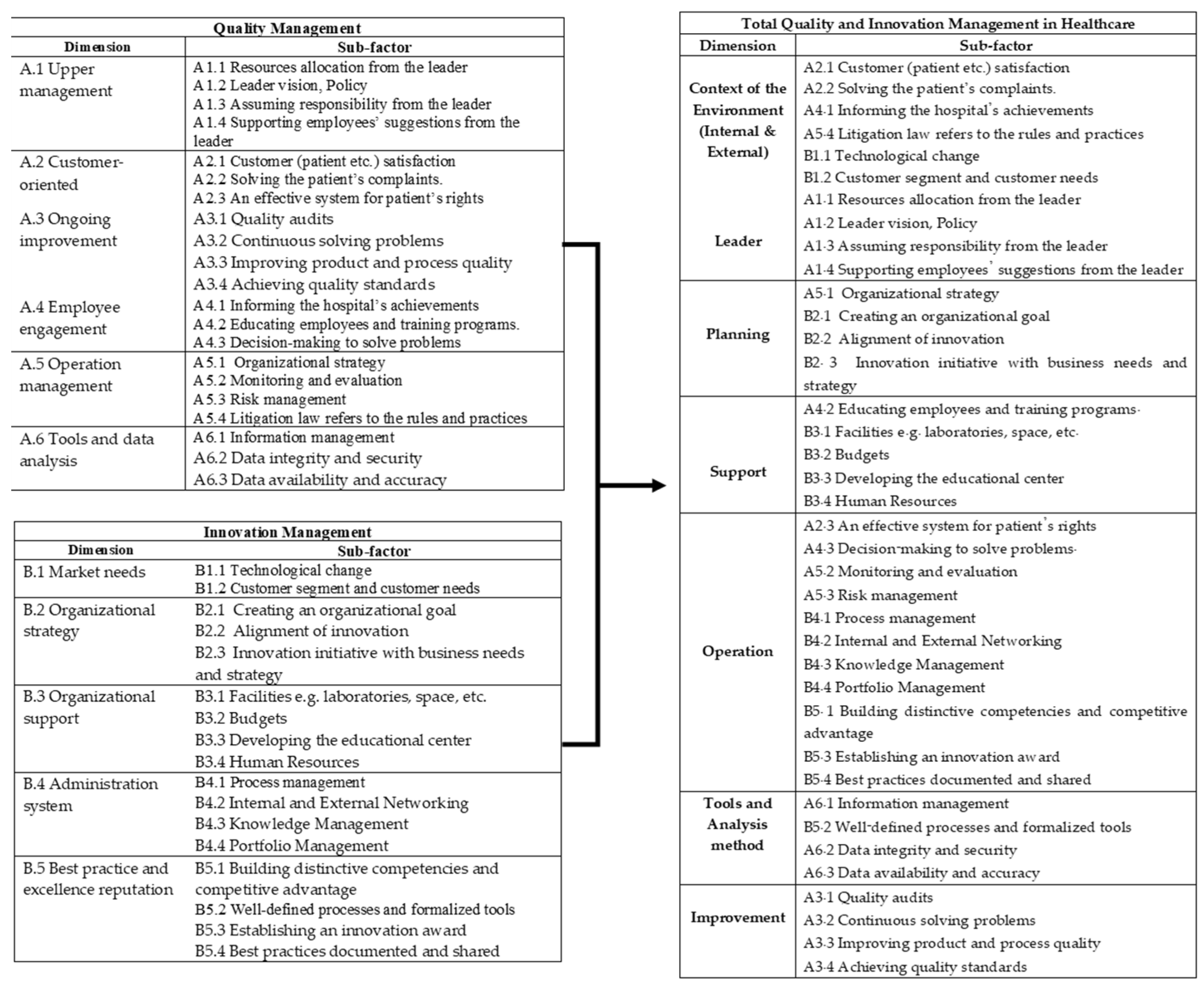
3.1. A Literature Review
3.2. Case Study Analysis
3.3. Delphi Study with Expert Panels
3.3.1. Delphi First Round
3.3.2. Delphi Second Round
3.3.3. Delphi Third Round
3.4. TAM
3.5. Triangulation with the Healthcare Innovation Development
Healthcare Innovation Situation
3.6. The Refined TQIM-H Conceptual Framework
4. Discussion and Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| TQIM-H | Importance Level | Performance Level | Quadrant | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| A2.1 Customer (patient, etc.) satisfaction | 8.67 | 0.55 | 7.83 | 0.91 | Q2 |
| A2.2 Solving the patient’s complaints. | 7.37 | 1.27 | 6.77 | 0.86 | Q3 |
| A4.1 Informing the hospital’s achievements | 6.9 | 1.58 | 7.1 | 1.03 | Q4 |
| A5.4 Litigation law refers to the rules and practices | 8.67 | 0.71 | 8.23 | 1.04 | Q2 |
| B1.1 Technological change | 8.03 | 0.76 | 7.1 | 1.47 | Q2 |
| B1.2 Customer segment and customer needs | 7.37 | 1.03 | 7.4 | 0.97 | Q4 |
| A1.1 Allocating resources. | 8.57 | 0.63 | 6.83 | 1.02 | Q1 |
| A1.2 Leader vision, Policy | 8.27 | 0.69 | 7.37 | 1.07 | Q2 |
| A1.3 Assuming responsibility. | 7.37 | 1.22 | 7.47 | 1.28 | Q4 |
| A1.4 Supporting employees’ suggestion | 6.97 | 1.4 | 6.37 | 1.22 | Q3 |
| A5.1 Organizational strategy | 8.33 | 0.88 | 6.87 | 1.17 | Q1 |
| B2.1 Creating an organizational goal | 7.3 | 1.02 | 6.83 | 0.87 | Q3 |
| B2.2 Alignment of innovation | 8.1 | 0.8 | 6.17 | 0.91 | Q1 |
| B2.3 Innovation initiative with business needs and strategy | 7.17 | 1.42 | 6 | 0.95 | Q3 |
| A4.2 Educating employees and training programs | 8.6 | 0.56 | 6.53 | 1.2 | Q1 |
| B3.1 Facilities, e.g., laboratories, space, etc. | 8.33 | 0.76 | 6.37 | 1.35 | Q1 |
| B3.2 Budgets | 8.4 | 0.86 | 6.37 | 1.25 | Q1 |
| B3.3 Developing the educational center | 7.33 | 0.96 | 6.2 | 0.89 | Q3 |
| B3.4 Human Resources | 7.23 | 1.01 | 6.33 | 1.15 | Q3 |
| A2.3 An effective system for patient’s rights | 7.3 | 1.06 | 7.93 | 1.14 | Q4 |
| A4.3 Decision-making to solve problems. | 7.2 | 1.58 | 6.13 | 1.25 | Q3 |
| A5.2 Monitoring and evaluation | 8.1 | 0.71 | 7.03 | 1.19 | Q2 |
| A5.5 Risk management | 8.5 | 0.63 | 7.53 | 0.9 | Q2 |
| B4.1 Process management | 8.5 | 0.51 | 6.87 | 0.97 | Q1 |
| B4.2 Internal and External Networking | 8.13 | 0.82 | 7.1 | 1.37 | Q2 |
| B4.3 Knowledge Management | 8.47 | 0.57 | 6.63 | 1.3 | Q1 |
| B4.4 Portfolio Management | 7.13 | 1.01 | 5.7 | 1.21 | Q3 |
| B5.1 Building distinctive competencies and competitive advantage | 8.17 | 0.75 | 6.13 | 1.43 | Q1 |
| B5.3 Establishing an innovation award | 8 | 0.74 | 6.9 | 1.16 | Q2 |
| B5.4 Best practices documented and shared | 7.33 | 1.03 | 5.97 | 1.1 | Q3 |
| A6.1 Information management | 8.43 | 0.77 | 7.27 | 1.11 | Q2 |
| B5.2 Well-defined processes and formalized tools | 8 | 0.95 | 6.4 | 1.04 | Q1 |
| A6.2 Data integrity and security | 8.47 | 0.82 | 7.43 | 1.5 | Q2 |
| A6.3 Data availability and accuracy | 8.53 | 0.78 | 7.5 | 1.04 | Q2 |
| A3.1 Quality audits | 8.17 | 0.83 | 7.33 | 1.06 | Q2 |
| A3.2 Continuous solving | 8.13 | 0.73 | 7.1 | 0.84 | Q2 |
| A3.3 Improving product and process quality | 7.4 | 0.81 | 7.27 | 1.14 | Q4 |
| A3.4 Achieving quality standards | 8.03 | 0.89 | 7.73 | 1.08 | Q2 |
References
- Alwashmi, M.F. The use of digital health in the detection and management of COVID-19. Int. J. Environ. Res. Public Health 2020, 17, 2906. [Google Scholar] [CrossRef]
- Fundin, A.; Lilja, J.; Lagrosen, Y.; Bergquist, B. Quality 2030: Quality management for the future. Total Qual. Manag. Bus. Excell. 2020, 1, 1–17. [Google Scholar] [CrossRef]
- van Kemenade, E.; Hardjono, T.W. Twenty-first century total quality management: The emergence paradigm. Total Qual. Manag. 2019, 31, 150–166. [Google Scholar] [CrossRef]
- Vandenbrande, W.W. Quality for a sustainable future. Total Qual. Manag. Bus. Excell. 2021, 32, 467–475. [Google Scholar] [CrossRef]
- Abbas, J. Crisis management, transnational healthcare challenges and opportunities: The intersection of COVID-19 pandemic and global mental health. Res. Glob. 2021, 3, 100037. [Google Scholar] [CrossRef]
- Mason, K.; Araujo, L. Implementing marketization in public healthcare systems: Performing reform in the English National Health Service. Br. J. Manag. 2021, 32, 473–493. [Google Scholar] [CrossRef]
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef]
- Sheth, J. Impact of Covid-19 on consumer behavior: Will the old habits return or die? J. Bus. Res. 2020, 117, 280–283. [Google Scholar] [CrossRef]
- Abi Younes, G.; Ayoubi, C.; Ballester, O.; Cristelli, G.; de Rassenfosse, G.; Foray, D.; Webster, E. COVID-19: Insights from innovation economists. Sci. Public Policy 2020, 47, 733–745. [Google Scholar] [CrossRef]
- Dutta, S.; Lanvin, B.; Wunsch-Vincent, S. The Global Innovation Index 2018: Energizing the World with Innovation; WIPO: Geneva, Switzerland, 2018. [Google Scholar]
- Heinonen, K.; Strandvik, T. Reframing service innovation: COVID-19 as a catalyst for imposed service innovation. J. Serv. Manag. 2020, 32, 101–112. [Google Scholar] [CrossRef]
- Lee, S.M.; Trimi, S. Convergence innovation in the digital age and in the COVID-19 pandemic crisis. J. Bus. Res. 2021, 123, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K. Integrating technology, innovation and policy: COVID-19 and HTA. Health Policy Technol. 2021, 10, 16–20. [Google Scholar] [CrossRef]
- Soltanisehat, L.; Alizadeh, R.; Hao, H.; Choo, K.-K.R. Technical, Temporal, and Spatial Research Challenges and Opportunities in Blockchain-Based Healthcare: A Systematic Literature Review. IEEE Trans. Eng. Manag. 2020, 67, 1–16. [Google Scholar] [CrossRef]
- Dana, L.-P.; Gurău, C.; Hoy, F.; Ramadani, V.; Alexander, T. Success factors and challenges of grassroots innovations: Learning from failure. Technol. Forecast. Soc. Change 2019, 164, 119600. [Google Scholar] [CrossRef]
- Kaplan, B.; Harris-Salamone, K.D. Health IT success and failure: Recommendations from literature and an AMIA workshop. J. Am. Med. Inform. Assoc. 2009, 16, 291–299. [Google Scholar] [CrossRef]
- Yu, L.; Li, H.; Wang, Z.; Duan, Y. Technology imports and self-innovation in the context of innovation quality. Int. J. Prod. Econ. 2019, 214, 44–52. [Google Scholar] [CrossRef]
- Arumugam, V.; Chang, H.W.; Ooi, K.B.; Teh, P.L. Self-assessment of TQM practices: A case analysis. Total Qual. Manag. 2009, 21, 46–58. [Google Scholar] [CrossRef]
- Choi, D.; Valikangas, L. Patterns of strategy innovation. Eur. Manag. J. 2001, 19, 424–429. [Google Scholar] [CrossRef]
- Choi, T.Y.; Eboch, K. The TQM paradox: Relations among TQM practices, plant performance, and customer satisfaction. J. Oper. Manag. 1998, 17, 59–75. [Google Scholar] [CrossRef]
- Elg, M.; Gremyr, I.; Hellström, A.; Witell, L. The role of quality managers in contemporary organisations. Total Qual. Manag. Bus. Excell. 2011, 22, 795–806. [Google Scholar] [CrossRef]
- Lee, D. The effect of operational innovation and QM practices on organizational performance in the healthcare sector. Int. J. Qual. Innov. 2015, 1, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Perlich, A.; Thienen, J.V.V.; Wenzel, M.; Meinel, C. Learning from success and failure in healthcare innovation: The story of tele-board MED. Des. Think. Res. 2018, 14, 327–345. [Google Scholar]
- Adinolfi, P. Total quality management in public health care: A study of Italian and Irish hospitals. Total Qual. Manag. Bus. Excell. 2003, 14, 141–150. [Google Scholar] [CrossRef]
- Alolayyan, M.N.F.; Mohd Ali, K.A.; Idris, F.; Ibrehem, A.S. Advance mathematical model to study and analyse the effects of total quality management (TQM) and operational flexibility on hospital performance. Total Qual. Manag. Bus. Excell. 2011, 22, 1371–1393. [Google Scholar] [CrossRef]
- Aoun, M.; Hasnan, N. Health-care technology management: Developing the innovation skills through implementing soft TQM among Lebanese hospitals. Total Qual. Manag. Bus. Excell. 2017, 28, 1–11. [Google Scholar] [CrossRef]
- Kaplan, H.C.; Provost, L.P.; Froehle, C.M.; Margolis, P.A. The Model for Understanding Success in Quality (MUSIQ): Building a theory of context in healthcare quality improvement. BMJ Qual. Saf. 2012, 21, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Leslie, M.; Paradis, E.; Gropper, M.A.; Reeves, S.; Kitto, S. Applying ethnography to the study of context in healthcare quality and safety. BMJ Qual. Saf. 2014, 23, 99–105. [Google Scholar] [CrossRef]
- Cunningham, F.C.; Ranmuthugala, G.; Plumb, J.; Georgiou, A.; Westbrook, J.I.; Braithwaite, J. Health professional networks as a vector for improving healthcare quality and safety: A systematic review. BMJ Qual. Saf. 2012, 21, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Suebsakul, T.; Natcha, T. A systematic literature review of TQM and innovation in healthcare. Proceedings of The International Society for Professional Innovation Management (ISPIM), Bangkok, Thailand, 1–3 April 2020; pp. 1–17. [Google Scholar]
- Howze, P.C.; Dalrymple, C. Consensus without all the meetings: Using the Delphi method to determine course content for library instruction. Ref. Serv. Rev. 2004, 32, 174–184. [Google Scholar] [CrossRef]
- Yousuf, M.I. Using expertsopinions through Delphi technique. Pract. Assess. Res. Evaluation. 2007, 12, 4. [Google Scholar]
- Gibson, C.B. Elaboration, generalization, triangulation, and interpretation: On enhancing the value of mixed method research. Organ. Res. Methods. 2017, 20, 193–223. [Google Scholar] [CrossRef] [Green Version]
- Lewis, M.W. Iterative triangulation: A theory development process using existing case studies. J. Oper. Manag. 1998, 16, 455–469. [Google Scholar] [CrossRef]
- Trevelyan, E.G.; Robinson, N. Delphi methodology in health research: How to do it? Eur. J. Integr. Med. 2015, 7, 423–428. [Google Scholar] [CrossRef]
- Joslin, R.; Müller, R. Identifying interesting project phenomena using philosophical and methodological triangulation. Int. J. Constr. Proj. Manag. 2016, 34, 1043–1056. [Google Scholar] [CrossRef]
- Vogel, S.; Kreimeyer, M.; Richter, R.; Spinler, S. The Missing Piece for Persistent Anchoring of Customer Integration in Organizational Processes. IEEE Trans. Eng. Manag. 2021, 1–17. [Google Scholar] [CrossRef]
- Guenzi, P.; Storbacka, K. The organizational implications of implementing key account management: A case-based examination. Ind. Mark. Manag. 2015, 45, 84–97. [Google Scholar] [CrossRef]
- Järvensivu, T.; Törnroos, J.-Å. Case study research with moderate constructionism: Conceptualization and practical illustration. Ind. Mark. Manag. 2010, 39, 100–108. [Google Scholar] [CrossRef]
- Turner, S.F.; Cardinal, L.B.; Burton, R.M. Research design for mixed methods: A triangulation-based framework and roadmap. Organ. Res. Methods 2017, 20, 243–267. [Google Scholar] [CrossRef]
- Fleiszer, A.R.; Semenic, S.E.; Ritchie, J.A.; Richer, M.C.; Denis, J.L. The sustainability of healthcare innovations: A concept analysis. J. Adv. Nurs. 2015, 71, 1484–1498. [Google Scholar] [CrossRef]
- Lennox, L.; Linwood-Amor, A.; Maher, L.; Reed, J. Making change last? Exploring the value of sustainability approaches in healthcare: A scoping review. Health Res. Policy Syst. 2020, 18, 1–24. [Google Scholar] [CrossRef]
- Vergunst, F.; Berry, H.L.; Rugkåsa, J.; Burns, T.; Molodynski, A.; Maughan, D.L. Applying the triple bottom line of sustainability to healthcare research a feasibility study. Int. J. Qual. Health Care 2020, 32, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Ghannadpour, S.F.; Zandieh, F.; Esmaeili, F. Optimizing triple bottom-line objectives for sustainable health-care waste collection and routing by a self-adaptive evolutionary algorithm: A case study from tehran province in Iran. J. Clean. Prod. 2021, 287, 125010. [Google Scholar] [CrossRef]
- Hussain, M.; Ajmal, M.M.; Gunasekaran, A.; Khan, M. Exploration of social sustainability in healthcare supply chain. J. Clean. Prod. 2018, 203, 977–989. [Google Scholar] [CrossRef]
- Moro Visconti, R.; Morea, D. Big data for the sustainability of healthcare project financing. Sustainability 2019, 11, 3748. [Google Scholar] [CrossRef] [Green Version]
- Leite, H.; Bateman, N.; Radnor, Z. Beyond the ostensible: An exploration of barriers to lean implementation and sustainability in healthcare. Prod. Plan. Control. 2020, 31, 1–18. [Google Scholar] [CrossRef]
- Doyle, C.; Howe, C.; Woodcock, T.; Myron, R.; Phekoo, K.; McNicholas, C.; Bell, D. Making change last: Applying the NHS institute for innovation and improvement sustainability model to healthcare improvement. Implement. Sci. 2013, 8, 127. [Google Scholar] [CrossRef] [Green Version]
- Flynn, R.; Mrklas, K.; Campbell, A.; Wasylak, T.; Scott, S.D. Contextual factors and mechanisms that influence sustainability: A realist evaluation of two scaled, multi-component interventions. BMC Health Serv. Res. 2021, 21, 1–17. [Google Scholar] [CrossRef]
- de Fátima Castro, M.; Mateus, R.; Bragança, L. A critical analysis of building sustainability assessment methods for healthcare buildings. Environ. Dev. Sustain. 2015, 17, 1381–1412. [Google Scholar] [CrossRef] [Green Version]
- Asif, M.; Searcy, C.; Garvare, R.; Ahmad, N. Including sustainability in business excellence models. Total Qual. Manag. Bus. Excell. 2011, 22, 773–786. [Google Scholar] [CrossRef]
- Kanji, G.K. Sustainable growth and business excellence. Total Qual. Manag. Bus. Excell. 2005, 16, 1069–1078. [Google Scholar] [CrossRef]
- Chen, R.; Lee, Y.-D.; Wang, C.-H. Total quality management and sustainable competitive advantage: Serial mediation of transformational leadership and executive ability. Total Qual. Manag. Bus. Excell. 2020, 31, 451–468. [Google Scholar] [CrossRef]
- Hassini, E.; Surti, C.; Searcy, C. A literature review and a case study of sustainable supply chains with a focus on metrics. Int. J. Prod. Econ. 2012, 140, 69–82. [Google Scholar] [CrossRef]
- Bai, C.; Dallasega, P.; Orzes, G.; Sarkis, J. Industry 4.0 technologies assessment: A sustainability perspective. Int. J. Prod. Econ. 2020, 229, 107776. [Google Scholar] [CrossRef]
- Lopes, C.M.; Scavarda, A.J.; De Carvalho, M.N.M.; Vaccaro, G.; Korzenowski, A.L. Analysis of sustainability in hospital laundry: The social, environmental, and economic (cost) risks. Resources 2019, 8, 37. [Google Scholar] [CrossRef] [Green Version]
- Maynard, D.d.C.; Vidigal, M.D.; Farage, P.; Zandonadi, R.P.; Nakano, E.Y.; Botelho, R.B.A. Environmental, social and economic sustainability indicators applied to food services: A systematic review. Sustainability 2020, 12, 1804. [Google Scholar] [CrossRef] [Green Version]
- Lindgreen, A.; Antioco, M.; Harness, D.; Van der Sloot, R. Purchasing and marketing of social and environmental sustainability for high-tech medical equipment. J. Bus. Ethics. 2009, 85, 445–462. [Google Scholar] [CrossRef]
- AnAaker, A.; Elf, M. Sustainability in nursing: A concept analysis. Scand. J. Caring Sci. 2014, 28, 381–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoyo-Castelazo, E.; Azapagic, A. Sustainability assessment of energy systems: Integrating environmental, economic and social aspects. J. Clean Prod. 2014, 80, 119–138. [Google Scholar] [CrossRef]
- Spangenberg, J.H. Economic sustainability of the economy: Concepts and indicators. Int. J. Sustain. Dev. 2005, 8, 47–64. [Google Scholar] [CrossRef]
- Dervitsiotis, K.N. The challenge of adaptation through innovation based on the quality of the innovation process. Total Qual. Manag. Bus. Excell. 2011, 22, 553–566. [Google Scholar] [CrossRef]
- Swain, S.; Kar, N.C. Hospital service quality as antecedent of patient satisfaction–a conceptual framework. Int. J. Pharm. Healthc. Mark. 2018, 12, 251–269. [Google Scholar] [CrossRef]
- Padma, P.; Rajendran, C.; Sai, L.P. A conceptual framework of service quality in healthcare: Perspectives of Indian patients and their attendants. Benchmarking 2009, 16, 157–191. [Google Scholar] [CrossRef]
- Omachonu, V.K.; Einspruch, N.G. Innovation in healthcare delivery systems: A conceptual framework. Innov. J. 2010, 15, 1–20. [Google Scholar]
- Gu, D.; Liang, C.; Kim, K.S.; Yang, C.; Cheng, W.; Wang, J. Which is more reliable, expert experience or information itself? weight scheme of complex cases for health management decision making. Int. J. Inf. Technol. Decis. Mak. 2015, 14, 597–620. [Google Scholar] [CrossRef]
- Plsek, P.E.; Wilson, T. Complexity, leadership, and management in healthcare organisations. BMJ. 2001, 323, 746–749. [Google Scholar] [CrossRef]
- Bordoloi, P.; Islam, N. Knowledge management practices and healthcare delivery: A contingency framework. Electron. J. Knowl. Manag. 2012, 10, 110–120. [Google Scholar]
- Hellsten, U.; Klefsjö, B. TQM as a management system consisting of values, techniques and tools. TQM mag. 2000, 12, 238–244. [Google Scholar] [CrossRef]
- Dargan, L.; Shucksmith, M. LEADER and innovation. Sociol. Rural. 2008, 48, 274–291. [Google Scholar] [CrossRef]
- Bouhali, R.; Mekdad, Y.; Lebsir, H.; Ferkha, L. Leader roles for innovation: Strategic thinking and planning. Procedia. Soc. Behav. Sci. 2015, 181, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Zhuhadar, L.; Thrasher, E.; Marklin, S.; de Pablos, P.O. The next wave of innovation—Review of smart cities intelligent operation systems. Comput. Hum. Behav. 2017, 66, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Afuah, A. Innovation Management-Strategies, Implementation, and Profits; Oxford University Press: New York, NY, USA, 2020. [Google Scholar]
- Gawer, A.; Cusumano, M.A. Industry platforms and ecosystem innovation. J. Prod. Innov. Manag. 2014, 31, 417–433. [Google Scholar] [CrossRef] [Green Version]
- Leede, J.D.; Looise, J.C.; Alders, B.C. Innovation, improvement and operations: An exploration of the management of alignment. Int. J. Technol. Manag. 2002, 23, 353–368. [Google Scholar] [CrossRef]


| Expert Categories | Required Qualification | Number of the Expert Panel |
|---|---|---|
| Academics | More than 5 years of experience in the academic area with a Ph.D. | 6 |
| CEO/Directors | Top management in healthcare and more than 5 years of experience in the healthcare position. | 6 |
| Healthcare quality assurancespecialist | Healthcare quality assurance specialist with healthcare quality certification and more than 5 years of experience in the healthcare position. | 6 |
| Innovation specialist in healthcare | Healthcare innovation specialist and more than 5 years of experience in the healthcare position. | 6 |
| Project development specialist in healthcare | Project manager/technical specialist and more than 5 years of experience in the healthcare position. | 6 |
| Total | 30 |
| Dimension | Sub-Factor | Reference |
|---|---|---|
| Context of the Environment (Internal and External) | A2.1 Customer (patient, etc.) satisfaction | [41,42,45,47,48,50,51,52,53,54,56,57,59,60,62] |
| A2.2 Solving the patient’s complaints. | [42,45,47,51,52,53,54,57,59,60,62] | |
| A4.1 Informing the hospital’s achievements | [41,48,50,52,53,56,60,61,62] | |
| A5.4 Litigation law refers to the rules and practices | [41,44,46,48,51,53,55,56,61,62] | |
| B1.1 Technological change | [42,43,45,46,47,49,50,53,55,56,59,60,61] | |
| B1.2 Customer segment and customer needs | [41,45,47,51,52,54,56,59,60,61] | |
| Leader | A1.1 Resources allocation from the leader | [41,43,44,45,46,47,48,49,50,51,52,53,55,56,58,59,60,62] |
| A1.2 Leader vision, Policy | [41,43,45,46,47,48,50,52,53,54,55,56,57,58,60,61] | |
| A1.3 Assuming responsibility from the leader | [43,45,47,48,50,54,55,57,58,61,62] | |
| A1.4 Supporting employees’ suggestions from the leader | [41,46,47,48,50,54,56,57,60,61] | |
| Planning | A5.1 Organizational strategy | [42,45,46,47,49,50,51,52,53,54,56,57,60] |
| B2.1 Creating an organizational goal | [42,43,46,50,51,52,56,57,59,60,61] | |
| B2.2 Alignment of innovation | [45,48,49,50,53,54,56,60] | |
| B2.3 Innovation initiative with business needs and strategy | [41,42,45,46,48,50,51,55,56,59,61,62] | |
| Support | A4.2 Educating employees and training programs. | [43,46,51,54,55,56,57,59,61,62] |
| B3.1 Facilities, e.g., laboratories, space, etc. | [41,42,43,46,49,50,52,53,54,56,57,60,61] | |
| B3.2 Budgets | [41,43,44,47,49,50,54,55,57,58,59,61,62] | |
| B3.3 Developing the educational center | [43,46,51,54,55] | |
| B3.4 Human Resources | [41,42,43,46,51,55,56,59,61] | |
| Operation | A2.3 An effective system for patient’s rights | [42,43,47,50,52,53,56,57,59,61,62] |
| A4.3 Decision-making to solve problems. | [44,45,47,49,50,52,54,55,56,59,61] | |
| A5.2 Monitoring and evaluation | [41,42,45,47,48,51,52,54,57,61,62] | |
| A5.3 Risk management | [41,42,45,46,47,48,50,51,55,57,58,60,61,62] | |
| B4.1 Process management | [41,42,43,44,46,49,50,51,52,53,54,56,57,59,60,61,62] | |
| B4.2 Internal and External Networking | [41,44,47,48,51,53,56,61,62] | |
| B4.3 Knowledge Management | [41,42,43,45,47,49,50,52,54,55,57,58,59,61] | |
| B4.4 Portfolio Management | [43,51,54,56,61] | |
| B5.1 Building distinctive competencies and competitive advantage | [41,43,44,46,47,48,49,51,55,59,60,62] | |
| B5.3 Establishing an innovation award | [41,46,50,56,61] | |
| B5.4 Best practices documented and shared | [41,44,49,50,55,57,59,61,62] | |
| Tools and Analysis method | A6.1 Information management | [42,45,46,49,50,52,56,59,60,61] |
| B5.2 Well-defined processes and formalized tools | [41,44,46,47,52,55,57,60,61,62] | |
| A6.2 Data integrity and security | [41,43,46,47,50,52,53,55,57,58,59,61,62] | |
| A6.3 Data availability and accuracy | [41,42,43,45,46,47,48,50,51,53,54,55,57,60,61,62] | |
| Improvement | A3.1 Quality audits | [41,42,43,45,47,48,50,51,52,53,56,57,58,59,61,62] |
| A3.2 Continuous solving problems | [41,42,43,47,48,50,51,52,53,55,56,57,58,59,61,62] | |
| A3.3 Improving product and process quality | [41,42,47,50,51,53,55,56,58,59,61,62] | |
| A3.4 Achieving quality standards | [41,42,46,47,49,51,53,54,57,59,60] |
| Dimension | Sub-Factor | Case Study | Sum | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |||
| Context of the Environment (Internal and External) | A2.1 Customer (patient etc.) satisfaction | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 14 | ||||||
| A2.2 Solving the patient’s complaints | / | / | / | / | / | / | 6 | |||||||||||||||
| A4.1 Informing the hospital’s achievements | / | / | / | / | / | / | / | / | / | / | 10 | |||||||||||
| A5.4 Litigation law refers to the rules and practices | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 15 | ||||||
| B1.1 Technological change | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 15 | ||||||
| B1.2 Customer segment and customer needs | / | / | / | / | / | / | / | / | / | / | / | 11 | ||||||||||
| Leader | A1.1 Resources allocation from the leader | / | / | / | / | / | / | / | / | / | / | / | / | / | 13 | |||||||
| A1.2 Leader vision, Policy | / | / | / | / | / | / | / | / | / | / | / | / | 12 | |||||||||
| A1.3 Assuming responsibility from the leader | / | / | / | / | / | / | / | / | / | 9 | ||||||||||||
| A1.4 Supporting employees’ suggestions from the leader | / | / | / | / | / | / | / | 7 | ||||||||||||||
| Planning | A5.1 Organizational strategy | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 13 | ||||||
| B2.1 Creating an organizational goal | / | / | / | / | / | / | / | 7 | ||||||||||||||
| B2.2 Alignment of innovation | / | / | / | / | / | / | / | / | / | / | / | 11 | ||||||||||
| B2.3 Innovation initiative with business needs and strategy | / | / | / | / | / | / | 6 | |||||||||||||||
| Support | A4.2 Educating employees and training programs. | / | / | / | / | / | / | / | / | / | / | 10 | ||||||||||
| B3.1 Facilities, e.g., laboratories, space, etc. | / | / | / | / | / | / | / | / | / | / | / | / | 12 | |||||||||
| B3.2 Budgets | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 16 | |||||
| B3.3 Developing the educational center | / | / | / | 3 | ||||||||||||||||||
| B3.4 Human Resources | / | / | / | / | / | / | / | / | / | / | / | / | / | 13 | ||||||||
| Operation | A2.3 An effective system for patient’s rights | / | / | / | / | / | / | / | / | 8 | ||||||||||||
| A4.3 Decision-making to solve problems. | / | / | / | / | / | / | / | 7 | ||||||||||||||
| A5.2 Monitoring and evaluation | / | / | / | / | / | / | / | / | / | / | / | / | 12 | |||||||||
| A5.5 Risk management | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 14 | |||||||
| B4.1 Process management | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 20 | |
| B4.2 Internal and External Networking | / | / | / | / | / | / | / | / | / | / | / | / | / | 13 | ||||||||
| B4.3 Knowledge Management | / | / | / | / | / | / | / | / | / | / | 10 | |||||||||||
| B4.4 Portfolio Management | / | / | / | / | / | / | 6 | |||||||||||||||
| B5.1 Building distinctive competencies and competitive advantage | / | / | / | / | / | / | / | / | / | / | / | 11 | ||||||||||
| B5.3 Establishing an innovation award | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 15 | ||||||
| B5.4 Best practices documented and shared | / | / | / | / | / | / | / | / | / | 9 | ||||||||||||
| Tools and Analysis method | A6.1 Information management | / | / | / | / | / | / | / | / | / | / | / | / | / | 13 | |||||||
| B5.2 Well-defined processes and formalized tools | / | / | / | / | / | / | / | / | / | / | / | / | 12 | |||||||||
| A6.2 Data integrity and security | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 16 | |||||
| A6.3 Data availability and accuracy | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 17 | ||||
| Improvement | A3.1 Quality audits | / | / | / | / | / | / | / | / | / | / | / | / | / | 13 | |||||||
| A3.2 Continuous solving problems | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 14 | |||||||
| A3.3 Improving product and process quality | / | / | / | / | / | / | / | / | / | 9 | ||||||||||||
| A3.4 Achieving quality standards | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / | 15 | ||||||
| Quadrant | Characteristic | TQIM-H Factor |
|---|---|---|
| 1 | “Concentrate here” (high importance and low performance). This quadrant shows that a company’s performance does not meet the importance level of its products and services. Therefore, management needs to focus on improving current products and services performance. | The first quadrant has 10 TQIM-H factors, including
|
| 2 | Quadrant 2: “Keep up the good work” presented high importance and high performance of each TQIM-H factor. Attributes plotted in this area show that the hospital must continue to perform well, as the attributes are considered important. The attributes in this quadrant may be viewed as a set of opportunities to continue doing a good job over competitors. | The second quadrant has 14 TQIM-H factors, including
|
| 3 | Quadrant 3: “Low priority” (low importance and low performance). Customers perceive attributes in this area as unimportant and communicate that the company is not performing well. | The third quadrant has nine TQIM-H factors, including
|
| 4 | Quadrant 4: “Possible overkill” (low importance and high performance). For each attribute in this area, customers evaluate its performance as exceeding its importance. Therefore, too much attention paid to this area could represent overkill concerning the use of resources that could be better directed to other areas, although high performance on an attribute in this area could be considered a strength in that it may enable the company to attract new customers (Gates and Amarani, 1992). | The fourth quadrant has five TQIM-H factors, including
|
| Respondents’ Demographics | Frequency | Percent |
|---|---|---|
| Gender | ||
| Men | 19 | 38 |
| Women | 31 | 62 |
| Total | 50 | 100 |
| Age | ||
| <30 years | 3 | 6 |
| 30–39 years | 11 | 22 |
| 40–49 years | 19 | 38 |
| 50–59 years | 12 | 24 |
| >60 years | 5 | 10 |
| Total | 50 | 100 |
| Level of education | ||
| Bachelor’s degree | 28 | 56 |
| Master’s degree | 16 | 32 |
| Doctorate | 6 | 12 |
| Total | 50 | 100 |
| Position | ||
| President/Director/Manager | 17 | 34 |
| Physician/Dentist/Pharmacist | 12 | 24 |
| Medical technician/Radiologist/Physiotherapist/Nutritionist | 2 | 4 |
| Nurse/Nursing Assistant | 8 | 16 |
| Customer service | 2 | 4 |
| Office workers/Support staff | 7 | 14 |
| Other | 2 | 4 |
| Total | 50 | 100 |
| Working Experience | ||
| <10 years | 8 | 16 |
| 10–20 years | 32 | 64 |
| >20 years | 10 | 20 |
| Total | 50 | 100 |
| The TQIM-H conceptual framework experience | ||
| Not used to | 50 | 100 |
| Used to | 0 | 0 |
| Total | 50 | 100 |
| Preference to use the TQIM-H conceptual framework | ||
| Acceptation | 50 | 100 |
| Rejection | 0 | 0 |
| Total | 50 | 100 |
| The Program Characteristic | Mean | SD |
|---|---|---|
| 1. Effective implementation of TQIM-H conceptual framework to develop quality innovation projects in healthcare | 4.67 | 0.52 |
| (1.1) Decreases time wasted in developing quality and innovation projects in healthcare | 4.75 | 0.65 |
| (1.2) Provides an effective process for developing quality and innovation projects in the healthcare | 4.62 | 0.54 |
| (1.3) Be comprehensive and completely cover the development of quality and innovation projects in the healthcare | 4.74 | 0.69 |
| (1.4) Is a modern and acceptable conceptual framework | 4.56 | 0.78 |
| 2. Ease of use | 4.61 | 0.92 |
| (2.1) The objective of using the TQIM-H conceptual framework is clear | 4.55 | 0.80 |
| (2.2) The operation procedure of the TQIM-H conceptual framework is clear and easy to understand | 4.64 | 0.48 |
| (2.3) The conceptual framework is easy to learn and understand. Self-study using the instructions TQIM-H conceptual framework is easy | 4.58 | 0.32 |
| (2.4) A healthcare innovator can easily use the TQIM-H conceptual framework to develop quality and innovation projects in the healthcare | 4.76 | 0.58 |
| (2.6) TQIM-H conceptual framework is easy to use. | 4.54 | 0.65 |
| 3. User Interface | 4.60 | 0.49 |
| (3.1) TQIM-H conceptual framework is attractive | 4.68 | 0.75 |
| (3.2) TQIM-H conceptual framework is up-to-date | 4.60 | 0.92 |
| (3.3) The diagram of the TQIM-H conceptual framework is appropriate | 4.52 | 0.81 |
| 4. The comparison of the quality and innovation project development in healthcare through the TQIM-H conceptual framework and the traditional developed innovation project in healthcare without the conceptual framework. | N/A | N/A |
| (4.1) The conceptual framework reduces time spent collecting, analyzing, and processing to develop quality and innovative projects in healthcare | N/A | N/A |
| Before the TQIM-H conceptual framework is used | 3.34 | 0.67 |
| After the TQIM-H conceptual framework is used | 4.54 | 0.83 |
| (4.2) The conceptual framework reduces skills, expertise and reduces decisions using experience to measure and evaluate develop quality and innovation projects in healthcare | N/A | N/A |
| Before the TQIM-H conceptual framework is used | 3.48 | 0.59 |
| After the TQIM-H conceptual framework is used | 4.76 | 0.68 |
| (4.3) The conceptual framework provides a systematic work process that is clear so using the program is convenient and easy. | N/A | N/A |
| Before the TQIM-H conceptual framework is used | 3.12 | 0.95 |
| After the TQIM-H conceptual framework is used | 4.82 | 0.87 |
| (4.4) The conceptual framework reduces work processes and eliminates the duplication of operations. | N/A | N/A |
| Before the TQIM-H conceptual framework is used | 3.26 | 0.75 |
| After the TQIM-H conceptual framework is used | 4.86 | 0.64 |
| 5. The practical concept of the TQIM-H conceptual framework | 4.66 | 0.38 |
| (5.1) TQIM-H conceptual framework can be applied to quality and innovation project development in healthcare effectively. | 4.70 | 0.96 |
| (5.2) TQIM-H conceptual framework leads to the improvement of processes involved in the development of quality and innovation projects in healthcare. | 4.62 | 0.94 |
| TQIM-H | Procedure |
|---|---|
| Context of the Environment (Internal and External) |
|
| Leader |
|
| Planning |
|
| Operation |
|
| Tools and Analysis method |
|
| Support |
|
| Improvement |
|
| Organizational performance measurement |
IPD service time was reduced by 48%. Nurse man-hours were reduced by 33%. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonjang, S.; Thawesaengskulthai, N. Total Quality and Innovation Management in Healthcare (TQIM-H) for an Effective Innovation Development: A Conceptual Framework and Exploratory Study. Appl. Syst. Innov. 2022, 5, 70. https://doi.org/10.3390/asi5040070
Tonjang S, Thawesaengskulthai N. Total Quality and Innovation Management in Healthcare (TQIM-H) for an Effective Innovation Development: A Conceptual Framework and Exploratory Study. Applied System Innovation. 2022; 5(4):70. https://doi.org/10.3390/asi5040070
Chicago/Turabian StyleTonjang, Suebsakul, and Natcha Thawesaengskulthai. 2022. "Total Quality and Innovation Management in Healthcare (TQIM-H) for an Effective Innovation Development: A Conceptual Framework and Exploratory Study" Applied System Innovation 5, no. 4: 70. https://doi.org/10.3390/asi5040070
APA StyleTonjang, S., & Thawesaengskulthai, N. (2022). Total Quality and Innovation Management in Healthcare (TQIM-H) for an Effective Innovation Development: A Conceptual Framework and Exploratory Study. Applied System Innovation, 5(4), 70. https://doi.org/10.3390/asi5040070







