Paleoecological Reconstruction Derived from an Age–Depth Model and Mollusc Data, Pécel, Hungary
Abstract
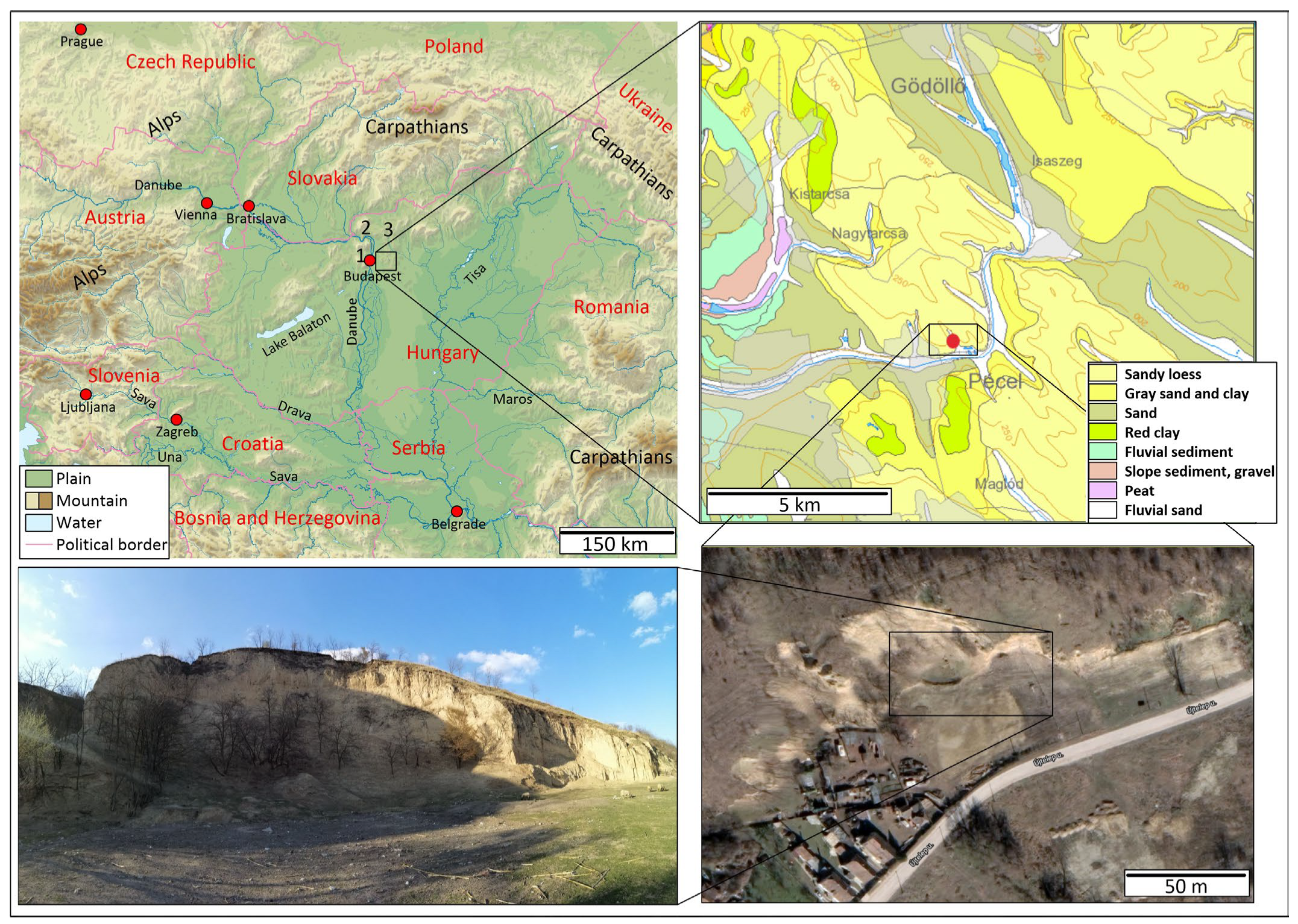
1. Introduction
2. Materials and Methods
2.1. Absolute Dating
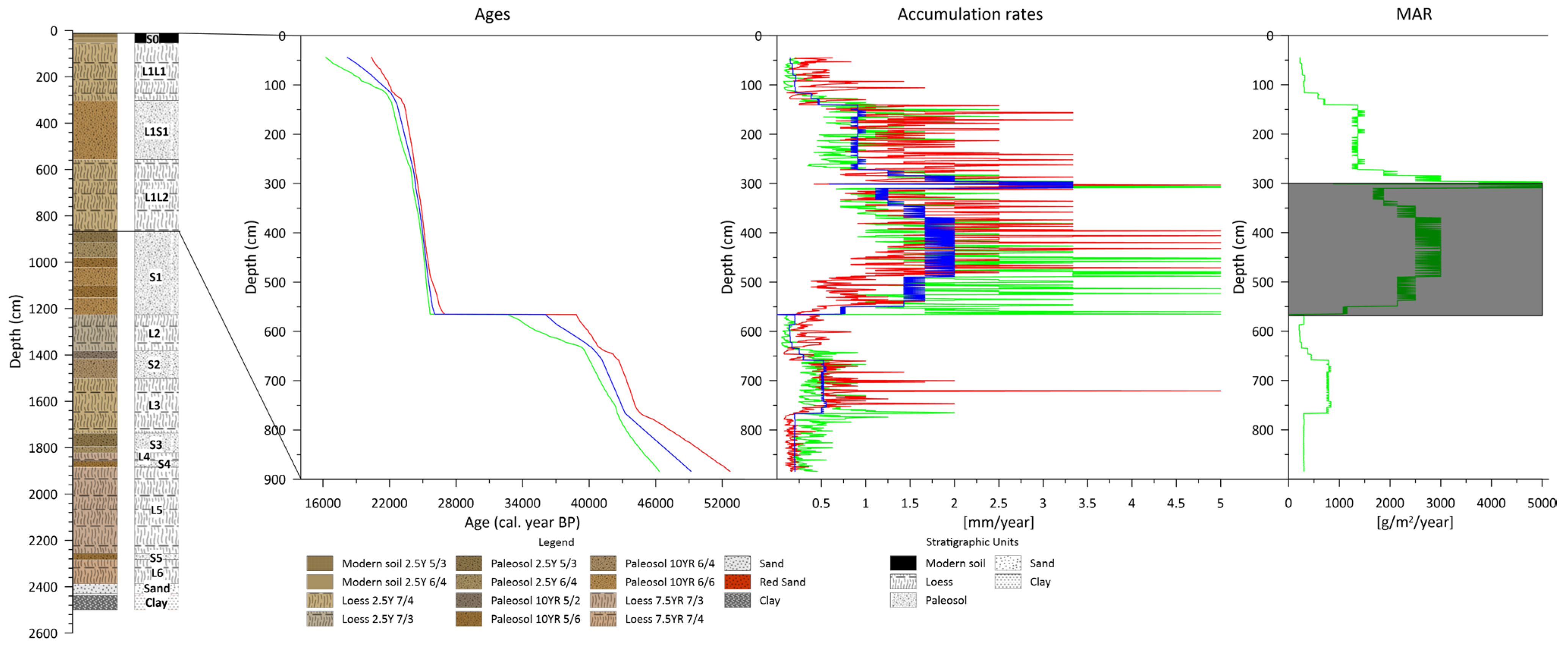
2.2. Age–Depth Model
2.3. Accumulation Rate, Mass Accumulation Rate
2.4. Magnetic Susceptibility Measurement
2.5. Malacological Examination Method
3. Results
3.1. Absolute Dating, AR, MAR
3.2. Magnetic Susceptibility
3.3. Malacological Examinations
3.4. Malaco-Zones
- PCMZ-1 (22.80–21.72 m): The lowest and presumably oldest zone is located in the PL5 loess layer. The gastropod fauna is characterised by an absolute dominance of the mesophilous species (~75%; Figure 5), the assemblage being mainly composed of Vallonia costata shells. In addition, V. pulchella and the warmth-loving Helicopsis striata and Pupilla triplicata represented a more significant proportion of the fauna. Cold-resistant species were only present in an indicative proportion (Pupilla sterrii ~1%), and cold-loving species were absent. The mean abundance of the zone was 41.5, higher than the overall mean abundance, with species richness per sample ranging from one to six.
- PCMZ-2 (21.72–18.00 m): The second malacological zone includes the upper part of the PL5 loess layer, the whole of PS4, PL4 paleosol and loess layers, and the lower part of the PS3 paleosol layer. The zone contains an approximately equal distribution of thermophilous (H. striata, P. triplicata, Chondrula tridens) and wide-tolerant species (V. costata, Pupilla muscorum); cold-resistant and cold-loving species were not found in this zone. The average abundance of the malaco-zone is low, only eight, while the frequency of species varied between one and five.
- PCMZ-3 (18.00–15.72 m): The third zone covers the upper part of the PS3 paleosol layer, the PL3 loess layer, and the smaller lower part of the PS2 paleosol. The upper part of the zone was found to be sterile, while the rest of the zone was characterised by a high distribution of thermophilous species, especially H. striata. In addition to H. striata, P. triplicata and the mesophilous P. muscorum and V. costata were more abundant, and the cold-resistant Succinella oblonga was also present in two samples. Cryophilous species were not present in the zone. The distribution of the richness of species per sample and the mean abundance values are similar to those of PCMZ-1 (1–7 and 41.3, respectively).
- PCMZ-4 (15.72–12.96 m): The zone is located in the upper part of the PS2 paleosol and the lower part of the PL2 loess layer. In the lower part, there is a rather high proportion of mesophilous species (Limacidae, V. pulchella, P. muscorum), and then, moving upwards, of more thermophilous species (H. striata, P. triplicata). In the lower part of the zone, the proportion of Limacidae is more pronounced. In one sample, the cold-loving Columella columella also appears. The average abundance of the zone, thanks also to the 10 sterile samples, is only 8.2, with species numbers ranging from zero to eight.
- PCMZ-5 (12.96–9.48 m): The fifth zone covers the upper part of the PL2 layer and the upper part of the PS1 paleosol. The zone is characterised by low abundance, with an average of only one specimen per sample and species richness ranging from zero to three. Fifteen samples in the zone did not contain any valuable snail shells. Despite the low values, it can be concluded that warmth-loving species were also the most abundant in the zone, with Granaria frumentum being the most abundant species. In addition, the shells of H. striata, P. triplicata and C. tridens were also found. P. muscorum and V. pulchella are subordinately represented in the wide-tolerant species, and shells of Clausilia dubia and Nesovitrea hammonis also appear for the first time in this zone. Among the cold-resistant species, S. oblonga and Discus ruderatus, which also appear here for the first time, are present, with no cryophilous element in the zone.
- PCMZ-6 (9.48–6.00 m; 49,172 (from 8.84 m) −33,235 cal BP): In the next zone, which includes the upper part of the PS1 layer and most of the PL1L2 loess layer, the most abundant species are the widely tolerant ones, with a high proportion of V. costata, P. muscorum and Limacidae fossils. The thermophilous species are represented, to a lesser extent, by the pair H. striata and P. triplicata, and of the cold-resistant species, only S. oblonga is present. The mean abundance is only 23.4, the species number varies from zero to five, and only one sample in the zone was sterile. Based on the age–depth model, the 884–600 cm section of the zone covers the interval 49,172–33,235 cal BP.
- PCMZ-7 (6.00–4.68 m; 33,235–25,359 cal BP): This small (only 132 cm) zone covers only 7876 cal BP ys, and includes the upper part of PL1L1 and the lower part of the PL1S1 paleosol layer. In general, it has the highest average abundance in the section (170.3) and is also the most species-rich (5–16 species) zone on average. The malacofauna is dominated by species with wide tolerances (75–80%), with the most significant occurrence of P. muscorum and V. costata, but other species of wide tolerance are also present in this zone, which have not been found so far (Clausilia pumila, Euconulus fulvus) or only sporadically (Vallonia pulchella, Clausilia dubia, Nesovitrea hammonis, Limacidae). The warmth-loving snail fauna, similar to PCMZ-1, comprised 15–20% of the snail community, with Cochlicopa lubricella and Fruticicola fruticum present along with H. striata and P. triplicata. The proportion and number of species of cold-resistant (Trochulus hispidus, S. oblonga and Pupilla sterrii) and cold-loving (Columella columella) snails are also noteworthy.
- PCMZ-8 (4.68–2.40 m; 25,359–23,796 cal BP): The zone covers the upper part of the PL1S1 paleosol layer and the lower part of the PL1L1 loess layer, covering about 1563 cal BP years according to the age–depth model. The average abundance of the zone is about twice the average for the whole section, 64.2, with species richness ranging from 2 to 10. The malacofauna of the zone is characterised by a significant thermophilous dominance, ranging from 50 to 90%. The highest proportions of H. striata and P. triplicata were found, but also G. frumentum, C. tridens and C. lubricella are remarkable in some samples. Mesophilous species are subordinate in the zone with a proportion of 20–50%, with the most significant presence of P. muscorum. Also remarkable are the proportions of Vallonia pulchella, C. dubia and Limacidae, and Punctum pygmaeum is also present in this zone. V. costata shells were not found in this zone. Among the cold-resistant species, Vestia turgida is the only one to occur here, and the cold-loving species are represented by two species (C. columella and Vertigo pygmaea, which appear here), but in low proportions.
- PCMZ-9 (2.40–1.20 m; 23,796–22,235 cal BP): The zone, which is only 120 cm thick and is exclusively assigned to the PL1L1 loess layer, covers a period of 1561 cal BP years according to the age–depth model. The abundance per sample here is only 13.3 specimens, and the species number varies from one to five. This zone is also characterised by the dominance of warmth-loving species (30–100%), with only H. striata and P. muscorum present, the former in several samples with proportions of around 50% and even 100%. Mesophilous species are represented in lower proportions but with more species. In addition to P. muscorum, V. costata reappears, with a predominant proportion of this pair. In addition, C. dubia, E. fulvus and Limacidae shells were also found in the zone. The zone contained neither cold-tolerant nor cold-loving species.
- PCMZ-10 (1.20–0 m; 22,235–18,090 (from 44 cm) cal BP): The uppermost and youngest malaco-zone of the section covers the upper third of the PL1L1 loess layer and the PS0 modern soil layer. The abundance per sample has increased slightly (25.4), as has the species richness (2–12) compared to PCMZ-2. As we move upwards in the zone, we observe gradually increasing warmth-loving (from 40% to 80%) and gradually decreasing mesophilous (from 75% to 30%) proportions, along with more significant cold-resistant proportions (20–25%). Among the thermophilous species, the proportion of H. striata has decreased; the most dominant thermophilous species are P. triplicata and G. frumentum (mainly in the upper part of the zone), and the presence of F. fruticum is also noticeable in the zone. Among the wide-tolerant species, in addition to V. costata and P. muscorum, Vitrea crystallina and Vitrina pellucida are also present in the upper part of the zone (PS0). Three species, P. sterrii, S. oblonga and T. hispidus, make up the larger proportion of cold-tolerant species. No cryophilous species were found in this zone.
3.5. Diversity and PCA
4. Discussion
4.1. Absolute Dating, AR, MAR
4.2. Malacology
- The snail fauna of PCMZ-1 (22.80–21.72 m) is predominantly composed of the mesophilous species Vallonia costata and Vallonia pulchella shells. V. costata tolerates moisture, V. pulchella requires moisture, so the subordinate proportion of drought-tolerant species and the absence of shade-loving species suggest that this zone represents an open, higher humidity, temperate climate. This is confirmed by the climate and humidity data as well. The zone covers the middle to lower part of the PL5 loess layer, and the correlation suggests an age of more than 400 kys, which is consistent with MIS 12 according to Marine Isotope Stages [64,65].
- The changes in the malacofauna of PCMZ-2 (21.72–18.00 m) (high proportion of thermophilous species) indicate a warming period. In addition, the presence of a high proportion of Helicopsis striata, Pupilla triplicata and Chondrula tridens, which prefer dry, open vegetation, and of the mesophilous V. costata and Pupilla muscorum, which prefer open vegetation, indicates a dry steppe environment, which, based on the remains of the shade-loving Limacidae, which appear at two levels, may have been interspersed periodically with small tree communities. This is also supported by the graphs indicating higher temperatures and lower humidity. The malaco-zone covers the upper part of PL5, the PS4, PL4 layers completely, and the PS3 paleosol layer almost completely, so the correlation suggests an age of approximately 400–280 kys. The period of formation is thus estimated to be between MIS 12-9 [64,65].
- The PCMZ-3 zone is defined between 18.00 and 15.72 m. Based on the additional high proportion of thermophilous species, this zone also marks a warmer period, dominated by species preferring open vegetation (H. striata, P. triplicata, P. muscorum). C. tridens and V. costata are not significant at this level. Hygrophilous and cold-tolerant Succinella oblonga (17.04–16.80 m, in negligible proportions) appeared at one level, perhaps confirming a minor cooling period. The zone was characterised by a warm, dry period, based on the calculated temperature and humidity curves, apart from the S. oblonga level. The zone includes the upper part of the PS3 paleosol, the PL3 loess layer, and the lower half of the PS2 paleosol layer, so that correlations suggest an age of the zone between 280 and 220 kys, which corresponds to MIS sections 8–7e [64,65].
- The PCMZ-4 zone represents the section between 15.72 and 12.96 m. The part of this zone in the paleosol PS2 is dominated by the shade-loving, wide-tolerant Limacidae species. Further along the zone, the proportion of Limacidae species decreases, with the wide-tolerant V. pulchella and P. muscorum and the thermophilous H. striata and P. triplicata appearing in smaller proportions. Entering the PL2 loess layer, the proportion of thermophilous and mesophilous species is approximately equal. In this layer (14.28–14.16 m), the cold-loving Columella columella, which prefers open vegetation, is present. Its presence and low proportion may indicate a minor cooling period. In the upper part of the zone, the high proportion of thermophilous and open vegetation-preferring species (especially H. striata) indicates a dry, open vegetation environment. The age of the zone can only be estimated by correlation, which ranges between 220 and 150 kys, covering the MIS 7 and part of the MIS 6 period [64,65].
- The short section of the PCMZ-5 malaco-zone (12.96–9.48 m) on PL2 loess layer is characterised by warmth-loving dry, open vegetation preferring Granaria frumentum, H. striata, P. triplicata and cold-resistant S. oblonga (only between 12.96 and 12.84 m) preferring humid, open vegetation. The distribution of the faunal composition indicates periodic moister phases, which are also reflected in the humidity graph. The shade-loving species Clausilia dubia and Nesovitrea hammonis, which are wide-tolerant, and Discus ruderatus, a cold-resistant species that prefers coniferous forests, are present in the PS1 layer of the zone. Their distribution in the zone (as well as in PS1) reveals three forest expansion events between 12.72 and 12.48 m C. dubia, between 1092 and 1056 cm N. hammonis and D. ruderatus, where coniferous trees were probably present, and between 9.96 and 9.72 m again marked by C. dubia. At the latter two levels, species preferring open vegetation appear in addition to shade-loving species, suggesting a mosaic character of the landscape, i.e., the area may have developed into a steppe-like environment with trees. Temperatures estimated in the zone, due to the relatively high number of sterile samples, were quite variable, typically lower in the forest cover levels. The age of the zone can still be correlated with the age of the PS1 paleosol falling within MIS 5, i.e., between 127 and 73 kys [64,65].
- The PCMZ-6 malaco-zone (9.48–6.00 m) already has absolute age data taken from the calculated age–depth model (from 8.84 m). The snail fauna of this zone is similar to PCMZ-2, but with a higher species richness and a higher diversity, especially of the cold-resistant and cryophilous species. Among the thermophilous species, the presence of open vegetation preferring species (H. striata, P. triplicata, C. tridens; G. frumentum has disappeared from the zone) dominates, but the proportion of mesophilous species is higher. In contrast to the predominance of P. muscorum, which prefers an open, dry environment, and V. costata, which prefers open vegetation, in the middle part of the zone (between 8.16 and 6.24 m in PL1L2 layer, ca. 45,714–38,579 cal BP), moisture-loving species such as Vallonia pulchella and V. enniensis, Euconulus fulvus and Limacidae species, as well as the cold-resistant S. oblonga (between 816 and 744 cm; 45,741–42,745 cal BP) occur, indicating a lower cooling period. The age of the zone is 49,172–33,235 cal BP, which falls within the MIS 3 interglacial period [64,65]. The moister period falls within the Greenland Stadial (GS) 9–12 and Greenland Interstadial (GI) 9–12 periods [66], i.e., covering the period of rapid alternation of stadials and interstadials.
- The PCMZ-7 zone (6.00–4.68 m) ranges from 33,235 to 25,359 cal BP, covering the MIS 2 stage [64,65], including the GS 6-3 and GI 5.2-3 periods [66]. The total length of the stadials is much longer than the interstadials, indicating a colder period on average. The evolution of the malacofauna is also consistent with this. In addition to a significant decrease in the proportion of thermophilous species, there is an increase in the proportion of cold-loving species, in this case, Columella columella, which prefers cold, moist grassland. However, the proportion of C. columella is rather low (1–3%), but several cold-tolerant species also appear in the zone: the largest proportion being the shade-loving Trochulus hispidus, which prefers a more humid environment, and the moisture-loving S. oblonga. There is also a relatively low dominance of Pupilla sterrii, which prefers open, dry vegetation, and Discus ruderatus, which prefers coniferous forests. The absolute predominance of the mesophilous species is represented by P. muscorum and V. costata, but several shade-loving (Clausilia dubia, C. pumila, Nesovitrea hammonis, Euconulus fulvus, Limacidae) and hygrophilous (Vallonia enniensis, V. pulchella) species also appear. According to the composition of the snail fauna, the study area alternated between cool to moderately dry steppe and more humid forest areas, providing a habitat for a wide range of snail species. The average abundance per sample is the highest in the whole section, and diversity and species richness are also at their maximum here. Lower average temperatures and higher humidity resulted in a much more diverse malacofauna.
- The PCMZ-8 zone was delineated between 4.68 and 2.40 m in the section, between 25,359 and 23,796 cal BP, falling entirely within the GS 3 stadial phase of the MIS 2 period [64,65,66]. The malacofauna of the Pécel loess–paleosol section shows a warmer and drier period during the stadial phase compared to the previous zone. The proportion of warmth-loving species was increased, with G. frumentum, C. tridens and Cochlicopa lubricella present in addition to the prominent proportions of H. striata and P. triplicata. All the thermophilous species present here prefer open vegetation, so the principal environment of the zone may have been dry grassland. Among the wide-tolerant species, P. muscorum prefers this environment, while the shade-loving species Euconulus fulvus, Punctum pygmaeum, and Clausilia dubia are also present in the lower part of the zone (468–396 cm; 25,359–24,965 cal BP). In addition to the cryophilous C. columella, Vertigo pygmaea, which also prefers open vegetation, was more abundant in the zone. The proportion of arboreal vegetation may have been higher in the first phase of the zone, but it gradually declined over time.
- In PCMZ-9 (2.40–1.20 m; 23,796–22,235 cal BP), cold-resistant and cryophilous species disappear from the zone, indicating a rather warm and dry period, except for the earliest phase. The zone covers the GI 2.2 and 2.1 interstadials [66], but it cannot trigger such warming. Among the warmth-loving species, H. striata is extremely dominant, with P. triplicata also found subordinate. Among the wide-tolerant species, P. triplicata and V. costata have the highest proportion, and Limacidae, C. dubia and E. fulvus shells were also sporadically found. These species are evidence of the occasional occurrence of small groups of trees in the dry steppe area during this period.
- PCMZ-10 (1.20–0 m) comprises the youngest malacofauna, from 22,235 cal BP, the GS 2.1 stadial period [66]. The latest calculated age date is 18,090 cal BP (44 cm). The snail fauna in this zone was also dominated by species preferring warm, dry, open vegetation (P. triplicata, G. frumentum, H. striata) and by the wide-tolerant P. muscorum and V. costata with similar ecological requirements. In the youngest part of the zone, the new species Vitrea crystallina, which prefers humid forests with wide tolerance, appears together with the cold-resistant Pupilla sterrii, Succinella oblonga and Trochulus hispidus. The co-occurrence of these species indicates a decrease in temperature and an increase in humidity, and thus the advance of arboreal vegetation.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pye, K. The nature, origin and accumulation of loess. Quat. Sci. Rev. 1995, 14, 653–667. [Google Scholar] [CrossRef]
- Krolopp, E.; Sümegi, P. Reconstruction of palaeoecological conditions during the deposition of Würm loess formations of Hungary, based on molluscs. Földtani Közlöny 1995, 125, 125–148. (In Hungarian) [Google Scholar]
- Gallet, S.; Jahn, B.; Torii, M. Geochemical characterization of the Luochuan loess-paleosol sequence, China, and paleoclimatic implications. Chem. Geol. 1996, 133, 67–88. [Google Scholar] [CrossRef]
- Banak, A.; Mandic, O.; Kovačić, M.; Pavelić, D. Late Pleistocene climate history of the Baranja loess plateau—Evidence from the Zmajevac loess-paleosol section (northeastern Croatia). Geol. Croat. 2012, 65, 411–422. [Google Scholar] [CrossRef]
- Marković, S.B.; Hambach, U.; Stevens, T.; Kukla, G.J.; Heller, F.; McCoy, W.D.; Oches, E.A.; Buggle, B.; Zöller, L. The last million years recorded at the Stari Slankamen (Northern Serbia) loess-palaeosol sequence: Revised chronostratigraphy and long-term environmental trends. Quat. Sci. Rev. 2011, 30, 1142–1154. [Google Scholar] [CrossRef]
- Marković, S.B.; Stevens, T.; Kukla, G.J.; Hambach, U.; Fitzsimmons, K.E.; Gibbard, P.; Buggle, B.; Zech, M.; Guo, Z.; Hao, Q.; et al. Danube loess stratigraphy—Towards a pan-European loess stratigraphic model. Earth-Sci. Rev. 2015, 148, 228–258. [Google Scholar] [CrossRef]
- Marković, S.B.; Stevens, T.; Mason, J.; Vandenberghe, J.; Yang, S.; Veres, D.; Újvári, G.; Timar-Gabor, A.; Guo, Z.; Hao, Q.; et al. Loess correlations—Between myth and reality. Palaeogr. Palaeoclimatol. Palaeoecol. 2018, 509, 4–23. [Google Scholar] [CrossRef]
- Újvári, G.; Kovács, J.; Varga, G.; Raucsik, B.; Marković, S.B. Dust flux estimates for the Last Glacial Period in East Central Europe based on terrestrial records of loess deposits: A review. Quat. Sci. Rev. 2010, 29, 3157–3166. [Google Scholar] [CrossRef]
- Újvári, G.; Varga, A.; Raucsik, B.; Kovács, J. The Paks loess-paleosol sequence: A record of chemical weathering and provenance for the last 800 ka in the mid-Carpathian Basin. Quat. Int. 2014, 319, 22–37. [Google Scholar] [CrossRef]
- Újvári, G.; Molnár, M.; Páll-Gergely, B. Charcoal and mollusc shell 14C-dating of the Dunaszekcső loess record, Hungary. Quat. Geochronol. 2016, 35, 43–53. [Google Scholar] [CrossRef]
- Makó, L.; Molnár, D.; Runa, B.; Bozsó, G.; Cseh, P.; Nagy, B.; Sümegi, P. Selected Grain-Size and Geochemical Analyses of the Loess-Paleosol Sequence of Pécel (Northern Hungary): An Attempt to Determine Sediment Accumulation Conditions and the Source Area Location. Quaternary 2021, 4, 17. [Google Scholar] [CrossRef]
- Makó, L.; Molnár, D.; Cseh, P.; Nagy, B.; Sümegi, P. Development history of the loess-paleosol profiles of Pécel, Kisdorog and Bonyhádvarasd, Hungary. Quaternary 2023, 6, 38. [Google Scholar] [CrossRef]
- Sümegi, P.; Náfrádi, K.; Molnár, D.; Sávai, S. Results of paleoecological studies in the loess region of Szeged-Öthalom (SE Hungary). Quat. Int. 2015, 372, 66–78. [Google Scholar] [CrossRef]
- Újvári, G.; Molnár, M.; Novothny, Á.; Páll-Gergely, B.; Kovács, J.; Várhegyi, A. AMS 14C and OSL/IRSL dating of the Dunaszekcső loess sequence (Hungary): Chronology for 20 to 150 ka and implications for establishing reliable age-depth models for the last 40ka. Quat. Sci. Rev. 2014, 106, 140–154. [Google Scholar] [CrossRef]
- Sümegi, P.; Gulyás, S.; Molnár, D.; Szilágyi, G.; Sümegi, B.P.; Törőcsik, T.; Molnár, M. 14C Dated Chronology of the Thickest and Best Resolved Loess/Paleosol Record of the LGM from SE Hungary Based on Comparing Precision and Accuracy of Age-Depth Models. Radiocarbon 2020, 62, 403–417. [Google Scholar] [CrossRef]
- Sümegi, P.; Gulyás, S.; Molnár, D.; Bozsó, G.; Fekete, I.; Makó, L.; Cseh, P.; Molnár, M.; Sümegi, B.P.; Almond, P.; et al. New chronology and extended palaeoenvironmental data to the 1975 loess profile of Madaras brickyard, South Hungary. J. Quat. Sci. 2021, 11, 1364–1381. [Google Scholar] [CrossRef]
- Sümegi, P.; Molnár, D.; Náfrádi, K.; Makó, L.; Cseh, P.; Törőcsik, T.; Molnár, M.; Zhou, L. Vegetation and land snail-based reconstruction of the palaeocological changes in the forest steppe eco-region of the Carpathian Basin during last glacial warming. Glob. Ecol. Conserv. 2022, 33, e01976. [Google Scholar] [CrossRef]
- Dövényi, Z. Cserhát-vidék in Magyarország Kistájainak Katasztere, 2nd ed.; MTA Földrajztudományi Kutatóintézet: Budapest, Hungary, 2010; pp. 705–708. (In Hungarian) [Google Scholar]
- Varga, A.; Újvári, G.; Raucsik, B. Tectonic versus climatic control on the evolution of a loess-paleosol sequence at Beremend, Haungary: An integrated approach based on paleoecological, clay mineralogical, and geochemical data. Quat. Int. 2011, 240, 71–86. [Google Scholar] [CrossRef]
- Sümegi, P.; Gulyás, S.; Persaits, G.; Páll, D.G.; Molnár, D. The loess-paleosol sequence of Basaharc (Hungary) revisited: Mollusc-based paleoecological results for the Middle and Upper Pleistocene. Quat. Int. 2011, 240, 181–192. [Google Scholar] [CrossRef]
- Sümegi, P.; Marković, S.B.; Molnár, D.; Sávai, S.; Szelepcsényi, Z.; Novák, Z. Črvenka loess-paleosol sequence revisited: Local and regional Quaternary biogeographical inferences of the southern Carpathian Basin. Open Geosci. 2016, 8, 309–404. [Google Scholar] [CrossRef]
- Bösken, J.; Obreht, I.; Zeeden, C.; Klasen, N.; Hambach, U.; Sümegi, P.; Lehmkuhl, F. High-resolution paleclimatic proxy data from the MIS3/2 transition recorded in northeastern Hungarian loess. Quat. Int. 2019, 502, 95–107. [Google Scholar] [CrossRef]
- Makó, L.; Molnár, D.; Cseh, P.; Sümegi, P. MAR comparisons between different chronometric methods for two profiles in the Bodrogkeresztúr area. Stud. Quat. 2021, 38, 67–73. [Google Scholar] [CrossRef]
- Krolopp, E. Biostratigraphic subdivision of Hungarian Pleistocene formation according to their mollusc fauna. Acta Geol. Hung. 1983, 26, 62–89. [Google Scholar]
- Molnár, M.; Janovics, R.; Major, I.; Orsovszki, J.; Gönczi, R.; Veres, M.; Leonard, A.G.; Castle, S.M.; Lange, T.E.; Wacker, L.; et al. Status report of the new AMS C-14 preparation lab of the Hertelendi Laboratory of Environmental Studies, Debrecen. Hungary. Radiocarb. 2013, 55, 665–676. [Google Scholar] [CrossRef]
- Molnár, M.; Rinyu, L.; Veres, M.; Seiler, M.; Wacker, L.; Synal, H.A. EnvironMICADAS: A mini 14C AMS with enhanced Gas Ion Source Interface in the Hertelendi Laboratory of Environmental Studies (HEKAL), Hungary. Radiocarbon 2013, 55, 338–344. [Google Scholar] [CrossRef]
- Reimer, P.J.; Austin, W.E.; Bard, E.; Bayliss, A.; Blackwell, P.G.; Ramsey, C.B.; Butzin, M.; Cheng, H.; Edwards, R.L.; Friedrich, M.; et al. The IntCal20 Northern Hemisphere Radiocarbon Age Calibration Curve (0–55 cal kBP). Radiocarbon 2020, 62, 725–757. [Google Scholar] [CrossRef]
- Stuiver, M.; Reimer, P.J. CALIB rev. 8. Radiocarbon 1993, 35, 215–230. [Google Scholar] [CrossRef]
- Blaauw, M.; Christen, A.J. Flexible paleoclimate age-depth models using an autoregressive gamma process. Bayesian Anal. 2011, 6, 457–474. [Google Scholar] [CrossRef]
- Kohfeld, K.E.; Harrison, S.P. DIRTMAP: The geological record of dust. Earth-Sci. Rev. 2001, 54, 81–114. [Google Scholar] [CrossRef]
- Zhou, L.P.; Oldfield, F.; Wintle, A.G.; Robinson, S.G.; Wang, J.T. Partly pedogenic origin of magnetic variations in Chinese loess. Nature 1990, 346, 737–739. [Google Scholar] [CrossRef]
- An, Z.; Kukla, G.J.; Porter, S.C.; Xiao, J. Magnetic Susceptibility Evidence of Monsoon Variation on the Loess Plateau of Central China during the last 130,000 Years. Quat. Res. 1991, 36, 29–36. [Google Scholar] [CrossRef]
- Rousseau, D.D.; Kukla, G. Late Pleistocene climate record in the Eustis loess section, Nebraska, based on land snail assemblages and magnetic susceptibility. Quat. Res. 1994, 42, 176–187. [Google Scholar] [CrossRef]
- Dearing, J.A.; Hay, K.L.; Baban, S.M.J.; Huddleston, A.S.; Wellington, E.M.H.; Loveland, P.J. Magnetic susceptibility of soil: An evaluation of conflicting theories using a national data set. Geophys. J. Int. 1996, 127, 728–734. [Google Scholar] [CrossRef]
- Sun, J.; Liu, T. Multiple origins and interpretations of the magnetic susceptibility signal in Chinese wind-blown sediments. Earth Planet. Sci. Lett. 2000, 180, 287–296. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, Q.; Jackson, M.J. Paleoenvironmental significance of the magnetic fabrics in Chinese loess-paleosols since the last interglacial (<130 ka). Earth Planet. Sci. Lett. 2004, 221, 55–69. [Google Scholar]
- Hlavatskyi, D.; Bakhmutov, V. Early-Middle Pleistocene Magnetostratigraphic and Rock Magnetic Records of the Dolynske Section (Lower Danube, Ukraine) and Their Applications to the Correlation of Loess-Palaeosol Sequences in Eastern and South-Eastern Europe. Quaternary 2021, 4, 43. [Google Scholar] [CrossRef]
- Bradák, B.; Seto, Y.; Stevens, T.; Újvári, G.; Fehér, K.; Költringer, C. Magnetic susceptibility in the European Loess Belt: New and existing models of magnetic enhancement in loess. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 569, 110329. [Google Scholar] [CrossRef]
- Sümegi, P. Az ÉK-Magyarországi Löszterületek Összehasonlító Őskörnyezeti Rekonstrukciója és Rétegtani Értékelése. Master’s Thesis, Kossuth Lajos Tudományegyetem, Debrecen, Hungary, 1996; 120p. (In Hungarian). [Google Scholar]
- Sümegi, P. Loess and Upper Paleolithic Environment in Hungary; Aurea Kiadó: Nagykovácsi, Hungary, 2005; 312p. [Google Scholar]
- Sümegi, P.A. Hajdúság Felső-Pleisztocén Fejlődéstörténete Finomrétegtani (Üledékföldtani, Őslénytani, Geokémiai) Vizsgálatok Alapján. Ph.D. Thesis, Kossuth Lajos Tudományegyetem, Debrecen, Hungary, 1989; 96p. (In Hungarian). [Google Scholar]
- Krolopp, E.; Sümegi, P. A Magyarországi Löszök Képződésének Paleoökológiai Rekonstrukciója Mollusca Fauna Alapján. In Fáciesanalitikai, Paleobiogeokémiai és Paleoökológiai Kutatások; Szöőr, G., Ed.; MTA Debreceni Akadémiai Bizottság: Debrecen, Hungary, 1992; pp. 247–263. (In Hungarian) [Google Scholar]
- Krolopp, E.; Sümegi, P. Palaeoecological Reconstruction of the Late Pleistocene, Based on Loess Malacofauna in Hungary. GeoJournal 1995, 36, 213–222. [Google Scholar] [CrossRef]
- Ložek, V. Quartarmollusken der Tschechoslowakei; Rozpravy Ústredniho Ústavu Geologického: Praha, Czech Republic, 1964; Volume 31, 374p. (In German) [Google Scholar]
- Antoine, P.; Rousseau, D.D.; Zöller, L.; Lang, A.; Munaut, A.V.; Hatté, C.; Fontugne, M. High-resolution record of the last Interglacial–glacial cycle in the Nussloch loess–palaeosol sequences, Upper Rhine Area, Germany. Quat. Int. 2001, 76–77, 211–229. [Google Scholar] [CrossRef]
- Alexandrowicz, W.P.; Dmytruk, R. Molluscs in Eemian-Vistulian deposits of the Kolodiiv section, Ukraine (East Carpathian Foreland) and their palaeoecological interpretation. Geol. Q. 2007, 51, 173–178. [Google Scholar]
- Rousseau, D.D. Biogeography of the Pleistocene pleniglacial malacofaunas in Europe. Stratigraphic and climatic implications. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1990, 80, 7–23. [Google Scholar] [CrossRef]
- Rousseau, D.D.; Puisségur, J.J. Climatic interpretation of terrestrial malacofaunas of the last interglacial in southeastern France. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1999, 151, 321–336. [Google Scholar] [CrossRef]
- Moine, O.; Rousseau, D.D.; Antoine, P. The impact of Dansgaard–Oeschger cycles on the loessic environment and malacofauna of Nussloch (Germany) during the Upper Weichselian. Quat. Res. 2008, 70, 91–104. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Springer: Dordrecht, The Netherlands, 1988; 179p. [Google Scholar]
- Southwood, T.R.E.; Henderson, P.A. Ecological Methods; Blackwell Science: Hoboken, NJ, USA, 2000; 575p. [Google Scholar]
- Rousseau, D.D. Statistical analyses of loess molluscs for paleoecological reconstructions. Quat. Int. 1990, 7, 81–89. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics software package for education and data analysis. Paleontol. Electr. 2001, 4, 1–9. [Google Scholar]
- Kukla, G. Loess stratigraphy in central China. Quat. Sci. Rev. 1987, 6, 191–207, 209–219. [Google Scholar] [CrossRef]
- Harnois, L. The CIW index: A new chemical index of weathering. Sediment. Geol. 1988, 55, 319–322. [Google Scholar] [CrossRef]
- Kukla, G.; An, Z.S.; Melice, J.L.; Gavin, J.; Xiao, J.L. Magnetic susceptibility record of Chinese Loess. Trans. R. Soc. Edinb. Earth Sci. 1990, 81, 263–288. [Google Scholar] [CrossRef]
- Ding, Z.L.; Derbyshire, E.; Yang, S.L.; Sun, J.M.; Liu, T.S. Stepwise expansion of desert environment across northern China in the past 3.5 Ma and implications for monsoon evolution. Earth Planet. Sci. Lett. 2005, 237, 45–55. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Liu, L.; Ji, J.; Balsam, W.; Sun, Y.; Lu, H. Zr/Rb ratio in the Chinese loess sequence and its implication for changes in the East Asian winter monsoon strength. Geochem. Cosmochim. Acta 2006, 70, 1471–1482. [Google Scholar] [CrossRef]
- Liang, L.; Sun, Y.; Beets, C.J.; Prins, M.A.; Wu, F.; Vandenberghe, J. Impact of grain size sorting and chemical weathering on the geochemistry of Jingyuan loess in the northwestern Chinese Loess Plateau. J. Asian Earth Sci. 2013, 69, 177–184. [Google Scholar] [CrossRef]
- Hupuczi, J.; Sümegi, P. The Late Pleistocene paleoenvironment and paleoclimate of the Madaras section (South Hungary), based on preliminary records from mollusks. Cent. Eur. J. Geosci. 2010, 2, 64–70. [Google Scholar] [CrossRef]
- Molnár, D.; Sümegi, P.; Fekete, I.; Makó, L.; Sümegi, B.P. Radiocarbon dated malacological records of two Late Pleistocene loess-paleosol sequences from SW-Hungary: Paleoecological inferences. Quat. Int. 2019, 504, 108–117. [Google Scholar] [CrossRef]
- Molnár, D.; Makó, L.; Cseh, P.; Sümegi, P.; Fekete, I.; Galović, L. Middle and Late Pleistocene loess-paleosol archives in East Croatia: Multi-proxy paleoecological studies on Zmajevac and Šarengrad II sequences. Stud. Quat. 2021, 38, 3–17. [Google Scholar]
- Molnár, D.; Sümegi, P.; Makó, L.; Cseh, P.; Zeeden, C.; Nett, J.; Lehmkuhl, F.; Törőcsik, T.; Sümegi, B.P. Palaeoecological background of the Upper Palaeolithic site of Ságvár, Hungary: Radiocarbon-dated malacological and sedimentological studies on the Late Pleistocene environment. J. Quat. Sci. 2021, 36, 1353–1363. [Google Scholar] [CrossRef]
- Bond, G.; Broecker, W.; Johnsen, S.; McManus, J.; Labeyrie, L.; Jouzel, J.; Bonani, G. Correlations between climate records from North Atlantic sediments and Greenland ice. Nature 1993, 365, 143–147. [Google Scholar] [CrossRef]
- Björck, S.; Walker, M.J.C.; Cwynar, L.C.; Johnsen, S.; Knudsen, K.L.; Lowe, J.J.; Wolfharth, B.; INTIMATE Members. An event statigraphy for the Last Termination in the North Atlantic region based on the Greenland Ice-core record: A proposal by the INTIMATE group. J. Quat. Sci. 1998, 13, 283–292. [Google Scholar] [CrossRef]
- Rasmussen, S.O.; Bigler, M.; Blockley, S.P.; Blunier, T.; Buchardt, S.L.; Clausen, H.B.; Gkinis, V. A stratigraphic framework for abrupt climatic changes during the Last Glacial period based on three synchronized Greenland ice-core records: Refining and extending the INTIMATE event stratigraphy. Quat. Sci. Rev. 2014, 106, 14–28. [Google Scholar] [CrossRef]




| Lab Code | Depth (cm) | Material | uncal. BP | σ | cal. BP | σ | Probability Distribution |
|---|---|---|---|---|---|---|---|
| DeA-34288 | 12–24 | G. frumentum | 1381 | 21 | 1297 | 16 | 92% |
| DeA-34278 | 108–120 | T. hispidus | 18,105 | 58 | 22,062 | 159 | 100% |
| DeA-34289 | 132–144 | H. striata | 19,698 | 63 | 23,781 | 74 | 65% |
| DeA-34279 | 264–276 | T. hispidus | 20,135 | 68 | 24,119 | 235 | 99% |
| DeA-34290 | 276–288 | H. striata | 20,361 | 69 | 24,442 | 240 | 100% |
| DeA-34280 | 300–312 | T. hispidus | 20,004 | 67 | 24,022 | 184 | 100% |
| DeA-34291 | 336–348 | H. striata | 20,556 | 66 | 24,773 | 261 | 99% |
| DeA-34281 | 360–372 | T. hispidus | 20,634 | 71 | 24,862 | 244 | 100% |
| DeA-34282 | 408–420 | T. hispidus | 20,775 | 72 | 25,043 | 220 | 100% |
| DeA-34292 | 432–444 | H. striata | 20,902 | 70 | 25,183 | 188 | 96% |
| DeA-34283 | 468–480 | T. hispidus | 21,113 | 70 | 25,546 | 124 | 100% |
| DeA-34293 | 480–492 | T. hispidus | 20,541 | 67 | 24,751 | 256 | 97% |
| DeA-34284 | 516–528 | T. hispidus | 34,640 | 206 | 39,937 | 593 | 100% |
| DeA-34294 | 540–552 | T. hispidus | 21,266 | 73 | 25,568 | 245 | 100% |
| DeA-34285 | 552–564 | T. hispidus | 21,008 | 73 | 25,385 | 232 | 100% |
| DeA-34295 | 576–588 | T. hispidus | 20,141 | 66 | 24,126 | 233 | 98% |
| DeA-34296 | 624–636 | H. striata | 35,214 | 214 | 40,349 | 519 | 100% |
| DeA-34286 | 648–660 | T. hispidus | 38,904 | 325 | 42,607 | 277 | 100% |
| DeA-34287 | 744–756 | T. hispidus | 39,385 | 334 | 42,803 | 343 | 100% |
| DeA-34297 | 756–768 | H. striata | 38,639 | 290 | 42,508 | 248 | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makó, L.; Cseh, P.; Nagy, B.; Sümegi, P.; Molnár, D. Paleoecological Reconstruction Derived from an Age–Depth Model and Mollusc Data, Pécel, Hungary. Quaternary 2025, 8, 37. https://doi.org/10.3390/quat8030037
Makó L, Cseh P, Nagy B, Sümegi P, Molnár D. Paleoecological Reconstruction Derived from an Age–Depth Model and Mollusc Data, Pécel, Hungary. Quaternary. 2025; 8(3):37. https://doi.org/10.3390/quat8030037
Chicago/Turabian StyleMakó, László, Péter Cseh, Balázs Nagy, Pál Sümegi, and Dávid Molnár. 2025. "Paleoecological Reconstruction Derived from an Age–Depth Model and Mollusc Data, Pécel, Hungary" Quaternary 8, no. 3: 37. https://doi.org/10.3390/quat8030037
APA StyleMakó, L., Cseh, P., Nagy, B., Sümegi, P., & Molnár, D. (2025). Paleoecological Reconstruction Derived from an Age–Depth Model and Mollusc Data, Pécel, Hungary. Quaternary, 8(3), 37. https://doi.org/10.3390/quat8030037








