Evidence of Chronic Tusk Trauma and Compensatory Scoliosis in Mammuthus meridionalis from Madonna della Strada (Scoppito, L’Aquila, Italy)
Abstract
1. Introduction
2. Materials and Methods
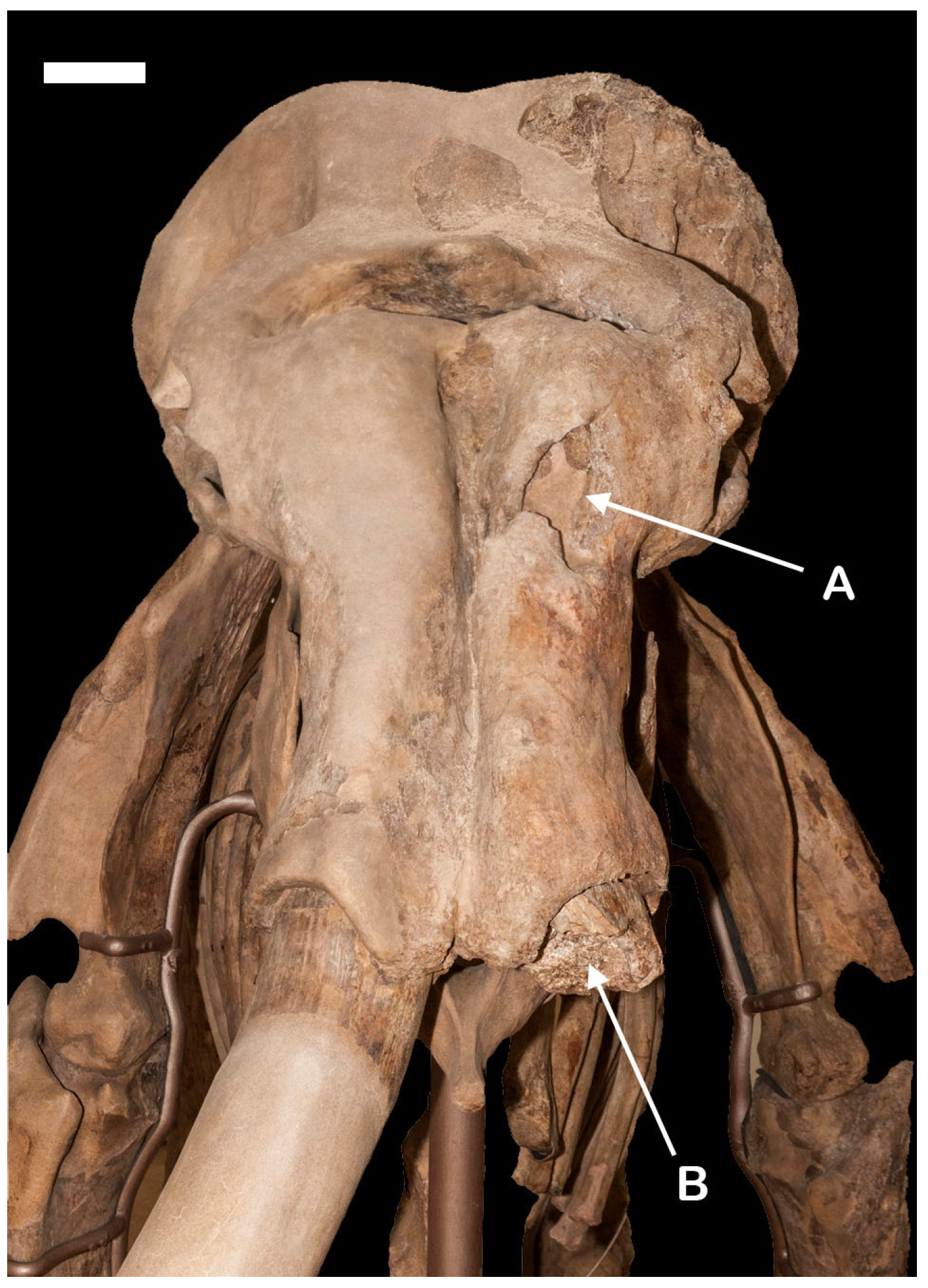
2.1. Paleopathological Evidence on the Skull
2.2. Ultrasound Investigation Conducted on the Left Premaxilla
- Right tusk (ivory), used to obtain the characteristic signal of ivory.
- Stump of the left tusk (ivory + mastic), to detect the signal behavior in the presence of composite materials.
- Reconstructed right premaxilla (mastic + iron), to determine if metal is present.
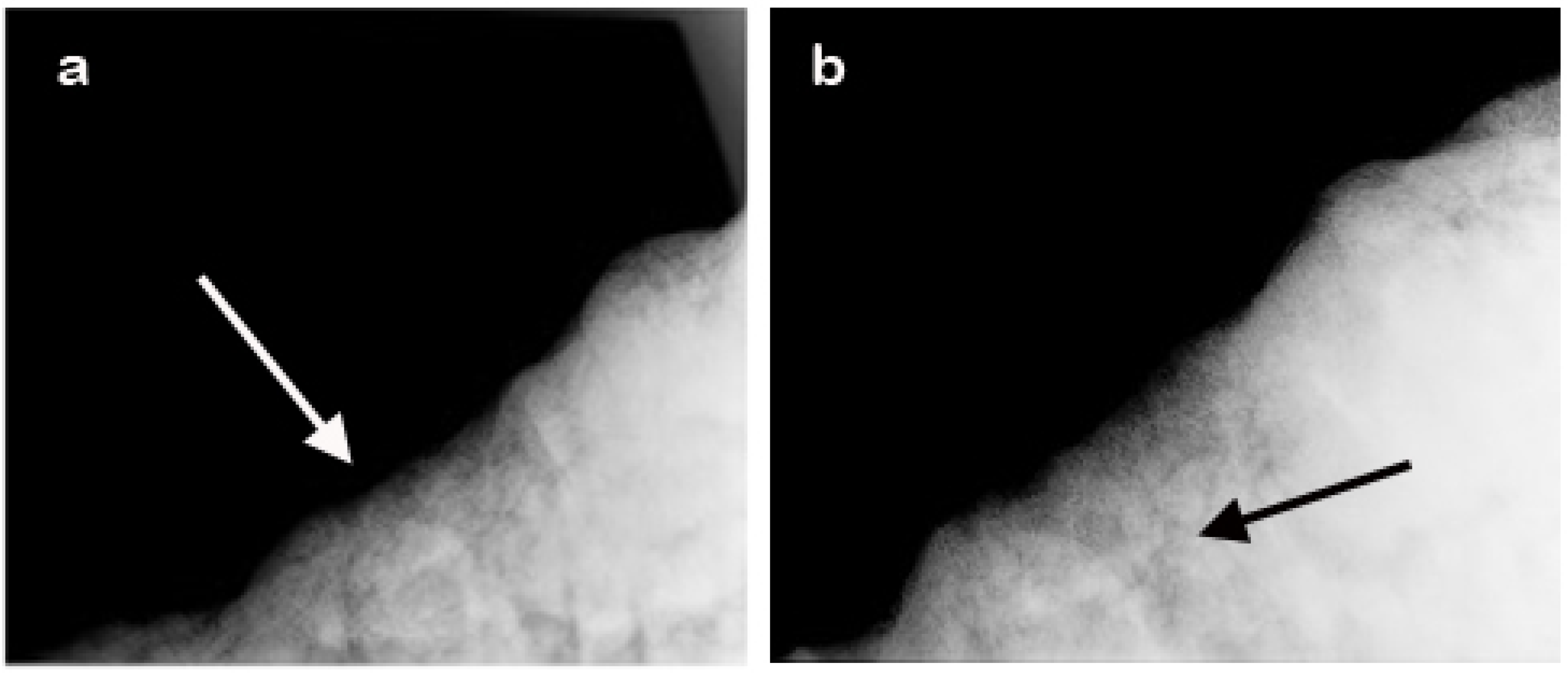
2.3. Radiographic Analysis
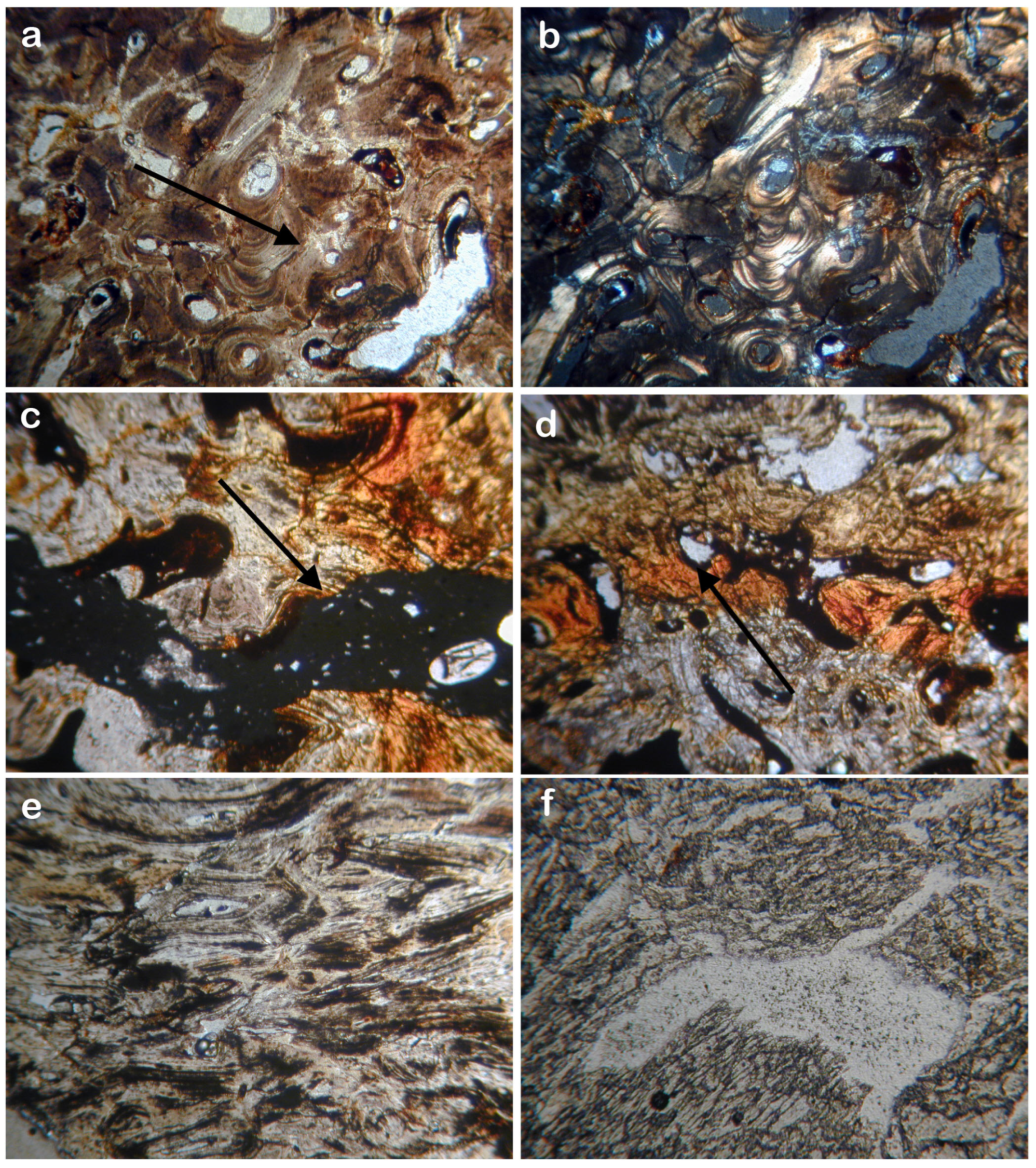
2.4. Polarizing Microscope Analysis
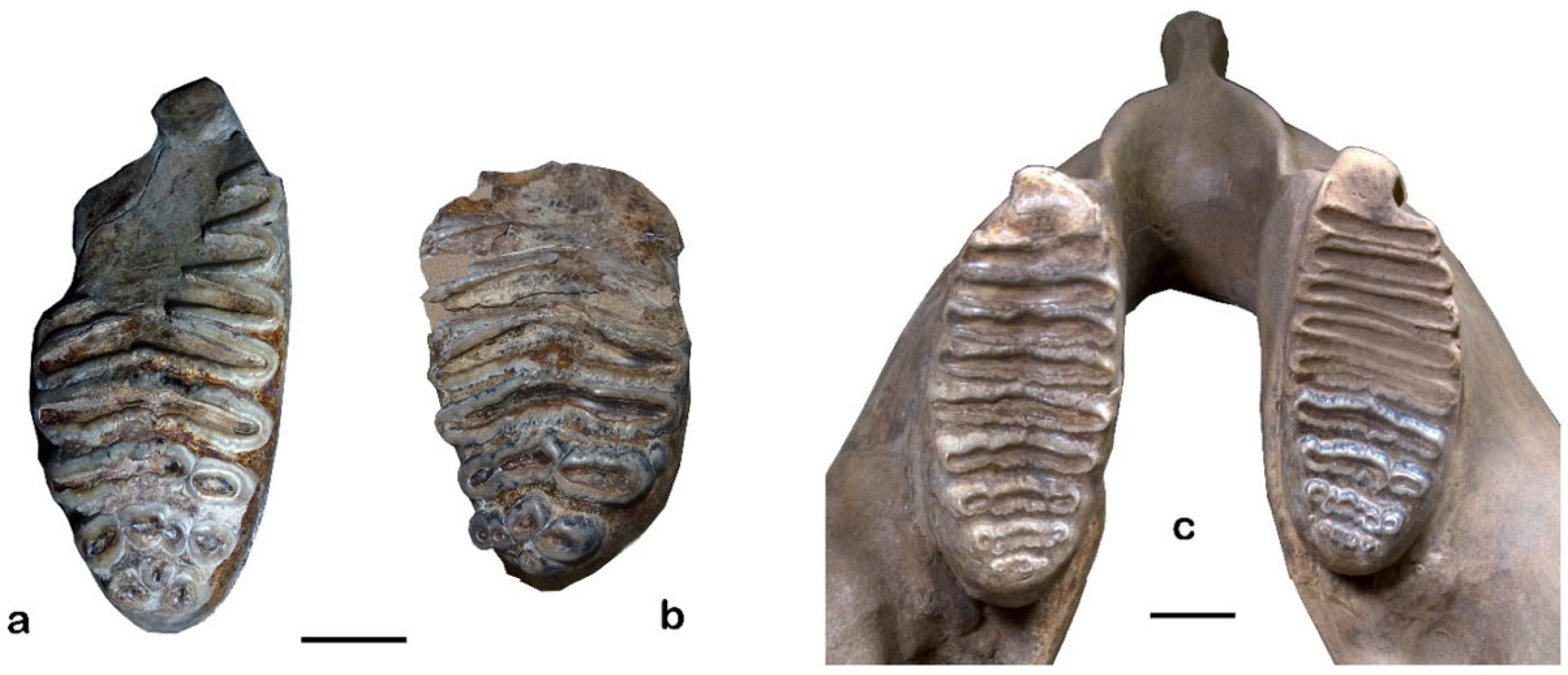
2.5. Molar Functional Morphology
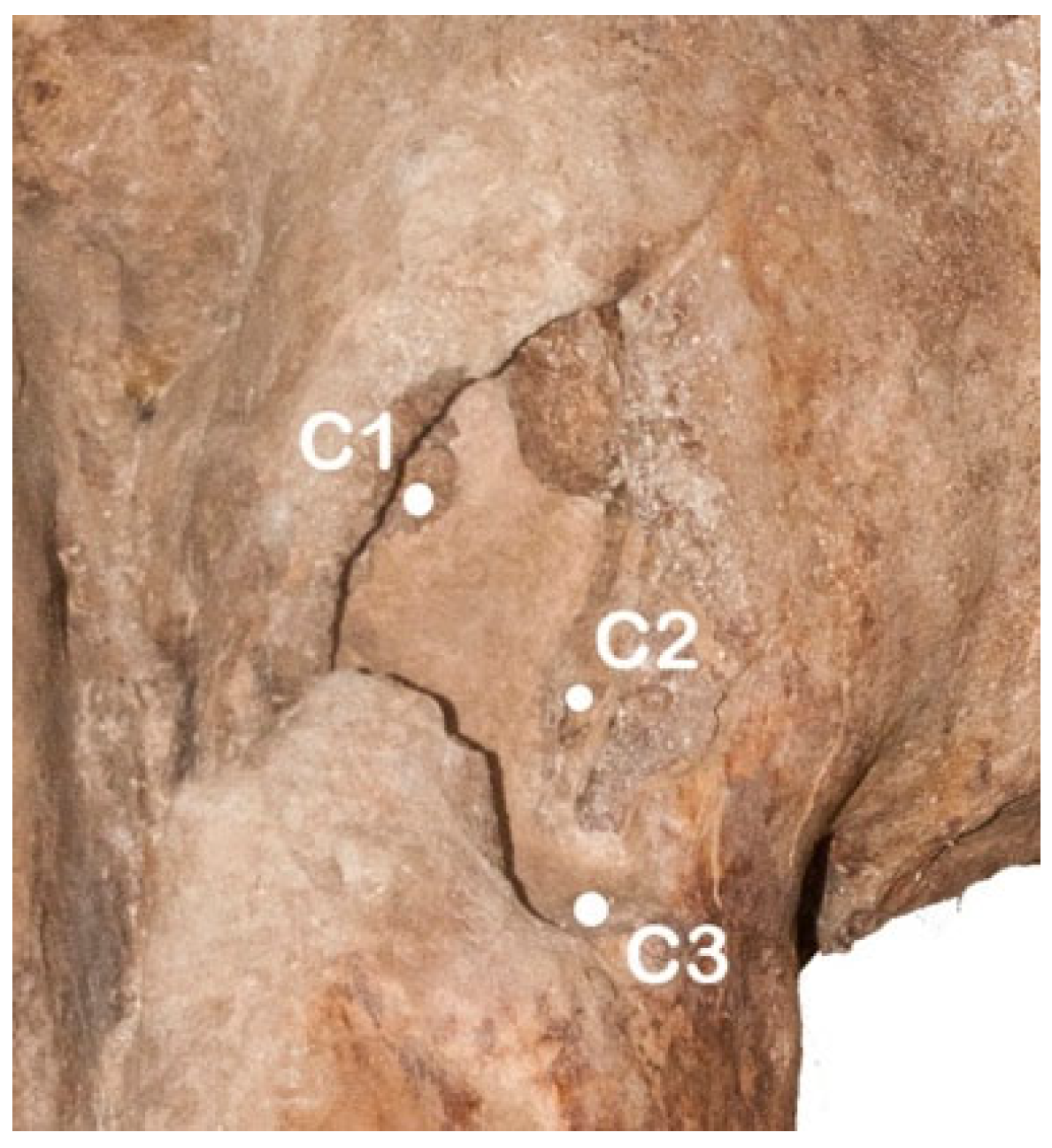
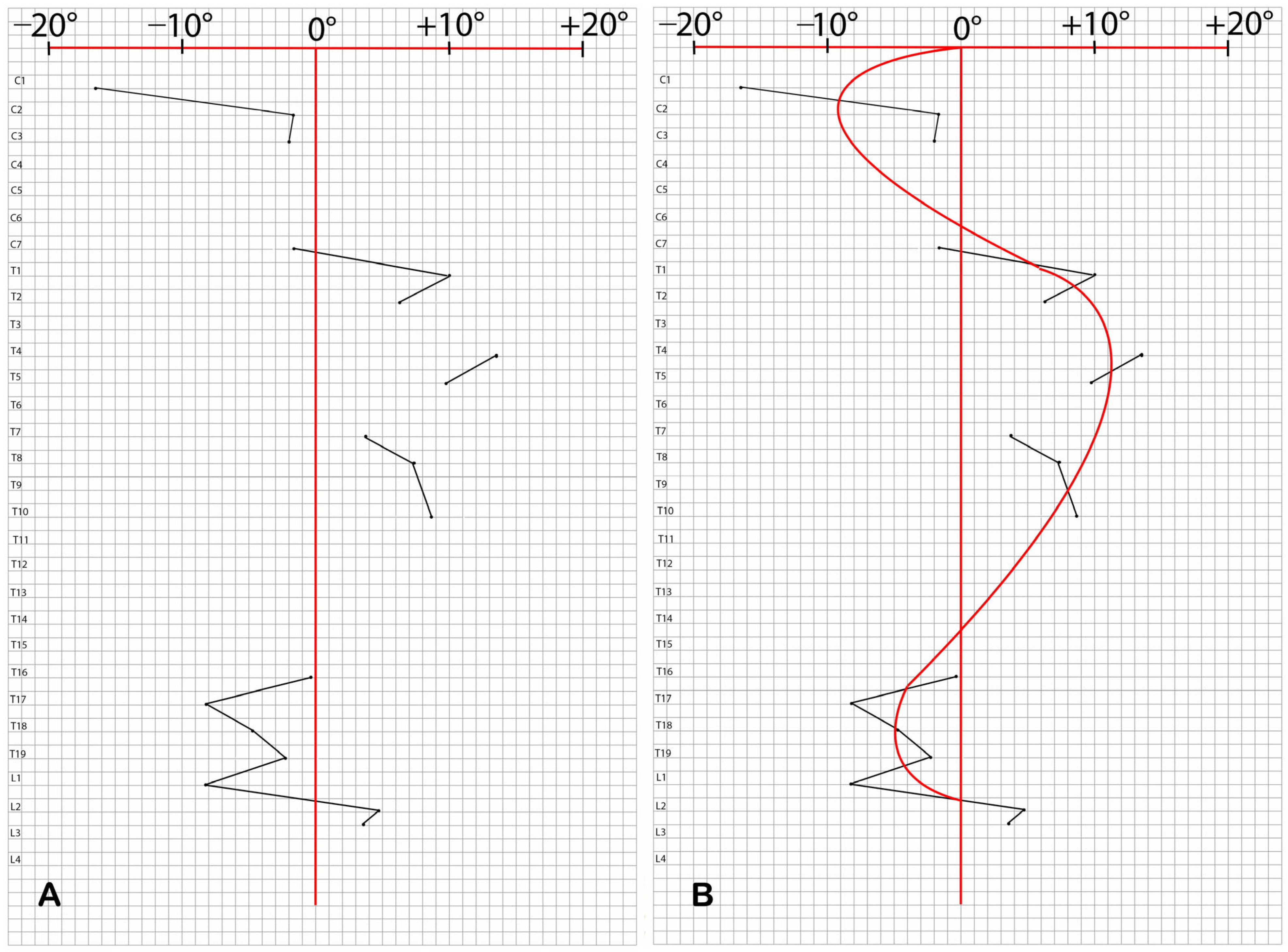
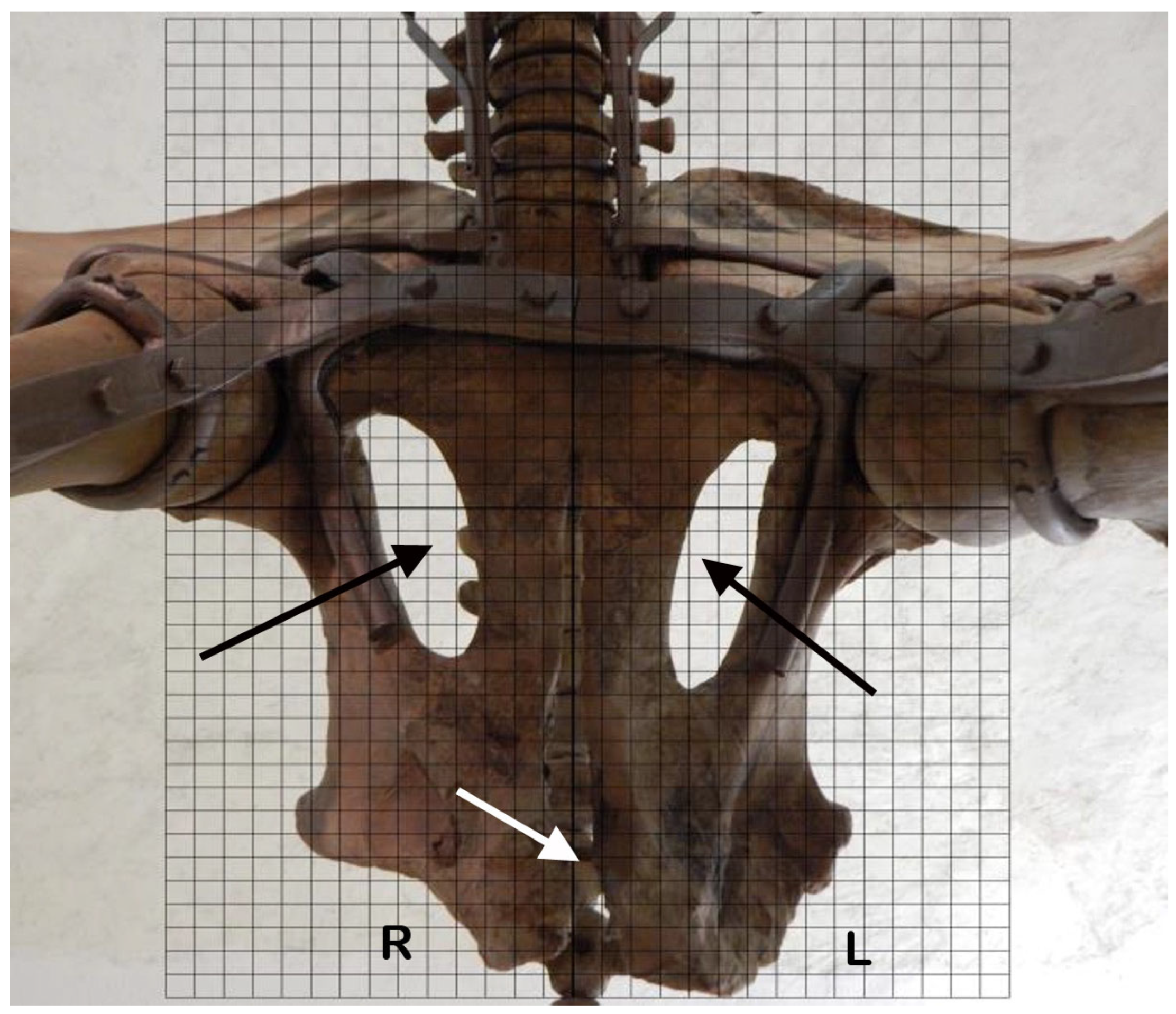
2.6. Morphological Asymmetries of the Skeleton
- Angle 1: between the spinous process axis (from the apex to neural arch or spinous base) and the perpendicular to the vertebral body’s maximum transverse diameter (Figure S2a);
- Angle 2: between the spinous process axis and the perpendicular to the line connecting the transverse processes (Figure S2b);
- Angle 3: between the spinous process axis and the perpendicular to the line connecting the anterior articular processes (Figure S2c).
3. Results
3.1. Paleopathological Evidence on the Skull
3.2. Ultrasound Investigation Conducted on the Left Premaxilla
3.3. Radiographic Findings
3.4. Histopathological Results
3.5. Molar Functional Morphology
3.6. Morphological Asymmetries of the Skeleton
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nesti, F. Sulla nuova specie di elephante fossile del Valdarno all’Illustrissimo sig. Dott. Prof. Ottaviano Targioni Tozzetti. Nuovo Giorn. Lett. 1825, 11, 195–216. [Google Scholar]
- Lister, A.M.; Sherb, A.V.; van Essen, H.; Wei, G. The pattern and process of mammoth evolution in Eurasia. Quat. Int. 2005, 126-128, 49–64. [Google Scholar] [CrossRef]
- Azzaroli, A. Evolutionary patterns of Villafranchian elephants in Central Italy. Atti Accad. Naz. Lincei Mem. Cl. Sci. Fis. 1977, 14, 149–168. [Google Scholar]
- Ferretti, M.P. Mammuthus meridionalis (Mammalia, Proboscidea, Elephantidae) from the “Sabbie Gialle” of Oriolo (Cava La Salita, Faenza, Northern Italy) and other European late populations of southern mammoth. Eclogae Geol. Helv. 1999, 92, 503–515. [Google Scholar]
- Ferretti, M.P.; Bellucci, L.; Rustioni, M. Cranial morphology and variation in the iconic Villafranchian proboscidean Mammuthus meridionalis (Nesti, 1825) from the type locality of Upper Valdarno (Tuscany, Italy). Boll. Soc. Paleontol. Ital. 2025, 64, 165–187. [Google Scholar]
- Palombo, M.R.; Ferretti, M.P. Elephant fossil record from Italy: Knowledge, problems, and perspectives. Quat. Int. 2005, 126, 107–136. [Google Scholar] [CrossRef]
- Agostini, S.; Palombo, M.R.; Rossi, M.A.; Di Canzio, E.; Tallini, M. Mammuthus meridionalis (Nesti, 1825) from Campo di Pile (L’Aquila, Abruzzo, Central Italy). Quat. Int. 2012, 276–277, 42–52. [Google Scholar] [CrossRef]
- Agostini, S.; Di Canzio, E.; Rossi, M.A. Abruzzo (Italy): The Plio-Pleistocene proboscidean-bearing sites. In La Terra Degli Elefanti. Atti del 1° Congresso Internazionale; Cavarretta, G., Mussi, M., Palombo, M.R., Eds.; CNR: Roma, Italy, 2001; pp. 163–166. [Google Scholar]
- Leuci, G.; Scorziello, R. Il Mammuthus meridionalis (Nesti) di Contrada «Tratturo» Giuliano Teatino (Chieti). Boll. Mus. Reg. Sci. Nat. Torino 1993, 11, 387–406. [Google Scholar]
- Barbosa, F.H.S.; Porpino, K.O.; Bergqvist, L.P.; Rothschild, B.M. Elucidating bone diseases in Brazilian Pleistocene sloths (Xenarthra, Pilosa, Folivora): First cases reported for the Nothrotheriidae and Megalonychidae families. Ameghiniana 2017, 54, 331–340. [Google Scholar] [CrossRef]
- Haynes, G.; Klimowicz, J. A preliminary review of bone and teeth abnormalities seen in recent Loxodonta and extinct Mammuthus and Mammut, and suggested implications. Quat. Int. 2015, 379, 135–146. [Google Scholar] [CrossRef]
- Krzeminska, A.; Wedzicha, S. Pathological changes on the ribs of woolly mammoths (Mammuthus primigenius). Quat. Int. 2015, 359–360, 186–194. [Google Scholar] [CrossRef]
- Krzeminska, A.; Wojtal, P.; Oliva, M. Pathological changes on woolly mammoth (Mammuthus primigenius) bones: Holes, hollows and other minor changes in the spinous processes of vertebrae. Quat. Int. 2015, 359–360, 178–185. [Google Scholar] [CrossRef]
- Pawłowska, K.; Chroszcz, A.; Poradowski, D.; Kubiak-Nowak, D.; Borawski, W. Diseases and Traumas of Pleistocene Megafauna: A perspective from Poland. Int. J. Osteoarchaeol. 2025, 35, e3387. [Google Scholar] [CrossRef]
- Labarca, R.; Pacheco, A. Palaeopathological analysis of a Chilean gomphothere (Proboscidea: Gomphotheriidae). Int. J. Paleopathol. 2019, 26, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Zorro-Luján, C.M.; Noé, L.F.; Gómez-Pérez, M.; Grouard, S.; Chaparro, A.; Torres, S. Vertebral lesions in Notiomastodon platensis, Gomphotheriidae, from Anolaima, Colombia. Quat. Res. 2022, 112, 1–15. [Google Scholar] [CrossRef]
- Luna, C.A.; Barbosa, F.H.S.; Gonzalez, R.; Miño-Boilini, Á.R.; Repetto, C.; Zurita, A.E. Bone diseases in a Pleistocene South American native ungulate species: The case of Toxodon platensis Owen, 1837 (Mammalia, Notoungulata, Toxodontidae). J. Quat. Sci. 2024, 39, 1206–1215. [Google Scholar] [CrossRef]
- Barbosa, F.H.S.; de Araújo-Júnior, H.I.; Mothé, D.; Avilla, L.S. Osteological diseases in an extinct Notiomastodon (Mammalia, Proboscidea) population from the Late Pleistocene of Brazil. Quat. Int. 2017, 443, 228–232. [Google Scholar] [CrossRef]
- Lister, A.M. Late-glacial mammoth skeletons (Mammuthus primigenius) from Condover (Shropshire, UK): Anatomy, pathology, taphonomy and chronological significance. Geol. J. 2009, 44, 447–479. [Google Scholar] [CrossRef]
- Grigoriev, S.E.; Fisher, D.E.; Obada, T.; Shirley, E.A.; Rountrey, A.N.; Savvinov, G.N.; Garmaeva, D.K.; Novgorodov, G.P.; Cheprasov, M.Y.; Vasilev, S.E.; et al. A woolly mammoth (Mammuthus primigenius) carcass from Maly Lyakhovsky Island (New Siberian Islands, Russian Federation). Quat. Int. 2017, 445, 89–103. [Google Scholar] [CrossRef]
- Rothschild, B.M.; Laub, R. Hyperdisease in the Late Pleistocene: Validation of an Early 20th Century Hypotesis. Sci. Nat. 2006, 93, 557–564. [Google Scholar] [CrossRef]
- Rothschild, B.M.; Surmik, D.; Bertozzo, F. Infectious Disease. In Modern Paleopathology: The Study of Diagnostic Approach to Ancient Diseases, Their Pathology and Epidemiology; Smith, J., Garcia, L., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 309–404. [Google Scholar]
- Garcês, A.; Pires, I.; Garcês, S. Ancient Diseases in Vertebrates: Tumours through the Ages. Animals 2024, 14, 1474. [Google Scholar] [CrossRef]
- Woital, P.; Wilczynski, J.; Haynes, G. A Gravettian Site in Southern Poland: Kraków Spadzista 1; Institute of Systematics and Evolution of Animals, Polish Academy of Sciences: Krakow, Poland, 2015. [Google Scholar]
- Stoffel, E.W. The Kyle Mammoth Project: An Archaeological, Paleoecological and Taphonomic Analysis. Master’s Thesis, University of Saskatchewan, Saskatoon, Canada, 2016. [Google Scholar]
- Magri, D.; Di Rita, F.; Palombo, M.R. An Early Pleistocene interglacial record from an intermontane basin of central Italy (Scoppito, L’Aquila). Quat. Int. 2010, 225, 106–113. [Google Scholar] [CrossRef]
- Maccagno, A.M. L’Elephas meridionalis Nesti di Contrada “Madonna della Strada” Scoppito (L’Aquila). Atti Accad. Sci. Fis. Mat. Napoli Mem. 1962, 4, 1–129. [Google Scholar]
- Rossi, M.A.; Agostini, S.; Palombo, M.R.; Angelini, I.; Caramiello, S.; Casarin, F.; Ghezzo, E.; Marano, F.; Molin, G.; Reggiani, P.; et al. Mammuthus meridionalis from Madonna della Strada (Scoppito, L’Aquila): Diagnostics and restoration. Boll. Soc. Paleontol. Ital. 2017, 56, 359–373. [Google Scholar]
- Assis, S.; Keenleyside, A.; Santos, A.L.; Alves Cardoso, F. Bone diagenesis and its implication to disease diagnosis: The relevance of bone microstructure analysis in past human remains. Microsc. Microanal. 2015, 21, 805–825. [Google Scholar] [CrossRef]
- Salazar, A.; Rodríguez, A.; Safont, G.; Vergara, L. Prospective of the Application of Ultrasounds in Archaeology. IOP Conf. Ser. Mate. Sci. Eng. 2012, 42, 012010. [Google Scholar] [CrossRef]
- Bochud, N.; Vallet, Q.; Minonzio, J.G.; Laugier, P. Predicting bone strength with ultrasonic guided waves. Sci. Rep. 2017, 7, 43628. [Google Scholar] [CrossRef] [PubMed]
- Zelditch, M.L.; Swiderski, D.L.; Sheets, H.D.; Fink, W.L. Geometric Morphometric for Biologists; Elsevier: New York, NY, USA, 2004. [Google Scholar]
- Pacciani, E.; Fariello, G.; Donnetti, L.; Urso, S. Diagnostica neuroradiologica nella scoliosi. Riv. Neuroradiol. 1999, 12, 179–184. [Google Scholar] [CrossRef]
- Slice, D.E. Geometric morphometrics. Ann. Rev. Anthropol. 2007, 36, 261–281. [Google Scholar] [CrossRef]
- Hackenberg, L. Stellenwert der Rückenformanalyse in der Therapie von Wirbersäulendeformitäten. Ph.D. Thesis, Westfälische Wilhelms-Universität Münster, Münster, Germany, 2003. [Google Scholar]
- Birchall, D.; Hughes, D.; Gregson, B.; Williamson, B. Demonstration of vertebral and disc mechanical torsion in adolescent idiopathic scoliosis using three-dimensional MR imaging. Eur. Spine J. 2005, 14, 123–129. [Google Scholar] [CrossRef]
- Stokes, I.A. Axial rotation component of thoracic scoliosis. J. Orthop. Res. 1989, 7, 702–708. [Google Scholar] [CrossRef]
- Goldberg, C.J.; Kaliszer, M.; Moore, D.P.; Fogarty, E.E.; Dowling, F.E. Surface topography, Cobb angles, and cosmetic change in scoliosis. Spine 2001, 26, E55–E63. [Google Scholar] [CrossRef]
- Stokes, I.A.; Bigalow, L.C.; Moreland, M.S. Measurement of axial rotation of vertebrae in scoliosis. Spine 1986, 11, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Ortner, D.J. Infectious diseases: Introduction, biology, osteomyelitis, periostitis, brucellosis, glanders, and septic arthritis. In Identification of Pathological Conditions in Human Skeletal Remains; Ortner, D.J., Ed.; Academic Press: San Diego, CA, USA, 2003; pp. 179–226. [Google Scholar]
- Barbosa, F.H.S.; Medeiros da Silva, L.H.; de Araújo-Júnior, H.I. Differentiating taphonomic and paleopathological features in Vertebrate Paleontology: A study case with Quaternary mammals. PalZ 2019, 94, 595–601. [Google Scholar] [CrossRef]
- Carini, F.; Longoni, S.; Amosso, E.; Carini, S.; Garavello, W.; Porcaro, G. Odontogenic maxillary sinusitis with oro-nasal fistula: A case report. Ann. Stomatol. 2014, 5, 37–39. [Google Scholar]
- Palombo, M.R. Quando a Scoppito vivevano gli elefanti. In Il Mammut del Castello: Settant’anni dalla sua Scoperta. Nuovi Dati nel Quadro Dell’evoluzione Ambientale del Pleistocene; Agostini, S., Rossi, M.A., Zalabra, F., Eds.; All’Insegna del Giglio: Firenze, Italy, 2025; pp. 61–75. [Google Scholar]
- Laws, R.M. Age criteria for the African elephant. Afr. J. Ecol. 1966, 4, 1–37. [Google Scholar] [CrossRef]
- Roth, V.L.; Shoshani, J. Dental identification and age determination in Elephas maximus. J. Zool. 1988, 214, 567–588. [Google Scholar] [CrossRef]
- Romano, M.; Manucci, F.; Antonelli, M.; Rossi, M.A.; Agostini, S.; Palombo, M.R. In vivo restoration and volumetric body mass estimate of Mammuthus meridionalis from Madonna della Strada (Scoppito, L’Aquila). Riv. Ital. Paleontol. Stratigr. 2022, 128, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Haynes, G. Mammoths, Mastodonts, and Elephants: Biology, Behavior and the Fossil Record; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Lister, A.; Bahn, P. Mammoths: Giants of the Ice Age, Revised ed.; University of California Press: Berkeley, CA, USA, 2007; p. 83. [Google Scholar]
- Mayhew, I.G.; Watson, A.G.; Heissan, J.A. Congenital occipitoatlantoaxial malformations in the horse. Equine Vet. J. 1978, 10, 103–113. [Google Scholar] [CrossRef]
- Enneking, W.F.; Harrington, P. Pathological changes in scoliosis. Bone Jt. Surg. Am. 1969, 51, 165–184. [Google Scholar] [CrossRef]
- Steenkamp, G.; Ferreira, S.M.; Bester, M.N. Tusklessness and tusk fractures in free-ranging African savanna elephants (Loxodonta africana). J. S. Afr. Vet. Assoc. 2007, 78, 75–80. [Google Scholar] [CrossRef]
- Whitehouse, A.M. Tusklessness in the elephant population of the Addo Elephant National Park, South Africa. J. Zool. 2002, 257, 249–254. [Google Scholar] [CrossRef]
- Weissengruber, G.E.; Egerbacher, M.; Forstenpointner, G. Structure and innervation of the tusk pulp in the African elephant (Loxodonta africana). J. Anat. 2005, 206, 387–393. [Google Scholar] [CrossRef]
- Bush, M.; Heese, D.W.; Gray, C.W.; James, A.E. Surgical repair of tusk injury (pulpectomy) in an adult, male forest elephant (Loxodonta cyclotis). J. Am. Dent. Assoc. 1976, 93, 372–375. [Google Scholar] [CrossRef] [PubMed]
- McGavin, M.D.; Walker, R.D.; Schroeder, E.C.; Patton, C.S.; McCraken, M.D. Death of an African elephant from probable toxemia attributed to chronic pulpitis. J. Am. Vet. Med. Assoc. 1983, 183, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Steenkamp, G. Oral biology and disorders of tusked mammals. Vet. Clin. N. Am. Exot. Anim. Pract. 2003, 6, 689–725. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, J.G. Veterinary Care of African Elephants; South African Veterinary Foundation and Novartis Animal Health: Johannesburg, South Africa, 2001; pp. 1–59. [Google Scholar]
- Schultz, M. Paleohistopathology of Bone: A New Approach to the Study of Ancient Diseases. Yearb. Phys. Anthropol. 2001, 44, 106–111. [Google Scholar] [CrossRef]
- Molnár, E.; Marcsik, A.; Bereczki, Z.; Schmidt-Schultz, T.H.; Schultz, M.; Pálfi, G. Malignant tumors in osteoarchaeological samples from Hungary. Acta Biol. Szeged. 2009, 53, 117–124. [Google Scholar]
- Subramanian, S.; Viswanathan, V.K. Lytic Bone Lesions. In StatPearls; StatPearls Publishing: Treasure Ilands, FL, USA, 2025. [Google Scholar]
- Ortner, D.J. (Ed.) Infectious diseases: Tuberculosis and leprosy. In Identification of Pathological Conditions in Human Skeletal Remains; Elsevier Science: Amsterdam, The Netherlands, 2003; pp. 227–271. [Google Scholar]
- Maslow, J.N.; Mikota, S.K. Tuberculosis in Elephants. A reemergent disease: Diagnostic dilemmas, the natural history of infection, and new immunological tools. Vet. Pathol. 2015, 52, 437–440. [Google Scholar] [CrossRef]
- Paudel, S.; Sreevatsan, S. Tuberculosis in elephants: Origins and evidence of interspecies transmission. Tuberculosis 2020, 123, 101962. [Google Scholar] [CrossRef]
- Schultz, M. Light microscopic analysis in skeletal paleopathology. In Identification of Pathological Conditions in Human Skeletal Remains; Ortner, D.J., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2003; pp. 73–108. [Google Scholar]
- Schultz, M.; Schmidt-Schultz, T.H. Is it possible to diagnose TB in ancient bone using microscopy? Tuberculosis 2015, 95, 580–586. [Google Scholar] [CrossRef]
- Hackett, C.J. Microscopical focal destruction (tunnels) in exhumed human bones. Med. Sci. Law 1981, 21, 243–265. [Google Scholar] [CrossRef]
- Barbosa, F.H.S.; Porpino, K.O.; Rothschild, B.M.; Cabral, U.G.; Bergqvist, L.P. Arthritic lesions and congenital fusion in foot bones of Panochthus sp. (Xenarthra, Cingulata). An. Acad. Bras. Cienc. 2019, 91, e20160812. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, B.M.; Martin, L.D. Skeletal Impact of Disease; New Mexico Museum of Natural History Press: Albuquerque, NM, USA, 2006; p. 226. [Google Scholar]
- Resnick, D. Diagnosis of Bone and Joint Disorders, 2nd ed.; Saunders: Philadelphia, PA, USA, 2002. [Google Scholar]
- Barbosa, F.H.S.; Porpino, K.O.; De Araújo-Júnior, H.I.; Bergqvist, L.P.; Rothschild, B.M. Articular and vertebral lesions in the Pleistocene sloths (Xenarthra, Folivora) from the Brazilian Intertropical Region. Hist. Biol. 2017, 29, 607–617. [Google Scholar] [CrossRef]
- Rothschild, B.M.; Woods, R.J. Spondyloarthropathy: Erosive arthritis in representative defleshed bones. Amer. J. Phys. Anthropol. 1991, 85, 125–134. [Google Scholar] [CrossRef]
- Mojtahedi, H.; Soleimanifar, N. Spondyloarthropathies and Environmental Stresses. In Ankylosing Spondylitis-Axial Spondyloarthritis Cellular, Molecular and Environmental Factors; Nicknam, M.H., Ed.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 171–182. [Google Scholar]
- Rothschild, B.M.; Rothschild, C. Trans-mammalian pandemic of inflammatory arthritis (Spondyloarthropathy variety): Persistence since the Pleistocene. Paleontol. Soc. Publ. 1996, 8, 330. [Google Scholar] [CrossRef]
- Rothschild, B.M. Osseotypes and spondyloarthropathy exposed. Curr. Rheumatol. Rev. 2005, 1, 57–63. [Google Scholar] [CrossRef]
- Barbosa, F.H.S.; Porpino, K.O.; Fragoso, A.B.L.; Oliveira, E.V. Arthritis in a Glyptodont (Mammalia, Xenarthra, Cingulata). PLoS ONE 2014, 9, e88646. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, B.M.; Wang, X.-M.; Shoshani, J. Spondyloarthropathy in proboscideans. J. Zoo Wildl. Med. 1994, 25, 360–366. [Google Scholar]
- Magerl, F.; Aebi, M.; Gertzbein, S.D.; Harms, J.; Nazarian, S. A comprehensive classification of thoracic and lumbar injuries. Eur. Spine J. 1994, 3, 184–201. [Google Scholar] [CrossRef]
- Tambusso, P.S.; Varela, L.; McDonald, H.G. Fusion of anterior thoracic vertebrae in Pleistocene ground sloths. Hist. Biol. 2018, 32, 244–251. [Google Scholar] [CrossRef]
- Tomassini, R.L.; Montalvo, C.I.; Garrone, M.C.; Domingo, L.; Ferigolo, J.; Cruz, L.E.; Sanz-Pérez, D.; Fernández-Jalvo, Y.; Cerda, I.A. Gregariousness in the giant sloth Lestodon (Xenarthra): Multi-proxy approach of a bonebed from the Last Maximum Glacial of Argentine Pampas. Sci. Rep. 2020, 10, 10955. [Google Scholar] [CrossRef]
- Dias, D.E.M.; Dantas, M.A.T.; Barbosa, F.H.S. Diagnosis of bone diseases in two representatives of the Pleistocene megafauna of Bahia, Brazil. Hist. Biol. 2020, 33, 3224–3227. [Google Scholar] [CrossRef]
- Barbosa, F.H.S.; De Araujo-Junior, H.I. Skeletal pathologies in the giant ground sloth Eremotherium laurillardi (Xenarthra, Folivora): New cases from the Late Pleistocene of Brazil. J. S. Am. Earth Sci. 2021, 110, 10337. [Google Scholar] [CrossRef]
- Reumer, J.W.F.; Ten Broek, C.M.A.; Galis, F. Extraordinary incidence of cervical ribs indicates vulnerable condition in Late Pleistocene mammoths. PeerJ 2014, 2, e318. [Google Scholar] [CrossRef]
- Maschenko, E.N.; Potapova, O.R.; Vershinina, A.; Shapiro, B.; Streletskaya, I.D.; Vasiliev, A.A.; Oblogov, G.E.; Kharlamova, A.S.; Potapov, E.; van der Plicht, J.; et al. The Zhenya Mammoth (Mammuthus primigenius (Blum.)): Taphonomy, geology, age, morphology and ancient DNA of a 48,000 year old frozen mummy from western Taimyr, Russia. Quat. Int. 2017, 445, 104–134. [Google Scholar] [CrossRef]
- Petrova, E.A.; Masutin, V.V.; Zhuykova, I.A. Two incomplete skeletons of woolly mammoth (Mammuthus primigenius) from the late Pleistocene in the Kirov Region, European Russia. Russ. J. Theriol. 2017, 16, 157–175. [Google Scholar] [CrossRef]
- Pruijs, J.E.; Hageman, M.A.; Keessen, W.; van der Meer, R.; van Wieringen, J.C. Variation in Cobb angle measurements in scoliosis. Skelet. Radiol. 1994, 23, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Deacon, P.; Flood, B.M.; Dickson, R.A. Idiopathic scoliosis in three dimensions. A radiographic and morphometric analysis. Bone Jt. J. 1984, 66, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Shpansky, A.V.; Sapunova, L.S.; Pilyukova, A.V. A traumatic case in Mammuthus trogontherii chosaricus Dubrovo (1966). Quat. Int. 2015, 379, 82–88. [Google Scholar] [CrossRef]
- Gray, J. Studies in Mechanics of the Tetrapod Skeleton. J. Exp. Biol. 1944, 20, 88–116. [Google Scholar] [CrossRef]
- Smit, T.H. The use of a quadruped as an in vivo model for the study of the spine–biomechanical considerations. Eur. Spine J. 2002, 11, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Schwab, F.; Patel, A.; Lafage, V.; Farcy, J.P. A porcine model for progressive thoracic scoliosis. Spine 2009, 34.11, 397–404. [Google Scholar] [CrossRef]
- Janssen, M.M.A.; de Wilde, R.F.; Kouwenhoven, J.W.; Castelein, R.M. Experimental animal models in scoliosis research: A review of the literature. Spine J. 2011, 11, 347–358. [Google Scholar] [CrossRef] [PubMed]



| Measurement | d (cm) | t (µs) | V (m/s) | Material | Observations |
|---|---|---|---|---|---|
| M1 | 22.5 | 267 | 843 | Ivory | Right tusk |
| M2 | 24 | 282 | 851 | Ivory | Right tusk |
| M3 | 14.5 | 248 | 585 | Ivory + Mastic | Left stump |
| M4 | 20 | 450 | 444 | Ivory + Mastic | Left stump |
| M5 | 13 | 248 | 524 | Bone | Left premaxilla |
| M6 | 14 | 254 | 551 | Bone | Left premaxilla |
| M7 | 20 | 218 | 917 | Mastic + Iron | Right premaxilla |
| VERTEBRAE | ANGLE 1 | ANGLE 2 | ANGLE 3 |
|---|---|---|---|
| C1 | −16.5° | / | / |
| C2 | −1.7° | / | / |
| C3 | −2° | / | / |
| C7 | −1.7° | 1.5° | / |
| T1 | 10.1° | 8.5° | 11.8° |
| T2 | 6.2° | 7° | 9.3° |
| T4 | 13.5° | / | 9.3° |
| T5 | 9.8° | / | 9.5° |
| T7 | 3.7° | / | 5.8° |
| T8 | 7.4° | / | 11.9° |
| T10 | 8.6° | −0.8° | 1.6° |
| T16 | −0.2° | / | −0.1° |
| T17 | −8.2° | / | −7.1° |
| T18 | −4.8° | −2.4° | / |
| T19 | −2.4° | −5.4° | −4.3° |
| L1 | −8.3° | / | 1.9° |
| L2 | 4.7° | / | / |
| L3 | 3.6° | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Salda, L.; Cuomo, A.; Antonucci, F.; Agostini, S.; Rossi, M.A. Evidence of Chronic Tusk Trauma and Compensatory Scoliosis in Mammuthus meridionalis from Madonna della Strada (Scoppito, L’Aquila, Italy). Quaternary 2025, 8, 46. https://doi.org/10.3390/quat8030046
Della Salda L, Cuomo A, Antonucci F, Agostini S, Rossi MA. Evidence of Chronic Tusk Trauma and Compensatory Scoliosis in Mammuthus meridionalis from Madonna della Strada (Scoppito, L’Aquila, Italy). Quaternary. 2025; 8(3):46. https://doi.org/10.3390/quat8030046
Chicago/Turabian StyleDella Salda, Leonardo, Amedeo Cuomo, Franco Antonucci, Silvano Agostini, and Maria Adelaide Rossi. 2025. "Evidence of Chronic Tusk Trauma and Compensatory Scoliosis in Mammuthus meridionalis from Madonna della Strada (Scoppito, L’Aquila, Italy)" Quaternary 8, no. 3: 46. https://doi.org/10.3390/quat8030046
APA StyleDella Salda, L., Cuomo, A., Antonucci, F., Agostini, S., & Rossi, M. A. (2025). Evidence of Chronic Tusk Trauma and Compensatory Scoliosis in Mammuthus meridionalis from Madonna della Strada (Scoppito, L’Aquila, Italy). Quaternary, 8(3), 46. https://doi.org/10.3390/quat8030046








