A Tropical Spiny Tree Rat (Rodentia, Echimyini) in the Late Quaternary of Southern South America (Argentina): Paleoenvironmental and Paleogeographic Implications
Abstract
1. Introduction
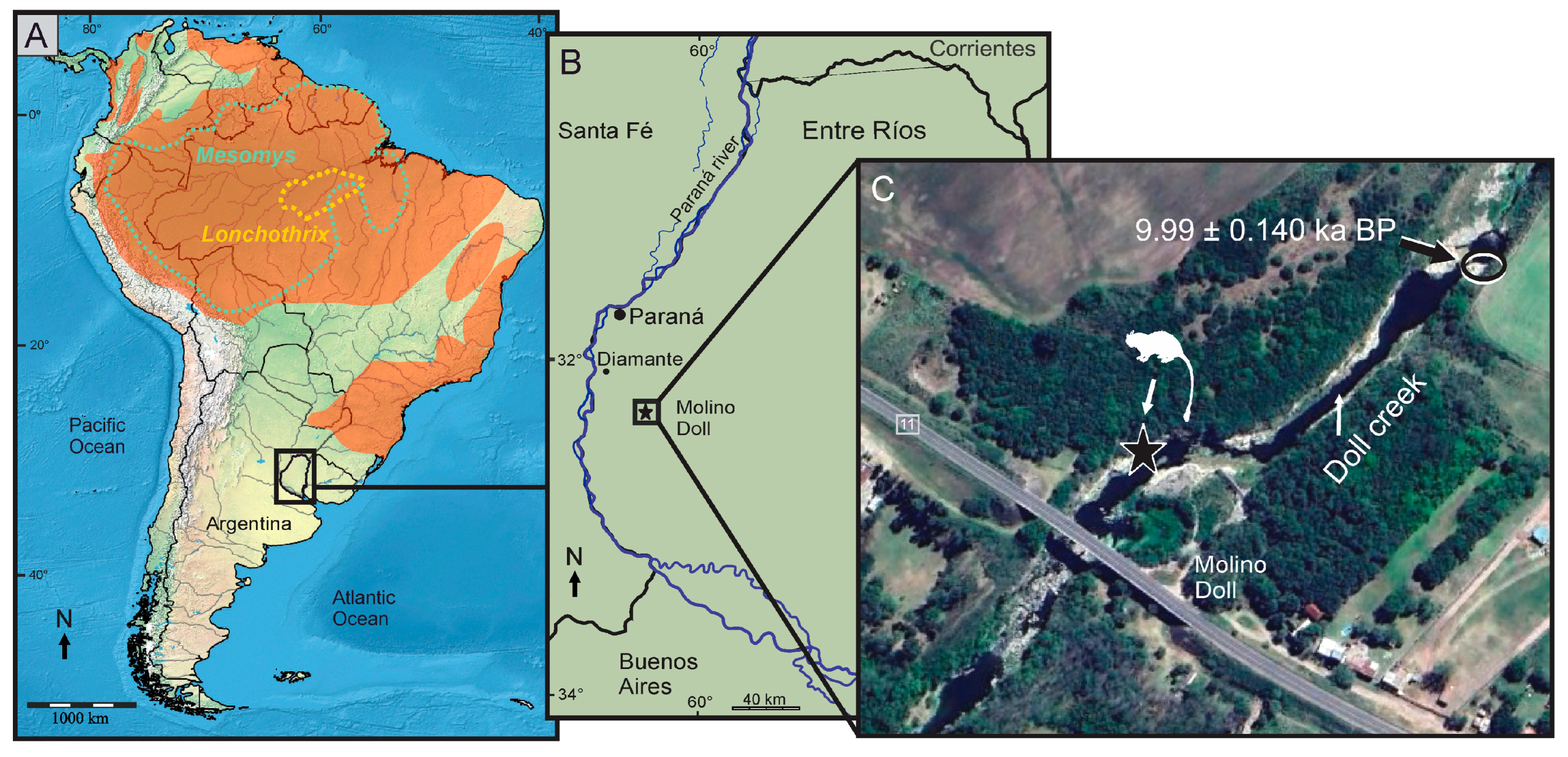
2. Geographical, Geological and Chronological Settings
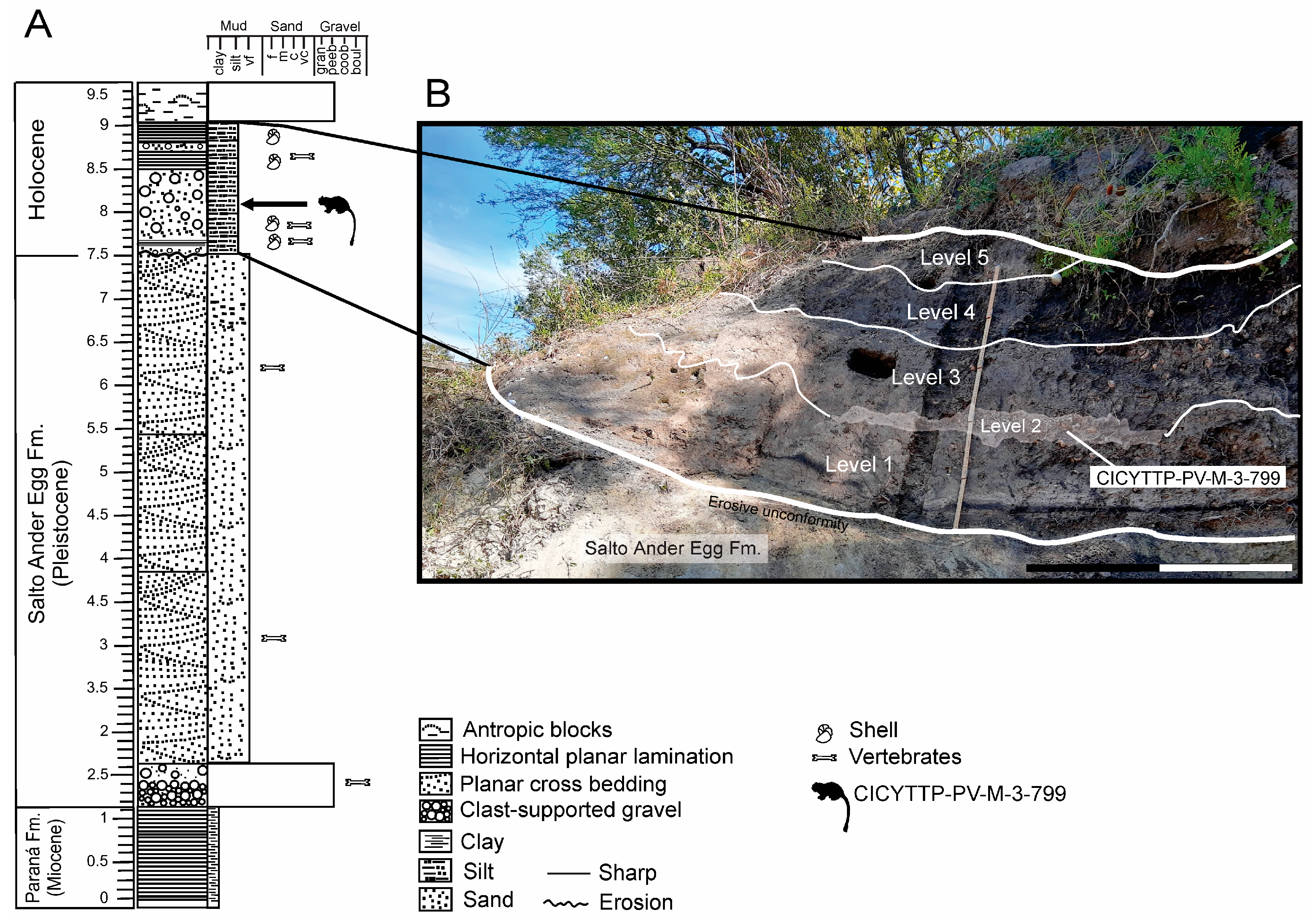
3. Materials and Methods
Institutional Abbreviations
4. Systematic Paleontology
4.1. Referred Material
4.2. Occurrence
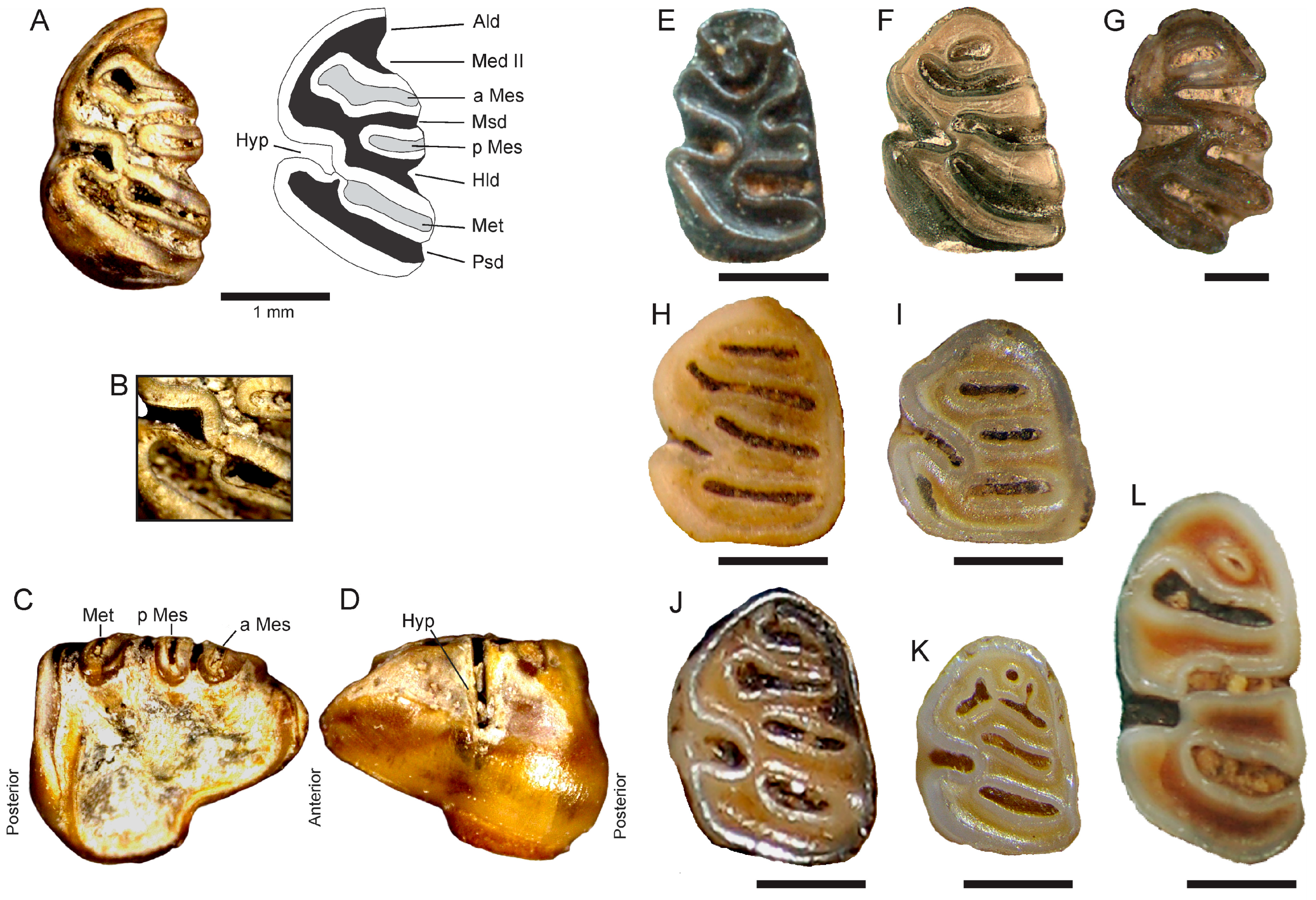
4.3. Description
4.4. Comparisons
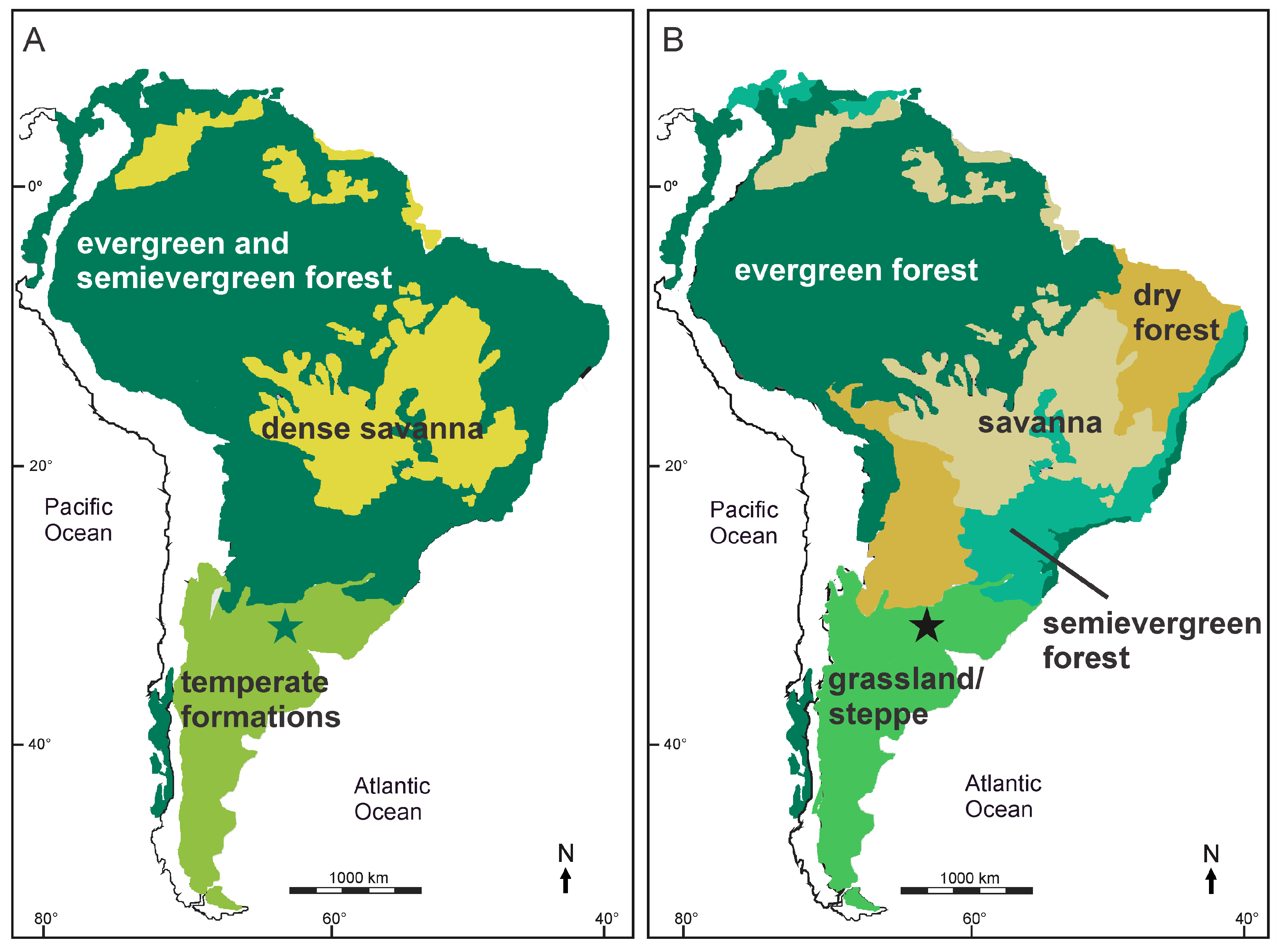
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eisenberg, J.F. The Mammalian Radiations. An Analysis of Trends in Evolution, Adaptation, and Behavior, 1st ed.; The University of Chicago Press: Chicago, IL, USA, 1981. [Google Scholar]
- Galewski, T.; Mauffrey, J.F.; Leite, Y.L.; Patton, J.L.; Douzery, E.J. Ecomorphological diversification among South American spiny rats (Rodentia; Echimyidae): A phylogenetic and chronological approach. Mol. Phylogenet. Evol. 2005, 34, 601–615. [Google Scholar] [CrossRef]
- Fabre, P.H.; Patton, J.L.; Leite, Y.L.R. Family Echimyidae. In Handbook of the Mammals of the World, 1st ed.; Wilson, D.E., Lacher, T.E., Jr., Mittermeier, R.A., Eds.; Lynx Edicions: Barcelona, España, 2016; Volume 6: Lagomorphs and rodents; pp. 552–641. [Google Scholar]
- Tavares, W.C.; Abi-Rezik, P.; Seuánez, H.N. Historical and ecological influence in the evolutionary diversification of external morphology of neotropical spiny rats (Echimyidae, Rodentia). J. Zool. Syst. Evol. Res. 2018, 56, 453–465. [Google Scholar] [CrossRef]
- Tavares, W.C.; Pessôa, L.M. Effects of size, phylogeny and locomotor habits on the pelvic and femoral morphology of South American spiny rats (Rodentia: Echimyidae). Biol. J. Linn. Soc. 2020, 131, 835–869. [Google Scholar] [CrossRef]
- Olivares, A.I.; Álvarez, A.; Verzi, D.H.; Perez, S.I.; De Santi, N.A. Unravelling the distinctive craniomandibular morphology of the Plio-Pleistocene Eumysops in the evolutionary setting of South American octodontoid rodents (Hystricomorpha). Palaeontology 2020, 63, 443–458. [Google Scholar] [CrossRef]
- Fernández Villoldo, J.A.; Verzi, D.H.; Olivares, A.I.; Dos Reis, S.F.; Lopes, R.T.; Perez, S.I. Exploring the palaeoneurology of the extinct spiny rat Eumysops chapalmalensis (Hystricognathi: Echimyidae): A comparative phylogenetic analysis of brain size and shape. Biol. J. Linn. Soc. 2025, 203, zlaf005. [Google Scholar] [CrossRef]
- Patton, J.L.; Pardiñas, U.F.J.; D’Elía, G. Mammals of South America, Rodents, 1st ed.; University of Chicago Press: Chicago, IL, USA, 2015. [Google Scholar]
- Fernández Villoldo, J.A.; Verzi, D.H.; Lopes, R.T.; Dos Reis, S.F.; Perez, S.I. Brain size and shape diversification in a highly diverse South American clade of rodents (Echimyidae): A geometric morphometric and comparative phylogenetic approach. Biol. J. Linn. Soc. 2023, 140, 277–295. [Google Scholar] [CrossRef]
- da Silva, D.; Verzi, D.H.; Martinez, P.A. Differential climatic niche diversification processes in South American rodents (Octodontoidea) across tropical and extratropical regions. Mamm. Rev. 2025, in press. [Google Scholar] [CrossRef]
- Emmons, L.H. A revision of the genera of arboreal Echimyidae (Rodentia: Echimyidae, Echimyinae), with descriptions of two new genera. In Mammalian Diversification: From Chromosomes to Phylogeography (a Celebration of the Career of James L. Patton), 1st ed.; Lacey, E.A., Myers, P., Eds.; University of California Press: Berkeley, CA, USA, 2005; pp. 247–309. [Google Scholar]
- Fabre, P.H.; Upham, N.S.; Emmons, L.H.; Justy, F.; Leite, Y.L.R.; Loss, A.C.; Orlando, L.; Tilak, M.-K.; Patterson, B.D.; Douzery, E.J.P. Mitogenomic phylogeny, diversification, and biogeography of South American spiny rats. Mol. Biol. Evol. 2017, 34, 613–633. [Google Scholar] [CrossRef] [PubMed]
- Upham, N.S.; Ojala-Barbour, R.; Brito, M.J.; Velazco, P.M.; Patterson, B.D. Transitions between Andean and Amazonian centers of endemism in the radiation of some arboreal rodents. BMC Evol. Biol. 2013, 13, 191. [Google Scholar] [CrossRef]
- Emmons, L.H.; Leite, Y.R.L.; Patton, J.L. Family Echimyidae. In Mammals of South America, Rodents, 1st ed.; Patton, J.L., Pardiñas, F.J., D’Elía, G., Eds.; University of Chicago Press: Chicago, IL, USA, 2015; Volume 2, pp. 877–880. [Google Scholar]
- Mammal Diversity Database, Version 2.0; Zenodo: Geneva, Switzerland, 2025. [CrossRef]
- Cirignoli, S. Kannabateomys amblyonyx. In Categorización 2019 de los Mamíferos de Argentina Según su Riesgo de Extinción. Lista Roja de los Mamíferos de Argentina, 1st ed.; SAyDS–SAREM, Ed.; SAREM: Buenos Aires, Argentina, 2019; Available online: https://cma.sarem.org.ar/es/especie-nativa/kannabateomys-amblyonyx (accessed on 10 May 2025).
- Leite, Y.R.L.; Loss, A.C. Genus Phyllomys. In Mammals of South America, Rodents, 1st ed.; Patton, J.L., Pardiñas, F.J., D’Elía, G., Eds.; University of Chicago Press: Chicago, IL, USA, 2015; Volume 2, pp. 915–928. [Google Scholar]
- Morrone, J.J. Cladistic biogeography of the Neotropical Region: Identifying the main events in the diversification of the terrestrial biota. Cladistics 2014, 30, 202–214. [Google Scholar] [CrossRef]
- Upham, N.S.; Patterson, B.D. Diversification and biogeography of the Neotropical caviomorph lineage Octodontoidea (Rodentia: Hystricognathi). Mol. Phyl. Evol. 2012, 63, 417–429. [Google Scholar] [CrossRef]
- Upham, N.S.; Patterson, B.D. Evolution of caviomorph rodents: A complete phylogeny and timetree for living genera. In Biology of Caviomorph Rodents: Diversity and Evolution, 1st ed.; Vassallo, A.I., Antenucci, D., Eds.; SAREM: Buenos Aires, Argentina, 2015; Volume 1, pp. 63–120. [Google Scholar]
- Verzi, D.H.; Olivares, A.I.; Hadler, P.; Castro, J.C.; Tonni, E.P. Occurrence of Dicolpomys (Echimyidae) in the late Holocene of Argentina: The most recently extinct South American caviomorph genus. Quat. Int. 2018, 490, 123–131. [Google Scholar] [CrossRef]
- Vucetich, M.G.; Mazzoni, M.M.; Pardiñas, U.F.J. Los roedores de la Formación Collón Curá (Mioceno medio), y la Ignimbrita Pilcaniyeu. Cañadón del Tordillo, Neuquén. Ameghiniana 1993, 30, 361–381. [Google Scholar]
- Solórzano, A.; Encinas, A.; Kramarz, A.G.; Carrasco, G.; Montoya-Sanhueza, G.; Reyes, M.; Bobe, R. Late Early Miocene caviomorph rodents from the Laguna del Laja (~37° S), Cura-Mallín Formation, south central Chile. J. South Am. Earth Sci. 2020, 102, 102658. [Google Scholar] [CrossRef]
- Piñero, P.; Olivares, A.I.; Verzi, D.H.; Contreras, V.H. Paralonchothrix gen. nov., the first record of Echimyini (Rodentia, Octodontoidea) in the Late Miocene of Southern South America. Earth Environ. Sci. Trans. R. Soc. Edinb. 2021, 112, 147–158. [Google Scholar] [CrossRef]
- Hadler, P.; Verzi, D.H.; Vucetich, M.G.; Ferigolo, J.; Ribeiro, A.M. Caviomorphs (Mammalia, Rodentia) from the Holocene of Rio Grande do Sul State, Brazil: Systematics and paleoenvironmental context. Rev. Bras. Paleontol. 2008, 11, 97–116. [Google Scholar] [CrossRef]
- Ferreira, T.M.F.; Olivares, A.I.; Kerber, L.; Parisi Dutra, R.; Santos Avilla, L. Late Pleistocene echimyid rodents (Rodentia, Hystricognathi) from northern Brazil. An. Acad. Bras. Ciênc. 2016, 88, 829–845. [Google Scholar] [CrossRef]
- Palazzesi, L.; Barreda, V. Major vegetation trends in the Tertiary of Patagonia (Argentina): A qualitative paleoclimatic approach based on palynological evidence. Flora 2007, 202, 328–337. [Google Scholar] [CrossRef]
- Olivares, A.I.; Verzi, D.H.; Contreras, V.H.; Pessôa, L. A new Echimyidae (Rodentia, Hystricomorpha) from the late Miocene of southern South America. J. Vertebr. Paleontol. 2017, 37, e1239204. [Google Scholar] [CrossRef]
- Peralta, M.J.; Bogan, S.; Ferrero, B.S.; Brunetto, E. An ichthyological assemblage from the Early Holocene in the distal Paraná River basin (Entre Ríos, Argentina): Systematics, taphonomy, and palaeoenvironmental reconstructions. Hist. Biol. 2025, 1–32. [Google Scholar] [CrossRef]
- Peralta, M.J. Vertebrados Fósiles de las Terrazas Fluviales Holocenas en el Sector Distal de la Cuenca del Río Paraná (Entre Ríos, Argentina): Sistemática, Tafonomía y Aspectos Paleoambientales. Ph.D. Thesis, Universidad Nacional de Córdoba, Córdoba, Argentina, 2024. [Google Scholar]
- Peralta, M.J.; Ferrero, B.S.; Fernández Ozuna, M.A. La fauna fósil de los paleocanales holocenos del Arroyo Doll en el sudoeste de Entre Ríos. Publ. Electron. Asoc. Paleontol. Argent. 2019, 19, R31. [Google Scholar]
- Peralta, M.J.; Ferrero, B.S. First fossil record of Bibimys Massoia, 1979 (Rodentia, Cricetidae) from the Early Holocene in Argentina. Hist. Biol. 2022, 36, 214–219. [Google Scholar] [CrossRef]
- Peralta, M.J.; Ferrero, B.S. First vertebrate assemblage of the Early Holocene from northeastern Argentina (Mesopotamian Region). Publ. Electron. Asoc. Paleontol. Argent. 2024, 24, 38–61. [Google Scholar] [CrossRef]
- Cohen, K.M.; Finney, S.C.; Gibbard, P.L.; Fan, J.-X. The ICS International Chronostratigraphic Chart. Episodes 2013, 36, 199–204. [Google Scholar] [CrossRef]
- Stieffel, R.H., Jr. Evidence for an Old Paraná Delta and Diachroneity in Global Highstands. Master’s Thesis, Louisiana State University, Baton Rouge, LA, USA, 2019. [Google Scholar]
- Brunetto, E.; Ferrero, B.S.; Noriega, J.I. Late Pleistocene lithostratigraphy and sequences in the southwestern Mesopotamia (Argentina): Evidences of the last interglacial stage. J. S. Am. Earth Sci. 2015, 58, 111–128. [Google Scholar] [CrossRef]
- Verzi, D.H.; Olivares, A.I.; Morgan, C.C.; Álvarez, A. Contrasting phylogenetic and diversity patterns in octodontoid rodents and a new definition of the family Abrocomidae. J. Mamm. Evol. 2016, 23, 93–115. [Google Scholar] [CrossRef]
- Verzi, D.H.; Olivares, A.I.; Morgan, C.C. Morphology of the lower deciduous premolars of South American hystricomorph rodents and age of the Octodontoidea. Hist. Biol. 2019, 31, 1170–1178. [Google Scholar] [CrossRef]
- Boivin, M.; Marivaux, L.; Antoine, P.-O. L’apport du registre paléogène d’Amazonie sur la diversification initiale des Caviomorpha (Hystricognathi, Rodentia): Implications phylogénétiques, macroévolutives et paléobiogéographiques. Geodiversitas 2019, 41, 143–245. [Google Scholar] [CrossRef]
- Pérez Pincheira, E.; Muñoz, N.; Carrevedo, M.L.; Nuñez Otaño, N.; Peralta, M.J.; Ferrero, B.S. Diatomeas en terrazas fluviales holocenas del sudoeste de Entre Ríos, Argentina. In Proceedings of the XVII Simposio Argentino de Paleobotánica y Palinología, Entre Ríos, Argentina, 30 July–3 August 2018. [Google Scholar]
- Zilli, F.L.; Peralta, M.J.; Pedersen, O.A.; Ferrero, B.S. Malacofauna holocena del sector distal de la cuenca del Río Paraná. Publ. Electron. Asoc. Paleontol. Argent. 2019, 20, R112. [Google Scholar]
- Peralta, M.J.; Ferrero, B.S. First Quaternary fossil record of a blind snake (Scolecophidia, Serpentes) from South America (Argentina). Hist. Biol. 2023, 36, 2108–2113. [Google Scholar] [CrossRef]
- Zapata, C.V.; Nuñez Otaño, N.B.; Peralta, M.J.; Ferrero, B.S.; Brunetto, E. Primer registro de Diplocladiella sp. para el Holoceno Temprano (Entre Ríos, Argentina). Valor paleoecológico y taxonómico para discutir la forma fósil Triporicellaesporites (Paleógeno-Neógeno). Bol. Soc. Argent. Bot. 2023, 58, 207. [Google Scholar]
- Holbrook, J.; Stieffel, R.; Kröhling, D.M.; Milana, J.P.; Roldan, J. The Paraná Delta (Argentina): A high-resolution record of the Holocene. In Proceedings of the INQUA Congress, Rome, Italy, 13–20 July 2023. [Google Scholar]
- Blum, M.D. Glacial-Interglacial Scale Fluvial Responses. In Encyclopedia of Quaternary Science, 2nd ed.; Elias, S.A., Mock, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 112–125. [Google Scholar] [CrossRef]
- Blum, M.D.; Törnqvist, T.E. Fluvial responses to climate and sea-level change: A review and look forward. Sedimentology 2000, 47, 2–48. [Google Scholar] [CrossRef]
- de Vivo, M.; Carmignotto, A.P. Holocene vegetation change and the mammal faunas of South America and Africa. J. Biogeogr. 2004, 31, 943–957. [Google Scholar] [CrossRef]
- Hermanowski, B.; Da Costa, M.L.; Behling, H. Environmental changes in southeastern Amazonia during the last 25,000 yr revealed from a paleoecological record. Quat. Res. 2012, 77, 138–148. [Google Scholar] [CrossRef]
- Maksic, J.; Shimizu, M.H.; De Oliveira, G.S.; Venancio, I.M.; Cardoso, M.; Ferreira, F.A. Simulation of the Holocene climate over South America and impacts on the vegetation. Holocene 2019, 29, 287–299. [Google Scholar] [CrossRef]
- Azevedo, V.; Strikis, N.M.; Novello, V.F.; Roland, C.L.; Cruz, F.W.; Santos, R.V.; Vuille, M.; Utida, G.; De Andrade, F.R.D.; Cheng, H.; et al. Paleovegetation seesaw in Brazil since the Late Pleistocene: A multiproxy study of two biomes. Earth Planet. Sc. Lett. 2021, 563, 116880. [Google Scholar] [CrossRef]
- Mottl, O.; Flantua, S.G.A.; Bhatta, K.P.; Felde, V.A.; Giesecke, T.; Goring, S.; Grimm, E.C.; Haberle, S.; Hooghiemstra, H.; Ivory, S.; et al. Global acceleration in rates of vegetation change over the past 18,000 years. Science 2021, 372, 860–864. [Google Scholar] [CrossRef]
- Maezumi, S.Y.; Power, M.J.; Smith, R.J.; McLauchlan, K.K.; Brunelle, A.R.; Carleton, C.; Kay, A.U.; Roberts, P.; Mayle, F.E. Fire-human-climate interactions in the Bolivian Amazon rainforest ecotone from the Last Glacial Maximum to late Holocene. Front. Environ. Archaeol. 2023, 2, 1208985. [Google Scholar] [CrossRef]
- Kraglievich, J.L. Speciation phylétique dans les rongeurs fossiles du genre Eumysops Amegh. (Echimyidae, Heteropsomyinae). Mammalia 1965, 29, 258–267. [Google Scholar] [CrossRef]
- Verzi, D.H.; Vucetich, M.G.; Montalvo, C.I. Un nuevo Eumysopinae (Rodentia, Echimyidae) del Mioceno tardío de la Provincia de La Pampa y consideraciones sobre la historia de la subfamilia. Ameghiniana 1995, 32, 191–195. [Google Scholar]
- Vucetich, M.G. Theridomysops parvulus (Rovereto, 1914), un primitivo Eumysopinae (Rodentia, Echimyidae) del Mioceno tardío de Argentina. Mastozool. Neotrop. 1995, 2, 167–172. [Google Scholar]
- Olivares, A.I.; Verzi, D.H.; Vucetich, M.G. Definición del género Eumysops Ameghino, 1888 (Rodentia, Echimyidae) y sistemática de las especies del Plioceno temprano de la Argentina central. Ameghiniana 2012, 49, 198–216. [Google Scholar] [CrossRef]
- Olivares, A.I.; Verzi, D.H. Systematics, phylogeny and evolutionary pattern of the hystricognath rodent Eumysops (Echimyidae) from the Plio–Pleistocene of southern South America. Hist. Biol. 2015, 27, 1042–1061. [Google Scholar] [CrossRef]
- Candela, A.M.; Cenizo, M.; Tassara, D.; Rasia, L.L.; Robinet, C.; Muñoz, N.A.; Cañón Valenzuela, C.; Pardiñas, U.F.J. A new echimyid genus (Rodentia, Caviomorpha) in central Argentina: Uncovered diversity of a Brazilian group of mammals in the Pleistocene. J. Paleontol. 2020, 94, 165–179. [Google Scholar] [CrossRef]
- Zachos, J.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 2001, 292, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Ortiz Jaureguizar, E.; Cladera, G.A. Paleoenvironmental evolution of southern South America during the Cenozoic. J. Arid. Environ. 2006, 66, 498–532. [Google Scholar] [CrossRef]
- Le Roux, J.P. A review of Tertiary climate changes in southern South America and the Antarctic Peninsula. Part 2: Continental conditions. Sediment. Geol. 2012, 247, 21–38. [Google Scholar] [CrossRef]
- Herbert, T.D.; Lawrence, K.T.; Tzanova, A.; Peterson, L.C.; Caballero-Gill, R.; Kelly, C.S. Late Miocene global cooling and the rise of modern ecosystems. Nat. Geosci. 2016, 9, 843–847. [Google Scholar] [CrossRef]
- Barreda, V.D.; Palazzesi, L. Role of climate and tectonism on the modernization of Patagonian floras: Evidence from the fossil record. Global Planet. Chang. 2021, 204, 103556. [Google Scholar] [CrossRef]
- Evangelinos, D.; Etourneau, J.; van de Flierdt, T.; Crosta, X.; Jeandel, C.; Flores, J.-A.; Harwood, D.M.; Ducassou, E.; Saurmilch, I.; Klocker, A.; et al. Late Miocene onset of the modern Antarctic Circumpolar Current. Nat. Geosci. 2024, 17, 165–170. [Google Scholar] [CrossRef]
- Musher, L.J.; Ferreira, M.; Auerbach, A.L.; McKay, J.; Cracraft, J. Why is Amazonia a ‘source’ of biodiversity? Climate mediated dispersal and synchronous speciation across the Andes in an avian group (Tityrinae). Proc. R. Soc. Lond. B. Biol. Sci. 2019, 286, 20182343. [Google Scholar] [CrossRef]
- Alley, R.B.; Ágústsdóttir, A.M. The 8k event: Cause and consequences of a major Holocene abrupt climate change. Quat. Sci. Rev. 2005, 24, 1123–1149. [Google Scholar] [CrossRef]
- Baker, P.A.; Seltzer, G.O.; Fritz, S.C.; Dunbar, R.B.; Grove, M.J.; Tapia, P.M.; Cross, S.L.; Rowe, H.D.; Broda, J.P. The History of South American Tropical Precipitation for the Past 25,000 Years. Science 2001, 291, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Mayle, F.E.; Power, M.J. Impact of a drier Early–Mid-Holocene climate upon Amazonian forests. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2008, 363, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- González-Pinilla, F.J.; Latorre, C.; Rojas, M.; Houston, J.; Rocuant, M.I.; Maldonado, A.; Santoro, C.M.; Quade, J.; Betancourt, J.L. High- and low-latitude forcings drive Atacama Desert rainfall variations over the past 16,000 years. Sci. Adv. 2021, 7, eabg1333. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peralta, M.J.; Olivares, A.I.; Ferrero, B.S.; Brunetto, E.; Verzi, D.H. A Tropical Spiny Tree Rat (Rodentia, Echimyini) in the Late Quaternary of Southern South America (Argentina): Paleoenvironmental and Paleogeographic Implications. Quaternary 2025, 8, 48. https://doi.org/10.3390/quat8030048
Peralta MJ, Olivares AI, Ferrero BS, Brunetto E, Verzi DH. A Tropical Spiny Tree Rat (Rodentia, Echimyini) in the Late Quaternary of Southern South America (Argentina): Paleoenvironmental and Paleogeographic Implications. Quaternary. 2025; 8(3):48. https://doi.org/10.3390/quat8030048
Chicago/Turabian StylePeralta, Matías J., A. Itatí Olivares, Brenda S. Ferrero, Ernesto Brunetto, and Diego H. Verzi. 2025. "A Tropical Spiny Tree Rat (Rodentia, Echimyini) in the Late Quaternary of Southern South America (Argentina): Paleoenvironmental and Paleogeographic Implications" Quaternary 8, no. 3: 48. https://doi.org/10.3390/quat8030048
APA StylePeralta, M. J., Olivares, A. I., Ferrero, B. S., Brunetto, E., & Verzi, D. H. (2025). A Tropical Spiny Tree Rat (Rodentia, Echimyini) in the Late Quaternary of Southern South America (Argentina): Paleoenvironmental and Paleogeographic Implications. Quaternary, 8(3), 48. https://doi.org/10.3390/quat8030048






