Paleoenvironments of the Last Interglacial–Glacial Transition on the East European Plain: Insights into Climate-Driven Ecosystem Dynamics
Abstract
1. Introduction
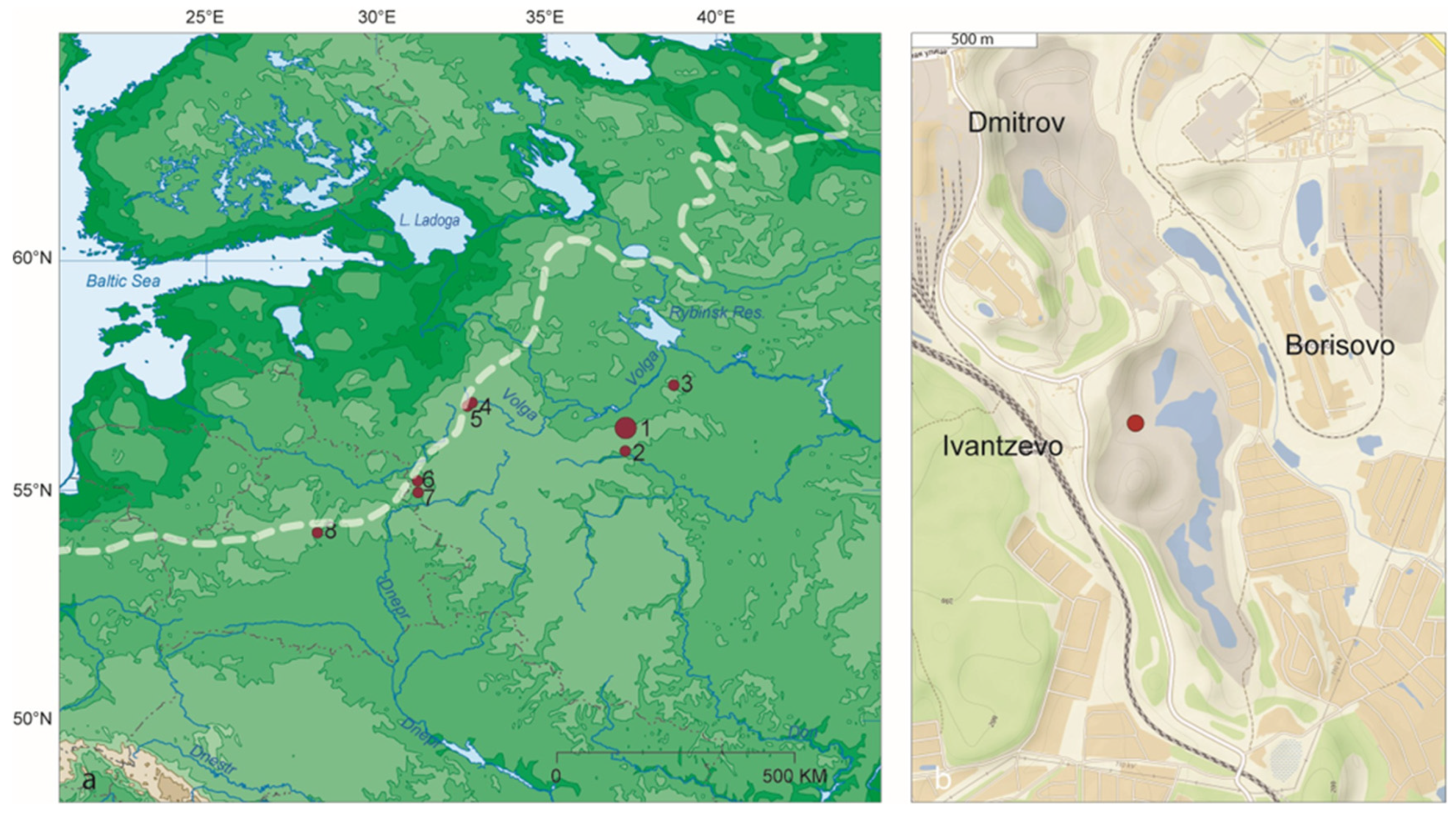
2. Regional Setting
3. Materials and Methods
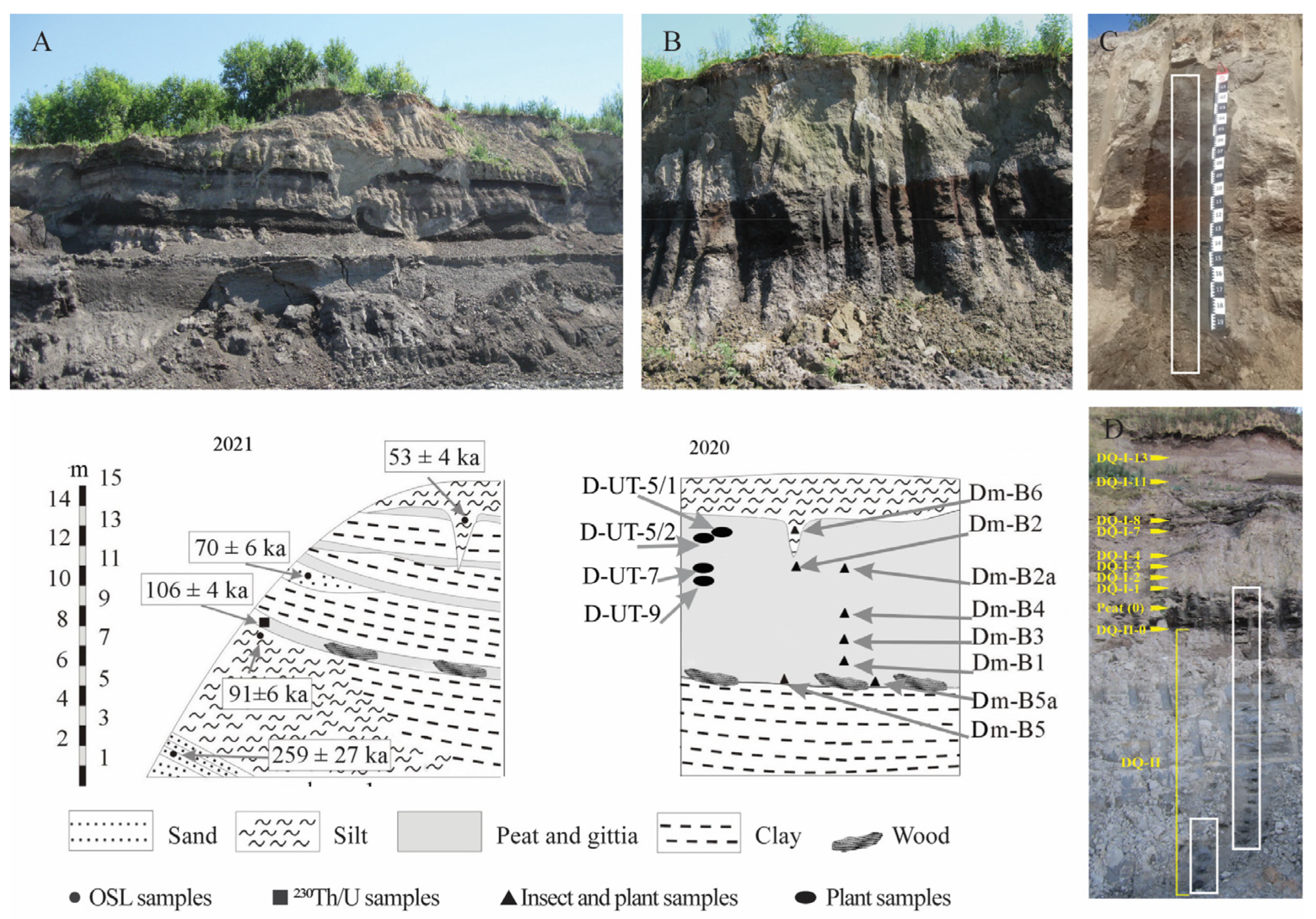
3.1. Stratigraphy and Sampling

3.2. Dating
3.2.1. Optically Stimulated Luminescence Dating
3.2.2. Uranium–Thorium Dating
3.3. Pollen Analysis
3.4. Biome Reconstruction
3.5. Climate Reconstruction
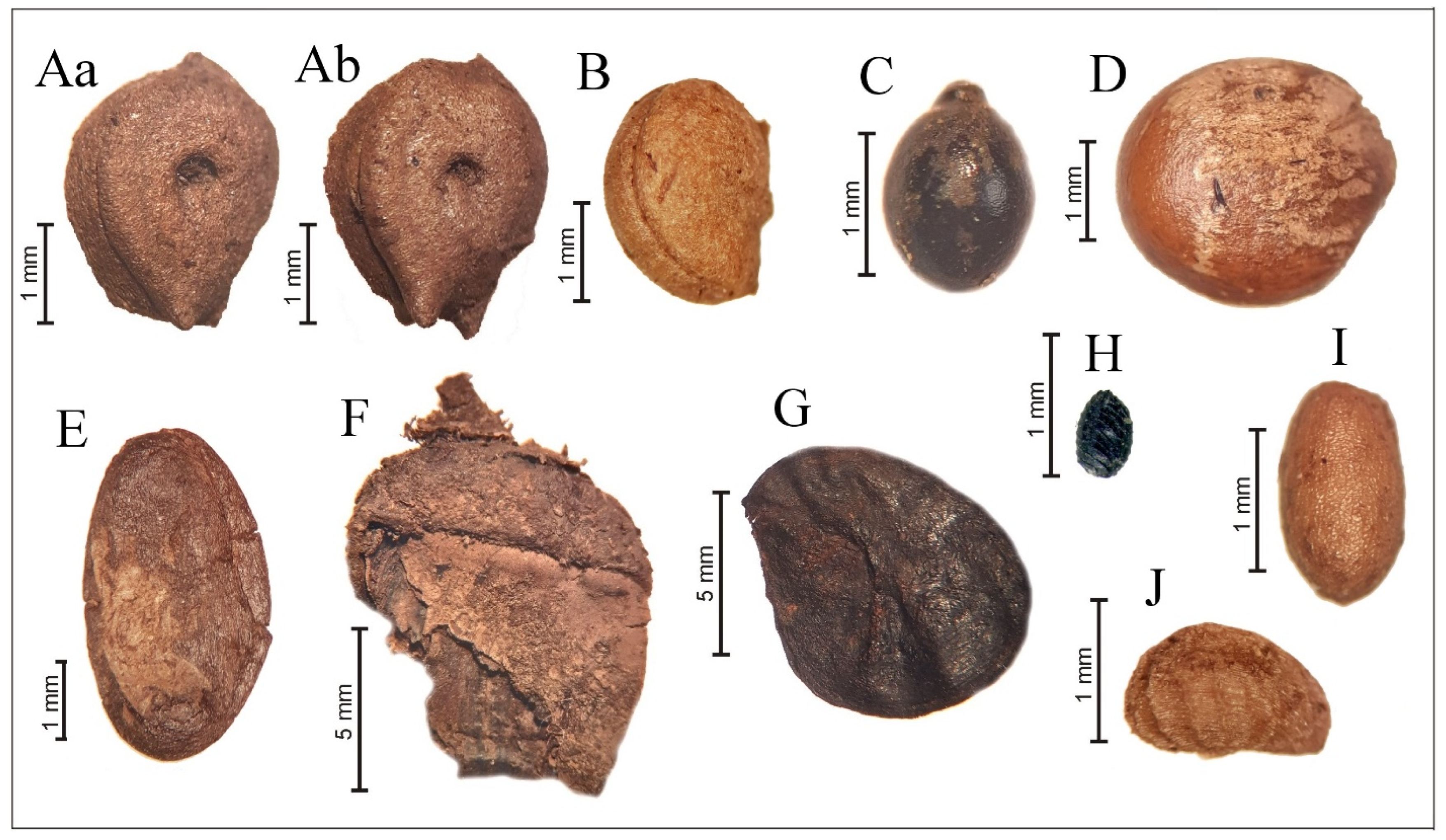
3.6. Analysis of Plant Macrofossils
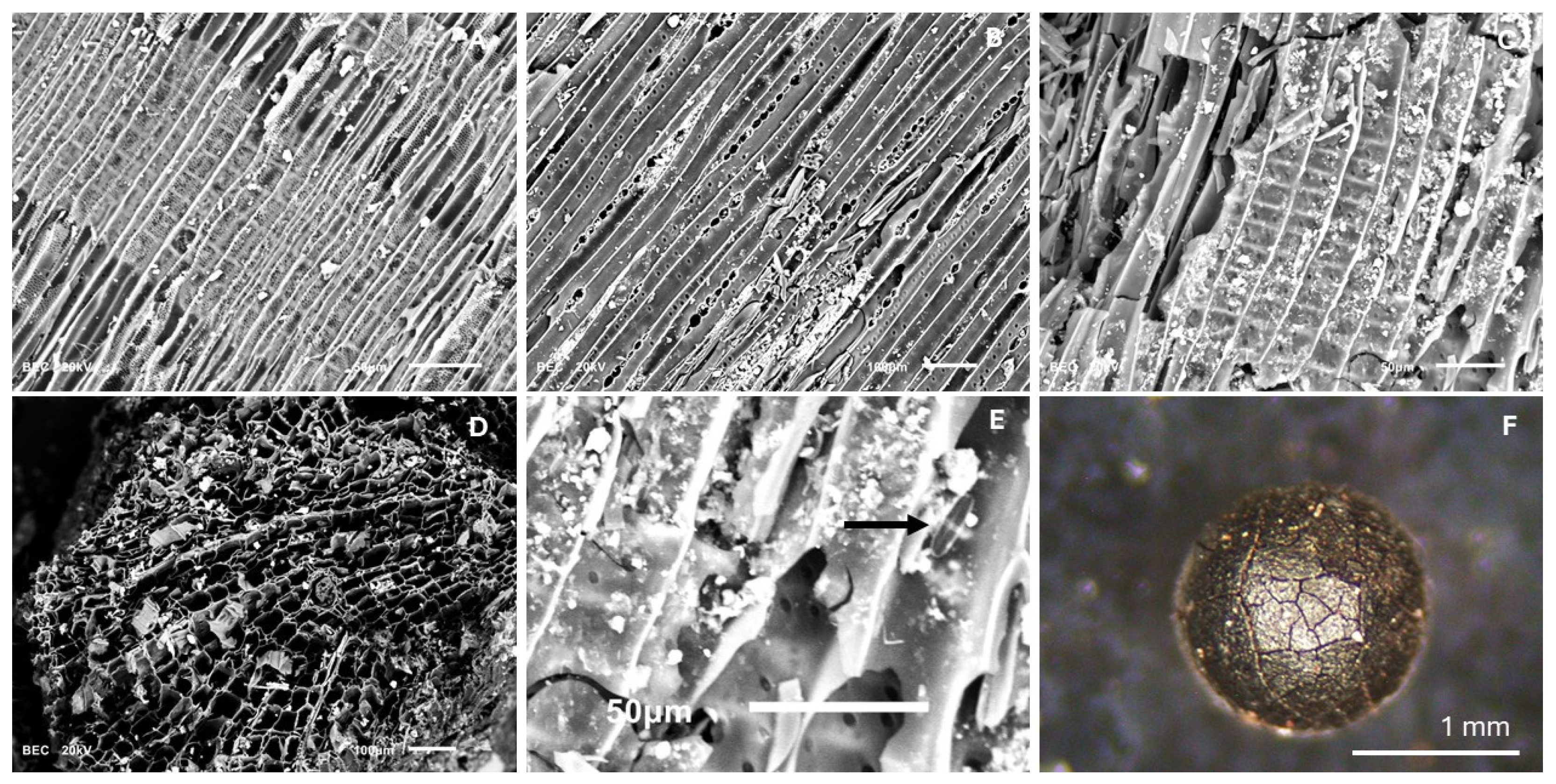
3.7. Anthracological Analysis
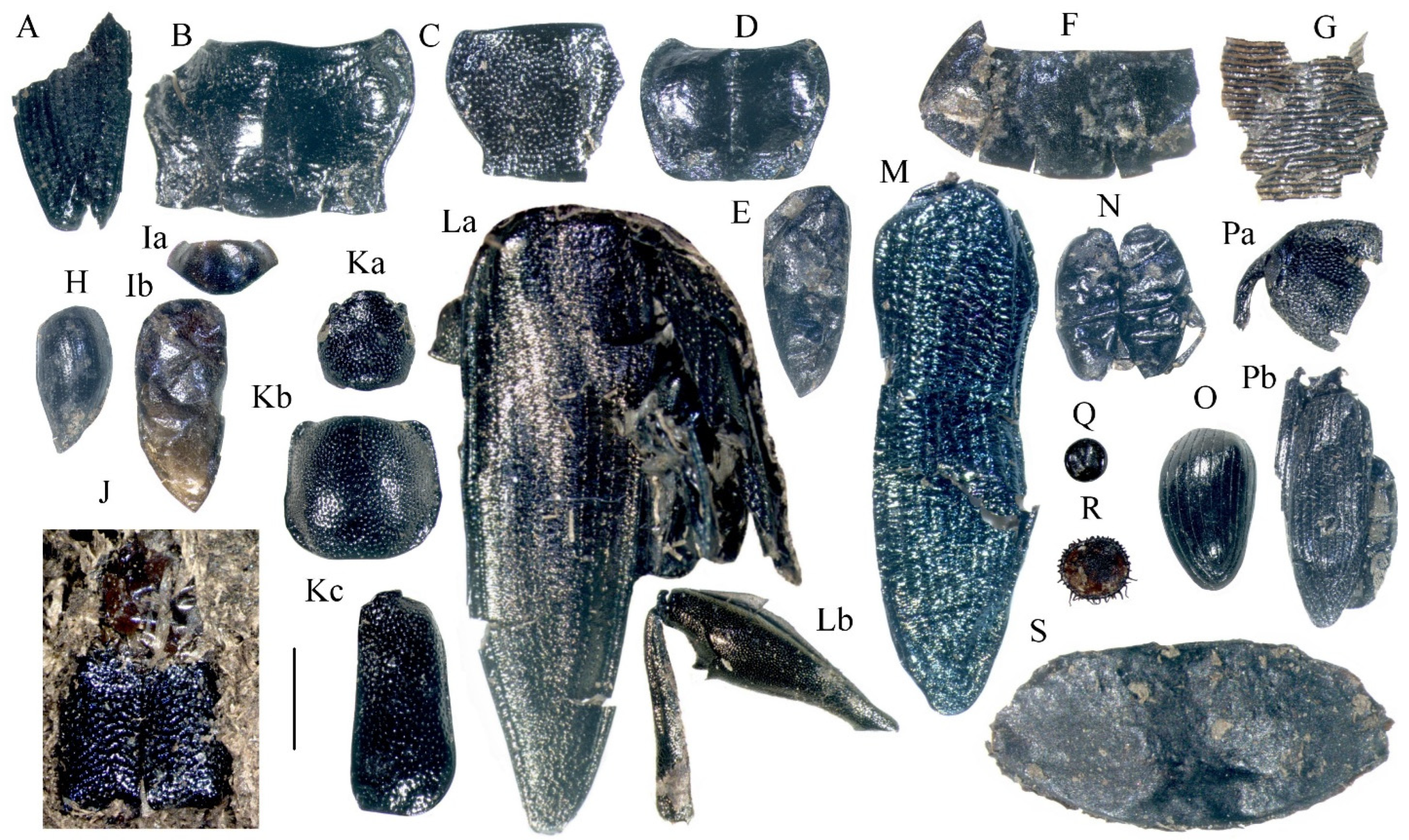
3.8. Analysis of Invertebrate Macrofossils
4. Results
4.1. Chronology
4.1.1. 230Th/U Chronology
4.1.2. OSL Dating
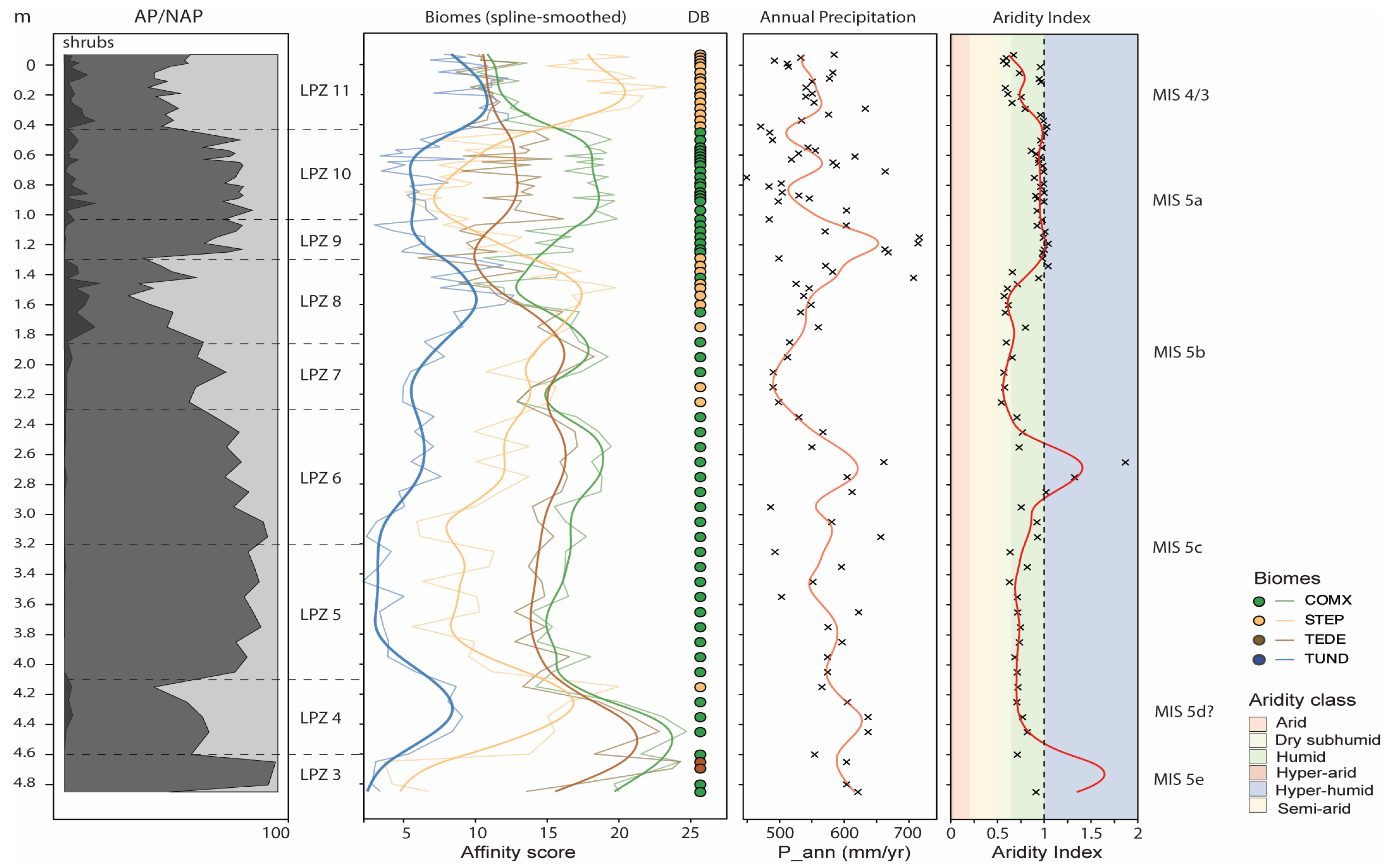
4.2. Pollen
4.3. Pollen-Based Biome Reconstruction
4.4. Pollen-Based Climate Reconstruction
4.5. Plant Macrofossils
4.6. Charred Plant Remains
4.7. Macrofossils of Invertebrates
4.8. Planktonic Crustaceans and Other Aquatic Microfauna
5. Discussion
5.1. Chronological Correlation of Peat Units with Mikulino Interglaciation
5.2. Regional Climate and Ecosystems Dynamics
- ▪
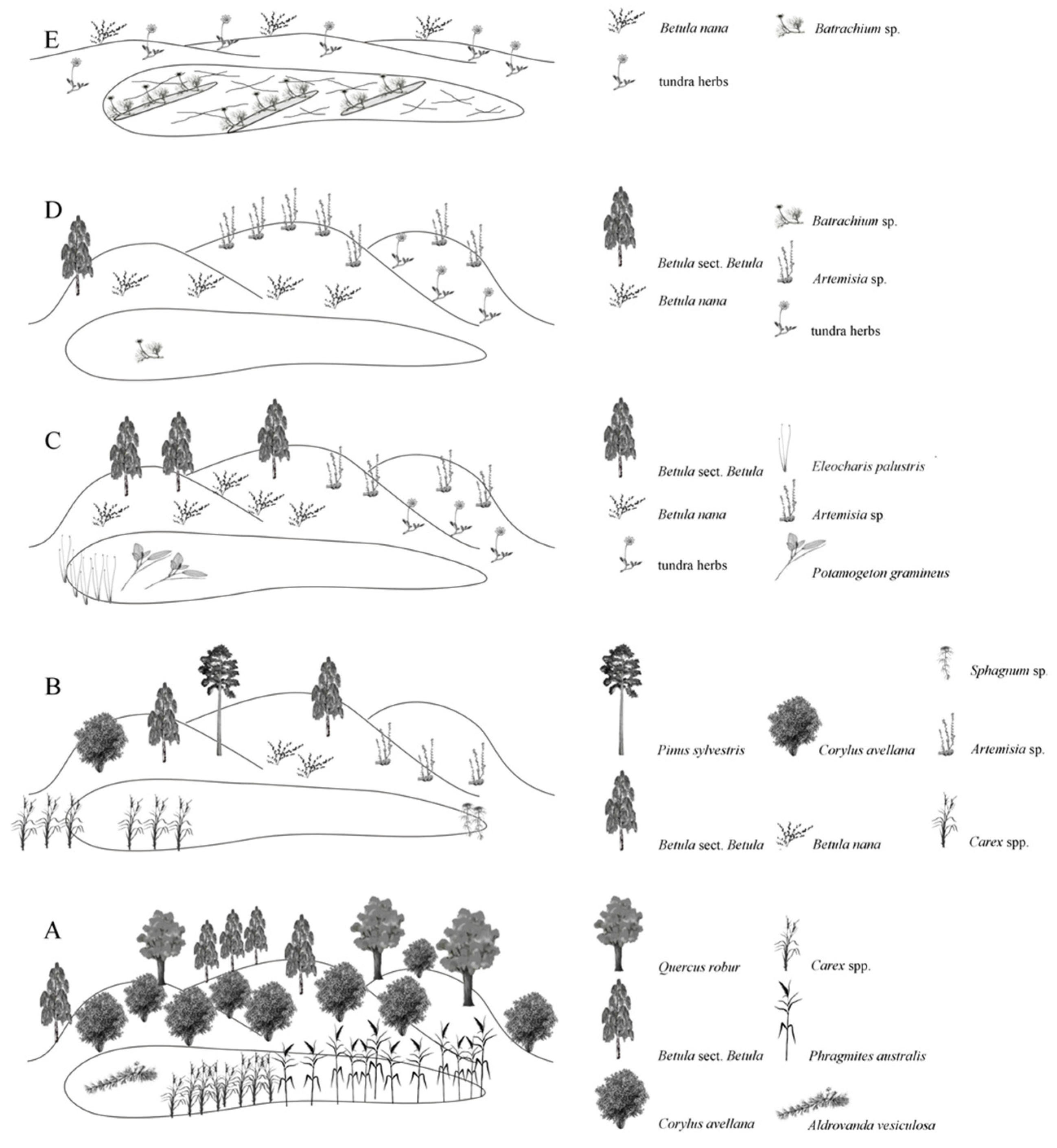
- Phase 1 (LPZ 1)—mixed forests (dominant biome COMX), reconstructed from only two samples from the lowest part of the section (grey silt/grey clay unit) with an OSL date of 259 ka at the basal level. It is characterized by dominance of tree birch and a proportion of conifers (Picea, Pinus, Abies, Larix) and broad-leaved trees (Tilia, Ulmus), with the presence of reworked pre-Quaternary spores. Such a mixture is characteristic of outwash fen deposits. The pollen spectrum may reflect the end of the previous interglacial period, supported by the OSL date. There is a time gap between phases 1 and 2, as the deposits between them do not contain pollen.
- ▪
- Phase 2 (LPZ 2)—cold steppes dominated by Artemisia and Amaranthaceae, with small patches of Betula and Picea (dominant biome STEP). This phase was reconstructed from only three samples from sandy loam and clay underlying the Mikulino horizon (lower peat). It presumably reflects the vegetation of the terminal stages of the Moscow Glaciation and the transition to the interglacial, corresponding to MIS 6a. The reconstructed annual precipitation for this phase is 500 mm, and Aridity Index (AI) is 0.75, parameters typical for the modern forest-steppe zone. There was a break between phases 2 and 3 due to discontinuous sampling.
- ▪
- Phase 3 (LPZ 3)—thermophilic broad-leaved forests with hazel undergrowth, documented in two samples from the lower peat, appear to represent the Mikulino thermal maximum, M4-5 according to Grichuk [84]. This phase corresponds to the Eemian Interglacial (MIS 5e). This interpretation is supported by the absolute dominance of Corylus and broad-leaved taxa, both in pollen and plant macrofossils, which is characteristic of stage M5 in all regional sections [14,16,17], as well as by the presence of the thermophilic aquatic carnivorous plant Aldrovanda vesiculosa. For this phase, the TEDE (Temperate deciduous forest) biome has been reconstructed, with annual precipitation of 600 mm and an AI increase to 1.7. Other phases of Mikulino (M2, M3, M5, and M6) are not represented in our pollen record, likely due to interruptions in sediment accumulation or the specifics of sample selection.
- ▪
- Phase 4 (LPZ 4) in its lower and central parts, represents a combination of broad-leaved groves and open grasslands. The reconstructed biome for this phase is COMX (cool mixed forest). Signs of forest decline appear at the end of this phase, coinciding with increased fire occurrence and droughts. The upper boundary of zone 4 is marked by a short stage of cold steppe, with a simultaneous drop of the aridity index to 0.70–0.75. This phase corresponds to the vegetation of the late Mikulino interglacial (M7–M8), and its end may mark the first post-Eemian (s. stricto) cooling phase, the Herning Stadial, during which much of Northern Europe became largely treeless. It corresponds to the end of MIS 5e and MIS 5d [83].
- ▪
- Phase 5 (LPZ 5-6)—reconstructed from pollen spectra from the peaty silt overlying the lower peat; it features forest recovery, initially of birch and later of mixed forests dominated by Pinus, Betula, and Picea. The reconstructed biome is COMX (cool mixed forest). Peaks in precipitation (up to 630 mm) and aridity index (up to 1.5) mark the end of the phase. This phase can be correlated with the first Valdai interstadial or the final phase of the Mikulino complex. It is similar to the Brørup Interstadial, which corresponds to MIS 5c, when birch and pine woodlands expanded across Northern and Central Europe [83].
- ▪
- Phase 6 (LPZ 7-8) reconstructed from the bottom of the upper peat. The pollen spectrum reflects gradual degradation of coniferous forests and subsequent expansion of tundra vegetation, dominated first by grasses and later by shrubs. The second half of the phase is marked by the maximum proportion of shrubs and the presence of Betula nana and Juniperus in both pollen and macrofossil assemblages. The dominant biomes are COMX and STEP, but at the end of this phase, the TUND (tundra) biome reaches its maximum value. Climate becomes drier, with P_ann 500–550 mm and AI 0.75. The phase may be correlated with the Rederstall Stadial (MIS 5b), characterized by initially grassy and later shrubby tundra over large areas of Europe [82,83].
- ▪
- Phase 7 (LPZ 9-10)—top of the upper peat, reflects the rapid establishment of boreal closed-canopy spruce forests. Picea, Betula, and Ericales are abundant in pollen spectra and plant macrofossils. Seeds of thermophilic water plants (Nuphar lutea and Ceratophyllum demersum) and the extinct species Potamogeton sukaczevii indicate a relatively warm and humid climate. The dominant biomes are COMX and TAIG; precipitation increases sharply to 650 mm, and AI to 1.0. This phase may represent the vegetation of the second post-Mikulino interstadial, correlating with the Odderade (MIS 5a), when boreal forests of pine, spruce, and birch were widespread in various combinations in central and northern Europe. The end of this interstadial in Europe is radiocarbon dated to ca. 60 ka [82,83].
- ▪
- Phase 8 (LPZ 11)—reconstructed from clayey silt above the peat, marks abrupt degradation of forest cover, near-complete disappearance of all tree taxa except Betula, and widespread fires, resulting in cold steppe and tundra landscapes. This zone shows peaks in the STEP and TUND biomes, with a noticeable proportion of shrubs. Climate reconstructions reflect decreased precipitation and increased aridity. Pollen-based reconstructions and the OSL date of 53 ± 4 ka place this phase within the Oerel Interstadial (MIS 3), radiocarbon-dated to 54–58 ka [83]. MIS 4 deposits are not detected in our section, likely due to cold and arid conditions that did not favor lacustrine or peat accumulation. Completely treeless episodes described for MIS 4 [89] are not represented in our pollen sequence.
5.3. Local Ecosystem Dynamics
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shackleton, N.J.; Sánchez-Goñi, M.F.; Pailler, D.; Lancelot, Y. Marine Isotope Substage 5e and the Eemian Interglacial. Glob. Planet. Change 2003, 36, 151–155. [Google Scholar] [CrossRef]
- Kukla, G.J. The last interglacial. Science 2000, 287, 987–988. [Google Scholar] [CrossRef]
- Aalbersberg, G.; Litt, T. Multiproxy Climate Reconstructions for the Eemian and Early Weichselian. J. Quat. Sci. 1998, 13, 367–390. [Google Scholar] [CrossRef]
- Bonelli, S.; Charbit, S.; Kageyama, M.; Woillez, M.-N.; Ramstein, G.; Dumas, C.; Quiquet, A. Investigating the evolution of major Northern Hemisphere ice sheets during the last glacial–interglacial cycle. Clim. Past 2009, 5, 329–345. [Google Scholar]
- Klotz, S.; Müller, U.; Mosbrugger, V.; de Beaulieu, J.-L.; Reille, M. Eemian to early Würmian climate dynamics: History and pattern of changes in Central Europe. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 211, 107–126. [Google Scholar] [CrossRef]
- Kühl, N.; Litt, T.; Schölzel, C.; Hense, A. Eemian and Early Weichselian temperature and precipitation variability in northern Germany. Quat. Sci. Rev. 2007, 26, 3311–3317. [Google Scholar] [CrossRef]
- Väliranta, M.; Birks, H.H.; Helmens, K.F.; Engels, S.; Piirainen, M. Early Weichselian interstadial (MIS 5c) summer temperatures were higher than today in northern Fennoscandia. Quat. Sci. Rev. 2009, 28, 777–782. [Google Scholar] [CrossRef]
- Novenko, E.Y. Vegetation and Climate Changes in Central and Eastern Europe During the Late Pleistocene and Holocene in Interglacials and Transitional Stages of Climatic Macrocycles; GEOS: Moscow, Russia, 2016; p. 228. (In Russian) [Google Scholar]
- Velichko, A.A.; Novenko, E.Y.; Pisareva, V.V.; Zelikson, E.M.; Boettger, T.; Junge, F.W. Vegetation and climate changes during the Eemian interglacial in Central and Eastern Europe: Comparative analysis of pollen data. Boreas 2005, 34, 207–219. [Google Scholar] [CrossRef]
- Borisova, O.K.; Novenko, E.Y.; Velichko, A.A.; Kremenetski, K.V.; Junge, F.W.; Boettger, T. Vegetation and Climate Changes During the Eemian and Early Weichselian in the Upper Volga Region. Quat. Sci. Rev. 2007, 26, 2574–2585. [Google Scholar] [CrossRef]
- Kuznetsov, V.Y.; Maksimov, F.E. Methods of Quaternary Geochronometry and Marine Geology; Nauka: Saint Petersburg, Russia, 2012; p. 190. (In Russian) [Google Scholar]
- Rusakov, A.; Nikonov, A.; Savelieva, L.; Simakova, A.; Sedov, S.; Maksimov, F.; Kuznetsov, V.; Savenko, V.; Starikova, A.; Korkka, M.; et al. Landscape evolution in the periglacial zone of Eastern Europe since MIS 5: Proxies from paleosols and sediments of the Cheremoshnik keysite (Upper Volga, Russia). Quat. Int. 2015, 365, 26–41. [Google Scholar] [CrossRef]
- Rusakov, A.; Sedov, S.; Sheinkman, V.; Dobrynin, D.; Zinovyev, E.; Trofimova, S.; Maksimov, F.; Kuznetsov, V.; Korkka, M.; Levchenko, S. Late Pleistocene paleosols in the extra-glacial regions of Northwestern Eurasia: Pedogenesis, post-pedogenic transformation, paleoenvironmental inferences. Quat. Int. 2019, 501, 174–192. [Google Scholar] [CrossRef]
- Karpukhina, N.V.; Pisareva, V.V.; Zyuganova, I.S.; Konstantinov, E.A.; Zakharov, A.L.; Baranov, D.V.; Utkina, A.O.; Panin, A.V. New data about the section stratigraphy near Kileshino (Tver oblast, Russia)—The key for understanding boundaries of glaciations on the Valdai Hills in the upper Pleistocene. News Russ. Acad. Sciences. Geogr. Ser. 2020, 84, 874–887. [Google Scholar] [CrossRef]
- Maksimov, F.E.; Savelieva, L.A.; Popova, S.S.; Zyuganova, I.S.; Grigoriev, V.A.; Levchenko, S.B.; Petrov, A.Y.; Fomenko, A.P.; Pankratova, L.A.; Kuznetsov, V.Y. Chronostratigraphic position of the Mikulinian deposits (case of the reference section near Nizhnyaya Boyarshchina Village, Smolensk Oblast). Izv. RAN. Seriya Geograficheskaya V 2022, 86, 447–469. [Google Scholar]
- Maksimov, F.E.; Savelieva, L.A.; Fomenko, A.P.; Popova, S.S.; Zyuganova, I.S.; Grigoriev, V.A.; Petrov, A.Y.; Boltramovich, S.F.; Kuznetsov, V.Y. Chronology and main stages of the vegetation development during the Mikulino Interglacial on the Russian Plain according to the results of buried lake and peat sediments study from Tver and Smolensk Province. Dokl. Earth Sci. 2023, 513 (Suppl. S1), S121–S139. [Google Scholar] [CrossRef]
- Maksimov, F.E.; Savelieva, L.A.; Fomenko, A.P.; Popova, S.S.; Zyuganova, I.S.; Grigoriev, V.A.; Petrov, A.Y.; Boltramovich, S.F.; Kuznetsov, V.Y. Chronology and main stages of vegetation development in the central region of the East European Plain during the Mikulino interglacial. Geomorfol. I Paleogeogr. 2024, 55, 147–174. [Google Scholar] [CrossRef]
- Sycheva, S.A. Paleocryogenic events in the periglacial area of the Central Russian Upland at the end of the Middle and Late Pleistocene. Earth Cryosphere 2012, 16, 45–56. (In Russian) [Google Scholar]
- Sycheva, S.A.; Khokhlova, O.S.; Pushkina, P.R. Structure of the Late Pleistocene climate rhythm inferred from the detailed soil-sedimentation archive of the extraglacial region of the East European Plain (Aleksandrovka Quarry). Stratigr. Geol. Correl. 2021, 29, 368–387. [Google Scholar] [CrossRef]
- Sycheva, S.A.; Khokhlova, O.S.; Ershova, E.G.; Myakshina, T.N.; Ukrainskiy, P.A. Cryogenic-lateral hypothesis of the formation of the parent rock of soddy-podzolic soils using the example of the Ryshkovo paleosol (MIS 5e) in the Taneyev quarry, Kursk region. Eur. Soil Sci. 2024, 57, 1308–1320. [Google Scholar] [CrossRef]
- Velichko, A.A.; Drenova, A.N.; Klimanov, V.A.; Kremenetski, K.V.; Nechaev, V.P.; Catto, N. Climate changes in East Europe and Siberia at the late Glacial–Holocene transition. Quat. Int. 2002, 91, 75–99. [Google Scholar] [CrossRef]
- Little, E.C.; Lian, O.B.; Velichko, A.A.; Morozova, T.D.; Nechaev, V.P.; Dlussky, K.G.; Rutter, N.W. Quaternary stratigraphy and optical dating of loess from the East European Plain (Russia). Quat. Sci. Rev. 2002, 21, 1745–1762. [Google Scholar] [CrossRef]
- Maksimov, F.E.; Savelyeva, L.A.; Levchenko, S.B.; Grigoriev, V.A.; Petrov, A.Y.; Fomenko, A.P.; Khrestievskym, V.V.; Kuznetsovm, V.Y. On the chronology of the Mikulino Interglacial in the northwest of the Russian plain. In Relief and Quaternary Formations of the Arctic, Subarctic, and Northwest Russia; Federal State Budgetary Institution: Petersburg, Russia, 2020; Volume 7, pp. 322–326. Available online: http://www.evgengusev.narod.ru/seminar5/conference.html (accessed on 3 November 2025). (In Russian)
- Stroeven, A.P.; Clas Hättestrand, C.; Kleman, J.; Heyman, J.; Fabel, D.; Fredin, O.; Goodfellow, B.W.; Harbor, J.M.; Jansen, J.D.; Olsen, L.; et al. Deglaciation of Fennoscandia. Quat. Sci. Rev. 2016, 147, 91–121. [Google Scholar] [CrossRef]
- Ershova, E.; Sycheva, S.; Kuzmina, S.; Zuganova, I.; Panin, P.; Meteleva, M. Preliminary results of a multidisciplinary study of the buried peatland and host sediments of the Moscow-Valdai (Dmitrov, Moscow region, Russia). In Proceedings of the EGU General Assembly, Virtual, 19–30 April 2021. [Google Scholar] [CrossRef]
- Kuzmina, S.A.; Sycheva, S.A.; Ershova, E.K.; Zyuganova, I.S.; Zakharov, A.L.; Gorbatov, E.S.; Kolesnikov, S.F.; Panin, P.G. Ivantsevo section of the buried Middle and Late Pleistocene lake-bog deposits near the town Dmitrov, Moscow Region. Geomorphology 2022, 53, 7–18. [Google Scholar] [CrossRef]
- Barber, K.E. Peatlands as Scientific Archives of Past Biodiversity. Biodivers. Conserv. 1993, 2, 474–489. [Google Scholar] [CrossRef]
- Birks, H.J.B.; Birks, H.H. Quaternary Palaeoecology; Edward Arnold: London, UK, 1980. [Google Scholar]
- Greiser, C.; Joosten, H. Archive value: Measuring the palaeoinformation content of peatlands in a conservation and compensation perspective. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2018, 14, 209–220. [Google Scholar] [CrossRef]
- Boyarskaya, T.D.; Nemtsova, G.M.; Sudakova, N.G. On the Pleistocene stratigraphy and paleogeography of the Klin-Dmitrov Upland (section at the Kunya River). In Nature-Population-Economy (Geogr. Aspects of Research); Golubchik, M.M., Ed.; Publishing House of Mordovian State University: Saransk, Russia, 1983; pp. 61–64. (In Russian) [Google Scholar]
- Nosov, A.A.; Skiba, L.A. Deposits of the Iksha interstadial near Dmitrov town. Bull. Comm. Quat. Period 1975, 44, 122–125. (In Russian) [Google Scholar]
- Sudakova, N.G.; Vvedenskaya, A.I.; Vaskovskaya, L.T.; Nemtsova, G.M.; Nosov, A.A.; Pisareva, V.V. New data about glaciations of Klin-Dmitrov Ridge considering glacial theory of P.A. Kropotkin. In Ideas of P.A. Kropotkin and Natural Science; Pirumova, N.M., Figurovskaya, N.K., Birukov, A.V., Eds.; Institute of Economy: Moscow, Russia, 2002; pp. 88–100. (In Russian) [Google Scholar]
- Sudakova, N.G.; Karpukhin, S.S.; Altynov, A.E. Palaeogeographic reconstructions of glacial morpholithogenic structures in the Moscow region based on satellite imagery. Bull. Mosc. Nat. Soc. 2015, 4, 76–89. (In Russian) [Google Scholar]
- Sudakova, N.G.; Vvedenskaya, A.I.; Vaskovskaya, L.T.; Pisareva, V.V. To the problem of stratigraphy of the Pleistocene of the Klin-Dmitrov Ridge. In Quaternary Geology and Paleogeography of Russia; Alexeev, M.N., Khoreva, I.M., Eds.; GEOS: Moscow, Russia, 1997; pp. 171–180. (In Russian) [Google Scholar]
- Alekseev, M.N.; Gablina, S.S.; Lavrushin, Y.A.; Hyutt, G.A.; Yakimenko, E.Y. Stratigraphy and Geological Events of the Middle and Late Pleistocene of the Moscow Region. In Quaternary Geology and Paleogeography of Russia; GEOS: Moscow, Russia, 2001; pp. 15–24. (In Russian) [Google Scholar]
- Antonov, S.; Rychagov, G.I.; Sudakova, N.G. Middle Pleistocene Glaciations in the Center of the Russian Plain: Problems of Stratigraphy and Paleogeography. Bull. Comm. Quart. Period. 2004, 65, 5–16. (In Russian) [Google Scholar]
- Gorbatov, E.S.; Kolesnikov, S.F. Ground disturbances in sediments of different ages at the Klinsko-Dmitrovskaya ridge. Geophys. Process. Biosph. 2022, 21, 137–146. (In Russian) [Google Scholar]
- Lazukov, G.I.; Sudakova, N.G.; Faustov, S.S. Analysis of glacial deposits of the Klin-Dmitrov Upland and the problems of stratigraphy and paleogeography. In Recent Tectonics, Recent Deposits, Man. 8; Naimark, A.A., Nikolaev, N.I., Eds.; Moscow State University: Moscow, Russia, 1982; pp. 86–101. (In Russian) [Google Scholar]
- Lian, O.B. Luminescence dating|Optically-Stimulated Luminescence, In Encyclopedia of Quaternary Science; Scott, A.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1491–1505. [Google Scholar] [CrossRef]
- Geyh, M.A.; Müller, H. Numerical 230Th/U dating and palynological review of the Holsteinian/Hoxnian Interglacial. Quat. Sci. Rev. 2005, 24, 1861–1872. [Google Scholar] [CrossRef]
- Maksimov, F.E.; Andreicheva, L.N.; Kuznetsov, V.Y.; Grigor’ev, V.A.; Petrov, A.Y.; Levchenko, S.B.; Marchenko-Vagapova, T.I.; Baranova, N.G. Age and chronostratigraphic position of lacustrine-bog deposits in the Chernaya River basin in the north of the Bolshezemelskaya tundra according to the results of their 230Th/U- and 14C-dating. Vestn. St. Petersburg Univ. Earth Sci. 2021, 66, 289–309. (In Russian) [Google Scholar] [CrossRef]
- Geyh, M.A. Selection of suitable data sets improves 230 Th/U dates of dirty material. Geochronometria 2008, 30, 69–77. [Google Scholar] [CrossRef]
- Maksimov, F.E.; Kuznetsov, V.Y. New version of the 230Th/U dating of the Upper and Middle Pleistocene buried organic-rich sediments. Bull. St. Petersburg State Univ. 2010, 7, 103–114. (In Russian) [Google Scholar]
- Böerner, A.; Hrynowiecka, A.; Kuznetsov, V.; Stachowicz-Rybka, R.; Maksimov, F.; Grigoriev, V.; Niska, M.; Moskal-del Hoyo, M. Palaeoecological investigations and 230Th/U dating of Eemian interglacial peat sequence of Banzin (Mecklenburg-Western Pomerania, NE-Germany). Quat. Int. 2015, 386, 122–136. [Google Scholar] [CrossRef]
- Moore, P.D.; Webb, J.A.; Collinson, M.E. Pollen Analysis, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1991; p. 216. [Google Scholar]
- Beug, H.J. Leitfaden der Pollenbestimmung für Mitteleuropa und Angrenzende Gebiete (Guideto the Pollen Analysis for Central Europe and the Adjacent Areas); Verlag Dr. Friedrich Pfeil: München, Germany, 2004. [Google Scholar]
- Severova, E.E.; Nilova, M.V.; Devyatov, A.G.; Volkova, O.A.; Maiorov, S.R.; Polevova, S.V.; Platonova, A.G.; Rud’ko, A.I.; Filin, V.R.; Fyrnin, D.M. Botany-Collection. Bio.Msu.Ru: Information System on Plant Morphology and Anatomy. Mosc. Univ. Biol. Sci. Bull. 2016, 71, 126–127. [Google Scholar] [CrossRef]
- Grimm, E. TILIA 1.11 and TILIA GRAPH 1.17; Research and Collection Center Illinois State Museum: Springfield, IL, USA, 1992; Available online: https://www.tiliait.com (accessed on 20 December 2024).
- R Core Team. R: A Language and Environment for Statistical Computing. In R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2023. [Google Scholar]
- Prentice, I.C.; Cramer, W.; Harrison, S.P.; Leemans, R.; Monserud, R.A.; Solomon, A.M. A global biome model based on plant physiology and dominance, soil properties and climate. J. Biogeogr. 1992, 19, 117–134. [Google Scholar] [CrossRef]
- Prentice, C.; Guiot, J.; Huntley, B.; Jolly, D.; Cheddadi, R. Reconstructing biomes from palaeoecological data: A general method and its application to European pollen data at 0 and 6 ka. Clim. Dyn. 1996, 12, 185–194. [Google Scholar] [CrossRef]
- Tarasov, P.E.; Webb, T., III; Andreev, A.A.; Afanas’eva, N.B.; Berezina, N.A.; Bezusko, L.G.; Blyakharchuk, T.A.; Bolikhovskaya, N.S.; Cheddadi, R.; Chernavskaya, M.M.; et al. Present-day and mid-Holocene biomes reconstructed from pollen and plant macrofossil data from the former Soviet Union and Mongolia. J. Biogeogr. 1998, 25, 1029–1053. [Google Scholar] [CrossRef]
- Tarasov, P.E.; Volkova, V.S.; Webb, T., III; Guiot, J.; Andreev, A.A.; Bezusko, L.G.; Bezusko, T.V.; Bykova, G.V.; Dorofeyuk, N.I.; Kvavadze, E.V.; et al. Last Glacial Maximum biomes reconstructed from pollen and plant macrofossil data from northern Eurasia. J. Biogeogr. 2000, 27, 609–620. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Juggins, S. Rioja: Analysis of Quaternary Science Data. 2024. R Package, Version 1.0-7. Available online: https://cran.r-project.org/web/packages/rioja/index.html (accessed on 15 September 2025).
- Zumaque, J.; de Verna, A.; Fréchette, B.; Davis, B.; Chevalier, M. A ready-to-use version of the Eurasian modern pollen database 2 (EMPD 2; Davis et al., 2020) for paleoclimatic reconstructions. Data Brief 2025, 61, 111657. [Google Scholar] [CrossRef]
- Zomer, R.J.; Trabucco, A.; Metzger, M.J.; Wang, M.; van Straaten, O. Global reference evapotranspiration and aridity index climate database v2. Sci. Data 2022, 9, 186. [Google Scholar] [CrossRef]
- Sassoon, D.; Combourieu-Nebout, N.; Peyron, O.; Bertini, A.; Toti, F.; Lebreton, V.; Moncel, M.-H. Pollen-based climatic reconstructions for the interglacial analogues of MIS 1 (MIS 19, 11, and 5) in the southwestern Mediterranean: Insights from ODP Site 976. Clim. Past 2025, 21, 489–515. [Google Scholar] [CrossRef]
- Sinopoli, G.; Peyron, O.; Masi, A.; Holtvoeth, J.; Francke, A.; Wagner, B.; Sadori, L. Pollen-based temperature and precipitation changes in the Ohrid Basin (western Balkans) between 160 and 70 ka. Clim. Past 2019, 15, 53–71. [Google Scholar] [CrossRef]
- Nikitin, V.P. Paleocarpological Method (Paleokarpologicheskij Metod); TGU: Tomsk, Russia, 1969; p. 82. (In Russian) [Google Scholar]
- Velichkevich, F.Y.; Zastawniak, E. Atlas of the Vascular Plant Macrofossils of Central and Eastern Europe, Part 1; W. Szafer Institute of Botany: Kraków, Poland, 2006; pp. 224–259. [Google Scholar]
- Velichkevich, F.Y.; Zastawniak, E. Atlas of the Vascular Plant Macrofossils of Central and Eastern Europe, Part 2; W. Szafer Institute of Botany: Kraków, Poland, 2008; p. 380. [Google Scholar]
- Schweingruber, F.H. Microscopic Wood Anatomy: Structural Variability of Stems and Twigsin Recent and Subfossil Woods from Central Europe, 2nd ed.; F. Fluck-Wirth: Teufen, Switzerland, 1982. [Google Scholar]
- Barefoot, A.C.; Hankins, F.W. Identification of Modern and Tertiary Woods; Oxford University Press: Oxford, UK, 1982. [Google Scholar]
- Wheeler, E.A.; Baas, P.; Gasson, P.E. (Eds.) IAWA List of Microscopic Features for Hardwood Identification by an IAWA Committee; IAWA: Leiden, Poland, 1989; Volume 10, pp. 219–332. [Google Scholar]
- Bienkowski, A.O. Aquatic Leaf Beetles (Coleoptera: Chrysomelidae: Donaciinae); Mukhamedov G.V.: Livny, Russia, 2014. (In Russian) [Google Scholar]
- Buckland, P.I. The Bugs Coleopteran Ecology Package (BugsCEP) database: 1000 sites and half a million fossils later. Quat. Int. 2014, 341, 272–282. [Google Scholar] [CrossRef]
- Gur’eva, E.L.; Kryzhanovsky, O.L. (Eds.) Guidebook to the Identification of the Insects of the European Part of the USSR. Volume 1. Coleoptera and Strepsiptera; Nauka: Leningrad/Moscow, Russia, 1965. (In Russian) [Google Scholar]
- Kiselev, S.V.; Nazarov, V.I. Late Cenozoic insects of Northern Eurasia. Paleontol. J. 2009, 43, 723–850. [Google Scholar] [CrossRef]
- Kryzhanovskij, O.L.; Belousov, I.A.; Kabak, I.I.; Kataev, B.M.; Makarov, K.V.; Shilenkov, V.G. A Checklist of the Ground-Beetles of Russia and Adjacent Lands (Insecta, Coleoptera, Carabidae); Pensoft: Sofia, Bulgaria; Moscow, Russia, 1995; p. 281. [Google Scholar]
- Sazhnev, A.S.; Lychkovskaya, I.Y.; Prokin, A.A. New data on the fauna of aquatic and semi-aquatic beetles (Coleoptera: Gyrinidae, Haliplidae, Noteridae, Dytiscidae, Hydrochidae, Hydrophilidae, Heteroceridae) of Ryazan Province. Eversmania 2018, 55, 47–51. (In Russian) [Google Scholar]
- Sushko, G.G. Weevils (Coleoptera: Apionidae, Curculionidae) of Belarus Poozerye raisedbogs. Vestn. VGU 2000, 2, 83–88. (In Russian) [Google Scholar]
- Zharov, A.A. Struktura i Zakonomernosti Formirovanija Tafocenozov Malyh Vodoemov. Unpublished Doctoral Dissertation, Koltzov Institute of Developmental Biology RAS: Moskva, Russia, 2021. Available online: https://www.dissercat.com/content/struktura-i-zakonomernosti-formirovaniya-tafotsenozov-malykh-vodoemov (accessed on 23 June 2025).
- Adamec, L. Biological Flora of Central Europe: Aldrovanda vesiculosa L. Perspect. Plant Ecol. Evol. Syst. 2018, 35, 8–21. [Google Scholar] [CrossRef]
- Gałka, M.; Bergonzini, L.; Williamson, D.; Majule, A.; Masao, C.A.; Huguet, A. Macrofossil evidence of Late Holocene presence of Aldrovanda vesiculosa L. in Central-Eastern Europe (Poland) and East Africa (Tanzania). Quat. Int. 2015, 386, 186–190. [Google Scholar] [CrossRef]
- Zyuganova, I.S. Early Valdai carpological assemblages from the Upper Volga Region. Paleontol. J. 2010, 44, 1368–1378. [Google Scholar] [CrossRef]
- Field, M.H.; Lewis, S.G. The first Pleistocene fossil records of Urtica kioviensis Rogow. (Urticaceae) and Potamogeton sukaczevii Wieliczk. (Potamogetonaceae) in the British Isles. Veg. Hist. Archaeobotany 2019, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kukla, G.J.; McManus, J.F.; Rousseau, D.-D.; Chuine, I. How long and how stable was the last interglacial? Quat. Sci. Rev. 1997, 16, 605–612. [Google Scholar] [CrossRef]
- Caspers, G.; Freund, H. Vegetation and climate in the Early and Pleni-Weichselian in northern Central Europe. J. Quat. Sci. 2001, 16, 31–48. [Google Scholar] [CrossRef]
- Guiot, J.; de Beaulieu, J.L.; Cheddadi, R.; David, F.; Ponel, P.; Reille, M. The climate in western Europe during the last glacial/interglacial cycle derived from pollen and insect remains. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1993, 103, 73–93. [Google Scholar]
- Helmens, K.F.; Väliranta, M.; Engels, S.; Shala, S. Large shifts in vegetation and climate during Early Weichselian (MIS 5d–c) inferred from multiproxy evidence at Sokli (northern Finland). Quat. Sci. Rev. 2012, 41, 22–38. [Google Scholar] [CrossRef]
- Helmens, K.F. The Last Interglacial-Glacial cycle (MIS 5-2) re-examined based on long proxy records from central and northern Europe. Quat. Sci. Rev. 2013, 86, 115–143. [Google Scholar]
- Grichuk, V.P. Fossil flora as a paleontological basis for the stratigraphy of Quaternary deposits. In Relief and Stratigraphy of Quaternary Deposits of the Northwestern Russian Plain; Markov, K.K., Ed.; Publishing House of the Academy of Sciences of the USSR: Moscow, Russia, 1961; Volume 78, pp. 25–71. (In Russian) [Google Scholar]
- Molodkov, A.N.; Bolikhovskaya, N.S. Palaeoenvironmental changes and their chronology during the latter half of MIS 5 on the south-eastern coast of the Gulf of Finland. Quat. Int. 2022, 616, 40–54. [Google Scholar] [CrossRef]
- Boettger, T.; Novenko, E.Y.; Velichko, A.A.; Borisova, O.K.; Kremenetski, K.V.; Knetsch, S.; Junge, F.W. Instability of climate and vegetation dynamics in Central and Eastern Europe during the final stage of the Last Interglacial (Eemian, Mikulino) and Early Glaciation. Quat. Int. 2009, 1, 137–144. [Google Scholar] [CrossRef]
- Velichko, A.A.; Kremenetsky, K.V.; Negendank, J.F.; Mingram, J.; Borisova, O.K.; Gribchenko, Y.N.; Zelikson, E.M.; Klimanov, V.A.; Novenko, E.Y.; Pirumovy, L.G.; et al. Late quaternary paleogeography of the North-East of Europe based (on the complex study of the Galich Lake sediments). In Proceedings of the Academy of Sciences; Geographical Series; GFZ: Potsdam, Germany, 2001; Volume 3, pp. 42–54. (In Russian) [Google Scholar]
- Degering, D.; Krbetschek, M.R. Dating of interglacial sediments by luminescence methods. In The Climate of Past Interglacials, Developments in Quaternary Science; Sirocko, F., Claussen, M., Sa’nchez Goni, M.F., Litt, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 157–172. [Google Scholar]
- Fletcher, W.J.; Goñi, M.F.S.; Allen, J.R.M.; Cheddadi, R.; Combourieu-Nebout, N.; Huntley, B.; Lawson, I.; Londeix, L.; Magri, D.; Margari, V.; et al. Millennial-scale variability during the last glacial in vegetation records from Europe. Quat. Sci. Rev. 2010, 29, 2839–2864. [Google Scholar] [CrossRef]
- Nazarov, V.I. The beetles from the Mikulian Interglacial peat at the Zapadnaya Dvina valley. Palaeontol. J. 1980, 1, 144–147. (In Russian) [Google Scholar]
- Nazarov, I.V. Reconstruction of Byelorussian Landscapes Based on Paleoentomological Data; Nauka: Moscow, Russia, 1984; p. 96. (In Russian) [Google Scholar]
- Kiselev, S.V.; Nazarov, V.I. Late Pleistocene insects. In Late Quaternary Environments of the Soviet Union; Velichko, A.A., Wright, H.E., Jr., Barnosky, C.W., Eds.; University of Minnesota Press: London, UK, 1984; pp. 223–228. [Google Scholar]
- Behre, K.-E. Biostratigraphy of the last glacial period in Europe. Quat. Sci. Rev. 1989, 8, 25–44. [Google Scholar] [CrossRef]
- Ponel, P. Rissian, Eemian and Würmian Coleoptera assemblages from La Grande Pile (Vosges, France). Palaeogeogr. Palaeoclimatol. Palaeoecol. 1995, 114, 1–41. [Google Scholar] [CrossRef]
- Walkling, A.P.; Coope, G.R. Climatic reconstructions from the Eemian/Early Weichselian transition in Central Europe based on the Coleopteran record from Gröbern, Germany. Boreas 1996, 25, 145–159. [Google Scholar] [CrossRef]
- Coope, G.R. A Late Pleistocene insect fauna from Chelford, Cheshire. Proc. R. Soc. Lond. B 1959, 151, 70–86. [Google Scholar]
- Coope, G.R. On the study of glacial and interglacial insect faunas. Proc. Linn. Soc. Lond. 1961, 172, 62–71. [Google Scholar] [CrossRef]
- Angus, R.B. Challenges and Rewards in the Identification of Pleistocene Fossil Beetles. Quat. Proc. 1997, 5, 5–14. [Google Scholar]
- Lemdahl, G.; Broström, A.; Hedenäs, L.; Arvidsson, K.; Holmgren, S.; Gaillard, M.-J.; Möller, P. Eemian and Early Weichselian environments in southern Sweden: A multiproxy study of till-covered organic deposits from the Småland peneplain. J. Quatern. Sci. 2013, 28, 705–719. [Google Scholar] [CrossRef]
- Lomnicki, A.M. Pleistcenskie Owady Z Boryslawia: (fauna Pleistocenica Insectorum Boryslaviensium). In Volume 4 of Muzeum imienia Dzieduszyckich we Lwowie; Zwiazkowej: Lviv, Ukraine, 1894; p. 127. [Google Scholar]
- Fridolin, V.V. Notice about fossil insects and molluscs from the peat of Sivoritsy Estate. Annu. Rep. Zool. Mus. Russ. Acad. Sci. 1922, 23, 129. (In Russian) [Google Scholar]
- Medvedev, L.N. About a new fossil beetle (Col., Chrysomelidae) from Lishlinsky Interglacial of Moscow region. In History of Vegetation of the Central Regions of the European Part of the USSR in the Antropogene; P’yavchenko, N.N., Ed.; Nauka: Moscow, Russia, 1968; pp. 124–128. (In Russian) [Google Scholar]
- Medvedev, L.N. About insect composition from the Holocene badger coproliths in the Moscow region. In History of the USSR Biogeocoenosis in the Holocene; Dinesman, L.G., Ed.; Nauka: Moscow, Russia, 1976; pp. 183–193. (In Russian) [Google Scholar]
- Panfilov, D.V. About subfossil insects from Serebryany Bor. Bull. Mosc. Nat. Soc. Biol. Sect. 1965, 70, 115–116. (In Russian) [Google Scholar]



| Age, ka | MIS | Correlation with Eemian [8,9,10] | Mikulino Stages, After [82] | 230Th/U Dates, ka [16] | Proposed Chronological Outline, ka [16] | 230Th/U Dates, ka | OLS Dates, ka | Lithology | Ivantzevo LPZ | Vegetation Phases (Pollen) | Vegetation | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 60 | MIS4/3 | 53 ± 4 | clayey silt | LPZ 11 | 8 | abrupt degradation of forest cover, formation of cold steppe and tundra | |||||||
| 70 | MIS5a | top of upper peat | LPZ 9-10 | 7 | boreal spruce forest | ||||||||
| 90 | MIS5b | 70 ± 6 | upper peat/gittya layer sand lens | LPZ 7-8 | 6 | gradual degradation of coniferous forests andexpansion of grassy and shrubby tundra | |||||||
| 100 | MIS5c | peaty clay | LPZ 5-6 | 5 | forest recovery, initially of birch and later of mixed forests dominated by Pinus, Betula, and Picea. | ||||||||
| 110 | MIS5d | E6-E7 | open landscapes, steppe elements | M8 | Pinus and Picea zone | peaty clay | LPZ 4 | 4 | forest decline, steppe elements | ||||
| E5 | forest decline | M7 | Picea zone (the upper maximum of Picea pollen) | ~100 | peaty clay | a combination of broad-leaved taxa/groves and open herbaceous communities | |||||||
| 116 | MIS5e | E4b | thermal optimum (Carpinus) | M6 | Carpinus zone | 108–97 | − | ||||||
| M5 | Tilia zone (end of the Corylus peak) | 112–108 | ~112 | 105 ± 4, 113 ± 3 | 90 ± 6 | lower peat | LPZ 3 | 3 | thermophilic broad-leaved forests with hazel | ||||
| E4a | M4b | Quercus and Ulmus zone (beginning of the Corylus peak) | 117–112 | − | |||||||||
| E3 | oak forest | M4a | 116–105 | − | |||||||||
| E2 | mixed forests with hazel | M3 | Pinus and Betula zone, pollen of broadleaved taxa is present | 118–112 | ~118 | − | |||||||
| E1 | boreal birch − pine forest | M2 | Pinus and Betula zone | 127–113 | ~126 | − | |||||||
| 130 | M1 | Picea zone (the lower maximum of Picea pollen); a transition from GL to IGL | 130–126 | ~130 | sandy loam | LPZ 2 | 2 | lower maximum of spruce pollen | |||||
| MIS6 | Late Saalian | Moscow Glaciation | clay | cold steppes dominated by Artemisia and Amaranthaceae, spruce appears | |||||||||
| 245–190 | MIS7 | Likhvin Interglacial | 259 ± 27 | sandy silt | LPZ 1 | 1 | mixed forests (birch and a small proportion of conifers) | ||||||
| Phase | Sample Code | Plant Macrofossils | Invertebrate Macrofossils | Aquatic Invertebrate Microfossils (# of Taxa) | |||
|---|---|---|---|---|---|---|---|
| Aquatic Taxa (A), (#) | Terrestrial Taxa (T), (#) | Charred Taxa (#) | A: T (MNI) | A: T (Taxa) | |||
| 8 | DM-B6 | 1 to 0 | 1 to 0 | Acroperus harpae, Biapertura (Alona) affinis, Bosmina longirostris, Chydorus sphaericus, Ephydatia sp.? Eubosmina (Bosmina) longispina, Planaria sp., Spongilla sp. (8) | |||
| Pollen-based vegetation reconstruction: abrupt degradation of forest cover, formation of cold steppe and tundra | |||||||
| 7 | D-UT 7, DM-B2, DM-B2a, D-UT 5/1, D-UT 5/2 | Batrachium, Nuphar lutea, Ceratophyllum demersum, Menyanthes trifoliata, Myriophyllum verticillatum, Hippuris vulgaris, Sparganium sp., Potamogeton filiformis, P. natans, P. rutilus, P. sukaczevii, Scheuchzeria palustris, Schoenoplectus lacustris (13) | Picea, Pinus, Betula sect. Betula, Chamaedaphne calyculata, Vaccinium oxycoccos, Rubus sp., Comarum palustre, Carex, Ranunculus sceleratus, Rorippa palustris, Chenopodium album (11) | Pinus, Picea, Juniperus, Ericaceae, Cenococcum (5), concentration of micro-charcoal | 26 to 44 | 9 to 27 | Acroperus harpae, Biapertura (Alona) affinis, Bosmina longirostris, Chydorus sphaericus, Cristatella mucedo (5) |
| Pollen-based vegetation reconstruction: boreal spruce forest | |||||||
| 6 | DM-B4, D-UT9 | Chara, Isoetes, Batrachium, Potamogeton rutilus, Najas flexilis, Menyanthes, Myriophyllum verticillatum, Hippuris, Potamogeton gramineus, P. cf. pusillus, Eleocharis (11) | Betula sect. Betula, Betula nana, Rorippa palustris, Comarum palustre, Carex, Ranunculus sceleratus, Chenopodium album, Chamaedaphne, Sambucus (9) | 3 to 5 | 3 to 4 | Acroperus harpae, Biapertura (Alona) affinis, Bosmina longirostris, Chydorus sphaericus, Eubosmina (Bosmina) longispina (5) | |
| Pollen-based vegetation reconstruction: gradual degradation of coniferous forests and expansion of grassy and shrubby tundra | |||||||
| 5 | DM-B3 | Potamogeton, Eleocharis (2) | Chamaedaphne, Carex (2) | Cenococcum sp. (1) | 0 to 13 | 0 to 8 | Acroperus harpae, Biapertura (Alona) affinis, Bosmina longirostris, Chydorus sphaericus, Eubosmina (Bosmina) longispina (6) |
| Pollen-based vegetation reconstruction: forest recovery, initially of birch and later forests dominated by Pinus, Betula, and Picea. | |||||||
| 4/5 | Pollen-based vegetation reconstruction: forest decline | ||||||
| 4 | DM-B1 | Menyanthes, Potamogeton rutilus (2) | Comarum, Carex (2) | Cenococcum sp. (1), micro-charcoal | 9 to 5 | 2 to 5 | Acroperus harpae, Biapertura (Alona) affinis, Bosmina longirostris, Chydorus sphaericus, Eubosmina (Bosmina) longispina (5) |
| Pollen-based vegetation reconstruction: a combination of broad-leaved groves and open herbaceous communities | |||||||
| 3 | DM-B5 | Aldrovanda vesiculosa, Menyanthes, Scheuchzeria, Eleocharis (4) | Quercus, Corylus, Acer, Sambucus, Picea, Chamaedaphne, Vaccinium, Carex (8) | 36 to 131 | 11 to 36 | Acroperus harpae, Biapertura (Alona) affinis, Bosmina longirostris, Chydorus sphaericus (4) | |
| Pollen-based vegetation reconstruction: thermophilic broad-leaved forests with hazel | |||||||
| 2 | Acroperus harpae, Biapertura (Alona) affinis, Bosmina longirostris, Chydorus sphaericus, Ephydatia sp.?, Eubosmina (Bosmina) longispina, Spongilla sp. (7) | ||||||
| Pollen-based vegetation reconstruction: cold steppes dominated by Artemisia and Amaranthaceae, spruce is present | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ershova, E.; Kuzmina, S.; Sycheva, S.; Zyuganova, I.; Izumova, E.; Zharov, A.; Kuznetsov, V.Y.; Maksimov, F.; Kolesnikov, S.; Lavrenov, N.; et al. Paleoenvironments of the Last Interglacial–Glacial Transition on the East European Plain: Insights into Climate-Driven Ecosystem Dynamics. Quaternary 2025, 8, 66. https://doi.org/10.3390/quat8040066
Ershova E, Kuzmina S, Sycheva S, Zyuganova I, Izumova E, Zharov A, Kuznetsov VY, Maksimov F, Kolesnikov S, Lavrenov N, et al. Paleoenvironments of the Last Interglacial–Glacial Transition on the East European Plain: Insights into Climate-Driven Ecosystem Dynamics. Quaternary. 2025; 8(4):66. https://doi.org/10.3390/quat8040066
Chicago/Turabian StyleErshova, E., S. Kuzmina, S. Sycheva, I. Zyuganova, E. Izumova, A. Zharov, V. Yu. Kuznetsov, F. Maksimov, S. Kolesnikov, N. Lavrenov, and et al. 2025. "Paleoenvironments of the Last Interglacial–Glacial Transition on the East European Plain: Insights into Climate-Driven Ecosystem Dynamics" Quaternary 8, no. 4: 66. https://doi.org/10.3390/quat8040066
APA StyleErshova, E., Kuzmina, S., Sycheva, S., Zyuganova, I., Izumova, E., Zharov, A., Kuznetsov, V. Y., Maksimov, F., Kolesnikov, S., Lavrenov, N., & Ponomarenko, E. (2025). Paleoenvironments of the Last Interglacial–Glacial Transition on the East European Plain: Insights into Climate-Driven Ecosystem Dynamics. Quaternary, 8(4), 66. https://doi.org/10.3390/quat8040066






