Possible Coevolution of Vampire Bats (Chiroptera: Phyllostomidae: Desmodus) and Large Xenarthrans (Cingulata, Pilosa) in North America and South America During the Quaternary
Abstract
1. Introduction
2. Methods and Materials
3. Fossil Record of Vampire Bats
4. Evolutionary History of Vampire Bats
4.1. South American
4.2. GABI–Late Miocene
4.3. GABI–Pliocene-Pleistocene
4.4. Holocene
4.5. Post-Columbian
5. Paleoecology of Vampire Bats
5.1. Diet of Living Vampire Bats
5.2. Diet of Extinct Vampire Bats
5.3. Evolution of Feeding Behavior in Vampire Bats
6. Extinction of Desmodus draculae and D. stocki
7. Future Research
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
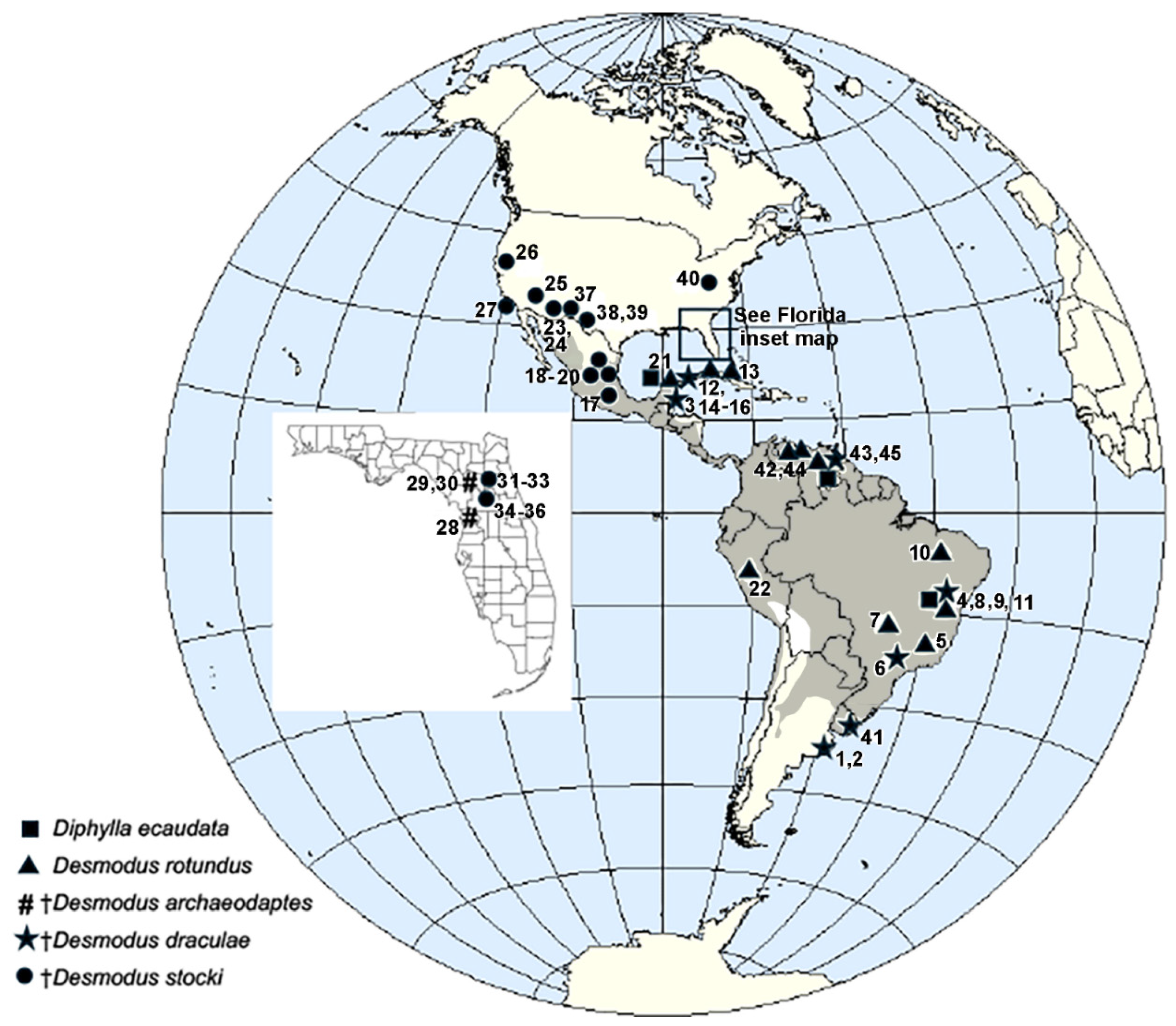
Appendix A
| Locality | Latitude, Longitude, & Elevation | Site Type | Age | Vampire Bat Species | Associated Large Mammals of South American Origin (Genus/Species) 1 | References |
| Argentina | ||||||
| 1. Centinela del Mar Buenos Aires Province | 38°26′ S 58°13′ W | open | L. Holocene | † Desmodus cf. draculae | None | [42] |
| 2. La Ballenera Buenos Aires Province | 38° 19′ S 57° 56′ W | cave | L. Pleistocene Lujanian | † Desmodus draculae | Mylodontidae Glyptodon reticulatus | [43] |
| Belize | ||||||
| 3. Cebada Cave Chiquibul Cave System Cayo District | cave | L Pleistocene Rancholabrean | † Desmodus draculae | None | [16] | |
| Brazil | ||||||
| 4. Gruta dos Brejões Bahia State | cave | L. Pleistocene; 14C date of 12,200 yrBP 2 | Desmodus rotundus | Catonyx cuvieri Eremotherium laurillardi, Glossotherium aff. lettsomi, Glossotherium robustum, Mylodon darwini, Mylodon ebseni, Ocnotherium giganteum Nothrotherium maquinense Myrmecophaga tridactyla Pampatherium humboldtii Coendou sp. Xenorhinotherium bahiense | [13,48] | |
| 5. Lapa da Lagoa do Sumidouro, near Lagoa Santa Minas Gerais State | 19°38′ S 43°53′ W | cave | L. Pleistocene or Holocene | Desmodus rotundus | [18,231] | |
| 6. Santana Cave Ribeira River Valley São Paulo State | 24°32′ S 48°42′ W | cave | L. Pleistocene | † Desmodus draculae | Eremotherium Scelidotherium (from other nearby caves) | [47] |
| 7. Serra da Mesa Goiás State | cave | L. Pleistocene or Holocene | Desmodus rotundus | none | [49] | |
| 8. Toca da Barriguda Bahia State | cave | Desmodus rotundus Diphylla ecaudata | Nothrotherium maquinense, Tamandua tetradactyla, Coendou magnus | [13] | ||
| 9. Toca da Boa Vista Bahia State | 600 m | cave | L. Pleistocene | †Desmodus draculae Desmodus rotundus Diphylla ecaudata | Catonyx cuvieri Nothrotherium maquinense Myrmecophaga tridactyla Euphractus sexcinctus Coendou prehensilis Cartelles coimbrafilhoi Caipora bambuiorum | [13,46,50,51,52,53] |
| 10. Toca do Gordo do Garrincho, Serra da Capivara Piauí State | cave | L. Pleistocene or Holocene | Desmodus rotundus | [54] | ||
| 11. Toca dos Ossos Bahia State | cave | L. Pleistocerne | † Desmodus draculae | Catonyx cuvieri, Eremotherium laurillardi, Glossotherium aff. lettsomi, Mylodonopsis ibseni, Nothrotherium maquinense, Ocnotherium giganteum, Glyptodon clavipes, Pampatherium humboldtii, Myrmecophaga tridactyla, Hydrochoerus hydrochaeris, Neochoerus sulcidens, Toxodon platensis, Trigonodops lopesi | [46,50,51,52,53] | |
| Cuba | ||||||
| 12. Cuevas Blancas Habana Province | 22°53′ N 82°19′ W | cave | Holocene 7864 ± 96 yrBP | Desmodus rotundus | None | [59,60] |
| 13. Cueva Centenario de Lenin Villa Clara Province | 22°24′ N 79°01′ W | cave | Holocene | Desmodus rotundus (=D. puntajudensis type locality) | [57,58,60] | |
| 14. Cueva Lamas Habana Province | 23°45′ N 82°32′ W | cave | L. Pleistocene or Holocene | Desmodus rotundus | Megalocnus rodens, Mesocnus torrei | [56,60] |
| 15. Cueva de los Nesophontes Habana-Matanzas Provinces | cave | L. Holocene | Desmodus rotundus | None | [61] | |
| 16. Cueva de Paredones Habana Province | cave | L. Pleistocene or Holocene | Desmodus rotundus | [58,60] | ||
| Mexico | ||||||
| 17. Cerro de Tlapacoya Estado de Mexico | 19°18′ N 98°55′ W 2240 m | cave | L. Pleistocene Rancholabrean | † Desmodus stocki | Neochoerus aesopi | [18,173,189,193] |
| 18. Cueva de la Boca Nuevo Leon | 25°25′ N 100°09′ W 540 m | cave | L. Pleistocene Rancholabrean | † Desmodus stocki | None | [40,175] |
| 19. Cueva de la Presita 3 San Luis Potosí | 23°30′ N 100°37′ W 1540 m | cave | L. Pleistocene Rancholabrean | † Desmodus stocki | Nothrotheriops shastensis | [16,18,173,188] |
| 20. Cueva de San Josecito (=San Josecito Cave) Nuevo León | 24°06′ N 99°49′ W 2250 m | cave | L. Pleistocene Rancholabrean | † Desmodus stocki (type locality) | Megalonyx jeffersonii, Nothrotheriops shastensis | [16,18,31,33,173,191] |
| 21. Gruta de Loltún (=Loltún Cave) Yucatán | 20°15′ N 89°28′ W 40 m | cave | L. Pleistocene | †Desmodus draculae Desmodus rotundus Diphylla ecaudata | None | [18,45,62,194] |
| Peru | ||||||
| 22. Jatun Uchco Departamento de Huánuco | cave | Pleistocene | Desmodus sp. | Diablotherium, Megatherium, Scelidodon | [55] | |
| United States | ||||||
| Arizona | ||||||
| 23. Arkenstone Cave Pima Co. | cave | L. Pleistocene Rancholabrean | † Desmodus stocki | None | [36] | |
| 24. La Tetera Cave Pima Co. | cave | L. Pleistocene Rancholabrean 23,745 yrBP | † Desmodus stocki | None | [37] | |
| 25. Rampart Cave Mohave Co. | 36°06′ N 113°56′ W | cave | L. Pleistocene Rancholabrean | † Desmodus stocki | Nothrotheriops shastensis | [16,18,181] |
| California | ||||||
| 26 Potter Creek Cave Shasta Co. | 40°47′ N 122°17′ W | cave | L. Pleistocene Rancholabrean | † Desmodus stocki | Nothrotheriops shastensis Megalonyx jeffersonii | [16,33,232] |
| 27. San Miguel Island, Santa Barbara Co | 34° N 120°20′ W | cave arch. site 3 | Holocene ~3–5000 yr BP | † Desmodus stocki | none | [38,39] |
| Florida | ||||||
| 28. Inglis 1A Citrus Co. | 29°01′ N 82°41′ W El.: sea level | karst fissure/ sinkhole | E. Pleistocene L. Blancan | † Desmodus archaeodaptes | Titanis walleri Eremotherium eomigrans Megalonyx leptostomus Paramylodon harlani Dasypus bellus Glyptotherium texanum Holmesina floridanus Neochoerus sp. | [10,11,23] |
| 29. Haile 16A Alachua Co. | 29°41′ N 82°34′ W El.: 30 m | karst fissure/ sinkhole | E. Pleistocene E. Irvingtonian | † Desmodus archaeodaptes | Eremotherium eomigrans Megalonyx wheatleyi Paramylodon harlani Dasypus bellus Holmesina floridanus Pachyarmatherium leiseyi | [10,11,23] |
| 30. Haile 21A Alachua Co. | 29°41′ N 82°35′ W El.: 30 m | karst fissure/ sinkhole | E. Pleistocene E. Irvingtonian | † Desmodus archaeodaptes (type locality) | Eremotherium eomigrans Dasypus bellus | [10,11,23] |
| 31. Arredondo 2A Alachua Co. | 29°37′ N 82°24′ W El.: 30 m | karst fissure/ sinkhole | L. Pleistocene Rancholabrean | † Desmodus stocki | Paramylodon harlani Dasypus bellus | [10,11,18,227] |
| 32. Haile 1A Alachua Co. | 29°41′ N 82°34′ W El.: 30 m | karst fissure/ sinkhole | L. Pleistocene Rancholabrean | † Desmodus stocki | none | [10,11,32] |
| 33. Haile 11B Alachua Co. | 29°41′N’ 82°34′W El.: 30 m | karst fissure/ sinkhole | L Pleistocene Rancholabrean | † Desmodus stocki | Dasypus bellus | [18,33] |
| 34. Reddick 1A Marion Co. | 29°22′ N 82°11′ W El.: 22 m | karst fissure/ sinkhole | L Pleistocene Rancholabrean | † Desmodus stocki (type locality of † D. magnus) | Megalonyx jeffersonii Paramylodon harlani Dasypus bellus Holmesina septentrionalis | [10,11,32,233] |
| 35. Reddick 1B Marion Co. | 29°22′ N 82°11′ W El.: 22 m | karst fissure/ sinkhole | L Pleistocene Rancholabrean | † Desmodus stocki | Megalonyx jeffersonii Paramylodon harlani Dasypus bellus | [10] |
| 36 Reddick 1C Marion Co. | 29°22′ N 82°11′ W El.: 22 m | karst fissure/ sinkhole | L Pleistocene Rancholabrean | † Desmodus stocki | none | [10] |
| New Mexico | ||||||
| 37. U-Bar Cave Hidalgo Co. | 31°29′ N 108°26′ W El.: 1570 m | cave | L. Pleistocene Rancholabrean 26,150–35,890 yrBP (14C dates) | † Desmodus stocki | Nothrotheriops shastensis | [16,18,35,183,184] |
| Texas | ||||||
| 38. Sierra Diablo Cave Hudspeth Co. | El.: 1660 m | cave | L. Pleistocene Rancholabrean ~35,000 yrBP | † Desmodus stocki | Nothrotheriops shastensis | [35] |
| 39 Terlingua Brewster Co. | 29°19′ N 103°31′ W | mine/ fissure | L. Pleistocene | † Desmodus stocki | none | [185,186] |
| West Virginia | ||||||
| 40 New Trout Cave Pendleton Co. | 38°39’ N 79°23′ W El.: 570 m | cave | L. Pleistocene Rancholabrean >29,400 yrBP | † Desmodus stocki | Megalonyx jeffersonii | [18,34,175,176,232] |
| Uruguay | ||||||
| 41. Kiyú locality Raigón Formation San José Department | open site | L. Pliocene or E. to M. Pleistocene | † Desmodus aff. draculae | medium to large ground sloths, glyptodonts, litopterns, notoungulates, dinomyid rodents, and large phorusrhacid birds | [24] | |
| Venezuela | ||||||
| 42. Cueva de la Brújula Miranda State | 10.45° N 66.77° W | cave | Holocene | Desmodus rotundus | none | [18,234] |
| 43. Cueva del Guácharo Monagas State | 10°10’ N’ 62°33′ W | cave | L. Pleistocene/Holocene | †Desmodus draculae (type locality) Desmodus rotundus Diphylla ecaudata | none | [18,23] |
| 44 Cueva de Quebrada Honda Aragua State | 09°56′ N 67°15′ W | cave | Holocene | Desmodus rotundus | none | [18,235] |
| 45. El Breal de Orocual Monagas State | open site tar pit | L. Pliocene to E. Pleistocene | cf. Desmodus sp. | Eremotherium sp., Megalonychidae, Propraopus sulcatus, Glyptodon sp., Hoplophorus sp., Pachyarmatherium cf. leiseyi, Holmesina occidentalis, Pampatherium humboldtii, cf. Myrmecophaga, cf. Chapalmatherium, Mixotoxodon larensis | [17,25] |
References
- Koopman, K.F. Systematics and Distribution. In Natural History of Vampire Bats; Greenhall, A.M., Schmidt, U., Eds.; CRC Press: Boca Raton, FL, USA, 1988; pp. 7–17. [Google Scholar]
- Greenhall, A.M.; Joermann, G.; Schmidt, U. Desmodus rotundus. Mammal. Spec. 1983, 202, 1–6. [Google Scholar] [CrossRef]
- Greenhall, A.M. Feeding behavior. In Natural History of Vampire Bats; Greenhall, A.M., Schmidt, U., Eds.; CRC Press: Boca Raton, FL, USA, 1988; pp. 111–131. [Google Scholar]
- Webb, S.D. Mammalian faunal dynamics of the Great American Interchange. Paleobiology 1976, 2, 220–234. [Google Scholar] [CrossRef]
- Webb, S.D. A history of savanna vertebrates in the New World. Part II: South America and the Great Interchange. Ann. Rev. Ecol. Syst. 1978, 9, 393–426. [Google Scholar] [CrossRef]
- Webb, S.D. Late Cenozoic mammal dispersals between the Americas. In The Great American Biotic Interchange; Stehli, F.G., Webb, S.D., Eds.; Plenum Press: New York, NY, USA, 1985; pp. 357–386. [Google Scholar]
- Webb, S.D. Ecogeography and the Great American Interchange. Paleobiology 1991, 17, 266–280. [Google Scholar] [CrossRef]
- Webb, S.D. The Great American Biotic Interchange: Patterns and processes. Ann. Missouri Bot. Gard. 2006, 93, 245–257. [Google Scholar] [CrossRef]
- Stehli, F.G.; Webb, S.D. (Eds.) The Great American Biotic Interchange; Plenum Press: New York, NY, USA, 1985. [Google Scholar]
- Morgan, G.S. Neotropical Chiroptera from the Pliocene and Pleistocene of Florida. Bull. Amer. Mus. Natl. Hist. 1991, 206, 176–213. [Google Scholar]
- Morgan, G.S. The Great American Biotic Interchange in Florida. Bull. Florida Mus. Nat. Hist. 2005, 45, 271–311. [Google Scholar] [CrossRef]
- Morgan, G.S. Vertebrate fauna and geochronology of the Great American Biotic Interchange in North America. New Mexico Mus. Nat. Hist. Sci. Bull. 2008, 44, 93–140. [Google Scholar]
- Czaplewski, N.J.; Cartelle, C. Pleistocene bats from cave deposits in Bahia, Brazil. J. Mammal. 1998, 79, 784–803. [Google Scholar] [CrossRef]
- Czaplewski, N.J.; Krejca, J.; Miller, T.E. Late Quaternary Bats from Cebada Cave, Chiquibul Cave System, Belize. Carib. J. Sci. 2003, 39, 23–33. [Google Scholar]
- McDonald, H.G. Paleoecology of extinct xenarthrans and the Great American Biotic Interchange. Bull. Florida Mus. Nat. Hist. 2005, 45, 313–333. [Google Scholar]
- McDonald, H.G.; Jefferson, G.T. Distribution of Pleistocene Nothrotheriops (Xenarthra, Nothrotheriidae) in North America. Nat. Hist. Mus. LA Co. Sci. Ser. 2008, 41, 313–331. [Google Scholar]
- Czaplewski, N.J.; Rincón, A.D. A giant vampire bat (Phyllostomidae, Desmodontinae) from the Pliocene-Pleistocene El Breal de Orocual asphaltic deposits (tar pits), Venezuela. Hist. Biol. 2020, 33, 2438–2443. [Google Scholar] [CrossRef]
- Ray, C.E.; Linares, O.J.; Morgan, G.S. Paleontology. In Natural History of Vampire Bats; Greenhall, A.M., Schmidt, U., Eds.; CRC Press: Boca Raton, FL, USA, 1988; pp. 19–30. [Google Scholar]
- Koopman, K.F. Zoogeography. In Biology of Bats of the New World Family Phyllostomatidae, Part 1; Baker, R.J., Jones, J.K., Jr., Carter, D.C., Eds.; Texas Tech Press: Lubbock, TX, USA, 1976; pp. 39–47. [Google Scholar]
- Koopman, K.F. Biogeography of the bats of South America. In Mammalian Biology in South America; Mares, M.A., Genoways, H.H., Eds.; Pymatuning Laboratory of Ecology at the University of Pittsburgh: Linesville, PA, USA, 1982; Volume 6, pp. 273–302. [Google Scholar]
- Baker, R.J.; Bininda-Emonds, O.R.P.; Mantilla-Meluk, H.; Porter, C.A.; van den Bussche, R.A. Molecular time scale of diversification of feeding strategy morphology in New World leaf-nosed bats (Phyllostomidae): A phylogenetic perspective. In Evolutionary History of Bats: Fossils, Molecules and Morphology; Gunnell, G.F., Simmons, N.B., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 385–409. [Google Scholar]
- Rojas, D.; Warsi, O.M.; Dávalos, L.M. Bats (Chiroptera: Noctilionoidea) challenge a recent origin of extant Neotropical diversity. Syst. Biol. 2016, 65, 432–448. [Google Scholar] [CrossRef]
- Morgan, G.S.; Linares, O.J.; Ray, C.E. New species of fossil vampire bats (Mammalia, Chiroptera, Desmodontidae) from Florida and Venezuela. Proc. Biol. Soc. Washington 1988, 101, 912–928. [Google Scholar]
- Ubilla, M.; Gaudioso, P.; Perea, D. First fossil record of a bat (Chiroptera, Phyllostomidae) from Uruguay (Plio-Pleistocene, South America): A giant desmodontine. Hist. Biol. 2021, 33, 137–145. [Google Scholar] [CrossRef]
- Rincón, A.D.; Parra, G.E.; Prevosti, F.J.; Alberdi, M.T.; Bell, C.J. A preliminary assessment of the mammalian fauna from the Pliocene-Pleistocene El Breal de Orocual locality, Monagas state, Venezuela. Mus. North. Arizona Bull. 2009, 65, 593–620. [Google Scholar]
- McNab, B.K. Energetics and the distribution of vampires. J. Mammal. 1973, 54, 131–144. [Google Scholar] [CrossRef]
- Kurtén, B.; Anderson, E. Pleistocene Mammals of North America; Columbia University Press: New York, NY, USA, 1980; 442p. [Google Scholar]
- Martin, R.A. Fossil mammals from the Coleman IIA Fauna, Sumter County. In Pleistocene Mammals of Florida; Webb, S.D., Ed.; University Presses of Florida: Gainesville, FL, USA, 1974; pp. 35–99. [Google Scholar]
- Morgan, G.S. The extinct free-tailed bat Tadarida constantinei and associated vertebrates from Pleistocene deposits in Slaughter Canyon Cave, Carlsbad Caverns National Park, southeastern New Mexico. New Mexico Geol. 2003, 25, 43. [Google Scholar]
- Morgan, G.S.; Lucas, S.G. Pleistocene vertebrates from southeastern New Mexico. New Mex. Geol. Surv. Guideb. 2006, 57, 317–335. [Google Scholar]
- Jones, J.K. Pleistocene bats from San Josecito Cave, Nuevo Leon, Mexico. Univ. Kansas Publ. Mus. Nat. Hist. 1958, 9, 389–396. [Google Scholar]
- Gut, H.J. A Pleistocene vampire bat from Florida. J. Mammal. 1959, 40, 534–538. [Google Scholar] [CrossRef]
- Hutchison, J.H. A Pleistocene vampire bat (Desmodus stocki) from Potter Creek Cave, Shasta County, California. PaleoBios 1967, 3, 1–6. [Google Scholar]
- Grady, F.; Arroyo-Cabrales, J.; Garton, E.R. The northernmost occurrence of the Pleistocene vampire bat Desmodus stocki Jones (Chiroptera: Phyllostomatidae: Desmodontinae) in Eastern North America. Smithson. Contrib. Paleobiol. 2002, 93, 73–75. [Google Scholar]
- Harris, A.H. Pleistocene vertebrates of Southwestern USA and Northwestern Mexico. Available online: www.utep.edu/leb/PleistNM/ (accessed on 1 July 2025).
- Czaplewski, N.J.; Peachey, W.D. Late Pleistocene bats from Arkenstone Cave, Arizona. Southwest. Natur. 2003, 48, 597–609. [Google Scholar] [CrossRef]
- Czaplewski, N.J.; Mead, J.I.; Peachey, W.D. Late Pleistocene vertebrate fauna and bat guano deposit of La Tetera Cave, Arizona, USA. J. Cave Karst Stud. 2025. [Google Scholar]
- Guthrie, D.A. Analysis of avifaunal and bat remains from midden sites on San Miguel Island. In The California Islands: Proceedings of a Multidisciplinary Symposium; Power, D.M., Ed.; Santa Barbara Museum of Natural History: Santa Barbara, CA, USA, 1980; pp. 689–702. [Google Scholar]
- Guthrie, D.A. Fossil Vertebrates from Pleistocene Terrestrial Deposits on the Northern Channel Islands, Southern California. In Contributions to the Geology of the Northern Channel Islands, Southern California; Pacific Section, American Association of Petroleum Geologists (AAPG): Bakersfield, CA, USA, 1998; pp. 187–192. [Google Scholar]
- Hall, E.R. The Mammals of North America, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1981; Volume 1, 1181p. [Google Scholar]
- Suzan, A.G. Common Vampire Bat, Desmodus rotundus. In Mammals of Mexico; Ceballos, G., Ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2014; pp. 688–689. [Google Scholar]
- Pardiñas, U.F.J.; Tonni, E.P. A giant vampire (Mammalia, Chiroptera) in the Late Holocene from the Argentinean pampas: Paleoenvironmental significance. Palaeogeo. Palaeoclimat. Palaeoecol. 2000, 160, 213–221. [Google Scholar] [CrossRef]
- Brizuela, S.; Tassara, D.A. New record of the vampire Desmodus draculae (Chiroptera) from the late Pleistocene of Argentina. Ameghiniana 2021, 58, 169–176. [Google Scholar] [CrossRef]
- Arroyo-Cabrales, J.; Alvarez, T. Restos Óseos de Murciélagos (Orden Chiroptera) Procedentes de las Excavaciones Arqueológicas en las Grutas de Loltún, Yucatán, México; Instituto Nacional de Antropología e Historia: Mexico City, Mexico, 1990; Volume 194, pp. 1–103. [Google Scholar]
- Arroyo-Cabrales, J.; Alvarez, T. A Preliminary Report of the Late Quaternary Mammal Fauna from Loltún Cave, Yucatán, Mexico. In Ice Age Cave Faunas of North America; Schubert, B.W., Mead, J.I., Graham, R.W., Eds.; Indiana University Press: Bloomington, IN, USA, 2003; pp. 262–272. [Google Scholar]
- Cartelle, C.; Abuhid, V.S. Chiroptera do Pleistoceno final-Holoceno da Bahia. Acta Geol. Leopold. 1994, 39, 429–440. [Google Scholar]
- Trajano, E.; de Vivo, M. Desmodus draculae Morgan, Linares and Ray, 1988, reported for Southeastern Brazil, with paleoecological comments (Phyllostomidae, Desmodontinae). Mammalia 1991, 55, 456–459. [Google Scholar]
- Barleto, E.A.; de Souza, H.N.; Lessa, A.G. Conservação do patrimônio paleontológico, arqueológico, e cultural na Apa Gruta de Brejões/Vereda do Romão Gramacho–BA. In Proceedings of the 29th Brazilian Congress of Speleology, Ouro Preto, Brazil, 7–10 June 2007; pp. 39–46. [Google Scholar]
- Fracasso, M.P.A.; Salles, L.O. Diversity of Quaternary bats from Serra da Mesa (State of Goiás, Brazil). Zootaxa 2005, 817, 1–19. [Google Scholar] [CrossRef]
- Cartelle, C. Edentata e Megamamíferos Herbívoros Extintos da Toca dos Ossos (Ourolândia, BA, Brasil). Ph.D. Thesis, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 1992. [Google Scholar]
- Cartelle, C. A fauna local de mamíferos pleistocênicos da Toca da Boa Vista (Laje dos Negros, BA). Ph.D. Thesis, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 1995. [Google Scholar]
- Cartelle, C. Pleistocene Mammals of the Cerrado and Caatinga of Brazil. In Mammals of the Neotropics: The Central Neotropics; Eisenberg, J.B., Redford, K.H., Eds.; University of Chicago Press: Chicago, IL, USA, 1999; pp. 27–46. [Google Scholar]
- Auler, A.S.; Piló, L.B.; Smart, P.L.; Wang, X.; Hoffmann, D.; Richards, D.A.; Edwards, R.L.; Neves, W.A.; Cheng, H. U-series dating and taphonomy of Quaternary vertebrates from Brazilian caves. Palaeogeog. Palaeoclimat. Palaeoecol. 2006, 240, 508–522. [Google Scholar] [CrossRef]
- Hadler, P.; Mayer, E.L.; Motta, F.; Ribeiro, A.M. Fossil bats from the Quaternary of Serra da Capivara, northeast Brazil. Quat. Internat. 2018, 464, 411–416. [Google Scholar] [CrossRef]
- Shockey, B.; Salas Gismondi, R.; Baby, P.; Guyot, L.P.; Baltazar, M.; Huamán, L.; Clack, A.; Stucchi, M.; Pujos, F.; Emerson, J.; et al. New Pleistocene cave faunas of the Andes of Central Perú: Radiocarbon ages and the survival of low latitude Pleistocene DNA. Palaeontol. Elect. 2009, 12, 15. [Google Scholar]
- Koopman, K.F. A fossil vampire bat from Cuba. Breviora 1958, 90, 1–4. [Google Scholar]
- Wołoszyn, B.W.; Mayo, N.A. Postglacial remains of a vampire bat (Chiroptera: Desmodus) from Cuba. Acta Zool. Cracoviensia 1974, 19, 253–265. [Google Scholar]
- Suárez, W. Taxonomic status of the Cuban vampire bat (Chiroptera: Phyllostomidae: Desmodontinae: Desmodus). Carib. J. Sci. 2005, 41, 761–767. [Google Scholar]
- Jiménez, O.; Condis, M.M.; García, E. Vertebrados post-glaciales en un residuario fósil de Tyto alba scopoli (Aves: Tytonidae) en el occidente de Cuba. Rev. Mexicana Mastozool. 2005, 9, 84–111. [Google Scholar]
- Orihuela, J. Skull variation of the vampire bat Desmodus rotundus (Chiroptera: Phyllostomidae): Taxonomic implications for the Cuban fossil vampire bat Desmodus puntajudensis. Chirop. Neotrop. 2011, 17, 963–976. [Google Scholar]
- Orihuela, J. Late Holocene fauna from a cave deposit in western Cuba: Post-Columbian occurrence of the vampire Desmodus rotundus (Phyllostomidae: Desmodontinae). Carib. J. Sci. 2012, 46, 297–312. [Google Scholar] [CrossRef]
- Arroyo-Cabrales, J.; Ray, C.E. Revisión de los vampiros fósiles (Chiroptera: Phyllostomidae: Desmodontinae) de México. In Homenaje al Profesor Ticul Álvarez; Arroyo-Cabrales, J., Polaco, O.J., Eds.; Instituto Nacional de Antropología e Historia (INAH): Mexico City, Mexico, 1997; Volume 357, pp. 69–86. [Google Scholar]
- Mendoza, Z.; Xiong, Z.; Escalera-Zamudio, M.; Runge, A.K.; Thézé, J.; Streicker, D.; Frank, H.K.; Loza-Rubio, E.; Liu, S.; Ryder, O.A.; et al. Hologenomic adaptations underlying the evolution of sanguivory in the common vampire bat. Nat. Ecol. Evol. 2018, 2, 659–668. [Google Scholar] [CrossRef]
- Blumer, M.; Brown, T.; Freitas, M.B.; Destro, A.L.; Oliveira, J.A.; Morales, A.E.; Schell, T.; Greve, C.; Pippel, M.; Jebb, D.; et al. Gene losses in the common vampire bat illuminate molecular adaptations to blood feeding. Sci. Adv. 2022, 8, eabm6494. [Google Scholar] [CrossRef] [PubMed]
- Teeling, E.C.; Springer, M.S.; Madsen, O.; Bates, P.; O’Brien, S.J.; Murphy, W.J. A molecular phylogeny for bats illuminates biogeography and the fossil record. Science 2005, 307, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.K. Review of the origins and biogeography of bats in South America. Chirop. Neotrop. 2009, 15, 391–410. [Google Scholar]
- Morgan, G.S.; Czaplewski, N.J. Evolutionary history of the Neotropical Chiroptera: The fossil record. In Evolutionary History of Bats: Fossils, Molecules, and Morphology; Gunnell, G.F., Simmons, N.B., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 105–161. [Google Scholar] [CrossRef]
- Simmons, N.B.; Gunnell, G.F.; Czaplewski, N.J. Fragments and Gaps: The fossil Record. In Phyllostomid Bats: A Unique Mammalian Radiation; Fleming, T.H., Dávalos, L.M., Mello, M.A.R., Eds.; University of Chicago Press: Chicago, IL, USA, 2020; pp. 63–86. [Google Scholar] [CrossRef]
- Morgan, G.S.; Czaplewski, N.J.; Rincon, A.F.; Bloch, J.I.; Wood, A.R.; MacFadden, B.J. A new early Miocene bat (Chiroptera: Phyllostomidae) from Panama confirms middle Cenozoic chiropteran dispersals between the Americas. J. Mammal. Evol. 2023, 30, 963–993. [Google Scholar] [CrossRef]
- Czaplewski, N.J. Colhuehuapian bats (Mammalia: Chiroptera) from the Gran Barranca, Chubut province, Argentina. In The Paleontology of Gran Barranca: Evolution and Environmental Change Through the Middle Cenozoic of Patagonia; Madden, R.H., Carlini, A.A., Vucetich, M.G., Kay, R.F., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 240–252. [Google Scholar]
- Baker, R.J.; Solari, S.A.; Cirranello, A.; Simmons, N.B. Higher level classification of phyllostomid bats with a summary of DNA synapomorphies. Acta Chirop. 2016, 18, 1–38. [Google Scholar] [CrossRef]
- McKenna, M.C.; Bell, S.K. Classification of Mammals Above the Species Level; Columbia University Press: New York, NY, USA, 1997; 631p. [Google Scholar]
- McDonald, H.G.; Vizcaíno, S.F.; Bargo, M.S. Fossil Vermilinguas-An Overview. In The Biology of the Xenarthra; Vizcaíno, S.F., Loughry, J., Eds.; University of Florida Press: Gainesville, FL, USA, 2008; pp. 64–78. [Google Scholar]
- Delsuc, F.; Gibb, G.C.; Kuch, M.; Billet, G.; Hautier, L.; JSouthon, J.; Rouillard, J.-M.; Fernicola, J.C.; Vizcaíno, S.F.; MacPhee, R.D.E.; et al. The phylogenetic affinities of the extinct glyptodonts. Cur. Biol. 2016, 26, R141–R156. [Google Scholar] [CrossRef]
- Mitchell, K.J.; Scanferla, A.; Soibelzon, E.; Bonini, R.; Ochoa, J.; Cooper, A. Ancient DNA from the extinct South American giant glyptodont Doedicurus sp. (Xenarthra: Glyptodontidae) reveals that glyptodonts evolved from Eocene armadillos. Molec. Ecol. 2016, 25, 3499–3508. [Google Scholar] [CrossRef]
- Fariña, R.A.; Vizcaíno, S.F.; Bargo, M.S. Body mass estimations in Lujanian (late Pleistocene-early Holocene of South America) mammal megafauna. Mastozool. Neotrop. 1998, 5, 87–108. [Google Scholar]
- Kramarz, A.G.; MacPhee, R.D.E. Did some extinct South American native ungulates arise from an afrothere ancestor? A critical reappraisal of Avilla and Mothé’s (2021) Sudamericingulata-Panameridungulata hypothesis. J. Mammal. Evol. 2022, 30, 67–77. [Google Scholar] [CrossRef]
- Lundelius, E.L.; Bryant, V.M., Jr.; Mandel, R.; Thies, K.J.; Thoms, A. The first occurrence of a toxodont (Mammalia, Notoungulata) in the United States. J. Vert. Paleontol. 2013, 33, 229–232. [Google Scholar] [CrossRef]
- O’Dea, A.; Lessios, H.A.; Coates, A.G.; Eytan, R.I.; Restrepo-Moreno, S.A.; Cione, A.L.; Collins, L.S.; de Queiroz, A.; Farris, D.W.; Norris, R.D.; et al. Formation of the Isthmus of Panama. Sci. Adv. 2016, 2, e1600883. [Google Scholar] [CrossRef] [PubMed]
- Woodburne, M.O. The Great American Biotic Interchange: Dispersals, tectonics, climate, sea level and holding pens. J. Mammal. Evol. 2010, 17, 245–264. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, S.E.; Webb, S.D. Plio-Pleistocene megalonychid sloths of North America. Bull. Fla. State Mus. Biol. Sci. 1968, 12, 213–296. [Google Scholar] [CrossRef]
- Hirschfeld, S.E. Pliometanastes protistus (Edentata: Megalonychidae) from Knight’s Ferry, California. PaleoBios 1981, 36, 1–16. [Google Scholar]
- McDonald, H.G.; Morgan, G.S. Ground sloths of New Mexico. New Mexico Mus. Nat. Hist. Sci. Bull. 2011, 53, 652–663. [Google Scholar]
- McDonald, H.G. Fossil Xenarthra of Mexico: A review. In Avances en los Estudios Paleomastozoológicos en México; Montellano Ballesteros, M., Arroyo-Cabrales, J., Eds.; Serie Arqueológica; Instituto Nacional de Antropología e Historia (INAH): Mexico City, Mexico, 2002; pp. 227–248. [Google Scholar]
- Webb, S.D. Osteology and Relationships of Thinobadistes segnis, the First mylodont Sloth in North America. In Advances in Neotropical Mammalogy; Redford, K.H., Eisenberg, J.F., Eds.; Sandhill Crane Press: Gainesville, FL, USA, 1989; pp. 469–532. [Google Scholar]
- Laurito, C.A.; Valerio, A.L. Primer registro fósil de Pliometanastes sp. (Mammalia, Xenarthra, Megalonychidae) para el Mioceno Superior de Costa Rica, América Central. Una nueva pista en la comprensión del Pre-GABI. Rev. Geol. Amér.Cent. 2012, 47, 95–108. [Google Scholar] [CrossRef]
- Valerio, A.L.; Laurito, C.; McDonald, H.G.; Rincón, A.D. Megalonychid sloths from the Early Late Hemphillian (Late Miocene), Curré Formation, San Gerardo de Limoncito, Costa Rica. Rev. Geol. Amér. Cent. 2022, 66, 1–15. [Google Scholar] [CrossRef]
- Laurito, C.A.; Valerio, A.L. Scirrotherium antelucanus, una nueva especie de Pampatheriidae (Mammalia, Xenarthra, Cingulata) del Mioceno Superior de Costa Rica, América Central. Rev. Geol. Amér. Cent. 2013, 49, 45–62. [Google Scholar] [CrossRef]
- McDonald, H.G.; Carranza-Castañeda, O. Increased xenarthran diversity of the Great American Biotic Interchange: A new genus and species of ground sloth (Mammalia, Xenarthra, Megalonychidae) from the Hemphillian (late Miocene) of Jalisco, Mexico. J. Paleontol. 2017, 91, 1069–1082. [Google Scholar] [CrossRef]
- Baskin, J.A.; Valenciano, A. Procyonidae (Mammalia, Carnivora) and the Great American Biotic Interchange. In Windows into Sauropsid and Synapsid Evolution; Essays in Honor of Prof. Louis L. Jacobs; Lee, Y.-N., Ed.; Dinosaur Science Center Press: Republic of Korea, 2023; pp. 341–365. [Google Scholar]
- Campbell, K.E.; Frailey, C.D.; Romero-Pittman, L. The Late Miocene Gomphothere Amahuacatherium peruvium (Proboscidea: Gomphotheriidae) from Amazonian Peru: Implications for the Great American Faunal Interchange; Série D, Estudios Regionales; Instituto de Geología, Minería y Metalurgia: Lima, Peru, 2000; Volume 23, pp. 1–152. [Google Scholar]
- Frailey, C.D.; Campbell, K.E. Two new genera of peccaries (Mammalia, Artiodactyla, Tayassuidae) from Upper Miocene deposits of the Amazon Basin. J. Paleontol. 2012, 86, 852–877. [Google Scholar] [CrossRef]
- Prothero, D.R.; Campbell, K.E.; Beatty, B.L.; Frailey, C.D. New late Miocene dromomerycine artiodactyl from the Amazon Basin: Implications for Interchange dynamics. J. Paleontol. 2014, 88, 423–443. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.G. The palaeobiogeography of South American gomphotheres. J. Palaeogeog. 2013, 2, 19–40. [Google Scholar]
- Mothé, D.; Avilla, L. Mythbusting evolutionary issues on South American Gomphotheriidae (Mammalia: Proboscidea). Quat. Sci. Rev. 2015, 110, 23–35. [Google Scholar] [CrossRef]
- Gasparini, G.M.; Parisi Dutra, R.; Perini, F.A.; Croft, D.A.; Cozzuol, M.A.; Missagia, R.V.; Lucas, S.G. On the supposed presence of Miocene Tayassuidae and Dromomerycinae (Mammalia, Cetartiodactyla) in South America. Amer. Mus. Novitates 2021, 3968, 1–27. [Google Scholar] [CrossRef]
- Campbell, K.E., Jr.; Heizler, M.; Frailey, C.D.; Romero-Pittman, L.; Prothero, D.R. Upper Cenozoic chronostratigraphy of the south-western Amazon Basin. Geology 2001, 29, 595–598. [Google Scholar] [CrossRef]
- Altenbach, J.S. Locomotor Morphology of the Vampire Bat, Desmodus Rotundus; Special publication No. 6; American Society of Mammalogists: Pittsburgh, PA, USA, 1979; pp. 1–137. [Google Scholar]
- Altenbach, J. Locomotion. In Natural History of Vampire Bats; Greenhall, A.M., Schmidt, U., Eds.; CRC Press: Boca Raton, FL, USA, 1988; pp. 71–83. [Google Scholar]
- Goodwin, G.G.; Greenhall, A.M. A review of the bats of Trinidad and Tobago. Bull. Amer. Mus. Nat. Hist. 1961, 122, 187–302. [Google Scholar]
- Koopman, K.F. Land bridges and ecology of bat distribution on islands of the northern coast of South America. Evolution 1958, 12, 429–439. [Google Scholar] [CrossRef]
- MacPhee, R.D.; Iturralde-Vinent, M. Orgin of the Greater Antillean land mammal fauna, 1: New Tertiary fossils from Cuba and Puerto Rico. Amer. Mus. Novitates 1995, 3141, 1–31. [Google Scholar]
- MacPhee, R.D.E.; Iturralde-Vinent, M. First Tertiary land mammal from Greater Antilles: An early Miocene sloth (Xenarthra, Megalonychidae) from Cuba. Amer. Mus. Novitates 1994, 3094, 1–42. [Google Scholar]
- Viñola-Lopez, L.W.; Suárez, E.E.C.; Vélez-Juarbe, J.; Milan, J.N.A.; Bloch, J.I. The oldest known record of a ground sloth (Mammalia, Xenarthra, Folivora) from Hispaniola: Evolutionary and paleobiogeographical implications. J. Paleontol. 2022, 96, 684–691. [Google Scholar] [CrossRef]
- Morgan, G.S. Patterns of Extinction in West Indian Bats. In Biogeography of the West Indies: Patterns and Perspectives, 2nd ed.; Woods, C.A., Sergile, F.E., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 369–407. [Google Scholar]
- Velazco, P.M.; O’Neill, H.; Gunnell, G.F.; Cooke, S.B.; Rimoli, R.; Rosenberger, A.L.; Simmons, N.B. Quaternary bat diversity in the Dominican Republic. Amer. Mus. Novitates 2013, 3779, 1–20. [Google Scholar] [CrossRef]
- Brodkorb, P. A giant flightless bird from the Pleistocene of Florida. Auk 1963, 80, 111–115. [Google Scholar] [CrossRef]
- Gould, G.C.; Quitmyer, I.R. Titanis walleri: Bones of contention. Bull. Florida Mus. Nat. Hist. 2005, 45, 201–229. [Google Scholar] [CrossRef]
- Baskin, J.A. The giant flightless bird Titanis walleri (Aves: Phorusrhacidae) from the Pleistocene coastal plain of south Texas. J. Vert. Paleontol. 1995, 15, 842–844. [Google Scholar] [CrossRef]
- MacFadden, B.J.; Labs-Hochstein, J.; Hulbert, R.C., Jr.; Baskin, J.A. Revised age of the Late Neogene terror bird (Titanis) in North America during the Great American Interchange. Geology 2007, 35, 123–126. [Google Scholar] [CrossRef]
- Shaw, C.A.; McDonald, H.G. First record of giant anteater (Xenarthra, Myrmecophagidae) in North America. Science 1987, 236, 186–188. [Google Scholar] [CrossRef]
- Webb, S.D.; Perrigo, S.C. Late Cenozoic vertebrates from Honduras and El Salvador. J. Vert. Paleontol. 1984, 4, 237–254. [Google Scholar] [CrossRef]
- Cisneros, J.C. New Pleistocene vertebrate fauna from El Salvador. Rev. Brasileira Paleontol. 2005, 8, 239–255. [Google Scholar] [CrossRef]
- Cisneros, J.C. The fossil mammals of El Salvador. New Mexico Mus. Nat. Hist. Sci. Bull. 2008, 44, 375–380. [Google Scholar]
- Dávila, S.L.; Stinnesbeck, S.R.; Gonzalez, S.; Lindauer, S.; Escamilla, J.; Stinnesbeck, W. Guatemala’s Late Pleistocene (Rancholabrean) fauna: Revision and interpretation. Quat. Sci. Rev. 2019, 219, 277–296. [Google Scholar] [CrossRef]
- Lucas, S.G.; Garcia, R.; Espinosa, E.; Alvarado, G.E.; Hurtado de Mendoza, L.; Vega, E. The fossil mammals of Nicaragua. New Mexico Mus. Nat. Hist. Sci. Bull. 2008, 44, 417–429. [Google Scholar]
- Lucas, S.G.; Alvarado, G.E.; Vega, E. The Pleistocene mammals of Costa Rica. J. Vert. Paleontol. 1997, 17, 413–427. [Google Scholar] [CrossRef]
- Lucas, S.G. Late Pleistocene mammals from El Hatillo, Panamá. Rev. Geol. Amér. Cent. 2014, 50, 139–151. [Google Scholar]
- Polaco, O.J.; Guzmán, A.F.; Ramírez, G.T. Occurrence of toxodonts in the Pleistocene of Mexico. Cur. Res. Pleist. 2004, 21, 113–115. [Google Scholar]
- Mead, J.I.; Baez, A.; Swift, S.L.; Carpenter, M.C.; Hollenshead, M.; Czaplewski, N.J.; Steadman, D.W.; Bright, J.; Arroyo-Cabrales, J. Tropical marsh and savanna of the Late Pleistocene in northeastern Sonora, Mexico. Southwest. Nat. 2006, 51, 226–239. [Google Scholar] [CrossRef]
- Carbot-Chanona, G.; Eng-Ponce, J.; Gomez-Perez, L.E. Description of Neochoerus specimens from the late Pleistocene (Rancholabrean) of Chiapas, and comments on the taxonomic identity of the fossil capybaras from other Mexican localities. Bol. Soc. Geol. Mexicana 2020, 72. [Google Scholar] [CrossRef]
- Ahearn, M.E. A Revision of the North American Hydrochoeridae. Master’s Thesis, University of Florida, Gainesville, FL, USA, 1981; 99p. [Google Scholar]
- Sanders, A.E. Additions to the Pleistocene mammal faunas of South Carolina, North Carolina, and Georgia. Trans. Amer. Phil. Soc. 2002, 92, 1–152. [Google Scholar] [CrossRef]
- Baskin, J.A.; Gervais, P.D.; Gervais, C.J. A late Pleistocene capybara (Rodentia, Caviidae, Hydrochoerinae) from near Houston, Texas, USA, with a brief review of North American fossil capybaras. Proc. Acad. Nat. Sci. Phila. 2020, 167, 57–68. [Google Scholar] [CrossRef]
- Carranza-Castañeda, O. Roedores caviomorphos (Rodentia Hydrochoeridae) del Blancano temprano-tardío–Irvingtoniano de los estados de Guanajuato, Jalisco y Sonora, México: Relación con Phugatherium dichroplax. Rev. Mexicana Cienc. Geol. 2016, 33, 297–315. [Google Scholar]
- Ahearn, M.E.; Lance, J.F. A new species of Neochoerus (Rodentia: Hydrochoeridae) from the Blancan (late Pliocene) of North America. Proc. Biol. Soc. Wash. 1980, 93, 435–442. [Google Scholar]
- Carranza-Castañeda, O.; Miller, W.E. Roedores caviomorfos de la Mesa Central de México, Blancano Temprano (Plioceno Tardío) de la Fauna Local Rancho Viejo, Estado de Guanajuato. Univ. Nac. Autó. México Inst. Geol. Rev. 1988, 7, 182–199. [Google Scholar]
- Hulbert, R.C., Jr. A new early Pleistocene tapir (Mammalia: Perissodactyla) from Florida, with a review of Blancan tapirs from the state. Bull. Fla. Mus. Nat. Hist. 2010, 49, 67–126. [Google Scholar] [CrossRef]
- Vucetich, M.G.; Deschamps, C.M.; Pérez, M.E. The first capybaras (Rodentia, Caviidae, Hydrochoerinae) involved in the Great American Biotic Interchange. Ameghiniana 2015, 52, 324–333. [Google Scholar] [CrossRef]
- Webb, S.D.; Perrigo, S.C. New Megalonychid Sloths from El Salvador. In The Evolution and Ecology of Armadillos, Sloths and Vermilinguas; Montgomery, G.G., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1985; pp. 113–120. [Google Scholar]
- McDonald, H.G.; Chatters, J.C.; Gaudin, T.J. A new genus of megalonychid ground sloth (Mammalia, Xenarthra) from the late Pleistocene of Quintana Roo, Mexico. J. Vert. Paleontol. 2017, 37, e1307206. [Google Scholar] [CrossRef]
- McDonald, H.G.; Arroyo-Cabrales, J.; Alarcón-Durán, I.; Espinosa-Martínez, D.V. First record of Meizonyx salvadorensis (Mammalia: Xenarthra: Pilosa) from the late Pleistocene of Mexico and its evolutionary implications. J. Syst. Palaeontol. 2020, 18, 1829–1851. [Google Scholar] [CrossRef]
- Stinnesbeck, S.R.; Frey, E.; Stinnesbeck, W. New insights on the paleogeographic distribution of the Late Pleistocene ground sloth genus Xibalbaonyx along the Mesoamerican Corridor. J. South Amer. Ear. Sci. 2018, 85, 108–120. [Google Scholar] [CrossRef]
- MacPhee, R.D.E.; White, J.L.; Woods, C.A. New megalonychid sloths (Phyllophaga, Xenarthra) from the Quaternary of Hispaniola. Amer. Mus. Novitates 2000, 3303, 1–32. [Google Scholar] [CrossRef]
- White, J.L.; MacPhee, R.D.E. The sloths of the West Indies: A Systematic and Phylogenetic Review. In Biogeography of the West Indies: Patterns and Perspectives; Woods, C.A., Sergile, F.E., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 201–236. [Google Scholar]
- Iturralde-Vinent, M.A.; MacPhee, R.D.E. New evidence for late Eocene-early Oligocene uplift of Aves Ridge and paleogeography of GAARlandia. Geol. Acta 2023, 21, 1–10. [Google Scholar] [CrossRef]
- McDonald, H.G. The Shasta Ground Sloth Nothrotheriops shastensis (Xenarthra, Megatheriidae) in the Middle Pleistocene of Florida. In The Evolution and Ecology of Armadillos, Sloths, and Vermilinguas; Montgomery, G.G., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1985; pp. 95–104. [Google Scholar]
- McDonald, H.G. Gravigrade xenarthrans from the early Pleistocene Leisey Shell Pit 1A, Hillsborough County, Florida. Bull. Fla. Mus. Nat. Hist. 1995, 37, 345–373. [Google Scholar] [CrossRef]
- Cassiliano, M.L. Biostratigraphy of Blancan and Irvingtonian mammals in the Fish Creek-Vallecito Creek section, southern California, and a review of the Blancan-Irvingtonian boundary. J. Vert. Paleontol. 1999, 19, 169–186. [Google Scholar] [CrossRef]
- Croxen, F.W., III; Shaw, C.A.; Sussman, D.R. Pleistocene geology and paleontology of the Colorado River Delta at Golfo de Santa Clara, Sonora, Mexico. In Proceedings of the 2007 Desert Symposium; Reynolds, R.E., Ed.; Studies Consortium and LSA Associates, Inc.: Santa Ana, CA, USA; California State University: Long Beach, CA, USA, 2007; pp. 84–89. [Google Scholar]
- De Iuliis, G.; McDonald, H.G.; Stanchly, N.; Spenard, J.; Powis, T.G. Nothrotheriops shastensis (Sinclair) from Actun Lak: First record of Nothrotheriidae (Mammalia, Xenarthra, Pilosa) from Belize. Ameghiniana 2015, 52, 153–171. [Google Scholar] [CrossRef]
- Morgan, G.S.; Woods, C.A. Extinction and the zoogeography of West Indian land mammals. Biol. J. Linn. Soc. 1986, 28, 167–203. [Google Scholar] [CrossRef]
- Steadman, D.W.; Martin, P.S.; MacPhee, R.D.E.; Jull, A.J.T.; McDonald, H.G.; Woods, C.A.; Iturralde-Vinent, M.; Hodgins, G.W. Asynchronous extinction of late Quaternary sloths on continents and islands. Proc. Natl. Acad. Sci. USA 2005, 102, 11763–11768. [Google Scholar] [CrossRef]
- MacPhee, R.D.E.; Iturralde-Vinent, M.A.; Jiménez Vázquez, O. Prehistoric sloth extinctions in Cuba: Implications of a new “Last” Appearance Date. Carib. J. Sci. 2007, 43, 94–98. [Google Scholar] [CrossRef]
- Bobrowiec, P.E.D.; Lemes, M.R.; Gribel, R. Prey preference of the common vampire bat (Desmodus rotundus, Chiroptera) using molecular analysis. J. Mammal. 2015, 96, 54–63. [Google Scholar]
- Carter, G.; Brown, B.; Razik, I.; Ripperger, S. Penguins, Falcons, and Mountain Lions: The Extraordinary Host Diversity of Vampire Bats. In 50 Years of Bat Research; Lim, B.K., Fenton, M.B., Brigham, R.M., Mistry, S., Kurta, A., Gillam, E.H., Russell, A., Ortega, J., Eds.; Springer: Cham, Switzerland, 2021; pp. 151–170. [Google Scholar] [CrossRef]
- Bennett, M.R.; Bustos, D.; Pigati, J.S.; Springer, K.B.; Urban, T.M.; Holliday, V.T.; Reynolds, S.C.; Budka, M.; Honke, J.S.; Hudson, A.M.; et al. Evidence of humans in North America during the Last Glacial Maximum. Science 2021, 373, 1528–1531. [Google Scholar] [CrossRef]
- Ríos-Solís, J.A.; López-Acosta, J.C.; MacSwiney, M.C. Potential attack of the common vampire bat (Desmodus rotundus) on nine-banded armadillo (Dasypus novemcinctus) in northern Oaxaca, México. Therya Not. 2021, 2, 147–150. [Google Scholar] [CrossRef]
- De Oliveira, M.B.; de Andrade, H.S.F.; Cordeiro, J.L.P.; de Oliveira, L.F.B. Potential feeding event of Priodontes maximus (Cingulata: Dasypodidae) by Desmodus rotundus (Chiroptera: Desmodontinae) in the Cerrado, Western Brazil. Not. Mamífer. Sudamer. 2022, 4, 2–10. [Google Scholar] [CrossRef]
- Kays, R. Candid Creatures: How Camera Traps Reveal the Mysteries of Nature; Johns Hopkins University Press: Baltimore, MD, USA, 2016; 261p. [Google Scholar]
- Carranza, J.; Campo, D.R. Incidencias del murciélago hematófago Desmodus rotundus sobre los indígenas Yanomami de Venezuela. Doñana Acta Vertebr. 1982, 7, 113. [Google Scholar]
- Carranza, J. Murciélago hematófago Desmodus rotundus parasitando a un chiguire Hydrochoerus hydrochaeris. Doñana Acta Vertebr. 1982, 9, 414–415. [Google Scholar]
- Gonçalves, F.M.; Magioli, M.; Bovendorp, R.S.; de Barros Ferraz, K.M.P.M.; Cagnoni, L.B.; Moreira, M.Z.; Galetti, M. Prey choice of the common vampire bat on introduced species in an Atlantic Forest land-bridge island. Acta Chiropt. 2020, 22, 167–174. [Google Scholar] [CrossRef]
- Mann Fischer, G. Biología del vampiro: Biol. Trabajo. Inst. Biol. “Juan Noe”, Santiago de Chile, Univ. Chile 1951, 12–13, 3–24. [Google Scholar]
- Catenazzi, A.; Donnelly, M.A. Sea lion Otaria flavescens as host of the common vampire bat Desmodus rotundus. Marine Ecol. Prog. Ser. 2008, 360, 285–289. [Google Scholar] [CrossRef]
- Barquez, R.M.; Mares, M.A.; Braun, J.K. The Bats of Argentina; Special publications no. 42; Museum of Texas Tech University: Lubbock, TX, USA, 1999; pp. 1–275. [Google Scholar]
- Galetti, M.; Pedrosa, F.; Keuroghlian, A.; Sazima, I. Liquid lunch–vampire bats feed on invasive feral pigs and other ungulates. Front. Ecol. Environ. 2016, 14, 505–506. [Google Scholar] [CrossRef]
- Gnocchi, A.P.; Srbek-Araujo, A.C. Common Vampire Bat (Desmodus rotundus) feeding on Lowland Tapir (Tapirus terrestris) in an Atlantic Forest remnant in southeastern Brazil. Biota Neotrop. 2017, 17. [Google Scholar] [CrossRef]
- Zortéa, M.; Silva, D.A.; Calaça, A.M. Susceptibility of targets to the vampire bat Desmodus rotundus are proportional to their abundance in Atlantic Forest fragments? Iheringia. Sér. Zool. 2018, 108, 2015–2018. [Google Scholar] [CrossRef]
- Castellanos, A.X.; Banegas, G.A. Vampire bats bite lowland tapirs in Yasuni National Park, Ecuador. Tapir Conserv. 2015, 24, 7. [Google Scholar]
- Voigt, C.C.; Kingston, T. Bats in the Anthropocene: Conservation of Bats in a Changing World; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Sánchez-Cordero, V.; Botello, F.; Magaña-Cota, G.; Iglesias, J. Vampire bats, Desmodus rotundus, feeding on white-tailed deer, Odocoileus virginianus. Mammalia 2011, 75, 91–92. [Google Scholar] [CrossRef]
- Tello-Mera, E.L.; Mandujano, S. Primer registro fotográfico de murciélagos hematófagos Desmodus rotundus (Chiroptera: Phyllostomidae) alimentándose de Odocoileus virginianus (Artiodactyla: Cervidae) en la Reserva de la Biosfera Tehuacán-Cuicatlán, México. Mammal. Notes 2016, 3, 17–19. [Google Scholar] [CrossRef]
- Silveira, L.; de Almeida Jácomo, A.T.; Malzoni Furtado, M.; Mundim Torres, N.; Sollmann, R.; Vynn, G. Ecology of the giant armadillo (Priodontes maximus) in the grasslands of central Brazil. Edentata 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Greenhall, A.M.; Schmidt, U. (Eds.) Natural History of Vampire Bats; CRC Press: Boca Raton, FL, USA, 1988; 246p. [Google Scholar]
- Borroto-Paez, R.; Mancina, C.A. Biodiversity and conservation of Cuban mammals: Past, present, and invasive species. J. Mammal. 2017, 98, 964–985. [Google Scholar] [CrossRef]
- Dondas, A.; Isla, F.I.; Carballido, J.L. Paleocaves exhumed from the Miramar Formation (Ensenadan Stage-age, Pleistocene), Mar del Plata, Argentina. Quat. Internat. 2009, 210, 44–50. [Google Scholar] [CrossRef]
- Frank, H.T.; Althaus, C.E.; Dario, E.M.; Tramontina, F.R.; Adriano, R.M.; de Lima Almeida, M.; Ferreira, G.F.; Nogueira, R.; Breier, R. Underground chamber systems excavated by Cenozoic ground sloths in the state of Rio Grande do Sul, Brazil. Rev. Bras. Paleontol. 2015, 18, 273–284. [Google Scholar] [CrossRef]
- Pereira Lopes, R.; Frank, H.T.; Sekiguchi de Carvalho Buchmann, F.; Caron, F. Megaichnus igen. nov.: Giant paleoburrows attributed to extinct Cenozoic mammals from South America. Ichnos 2016, 24, 133–145. [Google Scholar] [CrossRef]
- Audi, C.; Meyer, D.; Yeuw, T.T.; Barrionuevo Baraldo, K.; Fey, J.D.; Spanghero, N.F.; Schereiber Munhoz, M.; de Oliveira, B.J.; Sekiguchi de Carvalho Buchmann, F. Fotogrametria de um icnofóssil escavado por preguiças-gigantes (Megaichnus major). Rev. Bras. Paleontol. 2022, 25, 208–218. [Google Scholar] [CrossRef]
- de Lazaro, E. 100,000-year-old fossil of giant vampire bat found in Argentina. Sci News, 26 July 2021. Available online: https://www.sci.news./paleontology/desmodus-draculae-fossil-09898.html (accessed on 10 July 2025).
- Agenbroad, L.D. Giants and Pygmies: Mammoths of Santa Rosa Island, California (USA). Quat. Internat. 2012, 255, 2–8. [Google Scholar] [CrossRef]
- Arroyo-Cabrales, J.; Polaco, O.J. Fossil bats from Mesoamerica. Arch. Mus. Nac. Rio J. 2008, 66, 155–160. [Google Scholar]
- De Iuliis, G.; Cartelle, C. A new giant megatheriine ground sloth (Mammalia: Xenarthra: Megatheriidae) from the late Blancan to early lrvingtonian of Florida. Zool. J. Linn. Soc. 1999, 47, 495–515. [Google Scholar] [CrossRef]
- Grady, F.; Garton, E.R. Pleistocene fauna from New Trout Cave. Capital Area Cav. Bull. 1982, 1, 62–69. [Google Scholar]
- Grady, F.; Garton, E.R. Paleontology and historic field trip of the John Guilday Cave Preserve (Trout Rock). West Virginia Speleo. Surv. Bull. 2000, 14, 241–244. [Google Scholar]
- Guilday, J.E.; McCrady, S. Armadillo remains from Tennessee and West Virginia caves. Nat. Speleo. Soc. Bull. 1966, 28, 183–184. [Google Scholar]
- Wilson, R.W. Preliminary study of the fauna of Rampart Cave, Arizona. Contrib. Paleontol. Carnegie Inst. Publ. 1942, 530, 169–185. [Google Scholar]
- Mead, J.I. The last 30,000 years of faunal history within the Grand Canyon, Arizona. Quat. Res. 1981, 15, 311–326. [Google Scholar] [CrossRef]
- Mead, J.I.; Tweet, J.S.; Santucci, V.L.; Tobin, B.; Chambers, C.L.; Thomas, S.C.; Carpenter, M.C. Chapter 11. Pleistocene/Holocene cave fossils from Grand Canyon National Park: Ice Age (Pleistocene) flora, fauna, environments, and climate of the Grand Canyon, Arizona. In Grand Canyon National Park Centennial Paleontological Resource Inventory (Non-Sensitive Version); Santucci, V.L., Tweet, J.S., Eds.; Natural Resource Report. NPS/GRCA/NRR–2020/2103; U.S. Department of the Interior, National Park Service, Natural Resource Stewardship and Science: Fort Collins, CO, USA, 2020; pp. 403–463. [Google Scholar]
- Carpenter, M.C. Late Pleistocene Aves, Chiroptera, Perissodactyla, and Artiodactyla from Rampart Cave, Arizona. Master’s Thesis, Northern Arizona University, Flagstaff, AZ, USA, 2004. [Google Scholar]
- Hodnett, J.P.; White, R.S.; Carpenter, M.; Mead, J.I.; Santucci, V.L. Miracinonyx trumani (Carnivora: Felidae) from the Rancholabrean of the Grand Canyon, Arizona and its implications for the ecology of the “American Cheetah”. New Mexico Mus. Nat. Hist. Sci. Bull. 2022, 88, 157–185. [Google Scholar]
- Harris, A.H. Preliminary report on the vertebrate fauna of U-Bar Cave, Hidalgo County, New Mexico. New Mexico Geol. 1985, 7, 74–77+84. [Google Scholar] [CrossRef]
- Harris, A.H. Reconstruction of Mid-Wisconsin environments in southern New Mexico. Nat. Geog. Res. 1987, 3, 142–151. [Google Scholar]
- Ray, C.E.; Wilson, D.E. Evidence for Macrotus californicus from Terlingua, Texas. Occ. Pap. Mus. Texas Tech Univ. 1979, 57, 1–10. [Google Scholar]
- Cockerell, T.D.A. An apparently extinct Euglandina from Texas. Proc. Colorado Mus. Nat. Hist. 1930, 9, 52–53. [Google Scholar]
- Arroyo-Cabrales, J. Sinopsis de los murciélagos fósiles de Mexico. Rev. Soc. Mexicana Paleontol. 1992, 5, 1–14. [Google Scholar]
- Arroyo-Cabrales, J.; Polaco, O.J. Caves and the Pleistocene vertebrate paleontology of Mexico. In Ice Age Cave Faunas of North America; Schubert, B.W., Mead, J.I., Graham, R.W., Eds.; Indiana University Press: Bloomington, IN, USA, 2003; pp. 273–291. [Google Scholar]
- Alvarez, T. Nuevo registro para el vampiro del Pleistoceno Desmodus stocki de Tlapacoya, Mexico. An. Esc. Nac. Cienc. Biol. Mexico 1972, 19, 163–165. [Google Scholar]
- Arroyo-Cabrales, J.; Johnson, E.; Cruz, J.A. San Josecito Cave and its paleoecological contributions for Quaternary studies in Mexico. Quaternary 2021, 4, 34. [Google Scholar] [CrossRef]
- Stock, C. The Cave of San Josecito, Mexico: New Discoveries of the Vertebrate Life of the Ice Age. 1943. Available online: https://calteches.library.caltech.edu/87/1/Stock.pdf (accessed on 10 July 2025).
- Polaco, O.J.; Butrón, M.L. Mamíferos Pleistocenicos de la Cueva La Presita, San Luís Potosí, Mexico. In Homenaje al Profesor Ticul Alvarez; Arroyo-Cabrales, J., Polaco, O.J., Eds.; Instituto Nacional de Antropología e Historia: Mexico City, Mexico, 1997; pp. 279–296. [Google Scholar]
- Alvarez, T. Restos fósiles de mamíferos de Tlapacoya, Estado de Mexico (Pleistoceno-Reciente). Univ. Kansas Mus. Nat. Hist. Misc. Publ. 1969, 51, 93–112. [Google Scholar]
- Alvarez, T. Restos de mamíferos recientes y pleistocénicos procedentes de la Grutas de Loltún, Yucatán, Mexico. Inst. Nac. Antropol. Hist. Depart. Prehist. Cuad. Trab. 1982, 29, 7–35. [Google Scholar]
- Stinnesbeck, S.R.; Frey, E.; Olguín, J.A.; Stinnesbeck, W.; Zell, P.; Mallison, H.; González, A.G.; Núñez, E.A.; Morlet, A.V.; Mata, A.T.; et al. Xibalbaonyx oviceps, a new megalonychid ground sloth (Folivora, Xenarthra) from the late Pleistocene of the Yucatán Peninsula, Mexico, and its paleobiogeographic significance. PalZ 2017, 91, 245–271. [Google Scholar] [CrossRef]
- Stinnesbeck, S.R.; Stinnesbeck, W.; Frey, E.; Avíles Olguín, J.; González González, A. Xibalbaonyx exinferis n. sp. (Megalonychidae), a new Pleistocene ground sloth from the Yucatán Peninsula, Mexico. Hist. Biol. 2020, 33, 1952–1963. [Google Scholar] [CrossRef]
- Churcher, C.S. Pleistocene mammals from Extinction Cave, Belize. Canadian J. Ear. Sci. 2020, 57, 366–376. [Google Scholar] [CrossRef]
- Larmon, J.T.; McDonald, H.G.; Ambrose, S.; DeSantis, L.R.G.; Lucero, L.J. A year in the life of a giant ground sloth during the Last Glacial Maximum in Belize. Sci. Adv. 2019, 5, eaau1200. [Google Scholar] [CrossRef]
- Alves-Silva, L.; Cherkinsky, A.; Dantas, M.A.T. Late Pleistocene mammals from northeastern Brazil caves: Taxonomy, radiocarbon dating, isotopic paleoecology (δ13C), and paleoenvironment reconstruction (δ13C, δ18O). Quat. Internat. 2023, 668, 7–13. [Google Scholar] [CrossRef]
- Schutt, W.A., Jr.; Hermanson, J.W.; Chang, Y.H.; Cullinane, D.; Altenbach, J.S.; Muradali, F.; Bertram, J.E.A. Functional morphology of the common vampire bat, Desmodus rotundus. J. Exp. Biol. 1997, 200, 3003–3012. [Google Scholar] [CrossRef]
- Schutt, W.A., Jr. The Chiropteran Hindlimb Morphology and the Origin of Blood Feeding in Bats. In Bat Biology and Conservation; Kunz, T.H., Racey, P.A., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1998; pp. 157–168. [Google Scholar]
- Schutt, W.A., Jr.; Simmons, N.B. Quadrupedal Bats: Form, Function, and Evolution. In Functional and Evolutionary Ecology of Bats; Zubaid, A., McCracken, G.F., Kunz, T.H., Eds.; Oxford University Press: Oxford, UK, 2006; pp. 145–159. [Google Scholar]
- Riskin, D.K.; Hermanson, J.W. Biomechanics: Independent evolution of running in vampire bats. Nature 2005, 434, 292. [Google Scholar] [CrossRef]
- Riskin, D.K.; Parsons, S.; Schutt, W.A., Jr.; Carter, G.G.; Hermanson, J.W. Terrestrial locomotion of the New Zealand short-tailed bat Mystacina tuberculata and the common vampire bat Desmodus rotundus. J. Exp. Biol. 2006, 209, 1725–1736. [Google Scholar] [CrossRef]
- Crespo, R.F.; Burns, R.J.; Linhart, S.B. Load-lifting capacity of the vampire bat. J. Mammal. 1970, 51, 627–629. [Google Scholar] [CrossRef]
- Jones, M.F.; Hasiotis, S.T. Terrestrial behavior and trackway morphology of Neotropical bats. Acta Chiropt. 2018, 20, 229–250. [Google Scholar] [CrossRef]
- McDonald, H.G. An overview of the presence of osteoderms in sloths: Implications for osteoderms as a plesiomorphic character of the Xenarthra. J. Mammal. Evol. 2018, 25, 485–493. [Google Scholar] [CrossRef]
- Gillette, D.D. Evolution of feeding strategies in bats. Tebiwa 1975, 18, 39–48. [Google Scholar]
- Ferrarezzi, H.; Gimenez, E.A. Systematic patterns and the evolution of feeding habits in Chiroptera (Archonta: Mammalia). J. Comp. Biol. 1996, 1, 75–94. [Google Scholar]
- Rojas, D.; Vale, A.; Ferrero, V.; Navarro, L. When did plants become important to leaf-nosed bats? Diversification of feeding habits in the family Phyllostomidae. Mol. Ecol. 2011, 20, 2217–2228. [Google Scholar] [CrossRef]
- Dumont, E.R.; Dávalos, L.M.; Goldberg, A.; Voigt, C.C.; Rex, K.; Santana, S.E. Morphological innovation, diversification and the invasion of a new adaptive zone. Proc. Roy. Soc. London B 2012, 279, 1797–1805. [Google Scholar] [CrossRef]
- Yohe, L.R.; Velazco, P.M.; Rojas, D.; Gerstner, B.E.; Simmons, N.B.; Dávalos, L.M. Bayesian hierarchical models suggest oldest known plant-visiting bat was omnivorous. Biol. Let. 2015, 11, 20150501. [Google Scholar] [CrossRef]
- Dávalos, L.M.; Cirranello, A.L.; Geisler, J.H.; Simmons, N.B. Understanding phylogenetic incongruence: Lessons from phyllostomid bats. Biol. Rev. 2012, 87, 991–1024. [Google Scholar] [CrossRef]
- Slaughter, B.H. Evolutionary Trends of Chiropteran Dentitions. In About Bats; Slaughter, B.H., Walton, D.W., Eds.; Southern Methodist University Press: Dallas, TX, USA, 1970; pp. 51–83. [Google Scholar]
- Turner, D.C. The Vampire Bat: A Field Study in Behavior and Ecology; Johns Hopkins University Press: Baltimore, MD, USA, 1975; 145p. [Google Scholar]
- Hill, J.E.; Smith, J.D. Bats: A Natural History; British Museum (Natural History): London, UK, 1984; 243p. [Google Scholar]
- Fenton, M.B. Wounds and the origin of blood-feeding in bats. Biol. J. Linnean Soc. 1992, 47, 161–171. [Google Scholar] [CrossRef]
- Morrone, J.J. Neotropical Biogeography: Regionalization and Evolution; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Morrone, J.J.; Escalante, T.; Rodríguez-Tapia, G.; Carmona, A.; Arana, M.; Mercado-Gómez, J.D. Biogeographic regionalization of the Neotropical region: New map and shapefile. An. Acad. Bras. Cien. 2022, 94, e20211167. [Google Scholar] [CrossRef] [PubMed]
- Van de Vuurst, P.; Qiao, H.; Soler-Tovar, D.; Escobar, L.E. Climate change linked to vampire bat expansion and rabies virus spillover. Ecography 2023, 2024, e06714. [Google Scholar] [CrossRef] [PubMed]
- Gunnell, G.F.; Worsham, S.R.; Seiffert, E.R.; Simons, E.L. Vampyravus orientalis Schlosser (Chiroptera) from the early Oligocene (Rupelian), Fayum, Egypt—Body mass, humeral morphology and affinities. Acta Chirop. 2009, 11, 271–278. [Google Scholar]
- McDonald, H.G. Paleoecology of the extinct Shasta ground sloth, Nothrotheriops shastensis, (Xenarthra, Nothrotheriidae): The physical environment. New Mexico Mus. Nat. Hist. Sci. Bull. 2022, 88, 33–43. [Google Scholar]
- Medina, M.J.; Antic, D.; Borges, P.A.V.; Borko, S.; Fiser, C.; Lauritzen, S.-E.; Martín, J.L.; Oromi, P.; Pavlek, M.; Premate, E.; et al. Temperature variation in caves and its significance for subterranean ecosystems. Sci. Rep. 2023, 13, 20735. [Google Scholar] [CrossRef]
- Graham, R.W.; Lundelius, E.L., Jr. Coevolutionary disequilibrium and Pleistocene extinctions. In Quaternary Extinctions: A Prehistoric Revolution; Martin, P.S., Klein, R.G., Eds.; University of Arizona Press: Tucson, AZ, USA, 1984; pp. 223–249. [Google Scholar]
- Semken, H.A., Jr.; Graham, R.W.; Stafford, T.W., Jr. AMS 14C analysis of Late Pleistocene non-analog faunal components from 21 cave deposits in southeastern North America. Quat. Internat. 2010, 217, 240–255. [Google Scholar] [CrossRef]
- Webb, S.D. Chronology of Florida Pleistocene mammals. In Pleistocene Mammals of Florida; Webb, S.D., Ed.; University Press of Florida: Gainesville, FL, USA, 1974; pp. 5–31. [Google Scholar]
- Brodkorb, P. The Pleistocene avifauna of Arredondo, Florida. Bull. Fla. St. Mus. Biol. Sci. 1959, 4, 269–291. [Google Scholar]
- Morgan, G.S.; Emslie, S.D. Tropical and western influences in vertebrate faunas from the Pliocene and Pleistocene of Florida. Quat. Internat. 2010, 217, 143–158. [Google Scholar]
- Martin, P.S. Twilight of the Mammoths; University of California Press: Berkeley, CA, USA, 2007; 276p. [Google Scholar]
- Stafford, T.W., Jr.; Semken, H.A., Jr.; Graham, R.W.; Klippel, W.F.; Markova, A.; Smirnov, N.G.; Southon, J. First AMS 14C dates documenting contemporaneity of non-analog species in late Pleistocene mammal communities. Geology 1999, 27, 903–906. [Google Scholar] [CrossRef]
- Winge, H. Jordfundne og nulevende F1agermus (Chiroptera) fra Lagoa Santa, Minas Geraes, Brasilien: Med udsigt over F1agermusenes indbyrdes Slaegstkab. E Museo Lundii 1893, 2, 1–92. [Google Scholar]
- Paleobiology Database. Available online: https://paleobiodb.org/ (accessed on 22 February 2024).
- Olsen, S.J. Additional remains of Florida’s Pleistocene vampire. J. Mammal. 1960, 41, 458–462. [Google Scholar] [CrossRef]
- Linares, O.J. Quir6pteros subf6siles encontrados en las cuevas Venezolanas. Parte III. Desmodus rotundus en la Cueva de la Brújula (Mi. 1) Miranda. Bol. Soc. Venez. Espeleo. 1970, 3, 33–36. [Google Scholar]
- Linares, O.J. Quir6pteros subf6siles encontrados en las cuevas Venezolanas. Parte I. Dep6sito de la Cueva de Quebrada Honda (Designaci6n de Catastro Ar-l). Bol. Soc. Venez. Espeleo. 1968, 1, 119–145. [Google Scholar]



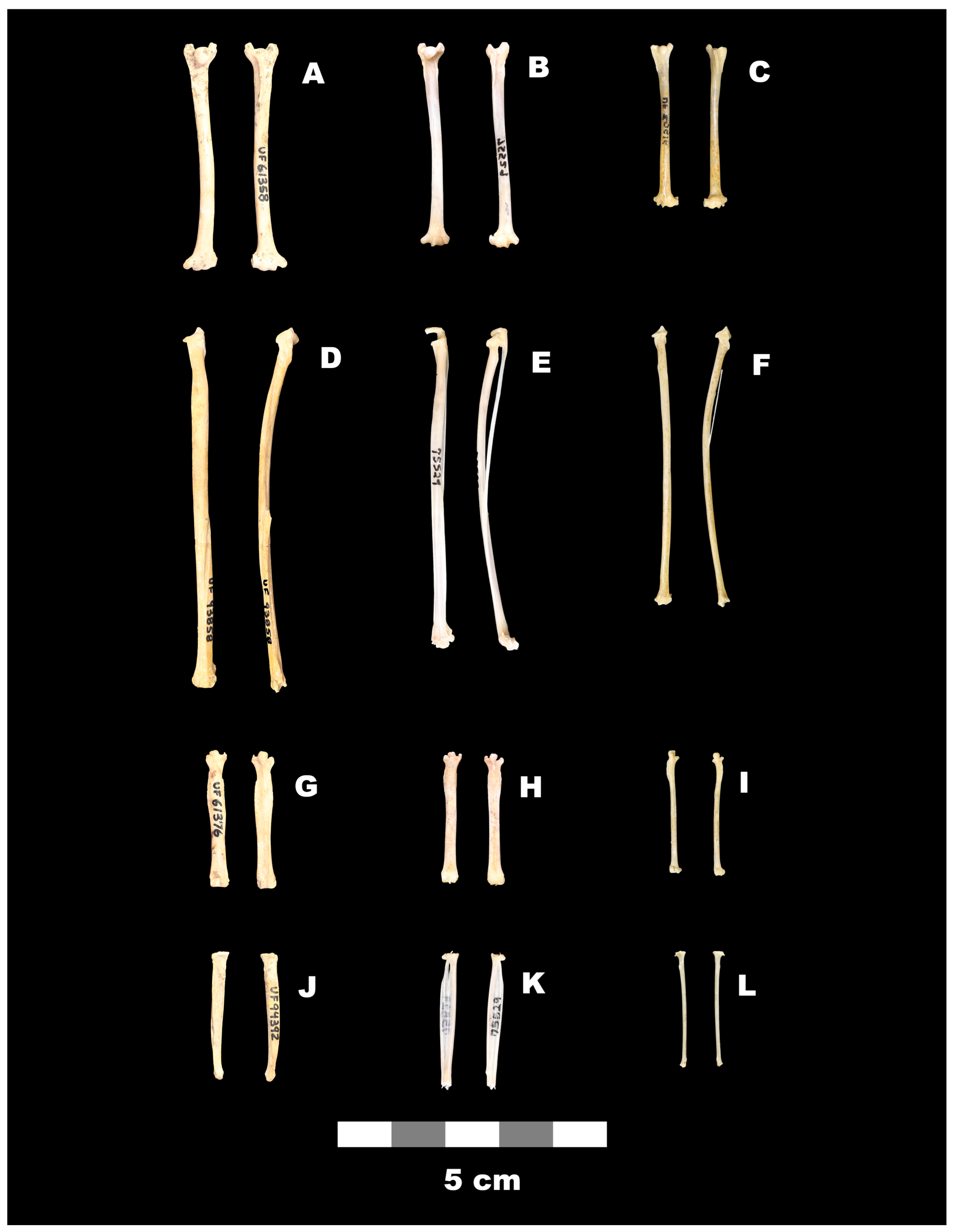
| Element, Species, and Locality | Total Length | Proximal Width | Distal Width | Shaft Width | |
|---|---|---|---|---|---|
| Humerus | |||||
| Desmodus rotundus | M | 37.0 | 4.9 | 5.4 | 2.1 |
| Neotropical Region | OR | 32.4–42.4 | 4.4–5.6 | 4.8–5.9 | 1.7–2.4 |
| Recent | SS | 19 | 19 | 19 | 19 |
| † Desmodus stocki 1 | M | 43.6 | 6.3 | 6.8 | 2.5 |
| San Josecito Cave | OR | 39.3–47.5 | 5.8–6.8 | 6.4–7.3 | 2.0–2.9 |
| Nuevo Leon, Mexico | SS | 42 | 47 | 52 | 56 |
| † Desmodus stocki 1 | M | 43.8 | 6.8 | 7.0 | 2.7 |
| Potter Creek Cave | OR | – | 6.7–6.8 | 6.5–7.1 | 2.5–2.8 |
| California | SS | 1 | 2 | 5 | 7 |
| † Desmodus stocki 2 Reddick 1, Florida | M OR SS | 41.8 39.4–44.3 15 | 6.3 6.0–6.7 38 | 6.8 6.4–7.2 42 | 2.6 2.2–2.7 42 |
| † Desmodus stocki 3 Reddick 1, Florida | M OR SS | 41.1 38.5–43.5 21 | 6.4 6.1–6.9 46 | 6.7 6.3–7.3 66 | 2.7 2.4–2.9 49 |
| † Desmodus draculae 4 Cueva del Guácharo Venezuela (part of holotype) | 51.0 | – | 8.5 | 3.3 | |
| † Desmodus aff. draculae 5 Kiyú, Uruguay | 48.7 | 7.2 | 8.5 | 2.9 | |
| † Desmodus archaeodaptes 4 | |||||
| Inglis 1A, Florida | 39.7 | 5.7 | 6.2 | 2.2 | |
| Haile 16A, Florida | – | – | 5.9 | – | |
| Haile 21A, Florida | – | 5.2 | – | 2.1 | |
| Radius-ulna | |||||
| Desmodus rotundus | M | 58.0 | 3.7 | 3.6 | 2.5 |
| Neotropical Region | OR | 54.3–65.5 | 3.3–4.3 | 3.3–4.1 | 2.1–2.9 |
| Recent | SS | 15 | 15 | 15 | 15 |
| † Desmodus stocki 2 Reddick 1, Florida | M OR SS | 65.3 64.0–66.6 2 | 4.5 4.3–4.7 22 | 4.4 4.2–4.6 9 | 2.9 2.7–3.1 10 |
| † Desmodus stocki 3 Reddick 1, Florida | M OR SS | 62.5 58.8–65.7 3 | 4.6 4.2–4.9 45 | 4.6 4.1–5.2 28 | 2.9 2.5–3.7 17 |
| Femur | |||||
| Desmodus rotundus | M | 24.3 | 3.7 | 3.2 | 2.2 |
| Neotropical Region | OR | 23.1–26.3 | 3.3–4.1 | 2.9–3.6 | 1.9–2.6 |
| Recent | SS | 16 | 16 | 16 | 16 |
| † Desmodus stocki 2 Reddick 1, Florida | M OR SS | 24.6 23.3–25.3 9 | 4.5 4.2–4.8 24 | 3.8 3.6–4.0 13 | 3.2 3.0–3.4 13 |
| † Desmodus stocki 3 Reddick 1, Florida | M OR SS | 24.5 23.6–25.5 7 | 4.6 4.2–4.8 12 | 3.7 3.5–3.9 11 | 3.4 3.2–3.7 8 |
| Tibia | |||||
| Desmodus rotundus | M | 24.4 | 2.8 | 1.6 | 1.6 |
| Neotropical Region | OR | 22.7–26.6 | 2.6–3.0 | 1.5–1.7 | 1.5–1.9 |
| Recent | SS | 16 | 16 | 16 | 16 |
| † Desmodus stocki 2 Reddick 1, Florida | M OR SS | 23.5 23.3–23.6 4 | 3.3 3.1–3.5 16 | 1.9 1.9–2.1 4 | 2.4 2.3–2.5 14 |
| † Desmodus stock 3 Reddick 1, Florida | M OR SS | 23.2 22.3–24.3 3 | 3.4 3.3–3.5 4 | 2.0 1.8–2.0 4 | 2.6 2.4–2.8 3 |
| Species | Humerus Shaft Width Observed Range (mm) | Sample Size | Predicted Body Weight Range (g) |
|---|---|---|---|
| Desmodus rotundus | 1.7–2.4 | 19 | 23.8–52.3 |
| D. archaeodaptes | 2.1–2.2 | 2 | 38.7–43.5 |
| D. stocki | 2.0–2.9 | 154 | 34.5–81.3 |
| D. aff. draculae | 2.9 | 1 | 81.3 |
| D. draculae | 3.3 | 1 | 110.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Morgan, G.; McDonald, H.G.; Czaplewski, N.J. Possible Coevolution of Vampire Bats (Chiroptera: Phyllostomidae: Desmodus) and Large Xenarthrans (Cingulata, Pilosa) in North America and South America During the Quaternary. Quaternary 2026, 9, 2. https://doi.org/10.3390/quat9010002
Morgan G, McDonald HG, Czaplewski NJ. Possible Coevolution of Vampire Bats (Chiroptera: Phyllostomidae: Desmodus) and Large Xenarthrans (Cingulata, Pilosa) in North America and South America During the Quaternary. Quaternary. 2026; 9(1):2. https://doi.org/10.3390/quat9010002
Chicago/Turabian StyleMorgan, Gary, H. Gregory McDonald, and Nicholas J. Czaplewski. 2026. "Possible Coevolution of Vampire Bats (Chiroptera: Phyllostomidae: Desmodus) and Large Xenarthrans (Cingulata, Pilosa) in North America and South America During the Quaternary" Quaternary 9, no. 1: 2. https://doi.org/10.3390/quat9010002
APA StyleMorgan, G., McDonald, H. G., & Czaplewski, N. J. (2026). Possible Coevolution of Vampire Bats (Chiroptera: Phyllostomidae: Desmodus) and Large Xenarthrans (Cingulata, Pilosa) in North America and South America During the Quaternary. Quaternary, 9(1), 2. https://doi.org/10.3390/quat9010002







