Middle Holocene Subsistence in Southwestern Transylvania: Bioarchaeological Data on the Multicultural Site of Șoimuș-Teleghi (Hunedoara County, Romania)
Abstract
1. Introduction
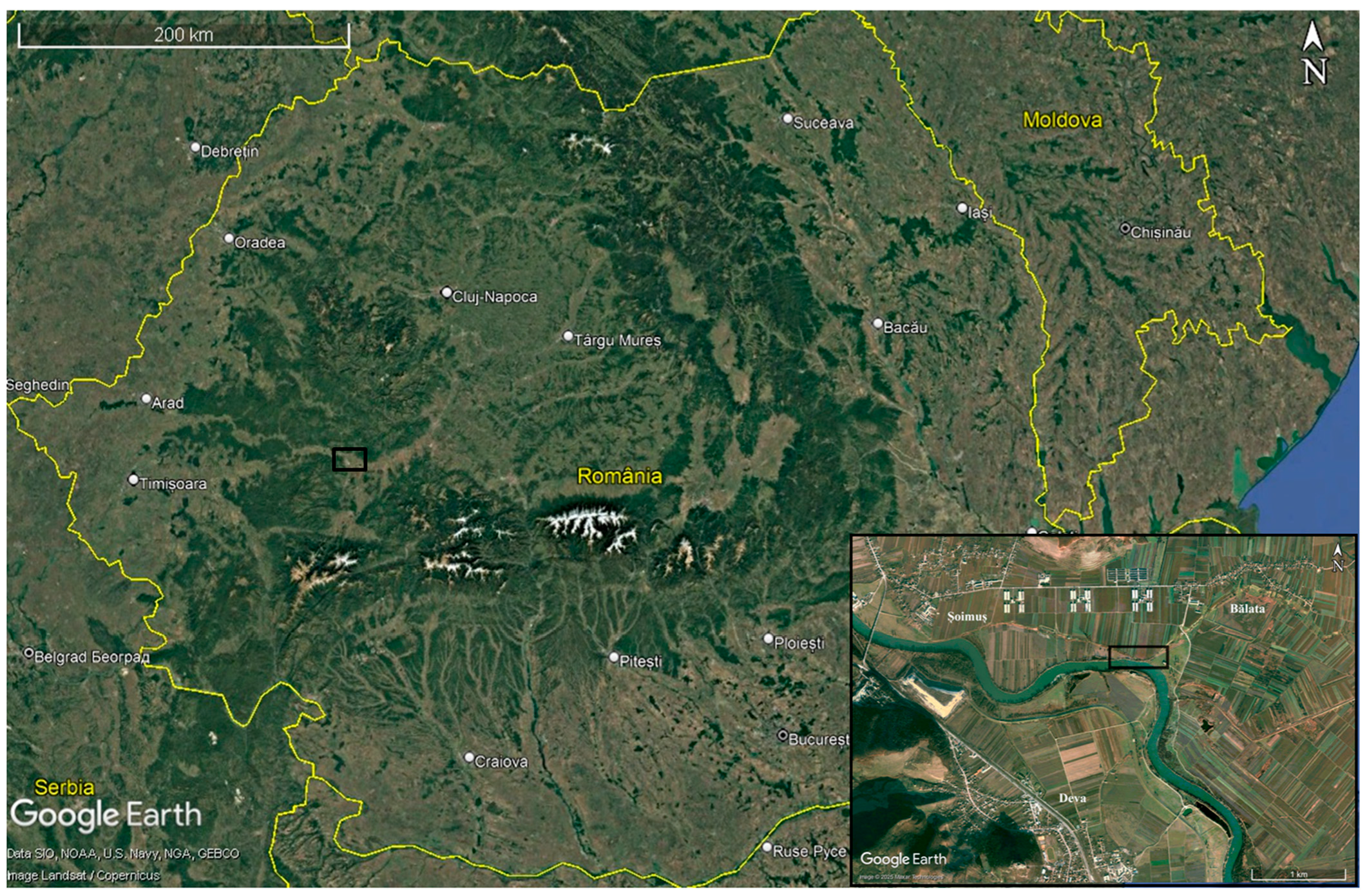
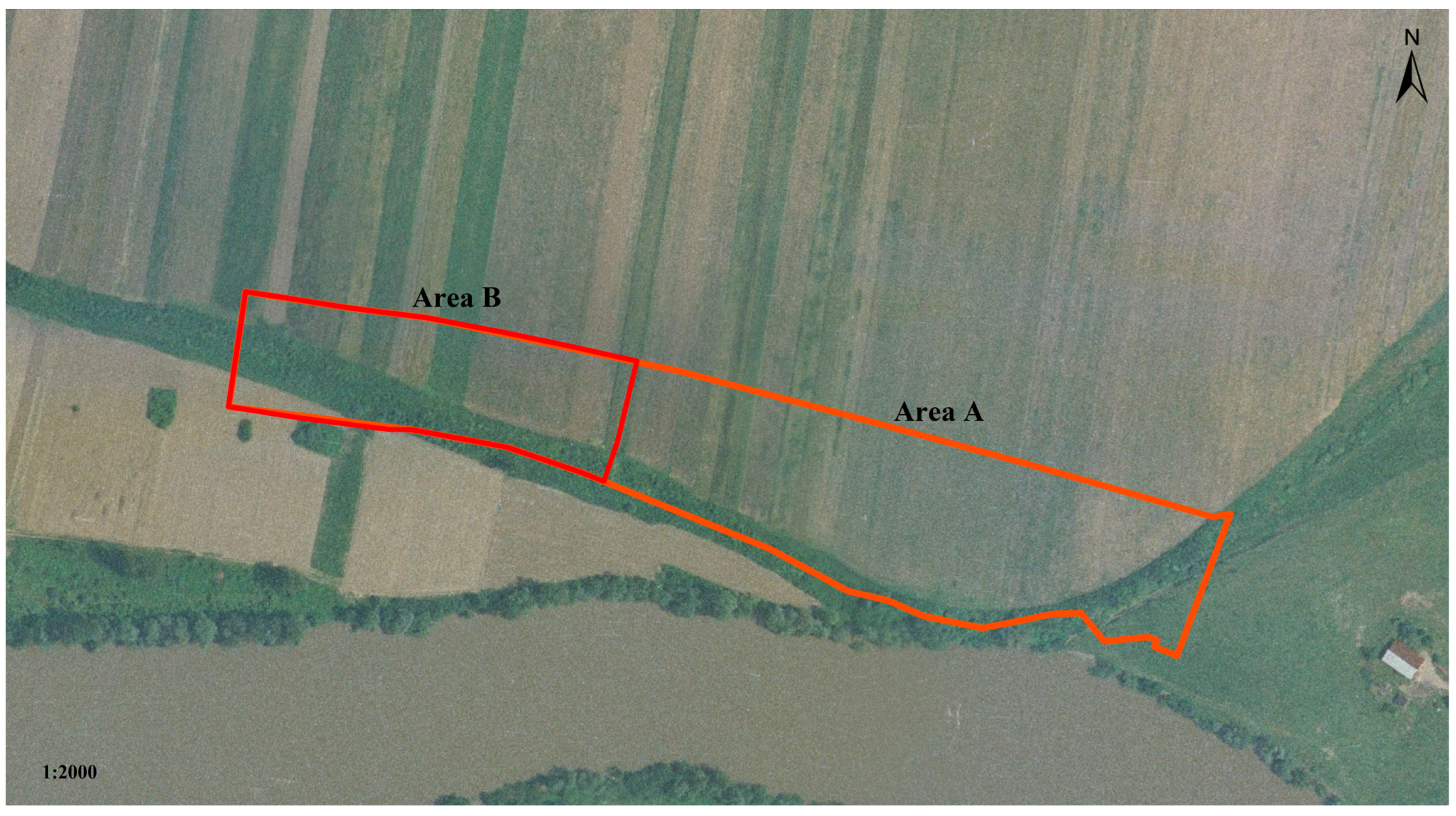
Archaeological Context and Chronology
2. Materials and Methods
2.1. Archaeozoology
2.2. Archaeobotany
3. Results and Discussion
3.1. Archaeozoology
3.1.1. Frequency of Taxa
3.1.2. Estimating Age at Death and Sex
3.1.3. Faunal Diversity in the Samples
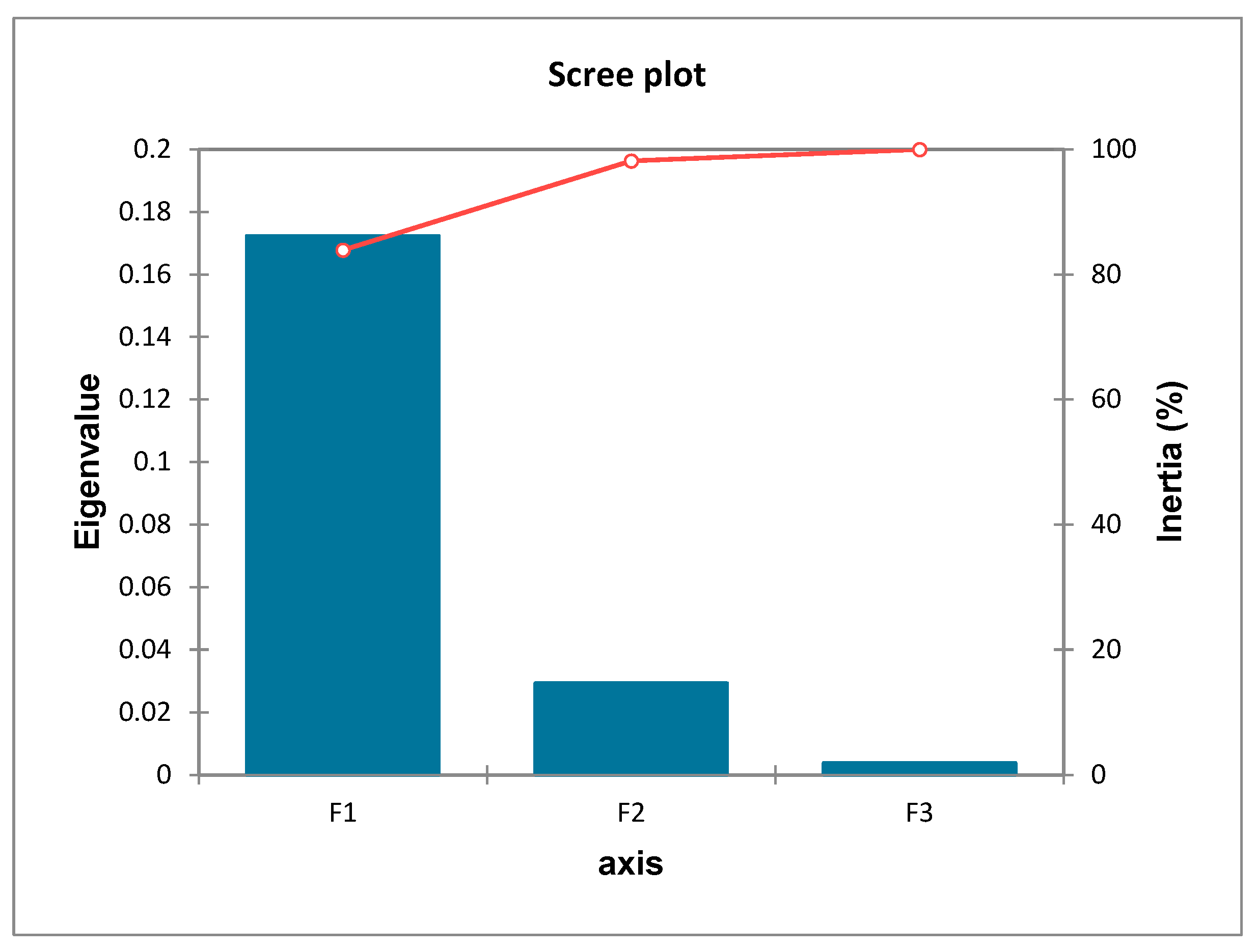
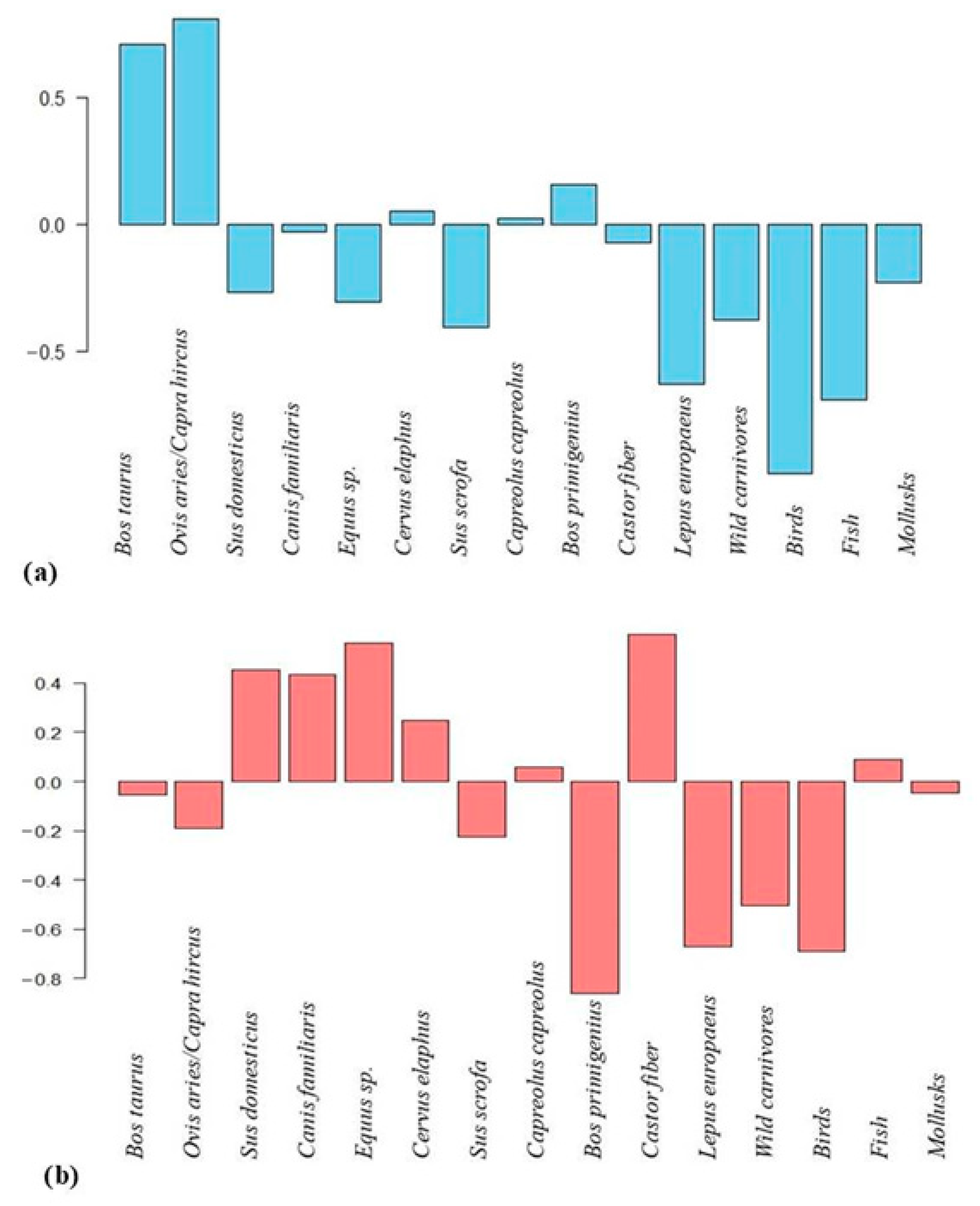
3.1.4. Correspondence Analysis
3.2. Archaeobotany
3.2.1. Phytolith Assemblages
3.2.2. Paleoenvironmental and Cultural Implications
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cramp, L.J.; Ethier, J.; Urem-Kotsou, D.; Bonsall, C.; Borić, D.; Boroneanţ, A.; Evershed, R.P.; Perić, S.; Roffet-Salque, M.; Whelton, H.L.; et al. Regional diversity in subsistence among early farmers in Southeast Europe revealed by archaeological organic residues. Proc. R. Soc. B. 2019, 286, 20182347. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, H.; Jongsma-Greenfield, T. Sedentary pastoral gatherers in the Early Neolithic—Architectural, botanical, and zoological evidence for mobile economies from Foeni-Salas, SW Romania. In Living Well Together? Settlement and Materiality in the Neolithic of South-East and Central Europe; Bailey, D.W., Whittle, A., Hofmann, D., Eds.; Oxbow Books: Oxford, UK, 2008; pp. 108–130. ISBN 978-1-84217-267-4. [Google Scholar]
- Evin, A.; Flink, L.G.; Bălăşescu, A.; Popovici, D.; Andreescu, R.; Bailey, D.; Mirea, P.; Lazăr, C.; Boroneanţ, A.; Bonsall, C.; et al. Unravelling the complexity of domestication: A case study using morphometrics and ancient DNA analyses of archaeological pigs from Romania. Philos. Trans. R. Soc. B 2015, 370, 20130616. [Google Scholar] [CrossRef] [PubMed]
- Andrițoiu, I. Contributions to the archaeological repertoire of Hunedoara County. Sargetia 1979, 14, 15–34. [Google Scholar]
- Damian, P.; Bocan, I.; Neagu, C.; Marius Paraschiv-Grigore, E.; Vasile, M.; Vleja, D.; Ene, E.-S.; Paraschiv-Grigore, I.; Bălos, A. Deva–Orăștie bypass, km. 0+000–32+500, Hunedoara County. In Cronica Cercetărilor Arheologice, Campania 2011; Institutul National al Patrimoniului: Târgu Mureş, Romania, 2012; pp. 278–279. [Google Scholar]
- Schuster, C.; Petcu, R.; Petcu, R.; Heroiu, A.; Rumega, V.; Creţu, A.P.; Dimache, M.; Irimuş, L.; Dobrotă, S.; Vasilescu, D.; et al. Şoimuş, Şoimuş Commune, Hunedoara County (Deva–Orăştie bypass), Point: Şoimuş 1 (Avicola) km. 29+750–30+300. In Cronica Cercetărilor Arheologice, Campania 2011; Institutul National al Patrimoniului: Târgu Mureş, Romania, 2012; pp. 291–292. [Google Scholar]
- Ștefan, C.E.; Petcu, R.; Petcu, R. Brief note on some Basarabian-type complexes from Şoimuș-La Avicola (Farm 2), Hunedoara County (Romania). Stud. Preist. 2013, 10, 49–66. [Google Scholar]
- Ștefan, C.E. Playing with Clay: Anthropomorphic Figurines from Şoimuş—La Avicola (Ferma 2), Hunedoara County. Dacia (Nouv. Série) 2016, 60, 31–66. [Google Scholar]
- Ștefan, C.E. Miniature Vessels from Şoimuş—La Avicola (Ferma 2), Hunedoara County. A Case Study. Dacia (Nouv. Série) 2017, 61, 7–69. [Google Scholar]
- Bărbat, A. Short Considerations About the Starčevo-Criș Figurines from the Şoimuș-Teleghi Archaeological Site, Feature 176A (Hunedoara County), 28th ed.; Editura Mega: Cluj-Napoca, Romania, 2018. [Google Scholar]
- Bărbat, A.I. Interaction of Early Neolithic communities with the environment. The Starčevo-Criş settlements from Şoimuş (Hunedoara County). Sargetia Acta Musei Devensis 2015, VI, 9–40. [Google Scholar]
- Malaxa, D.I.; Stanc, S.; Bărbat, A.; Gâza, O.; Păceșilă, D.; Bejenaru, L.; Danu, M. Farming Beginning in Southwestern Transylvania (Romania). Subsistence Strategies in Mureș Valley during the Early Neolithic. Diversity 2022, 14, 894. [Google Scholar] [CrossRef]
- Rișcuța, N.C.; Marc, A.T. Cultic Discoveries from Late Bronze Age Settlement from Şoimuş–Teleghi (Hunedoara County). In Representations, Signs and Symbols, Proceedings of the Symposium on Religion and Magic, Deva, Romania, 27–29 March 2014; Rișcuța, N.C., Ferencz, I.V., Tutilă Bărbat, O., Eds.; Editura Mega: Cluj-Napoca, Romania, 2015; pp. 139–170. [Google Scholar]
- Rotea, M. Penetration of Ottoman culture in Transylvania. Between reality and chimera. Apulum 1994, 31, 39–57. [Google Scholar]
- Rișcuța, N.C.; Marc, A.T. Children burials in the Bronze Age settlement from Soimus–Teleghi (Hunedoara County). In Settlements of Life and Death. Studies from Prehistory to Middle Ages, Proceedings of an International Colloquium Tulcea, Cluj-Napoca, Romania, 25–28 May 2016; Editura Mega: Cluj-Napoca, Romania, 2016; pp. 201–234. [Google Scholar]
- Popa, C.I.; Totoianu, R. New opinions regarding the cultural evolution of the Late Bronze Age in the central and southwestern Transylvanian area. In Aspecte ale Epocii Bronzului în Transilvania (Între Vechile și Noile Cercetări); Popa, C.I., Totoianu, R., Eds.; Altip: Alba Iulia, Romania, 2010; pp. 171–292. [Google Scholar]
- Brown, L.D.; Cai, T.T.; DasGupta, A. Interval estimation for a binomial proportion. Stat. Sci. 2001, 16, 101–133. [Google Scholar] [CrossRef]
- Altman, D.; Machin, D.; Bryant, T.; Gardner, M. (Eds.) Statistics with Confidence: Confidence Intervals and Statistical Guidelines; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Schmid, E. Atlas of Animal Bon; Elsevier Publishing Company: Amsterdam, The Netherlands, 1972. [Google Scholar]
- Grant, A. The use of tooth wear as a guide to the age of domestic ungulates. In Ageing and Sexing Animal Bones from Archaeological Sites; Wilson, B., Grigson, C., Payne, S., Eds.; B.A.R.: Oxford, UK, 1982; pp. 97–108. [Google Scholar]
- Udrescu, M.; Bejenaru, L.; Hriscu, C. Introduction in Archaeozoology; Editura Corson: Iași, Romania, 1999. [Google Scholar]
- Kirby, M.; Simons, A. binom: Binomial Proportions Confidence Intervals (R package version 1.1.0) [Software]. 2018. Available online: https://CRAN.R-project.org/package=binom (accessed on 30 July 2025).
- Reitz, E.J.; Wing, E.S. Zooarchaeology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- VanDerwarker, A.M. Simple measures for integrating plant and animal remains. In Integrating Zooarchaeology and Paleoethnobotany: A Consideration of Issues, Methods, and Cases; Springer: New York, NY, USA, 2010; pp. 65–74. [Google Scholar]
- Ortiz-Burgos, S. Shannon-Weaver Diversity Index. In Encyclopedia of Estuaries. Encyclopedia of Earth Sciences Series; Kennish, M.J., Ed.; Springer: Dordrecht, The Netherlands, 2016; pp. 572–573. [Google Scholar] [CrossRef]
- Greenacre, M.; Hastie, T. The Geometric Interpretation of Correspondence Analysis. J. Am. Stat. Assoc. 1987, 82, 437–447. [Google Scholar] [CrossRef]
- UCLA: Statistical Consulting Group. Principal Components (PCA) and Exploratory Factor Analysis (EFA) with SPSS. Available online: https://stats.oarc.ucla.edu/spss/seminars/efa-spss/#top (accessed on 20 September 2025).
- Good, I.J. Permutation Tests: A Practical Guide to Resampling Methods for Testing Hypotheses; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Grove, M.; Blinkhorn, J. Testing the integrity of the Middle and Later Stone Age cultural taxonomic division in eastern Africa. J. Paleolit. Archaeol. 2021, 4, 14. [Google Scholar] [CrossRef]
- Lentfer, C.J.; Boyd, W.E. A comparison of three methods for the extraction of phytoliths from sediments. J. Archaeol. Sci. 1998, 25, 1159–1183. [Google Scholar] [CrossRef]
- International Committee on Phytolith Taxonomy (ICPT). International code for phytolith nomenclature (ICPN) 2.0. Ann. Bot. 2019, 124, 189–199. [Google Scholar]
- Alexandre, A.; Meunier, J.D.; Lézine, A.M.; Vincens, A.; Schwartz, D. Phytoliths: Indicators of grassland dynamics during the late Holocene in intertropical Africa. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1997, 136, 213–229. [Google Scholar] [CrossRef]
- Barboni, D.; Bremond, L.; Bonnefille, R. Comparative studies of modern phytolith assemblages from inter-tropical Africa. Paleogeography Palaeoclimatol. Palaeoecol. 2007, 246, 454–470. [Google Scholar] [CrossRef]
- Piperno, D.R. The Application of Phytolith Analysis to the Reconstruction of Plant Subsistence and Environments in Prehistoric Panama. Ph.D. Dissertation, Temple University, Philadelphia, PA, USA, 1983. [Google Scholar]
- Strömberg, C.A.E. The Origin and Spread of Grass-Dominated Ecosystems During the Tertiary of North America and How It Relates to the Evolution of Hypsodonty in Equids. Ph.D. Thesis, University of California, Berkeley, CA, USA, 2003. [Google Scholar]
- Piperno, D.R. Phytolith Analysis: An Archaeological and Geological Perspective; Academic Press: San Diego, CA, USA, 1988. [Google Scholar]
- Esau, K. Plant Anatomy, 2nd ed.; Wiley: New York, NY, USA, 1965. [Google Scholar]
- An, X.-H. Morphological characteristics of phytoliths from representative conifers in China. Palaeoworld 2016, 25, 116–127. [Google Scholar] [CrossRef]
- Blinnikov, M.; Busacca, A.; Whitlock, C. Reconstruction of the late Pleistocene grassland of the Columbia basin, Washington, USA, based on phytolith records in loess. Paleogeography Palaeoclimatol. Palaeoecol. 2002, 177, 77–101. [Google Scholar] [CrossRef]
- Carnelli, A.L.; Theurillat, J.-P.; Madella, M. Phytolith types and type-frequencies insubalpine–alpine plant species of the European Alps. Rev. Palaeobot. Palynol. 2004, 129, 39–65. [Google Scholar] [CrossRef]
- Novello, A.; Barboni, D.; Berti-Equille, L.; Mazur, J.-C.; Poilecot, P.; Vignaud, P. Phytolith signal of aquatic plants and soils in Chad, Central Africa. Rev. Palaeobot. Palynol. 2012, 178, 43–58. [Google Scholar] [CrossRef]




| Cultural Level | Early Neolithic | Middle Neolithic | Middle Bronze Age | Late Bronze Age | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Starčevo-Criș [6] | Vinča | W II | W III | LBA I | LBA II | ||||||||
| Classes | NISP | % | NISP | % | NISP | % | NISP | % | NISP | % | NISP | % | |
| Molluscs | 8 | 0.71 | 215 | 14.12 | 2250 | 44.19 | 2433 | 54.82 | 1299 | 19.48 | 14 | 8.05 | |
| Fish | - | - | - | - | 6 | 0.12 | - | - | 4 | 0.06 | - | - | |
| Amphibians | - | - | - | - | 7 | 0.14 | - | - | - | - | - | - | |
| Reptiles | - | - | - | - | - | - | - | - | 1 | 0.01 | - | - | |
| Birds | - | - | 1 | 0.06 | 36 | 0.71 | 1 | 0.02 | 9 | 0.13 | - | - | |
| Mammals | 1114 | 99.29 | 1307 | 85.82 | 2793 | 54.85 | 2004 | 45.16 | 5356 | 80.31 | 160 | 91.95 | |
| Total sample | 1122 | 100 | 1523 | 100 | 5092 | 100 | 4438 | 100 | 6669 | 100 | 174 | 100 | |
| Contexts | Pits | Dwellings | Other Contexts | Total | |||
|---|---|---|---|---|---|---|---|
| Cultural Layer | NISP | % | NISP | % | NISP | % | NISP |
| Starčevo-Criș | 0 | 0 | 0 | 0 | 8 | 100 | 8 |
| Vinča | 154 | 71.63 | 31 | 14.42 | 30 | 13.95 | 215 |
| W II | 2231 | 99.15 | 19 | 0.85 | 0 | 0 | 2250 |
| W III | 2433 | 100 | 0 | 0 | 0 | 0 | 2433 |
| LBA I | 480 | 36.95 | 474 | 36.5 | 345 | 26.56 | 1299 |
| Cultural Level | Starčevo-Criș [6] | Vinča | W II | W III | LBA I | LBA II | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NISP | % | NISP | % | NISP | % | NISP | % | NISP | % | NISP | % | |
| Burn marks | 23 | 2.06 | 84 | 7.54 | 48 | 4.31 | 55 | 4.94 | 82 | 7.36 | 5 | 0.45 |
| Butchery marks | 63 | 5.66 | 74 | 6.64 | 61 | 5.48 | 234 | 21.01 | 487 | 43.72 | 41 | 3.68 |
| Gnawing marks | 29 | 2.60 | 41 | 3.68 | 68 | 6.10 | 32 | 2.87 | 172 | 15.44 | 7 | 0.63 |
| Processing marks | 4 | 0.36 | 1 | 0.09 | 8 | 0.72 | 3 | 0.27 | 12 | 1.08 | 1 | 0.09 |
| Pathologies | - | - | 2 | 0.18 | 1 | 0.09 | 1 | 0.09 | 7 | 0.63 | - | - |
| Unidentified mammal remains | 460 | 41.29 | 498 | 38.10 | 1233 | 44.15 | 670 | 33.43 | 2463 | 45.99 | 57 | 35.63 |
| Total mammal remains | 1114 | 100 | 1307 | 100 | 2793 | 100 | 2004 | 100 | 5356 | 100 | 160 | 100 |
| Cultural Level | Early Neolithic | Middle Neolithic | Middle Bronze Age | Late Bronze Age | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Starčevo-Criș [6] | Vinča | W II | W III | LBA I | LBA II | ||||||||
| Mammal Groups | NISP | % | NISP | % | NISP | % | NISP | % | NISP | % | NISP | % | |
| Domestic mammals | 606 | 54.40 | 695 | 53.18 | 1200 | 42.96 | 1123 | 56.04 | 2461 | 45.95 | 90 | 56.25 | |
| Wild mammals | 48 | 4.31 | 114 | 8.72 | 360 | 12.89 | 211 | 10.53 | 432 | 8.07 | 13 | 8.13 | |
| Unidentified mammals | 460 | 41.29 | 498 | 38.10 | 1233 | 44.15 | 670 | 33.43 | 2463 | 45.99 | 57 | 35.63 | |
| Total | 1114 | 100 | 1307 | 100 | 2793 | 100 | 2004 | 100 | 5356 | 100 | 160 | 100 | |
| Cultural Level | Starčevo-Criș [6] | Vinča | W II | W III | LBA I | LBA II | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taxon | NISP | % | MNI | % | NISP | % | MNI | % | NISP | % | MNI | % | NISP | % | MNI | % | NISP | % | MNI | % | NISP | % | MNI | % |
| Cattle (Bos taurus) | 353 | 53.98 | 10 | 27.78 | 323 | 39.93 | 9 | 22.5 | 446 | 28.59 | 10 | 16.39 | 460 | 34.48 | 9 | 21.43 | 1013 | 35.02 | 13 | 16.25 | 25 | 24.27 | 3 | 21.43 |
| Sheep (Ovis aries)/Goat (Capra hircus) | 247 | 37.77 | 10 | 27.78 | 221 | 27.32 | 9 | 22.5 | 313 | 20.06 | 11 | 18.03 | 262 | 19.64 | 5 | 11.90 | 558 | 19.29 | 13 | 16.25 | 41 | 39.81 | 3 | 21.43 |
| Pig (Sus domesticus) | 5 | 0.76 | 2 | 5.56 | 119 | 14.71 | 4 | 10 | 378 | 24.23 | 16 | 26.23 | 333 | 24.96 | 8 | 19.05 | 746 | 25.79 | 19 | 23.75 | 16 | 15.53 | 2 | 14.29 |
| Horse (Equus caballus) | - | - | - | - | - | - | - | - | 25 | 1.60 | 2 | 3.28 | 21 | 1.57 | 1 | 2.38 | 54 | 1.87 | 4 | 5 | 2 | 1.94 | 1 | 7.14 |
| Donkey (Equus asinus) | - | - | - | - | - | - | - | - | - | - | - | - | 2 | 0.15 | 1 | 2.38 | - | - | - | - | - | - | - | - |
| Dog (Canis familiaris) | 1 | 0.15 | 1 | 2.78 | 32 | 3.96 | 1 | 2.5 | 38 | 2.44 | 3 | 4.92 | 45 | 3.37 | 4 | 9.52 | 90 | 3.11 | 5 | 6.25 | 6 | 5.83 | 1 | 7.14 |
| Domestic mammals | 606 | 92.66 | 23 | 63.89 | 695 | 85.91 | 23 | 58 | 1200 | 76.92 | 42 | 68.85 | 1123 | 84.18 | 28 | 66.67 | 2461 | 85.07 | 54 | 67.5 | 90 | 87.38 | 10 | 71.43 |
| Red deer (Cervus elaphus) | 36 | 5.50 | 5 | 13.89 | 44 | 5.44 | 3 | 7.5 | 165 | 10.58 | 5 | 8.20 | 103 | 7.72 | 4 | 9.52 | 278 | 9.61 | 11 | 13.75 | 8 | 7.77 | 1 | 7.14 |
| Wild boar (Sus scrofa) | 5 | 0.76 | 3 | 8.33 | 31 | 3.83 | 3 | 7.5 | 117 | 7.50 | 4 | 6.56 | 70 | 5.25 | 2 | 4.76 | 93 | 3.21 | 3 | 3.75 | 3 | 2.91 | 1 | 7.14 |
| Roe deer (Capreolus capreolus) | 2 | 0.31 | 2 | 5.56 | 21 | 2.60 | 4 | 10 | 32 | 2.05 | 3 | 4.92 | 17 | 1.27 | 1 | 2.38 | 38 | 1.31 | 6 | 7.5 | 1 | 0.97 | 1 | 7.14 |
| Aurochs (Bos primigenius) | 4 | 0.61 | 2 | 5.56 | 2 | 0.25 | 1 | 2.5 | 18 | 1.15 | 1 | 1.64 | - | - | - | - | 6 | 0.21 | 1 | 1.25 | - | - | - | - |
| Wild horse (Equus ferus) | - | - | - | - | 5 | 0.62 | 1 | 2.5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Wild donkey (Equus hydruntinus) | - | - | - | - | 1 | 0.12 | 1 | 2.5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Hare (Lepus europaeus) | - | - | - | - | 4 | 0.49 | 1 | 2.5 | 18 | 1.15 | 2 | 3.28 | 3 | 0.22 | 1 | 2.38 | 5 | 0.17 | 1 | 1.25 | - | - | - | - |
| Beaver (Castor fiber) | - | - | - | - | 1 | 0.12 | 1 | 2.5 | 3 | 0.19 | 1 | 1.64 | - | - | - | - | 4 | 0.14 | 1 | 1.25 | - | - | - | - |
| Wolf (Canis lupus) | - | - | - | - | 3 | 0.37 | 1 | 2.5 | - | - | - | - | 2 | 0.15 | 1 | 2.38 | 2 | 0.07 | 1 | 1.25 | - | - | - | - |
| Fox (Vulpes vulpes) | - | - | - | - | - | - | - | - | 3 | 0.19 | 1 | 1.64 | 1 | 0.07 | 1 | 2.38 | - | - | - | - | - | - | - | - |
| Bear (Ursus arctos) | - | - | - | - | 2 | 0.25 | 1 | 2.5 | 3 | 0.19 | 1 | 1.64 | 3 | 0.22 | 1 | 2.38 | 2 | 0.07 | 1 | 1.25 | - | - | - | - |
| Polecat (Mustela putorius) | 1 | 0.15 | 1 | 2.78 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Badger (Meles meles) | - | - | - | - | - | - | - | - | 1 | 0.06 | 1 | 1.64 | 11 | 0.82 | 2 | 4.76 | 4 | 0.14 | 1 | 1.25 | 1 | 0.97 | 1 | 7.14 |
| Lynx (Lynx lynx) | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 0.07 | 1 | 2.38 | - | - | - | - | - | - | - | - |
| Wild mammals | 48 | 7.34 | 13 | 36.11 | 114 | 14.09 | 17 | 43 | 360 | 23.08 | 19 | 31.15 | 211 | 15.82 | 14 | 33.33 | 432 | 14.93 | 26 | 32.5 | 13 | 12.62 | 4 | 28.57 |
| Total identified mammals | 654 | 100 | 36 | 100 | 809 | 100 | 40 | 100 | 1560 | 100 | 61 | 100 | 1334 | 100 | 42 | 100 | 2893 | 100 | 80 | 100 | 103 | 100 | 14 | 100 |
| Bos taurus | |||||
|---|---|---|---|---|---|
| Age Cultural Layer | <1 year (MNI) | 1–2 years (MNI) | 2–3 years (MNI) | 3–4 years (MNI) | >4 years (MNI) |
| Starčevo-Criș | 1 | 2 | 3 | 0 | 0 |
| Vinča | 2 | 0 | 2 | 1 | 1 |
| W II | 1 | 0 | 1 | 1 | 2 |
| W III | 0 | 0 | 2 | 1 | 0 |
| LBA I | 4 | 0 | 3 | 2 | 4 |
| LBA II | 0 | 0 | 1 | 0 | 0 |
| Ovis aries/Capra hircus | |||||
| Age Cultural Layer | <1 year (MNI) | 1–2 years (MNI) | 2–3 years (MNI) | >3 years (MNI) | |
| Starčevo-Criș | 1 | 1 | 0 | 1 | |
| Vinča | 1 | 2 | 3 | 1 | |
| W II | 2 | 1 | 3 | 0 | |
| W III | 1 | 0 | 3 | 1 | |
| LBA I | 2 | 1 | 3 | 3 | |
| LBA II | 1 | 1 | 1 | 0 | |
| Sus domesticus | |||||
| Age Cultural layer | <1 year (MNI) | 1–2 years (MNI) | 2–3 years (MNI) | >3 years (MNI) | |
| Starčevo-Criș | 1 | 0 | 0 | 0 | |
| Vinča | 2 | 0 | 1 | 1 | |
| W II | 2 | 1 | 5 | 0 | |
| W III | 2 | 1 | 1 | 1 | |
| LBA I | 7 | 2 | 7 | 3 | |
| Species | Cultural Layer | Mature (MNI) | Immature (MNI) | Mature Proportion | Immature Proportion | CI Lower | CI Upper |
|---|---|---|---|---|---|---|---|
| Bos taurus | Starčevo-Criș | 3 | 3 | 0.5 | 0.5 | 0.08 | 0.92 |
| Vinča | 4 | 2 | 0.667 | 0.333 | 0.249 | 0.973 | |
| W II | 4 | 1 | 0.8 | 0.2 | 0.358 | 0.976 | |
| W III | 2 | 1 | 0.667 | 0.333 | 0.123 | 0.955 | |
| LBA I | 9 | 4 | 0.692 | 0.308 | 0.421 | 0.897 | |
| LBA II | 1 | 0 | 1 | 0 | 0.272 | 1 | |
| Ovis aries/Capra hircus | Starčevo-Criș | 2 | 1 | 0.667 | 0.333 | 0.073 | 0.986 |
| Vinča | 5 | 2 | 0.714 | 0.286 | 0.334 | 0.957 | |
| W II | 4 | 2 | 0.667 | 0.333 | 0.28 | 0.919 | |
| W III | 4 | 1 | 0.8 | 0.2 | 0.345 | 0.977 | |
| LBA I | 7 | 2 | 0.778 | 0.222 | 0.444 | 0.944 | |
| LBA II | 2 | 1 | 0.667 | 0.333 | 0.082 | 0.974 | |
| Sus domesticus | Starčevo-Criș | 0 | 1 | 0 | 1 | 0 | 1 |
| Vinča | 2 | 2 | 0.5 | 0.5 | 0.123 | 0.877 | |
| W II | 6 | 2 | 0.75 | 0.25 | 0.381 | 0.973 | |
| W III | 3 | 2 | 0.6 | 0.4 | 0.186 | 0.915 | |
| LBA I | 12 | 7 | 0.632 | 0.368 | 0.425 | 0.803 |
| Period | Sample | Richness (S) | Shannon Index (H′) | Equitability (J) |
|---|---|---|---|---|
| Early Neolithic | Starčevo-Criș | 10 | 1.06 | 0.46 |
| Middle Neolithic | Vinča | 16 | 1.81 | 0.65 |
| Middle Bronze Age | W II | 19 | 1.51 | 0.51 |
| W III | 18 | 1.27 | 0.44 | |
| Late Bronze Age | LBA I | 19 | 1.79 | 0.61 |
| LBA II | 10 | 1.8 | 0.78 |
| F1 | F2 | F3 | |
|---|---|---|---|
| Eigenvalue | 0.172 | 0.029 | 0.004 |
| Inertia (%) | 83.909 | 14.246 | 1.846 |
| Cumulative % | 83.909 | 98.154 | 100.000 |
| Contribution of taxa | |||
| F1 | F2 | F3 | |
| Bos taurus | 0.311 | 0.035 | 0.011 |
| Ovis aries/Capra hircus | 0.223 | 0.106 | 0.007 |
| Sus domesticus | 0.002 | 0.572 | 0.012 |
| Canis familiaris | 0.003 | 0.070 | 0.236 |
| Equus sp. | 0.000 | 0.051 | 0.020 |
| Cervus elaphus | 0.012 | 0.061 | 0.130 |
| Sus scrofa | 0.001 | 0.003 | 0.104 |
| Capreolus capreolus | 0.001 | 0.009 | 0.342 |
| Bos primigenius | 0.000 | 0.013 | 0.005 |
| Castor fiber | 0.000 | 0.004 | 0.003 |
| Lepus europaeus | 0.001 | 0.001 | 0.068 |
| Wild carnivores | 0.000 | 0.001 | 0.047 |
| Birds | 0.004 | 0.001 | 0.001 |
| Fish | 0.000 | 0.001 | 0.009 |
| Molluscs | 0.440 | 0.071 | 0.003 |
| Contribution of periods | |||
| F1 | F2 | F3 | |
| Early Neolithic | 0.409 | 0.433 | 0.110 |
| Middle Neolithic | 0.126 | 0.000 | 0.798 |
| Middle Bronze Age | 0.341 | 0.100 | 0.000 |
| Late Bronze Age | 0.124 | 0.467 | 0.092 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanc, M.S.; Malaxa, D.I.; Bărbat, I.A.; Marc, A.T.; Popovici, M.; Bejenaru, L.; Danu, M. Middle Holocene Subsistence in Southwestern Transylvania: Bioarchaeological Data on the Multicultural Site of Șoimuș-Teleghi (Hunedoara County, Romania). Quaternary 2025, 8, 60. https://doi.org/10.3390/quat8040060
Stanc MS, Malaxa DI, Bărbat IA, Marc AT, Popovici M, Bejenaru L, Danu M. Middle Holocene Subsistence in Southwestern Transylvania: Bioarchaeological Data on the Multicultural Site of Șoimuș-Teleghi (Hunedoara County, Romania). Quaternary. 2025; 8(4):60. https://doi.org/10.3390/quat8040060
Chicago/Turabian StyleStanc, Margareta Simina, Daniel Ioan Malaxa, Ioan Alexandru Bărbat, Antoniu Tudor Marc, Mariana Popovici, Luminița Bejenaru, and Mihaela Danu. 2025. "Middle Holocene Subsistence in Southwestern Transylvania: Bioarchaeological Data on the Multicultural Site of Șoimuș-Teleghi (Hunedoara County, Romania)" Quaternary 8, no. 4: 60. https://doi.org/10.3390/quat8040060
APA StyleStanc, M. S., Malaxa, D. I., Bărbat, I. A., Marc, A. T., Popovici, M., Bejenaru, L., & Danu, M. (2025). Middle Holocene Subsistence in Southwestern Transylvania: Bioarchaeological Data on the Multicultural Site of Șoimuș-Teleghi (Hunedoara County, Romania). Quaternary, 8(4), 60. https://doi.org/10.3390/quat8040060







