Abstract
The timing, cause, and magnitude of mammalian extinctions during the African Middle Pleistocene remain largely unresolved. The demise of Elephas/Palaeoloxodon recki, a lineage that had a great geographic and temporal span, represents a particularly enigmatic case of megafaunal extinction. Previous studies of Early Pleistocene fossil material have proposed that this lineage was a strict C4-grazer, with its dietary specialization causing its extinction during a period of climatic instability that coincided with the Late Acheulean. Others have associated its disappearance with overhunting by hominins during the same period. We contribute to this debate by analyzing carbon and oxygen isotope data from the tooth enamel of late Early and Middle Pleistocene Palaeoloxodon specimens from various localities in the Afar Rift. To contextualize the isotopic data of Palaeoloxodon within its broader ecosystem, we also provide data from non-elephant species. Carbon isotope values indicate that while C4 plants dominated diets, varying amounts of C3 vegetation were also consumed throughout this period. Oxygen isotope values reflect an initial focus on stable water sources that were later broadened to include transient sources. Serially sampled teeth of P. cf. recki recki from Late Acheulean contexts in the Megenta research area show no significant seasonal shifts in δ13C or δ18O values, even during a period of heightened climatic instability regionally. Taken together, our results suggest that Palaeoloxodon was capable of flexibility in diet and drinking habits which belies its morphological specializations. Our results do not support the idea that an inability to adapt to climatic instability caused the extinction of P. recki recki during the Late Acheulean. There is also currently no solid evidence that hominin hunting activities were the cause. However, we cannot discount the potential cumulative impact of climatic-induced environmental pressures and advancements in hominin hunting technologies during the early Middle Stone Age on the eventual extinction of the Palaeoloxodon lineage during the Middle–Late Pleistocene interface.
Keywords:
proboscideans; Elephas; Palaeoloxodon recki; Palaeoloxodon jolensis; isotope; palaeodiet; Afar 1. Introduction and Background
The African Middle Pleistocene witnessed the extinction of numerous mammalian species and the emergence of others, including our own species [1,2,3,4]. Although accumulating data continue to offer a clearer view of the magnitude and spatiotemporal extent of faunal turnovers, the factors driving these processes remain a topic of intense debate [5,6,7,8,9]. One reason for this is the lack of detailed taxa-specific data needed to effectively test relevant hypotheses. This is especially true for African Pleistocene elephants whose evolutionary record is dominated by what are collectively termed as the “Elephas recki complex” [10,11].
The “E. recki complex” originated in Africa during the mid-Pliocene and represents a proboscidean lineage characterized by the gradual development of increasingly efficient dental adaptations optimized for grazing. Key progressive trends include increased crown height, higher lamellar frequency, more pronounced enamel folding, greater cementum deposition, and a higher number of plates, accompanied by a reduction in enamel thickness [12,13,14]. This lineage would numerally and ecologically dominate the African Plio-Pleistocene landscape until its eventual demise across the Middle–Late Pleistocene interface. Notably, it is hypothesized to have given rise to the ancestors of the extant Asian elephant, Elephas maximus, before its disappearance from Africa.
Despite over a century of research primarily in eastern Africa [15,16], the “E. recki complex” remains unresolved in terms of the patterns of evolution (anagenesis vs. cladogenesis) and taxonomic attributions (e.g., Palaeoloxodon vs. Elephas). The classification of later members of this lineage is particularly debated, with numerous studies offering different perspectives on lineage and generic affiliations [10,11,17,18,19,20,21,22,23,24]. While the debates surrounding taxonomic systematics are beyond the scope of this paper and have little bearing on the results presented, it is reasonable to clarify the views we adopt in the context of our research and pending future studies. Recent comprehensive analyses [10,23] advocate restricting the genus Elephas to the older stages of the lineage while assigning the more recent members (P. recki ileretensis and P. recki recki) to Palaeoloxodon. We adopt this taxonomic framework for all specimens analyzed in our study, the oldest of which is <1.2 Ma [25]. In the absence of independent morphological revisions, we also opt to retain the taxonomic identification of most specimens included in our study as assigned by the late Professor Haruo Saegusa, to whom we dedicate this work.
The extinction of the “E. recki complex” is shrouded in uncertainty and complicated by the debated and poorly understood relationship between P. recki recki and the chronologically younger P. jolensis. Palaeoloxodon jolensis shares notable similarities in cheek tooth morphology with P. recki recki, indicating a close relationship. However, it is unclear whether P. jolensis represents a distinct species that evolved from P. recki recki, thereby serving as the terminal representative of the “E. recki complex”, or is simply an advanced evolutionary stage of P. recki recki [11,26]. Clarifying this relationship would determine whether P. recki recki underwent a gradual evolutionary transformation into P. jolensis, signaling a slow and progressive decline toward extinction rather than an abrupt disappearance.
Fossil evidence indicates that P. recki recki persisted well into the Middle Pleistocene, with remains found at sites such as the Masek Beds at Olduvai Gorge (1.2–0.5 Ma), Olorgesailie (ca. 1.2–0.6 Ma), and several southern African localities, including Kathu Pan, Power’s Site, and Namib IV, all associated with Acheulean stone tool industries and dated between 1 Ma and 0.5 Ma [3,27,28]. Significantly, P. recki recki is absent from horizons containing transitional Acheulean/Middle Stone Age (MSA) industries, as well as from MSA contexts [3,26]. Its disappearance thus likely coincides broadly with the end of the Acheulean. By contrast, P. jolensis appears mostly in stratigraphic contexts associated with transitional Acheulean/MSA and MSA technologies. For example, evidence from the Vaal River sequence in southern Africa (ca. 286–276 ka) suggests an overlap between P. jolensis and the transitional Acheulean/MSA Fauresmith industry. In eastern Africa, P. jolensis persisted to the end of the Middle Pleistocene at Natodomeri, Kenya, where it is associated with horizons dated to ca. 130 ka in the Kibish Formation [26].
The disappearance of P. recki recki, or the suite of morphological traits associated with it, coincided with the onset of a period marked by significant technological advancements, raising the assumption that improved hunting technologies, such as hafted Levallois spear points [29], played a role [30]. However, this remains speculative due to limited supporting evidence [5,27]. More concretely, the extinction of P. recki recki occurred alongside the decline of other large-bodied specialized grazing species and aligns with a period of pronounced climatic volatility and ecological transformation [3,31]. A detailed multiproxy record, derived from marine, aquatic, and terrestrial data, records a long-term trend of progressive aridification throughout the African Middle Pleistocene [32,33,34]. Superimposed on this were alternating phases of climate variability, where humid episodes were interspersed with dry periods. By the late Middle Pleistocene, coinciding with the end of the Acheulean, this pattern of climatic cyclicity intensified, with dry periods becoming progressively more severe due to the overarching trend of increasing aridity [35,36]. The effect of this on food and water source distributions, particularly for herbivores, would have been profound. A likely scenario is that as landscapes became increasingly fragmented, dietary diversification became essential, forcing herbivores to incorporate foods they might not typically consume. At the same time, permanent or seasonal water sources became more dispersed and unreliable, compelling obligate drinkers like elephants to exploit a wider range of transient alternatives [36]. These environmental pressures arguably placed niche specialists, which Palaeoloxodon presumably was, under tremendous pressure, forcing them either to adapt, disperse to areas where their preferred habitats still existed, or go extinct [37].
Faunal records from such a pivotal period in Africa’s Middle Pleistocene climatic history are sparse. This limits our understanding of the evolutionary responses, both morphological and behavioral, of members of the African faunal community to climatic volatility. Comparisons of the limited available faunas from Late Acheulean and early MSA contexts have determined that the late Middle Pleistocene was not simply a time of extinction, but of major faunal turnovers [3]. Some lineages, such as Equus (zebra) and Hippopotamus (hippopotamus), survived this period of climatic and environmental instability by evolving forms with smaller bodies and an ability to consume a diversity of foods [38,39,40]. By contrast, other lineages such as Palaeoloxodon, which survived the initial stages of this transitional period, ultimately went extinct. In some cases, these lineages were replaced by unrelated yet ecologically similar ones. In the case of Palaeoloxodon, this was Loxodonta, which had coexisted with it, but eventually came to dominate the African proboscidean niche [41].
The advanced suite of dental traits seen in P. jolensis, such as extreme hypsodonty, pronounced enamel folding, high lamellar frequency, anteroposteriorly thickened plates, and a relatively modest number of plates [26], suggest that Palaeoloxodon intensified its evolutionary focus on grazing at a time when other herbivores were shifting towards more generalized diets. While it could be argued that this apparent dietary specialization was the reason for the eventual demise of the lineage, such a conclusion is possibly too simplistic. There is a growing realization that dental morphology does not always accurately reflect diet within species or lineages [42,43]. It has also become clear that major changes in diets preceded changes in dental morphology, often by millions of years, in the evolutionary record of proboscideans [13,14]. Thus, the retention and even refinement of the suite of dental traits that initially enabled it to exploit expanding C4 grasslands should not have precluded Palaeoloxodon from expanding its diet to include browse as a supplement during periods of climatic instability. If it can be shown that Palaeoloxodon successfully navigated these conditions by exploiting a diversity of food resources and water sources, the cause of its ultimate demise should be sought in other factors. One such cause, suggested as a possibility is overhunting by hominins during the Middle Pleistocene [30,44,45,46,47].
As a contribution to ongoing discussions regarding the trophic adaptations and extinction trajectory of the “Elephas recki complex” during the Middle Pleistocene, we report and contextualize δ13C and δ18O values of P. recki and P. jolensis tooth enamel from various localities in the Afar Rift of Ethiopia (Figure 1). The contextualization is provided through δ13C and δ18O values for species such as Equus sp. and Giraffa sp., representing the C3 and C4 endpoints, along with hippopotamus, which serves as a low δ18O endpoint. This eastern African region provides a unique opportunity to investigate the ecological dynamics underlying the final stages of this elephant lineage within a single basin. One of a few African contexts with a relatively continuous record of Palaeoloxodon spanning the late Early to Middle Pleistocene (1.2 to ~0.129 Ma), the studied region documents the lineage’s sustained presence over an extended period. This record is further enriched by fossils from a diverse faunal community and extensive archeological record, which offer valuable insights into the environmental context, habitat diversity, and hominin behavior that characterized the region during this time.
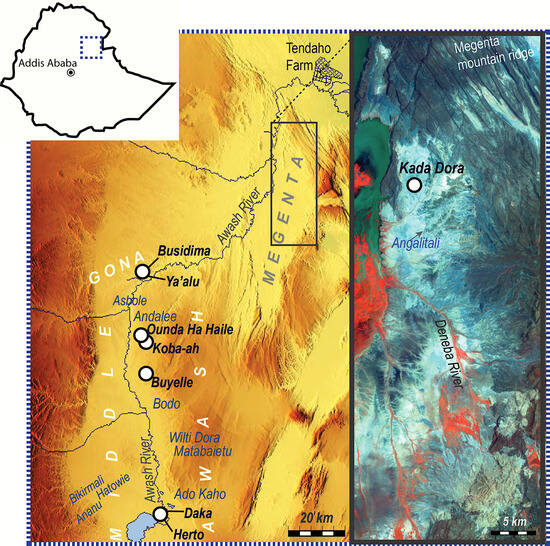
Figure 1.
A map of the Middle and Lower Awash basins showing the localities and project areas from which the studied samples are derived. White circles represent localities from which the studied elephant specimens are derived; localities with comparative samples are indicated by blue text.
Stable isotope analysis of fauna, flora, and soils is a widely used tool in research on both modern and ancient environments [48,49]. The δ13C values of faunal tissues reflect dietary sources, particularly the proportions of C3 and C4 plants consumed. In C3-dominated environments, cooler and wetter conditions typically result in more negative plant δ13C values [50,51], which can help identify variations in microhabitat use among co-existing herbivores [52]. Similarly, δ18O values provide insights into an animal’s water sources, drinking habits, and physiology [53,54,55,56,57]. These values have also been employed as indicators of aridity [58,59]. This aridity index method has played a key role in reconstructing past environmental conditions; however, it is important to acknowledge that it is subject to significant limitations [60].
The failure of large-bodied dietary specialists to meet essential nutrient and water requirements amid heightened climate instability has been suggested as the driver of their extinction during the African late Middle Pleistocene [3]. The δ13C and δ18O values generated in this study are used to reconstruct the dietary and drinking behaviors of the Afar Rift Palaeoloxodon, thereby enabling the evaluation of the following scenarios:
(1) P. recki recki went extinct because of climate-driven environmental shifts during the late Middle Pleistocene (coincident with the terminal Acheulean) and was replaced by its descendent species P. jolensis, which persisted until its extinction during the Middle–Late Pleistocene interface. Under this scenario, we anticipate limited evidence of dietary flexibility, as late-occurring P. recki recki likely adhered strictly to a grass diet despite fluctuating availability. Regarding drinking behavior, we also expect late-occurring P. recki recki to have shown little flexibility in utilizing alternative, transient water sources that, due to unpredictable rainfall patterns, would have been abundantly available. In the case of P. jolensis, we anticipate a similar strict reliance on grass and preference for stable water sources. Its reluctance or inability to adapt under prolonged fluctuating climatic conditions would have ultimately resulted in its extinction, marking the end of the “E. recki complex”.
(2) P. recki recki underwent a gradual evolutionary transformation into P. jolensis, signaling a slow and progressive decline of the lineage toward eventual extinction at the end of the Middle Pleistocene. Under this scenario, we expect to see the emergence of dietary flexibility in late-occurring P. recki recki, despite exhibiting specialized dental adaptations for grazing. Regarding drinking behavior, we similarly expect to see flexibility, with individuals utilizing both stable and transient water sources as the latter became more freely available. In the case of P. jolensis, we also anticipate evidence of dietary flexibility and an ability to utilize stable and transient water sources. Under this scenario, P. jolensis would eventually go extinct despite exhibiting flexibility in diet and drinking behavior, bringing an end to the “E. recki complex”.
2. Materials and Methods
We measured the δ13C and δ18O values from the tooth enamel of specimens representing later evolutionary stages of the “Elephas recki complex”. To provide a comprehensive isotopic profile of the ecosystem, we included measurements from Equus sp., Giraffa sp., two bovid tribes (Tragelaphini and Antilopini), and a hippopotamus species (H. cf. gorgops).
2.1. Fossil Assemblages
The proboscidean assemblages sampled by this study are derived from various late Early and Middle Pleistocene localities in three paleoanthropological study areas in the Afar Rift (Figure 1). Isolated molar and dentulous mandibular remains from the Middle Awash research area sample discrete time intervals ranging from the Upper Herto (160–154 ka) to the Andalto (Middle Pleistocene) and Daka (~1.0 Ma) members [21,23,61]. Two molars from Gona come from the Upper Busidima Formation, dated to <1.2 Ma [62]. Additional dental remains from the Gona study area sample the Asbole section of the same formation, dating to the Middle–Late Pleistocene interface < 0.15 Ma [63]. Several samples, including nearly complete dentulous mandibles from the Megenta research project area, come from the fossiliferous sand unit spanning 0.5 to ~0.35 Ma [61]. Table 1 provides the localities, age estimates, and taxonomic attributions of each studied elephant specimen.

Table 1.
Contextual data for elephant specimens sampled by the present study. The respective project areas of the listed localities per Figure 1 and Supplementary Table S1.
The Megenta specimens representing the transitional period, which straddles the last appearance of P. recki recki and the first appearance of P. jolensis, exhibit a mosaic of dental features. For most of these specimens, the crown heights could not be reliably collected due to mandibular bone cover and considerable wear. Therefore, we designate these ‘P. cf. recki recki’ to highlight their mixed features, pending detailed taxonomic descriptions. Because these specimens are temporally and geographically relatively constrained, our tentative classification of them as ‘transitional’ between P. recki recki and P. jolensis will not affect the dietary and evolutionary interpretations that make up the focus of this paper.
In addition to the elephant fossils, we sampled several other taxa from Megenta and the Middle Awash for a better understanding of the isotopic compositions of grazers and browsers from the broader period and a contextualization of the Palaeoloxodon isotopic data. For grazers, we included Hippopotamus and Equus samples, and for browsers, Tragelaphus and Giraffa specimens based on their commonly recognized dietary adaptations and isotopic values (OSM Table 1).
2.2. Sample Collection
Teeth were sampled in the National Museum of Ethiopia, Addis Ababa, where the specimens are permanently housed. Dentulous specimens were selected for species identification and tooth enamel sampling while mandibular remains were assessed for bone surface modification. Bulk sampling for carbon and oxygen isotopes was conducted using a handheld drill with diamond powder-tipped bits of even shape. The outer surface of the tooth was first cleaned by gentle abrasion to remove a very thin layer of possible unwanted inclusions, such as a sedimentary matrix. A gentle and even abrasion was applied along the growth axis of each tooth, from the cervix (at the base of the tooth) to the apex, near the occlusal surface of the tooth. Weighing paper was used to collect the resultant tooth enamel powder, which was then transferred into a 2 mL centrifuge tube. Low-power drilling was conducted to avoid heating. Used drill bits were cleaned with diluted HCl and weighing papers were changed for each new sampling.
Elephant molars undergo a long process of formation and mineralization, which continues throughout an elephant’s life. As an elephant matures and teeth wear, new molars emerge periodically to replace worn ones. The mineralization process incorporates various elements such as oxygen and carbon from the animal’s diet and environment. Because this process takes several years [64,65], such dental remains serve as an excellent archive for studying seasonal changes in the isotopic composition of the environment. The crown formation times of molar plates from the extant African elephant (Loxodonta africana) and the mammoth (Mammuthus columbi) were found to be approximately 5 to 6 years and 11 years, respectively [64]. These isotopic variations—such as those from shifts in water sources or diet (in other words, vegetation)—are reflected in the tooth enamel as it forms. Since each molar retains a record of the elephant’s diet and environmental conditions during the 5 to 11 years of crown formation, we can analyze the isotopic ratios within the teeth to trace seasonal patterns over a span of several years. A confounding factor is that during the enamel maturation phase, enamel density increases as organic material and water are replaced by bioapatite. This maturation process dampens the primary isotopic input signal recorded during the secretory phase. Consequently, the distal end of the molar contains a higher concentration of inorganic carbonate, while the proximal end is less affected by this maturation process [64]. To track these seasonal/decadal patterns, our study included the collection of serial samples from three Palaeoloxodon molars from Megenta. The locations of the samples taken are presented in Table 1.
Examination of bone surface modification was conducted in the museum on all sampled specimens with preserved mandibular bone surfaces. In addition, 39 specimens representing different postcranial elements, mostly limb bones, ribs, and vertebrae of Middle Pleistocene elephants from several sites, were investigated in the field. Different projects use different collection protocols, with most collecting only dental and cranial remains of elephants while leaving postcrania in the field due to logistic and research focus. For this reason, uncollected postcranial elements were investigated in their discovery locations out in the field. The examination included the commonly used methods of identifying bone surface modification (i.e., linear scores with V-shaped grooves, grooves with shouldering effect, spiral fractures, hammerstone marks/percussion pits, and other signs such as green bone fracture, functional association with stone artifacts, and the absence of carnivore tooth marks) [66,67].
2.3. Sample Pre-Treatment
Bulk samples were taken from all the specimens; three of the elephant specimens were additionally serially sampled. For the bulk samples, enamel powder was drilled from the entire height of the teeth from the occlusal surface to the enamel–root junction to integrate the whole period of crown formation and average out seasonal variations. This did not account for the enamel lost through wear, which was unavoidable. Enamel was pretreated as described by Sponheimer and Lee-Thorp [68,69] with modifications as given by Luyt and Sealy [70]. Briefly described, the procedure involved treatment of the powdered enamel with 1 mL of ~1.75% v/v sodium hypochlorite at room temperature for 45 min to remove organics. Treated samples were then centrifuged and rinsed 3 times with Ultrapure Milli Q water. Next, they were treated with 1 mL of 0.1 M acetic acid at room temperature for 15 min to remove any soluble mineral components including adsorbed carbonates, which are more soluble than structural carbonates [71]. The samples were centrifuged and rinsed 3 times with Ultrapure Milli Q water before being freeze-dried.
2.4. Sample Isotopic Analysis
About 2 mg of dry, pre-treated enamel powder was weighed and placed into a 12 mL borosilicate glass tube with a screw-top lid and septum. The tubes were then loaded into a Thermo Model II gas bench equipped with a CTC Analytics A200S autosampler. The sample tray was maintained at a controlled temperature of 72 °C. After flushing the tubes with helium, five to seven drops of pure H3PO4 (depending on sample size) were manually added to each sample tube via syringe. The samples were left for at least two and a half hours to allow the carbonate in the apatite to react with the acid, releasing CO2. This evolved gas was collected by the auto sampler and passed through a Nafion water removal unit, a “Poraplot Q” gas chromatography column, and a second Nafion water trap. Finally, the purified CO2 was analyzed using a Delta Plus XP isotope ratio mass spectrometer (IRMS) (Thermo, Bremen, Germany) managed by Isodat software (Version 3.0.94.12, Thermo, Bremen, Germany) and housed in the Stable Light Isotope Laboratory at the University of Cape Town.
Measurement precision was monitored through repeated analyses of internationally recognized standards (IAEA-CO8; δ13C = -5.8‰; δ18O = −22.7‰ [72] and Carrara Z; δ13C = 2.12‰; δ18O = −2.13‰; IVA, Meerbusch, Germany) and an internal standard (Cavendish marble; δ13C = 0.34‰; δ18O = −8.95‰) included in each run. Ratios of 13C/12C and 18O/16O were reported in δ notation relative to PeeDee Belemnite (PDB, a marine sedimentary carbonate) in per mil (‰). Repeated measurements of the standards showed a reproducibility of ≤0.2‰ for both carbon and oxygen.
2.5. Data Analysis
Data analyses were conducted using SPSS (Version 29). The results of these tests are provided in Supplementary Table S1. The data were non-normal and, as such, non-parametric tests were used in evaluating the data. Kruskal–Wallis H one-way tests were run for each of the carbon and oxygen datasets. Pairwise comparisons with adjusted p-values were used to demonstrate which taxa were different. If only two groups existed, then Mann–Whitney tests were used. If there were more than two groups, Kruskal–Wallis one-way tests were used to determine if groups were different, while the Wilcoxon test was used to indicate which groups these were. A p-value of <0.05 indicated a significant difference.
Since a consumer’s tissues originate from their diet, stable isotope mixing models can estimate an individual or group’s assimilated diet based on the isotopic ratios of their tissues and food sources. However, this method relies on certain assumptions and should be interpreted with those in mind. Assumptions include the δ13C of the food consumed, the enrichment factors, and the C3/C4 endpoints. A simple mixing model was run to quantify consumer diets by determining the proportion of C3 vs. C4 plants consumed based on the sample δ13C. The equation used and the outcome of the model are reported in Supplementary Table S1.
3. Results
δ13C and δ18O values for all enamel samples measured in this study are listed in Supplementary Table S1 (bulk samples) and Supplementary Table S2 (serial samples), respectively. Table 2 gives summary statistics for each taxon, while Figure 2 explores the relationship between δ13C and δ18O for each specimen.

Table 2.
δ13C and δ18OvPDB summary statistics for each taxa.

Figure 2.
Scatter plot of δ13C against δ18OvPDB for all species.
3.1. Bulk Samples
3.1.1. δ13C (Carbon Isotope Ratios)
The δ13C values provide insight into the types of vegetation these animals consumed, as C3 and C4 plants differ in their carbon isotopic compositions. δ13C values of fauna have been used mainly to assess diets, particularly the proportions of C3 and C4 plants consumed [73,74,75,76].
Elephantidae (n = 22): The range for P. jolensis is −2.97‰ to 0.74‰ and the mean δ13C is −0.50‰. Refer to Table 2 and Figure 3. The range for P. recki recki is −1.94‰ to 1.18‰ with a mean δ13C of −0.41‰. Both P. jolensis and P. recki recki have overlapping δ13C ranges, although δ13C values for P. jolensis are more tightly clustered, suggesting similar general feeding behaviors. The groups are not significantly different (Mann–Whitney U, p = 0.562), confirming this similar diet. The four P. cf. recki recki specimens from the Megenta locality were isolated to assess whether they fall within the established range of P. recki recki. The four Megenta P. cf. recki recki specimens were found not to be significantly different from the overall P. cf. recki recki group (Mann–Whitney U, p = 0.527).

Figure 3.
δ13C (carbon isotope ratios) in parts per mille (‰) of all species sampled. Open circles indicate individual sample values. Bold horizontal lines indicate the median, with surrounding boxes representing the interquartile range (first quartile to third quartile). The whiskers indicate the range of values.
Giraffidae (n = 19): The mean δ13C value for Giraffa sp. is −8.14‰ (median = −9.02‰). The giraffids are statistically significantly different from the Equid group [Kruskal–Wallis H (7) = 25.355, padj < 0.05], as well as all three of the elephant groups (P. jolensis [Kruskal–Wallis H (7) = −32.314, padj < 0.001], P. recki recki [Kruskal–Wallis H (7) = −32.189, padj < 0.001], and the P. cf. recki recki from Megenta [Kruskal–Wallis H (7) = −34.105, padj < 0.001]).
Equidae (n = 6): the mean δ13C value for Equus sp. is −1.38‰, consistent with a C4 grazing diet with some mixed feeding behavior evident in the range of values (median = −0.95‰).
Bovidae (n = 6): One Antilopini specimen was analyzed, yielding a δ13C value of 0.26‰. The mean δ13C for Tragelaphini is −2.58‰ (median −1.66‰), although this includes a very negative outlier of –8.63‰. Without the single outlier, the δ13C median/mean would be higher, indicating a predominantly C4 diet.
Hippopotamidae (n = 6): the mean δ13C value for the H. cf. gorgops specimens is −2.07‰ (median −2.36‰). This indicates a predominantly grazing diet, dominated by C4 plants.
3.1.2. δ18O (Oxygen Isotope Ratios)
The δ18O values are influenced by drinking water and environmental conditions like temperature and humidity. δ18O values can also give clues regarding the water sources utilized by the animal and its drinking behavior and physiology. Refer to Table 2 and Figure 4.

Figure 4.
δ18OvPDB (oxygen isotope ratios) in parts per mille (‰) of all species sampled. Open circles indicate individual sample values. Bold horizontal lines indicate the median, with surrounding boxes representing the interquartile range (first quartile to third quartile). The whiskers indicate the range of values.
Elephantidae (n = 22): The range of δ18O for P. jolensis is from −6.50‰ to 1.47‰, while the mean δ18O is −2.92‰. The range of δ18O for P. recki recki is −7.35 to −1.27‰ and the mean δ18O is −3.67‰. The slightly more negative mean δ18O for P. recki recki could suggest potential ecological or microhabitat differences, where the two species might have had access to different water sources, or P. recki recki living in less arid conditions. However, the δ18O are not statistically significantly different (Mann–Whitney U, p = 0.346). The P. jolensis species exhibits a significantly broader range of values, suggesting it may have accessed a more diverse array of water sources compared to P. recki. Additionally, the Megenta P. cf. recki recki specimens also display a much wider range (6.08‰) than other P. recki recki specimens, which are more tightly clustered with a range of just 1.89‰.
Giraffidae (n = 19): The mean δ18O value for Giraffa sp. is −0.28‰ (median similarity high at −0.96‰). While this is a high value, the range is quite large, from −6.76‰ to +5.31‰. Giraffids obtain much of their moisture from their food; this pattern aligns with previous studies, which have shown that animals consuming evaporatively enriched leaves exhibit more positive δ18O values [57,58,59,77,78,79].
Equidae (n = 6): The mean δ18O value for Equus sp. is −3.47‰ (median −3.31‰). Animals that are reliant on drinking water tend to have lower δ18O values, closer to the meteoric water δ18O.
Bovidae (n = 6): One Antilopini specimen was analyzed, yielding a δ18O value of −3.49‰. This is the same as the other grazer in the sample set, Equus sp., possibly indicating a reliance on drinking water. The mean δ18O for Tragelaphini is −1.94‰. In this case, the median of −3.2‰ is probably more accurate as this group includes a very positive outlier. The outlier is the same individual with the more negative δ13C value.
Hippopotamidae (n = 6): The mean δ18O value for the H. cf. gorgops specimens is −6.52‰ (median –6.89‰). Since hippopotami are semi-aquatic, their δ18O values have been used as a proxy for the δ18O of the local meteoric water [78]. The Hippopotamidae group is the only group that differs significantly from another group, namely the Giraffidae group (Kruskal–Wallis H (7) = 35.070, padj <0.001).
3.2. Serial Samples
Three specimens (M-KDR-1/5; M-KDR-1/53, and M-KDR-1/54) were serially sampled to investigate seasonal signals (Figure 5). Figure 6 illustrates the locations on the teeth where samples were collected. Supplementary Table S3a and S3b list the δ13C and δ18O results, respectively.
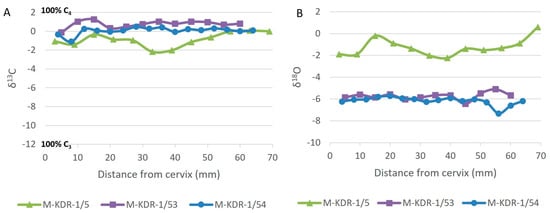
Figure 5.
Carbon and oxygen isotope values for the serially sampled P. cf. recki recki teeth. (A): δ13C and (B): δ18OvPDB in parts per mille (‰).

Figure 6.
Photograph showing sample locations on the serially sampled P. cf. recki recki specimens (M-KDR-1/54, M-KDR-1/53 and M-KDR-1/5).
The δ13C values for all three P. cf. recki recki specimens indicate a consistent dietary pattern, with no significant seasonal shifts evident in the carbon isotopic signatures. M-KDR 1/5 exhibits the widest range, possibly incorporating a small amount of browse. However, the values at −2‰ still fall within the overall C4 range, indicating that the contribution is likely to be less than 20% (refer to OSM Table 1). The stable δ13C values suggest that these species maintained a relatively uniform diet throughout the year, rather than exhibiting seasonal changes in the proportions of C3 and C4 plants consumed. This finding implies that the dietary resources available to these taxa were sufficiently stable across seasons, minimizing the need for dietary adjustments. A dampening effect during the enamel mineralization process in elephants may reduce the magnitude of δ13C values in their teeth, making them less reflective of the original δ13C input. As a result, what appears to be a relatively stable seasonal or annual isotopic signal could, in reality, represent considerably more variability. Considering this, it is important to note that two specimens (M-KDR 1/5 and M-KDR 1/54) were sampled posteriorly, while one (M-KDR 1/53) was sampled anteriorly. Since the mineral content in posteriorly sampled teeth may be slightly higher than in anteriorly sampled ones, the former could exhibit a greater degree of dampening, while the latter might show less pronounced effects. Unfortunately, the precise extent of this dampening effect cannot be determined without further testing.
Similarly, the δ18O values exhibit limited variability for each individual, indicating a lack of seasonal fluctuation in the δ18O of precipitation (or water sources) or substantial changes in ambient temperature. The M-KDR 1/5 specimen exhibits a bigger seasonal variation and a more positive sequence, ranging from −2‰ to +0.3‰, compared to the other two specimens. Several factors could account for this absolute difference, but since the primary determinant of δ18O in elephant enamel is likely the substantial quantities of water they consume, it is probable that two of the specimens accessed a different water source than the third. Oxygen isotope ratios in tooth enamel are typically influenced by factors such as seasonal variations in rainfall, humidity, and temperature, which alter the isotopic composition of available drinking water. It is likely that the two specimens with lower δ18O values consumed water from tributaries of the paleo-Awash River, while the third drank from the more evaporatively enriched main channel of the river. Although these specimens originate from the same designated locality, they are spatially distributed across a relatively wide area. Furthermore, it is highly likely that the sandstone sedimentary unit was deposited over thousands of years. Unfortunately, we cannot determine the precise time span represented by this unit since the range for the fossil- and artifact-bearing Megenta package is estimated to be between 0.5 and 0.35 Ma.
The relatively stable δ18O values in these three individuals (regardless of the absolute value) suggest they consistently accessed stable water sources throughout the year, likely derived from perennial sources such as the palaeo-Awash River. These results imply that seasonal temperature changes were not extreme enough to leave a detectable isotopic signal in the enamel, supporting the interpretation of a relatively stable and predictable climate within the study area during the period of enamel formation. All three teeth exhibit similar maturation and wear, suggesting that enamel thickness is unlikely to contribute to differences in dampening factors among the specimens. However, there is no definitive method to quantify the dampening effect, other than acknowledging it as a potential factor.
3.3. Bone Surface Modification
There were no conclusive bone surface modification marks identified on the mandibular and postcranial remains investigated, both in the field and the laboratory. This absence of marks persisted even on well-preserved bone surfaces and in contexts containing remains of other megafauna, such as hippopotamus, which showed evidence of stone tool-inflicted chop and percussion marks [1].
4. Discussion
The ecological dynamics that served as a backdrop to the final stages of the “Elephas recki complex” remain poorly understood. As a prominent and long-lived lineage, unraveling the factors behind its decline contributes to broader questions about the causes and mechanisms that drove the extinctions of large-bodied grazers during the Middle Pleistocene. This includes, amongst others, understanding the roles of climatic and environmental instability along with the compounding pressures exerted by increasingly complex hominin hunting behavior. In this study, we examined the δ13C and δ18O values of Palaeoloxodon tooth enamel from multiple sites within the Afar Rift, Ethiopia, spanning the late Early and Middle Pleistocene (<1.2 to <0.15 Ma). We also assessed mandibular bone and postcranial remains from the period of focus for possible modification marks.
4.1. Carbon and Oxygen Isotope Ratios of the Non-Proboscidean Fauna
Giraffa sp. has the most negative δ13C (mean = −8.14‰; median = −9.02‰) values in our sample, reflecting a strong reliance on C3 vegetation. This is in line with the behavior of extant giraffes, which are obligate browsers in open environments. The consistency between the mean and median values for this taxon in our dataset, combined with the fact that our comparative sample spans much of the Pleistocene, points to the persistent presence of wooded elements. However, the standard deviation (2.63‰) and a broad range of 9.08‰ suggest variable access to browse, possibly due to any combination of geographical variability, seasonal shifts in availability, and long-term environmental fluctuations. The mean δ13C (−2.58‰) value for Tragelaphini indicates a mixed C3/C4 plant diet. However, this is due to one outlier with a δ13C value of −8.6‰. Thus, the median value for the Tragelaphini group is a more accurate reflection of the group (−1.66‰) and leans towards the C4 end of the spectrum. The genus Tragelaphus exhibits considerable dietary variation in eastern Africa. For example, Levin et al. [80] reported that the fossil assemblage of Tragelaphini from the Sagantole Formation (Middle Awash) displays values (−0.1‰) that fall outside the range observed in their extant counterparts (−7‰). Tragelaphini exhibiting C4 isotopic signals have also been recorded at several Plio-Pleistocene sites in eastern and northern Africa, with some interpretations suggesting a diet primarily consisting of C4 browse, such as shrubs from the Amaranthaceae family [81,82]. Conducting meso- and microwear analyses on this species will be crucial to addressing this dietary hypothesis. The mean δ13C (−1.38‰) and the median δ13C (−0.95‰) values for Equus sp. indicate a diet dominated by C4 plants, which is not surprising for a taxon considered a dedicated grazer. However, the standard deviation (1.83‰; range = 4.88‰) indicates moderate variability in δ13C values among individuals, with some including a small amount of C3 vegetation in their diets, either in the form of C3 grasses [83] or C3 browse. This narrower range is consistent with findings for fossil and extant assemblages in the region, indicating a less flexible diet [80]. The data for Antilopini is limited to a single individual from Megenta. Nevertheless, with a δ13C value of 0.26‰, this individual relied heavily on C4 vegetation. The mean (−2.07‰) and median (−2.36‰) δ13C values for the hippopotamus H. cf. gorgops suggest a diet dominated by C4 plants but perhaps supplemented by C3 vegetation. This is similar to what was found in the older fossil assemblage of the Adu-Asa and Sangantole formations which had mean values of –2‰ and –2.8‰, respectively [39,80,84,85]. The same study reported more mixed feeding δ13C values for the extant hippopotamuses with δ13C values of −4.2‰ and −4.1‰ at two national parks in Ethiopia [80]. Being semi-aquatic, these animals likely fed along the paleo-Awash River and its tributaries, in a mosaic environment containing riparian forests, floodplains, and grasslands. The low standard deviation (0.88‰) and range (2.11‰) indicate minimal dietary variability.
The mean (−0.28‰) and median (−0.96‰) δ18O values for Giraffa sp. are more positive compared to the other taxa, indicating a stronger reliance on water obtained from evaporatively enriched C3 plants, rather than direct consumption. By contrast, the high standard deviation (3.42‰) (and broad range of 12.5‰) likely indicates that the sampled individuals accessed plants from habitats with differing levels of water availability and/or exposure to evaporation stress. This variability is not surprising given the wide temporal and geographical origins of the sample. The median δ18O of −3.20‰ indicates a reliance on water from more stable sources such as springs, lakes, and/or perennial rivers. The outlier with an unusually high δ18O is the same individual with a much lower δ13C value, perhaps indicating a reliance on C3 shrubland with evaporatively enriched leaves. Similarly, Equus sp. exhibits relatively lower mean (−3.47‰) and median (−3.31‰) δ18O values indicative of a reliance on stable water sources. However, the wide range (7.95‰) and high standard deviation (3.07‰) highlight variability, suggesting that some individuals also accessed more transient sources. The single Antilopini in the sample has a δ18O value of −3.49‰. This value is consistent with access to a stable water source. The mean (−6.52‰) and median (−6.89) δ18O values for H. cf. gorgops are not surprising, given that these are semi-aquatic mammals [78,80]. These low values indicate that these hippopotamids had constant access to a stable water source with low evaporation rates—most likely the paleo-Awash River. The comparatively low range value (3.17‰) reflects minor differences in water sources or environmental conditions between individuals.
4.2. Carbon and Oxygen Isotope Ratios of the Palaeoloxodon Sample
A mean δ13C value of −0.41‰ indicates that P. recki recki had a diet focused primarily on C4 vegetation and thus could have preferred open habitats like grasslands or consumed some C4 dicots such as shrubs of the family Amaranthaceae. However, since the species had a dental morphology adapted to a grass-based diet, the likelihood of it consuming a C4 dicot like Amaranthaceae is low. The δ13C values reflect dietary consistency across individuals in the sample and suggest reliable access to C4 vegetation (between 72 and 94% C4 plants, refer to OSM Table 1). However, the standard deviation (1.24‰) and range (3.12‰) could possibly indicate a measure of dietary variability among individuals, with some foraging exclusively in open C4-dominated habitats, while others also utilized habitats that contained C3 vegetation, possibly riparian forests or woodlands. These findings suggest that P. recki recki exploited a range of habitats, although it showed a clear preference for those dominated by C4 vegetation, probably grasslands. Palaeoloxodon cf. recki recki from Megenta exhibits a mean δ13C value of −0.19‰, indicating that its diet was perhaps more strongly dominated by C4 plants. These results imply that P. cf. recki recki may have occupied an environment characterized by lower habitat diversity and fewer closed areas, or it may have favored open C4 grass-dominated habitats. Palaeoloxodon jolensis has a mean δ13C value of −0.50‰, which suggests a diet dominated by C4 vegetation. Its mean value is more closely aligned with that of P. recki recki (−0.41‰) than P. cf. recki recki (−0.19‰). Unlike the narrow dietary range of P. cf. recki recki (1.17‰), the P. jolensis samples show a broader range of 3.71‰, suggesting moderate dietary variability likely caused by the inclusion of a small percentage of C3 vegetation (between 9 to 36% C3 consumption for individuals, refer to OSM Table 1). It is, however, possible that the observed differences might also be a result of the small sample size for P. cf. recki recki, which might not have fully captured its dietary diversity.
Palaeoloxodon recki recki has somewhat lower mean (−3.22‰) and median (−3.24‰) δ18O values, suggesting a reliance on stable water sources such as springs, lakes, or perennial rivers. The close alignment of the mean and median indicates little variability in water source and/or stable water conditions across its habitat. A standard deviation of 0.8‰, while modest, hints at minor variability in water availability. The narrow range of 1.89‰ further supports the idea that P. recki recki inhabited areas with stable water sources. In comparison, P. cf. recki recki exhibits notably more negative mean (−5.39‰) and median (−6.48‰) δ18O values, similarly indicating a primary dependence on stable water sources like rivers, fountains, and large lakes. However, the higher standard deviation (2.79‰) and broader range (6.08‰) suggest that P. cf. recki recki likely accessed a more diverse array of water sources compared to P. recki recki. Thus, in addition to stable water bodies like rivers and lakes, it would also have utilized evaporation-prone sources such as rain-fed pools and ephemeral streams. Like P. cf. recki recki, P. jolensis also displays a relatively high standard deviation (2.22‰) (and a broad range of 7.97‰), indicating substantial variability in δ18O values and pointing to the additional use of more transient or seasonal water sources.
4.3. Paleoenvironmental Implications of the Carbon and Oxygen Isotope Ratios
Given that samples vary widely with respect to spatial and temporal contexts, their isotopic values can offer only a general overview of late Early and Middle Pleistocene environmental conditions in the studied region. Nevertheless, we can still infer key aspects of the regional plant community and hydrological structure. Additionally, although isotopic ratios between taxa often only exhibit slight variations, environmental changes typically occur gradually, leading to small shifts in isotopic values. While these changes may not be statistically significant, they can still have ecological meaning.
The δ13C values of the analyzed taxa indicate that, except for Giraffa sp., most taxa relied primarily on C4 plants. Taxa with the highest δ13C values, such as elephantid and antilopins, show a strong association with what were almost certainly grassland ecosystems. Interestingly, mixed feeders like tragelaphins, which today primarily consume C3 browse [86], incorporated a substantial amount of C4 plants, indicating a greater level of dietary flexibility than expected. Meso- and microwear analyses will allow us to say with certainty whether this was C4 browse or C4 grasses. At face value, these findings align with an environment dominated by open grasslands. However, the δ13C values also reveal variability within taxa, suggesting that C3 vegetation was still a significant component of the landscape. As anticipated, giraffids exhibit the lowest δ13C values, resembling the diets of modern giraffes that feed in open woodlands and riparian forests [87,88]. Similarly, tragelaphins consumed more C4 plants than typically observed in modern environments, but C3 vegetation remained a substantial part of their diets (ranging between 13 and 76% (refer to OSM Table 1)). While equids, typically dedicated grazers in modern environments, also incorporated C3 plants into their diets, the nature of the C3 component in the diets of assumed grazers like equids (whether browse or graze) would also have to be confirmed in the future using meso- and microwear analyses.
While taxa can generally be classified according to their primary dietary preference, the δ13C values highlight considerable within-species variability. Some individuals within both browser and grazer categories consumed plant resources that were not strictly aligned with their assumed diets. For example, certain Giraffa sp. specimens display unusually high δ13C values (BOU-VP-1/229 = −3.47‰; KL 42 = −1.91‰; KL 129-1 = −4.27‰). This variability underscores the adaptability of the local herbivore community to a dynamic environment, where dietary choices may have fluctuated according to seasonal resource availability and regional microhabitat variations. These results suggest that taxa had access to diverse habitats and adjusted their diets to the specific geographical and seasonal conditions they encountered, further supporting the notion of a heterogeneous vegetation community.
The lack of fine-scale temporal resolution of the fossils in our study limits our ability to fully compare conditions in the region throughout the Middle Pleistocene. This period, which is of particular interest in this study, was characterized by climatic instability and increasing aridity, particularly in its later stages [36]. However, these limitations prevent us from clearly evaluating how these changes affected the environment in the study area. Similarly, other studies have provided limited detailed information about the Middle Pleistocene environment in this region, resulting in a major gap in our understanding. Despite these uncertainties, it is likely that the region was buffered from the most extreme climatic fluctuations due to the presence of the Awash River which is fed by water from the Ethiopian plateaus. For instance, the presence of H. cf. gorgops fossils at the Megenta localities suggests that the paleo-Awash River and associated habitats, such as riparian forest and wetlands, remained important and persistent features of the local landscape throughout the early Middle Pleistocene. The ongoing presence of a permanent water source, likely the paleo-Awash, is also indicated by the δ18O values of the Palaeoloxodon samples, which span the late Early and Middle Pleistocene. Moreover, the dynamic geology and varied topography of the Afar Rift would have supported seasonal groundwater recharge and the formation of transient streams and pools, which are processes that continue to shape the regional water supply today. The utilization of such sources is reflected in the elevated δ18O values of some obligate drinkers like equids and elephantids.
The limited paleoenvironmental data for the early Middle Pleistocene of the region comes from Asbole, dated 0.8–0.64 Ma (Figure 1) [89]. Faunal and isotopic evidence from this site suggests a heterogenous vegetation community that included wetlands, grasslands, and forests, with open C4 grassland habitats dominating the region and isolated riparian forests flanking the paleo-Awash and its tributary streams [90]. Taxonomic analyses of primate assemblages from the Middle Awash localities of interest also point to a heterogeneous environment during the later Early and Middle Pleistocene. Specifically, the relative rarity of cercopithecoid remains and the predominance of Theropithecus oswaldi—a specialized grazer—in the Daka Member of the Bouri Formation suggests the presence of more open, grassy environments [91] in contrast to those at Andalee and Asbole, downstream [92,93]. At Megenta, the predominance of hippopotamid and crocodilian remains suggests water bodies a few kilometers east of the paleo-Awash River, while other taxa present point to a relatively open grassland environment [61].
4.4. Ecological Dynamics of Palaeoloxodon in the Awash Basin
The δ13C values generated in this study emphasize a strong reliance on C4 grass across all three Palaeoloxodon sample groups. Palaeoloxodon recki recki and P. jolensis also consumed a small amount of C3 vegetation to supplement their C4 grass diets. This pattern is similarly evident among the non-proboscidean taxa that shared the landscape in the studied localities and reflects the sustained heterogeneous nature of the wider region’s vegetation community. Palaeoloxodon cf. recki recki from Megenta exhibits the lowest reliance on C3 vegetation, suggesting a diet almost exclusively dominated by C4 grass. This population could have lived exclusively in a grassland environment. Alternatively, or in addition, it may reflect increased climatic variability and/or aridity during the period 0.5–0.35 Ma. Considering that the P. cf. recki recki sample is intermediate in age between P. recki recki and P. jolensis and that the Megenta localities are geographically constrained by a prominent mountain ridge and the Awash River, the scenario of heightened climatic stress seems more plausible—provided the sample size (n = 4) is deemed sufficient for reliable interpretation.
The δ18O values highlight notable differences in water consumption patterns between P. recki recki and the two later Middle Pleistocene samples. Palaeoloxodon recki recki demonstrates a strong reliance on stable water sources, which likely included the paleo-Awash River. Its relatively low mean and median δ18O values, narrow range, and low standard deviation indicate reliable access to such water sources. Palaeoloxodon cf. recki recki and P. jolensis also exhibit low mean and median δ18O values, indicating similar reliance on stable water sources. However, their broad ranges and higher standard deviations suggest that some individuals in these samples also accessed more transient water sources, such as rain-fed pools or ephemeral streams. This is consistent with the increase in aridity suggested for the Middle Pleistocene [36].
The serially sampled δ13C and δ18O values from the three P. cf. recki recki individuals (M-KDR-1/5, -1/53, and -1/54) (Figure 5) provide finer-resolution dietary and water consumption patterns during a period of increased climatic fluctuation. The δ13C values strongly suggest diets dominated by C4 grasses in all three individuals. M-KDR-1/5, and to a lesser extent M-KDR-1/54, included a minor amount of C3 vegetation. Most importantly, all three individuals had access to C4 vegetation year-round, pointing to a landscape where grasslands were abundant and consistently productive throughout the season. The limited fluctuations seen between individuals likely reflect local variations in vegetation types but do not suggest any major dietary shifts driven by climate or heightened seasonality. The δ18O values of M-KDR-1/53 and M-KDR-1/54 are much lower and show minimal variation throughout the season, indicating an almost exclusive reliance on a stable, perennial water source like the paleo-Awash River. On the other hand, M-KDR-1/5 exhibits markedly more positive δ18O values indicative of the mixed use of stable and transient water sources throughout the season.
The time interval that the fossil-bearing sandstone layer at this locality represents cannot be more precisely determined, and the sandstone containing these specimens was either deposited over a short interval or represents a more extended period within the 0.5 to 0.35 Ma range. The first scenario corresponds to a relatively narrow timeframe in which the variation among these three individuals suggests that P. cf. recki recki had the ability to alternate between permanent and temporary water sources, enabling them to meet their water needs regardless of climatic conditions. Conversely, if the sandstone represents a longer time span, two of the sampled specimens utilized a different water source than the third.
The greater seasonal variation observed in the δ18O values of the third specimen (M-KDR-1/5) may mean that there were more acute fluctuations between rainy and dry seasons at the time that individual lived. This specimen, sampled from the posterior lamella of the molar, would have been particularly influenced by dampening, suggesting that the variations in input could be even greater than what is reflected in the reported δ18O values. However, MKDR 1/54, which was also sampled posteriorly, shows much less variation, suggesting that the dampening effect is likely less significant and that behavioral factors are the predominant influence. The Awash River is a perennial water source throughout the Afar Rift, sustained by rainfall from the highlands, including the Addis region. Most of this rainfall occurs during the northern summer months (June, July, and August), leading to the swelling of the main Awash stream and its tributaries. During the dry season (November to May), most animals in the Afar rely heavily on the main Awash River for water. Additionally, the presence of more permanent ponds during certain periods may have provided alternative sources of non-meteoric water, which could also explain the elevated δ18O value observed in the third specimen. Overall, the serially sampled isotopic data show that P. cf. recki recki was flexible enough to adapt to seasonal fluctuations in food and water sources, even during a period of increased climatic instability.
Based on our results, Palaeoloxodon in the Awash basin appeared to have lived in a landscape where resources were stable enough to support a C4 grass-based diet and a stable water supply. However, our results also suggest that these elephants were adaptable enough to cope with environmental changes by including C3 vegetation when necessary/available and utilizing transient water sources when rainfall patterns changed. This adaptability suggests that Palaeoloxodon was not strictly bound by its grazing specializations but, like other large-bodied herbivores, retained the physiological ability to digest a diversity of foods. Their large body size and increased gut capacity would have allowed prolonged retention of ingesta, facilitating the digestion of both grass and browse [94,95]. Such traits likely enabled Palaeoloxodon to expand its dietary niche under fluctuating conditions, making the lineage more resilient than its specialized dental morphology might suggest. In this respect, our results are more consistent with the second scenario outlined in the introduction, which sees P. recki recki slowly evolve into P. jolensis.
If this was the case, then why did the lineage not adapt to fluctuating resource availabilities by shifting towards more generalized dental morphologies like several other taxa? As mentioned in the introduction, researchers are realizing that dental morphology does not always accurately reflect diet within species or lineages [42]. For instance, hypsodonty has traditionally been regarded as an adaptation to grazing; however, this trait is also observed in some browsers like the pronghorn Antilocapra americana [96]. Therefore, hypsodonty should not necessarily be regarded as a trait that restricts diets to grazing; rather, it is a trait that potentially increases dietary flexibility by enabling species to graze, particularly in arid conditions, while still retaining the ability to browse [97]. The same goes for many of the other “grazing” traits that continued to evolve in Paleoloxodon dentition. Also, from the evolutionary record of proboscideans, it is clear that major changes in diets preceded changes in dental morphology, sometimes by millions of years [14]. Its ability to make use of a variety of food and water resources likely played a crucial role in Palaeoloxodon’s prolonged survival in the face of climatic instability, raising questions about the reasons behind its eventual demise.
4.5. Were Hominins Part of the Equation
The rise of our lineage during the African Middle Pleistocene broadly coincided with the Acheulean–MSA transition and the ultimate demise of the “E. recki complex” [3,98]. This has revived the suggestion that megafauna, particularly Palaeoloxodon, succumbed to hominin overhunting during this period [30,44]. However, unlike much of the New World, where debates on the overkill hypothesis rely on a plethora of megafaunal and associated archeological remains from more exhaustive researched contexts [99], the African Middle Pleistocene record presents an enigma whose resolution requires evidence from various sources.
The limited claims of elephant butchery in the Awash basin are based on the association of skeletal remains and Acheulean artifacts. However, there is no clear evidence of bone modifications that would indicate hunting or even scavenging (e.g., perimortem trauma marks, spiral fractures, and chop-, percussion-, or cutmarks). At Barogali, along the Lake Abhe shores on the Djibouti side, a 1.3 Ma P.recki ileretensis skeleton discovered in association with stone tools has been interpreted as evidence of butchery [100]. However, poor surface preservation on this specimen prevents a definitive conclusion regarding carcass processing. The recent invalidation [101] of similar butchery claims of Middle Pleistocene Palaeoloxodon, based on bone artifact association and skeletal element dispersion [102], highlights the need to revisit previous claims for megafaunal butchery using current knowledge and methodological advances [66,103,104,105].
At Daka, several remains of P. recki recki with good bone surface preservation were discovered in association with Acheulean tools from ~1.0 Ma [23]. However, unlike other taxa with clear bone modification marks from the locality, no elephant bone with such marks was identified ([91], p.6). Our investigation of additional elephantid postcranial bones from relevant sites in the Megenta and Middle Awash study areas did not provide any conclusive evidence of elephant carcass processing. While recognizing that the absence of evidence here cannot be used as evidence of absence, building a strong case for elephant hunting, even scavenging, requires the identification of bone modification marks while ruling out other evidence of modification such as carnivore tooth marks [66,106].
The assessment of the overhunting hypothesis relevant in our case requires several caveats. On the one hand, there is convincing evidence of megafauna butchery during the Early and Middle Pleistocene [1,66,107,108,109]. On the other hand, even when conclusive evidence of elephant butchery, inferred from bone modification marks, exists, it does not necessarily imply hunting and primary access to carcasses [99]. In fact, one would expect that primary access to such large carcasses would leave little, if any, marks on the bone as there would be excess meat that must be consumed within a short period of time before the kill attracts other carnivores. In the eastern African savanna environment, such large carcasses would also spoil faster than in the equatorial forests where ethnohistorical evidence for rare elephant hunting exists. Coupled with the meager claim of elephant butchery, which contrasts sharply with that of hippopotamids [1,66] it remains difficult to assess whether hominin overhunting contributed to the extinction of P. recki recki in Africa. In any event, the likelihood that P. recki recki, in the form of P. jolensis, persisted into the Middle–Late Pleistocene interface [26] renders this hypothesis moot in the African context.
The limited ethnographic evidence of elephant hunting for meat shows that it was an extremely dangerous activity that was conducted in groups and involved meat sharing among many members of villages and/or some form of meat exchange with neighboring groups [110]. Because African ethnographic cases usually involve metal-tipped hunting spears and poison, their relevance does not extend to the Middle Pleistocene [111,112]. From a technological point of view, one would expect to see evidence of the ability to throw at a distance with a force and accuracy that would allow the spear to successfully break the thick skin of the elephant. Such an ability to effectively hunt with a stone-tipped throwing spear from distances of >20m likely did not appear before 0.3 Ma in Africa [113,114]. Contact/ambush hunting in which even a single hunter delivered a fatal wound through the thin skin cover around the elephant’s neck/ear could have been effective. However, proving such hunting mechanisms archeologically would be difficult.
5. Conclusions
The uncertainty around the taxonomic significance of the final evolutionary stages of the “E. recki complex” and the reasons for its ultimate demise in Africa at the end of the Middle Pleistocene present an enigmatic puzzle in megafaunal extinction, often at the center of intense debate. The notable absence of cranial remains continues to hinder the taxonomic classification of some of the last representatives of this lineage. However, the ongoing discovery of fossils crucial to this debate offers hope for new insights [26]. The recent discovery of molars tentatively attributed to very late-occurring P. cf. recki recki, from sediments dating to this critical period in the Megenta study area, has enabled us to examine seasonal variation in diets and water consumption patterns. Those data, when combined with our comparison of broad dietary and water consumption patterns of Early and Middle Pleistocene Palaeoloxodon in the Awash basin, contribute to a more nuanced understanding of the ecological and behavioral adaptations of this proboscidean lineage during the period leading up to its extinction.
Previous studies have proposed that the “E. recki complex” was a lineage of highly specialized grazers, whose extinction was a result of their inability to adapt to climate-induced resource fluctuations and/or shifts during the Middle Pleistocene [14,74]. However, this hypothesis is based on the results of studies examining samples dating from 4.0 to 1.0 Ma in the Turkana Basin of Kenya and does not account for data from the critical period of climatic deterioration or from other regions. Recent data presented by Manthi et al., [26] have challenged this hypothesis, demonstrating that P. jolensis survived until ~0.13 Ma in eastern Africa, despite retaining its adaptations to grazing.
Our study of Palaeoloxodon samples that span the period between 1.2 and <0.15 Ma within the Awash basin has enabled us to closely test the hypothesis around patterns of dietary adaptation in the context of changing environmental conditions. We show that while C4 grasses continued to dominate Palaeoloxodon diets for the duration of the Middle Pleistocene, C3 plants were also consumed. It is possible that the δ13C values underestimate the consumption of C3 plants, likely due to the dampening effect that occurs during the maturation process of elephant teeth. In addition, δ18O values reveal a clear shift in water source usage, from a focus on stable water sources during the early Middle Pleistocene to the inclusion of more transient sources in the later stages. This is possibly due to a combination of factors, including unpredictable weather patterns and fragmented landscapes.
The serially sampled δ13C and δ18O values from three individuals from Megenta provide insights into seasonal dietary and water consumption patterns during a period of significant climatic fluctuations. The δ13C values suggest a diet primarily composed of C4 grasses, with minimal inclusion of C3 vegetation in two individuals. All three individuals had access to C4 grass year-round, suggesting consistent grassland productivity. The δ18O values show that two individuals relied on stable water sources, such as the paleo-Awash River, while the third individual used a mix of stable and transient water sources. Overall, the serially sampled isotopic data demonstrate that Palaeoloxodon adapted to seasonal fluctuations in food and water sources, even during a period of increased climatic instability. While our study raises questions about the role of climatic and environmental instability in the demise of the “E. recki complex”, we would like to caution that the Awash basin only represents one region in which this lineage occurred. In addition, it also appears to have been buffered from the worst effects of the climatic extremes of the period.
Our results favor the scenario where P. recki recki did not die out but adapted, in the form of P. jolensis, to changing climatic and environmental conditions coinciding with the Acheulean–MSA transition. If hominins were not responsible for the extinction of P. recki recki, at the end of the Acheulean, it is possible that they were ultimately responsible for the final demise of Palaeoloxodon during the MSA [30]. However, we note that there is no clear evidence of elephant butchery in any of the sites sampled for this study, or for that matter, any other Awash basin sites of the period. Neither is there conclusive evidence from the Pleistocene elsewhere in Africa to support such a hominin impact of catastrophic proportions on Palaeoloxodon. Establishing a causal relationship will be extremely difficult because bone modification marks and functional associations cannot definitively differentiate between scavenging and hominin predation.
Identifying the causes for the demise of a lineage that for a long time successfully dominated the African Pleistocene landscape would resolve an age-old debate and mystery. As tempting as that appears, we argue that there is currently no smoking gun that will solve the case. Ensuing research must rely on redoubled field and laboratory research in which the long evolutionary, ecological, and adaptive histories of both Palaeoloxodon [11,13] and hominins are carefully examined. Our work represents one such effort toward an integrative investigation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/quat8010016/s1. Supplementary Table S1. δ13C and δ18OvPDB of bulk enamel samples of all species. Results of % C3 and % C4 consumed using a simple mixing model are given alongside each sample based on Cerling et al. [115]) C3/C4 end points. Asterisked locality names denote the ‘upper (i.e., younger) sedimentary sections of the respective localities. The KL (Kalb Localities) collections are all from what is today the Middle Awash study area. They were collected by the late Jon Kalb as part of his Rift Valley Research Mission in Ethiopia (RVRME). Some of the locality names and spellings have since been revised by the Middle Awash project. We use the original (e.g., [91]) locality names and spellings. Supplementary Table S2. δ13C and δ18OvPDB results for three serially sampled P. cf. recki recki specimens from Megenta. Supplementary Table S3. Outcome of Kruskall–Wallis pairwise comparison tests (Table S3a is for δ13C and Table S3b is for δ18O). Supplementary Figure S1. Photographs depicting a selection of specimens sampled for the bulk SLI analysis: (a, b) elephants, (c, d) hippopotamus, (e) equid, and (f) giraffe.
Author Contributions
Conceptualization, J.L., Y.S. and D.S. Methodology, J.L. and Y.S.; Formal analysis, J.L.; investigation, Y.S.; data curation, J.L.; writing—original draft preparation, J.L., Y.S. and D.S.; writing—review and editing, J.L., Y.S. and D.S.; visualization, Y.S. and J.L.; project administration, Y.S.; funding acquisition, Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work is based on the research supported by the National Research Foundation of South Africa (Grant Number: 150524) and the Fritz Thyssen Stiftung (10.22.1.020AA) to Y.S.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the Ethiopian Heritage Authority for access to fossil specimens and museum laboratory facilities. We are grateful to the Gona and Middle Awash projects for permission to sample some of the specimens included in our study. We thank Malefeu Lethuba for assistance in the isotope laboratory. We would also like to thank Tomas Getachew, William Sanders, Steven Zhang, and Judith Sealy for insights regarding some aspects of the specimens and analyses included in this paper. Finally, we are greatly indebted to Haruo Saegusa whose initial comments on the taxonomic aspects of this paper have been helpful. We dedicate this work to him.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Correction Statement
This article has been republished with a minor correction to the readability of Figure 1. This change does not affect the scientific content of the article.
References
- Sahle, Y.; Beyene, Y.; Defleur, A.; Asfaw, B.; WoldeGabriel, G.; Hart, W.K.; Morgan, L.E.; Renne, P.R.; Carlson, J.P.; White, T.D. Revisiting Herto: New evidence of Homo sapiens from Ethiopia. In Modern Human Origins and Dispersals; Sahle, Y., Reyes-Centeno, H., Bentz, C., Eds.; Kerns Verlag: Tübingen, Germany, 2019; pp. 105–137. [Google Scholar]
- McKee, J.K. Faunal turnover rates and mammalian biodiversity of the late Pliocene and Pleistocene of Eastern Africa. Paleobiology 2001, 27, 500–511. [Google Scholar] [CrossRef]
- Potts, R.; Behrensmeyer, A.K.; Faith, J.T.; Tryon, C.A.; Brooks, A.S.; Yellen, J.E.; Deino, A.L.; Kinyanjui, R.; Clark, J.B.; Haradon, C.M.; et al. Environmental dynamics during the onset of the Middle Stone Age in eastern Africa. Science 2018, 360, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.M.; Lane, C.S.; Asrat, A.; Barfod, D.N.; Mark, D.F.; Tomlinson, E.L.; Tadesse, A.Z.; Yirgu, G.; Deino, A.; Hutchison, W.; et al. Age of the oldest known Homo sapiens from eastern Africa. Nature 2022, 601, 579–583. [Google Scholar] [CrossRef]
- Faith, J.T.; Du, A.; Behrensmeyer, A.K.; Davies, B.; Patterson, D.B.; Rowan, J.; Wood, B. Rethinking the ecological drivers of hominin evolution. Trends Ecol. Evol. 2021, 36, 797–807. [Google Scholar] [CrossRef]
- Lupien, R.L.; Russell, J.M.; Subramanian, A.; Kinyanjui, R.; Beverly, E.J.; Uno, K.T.; de Menocal, P.; Dommain, R.; Potts, R. Eastern African environmental variation and its role in the evolution and cultural change of Homo over the last 1 million years. J. Hum. Evol. 2021, 157, 103028. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.S.; Du, A.; Rowan, J.; Yost, C.L.; Billingsley, A.L.; Campisano, C.J.; Brown, E.T.; Deino, A.L.; Feibel, C.S.; Grant, K.; et al. Plio-Pleistocene environmental variability in Africa and its implications for mammalian evolution. Proc. Natl. Acad. Sci. USA 2022, 119, e2107393119. [Google Scholar] [CrossRef]
- Bibi, F.; Cantalapiedra, J.L. Plio-Pleistocene African megaherbivore losses associated with community biomass restructuring. Science 2023, 380, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Faith, J.T.; Rowan, J.; Du, A. Late Cenozoic Faunal and Ecological Change in Africa. Annu. Rev. Earth Planet. Sci. 2024, 52, 379–407. [Google Scholar] [CrossRef]
- Zhang, H. Evolution and Systematics of the Elephantidae (Mammalia, Proboscidea) from the Late Miocene to Recent. Ph.D. Thesis, University of Bristol, Bristol, UK, 2020. Unpublished. [Google Scholar]
- Sanders, W.J. Evolution and Fossil Record of African Proboscidea, 1st ed.; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar]
- Sanders, W.J.; Gheerbrant, E.; Harris, J.M.; Saegusa, H.; Delmer, C. Proboscidea. In Cenozoic Mammals of Africa; Werdelin, L., Sanders, W.J., Eds.; University of California Press: Berkeley, CA, USA, 2010; pp. 163–251. [Google Scholar]
- Lister, A.M. The role of behaviour in adaptive morphological evolution of African proboscideans. Nature 2013, 500, 331–334. [Google Scholar] [CrossRef]
- Saarinen, J.; Lister, A.M. Fluctuating climate and dietary innovation drove ratcheted evolution of proboscidean dental traits. Nat. Ecol. Evol. 2023, 7, 1490–1502. [Google Scholar] [CrossRef]
- Dietrich, W.O. Elephas antiquus recki n.f. aus dem Diluvium Deutch-Ostafrikas. In Wissenschaftliche Ergebnisse; Reck, H., Ed.; Oldoway Expedition: Berlin, Germany, 1915; pp. 1–80. [Google Scholar]
- Arambourg, C. L’Elephas recki Dietrich, sa position systematique et ses affinites. Bull. De La Société Géologique De Fr. 1942, 5, 73–89. [Google Scholar] [CrossRef]
- Arambourg, C.; Chavaillon, J.; Coppens, Y. Résultatsde la Nouvelle Mission de l’Omo (2e Campagne 1968). Comptes Rendus Des Séances L’académie Des Sci. 1969, 268, 759–762. [Google Scholar]
- Maglio, V.J. Origin and evolution of the Elephantidae. Trans. Amer. Philos. Soc. 1973, 63, 1–144. [Google Scholar] [CrossRef]
- Beden, M. Les Eléphants (Loxodonta et Elephas) d’Afrique Orientale. Systématique, Phylogénie, Intéret Biochronologie. Ph.D. Thesis, Faculté de Sciences, Université de Poitiers, Poitiers, France, 1979. [Google Scholar]
- Beden, M. Family Elephantidae. In Koobi Fora Research Project 2; Harris, J.M., Ed.; Clarendon Press: Oxford, UK, 1983; pp. 40–129. [Google Scholar]
- Kalb, J.E.; Mebrate, A. Fossil elephantoids: From the hominid-bearing Awash group, middle Awash valley, afar depression, Ethiopia. Trans. Am. Philos. Soc. 1993, 83, i–114. [Google Scholar] [CrossRef]
- Todd, N.E. Reanalysis of African Elephas recki: Implications for time, space and taxonomy. Quat. Int. 2005, 126, 65–72. [Google Scholar] [CrossRef]
- Saegusa, H.; Gilbert, W.H. Elephantidae. In Homo Erectus: Pleistocene Evidence from the Middle Awash, Ethiopia; Gilbert, W.H., Asfaw, B., Eds.; University of California Press: Berkeley, CA, USA, 2009; pp. 193–226. [Google Scholar]
- Sanders, W.J.; Haile-Selassie, Y. A new assemblage of mid-Pliocene proboscideans from the Woranso-Mille area, Afar region, Ethiopia: Taxonomic, evolutionary, and paleoecological considerations. J. Mamm. Evol. 2012, 19, 105–128. [Google Scholar] [CrossRef]
- Larramendi, A.; Zhang, H.; Palombo, M.R.; Ferretti, M.P. The evolution of Palaeoloxodon skull structure: Disentangling phylogenetic, sexually dimorphic, ontogenetic, and allometric morphological signals. Quat. Sci. Rev. 2020, 229, 106090. [Google Scholar] [CrossRef]
- Manthi, F.K.; Sanders, W.J.; Plavcan, J.M.; Cerling, T.E.; Brown, F.H. Late Middle Pleistocene Elephants from Natodomeri, Kenya and the Disappearance of Elephas (Proboscidea, Mammalia) in Africa. J. Mamm. Evol. 2020, 27, 483–495. [Google Scholar] [CrossRef]
- Klein, R.G. Why anatomically modern people did not disperse from Africa 100,000 years ago. In Neandertals and Modern Humans in Western Asia; Akazawa, T., Aoki, K., Bar-Yosef, O., Eds.; Springer: Boston, MA, USA, 1998; pp. 509–521. [Google Scholar]
- Porat, N.; Chazan, M.; Grün, R.; Aubert, M.; Eisenmann, V.; Horwitz, L.K. New radiometric ages for the Fauresmith industry from Kathu Pan, southern Africa: Implications for the Earlier to Middle Stone Age transition. J. Archaeol. Sci. 2010, 37, 269–283. [Google Scholar] [CrossRef]
- Wilkins, J.; Schoville, B.J.; Brown, K.S.; Chazan, M. Evidence for early hafted hunting technology. Science 2012, 338, 942–946. [Google Scholar] [CrossRef]
- Ben-Dor, M.; Barkai, R. A matter of fat: Hunting preferences affected Pleistocene megafaunal extinctions and human evolution. Quat. Sci. Rev. 2024, 331, 108660. [Google Scholar] [CrossRef]
- Cerling, T.E.; Andanje, S.A.; Blumenthal, S.A.; Brown, F.H.; Chritz, K.L.; Harris, J.M.; Hart, J.A.; Kirera, F.M.; Kaleme, P.; Leakey, L.N.; et al. Dietary changes of large herbivores in the Turkana Basin, Kenya from 4 to 1 Ma. Proc. Natl. Acad. Sci. USA 2015, 112, 11467–11472. [Google Scholar] [CrossRef] [PubMed]
- Wynn, J.G. Influence of Plio-Pleistocene aridification on human evolution: Evidence from paleosols of the Turkana Basin, Kenya. Am. J. Phys. Anthropol. 2004, 123, 106–118. [Google Scholar] [CrossRef]
- Owen, R.B.; Muiruri, V.M.; Lowenstein, T.K.; Renaut, R.W.; Rabideaux, N.; Luo, S.; Deino, A.L.; Sier, M.J.; Dupont-Nivet, G.; McNulty, E.P.; et al. Progressive aridification in East Africa over the last half million years and implications for human evolution. Proc. Natl. Acad. Sci. USA 2018, 115, 11174–11179. [Google Scholar] [CrossRef] [PubMed]
- Caley, T.; Souron, A.; Uno, K.T.; Macho, G.A. Climate and Human Evolution: Insights from Marine Records. Annu. Rev. Mar. Sci. 2024, 17, 23–53. [Google Scholar] [CrossRef]
- Castañeda, I.S.; Caley, T.; Dupont, L.; Kim, J.H.; Malaizé, B.; Schouten, S. Middle to Late Pleistocene vegetation and climate change in subtropical southern East Africa. Earth Planet. Sci. Lett. 2016, 450, 306–316. [Google Scholar] [CrossRef]
- Foerster, V.; Asrat, A.; Bronk Ramsey, C.; Brown, E.T.; Chapot, M.S.; Deino, A.; Duesing, W.; Grove, M.; Hahn, A.; Junginger, A.; et al. Pleistocene climate variability in eastern Africa influenced hominin evolution. Nat. Geosci. 2022, 15, 805–811. [Google Scholar] [CrossRef]
- Potts, R. Environmental hypotheses of hominin evolution. Am. J. Phys. Anthropol. 1998, 107, 93–136. [Google Scholar] [CrossRef]
- Orlando, L.; Metcalf, J.L.; Alberdi, M.T.; Telles-Antunes, M.; Bonjean, D.; Otte, M.; Martin, F.; Eisenmann, V.; Mashkour, M.; Morello, F.; et al. Revising the recent evolutionary history of equids using ancient DNA. Proc. Natl. Acad. Sci. USA 2009, 106, 21754–21759. [Google Scholar] [CrossRef]
- Boisserie, J.R.; Zazzo, A.; Merceron, G.; Blondel, C.; Vignaud, P.; Likius, A.; Brunet, M. Diets of modern and late Miocene hippopotamids: Evidence from carbon isotope composition and micro-wear of tooth enamel. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 221, 153–174. [Google Scholar] [CrossRef]
- Malherbe, M. Dental Occlusal Form and Function in Equus Capensis: Evaluating a Controvertible Taxonomic Status. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 2020. [Google Scholar]
- Assefa, Z.; Yirga, S.; Reed, K.E. The large-mammal fauna from the Kibish Formation. J. Hum. Evol. 2008, 55, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Mihlbachler, M.C.; Solounias, N. Coevolution of tooth crown height and diet in oreodonts (Merycoidodontidae, Artiodactyla) examined with phylogenetically independent contrasts. J. Mamm. Evol. 2006, 13, 11–36. [Google Scholar] [CrossRef]
- Souron, A. Diet and ecology of extant and fossil wild pigs. In Ecology, Conservation and Management of Wild Pigs and Peccaries; Cambridge University Press: Cambridge, UK, 2017; pp. 29–38. [Google Scholar]
- Martin, P.S. Africa and Pleistocene overkill. Nature 1966, 212, 339–342. [Google Scholar] [CrossRef]
- Martin, P.S. Prehistoric overkill. In Pleistocene Extinctions: The Search for a Cause; Martin, P.S., Wright, H.E., Jr., Eds.; Yale University Press: New Heaven, CT, USA, 1967; pp. 75–120. [Google Scholar]
- Surovell, T.A.; Waguespack, N.M.; Brantingham, P.J. Global archaeological evidence for proboscidean overkill. Proc. Natl. Acad. Sci. USA 2005, 102, 6231–6236. [Google Scholar] [CrossRef] [PubMed]
- Cantalapiedra, J.L.; Sanisidro, O.; Zhang, H.; Alberdi, M.T.; Prado, J.L.; Blanco, F.; Saarinen, J. The rise and fall of proboscidean ecological diversity. Nat. Ecol. Evol. 2021, 5, 1266–1272. [Google Scholar] [CrossRef]
- Rundel, P.W.; Ehleringer, J.R.; Nagy, K.A. (Eds.) Stable Isotopes in Ecological Research; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Ben-David, M.; Flaherty, E.A. Stable isotopes in mammalian research: A beginner’s guide. J. Mammal. 2012, 93, 312–328. [Google Scholar] [CrossRef]
- Diefendorf, A.F.; Mueller, K.E.; Wing, S.L.; Koch, P.L.; Freeman, K.H. Global patterns in leaf 13C discrimination and implications for studies of past and future climate. Proc. Natl. Acad. Sci. USA 2010, 107, 5738–5743. [Google Scholar] [CrossRef]
- Kohn, M.J. Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo)ecology and (paleo)climate. Proc. Natl. Acad. Sci. USA 2010, 107, 19691–19695. [Google Scholar] [CrossRef]
- Groenewald, P.A.; Sealy, J.; Stynder, D.; Smith, K.M. Dietary resource partitioning among three coeval proboscidean taxa (Anancus capensis, Mammuthus subplanifrons, Loxodonta cookei) from the South African Early Pliocene locality of Langebaanweg E Quarry. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 543, 109606. [Google Scholar] [CrossRef]
- Bryant, J.D.; Froelich, P.N. A model of oxygen isotope fractionation in body water of large mammals. Geochim. Cosmochim. Acta 1995, 59, 4523–4537. [Google Scholar] [CrossRef]
- Bryant, J.D.; Froelich, P.N.; Fricke, H.C.; O’Neil, J.R.; Lynnerup, N. Oxygen isotope composition of human tooth enamel from medieval Greenland. Geology 1996, 24, 477–479. [Google Scholar] [CrossRef]
- Sponheimer, M.; Lee-Thorp, J.A. The oxygen isotope composition of mammalian enamel carbonate from Morea Estate, South Africa. Oecologia 2001, 126, 153–157. [Google Scholar] [CrossRef]
- Lee-Thorp, J.A.; Sponheimer, M. Opportunities and constraints for reconstructing palaeoenvironments from stable light isotope ratios in fossils. Geol. Q. 2005, 49, 195–204. [Google Scholar]
- Lee-Thorp, J.A. On Isotopes and Old Bones. Archaeometry 2008, 50, 925–950. [Google Scholar] [CrossRef]
- Levin, N.E.; Cerling, T.E.; Passey, B.H.; Harris, J.M.; Ehleringer, J.R. A stable isotope aridity index for terrestrial environments. Proc. Natl. Acad. Sci. USA 2006, 103, 11201–11205. [Google Scholar] [CrossRef]
- Blumenthal, S.A.; Levin, N.E.; Brown, F.H.; Brugal, J.P.; Chritz, K.L.; Harris, J.M.; Jehle, G.E.; Cerling, T.E. Aridity and hominin environments. Proc. Natl. Acad. Sci. USA 2017, 114, 7331–7336. [Google Scholar] [CrossRef] [PubMed]
- Faith, J.T. Paleodietary change and its implications for aridity indices derived from δ18O of herbivore tooth enamel. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 490, 571–578. [Google Scholar] [CrossRef]
- Sahle, Y.; Giusti, D.; Tourloukis, V. Newly discovered Middle Pleistocene hominid-bearing deposits from the Lower Awash basin, Ethiopia. Anthropol. Sci. 2019, 127, 141–147. [Google Scholar] [CrossRef]
- Semaw, S.; Rogers, M.J.; Simpson, S.W.; Levin, N.E.; Quade, J.; Dunbar, N.; McIntosh, W.C.; Cáceres, I.; Stinchcomb, G.E.; Holloway, R.L.; et al. Co-occurrence of Acheulian and Oldowan artifacts with Homo erectus cranial fossils from Gona, Afar, Ethiopia. Sci. Adv. 2020, 6, eaaw4694. [Google Scholar] [CrossRef]
- Stinchcomb, G.E.; Quade, J.; Levin, N.E.; Iverson, N.; Dunbar, N.; McIntosh, W.; Arnold, L.J.; Demuro, M.; Duval, M.; Grün, R.; et al. Fluvial response to Quaternary hydroclimate in eastern Africa: Evidence from Gona, Afar, Ethiopia. Quat. Sci. Rev. 2023, 309, 108083. [Google Scholar] [CrossRef]
- Uno, K.T.; Fisher, D.C.; Wittemyer, G.; Douglas-Hamilton, I.; Carpenter, N.; Omondi, P.; Cerling, T.E. Forward and inverse methods for extracting climate and diet information from stable isotope profiles in proboscidean molars. Quat. Int. 2020, 557, 92–109. [Google Scholar] [CrossRef]
- Yang, D.; Bowen, G.J.; Uno, K.T.; Podkovyroff, K.; Carpenter, N.A.; Fernandez, D.P.; Cerling, T.E. BITS: A Bayesian Isotope Turnover and Sampling model for strontium isotopes in proboscideans and its potential utility in movement ecology. Methods Ecol. Evol. 2023, 14, 2800–2813. [Google Scholar] [CrossRef]
- Sahle, Y.; El Zaatari, S.; White, T.D. Hominid butchers and biting crocodiles in the African Plio–Pleistocene. Proc. Natl. Acad. Sci. USA 2017, 114, 13164–13169. [Google Scholar] [CrossRef]
- Haynes, G. Late quaternary proboscidean sites in Africa and Eurasia with possible or probable evidence for hominin involvement. Quaternary 2022, 5, 18. [Google Scholar] [CrossRef]
- Sponheimer, M.; Lee-Thorp, J.A. Alteration of Enamel Carbonate Environments during Fossilization. J. Archaeol. Sci. 1999, 26, 143–150. [Google Scholar] [CrossRef]
- Sponheimer, M.; Lee-Thorp, J.A. Enamel diagenesis at South African Australopith sites: Implications for paleoecological reconstruction with trace elements. Geochim. Cosmochim. Acta 2006, 70, 1644–1654. [Google Scholar] [CrossRef]
- Luyt, J.; Sealy, J. Inter-tooth comparison of δ13C and δ18O in ungulate tooth enamel from south-western Africa. Quat. Int. 2018, 495, 144–152. [Google Scholar] [CrossRef]
- Webb, E.C.; White, C.D.; Longstaffe, F.J. Investigating inherent differences in isotopic composition between human bone and enamel bioapatite: Implications for reconstructing residential histories. J. Archaeol. Sci. 2014, 50, 97–107. [Google Scholar] [CrossRef]
- Stichler, W. Interlaboratory comparison of new materials for carbon and oxygen ratio measurements. In Proceedings of the Consultants’ Meeting, Vienna, Austria, 1–3 December 1993; IAEA-TECDOC-825; IAEA: Vienna, Austria, 1995; pp. 67–74. [Google Scholar]
- Vogel, J.C. Isotopic assessment of the dietary habits of ungulates. S. Afr. J. Sci. 1978, 74, 298. [Google Scholar]
- Cerling, T.E.; Harris, J.M.; MacFadden, B.J.; Leakey, M.G.; Quade, J.; Eisenmann, V.; Ehleringer, J.R. Global vegetation change through the Miocene/Pliocene boundary. Nature 1997, 389, 153–158. [Google Scholar] [CrossRef]
- Sponheimer, M.; Lee-Thorp, J.A.; DeRuiter, D.J.; Smith, J.M.; Van Der Merwe, N.J.; Reed, K.; Marcus, W. Diets of southern African Bovidae: Stable isotope evidence. J. Mammal. 2003, 84, 471–479. [Google Scholar] [CrossRef]
- Luyt, J.; Faith, J.T.; Sealy, J. Large herbivore δ18O as a proxy for aridity in the South African winter and year-round rainfall zone. Quat. Res. 2024, 122, 92–105. [Google Scholar] [CrossRef]
- Braun, D.R.; Levin, N.E.; Stynder, D.; Herries, A.I.; Archer, W.; Forrest, F.; Roberts, D.L.; Bishop, L.C.; Matthews, T.; Lehmann, S.B.; et al. Mid-Pleistocene hominin occupation at Elandsfontein, Western Cape, South Africa. Quat. Sci. Rev. 2013, 82, 145–166. [Google Scholar] [CrossRef]
- Lehmann, S.B.; Braun, D.R.; Dennis, K.J.; Patterson, D.B.; Stynder, D.D.; Bishop, L.C.; Forrest, F.; Levin, N.E. Stable isotopic composition of fossil mammal teeth and environmental change in southwestern South Africa during the Pliocene and Pleistocene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 457, 396–408. [Google Scholar] [CrossRef]
- Robinson, J.R.; Rowan, J.; Faith, J.T.; Fleagle, J.G. Paleoenvironmental change in the late middle Pleistocene–Holocene Kibish Formation, southern Ethiopia: Evidence from ungulate isotopic ecology. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 450, 50–59. [Google Scholar] [CrossRef]
- Levin, N.E.; Simpson, S.W.; Quade, J.; Cerling, T.E.; Frost, S.R. Herbivore enamel carbon isotopic composition and the environmental context of Ardipithecus at Gona, Ethiopia. In The Geology of Early Humans in the Horn of Africa; Geological Society of America Special Paper; Geological Society of America: Boulder, CO, USA, 2008; Volume 446, pp. 215–234. [Google Scholar]
- Blondel, C.; Rowan, J.; Merceron, G.; Bibi, F.; Negash, E.; Barr, W.A.; Boisserie, J.R. Feeding ecology of Tragelaphini (Bovidae) from the Shungura Formation, Omo Valley, Ethiopia: Contribution of dental wear analyses. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 496, 103–120. [Google Scholar] [CrossRef]
- Ramírez-Pedraza, I.; Tornero, C.; Aouraghe, H.; Rivals, F.; Patalano, R.; Haddoumi, H.; Sala-Ramos, R. Arid, mosaic environments during the Plio-Pleistocene transition and early hominin dispersals in northern Africa. Nat. Commun. 2024, 15, 8393. [Google Scholar] [CrossRef]
- Souron, A. Morphology, diet, and stable carbon isotopes: On the diet of Theropithecus and some limits of uniformitarianism in paleoecology. Am. J. Phys. Anthropol. 2018, 166, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Souron, A.; Balasse, M.; Boisserie, J.R. Intra-tooth isotopic profiles of canines from extant Hippopotamus amphibius and late Pliocene hippopotamids (Shungura Formation, Ethiopia): Insights into the seasonality of diet and climate. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 342, 97–110. [Google Scholar] [CrossRef]
- Chritz, K.L.; Blumenthal, S.A.; Cerling, T.E.; Klingel, H. Hippopotamus (H. amphibius) diet change indicates herbaceous plant encroachment following megaherbivore population collapse. Sci. Rep. 2016, 6, 32807. [Google Scholar] [CrossRef]
- Gagnon, M.; Chew, A.E. Dietary preferences in extant African Bovidae. J. Mammal. 2000, 81, 490–511. [Google Scholar] [CrossRef][Green Version]
- Berry, P.S.; Bercovitch, F.B. Seasonal and geographical influences on the feeding ecology of giraffes in the Luangwa Valley, Zambia: 1973–2014. Afr. J. Ecol. 2017, 55, 80–90. [Google Scholar] [CrossRef]
- Cerling, T.E.; Andanje, S.A.; Gakuya, F.; Kariuki, J.M.; Kariuki, L.; Kingoo, J.W.; Khayale, C.; Lekolool, I.; Macharia, A.N.; Anderson, C.R.; et al. Stable isotope ecology of black rhinos (Diceros bicornis) in Kenya. Oecologia 2018, 187, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Wynn, J.G.; Roman, D.C.; Alemseged, Z.; Reed, D.; Geraads, D.; Munro, S. Stratigraphy, depositional environments, and basin structure of the Hadar and Busidima Formations at Dikika, Ethiopia. Geol. Soc. Am. Spec. Pap. 2008, 446, 87–118. [Google Scholar]
- Bedaso, Z.; Wynn, J.G.; Alemseged, Z.; Geraads, D. Paleoenvironmental reconstruction of the Asbole fauna (Busidima Formation, Afar, Ethiopia) using stable isotopes. Geobios 2010, 43, 165–177. [Google Scholar] [CrossRef]
- Gilbert, W.H.; Frost, S.R. Cercopithecidae. In Homo erectus: Pleistocene Evidence from the Middle Awash, Ethiopia; Gilbert, W.H., Asfaw, B., Eds.; University of California Press: Berkeley, CA, USA, 2009; pp. 115–132. [Google Scholar]
- Kalb, J.E.; Jaegar, M.; Jolly, C.J.; Kana, B. Preliminary geology, paleontology and paleoecology of a Sangoan site at Andalee, Middle Awash Valley, Ethiopia. J. Archaeol. Sci. 1982, 9, 349–363. [Google Scholar] [CrossRef]
- Geraads, D.; Alemseged, Z.; Reed, D.; Wynn, J.; Roman, D.C. The Pleistocene fauna (other than Primates) from Asbole, lower Awash Valley, Ethiopia, and its environmental and biochronological implications. Geobios 2004, 37, 697–718. [Google Scholar] [CrossRef]
- Parra, R. Comparison of foregut and hindgut fermentation in herbivores. In The Ecology of Arboreal Folivores; Montgomery, G.G., Ed.; Smithsonian Inst. Press: Washington, DC, USA, 1978; pp. 209–229. [Google Scholar]
- Demment, M.W.; Van Soest, P.J. A nutritional explanation for body-size patterns of ruminant and nonruminant herbivores. Am. Nat. 1985, 125, 641–672. [Google Scholar] [CrossRef]
- Semprebon, G.M.; Rivals, F. Was grass more prevalent in the pronghorn past? An assessment of the dietary adaptations of Miocene to recent Antilocapridae (Mammalia: Artiodactyla). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 253, 332–347. [Google Scholar] [CrossRef]
- Feranec, R.S. Stable isotopes, hypsodonty, and the paleodiet of Hemiauchenia (Mammalia: Camelidae): A morphological specialization creating ecological generalization. Paleobiology 2003, 29, 230–242. [Google Scholar] [CrossRef]
- Brooks, A.S.; Yellen, J.E.; Potts, R.; Behrensmeyer, A.K.; Deino, A.L.; Leslie, D.E.; Ambrose, S.H.; Ferguson, J.R.; d’Errico, F.; Zipkin, A.M.; et al. Long-distance stone transport and pigment use in the earliest Middle Stone Age. Science 2018, 360, 90–94. [Google Scholar] [CrossRef]
- Faith, J.T.; Rowan, J.; Du, A.; Barr, W.A. The uncertain case for human-driven extinctions prior to Homo sapiens. Quat. Res. 2020, 96, 88–104. [Google Scholar] [CrossRef]
- Berthelet, A.; Chavaillon, J. The early Palaeolithic butchery site of Barogali (Republic of Djibouti). In The World of Elephants. International Congress Proceedings, Consiglio Nazionale Delle Ricerche; Cavarretta, G., Gioia, P., Mussi, M., Palombo, M.R., Eds.; Consiglio Nazionale delle Ricerche: Rome, Italy, 2001; pp. 176–179. [Google Scholar]
- Wright, D.K.; Thompson, J.; Mackay, A.; Welling, M.; Forman, S.L.; Price, G.; Zhao, J.X.; Cohen, A.S.; Malijani, O.; Gomani-Chindebvu, E. Renewed geoarchaeological investigations of mwanganda’s village (elephant butchery site), Karonga, Malawi. Geoarchaeology 2014, 29, 98–120. [Google Scholar] [CrossRef]
- Clark, J.D.; Haynes, C.V., Jr. An elephant butchery site at Mwanganda’s Village, Karonga, Malawi, and its relevance for Palaeolithic archaeology. World Archaeol. 1970, 1, 390–411. [Google Scholar] [CrossRef]
- Domínguez-Rodrigo, M.; Pickering, T.R.; Bunn, H.T. Configurational approach to identifying the earliest hominin butchers. Proc. Natl. Acad. Sci. USA 2010, 107, 20929–20934. [Google Scholar] [CrossRef]
- Cifuentes-Alcobendas, G.; Domínguez-Rodrigo, M. More than meets the eye: Use of computer vision algorithms to identify stone tool material through the analysis of cut mark micro-morphology. Archaeol. Anthropol. Sci. 2021, 13, 167. [Google Scholar] [CrossRef]
- Egeland, C.P.; Pobiner, B.L.; Merritt, S.R.; Kunitz, S. Actualistic butchery studies in zooarchaeology: Where we’ve been, where we are now, and where we want to go. J. Anthropol. Archaeol. 2024, 73, 101565. [Google Scholar] [CrossRef]
- Domínguez-Rodrigo, M.; Bunn, H.T.; Yravedra, J. A critical re-evaluation of bone surface modification models for inferring fossil hominin and carnivore interactions through a multivariate approach: Application to the FLK Zinj archaeofaunal assemblage (Olduvai Gorge, Tanzania). Quat. Int. 2014, 322, 32–43. [Google Scholar] [CrossRef]
- Pante, M.C. The larger mammal fossil assemblage from JK2, Bed III, Olduvai Gorge, Tanzania: Implications for the feeding behavior of Homo erectus. J. Hum. Evol. 2013, 64, 68–82. [Google Scholar] [CrossRef]
- Smith, G.M.; Ruebens, K.; Gaudzinski-Windheuser, S.; Steele, T.E. Subsistence strategies throughout the African Middle Pleistocene: Faunal evidence for behavioral change and continuity across the Earlier to Middle Stone Age transition. J. Hum. Evol. 2019, 127, 1–20. [Google Scholar] [CrossRef]
- Yravedra, J.; Rubio-Jara, S.; Courtenay, L.A.; Martos, J.A. Mammal butchery by Homo erectus at the Lower Pleistocene acheulean site of Juma’s korongo 2 (JK2), bed III, Olduvai Gorge, Tanzania. Quat. Sci. Rev. 2020, 249, 106612. [Google Scholar] [CrossRef]
- Ichikawa, M. Elephant hunting by the Mbuti hunter-gatherers in the Eastern Congo Basin. In Tuebingen Paleoanthropology Book Series—Contributions in Paleoanthropology Band 1: Human-Elephant Interactions: From Past to Present; Konidaris, G., Barkai, R., Tourloukis, V., Harvati, K., Eds.; University of Tubingen Press: Tübingen, Germany, 2021; pp. 454–467. [Google Scholar]
- Bradfield, J. Identifying bone-tipped arrow types in the archaeological record of southern Africa: The contribution of use-trace studies. J. Afr. Archaeol. 2015, 13, 135–147. [Google Scholar] [CrossRef]
- Lupo, K.D.; Schmitt, D.N. Reframing prehistoric human-proboscidean interactions: On the use and implications of ethnohistoric records for understanding the productivity of hunting megaherbivores. J. Archaeol. Method Theory 2024, 31, 369–413. [Google Scholar] [CrossRef]
- Sahle, Y.; Hutchings, W.K.; Braun, D.R.; Sealy, J.C.; Morgan, L.E.; Negash, A.; Atnafu, B. Earliest stone-tipped projectiles from the Ethiopian Rift date to> 279,000 years ago. PLoS ONE 2013, 8, e78092. [Google Scholar] [CrossRef] [PubMed]
- Sahle, Y.; Lombard, M. The Evolution of Long-Range Hunting with Stone-Tipped Weapons During the Afrotropic Middle Stone Age: A Testable Framework Based on Tip Cross-Sectional Area. Quaternary 2024, 7, 50. [Google Scholar] [CrossRef]
- Cerling, T.E.; Manthi, F.K.; Mbua, E.N.; Leakey, L.N.; Leakey, M.G.; Leakey, R.E.; Brown, F.H.; Grine, F.E.; Hart, J.A.; Kaleme, P.; et al. Stable isotope-based diet reconstructions of Turkana Basin hominins. Proc. Natl. Acad. Sci. USA 2013, 110, 10501–10506. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).