Abstract
As humans expanded across the globe, the Americas were the last continents to be colonized. While debates persist regarding the timing and mechanisms of this process, it is widely accepted that by the Pleistocene–Holocene transition, the New World was populated from Alaska to Tierra del Fuego. During this period, hunter-gatherer societies demonstrated remarkable cultural and adaptive diversity, particularly in subsistence strategies and technological innovations. The colonization of the Americas offers valuable insights into population dynamics, human–environment interactions, species extinctions, and adaptive capacities. From an interdisciplinary perspective that combines an isotopic analysis of megafaunal remains with archaeological evidence, this study examines human interactions with Pleistocene fauna in the south–central region of South America’s Southern Cone. Isotopic analyses provide information about the diets, adaptations, and climatic challenges faced by megafaunal communities. Archaeological evidence reveals that humans utilized megafauna and other Pleistocene species for food and tool production. These findings are supported by evidence such as cut marks and bone tools, but also by sealed sediment layers and/or indisputable associations of lithic artifacts. This research contributes to our understanding of human dispersal in the Southern Cone during the colonization of the Americas, shedding light on the regional environments and adaptive strategies of early populations.
1. Introduction
Lasting approximately 2.6 million years ago in geological time, the Quaternary represents the last geological period, characterized by repeated glaciations. Its first epoch, the Pleistocene, holds significant scientific interest due to the multitude of climate fluctuations experienced and its importance in the evolution of hominids [1,2,3]. As a segment of the Late Pleistocene, the Last Glacial Maximum (LGM) occurred approximately 23.0–19.0 cal. kya (23,000–19,000 calibrated radiocarbon years before present (BP)) [4], followed by the Late Glacial or Post-Glacial (PG) period, often denoted as the end of the Ice Age, and precedes the Holocene, which commenced ~10 kya (~10.000 uncalibrated radiocarbon years BP) or ~11.7 cal. kya [5]. The period between 23.0 and 11.7 cal. kya is a topic of great interest from different scientific points of view (e.g., [6,7,8,9]), as it was the epigone when great environmental and socio-cultural changes happened, followed by the beginning of the Holocene. Particularly, from archaeological, anthropological, and paleontological perspectives, this PG period is linked to the expansion of colonization of the Americas and the extinction of the megafauna (mammalian species over 45 kg, sensu [10]). As humans dispersed across the globe, the Americas were the last continents to be colonized. While there exists a vigorous debate regarding the timing and mechanisms of this peopling process [11,12], there is a consensus that during the Pleistocene–Holocene transition, the New World was inhabited from Alaska in the extreme north to Tierra del Fuego in the extreme south. At that time, hunter-gatherers had significant cultural and adaptive diversity, especially considering subsistence and technology (e.g., [13,14,15,16]). This socio-cultural material evidence would be reflecting one of the possible waves of human colonizers that, during the last millennium of the Pleistocene, rapidly expanded throughout America. These human groups shared traditional technological knowledge of Eurasian Paleolithic and North American ancestors [17,18,19,20], but they also faced new environmental challenges, including encounters with a new and diverse megamammal community.
We focus on the South American sub-continent. The Southern Cone is the territory south of the 22nd parallel, occupied by the south of Brazil, Paraguay, Argentina, Chile, and Uruguay. At this point, it is worth mentioning that a little further north of this latitude, early in the 19th century (between 1835 and 1844), P. Lund conducted significant explorations in the Lagoa Santa region, Minas Gerais State, Brazil [21,22]. There, he discovered the remains of extinct fauna associated with human remains [23,24]. Later investigations in the region have reinforced Lagoa Santa’s significance as a key site for understanding early human occupations in South America [24,25,26,27,28].
Particularly in the Southern Cone, the historic archaeological record continues in the late 19th century with a meaningless theory on the local origin of humankind, the proposals for the exploitation of Pleistocene fauna by F. Ameghino [29,30] in Argentina, and the emerging discussions due to his positions [31]. It was not until the 1930s that Junius Bird (American Museum of Natural History, New York, NY, USA) discovered evidence of human groups coexisting with Pleistocene fauna in the Fell and Pali Aike caves in southern Chile (e.g., [32]) near the Straits of Magellan and close to the Argentine border at the Pali Aike Volcanic Field [33]. These findings occurred a few years later than the historic discoveries of Blackwater Draw, Folsom, and Lindenmeier in North America; sites that reliably demonstrated the exploitation of megafauna by human groups that used Clovis and Folsom points in their armature tips [34,35,36,37]. However, for many years in the history of archeology in the Southern Cone, despite Bird’s findings and the significant discoveries at Tagua Tagua in central Chile approximately three decades later [38], these early sites did not have a great impact on the discussion of the coexistence and exploitation of Pleistocene fauna by humans. Unlike North America, where the increasing number of sites clearly demonstrated how extinct fauna coexisted with and were exploited by humans (e.g., [39,40,41,42,43,44]), the Southern Cone of South America and other parts of the continent lacked explicit evidence for many years. Archaeological sites have been emerging over the last half-century; nevertheless, their cultural remains were much lower in abundance than in North America. Even though notable advances were being made in the 1980s (e.g., [45,46,47,48,49,50]), passionate questions about this topic still persisted up to the beginning of the 1990s [51]. In the last decades, numerous megamammals were identified in archaeological sites, exhibiting signs of human predation. Most of these sites span between the last millennia of the Pleistocene and the first of the Holocene (~14.0 to 11.0 cal. kya), and are located in the south–central portion of the Southern Cone: the Pampean and Patagonian regions and their surrounding areas [52,53,54,55,56,57,58].
Consequently, upon arrival in South America, the early people encountered a remarkably diverse fauna akin to that found in present-day Africa, though the last one is higher [2,3]. This community comprised 49 megamammals, of which 100% of species over 1000 kg and 80% over 45 kg became extinct at the Pleistocene–Holocene transition [59]. South American megafauna were peculiar due to their particular characteristics, gained during the isolation of this continent until 3 million years ago, when the Great American Biotic Interchange (GABI) event occurred after the formation of the Isthmus of Panama joined the two American continents [60]. The entrance of Holarctic species implied the development of new adaptive strategies, such as the evolution of gigantism and defensive structures [61,62]. Although the causes of extinction of the megafauna in a geologically short period at the end of the Pleistocene are still debated currently, after four decades of intense work, there is no doubt that humans coexisted and preyed on this community since the earliest human dispersal in the south–central region of the Southern Cone ([54,57,59,63], among others). Among the most exploited taxa by humans, several Xenarthra species, such as terrestrial ground sloths Mylodontinae/Mylodon darwini, Megatherium americanum, Glossotherium robustum, and the cingulate Doedicurus clavicaudatus, and Equidae species, such as Hippidion saldiasi and Equus neogeus, can be highlighted [59]. Xenarthra is the most conspicuous native South American magnaorder, comprising two orders: Cingulata (armored shape like armadillos, pampatheres, and glyptodons) and Pilosa. This last category is subdivided in Folivora, which includes extinct and extant sloths, and Vermilingua (restricted to the anteaters) [64]. The Equidae family originated in North America and was one of the several groups expanding into South America with the GABI, also becoming extinct during the Pleistocene–Holocene transition. Hippidion saldiasi (266 kg) was distributed in the Patagonian region, while Equus neogeus (378 kg) was found in the Pampean region [65,66]. Gomphotheriidae, another Holarctic immigrant, was less exploited, even though it appears in several archaeological sites in central Chile [67].
As a multidisciplinary research field, studies on the earliest settlement of the Americas have evolved at the intersection of various disciplines; these include biological anthropology, paleontology, archaeology, and geology. Increasingly, the field is expanding to encompass a wider range of investigations, incorporating detailed studies such as isotopic analysis, bioecological modeling, and many others, which build on insights from these diverse areas. Considering this multidisciplinary perspective, this article combines two fields of study: isotopic analysis, which provides insights into Late Pleistocene environments; and archaeology, which offers detailed evidence from various viewpoints on the topic of human settlement in the region. We focus on the period between ~23.0 and 11.0 cal. kya, due to the several climatic and biological events occurring during that time: LGM, PG amelioration, human entrance, and extinction of megafauna [68]. A systematic review of published information of δ13C and δ15N stable isotopes from native and Holarctic groups (Xenarthra and Equidae, respectively) was performed. These isotopes provided a better understanding of niche partitioning before and after human colonization, firmly dated at ~14/13 cal. kya. Three archaeological locales with secure associations between humans, lithic technology, and megafauna were examined. They represent key areas in Pampa and Patagonia, and a neighboring region close to northern Patagonia. In them, association of projectile points, stone and bone tools, as well as the remains of megafauna has been clearly established: Cueva del Medio (CM) in South Patagonia, and Tagua Tagua (TT) in central Chile, along with Campo Laborde (CL) in the Pampean region of Argentina. The locations of these sites coincide with the corners of a triangle that accounts for the majority of the early localities in the region. In this way, isotopy and archaeology provide a general approach to deciphering the local landscapes where humans and megamammals coexisted.
2. An Analytical Perspective
The study of the earliest human settlements in the Americas is inherently multidisciplinary, encompassing a wide array of scientific fields. As research evolves, it increasingly incorporates diverse methodologies, including isotopic analysis, which deepens our understanding of the intricate interactions between humans, mammals, and their environments. While traditional archaeology relies on the study of artifacts, cultural remains, and stratigraphic contexts, isotopic analysis offers a methodology that explores biochemical aspects not directly visible in the material record. This complementarily expands the tools available to archaeologists, providing a richer and more detailed understanding of the past. For example, an isotopic analysis of human and faunal remains can reveal dietary patterns and mobility, while the archaeological context provides the cultural and technological background. The combination of these approaches has been instrumental in identifying the migration routes, subsistence strategies, and environmental adaptations of early populations. Case studies such as those by Dillehay and colleagues [69,70] illustrate how isotopic data, when integrated with archaeological evidence, have clarified aspects of early human behavior that would remain elusive through a single-disciplinary approach. The use of specific examples in which archaeology and isotopy have worked together to address complex questions about the past is justified, demonstrating how these disciplines complement each other to reveal information that would be inaccessible through a single approach. The importance of multidisciplinary research in the field of modern archaeology is emphasized. The combination of archaeological and isotopic approaches not only enriches interpretation but also represents a current trend in research that seeks to integrate diverse data sources for a more integral understanding (e.g., [69,70]). In summary, the justification focuses on how isotopic analysis not only complements but enhances archaeological research, allowing for a more detailed and nuanced understanding of humankind through the convergence of contextual and biochemical data. This section seeks to integrate findings from an isotopic analysis, illuminating Late Pleistocene ecosystems, with archaeological evidence to offer a holistic view of early settlement patterns in the region.
2.1. The Relevance of an Isotopic Analysis
Advancements in the understanding of regional Pleistocene fauna through the use of isotopic analyses have been significant in recent years. In the Pampean and Patagonian regions, several research projects had obtained a hard database of δ13C, δ15N, and δ18O in several extinct and extant species. Most of the analyses concerned the apatite or bioapatite of the bone ([58,71,72,73,74], among others), due to protein conservation issues [68,75,76,77,78,79,80,81]. This research was regionally scaled or was performed to understand the feeding behavior of a specific taxon, and it had shed light on understanding the diets, ecology, and paleoenvironment of megamammals. As isotopes are a link between diets and paleoenvironments, they can be interpreted at an ample scale to understand how trophic chains were before human irruption and after its presence, despite the detailed small-scale information. Although isotopic information is irregularly distributed along time and space, it opens a new window to understand megamammals, environments, and humans just before the extinction of the megamammals, changes in environments, and human colonization of the Southern Cone. At this point, the late Pleistocene megafauna record is still patchy and scarce; thus, its paleoecology, chronology, and taxonomy are still subjects of debate.
In this work, we compiled δ13C and δ15N values to evaluate how Xenarthra and Equidae adapted to the variable Pampean and Patagonian landscapes since LGM times. How these species behaved with different environmental conditions can clarify their capacity of adaptability in their last millennia of habitation in the different South American environments. δ13C and δ15N values are among the direct proxies to understand this adaptation, as they inform directly on the individual being measured. This information can be combined with other ecological information, like their geographical distribution, to give a general picture of the tolerance of megamammals to changing environments.
Based on previous studies, δ13C and δ15N inform about diets and environments, as plants fix stable isotopes of carbon and nitrogen, which are transferred by isotopic discrimination to herbivorous, carnivorous, and omnivorous organisms. They also relate to environmental variables such as the average annual precipitation, temperature, or altitude (see [68,71,77,80,81] and the references cited therein). For the purposes of this work, we highlight that in the interpretation of δ13C, the photosynthetic pathway is important. Plants with C3 photosynthesis include all trees and shrubs, as well as herbaceous plants adapted to cold areas with high-altitude and/or high-latitude conditions [82,83]. On the contrary, those with C4 photosynthesis processes are related to herbaceous plants adapted to dry tropical climates, such as grasses. Thus, δ13C values around –27‰ will reflect the consumption of C3 plants related to a browsing diet, while isotopic ratios around –13‰ will indicate C4-type plants, which would be related to a grazing diet. δ15N is generally used as an indicator of the trophic position of the species, since each level tends to increase between 3‰ and 5‰ over the previous one [83]. However, in plants, δ15N is also related to environmental factors, and herbivores consuming these plants will reflect the local aridity and humidity. Higher δ15N values can be associated with more open and arid environments [84]. Consequently, increasing mean annual precipitation and decreasing mean annual temperature result in decreasing plant δ15N values [83]. Thus, δ13C and δ15N can be good proxies for indirect information on diet preferences, influencing climatic factors, and niche partitioning among the Equidae and Xenarthra communities distributed through the Pampean and Patagonian regions.
Regarding the general environmental trends based on previous isotopic studies, the Pampean region biapatite δ18O and δ13C values have shown an arid–temperate regime during LGM times, with an increase in humid conditions in PG environments [73], especially during the transition to Holocene times [74]. Bocherens and associates [77] have also pointed to aridification influencing δ15N values. A Patagonian isotopic analysis presented how a longitudinal climatic gradient influenced the isotopic variability from the humid western forest to the eastern arid steppe [80]. In the Nahuel Huapi region, the environment evolved from a glacial and dry open environment during the LGM to more humid conditions toward the postglacial transition [68]. A mix of C3 and C3-C4 plants was found in northern Patagonia during PG times, specifically in the Mendoza area [85]. More temperate conditions were described on the eastern side of the Andes [72]. At TT, samples from Gomphotheriidae have shown a closed environment, similar to the current one [58,86]. C3 plant consumption predominates in the southern regions of Patagonia [76,79]. An increase in moisture after LGM times was registered, interrupted by the Antarctic Cold Reverse episode between ~14.5 and 12.8 cal. kya [78,79,87].
2.2. Overview of Archaeological Studies
Archaeologists are tasked with navigating scattered records influenced by a myriad of natural and cultural processes that affect the quality of evidence. Using various methods, particularly excavations, hunter-gatherer archaeology and its derived sub-disciplines (such as lithic analysis and archaeofaunal studies) serve as a primary source of data that interrelate with and enrich other scientific disciplines, including paleontology, various dating techniques, and geoarchaeology, among many others (e.g., [65,88,89]). For instance, the study of ancient animal remains through archaeozoology provides crucial insights into past ecosystems and human diets, revealing which species were hunted and consumed [54,55,56,63,90,91,92]. Similarly, lithic analysis reveals information about tool-making techniques and aids in understanding human population expansion patterns, which are vital for dating methods that depend on the chronological context of artifacts [20]. Archaeology also uncovers the material culture of past societies, including artifacts, tools, and human burial practices, all of which offer valuable insights into their social structures, beliefs, and interactions with the environment. The analysis of bone remains can provide information about human diets, burial practices, and even health conditions, contributing to our understanding of humankind’s evolution and socio-cultural practices. Through these discoveries, we gain a deeper appreciation of human diversity and adaptability throughout the ages. This interconnectedness allows archaeology to function as a true interdisciplinary science, positively feeding back into these fields and enhancing our overall understanding of the past.
Thanks to this interrelated systematic work over the past four decades, a continuous and uninterrupted series of archaeological fieldwork endeavors have significantly advanced our understanding of Late Pleistocene human occupations in the Southern Cone (e.g., [53,57,70,93]). The expansion of human populations was well-documented across the Southern Cone of South America and characterized by small, mobile hunter-gatherer groups with extensive foraging ranges [11]. Foragers in the Southern Cone of South America during this period used “fishtail” or Fell projectile points (FPs). The Camelidae was the most consumed genera before and after the extinction of the megafauna community [94,95,96]. In this category, we consider Lama guanicoe, Lama gracilis, and Lama cf. owenii, even though only the first species survived into Holocene times. Lama cf. owenii especially was exploited in the southern Patagonian sites.
In this early panorama, archaeological evidence on the earliest human occupations exploiting Pleistocene fauna in the region is scattered [53,97], but it had unequivocally demonstrated that Pleistocene fauna not only coexisted with humans but were also actively exploited and utilized as food sources [52,55,56,58,63,92]. Consequently, our purpose is not to make a synopsis of all the sites in which extinct fauna and archaeological remains have been found, a topic that has been discussed with some frequency (e.g., [55,63], among others), but rather a few sites in which the record presents several aspects whose formation suggests that humans exploited and employed megafauna during a delimited timeframe at the terminal period of the Pleistocene and its transition to the Holocene. Thus, the examples of locales referred to in this work (TT, CL, and CM) that have associations of projectile points and/or their lithic assemblages with extinct fauna bones are reliable, considering various supporting factors. These factors include archaeological levels sealed by layers of sediments, conglomerates, and/or rock blocks; indisputable associations of a significant number of lithic artifacts; bone fractures and traces suggesting the exploitation and consumption of Pleistocene fauna; and robust correlations between cultural artifacts and paleontological remains. Preceding these sites are others with promising possibilities of being pre-Clovis and pre-Fell in North and South America, respectively (e.g., [53,55,70,98,99,100,101,102,103,104], among others).
3. Isotopic Analysis: Sampling Sites, Materials, Methodologies and Results
3.1. Isotopic Methodology
Bone samples dated between ~11.5 and 23.0 cal. kya from the Xenarthra, Mylodontinae/Mylodon darwinii, Megatherium americanum, Glossotherium robustum, Doedicurus clavicaudatus, and the equids Hippidion saldiasi and Equus neogeus from archaeological and paleontological sites were considered. The osteoderms classified as Mylodontinae in Patagonia were grouped with Mylodon darwinii, as this is the species most identified in the Patagonian record of Pleistocene ground sloths [94]. Published stable isotopic information (δ13C and δ15N) was classified according to the location in North and South Patagonian and Pampean regions that shape the environmental context of TT, CM and CL correspondingly. We differentiated South and North Patagonia due to environmental and geographical issues [105]. Thus, for southern Patagonia, we considered isotopic information from areas including Última Esperanza, Pali Aike, Tierra del Fuego, and the Central Plateau of Santa Cruz in Argentina and Chile. For the North, we considered information from the Nahuel Huapi region and Mendoza province (Argentina). The southern region of Mendoza is usually considered part of northern Patagonia due to various ecological factors [106]. Although TT is in the extra-Andean region of Chile, a different geographical area, it is located near Northern Patagonia and at a similar latitude to the Pampean region (Figure 1). Information from the Pampean region is from the southwest of Buenos Aires province (Argentina), where several archaeological and paleontological sites were studied.
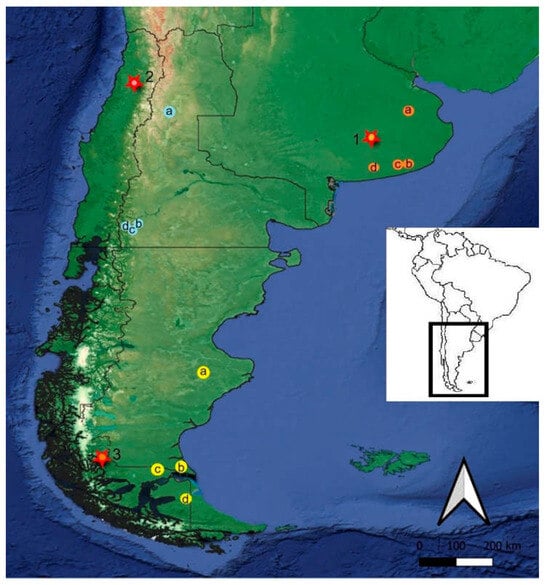
Figure 1.
Geographical location of the study region. References 1, Pampean region, the star indicates the archaeological site of Campo Laborde and the Arroyo Tapalque site. The orange circles indicate the following sites: a, Río Salado, b, Lobería, c, Paso Otero and Río Quequén Grande, and d, Arroyo Seco 2. 2, North Patagonia, the star indicates the archaeological site of Tagua Tagua. The blue light circles indicate the following sites: a, Manqui-Malal, b, Arroyo Corral 1, c, El Trébol, d, and Lake Nahuel Huapi. 3, South Patagonia, the star indicates the archaeological site of Cueva del Medio and the sites of Cueva Lago Sofía, Cueva Escondida, Arroyo Bloques, Cueva Chica, and Cueva del Milodón. The yellow circles indicate the following sites: a, Piedra Museo, b, Cueva de los Chingues, c, Cueva Fell, and d, Tres Arroyos.
In addition, we contemplate for comparison published δ13C collagen data lacking the δ15N and δ13C apatite values [73,74]. Only δ13C values were considered reliable in the published research [87,97,107,108]. Two earlier Pampean sites were also considered—Camet Norte and Arroyo del Vizcaíno dated ~28.0 to 32.0 kya [77,78,79,80,81]—as δ13C and δ15N values of the species analyzed here were measured. These samples provide knowledge of how δ13C and δ15N, or just δ13C, behaved in the considered time period or previously. In this way, the coherence of the samples can be evaluated; especially, this addition helped us to graph species for which we originally counted only two samples: Equus neogeus, Glossotherium robustum, and Doedicurus clavicaudatus.
Statistics, scatter plots, and box plots graphics were used to analyze the distributions of the isotopic values per species and/or region and chronology. Means, standard deviations, minima and maxima of species with more than 2 values served to indicate if δ13C and δ15N values clustered or were dispersed in relation to the mean. A conservative approach was applied; consequently, for individuals with two measurements or for samples with similar chronology and isotopic values, we averaged that information and counted the result as one sample (Table S1). Calibration was performed in https://c14.arch.ox.ac.uk/oxcal/OxCal.html (accessed on 7 April 2024) using Southern Hemisphere curve SHCal20 [109]. Paleoecological information (niche modelling, morphometrics, and use wear analysis) helped to contextualize δ13C and δ15N values at a regional scale. Archaeological evidence (other than from CM, TT, and CL provided direct evidence, such as cut marks or the usage of bones for tools or fuel, of the anthropological spectra of manipulation of the taxa considered in this analysis.
3.2. Isotopic Results
A database of 33 δ13C and δ15N values, with a minimum of two registered for each taxon was obtained (Table 1). Twenty records were registered in South Patagonia: Hippidion saldiasi (n: 13) and Mylodontinae/Mylodon darwinii (n: 7). Five other records were registered in North Patagonia, consisting of Mylodontinae (n: 3) and Megatherium americanum (n: 2). More variability of megafauna was registered in the Pampean region; for that region, eight registers with low quantity per species were counted: two records for Megatherium americanum, two for Equus neogeus, two for Glossotherium robustum, and two for Doedicurus clavicaudatus. Isotopic information from megamammals is irregular due to taphonomic and sampling issues. Erosive processes predominantly affect Patagonia, where the majority of the paleontological record is derived from archaeological sites located in caves with good conservation [94]. Conversely, in the Pampas, depositional processes prevail, resulting in a plethora of widespread and numerous open air paleontological sites and less conservation of the collagen fractions. The irregular isotopic information can also be related to research interests that have oriented the studies in Patagonia and Pampa [94], but the abundance of the different species in the landscape cannot be discarded. Niche modelling has shown a richer and variable fauna in Pampa than in Patagonia [66,110].

Table 1.
Details of the samples. Abbreviations: TS, type of sample; A, archaeological; P, paleontological; CPSC, Central Plateau of Santa Cruz; UE, Última Esperanza; PA, Pali Aike; TF, Tierra del Fuego; NH, Nahuel Huapi; PE, Pedemonte; BA, Buenos Aires; Ar, Argentina; Ch, Chile.
Table 2 details the characteristics of the comparative samples and Table 3 depicts the total n of each species from Table 1 and Table 2. These 16 extra samples, half (n: 8) presented only δ13C, as published for Cueva del Milodón, Paso Otero, El Chacay, and Gruta del Indio; half presented δ13C and δ15N (Camet Norte and Arroyo del Vizcaíno) (details in Table 2). Hippidion saldiasi and Mylodontinae/Mylodon darwinii have similar sampling quantities (n: 13 and 10, respectively) and Megatherium americanum only has 4. When comparative samples are added, Mylodontinae/Mylodon darwinii increases to sixteen and Megatherium americanum to nine. Species with originally two values, Equus neogeus, Glossotherium robustum, and Doedicurus clavicaudatus, increase to three, four, and four, respectively. Notice that no additional samples were counted for Hippidion saldiasi.

Table 2.
Details of the comparative samples. Abbreviations: Ar, Argentina; Ch, Chile.

Table 3.
Total number of radiocarbon samples from 11 to 23 cal. kya with δ13C and δ15N, and comparative samples.
Table 4 presents the statistics for Hippidion saldiasi, Mylodontinae/Mylodon darwinii, Megatherium americanum and the comparison samples. Hippidion saldiasi, Mylodontinae/Mylodon darwinii δ13C and δ15N, and Megatherium americanum δ13C fall equal to or within less than 2σ (95%). The δ13C from Hippidion saldiasi and Mylodontinae/Mylodon darwinii falls within 1σ (68%), indicating they are the most clustered around their means of –20.1‰ and –20.8‰, respectively. Megatherium americanum δ15N has a high standard deviation (3.7), indicating that the values measured for this isotope are more dispersed around the mean of 7.8‰. For Mylodontinae/Mylodon darwinii, when the six comparative δ13C values are added, the mean falls to –22.4‰ and the standard deviation increases to three, indicating that these samples have disparate measures of this isotope. The same behavior is seen for δ15N of this taxon and for δ13C and δ15N of Megatherium americanum, even though the dispersion is less acute. With the comparative samples, the statistics of Equus neogeus, Glossotherium robustum, and Doedicurus clavicaudatus isotopic values were calculated. For the equid, only one extra sample was registered from Arroyo del Vizcaíno, and thus the statistic shows only the differences between both isotopes. The δ13C standard deviation is less than 1σ and δ15N is more than 2σ, while for the other two species, the values are very much clustered around their means (equal or less than 1σ).

Table 4.
Statistics of Hippidion saldiasi, Mylodontinae/Mylodon darwinii, and Megatherium americanum and comparative samples (Mylodontinae/Mylodon darwinii, Megatherium americanum, Equus neogeus, Glossotherium robustum, and Doedicurus clavicaudatus).
Figure 2 and Figure 3 depict the box plots of δ13C and δ15N, both with and without the comparative samples. The graphics show how Mylodontinae/Mylodon darwinii δ13C falls with the additional samples and how the maximum δ15N range increases (Figure 2 and Figure 3). Megatherium americanum δ13C in Figure 2a barely matches the higher values of Hippidion saldiasi and Mylodontinae/Mylodon darwinii. When the comparative samples are added, the lower values of Megatherium americanum expand, superposing with the higher values of Hippidion saldiasi and Mylodontinae/Mylodon darwinii (Figure 2b), while the additional δ15N Megatherium americanum do not influence the maximum and the minimum, as they fall into the ranges of the original sample (Figure 3a,b, Table 4). The box plot also shows how the δ13C values of Glossotherium robustum and Doedicurus clavicaudatus fall into the same values with the rest of the species (Figure 2b), while δ15N are in their higher ranges (Figure 3b).
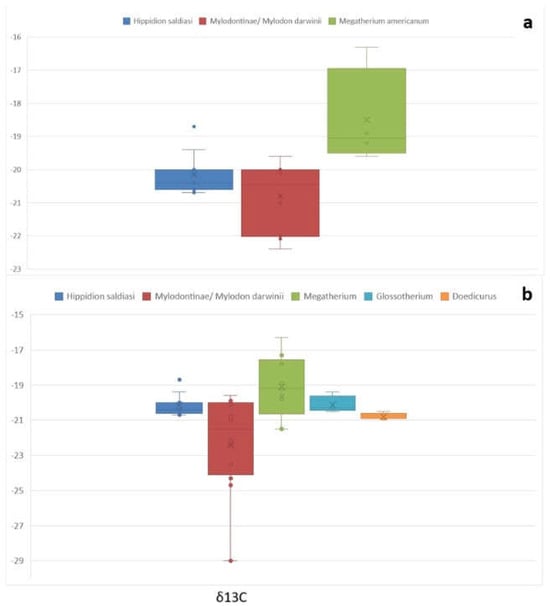
Figure 2.
Box plots showing the δ13C values of (a) Hippidion saldiasi, Mylodontinae/Mylodon darwinii, Megatherium americanum, and (b) the comparative samples.
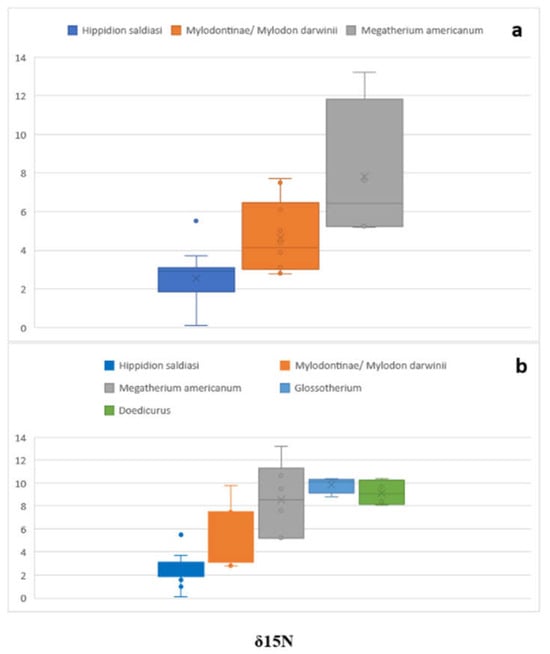
Figure 3.
Box plots showing the δ15N values of (a) Hippidion saldiasi, Mylodontinae/Mylodon darwinii, Megatherium americanum, and (b) the comparative samples.
The scatterplot of the 33 values of δ13C and δ15N (Figure 4) shows clustered measures of Hippidion saldiasi, Glossotherium robustum, and Doedicurus clavicaudatus, while Mylodontinae/Mylodon darwinii and Megatherium americanum are dispersed, as also seems to be the case for Equus neogeus. When comparative samples are added, the scatterplot of δ13C values shows similar measures for different species at 32.0, 28.0, and 23.0 cal. kya. During the early PG period, Mylodontinae/Mylodon darwinii and Megatherium americanum continued to be dispersed with respect to older ones and among South Patagonia, North Patagonia, and Pampa, even though most of the species were near –20‰ (e.g., Glossotherium robustum superpose with Hippidion salidiasi) (Figure 5). δ15N values were also clustered for 32.0, 28.0, and 23.0 cal. kya for various species, and during early PG times, were distributed in an ample range: Hippidion saldiasi among the lower range (between 2 and 4‰), Glossotherium robustum and Doedicurus clavicaudatus among the higher range (between 8 and 10‰), and Mylodontinae/Mylodon darwinii and Megatherium americanum distributed at intermediate and high ranges. Equus neogeus also showed a dispersed behavior for this isotope (Figure 6).
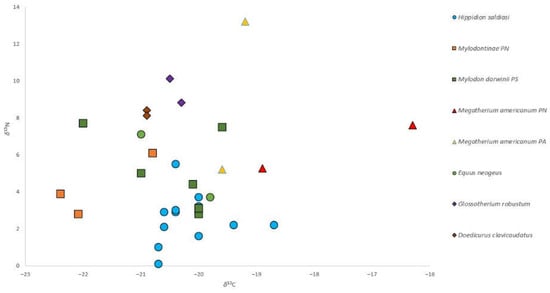
Figure 4.
Scatterplot of the δ13C and δ15N values of the 33 samples. North Patagonia, South Patagonia, and Pampean regions were differentiated. Abbreviations: Mylodontinae PN, North Patagonia; Mylodon darwinii PS, South Patagonia, Megatherium americanum PN, North Patagonia, Megatherium americanum PA, Pampa.
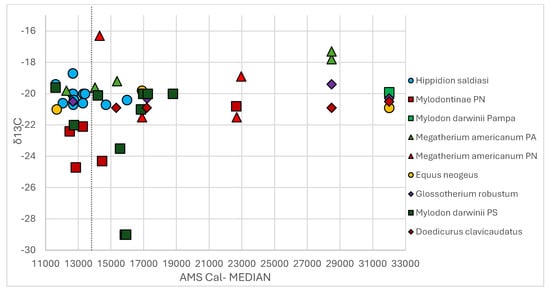
Figure 5.
Scatterplot of δ13C values of the 33 samples plus the comparative ones. The black dotted line indicates 14 cal. kya.
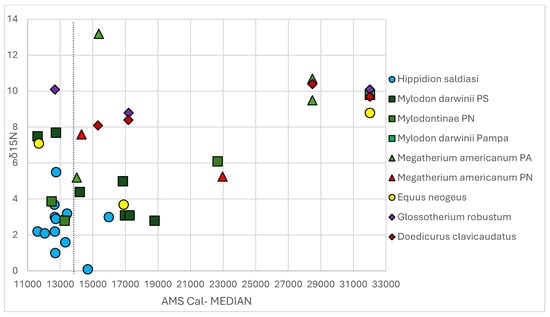
Figure 6.
Scatterplot of δ15N values of the 33 samples plus the comparative ones. The black dotted line indicates 14 cal. kya.
Based on these observations, some general trends can be seen for each species.
3.2.1. Hippidion saldiasi
Hippidion saldiasi values span from ~16.0 to 11.6 cal. kya in South Patagonia (Table 1), and this taxon has the lower values of δ13C and δ15N (Figure 2a, Figure 3a and Figure 4). δ13C values fall between –20 and –20.6‰ (mean of –20.1‰ ± 0.5‰) with a minimum of –20.7‰ at Tres Arroyos and a maximum of –18.7‰ (represented as an outlier in Figure 2) at Piedra Museo, both at ~12.7 cal. kya (Table 4). This taxon registers the lowest δ15N values among all of the samples, with a minimum of 0,1‰ at ~14.6 cal. kya in Tres Arroyos and a maximum of 5.5‰ in CM at ~12.7 cal. kya (outlier in Figure 3, Table 4), while all the values accommodate between 2 and 3‰ (mean 2.5‰ ± 1.3‰), showing that δ15N values were maintained in the lower ranges since early PG times to the transition to the Holocene (Figure 6).
This species was assigned into the browsing range of South American equids [65], with the consumption of C3-C4, occupying the south of the Patagonian region during this time period [65,66]. Human exploitation of this species was registered in Los Toldos (Cueva 3), El Ceibo (Cueva 7), as well as in the site AEP-1 in the Piedra Museo locality [92], CM (see Section 4.1), and the Late Pleistocene component of Cueva Bombero [96], even though this site yielded a date of 8.850 ± 80 uncalibrated radiocarbon years BP [111]. However, additional assays are required to verify the reliability of these outsider Early Holocene dates (e.g., [56]).
3.2.2. Mylodontinae/Mylodon darwinii
Mylodontinae/Mylodon darwinii results are from South and North Patagonia (seven and three samples, respectively), ranging between ~22.6 to 11.6 cal. kya (Table 1); according to Figure 2a and Figure 3a, it registers intermediate values of δ13C and δ15N. Even though this taxon has lower δ13C values than Hippidion saldiasi, with a minimum of –22.4‰ registered at El Trébol at ~12.4 cal. ka (Table 4), the mean is –20.8‰ ± 1‰ and 68% of the samples fall in this range. The maximum is –19.6‰ in Piedra Museo at ~11.6 cal. ka (Table 4). The acute decrease registered when adding the comparative samples (Figure 2b, Table 4) is due to lower values of δ13C (Table 2), such as –29‰ at 15.8/15.9 cal. kya and –23.5‰ at 15.5 cal. kya in Cueva del Milodón [87], or –24.3‰ at 14.4 cal. kya and –24.7‰ at 12.8 cal. kya at Gruta del Indio in Mendoza [107]. Only the value of –19.9‰ from Arroyo del Vizcaíno at ~32.0 cal. kya is similar to the maximum from Piedra Museo. This distribution explains the ample dispersion observed of δ13C observed for this taxon (Figure 2b and Figure 5).
The minimum and maximum δ15N values are registered for the same chronological period: 2.8‰ at El Trébol at ~13.2 cal. kya and 7.7‰ in Cueva Chica at ~12.7 cal. kya (Table 1 and Table 4). The values are dispersed (mean 4.6 ± 1.8), superposing with the higher range of Hippidion saldiasi and with the lowest range of Megatherium americanum (Figure 3a). For example, a minimum of 2.8‰ was also registered at Arroyo Bloques at 18.7 cal. kya (Table 1). At Cueva Chica, besides the 7.7‰ registered at ~13.2 cal. kya, another individual of M. darwinii dated at ~17.2 cal. kya had a value of 3.1‰ (Table 1). The younger individual dated in Piedra Museo at ~11.6 cal. kya had a value of 7.5‰; when the sample from Arroyo del Vizcaíno was added, the δ15N obtained a maximum of 9.8‰, with a higher mean and standard deviation 5.1 ± 2.3‰ (Table 2 and Table 4, Figure 4 and Figure 6). In general terms, δ15N presents a higher variability, which had already been noted in the Nahuel Huapi region and is observed in the Central Plateau of Santa Cruz [68,79]. This giant ground sloth is characterized as a mixed or selective feeder and foregut fermenter [112,113], an interpretation that is also supported by dung analysis from Cueva del Milodón [114]. Its ample geographical distribution was related to a great environmental tolerance [115]. Morphological studies had evidenced that this species had a narrow muzzle to select certain parts of plants [113]. Consequently, this species had an ample diet spectrum (from digger to selective feeder), and its presence was notable both in arid/cold and warmer fluctuations. Anthropic evidence found in El Trébol were cutmarked osteoderms [116], AEP-1 in Piedra Museo with cutmarked bones [92], and the use of bones for fuel in Paso Otero 5 [90]. The usage of caves by Mylodons and later on by humans was detected in the entire southern Andean zone, from Última Esperanza to the Nahuel Huapi region.
3.2.3. Megatherium americanum
Megatherium americanum values were sparser, as two individuals were recognized for North Patagonia and two for the Pampean region between ~23.0 and 14.0 cal. kya (Table 1). The δ13C values accommodated the mean of –18.5‰ ±1.4‰ (Table 4). According to Figure 2a and Figure 3a, this species had the lowest δ13C values and the highest δ15N values. The minimum value of δ13C (–19.6‰) from the archaeological site of Arroyo Seco at ~14.0 cal. kya superposes with the maximum of Mylodontinae/Mylodon darwinii (–19.6‰) and the outlier of Hippidion saldiasi (–18.5‰) (Figure 2a), both from Piedra Museo. The highest values (–16.3‰) correspond to the individual from Manqui Malal, in the North Patagonia at ~14.2 cal. kya (Table 1 and Table 4). When adding the other five samples, the mean is slightly lower (–19.1‰ ±1.7‰) due to the lower Pampean values (–19.8‰ in Paso Otero at 12.2 cal. kya, –17.8‰ and –17.3‰ at Camet Norte ~28.0 cal. kya) (Table 2 and Table 4), while the values from El Chacay (~at 16.8 and 22.6 cal. kya), located approximately 300 km north to Manqui Malal, were measured at –21‰ [108] (Table 2), expanding the minimum range for this species (Figure 2b and Figure 5). In addition, apatite measurements were obtained in Salto de Piedra, Campo Laborde [74], and Santa Rosa (La Pampa) [73], dated from ~28.0 to 11.0 cal. kya, in which a C3 to a C3-C4 diet was defined.
The δ15N presents the highest standard deviation of 3.7‰; thus, the values are very much dispersed along the mean of 7.8‰ (Table 4). Among the four samples, Rio Salado 1 has the highest value of 13.2‰ at ~15.3 cal. ka, and the lowest value was registered one millennium later (~5.2‰) at Arroyo Seco (Table 1). This last reading superposes with the higher values of Mylodontinae/Mylodon darwinii and with the outlier of Hippidion saldiasi (5.5) (Table 4, Figure 3a). The intermediate values correspond to the sample from Lake Nahuel Huapi at the LGM and the Manqui–Malal specimen (5.3‰ and 7.6‰, respectively). δ15N was measured only in the individual from Camet Norte and was located in the highest range of 10.7‰ and 9.5‰ (Table 2, Figure 3b).
Other ecomorphological studies characterized this species as a generalized selective feeder and partial browser of soft food (including carrion) [64]. The dental occlusal area showed high oral processing and/or a low fermentation capacity, or even higher metabolic requirements [112]. Consequently, this species inhabited arid and/or dry open landscapes with wooden shrubs and/or patches of trees. Evidence of human exploitation was registered in several individuals of the Pampean region; their bones were used as an informal tool (CL, see Section 4.3) [56], used for fuel (Paso Otero 5) [90], and even an atlas was registered with cut marks (Figure 7a) for its separation from the postcranial skeleton [91].

Figure 7.
Examples of megafauna bones exhibiting cut marks on their surfaces. (a) Atlas of Megatherium americanum from the Río de la Plata region, (b) Equus sp. bone exhumed at TT 1 during the Montané expedition (the scale is approximate), (c) Megatherium rib from the Campo Laborde site. (a) Photos by: K. Chichkoyan, (b) H. G. Nami, (c) modified after ([56]: Figure 4).
3.2.4. Equus neogeus, Glossotherium robustum, and Doedicurus clavicaudatus
Statistics of the Pampean Equus neogeus, Glossotherium robustum, and Doedicurus clavicaudatus were not calculated, as they only had two values in each case. Equus neogeus has similar δ13C values at Arroyo Tapalqué and Paso Otero at ~11.6 and ~16.8 cal. kya, respectively (–21‰ and –19.8‰), but different δ15N values (7.1‰ and 3.7‰) (Table 1). Only one sample from Arroyo del Vizcaíno can be added that is in the same range as the Arroyo Tapalqué individual (Table 1 and Table 2). Isotopic δ13C values from bioapatite [74] and the morphology of the bone point to grazing habits [65]. This species was exploited by humans in Arroyo Seco 2, where a radius was found broken for marrow extraction [55], and as fuel in Paso Otero 5 [90]. A bone of Equus sp. was found cutmarked in TT1 (see Section 4.2) (Figure 7b).
Glossotherium robustum has similar δ13C and δ15N values at ~17.1 and ~12.6 cal. kya (Table 1, Figure 4). This giant sloth was characterized as a bulk feeder [113] with digging capabilities in order to, among other things, search for food [64,113]. The occlusal surface of the bone presented an increased digestive efficiency and a very low basal metabolism [112]. Glossotherium robustum was exploited by humans at Arroyo Seco 2 [55] and for fuel in Paso Otero 5 [90]. Finally, Doedicurus clavicaudatus has similar high measurements for records dated in ~17.1 and ~15.3 cal. kya (Table 1, Figure 4). The diet of this Cingulate was also interpreted as bulk feeding in open environments, with high oral processing of food [117]. This species was exploited by humans at the archaeological site of La Moderna [56].
Glossotherium robustum and Doedicurus clavicaudatus were also measured at Camet Norte and Arroyo del Vizcaíno (Table 2). By ~30 cal. kya, these species had the same values as the postglacial individuals, for they were clustered around their means: δ13C values of –20.1 ± 0.4 and –20.8 ± 0.2 and δ15N values of 9.8 ± 0.7 and 9.1 ± 1.08 for Glossotherium robustum and Doedicurus clavicaudatus, respectively (Table 4, Figure 2b, Figure 3b, Figure 5 and Figure 6).
4. Notable Archaeological Locales
The purpose of this section is to describe CM, TT, and CL, where the remains of megafauna exhibit clear associations with the hunter-gatherers who coexisted with them. Their position enclosing the south–central portion of the Southern Cone, where the reviewed Xenarthra and Equidae specimens were also located, provide critical anthropological context and are particularly valuable for discussing the isotopic studies presented in this article.
4.1. Cueva del Medio
The CM is an archaeological and paleontological site situated on the slopes of Benitez Hill in the Mylodon Cave National Park, Última Esperanza, in southern Chilean Patagonia. A thick piece of hide with embedded dermal ossicles and long reddish hairs was discovered in the Cueva del Milodón in 1895, causing worldwide scientific interest. This hide was not associated with any known living species. Over the next two years, additional bones, skin, and dung that were assumed to belong to the same unknown species were discovered in the area. It was identified as a ground sloth, known as the Mylodon, an extinct herbivorous mammal from the Pleistocene period; nevertheless, there was some speculation that it might still be living in some areas of Andean Patagonia. Rodolfo Hauthal was one of the scientists who visited Benitez Hill at the time that he was working at the Museum of La Plata, Argentina. The first excavations at the CM were among the activities he carried out on Benitez Hill for scientific purposes. At the same time, the Daily Express in London organized an expedition with the aim of finding some living exemplars of this megamammal. Then, during the period spanning 1899–1902, like the other caves in the hill, the CM was visited by relic hunters looking for paleontological remains who destroyed a significant portion of the cave deposits. In this unscientific way, a large number of paleontological specimens were recovered and mostly sold to European museums.
In the Benitez Hills, near the CM, the famous Mylodon Cave was the center of paleontological and archaeological interest for decades ([118,119,120], among many others). However, since the rediscovery of CM, intense research activity including both disciplines has been carried out at that site (e.g., [121,122,123,124,125,126]).
Formed within the Benítez Hill conglomerate, CM is a large cave that is ~90 m long by ~50 m wide at its entrance. Due to its size and for purely descriptive and analytical purposes, the cave was divided into three portions as follows: the entrance, the interior, and the back [48]. They are, respectively, located in its mouth sector and drip line, and around and behind the large and important conglomerate blocks that had fallen from the cave walls and ceiling in the center. The site was widely disturbed by looters and the initial excavations carried out at the turn of the 20th century, and irregular digging left a lot of holes that destroyed a great portion of the sedimentary deposits containing archaeological and paleontological vestiges (Figure 8). Hence, in diverse sections of the cave’s sedimentary infilling, important accumulations of sediments had been removed and reformed. To investigate the archaeological and paleontological remains that were still potentially present, the disturbed sediments were carefully screened, and a number of interesting remains were recovered. After this removal, it was observed that in the front, there were several intact sections of deposits underlying the disturbed material. Also, surrounding the central large blocks, a layer of pebbles that had detached from them had sealed the sediments, making that area impossible to have been excavated by the looters (Figure 8b,e). Furthermore, a small section at the end of the cave was also undamaged. Our excavations were carried out in the deposits in these sectors, mainly in the center area (Figure 8f).

Figure 8.
Different images showing the Cerro Benitez and CM site. (a,b), Entrance of the cave, depicting the frontal and inner portions, (c,d) General views of the Cerro Benitez (c), and Cueva del Medio entrance (d), (e,f) the excavations of the different field-seasons. (e) Test pit made during the first field season. Several looters’ pits can be seen around it, one of which was used to start the excavation. (f) Paleoindian level exposed during the third expedition.
With regard to its stratigraphy, the sediments that formed the deposits of CM have have exogen and endogen origins [127]. At the entrance and in the central section, the stratigraphic sequence containing the archaeological record showed four strata, as follows: The first layer encountered (layer 1) is a fine a gray powdery surface level. The second (layer 2) is below the former in the eastern part of the cave; it can be observed from the surface toward the west and is composed of clasts and pebbles of various sizes mixed with fragments of conglomerates that originated from the wall and ceiling. In some places, this level is highly compacted and acts as a seal for the Paleoindian archaeological level. Underlying this stratum and still containing some pebbles, there is a sandy reddish/yellowish level (layer 3, [127]: Figure 7) that is not present over the totally excavated surface ([127]: Figure 7); hence, in a large part of the excavation, the stratum of pebbles and clasts of different sizes (layer 2) overlies layer 4. Under a gray sandy layer and below a humid clayed deposit with rounded clasts and pebbles, layer 5 was identified [48]. Below the archaeological excavations, a redeposited volcanic ash layer from the Reclús volcano that originated in the R1 eruption, which occurred at ~12.6 kya, was recently identified [126].
Different from the pre-Holocene deposit (layers 4 and 5), the sedimentary section containing the archaeological remains witnessed a very low deposition rate and unconformities due to episodic sedimentation [127]. Thus, the ~50 cm deposit registered a discontinuous sedimentary and archaeological record with gaps since the last millennium of the Pleistocene and Holocene. Originating in this way, there is a mix of diverse kinds of archaeological and paleontological materials in some sectors. However, in a great portion of the excavation, the Paleoindian level, located on the top of layer 4, was sealed by a significant layer of pebbles and cobbles (layer 2) [48,127]. This is the most reliable sector to discuss the lithic assemblages accompanying the FPs. In the portion of the excavation where it is present, the overlying level 3 ([127]: Figure 7a) provided stemless triangular points and unifacial tools with shapes and technology different from those in the underlying layer 4 [48,128].
Concerning chronology at the CM, more than 50 radiocarbon dates on diverse paleontological and archaeological materials were obtained (see [127] and the references cited therein). The samples for the radiocarbon analysis came from the archaeological excavations, as well as bone specimens from the looters’ pits. Except for the three samples coming from the apparently mixed deposit containing stemless triangular points and extinct fauna, the remaining ones all belong to the Paleoindian level. A detailed list of radiocarbon assays spanning the Late Pleistocene ages and the oldest human occupations is shown elsewhere ([127]. Table 1, Figure 11). The bulk of assays linked to human occupations during the Early Holocene and last millennium of the Pleistocene range from ~10.6–11.2 to 12.7–13.2 cal. kya, with the highest frequency falling between ~11.8–12.4 and 12.4–13.1 cal. kya.
At CM, with the exception of the projectile points and a few fractured bifacial specimens, one of which was possibly broken during the early manufacturing stage (Figure 9) made by bifacial reduction, the remaining early tools (n = 27) were unifaces of varied sizes and shapes [127]. However, as seen in Figure 9a–f,h–m, the remarkable ones are those that are larger in dimension, mainly side scrapers and knives (see [127]: Table 2).
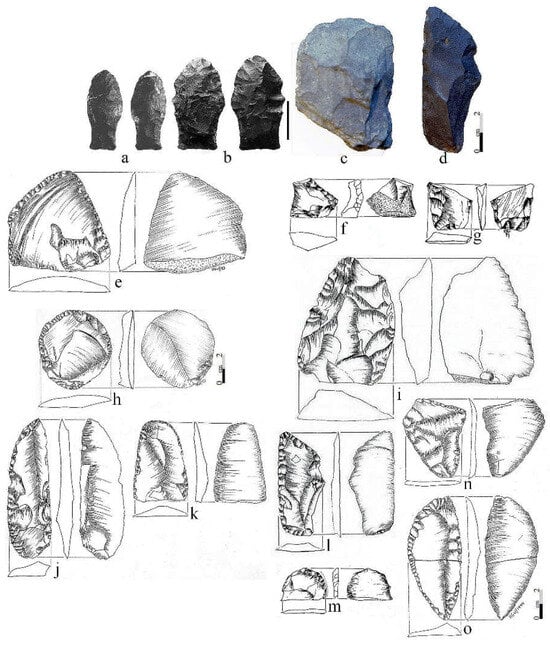
Figure 9.
Paleoindian lithic artifacts from CM (a–m) and Fell’s Cave (n,o) in southern Patagonia. (a,b) Fell points, (c–f,h–o) unifacial tools, and (g) bifacial preform. Photos and drawings by Hugo G. Nami.
Faunal remains unearthed at CM comprise Felis onça, Mylodon (?) listai, Dusicyum avus, Smilodon, horse (Hippidion saldiasi), Lama guanicoe, Lama gracilis, and a large camelid, initially classified as a Lama morphotype cf. L. owenii [121,122,123,124,129]. Presently, the latter is recognized as belonging to the Camelidae family [130] and has been identified in various archaeological and paleontological sites throughout the Chilean Cordillera [131]. Initial investigations at CM indicate that during the Late Pleistocene, marked faunal diversity existed in southern Patagonia [122,123,129,132], and the earliest hunter-gatherers exploited certain species from this fauna (Figure 10 and Figure 11). As evidenced by archaeological excavations and bone analyses, the greatest accumulation of remains is found in layer 4, predominantly comprising large-sized camelids, though Hippidion saldiasi was also consumed by these early hunter-gatherers [48,123,124,130,132]. Numerous dermal ossicles, vertebrae (Figure 11), and other Mylodon elements were unearthed primarily in the NW sector of the main excavation site (grids 27/11, 28/10 to 28/13, 29/12, and 29/13); however, no evidence of their consumption has been observed [122,133].

Figure 10.
Images of two hearths found at CM. (a–d) Frontal and upper views of one of the hearths with abundant Hippidion saldiasi bones associated with a Fell point indicated by the arrow and red circles in (b,d). Note the pebble layer sealing the archaeological remains, indicated with yellow arrows, (e,f) the pile of bones placed (f) over a typical Paleoindian hearth with concave base, and a mandible of an infant horse placed on it (g). Photos by Hugo G. Nami.
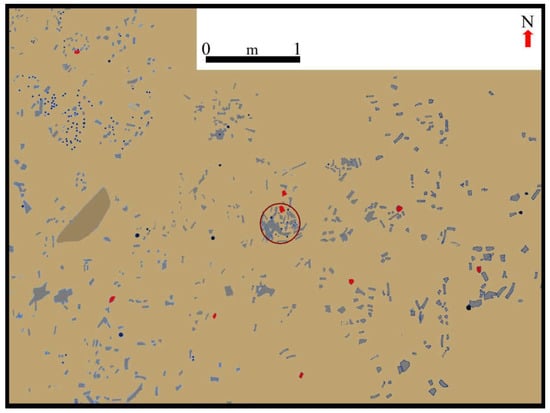
Figure 11.
Plan view of a section of the CM excavation, showing a significant part of the undisturbed archaeological level located in layer 4, which was covered by pebbles and blocks that fell from the cave’s roof. The circle indicates the concave base of the hearth depicted in Figure 10a–d. Lithic tools are indicated in red and flaking debris with black dots. Plan drawn by H. G. Nami and K. Chichkoyan.
4.2. Tagua Tagua Archaeological Locality
In central Chile, in the O’Higgins Region in the valley of the Rio Cachapoal, the Tagua Tagua lagoon occupies a basin surrounded by the Cordillera de la Costa, rising to an elevation of 1000 masl. In 1841, this pool was intentionally dried, and this action exposed extinct Pleistocene fauna remains. In this way, this locale became one of the richest paleontological reserves in the Andean country. Chilean scientists organized a program for systematic excavation of the site in 1967, after more than a century. The area was chosen because low hills surrounded the lake, making it a great place to hunt animals as they came to drink. As soon as the fieldwork began, the team found a stone flake associated with the lower mandible of a mastodon.
Remains of a variety of Ice Age fauna were eventually exposed in these archaeological excavations at TT1. In accordance with the nomenclature of [67], fragmentary and dispersed bones of mastodon (Notiomastodon platensis), deer (Antifer ultra), and horse (Equus sp.) were identified, suggesting human intervention (Figure 7b). During the first four months of excavation, fifty artifacts were encountered that were closely associated with the bones. Lithic flakes were the most abundant, along with debitage; however, several unifacially flaked artifacts were also recovered along with well-shaped bone instruments. Later, further research led by Lautaro Nuñez identified another site at 700 m south, labeled as TT2, while the site excavated by Montané was referred to as TT1 [52]. Then, new excavations with significant occupation levels were carried out at both sites. At TT1, Nuñez replicated the finds reported in the 1960s both in faunal and archaeological remains, and expanded the number of Paleoindian finds (Figure 12). In this way, in the Nuñez expedition, three FPs and diverse unifacial tools were recovered ([52]: Table 1).
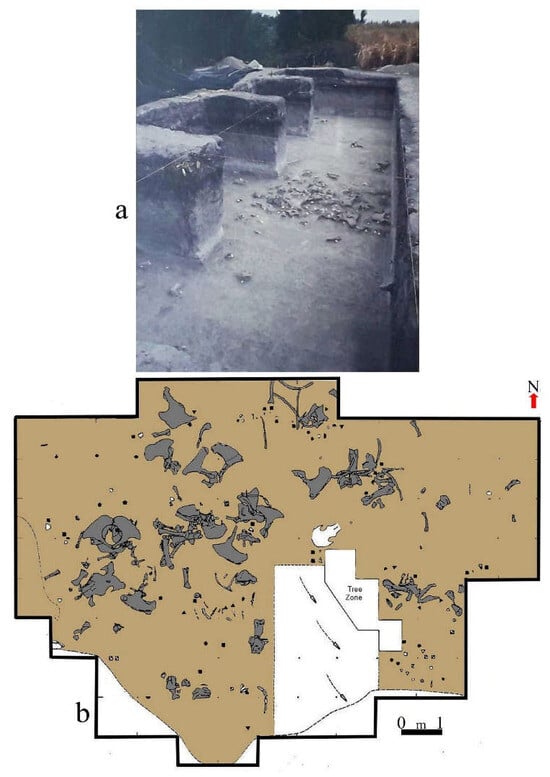
Figure 12.
(a) Archaeological excavations at TT2 showing the megafauna bones associated with cultural materials. Photo courtesy of L. Nuñez. (b) Plan floor drawing from TT2 (remade and designed by B. Chichkoyan, after [52]: Figure 4).
Remarkably, several unifacial implements, including large lateral scrapers and knives, are in the Tagua Tagua collection; in a nutshell, these are multipurpose tools. During the 1980s, one of us documented some of the Montané finds of megafaunal bones at the Santiago de Chile Natural History Museum (Figure 13b). Additionally, bone tools of excellent craftsmanship and mastodon bones continued to be discovered in the Tagua Tagua area. In summary, the Tagua Tagua Basin presents an exceptional Paleoindian record with outstanding sites demonstrating the exploitation of megamammals. Interestingly, during the initial excavation at TT1, only unifacial tools were found. However, almost three decades later, typical FPs associated with mastodon and other extinct fauna bones were discovered at TT2 (Figure 12 and Figure 13). Moreover, an additional fluted stem of an FP was found at the Santa Inés site in the Tagua Tagua Basin [134,135]. As in other contemporaneous sites [136], excellently made bone tools were discovered [52]. Particularly, an ivory artifact beautifully decorated with incised motifs stands out for its exquisite design ([137]; Nami pers. obs., 1992) (Figure 13b).
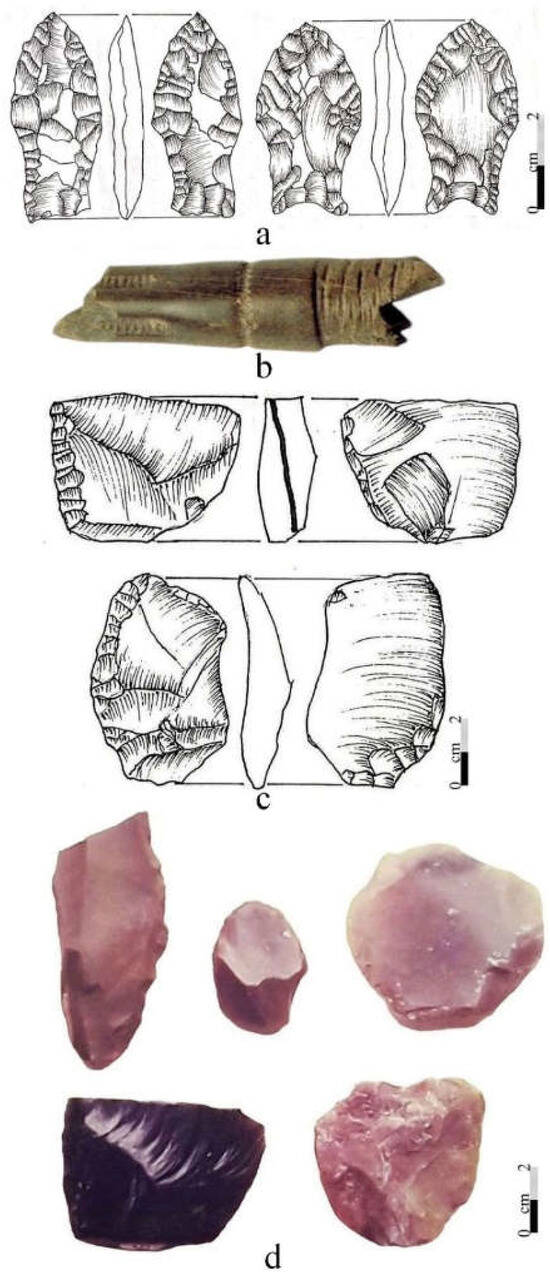
Figure 13.
Examples of artifacts from the Tagua Tagua locality. Fell points and the ivory foreshaft exhumed at TT2, as well as unifacial tools found at TT1. (a) Redrawn by H. G. Nami after [138]: Figures 28h and 29a,b. (b) Photos courtesy of V. Cortegoso and C. Méndez (after [139]); (d) photographs: H. G. Nami (the scale is approximate in (b,c)).
The calibrated radiocarbon assays obtained on the Paleoindian level of TT2 yielded dates ranging between ~11.8–11.2 and 13–12.7 cal. kya [140].
Lastly, it is worth mentioning that placed in the same Tagua Tagua Basin, close by TT1 as well as TT2 and 2 km from Santa Inés site, the recently reported TT3 site has yielded a well recorded archaeological level (12,440–12,550 calibrated radiocarbon years BP), coeval with the previously described records from this area. There, diverse kinds of lithic remains were exhumed in association with extinct fauna. Of importance is the hunting and primary processing of a young Gomphotheriidae, showing evident cut marks [58].
Direct evidence of human activity on extinct fauna in this locality, as well as other contemporary sites like Santa Julia and Valiente, excavated in northern Chile and analyzed by Jackson and colleagues [140] includes cut marks, fractures, and heat alterations of the bones of mastodons (Notiomastodon platensis), horses (Equus sp.), certain artiodactyls, and foxes (Lycalopex). For other species, such as Xenarthra (possibly Mylodontinae) at Quebrada Santa Julia and deer (Hippocamelus bisulcus, Antifer ultra) at TT1 and TT2, only fractures are observed. However, these remains likely became part of the archaeological contexts due to human activities, as suggested by their spatial relationships. The presence of cut marks in Myocastor coypus, birds, and Anura indicates that small fauna were also important at TT1 [141].
The evidence of both extinct and extant fauna shows various levels of human involvement with faunal remains. This pattern suggests insights into hunting behaviors and processing practices at the end of the Pleistocene in central–northern Chile. While the data are incomplete for a thorough understanding of early hunting methods, they clearly indicate that prey selection and processing varied based on ecological factors and the organization of the hunter-gatherers. The mentioned sites add to the well-described Monte Verde II site, dated at ~14.5 cal. kya, where Gomphotheriidae was also exploited [142].
4.3. Campo Laborde
One of the main sites that yielded an important record of megamammal exploitation by humans in the Pampean region is CL [56]. It is located in the upper basin of Tapalqué Creek, at ~15 km north-northeast of Olavarría city. Discovered in 2000, the original test pits were expanded to a total area of a 50 m2 excavation during two field programs carried out during 2001–2003 and 2016–2017. This is a unique site at which a Megatherium americanum giant ground sloth was hunted and killed (Figure 14). Most notably its bones are associated with lithic remains, witnessing that stone tools were employed to butcher the megamammal. Remarkably, as seen in Figure 7c, several cut marks were identified on the rib of the animal [56]. The AMS assays produced three radiocarbon ages ranging between 11,924–12,732 calibrated years BP and 11,304–13,207 calibrated years BP ([56]: Table 1).
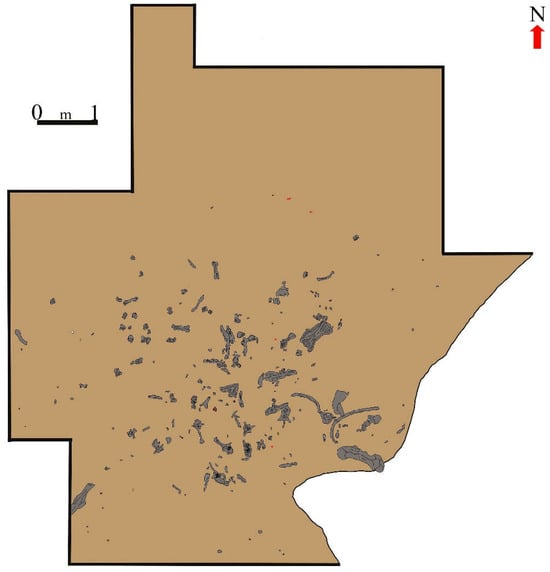
Figure 14.
Plan map for levels 0.90–1.35 of the CL excavations, showing the lithic artifacts and Megatherium americanum bones in situ (modified after [56]: Figure 2).
Debitage, as well as a few bifacial and unifacial stone tools, were associated with the carcass remains. Despite that, the tools were not numerous; notably, one of them was a broken and refitted unifacial large instrument shaped by direct short retouches on a relatively thin flake, associated with two bifacial pieces (Figure 15). One of them was a biface that displays a hump and an end thinning flake scar, probably to remove it [143]. This sort of biface might be an early stage of manufacture of a regional Paleoindian projectile point [144,145]; but, as experimentally observed on elephants, as versatile artifacts, the biface and the uniface adequately accomplish the task of processing megamammals during the butchering and dismembering process [143,146,147,148]. These actualistic observations support the excavator’s interpretation that the associated tools were employed by the hunters to cut the ground sloth meat [56]. Another significant bifacial artifact is a triangular fractured specimen finished by pressure flaking that artifact analysts interpreted as the basal part or stem of a projectile point ([56]: Figure 3a). This classification might be supported because at the tip of the triangle opposite to the fracture, at the junction of the retouched edges, there is a small flake scar showing micropolishing suggesting hafting ([56]: Figure 3b). However, despite this feature, from a macro-morphological viewpoint, this specimen might be seen from another perspective [149]. Indeed, the comprehension of stone tools, especially projectile points and their fragments, can pose a challenging and intricate inquiry stemming from various factors [150,151], including the researchers’ interpretation of lithic artifacts [152]. An alternative interpretation might be offered by viewing this piece from an inverse vertical perspective with a 180° rotation. Instead of being a basal portion, it could be interpreted as the distal tip of a projectile point that displays a collision breakage, likely an impact cone-initiating fracture ([19]: Figure 3e,f,k). By overlapping this piece with the commonly used fishtail point during the CL chronology, it might be possible to suggest that this specimen might be a broken distal portion of an FP displaying a transversal breakage during collision [19]. This other interpretation is depicted in the exercise made by overlapping the piece in query after normalizing its scale with one of the many FPs from the Southern Cone, particularly with two specimens coming from Uruguay [19,153]. Consequently, another explanation is that the giant ground sloth was exploited by foragers who used FPs in their weaponry. In this case, this is a possibility that might be considered. This preliminary elucidation might be further assessed with complementary data regarding unknown reliable stemmed pieces coeval with FPs in the region.
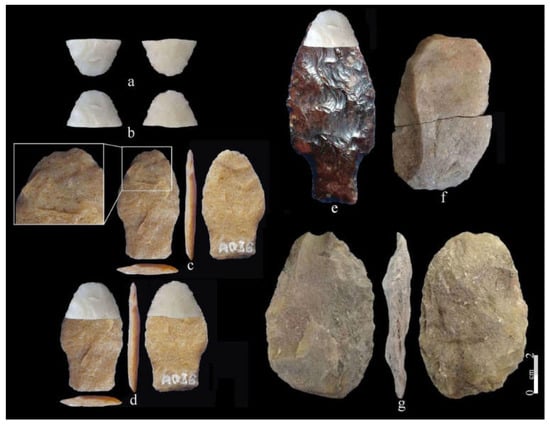
Figure 15.
(a,b) Bifacially flaked piece found at the CL site and (a) identified as a stem; (b) the same specimen observed with an inverse perspective. (c) Fell points from the San Gregorio de Polanco area, (d,e) overlapped images of the pieces depicted in (b,c), and a Fell point possibly found at the Solis Creek. (f) Unifacial tool and (g) bifacial tool. Figure made and designed by [149]. Photographs after [19]: Figures 3e and 13b, and [56]: Figure 3.
5. Discussion
5.1. Paleoecology of Extinct Faunas
Hippidion saldiasi is restricted geographically to the South Patagonian region [66]. The δ13C value is in the range of C3 plants, with similar δ13C measurements at long distance (e.g., –20‰ at Tres Arroyos and Cueva Fell, or Cueva Los Chingues at ~12.7 cal. kya) (Table 1) or at different moments of PG times (–20‰ at ~12.0 cal. kya and ~16.0 cal. kya), a variation which explains the 1σ of the standard deviation and the short interquartile range in the box plot (Table 4, Figure 2a). Higher values were registered at Piedra Museo: –19.4 and –18.7‰ at 11.6 and 12.6 cal. kya, respectively (represented as the maximum and outlier in the box plot of Figure 2a, also as the highest values in the scatterplot in Figure 5). This is the only site from the Central Plateau with Hippidion saldiasi values (Figure 1) and characterized as arid, which is a factor influencing enrichment of CO2 of plants (and animals that consume them). This climatic characteristic contributes to the slight difference in the eastern equids but also to the –19.4‰ value measured for Mylodon sp. at this site at 11.6 cal. kya (Table 1, Figure 5). A mean of 2.5‰ ± 1.3‰ for δ15N (Table 4) and its representation in the lowest ranges (Figure 3 and Figure 6) relates Hippidion saldiasi to humid environments. These low values depict a wooded landscape suitable for browsers over time and in line with other paleoecological studies. But, the highest values were also present in some individuals from Última Esperanza caves, such as CM (5.5‰ and 3.2‰ at ~12.7 and ~13.4 cal. kya, respectively), Cueva del Milodón (3‰ at ~15.9 cal. kya), and Cueva Los Chingues (Pali Aike) (3.7‰ and 2.9‰ at ~12 and 12.7 cal. kya, respectively) (Table 1, Figure 6). The δ15N values remain in similar ranges in samples from the same site but with different chronologies (e.g., Piedra Museo, Cueva Lago Sofía, or Tres Arroyos, with low or intermediate values) (Table 1, Figure 6). Between ~16.0 and 12.00 cal. kya, Hippidion shared with Mylodon darwinii several caves in Ultima Esperanza (Cueva del Medio, Cueva del Milodón, Cueva Escondida, and Cueva Lago Sofía). It was coeval with Mylodon sp. at ~11.6 cal. kya in Piedra Museo, where both species were exploited by humans. In these sites, δ15N values from the giant ground sloth were always higher than the Hippidion saldiasi, regardless of the chronology (Figure 5); the only exception is Cueva del Milodón, where both were measured as ~3‰, as seen in the superposition of Mylodon darwinii and Hippidion saldiasi in Figure 4 (see also Table 1).
Mylodon darwinii had an ample geographical range, identified from the Pampean region to the southern sites from Patagonia (including osteoderms from North Patagonia, which were classified at subfamily level). The small quantities of measurements (n: 10) between ~22.6 and 11.6 cal. kya are represented in the large interquartile range in Figure 2a, where the higher values superpose with the lower ranges of Hippidion saldiasi (see Section 3.2.1). Regardless, in general terms, the δ13C values reflect a C3 diet due to the 1σ of the standard deviation (Table 4 and the distribution in the scatterplot in Figure 4). When the comparison samples are added (from Cueva del Milodón at ~13.2 cal. kya and Gruta del Indio at ~10.9 and 12.3 cal. kya), values lower than –22 are present (Table 2, Figure 2b), a result that was marked as the threshold to indicate foraging in close canopy forest [77]. Consequently, taking together the 16 samples, it can be observed how the δ13C value of this ground sloth can be extremely dispersed in South and North Patagonia, ranging from –29‰ at ~16.0 cal. kya in Cueva del Milodón, –22‰ between ~14.5 and 12.5 cal. kya in North Patagonia, to –19.6‰ in Piedra Museo ~11.6 cal. ka (Table 1 and Table 2, Figure 5, and the box plot in Figure 2b). This variability might be reflecting the ample diet proposed by the morphological studies, with a low selection of plants for mastication, but also capable of selecting certain parts of plants. This capacity allowed a great distribution with ample environmental tolerance and/or to confront humid and/or dry pulses, which can also be reflected in the disparate δ13C values. In this sense, Mylodontinae from the Nahuel Huapi region dated at ~13.5 and 12.5 cal. kya showed lower values than the Mylodontinae dated to the LGM. This decrease was related to a humid trend in the last millennia of the Late Pleistocene [68]. The δ15N value also varies in an ample range, and contrasting with the δ13C value, it has a standard deviation of 2σ, which also is reflected in a larger interquartile range, superposing with Hippidion saldiasi and Megatherium americanum (see Section 3.2.2, Table 4 and Figure 3a). In the Nahuel Huapi region, for example, δ15N values from ~12.0/13.0 cal. kya (2.8 and 3.9‰) are much lower than the Mylodontinae measured during LGM times (6.1‰). This lowering implies the superposition with Hippidion saldiasi from South Patagonia during the last millennia of the Late Pleistocene and a similarity with Megatherium americanum during the LGM (Figure 4 and Figure 6). At Piedra Museo at 11.6 cal. kya, the Mylodon sp. and Hippidion saldiasi δ15N values diverge (δ15N of 7.5‰ and 2.2‰, respectively) (Table 1, Figure 6). When adding the additional sample from A. Vizcaíno at ~32.0 cal. kya, the highest value is registered (9.8‰) (Table 2, Figure 6). Consequently, δ15N behaves much more disparately than δ13C. On the one hand, the low number of samples (including the sample from A. Vizcaíno) complicates the analysis of this isotope in different Pampean and Patagonian landscapes, but some observations have tried to explain the variability seen at shorter spatial scales. At Piedra Museo, the differences observed in Hippidion saldiasi and Mylodon sp. might be related to the diet preference of both species [79] and to a marked niche partitioning between equids and giant ground sloths. At Nahuel Huapi, the difference noticed between the Mylodontinae from the LGM and the one from 12.0/13.0 cal. kya might be due to paleoenvironmental variability in this region during PG times [68]. At Arroyo del Vizcaíno, the general trend of high δ15N values in all of the megamammal community have suggested a probable niche partitioning between ground sloths and other herbivores in the Pampean Pleistocene fauna. As observed for δ13C, the plasticity of the diet and tolerance to different environmental conditions [113,115] might have influenced the irregular trend of the Mylodontinae/Mylodon darwinii δ15N isotope. This tolerance might have helped this Mylodon sloth to coexist in the Pampean and Patagonian areas with several megamammal species: in Arroyo del Vizcaíno, it shared the space with Glossotherium robustum, Doedicurus clavicaudatus, and Equus neogeus as early as 32.0 cal. kya. Mylodontinae osteoderms were also identified in the Nahuel Huapi region, coexisting with Megatherium americanum by LGM times. As mentioned, remains of these sloths are also found in caves with Hippidion saldiasi in South Patagonia. At these high latitudes, its hibernation capacity might have helped it cope with extreme climatic conditions [94].
Megatherium americanum isotopic information is very sparse, with n: 4 between ~23.0 and 14.0 cal. kya, and the distribution extending from the North Patagonia and Pampa regions, thus influencing the large interquartile range in Figure 2a and Figure 3a, the highest standard deviations (2σ or more) of δ13C and δ15N values (Table 4), and the extreme distribution in the scatterplot for this species (in Figure 4). This distribution is a reflection of the different measures obtained in each individual. For example, by ~14 cal. kya, the individual from Manqui–Malal was adapted to a C3-C4 diet, while the one from Arroyo Seco had a C3 value, suggesting a drier environment for North Patagonia. The original line meant this animal reflects an “spectra” of values from C3 to C3-C4. Thus, I think the word”transition” is not appropiated in this paraphrased sentence. This type of diet is similar to the results from the apatite analysis of Pampean samples, reflecting the transition from a C3 diet to a C3-C4 diet. When adding the comparative samples, more variability is seen. For example, values from El Chacay maintained their lowest values during LGM and PG times (see Section 3.2.3), while the older values from Camet Norte are in the highest range, differentiating them from PG samples in the Pampean region (Figure 5). The δ15N isotope also presents high variability in both regions, with high and low values (Figure 4). The values from Camet Norte are in the highest range, but lower than Rio Salado specimen (Figure 6). The high values of Megatherium americanum (Figure 3b and Figure 6) reflect the glacial landscape of LGM times in the Nahuel Huapi area but also the arid trends of the Pampean region. Both δ13C and δ15N values show a particular dynamic for this giant ground sloth, as the diet and ecology of this species are still very much discussed [64,81]. It seems that Megatherium americanum was capable of adapting to the colder and arid conditions of the LGM and to warming postglacial times, inhabiting disparate environments. Its dietary habits, between generalized selective to partial browser (or carrion eater) [64], might have allowed a non-conservative niche, being capable of adapting and switching to local humid and/or arid conditions.
Overall, Equus neogeus, Glossotherium robustum, and Doedicurus clavicaudatus have similar δ13C and δ15N values, indicating C3 intake under open–arid conditions (Table 1, Figure 2 and Figure 3). For Xenarthra, it must be further investigated if metabolic issues (e.g., larger oral processing in Cingulate and the digging capacities for searching for food in Glossotherium robustum) might have served as a niche-partitioning strategy among both megamammals. Regarding Equus neogeus, the δ15N values are slighter lower than the Xenarthra, but the value from Paso Otero of 3.7‰ is in its lower range, a factor that explains the dispersion observed for this isotope (contrasting the behavior of δ13C). This feature might indicate a more browsing feeding habit or probably it is an outlier, as several analyses have pointed to a grazing habit [65,74]. This equid has a Pampean geographical range, not superposing with Hippidion saldiasi [66].
Resuming, δ13C values of Equids and Xenarthra are in the range of C3 to C3-C4 for most grazers to most browser species. Megatherium americanum seems to switch among both feeding habits, according to geographical and local conditions. Something similar occurred with Mylodontinae/Mylodon darwinii, even though the data indicate a mostly C3 diet. Hippidion saldiasi, Equus neogeus, Glossotherium robustum, and Doedicurus clavicaudatus from Pampa and Patagonia have a conservative value in the range of C3 plants (Figure 2). The δ15N isotope is where more variability is observed, and this feature might be responding to particular dietary and ecological issues of this Late Pleistocene megamammal community.
5.2. Megamammal Exploitation in the Southern Cone in an Isotopic Context
As shown above and summarized in Table 5, the earliest postglacial human settlements, occurring almost contemporaneously in the triangle of south–central Argentina and Chile, engaged in the exploitation and consumption of animals from the Pleistocene faunal assemblage. This activity took place within well-developed and locally distinct niche partitioning during the PG period. While in CM, Hippidion saldiasi was consumed, at TT, a large range of species were hunted for consumption and material for tool making. In parallel, at CL, a similar behavior was registered for Megatherium americanum. In all of these sites, a similar lithic technology was used. To this evidence, Pampean sites such as Paso Otero and Arroyo Seco, and Patagonian sites, such as El Trébol and Piedra Museo, can be added. The selective cases from archaeological and paleontological sites in the central Southern Cone of South America demonstrate how isotopic and archaeological findings converged. Megafauna from these settlements not only survived but thrived amid climatic fluctuations and environmental challenges linked from the LGM to the Pleistocene–Holocene transition.

Table 5.
Summary of the chronology (not calibrated) and extinct megamammal species from CM, TT, and CL.
Figure 5 and Figure 6 show how the megamammal community was isotopically distributed by the time of the first widespread human dispersal in the Southern Cone at ~14.0–13.0 cal. kya. Homo sapiens was a novel species into the several millennia of coexistence among Xenarthra and Holartic species such as Equidae. During the Late Pleistocene, the local fauna developed different defensive strategies for coadaptation with the immigrants. Megatherium americanum and Mylodontinae/Mylodon darwinii adapted to different Pampean and Patagonian conditions, as reflected in variable isotopic δ13C and δ15N measures and ample geographical expansion. This variability is registered among individuals of the same group and among both species. Meanwhile, Hippidion saldiasi, Equus neogeus, Glossotherium robustum, and Doedicurus clavicaudatus had similar measurements over time and were restricted to one region. Consequently, when humans dispersed in the Southern Cone, the megafauna community had a mature niche partitioning, in which the geographical distribution, ecological features, and dietary preferences allowed the coexistence of a wide array of species.
According to the information compiled here, between ~23.0 and 14.0 cal. kya, while δ13C was maintained at ~–20‰ in Equidae and Xenarthra at both regions, δ15N had a greater amplitude. By LGM times, the information from North Patagonia shows the coexistence of Mylodontinae and Megatherium americanum during these extreme conditions, with relatively high δ15N values. In the PG period, Megatherium americanum, Glossotherium robustum, and Doedicurus clavicaudatus were located in the highest δ15N range, while Mylodon darwinii specimens from Última Esperanza caves and Hippidion saldiasi from Cueva Fell were in the lowest range. Equus neogeus from Paso Otero was also located in this range; but as stated, it is not clear if this measure is an outlier. Aridity might have been the main climatic variable in the Pampean region (as also seen as a dominant variable for the earlier sites of Camet Norte and Arroyo del Vizcaíno), with C3 to C3-C4 vegetation that supported a well niche-partitioned megafauna with grazing to browsing habits [73,74,77,81], while the Patagonian Mylodon darwinii and Hippidion saldiasi relate to a humid but colder environment.
After ~14.0–13.0 cal. kya, human expansion into high latitudes occurred in a geologically instantaneous timeframe. More isotopic information between ~14.0 and 11.5 cal. kya was obtained, as much archaeological work was carried out in order to clarify the chronology and environment of this new species. The Antarctic Cold Reverse episode between ~14.5 and 12.8 cal. kya [78,79,87] and the following transition to the Holocene period by ~11.7 cal. kya [5] were also coeval. Consequently, these biotic and climate events might have been determinants of megafauna extinctions [59].
At the southern portion of the triangle of the Southern Cone, by ~11,8–12.4 and ~12.4–13.1 cal. kya, humans appeared, exploiting camelids and Hippidion saldiasi at CM with a well-diversified lithic technology and FPs. The archaeological composition indicates extensive human activity in coexistence with several extinct species (even though consumption was not observed for all the taxa). This equid had maintained similar isotopic values at least since ~16.0 cal. kya, suggesting the persistence of the same feeding behavior despite the changing environmental conditions and the presence of humans. In South Patagonia, humans also exploited mylodons that were affected isotopically at the microenvironmental level (e.g., the Mylodon sp. living in the dry central Deseado plateau). The contrast in variability in δ15N values between Mylodontinae/Mylodon darwinii to the more stable Hippidion saldiasi might be confirming the niche partitioning between giant ground sloths and equids. Thus, when humans appeared in Ultima Esperanza, they exploited horses and mylodons, which had been in the region for at least several millennia and that had developed different strategies to cope with climatic fluctuations.
In North Patagonia, the eastern and western sides of the Andes present evidence of megafauna manipulation. The locality of TT composed of three archaeological sites dated between ~11.8–11.2 and ~13–12.7 cal. kya present strong evidence of hunting of Gomphotheriidae, Equus sp., and Antifer, and the production of bone tools and ivory artifacts. In addition to TT, in the sites of Santa Julia and Valiente, equids and probably Xenarthra were exploited. Monte Verde II, where the exploitation of Gomphotheriidae was registered almost 2000 years earlier, temporally and spatially extends human manipulation over extinct megafauna. On the western side of the Andes, El Trébol presents human exploitation of Mylodontinae. Human entrance to this site area was relatively fast, one millennium after the first occupation of the cave by this ground sloth, due to the postglacial emersion of the cave [68]. Paleontological and archaeological sites from North Patagonia present a patchy humid to dry landscape on both sides of the Andean mountains. For TT3, warm and Mediterranean conditions were described after the apatite analysis of the hunted Gomphotheriidae [58]. The expansion of forest after 13.5 ka influenced the diminution of δ15N value in the Mylodontinae individual from the Nahuel Huapi region [68], while the information from Mendoza presents dry to some humid microenvironments (e.g., Manqui Malal, El Chacay, and Gruta del Indio) at a short distance. Giant ground sloths (Megatherium americanum and Mylodons) and Gomphotheriidae had inhabited this region since LGM times, thriving with abrupt environmental gradients until their extinction.
Located at a similar latitude as TT, CL presents similar trends in human–megafauna interactions. The hunting of a Megatherium americanum individual for food and bone tool production in a humid context was clearly established [56,74]. Nevertheless, since ~30 cal. kya, dry conditions predominated, maintaining δ15N values at highest ranges also for PG times (e.g., Megatherium americanum, Glossotherium robustum, and Doedicurus clavicaudatus dated at ~17.1, ~15.3, and ~12.6 cal. kya) (Table 1, Figure 6), but also with humid conditions toward the Holocene. In this region, several species of megafauna were manipulated by humans for consumption, tool making, and for fire production.
6. Paleoenvironmental Perspective of the Landscape Usage
Undoubtedly, the evidence presented above is part of the Pleistocene as a geological epoch in general and as the period of the post-LGM in particular. During the terminal Pleistocene, the millennia before the onset of the Holocene were key for human settlement and dispersion in the South American Southern Cone. The Pleistocene record from this region is not different from that of the foragers that inhabited the rest of the world during that time (e.g., [155]). This similarity is expressed by the consumption and exploitation of megafauna, as well as in the traditional technological knowledge used for this purpose. The lithic technology, although impoverished in terms of morphological diversity compared to that of the North American hunter-gatherers, had a refined technology that included sophisticated strategies and methods of bifacial thinning, pressure retouching, the production of various unifacial instruments, prepared flake cores, heat treatment of raw materials, basal fluting of bifacial points, and the notable manufacture of bone objects. Like other Paleoindian lithic technologies from North America, the material culture shows notable reminiscences with that of the final Eurasian Upper Paleolithic concerning the lithic technology as well as bone manufacturing techniques and shapes [18,136,156], and even those of the Middle Upper Paleolithic in relation to unifacial instruments [149,157]. In this sense, the American peopling does not differ from euroasiatic Middle/Late Paleolithic dispersion, characterized by technologically large-sized lithic tools and well-developed bifacial reduction techniques representing a kind of socio-cultural process through which certain tools, despite exhibiting morphological variability, have persisted over time (in some cases, such as side scrapers, with virtually no significant changes). Also, America is the last part of the worldwide trend of hominid dispersal across continents following rich water and fauna resources [20,57,158,159], as can be exemplified with the overlap between megamammals and humans recorded in several caves in Patagonia. Here, humans arrived soon after the amelioration of the climate and while the Pleistocene fauna occupied the territory, as registered in El Trébol and other Patagonian caves, such as Cerro Casa de Piedra 7 [160].
But, the early human colonization of the Americas also offers significant avenues for investigating population expansion, human–environment interactions, human impact on extinctions, and human adaptive capabilities. These first hunter-gatherers viewed a variable fauna not seen before and later, during Holocene times. The American megafauna community was a novel environmental challenge in the worldwide human dispersal. The presence of megafauna was one of the resources exploited, as also happened with the smaller guanaco, cervids, and even carnivores. This broad and diverse spectrum of exploited fauna shows the adaptive versatility of the human groups using FPs as weapon tips.
This paper, with a primary focus on paleontological and archaeological perspectives, examines the interactions between humans and the Pleistocene faunal assemblage during its final millennia. Isotopic evidence shows a clear niche partitioning among Equidae and Xenarthra, and among species forming each community. According to dietary preferences, species like Hippidion saldiasi were distributed in South Patagonia in a relative humid and cold environment, while Equus neogeus was distributed over the arid Pampean landscape. Among Xenarthra, Mylodontinae/Mylodon darwinii showed a great climatic tolerance, adapting to both LGM conditions and postglacial times. For that adaptation, it was coeval with equids from the Pampean and Patagonian regions. Megatherium americanum was found in North Patagonia, coexisting with Mylodontinae by the LGM. Nevertheless, most of the registers are from the PG period in Mendoza and the Pampean region, supporting an arid and warmer climate. The most geographically localized Xenarthra were Glossotherium americanum and Doedicurus clavicaudatus. The conservative isotopic values and their restriction to the Pampean region might indicate less climate tolerance and a similar dietary habit. The first human population expanded through these niches, from open and dry Pampa to the more humid and cold Patagonian landscapes, interacting through different exploitation strategies (scavenge and/or hunt) with very well adapted megafaunal species. While the isotopic analysis provided insights into the diets, niche partitioning, and environmental challenges faced by megamammal communities inhabiting the south–central region of South America’s Southern Cone, archaeological evidence derived from diverse methodologies reveals that humans utilized megamammals for multiple purposes. Evidence substantiating these claims include well-defined archaeological contexts supported by various analyses demonstrating associations between human activity and paleontological materials: indications of carcass and bone manipulation of Pleistocene animals, such as the production of bone tools, discernible cut marks, charred bones, and hearths containing bones alongside diagnostic artifacts, among other notable features. Additionally, in several parts of South America, rock paintings appear to depict extinct Pleistocene species, including sloths, horses, mastodons, and camelids, further suggesting human coexistence with these faunas [161]. Archaeological findings provide insights into the methods and extent of this exploitation, revealing a previously unforeseen aspect of human adaptation and survival strategies in the region. Just a few decades ago, the idea of such intricate ecological interactions between ancient humans and their surroundings would have been deemed almost unimaginable.
7. Conclusions
The megafauna developed ecological traits after the GABI event, leading to a distinct niche partitioning in the Pampean and Patagonian regions, which was isotopically observable after 23 cal. kya. The arrival of humans around ~14.0–13.0 cal. kya. took advantage of these pre-established ecological networks, settling in resource-rich areas such as CM, TT, and CL. These archaeological sites exemplify how human populations occupied key bioenvironmental spaces, a pattern consistent with Pleistocene hominid behavior worldwide.
Equids and Xenarthra adapted to the PG climatic fluctuations, developing strategies that allowed them to persist in changing environments. During the Pleistocene–Holocene transition, they began to be exploited by hunter-gatherers who incorporated them into their subsistence strategies using FPs in their weaponry.
In conclusion, a previously mature megamammal trophic network, as evidenced by isotopic analyses, was now joined by an additional predator (humans) who, at least sporadically, consumed these large animals. This dynamic synergy may have played a role, alongside broader climatic and environmental factors, in the extinction of South American megafauna.
Isotopic analyses, particularly those based on δ13C and δ15N from megafauna bones, provided critical insights into niche partitioning and environmental conditions in the Southern Cone of South America during the LGM to postglacial period (~23.0–11.5 cal. kya). Archaeological remains showed the coexistence and exploitation of Pleistocene fauna by human groups. For that, this research included detailed information about excavations, findings illustrated with figures that depicted the context of the discovery, and situated each site within its environmental context. Our aim was to show that this evidence was both compelling and diverse. The illustrations highlighted findings related to extinct fauna, associated toolkits, cut marks on megafauna bones, and other artifacts, unequivocally demonstrating the exploitation and consumption of these animals by the hunter-gatherers who coexisted with them. This holistic and multidisciplinary perspective would have been scarcely conceivable decades ago.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/quat8010014/s1: Table S1. Details of the averaged samples.
Author Contributions
K.V.C.: investigation, methodology, formal analysis, writing—original draft, visualization. H.G.N.: conceptualization, investigation, methodology, formal analysis, writing—original draft, visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary Materials.
Acknowledgments
We are indebted to CONICET, UBA, and Sepkoski Grant 2020 (Paleontological Society International Research Program) for their support of our investigations; to G. Politis for his kindness in providing us with the CL figures and allowing us to reproduce several of the pictures published in this paper; to L. Nuñez for his gentleness and willingness in providing the photo of the TT2 excavation; to V. Cortegoso and C. Mendez for allowing to reproduce the specimens illustrated in Figure 13; to Sonia Lanzelotti and Luis Coll for their help with Figure 1; to Brises Chichkoyan for help with Figure 9; and to R. Bryan for her observations and impeccable work during the English editing of this article. We are grateful to the four anonymous reviewers for their valuable comments and suggestions, which have helped improve the quality of our manuscript. Finally, we are grateful to all the institutions and colleagues who allowed us to study their collections and provided financial support for the research carried out over the years.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dawson, A.G. Pleistocene. In The Oxford Companion to Archaeology; Fagan, B.M., Ed.; Oxford University Press: New York, NY, USA, 1996; pp. 570–573. [Google Scholar]
- Bibi, F.; Pante, M.; Souron, A.; Stewart, K.; Varela, S.; Werdelin, L.; Boisserie, J.-R.; Fortelius, M.; Hlusko, L.; Njau, J. Paleoecology of the Serengeti during the Oldowan-Acheulean transition at Olduvai Gorge, Tanzania: The mammal and fish evidence. J. Hum. Evol. 2017, 120, 48–75. [Google Scholar] [CrossRef]
- Faith, J.T.; Rowan, J.; Du, A. Early hominins evolved within non-analog ecosystems. Proc. Natl. Acad. Sci. USA 2019, 116, 21478–21483. [Google Scholar] [CrossRef]
- Hughes, P.D.; Gibbard, P.L. A stratigraphical basis for the Last Glacial Maximum (LGM). Quat. Int. 2015, 383, 174–185. [Google Scholar] [CrossRef]
- Stone, P.; Millward, D.; Young, B.; Merritt, J.; Clarke, S.; McCormac, M.; Lawrence, D. British Regional Geology: Northern England, 5th ed.; British Geological Survey: Nottingham, UK, 2010; p. 294.
- Clark, P.U.; Dyke, A.S.; Shakun, J.D.; Carlson, A.E.; Clark, J.; Wohlfarth, B.; Mitrovica, J.X.; Hostetler, S.W.; McCabe, A.M. The Last Glacial Maximum. Science 2009, 325, 710–714. [Google Scholar] [CrossRef]
- Elias, S.A.; Brigham-Grette, J. Late Pleistocene glacial events in Beringia. In Encyclopedia of Quaternary Science, 2nd ed.; Elias, S.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 191–201. [Google Scholar]
- Lambeck, K.; Roubya, H.; Purcella, A.; Sun, Y.; Sambridge, M. Sea level and global ice volumes from the Last Glacial Maximum to the Holocene. Proc. Natl. Acad. Sci. USA 2015, 111, 15296–15303. [Google Scholar] [CrossRef]
- Tallavaara, M.; Luoto, M.; Korhonen, N.; Järvinen, H.; Seppä, H. Human population dynamics in Europe over the Last Glacial Maximum. Proc. Natl. Acad. Sci. USA 2015, 112, 8232–8237. [Google Scholar] [CrossRef] [PubMed]
- Vizcaíno, S.F.; Bargo, M.S.; Toledo, N.; De Iuliis, G. Conceptual challenges for the paleoecological reconstruction of the Pleistocene Pampean megafauna and the consequences of its extinction. PE-APA 2023, 23, 317–330. [Google Scholar]
- Dillehay, T.D. Profiles in Pleistocene Prehistory. In Handbook of South American Archaeology; Silverman, H., Isbell, W.H., Eds.; Springer: New York, NY, USA, 2008; pp. 29–44. [Google Scholar]
- Meltzer, D.J. First Peoples in a New World: Colonizing Ice Age America; University of California Press: Berkeley, CA, USA, 2009; p. 500. [Google Scholar]
- Chauchat, C.; Bonavia, D. Débuts de l’exploitation de la mer sur la côte du Pérou. In L’Homme Préhistorique et la mer, 120e Congrès National des Sociétés Historiques et Scientifiques, Aix-en Provence, France, 23–26 October 1995; Camps, G., Ed.; CTHS: Paris, France, 1995; pp. 427–436. [Google Scholar]
- Kipnis, R. Early Hunter-Gatherers in the Americas: Perspectives from Central Brazil. Antiquity 1998, 72, 581–592. [Google Scholar] [CrossRef]
- De France, S.; Keefer, D.K.; Richardson, J.B.; Álvarez, U. Late Paleo-indian Coastal Foragers: Specialized Extractive Behavior at Quebrada Tacahuay, Peru. Lat. Am. Antiq. 2001, 12, 413–426. [Google Scholar] [CrossRef]
- de France, S.; Umire, A. Quebrada Tacahuay: Un sitio marítimo del Pleistoceno Tardío en la costa sur del Perú. Chungara 2004, 36, 257–278. [Google Scholar]
- Goebel, T.; Waters, M.R.; O’Rourke, D.H. The Late Pleistocene Dispersal of Modern Humans in the Americas. Science 2008, 319, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Haynes, G. The Early Settlement of North America. The Clovis Era; Cambridge University Press: Cambridge, UK, 2002; p. 345. [Google Scholar]
- Nami, H.G. New morpho-technological studies to enhance the knowledge of Fell point variability in southeastern South America. J. Archaeol. Sci. Rep. 2021, 40, 103205. [Google Scholar] [CrossRef]
- Nami, H.G. Fishtailed projectile points in the Americas: Remarks and hypotheses on the peopling of northern South America and beyond. Quat. Int. 2021, 578, 47–72. [Google Scholar] [CrossRef]
- Lund, P.W. Memórias Sobre a História Natural do Brasil; Typographia de Laemmert: Rio de Janeiro, Brazil, 1845. [Google Scholar]
- Luna, P. The Man Who Faced the Saber-Toothed Cat. Peter Wilhelm Lund’s Forgotten Encounters with the Brazilian Deep Past and the Colonial Present. In Fugitive Knowledge. The Preservation and Loss of Knowledge in Cultural Contact Zones; Andreas, B., Mackenthun, G., Eds.; Münster: Waxmann, Germany, 2014; pp. 163–179. [Google Scholar]
- Lund, P.W. Notice sur des Ossements Humains Fossiles, Trouvés Dans Une Caverne de Brésil. Extract d’une letter de M.P.W Lund to M.C.C. Rafn, Sécretaire de la Société. Memoires de la Société Royale des Antiquitaires du Nord. Copenhagen 1845, 47, 49–77. [Google Scholar]
- Da-Gloria, P.; Neves, W.A.; Hubbe, M. (Eds.) Archaeological and Paleontological Research in Lagoa Santa: The Quest for the First Americans; Springer: Cham, Switzerland, 2017; p. 401. [Google Scholar]
- Laming-Emperaire, A. Mission archéologique et ethnologique française au Brésil: Les fouilles de la grotte Lapa Vermelha. J. Soc. Am. 1979, 66, 7–28. [Google Scholar]
- Neves, W.A.; Prous, A. Lapa Vermelha IV Hominid 1: Morphological affinities of the earliest known American. Am. J. Phys. Anthropol. 1998, 107, 285–298. [Google Scholar] [CrossRef]
- Neves, W.A.; Hubbe, M. Cranial morphology of early Americans from Lagoa Santa, Brazil: Implications for the settlement of the New World. Proc. Natl. Acad. Sci. USA 2005, 102, 18309–18314. [Google Scholar] [CrossRef]
- Moreno da Sousa, J.C. Americas, South: Peopling Stage. In Encyclopedia of Archaeology, 2nd ed.; Nikita, E., Rehren., T., Eds.; Academic Press: Cambridge, MA, USA, 2024; Volume 3, pp. 252–265. [Google Scholar]
- Ameghino, F. La Antigüedad del Hombre en el Plata; G. Masson-Igon Hermanos: Paris, France; Buenos Aires, Argentina, 1880. [Google Scholar]
- Ameghino, F. Formaciones Sedimentarias del Cretácico Superior y Terciario de la Patagonia; Anal. Mus. Nac. XIV; Imprenta de Juan A. Alsina: Buenos Aires, Argentina, 1906. [Google Scholar]
- Hrdlička, A. Early Man in South America; Bureau of American Ethnology. Bulletin 52; Smithsonian Institution: Washington, DC, USA, 1912; p. 405. [Google Scholar]
- Bird, J. Before Magellan. Nat. Hist. 1938, 41, 16–28. [Google Scholar]
- Corbella, H. El campo volcánico-tectónico de Pali Aike. In Relatorio XV Congreso Geolόgico Argentino, el Calafate: Geología y Recursos Naturales de Santa Cruz; Haller, M.J., Ed.; Asociaciόn Geolόgica Argentina: Buenos Aires, Argentina, 2002; pp. 285–301. [Google Scholar]
- Figgins, J.D. The Antiquity of Man in America. Nat. Hist. 1927, 27, 229–239. [Google Scholar]
- Howard, E.B. Association of Artifacts with Mammoth and Bison in Eastern New Mexico. Science 1933, 78, 524. [Google Scholar]
- Roberts, F.H.H., Jr. A Folsom complex, preliminary report on investigations at the Lindenmeier site in northern Colorado. Smithson. Misc. Collect. 1935, 94, 1–35. [Google Scholar]
- Cotter, J.L. The Occurrence of Flints and Extinct Animals in Pluvial Deposits Near Clovis, New Mexico, Part IV-Report on Excavations at the Gravel Pit, 1936. Proc. Acad. Nat. Sci. Phila. 1937, 89, 2–16. [Google Scholar]
- Montané, J. Paleo-Indian Remains from Laguna de Tagua Tagua, Central Chile. Science 1968, 161, 1137–1138. [Google Scholar] [CrossRef] [PubMed]
- Wheat, J.B. The Olsen-Chubbuck Site: A Paleo-Indian Bison Kill Memoirs 26; Society for American Archaeology: Washington, DC, USA, 1972. [Google Scholar]
- Stanford, D.J. The Jones-Miller Site: An Example of Hell Gap Bison Procurement Strategy. Plains Anthropol. Mem. 1978, 14, 90–97. [Google Scholar] [CrossRef]
- Todd, L.C.; Hofman, J.L.; Schultz, C.B. Faunal analysis and Paleoindian studies: A reexamination of the Lipscomb bison bonebed. Plains Anthropol. 1992, 37, 137–165. [Google Scholar] [CrossRef]
- Frison, G.C. Survival by Hunting: Prehistoric Human Predators and Animal Prey; University of California Press: Berkeley, CA, USA, 2004; p. 288. [Google Scholar]
- Frison, G.C. (Ed.) The Casper Site: A Hell Gap Bison Kill on the High Plains; Percheron Press; Eliot Werner Publications, Clinton Corners: New York, NY, USA, 2013; p. 296. [Google Scholar]
- Bamforth, D.B. Origin Stories, Archaeological Evidence, and Postclovis Paleoindian Bison Hunting on the Great Plains. Am. Antiq. 2011, 76, 24–40. [Google Scholar] [CrossRef]
- Dillehay, T.D. A Late Ice-age Settlement in Southern Chile. Sci. Am. 1984, 251, 106–117. [Google Scholar] [CrossRef]
- Massone, M. Los cazadores paleoindios de Tres Arroyos (Tierra del Fuego). An. Inst. Pat. (Ser. Cs. Soc.) 1987, 17, 47–60. [Google Scholar]
- Nami, H.G. Excavación arqueológica y hallazgo de una punta de proyectil “Fell I” en la Cueva del Medio, Seno de Ultima Esperanza, Chile. Informe preliminar. An. Inst. Patagon. 1985, 16, 103–109. [Google Scholar]
- Nami, H.G. Cueva del Medio: Perspectivas Arqueológicas para la Patagonia Austral. An. Inst. Patagon. 1987, 17, 71–106. [Google Scholar]
- Prieto, A. Cazadores tempranos y tardíos en la Cueva del Lago Sofía. An. Inst. Pat. 1991, 20, 75–99. [Google Scholar]
- Miotti, L. Paleoindian occupations at Piedra Museo Locality, Santa Cruz province, Argentina. Cur. Res. Pleist. 1992, 9, 30–32. [Google Scholar]
- Lynch, T. Glacial-Age Man in South America: A Critical Review. Am. Antiq. 1990, 55, 12–36. [Google Scholar] [CrossRef]
- Nuñez, L.; Schiappacasse, V.; Niemeyer, H.; Villagrán, C. Cuenca de Taguatagua en Chile: El ambiente del 159 Pleistoceno y ocupaciones humanas. Rev. Chil. Hist. Nat. 1994, 67, 503–519. [Google Scholar]
- Dillehay, T.D. Monte Verde: A Late Pleistocene Settlement in Chile. Vol. 2, The Archaeological Context; Smithsonian Institution Press: Washington, DC, USA, 1997; p. 1071. [Google Scholar]
- Politis, G.; Prates, L.; Perez, S. El Poblamiento de América. Arqueología y Bío-Antropología de los Primeros Americanos; EUDEBA: Buenos Aires, Argentina, 2008; p. 196. [Google Scholar]
- Politis, G.; Gutierrez, M.; Rafuse, D.J.; Blasi, A. The arrival of Homo sapiens into the Southern Cone at 14,000 years ago. PLoS ONE 2016, 11, e0162870. [Google Scholar] [CrossRef]
- Politis, G.G.; Messineo, P.G.; Stafford, T.W., Jr.; Lindsey, E.L. Campo Laborde: A Late Pleistocene Giant Ground Sloth Kill and Butchering Site in the Pampas. Sci. Adv. 2019, 5, eaau4546. [Google Scholar] [CrossRef]
- Nami, H.G. Arqueología del último milenio del Pleistoceno en el Cono Sur de Sudamérica, puntas de proyectil y observaciones sobre tecnología Paleoindia en el Nuevo Mundo. In Peuplement et Modalités D’occupation de L’amérique du Sud: L’apport de la Technologie Lithique; Farias, M., Lourdeau, A., Eds.; @rchéo-éditions.com: Prigonrieux, France, 2014; pp. 279–336. [Google Scholar]
- Labarca, R.; Frugone-Alvarez, M.; Vilches, L.; Blanco, J.B.; Peñaloza, A.; Godoy-Aguirre, C.; Lizama-Catalán, A.; Oyarzo, C.; Tornero, C.; González-Guarda, E.; et al. Taguatagua 3: A new late Pleistocene settlement in a highly suitable lacustrine habitat in central Chile (34˚S). PLoS ONE 2024, 19, e0302465. [Google Scholar]
- Cione, A.L.; Tonni, E.P.; Soibelzon, L.H. Did humans cause large mammal Late Pleistocene-Holocene extinction in South America in a context of shrinking open areas? In American Megafaunal Extinctions at the End of the Pleistocene; Haynes, G., Ed.; Vertebrate Paleobiology and Palaeontology Series; Springer: Cham, Switzerland, 2009; pp. 125–144. [Google Scholar]
- Prado, J.L.; Alberdi, M.T.; Bellinzoni, J. Pleistocene mammals from Pampean Region (Argentina). Biostratigraphic, biogeographic, and environmental implications. Quaternary 2021, 4, 15. [Google Scholar] [CrossRef]
- Zurita, A.E.; Soibelzon, L.H.; Soibelzon, E.; Gasparini, G.M.; Cenizo, M.M.; Arzani, H. Accessory protection structures in Glyptodon Owen (Xenarthra, Cingulata, Glyptodontidae). Ann. Paleontol. 2010, 96, 1–11. [Google Scholar] [CrossRef]
- Vizcaíno, S.F.; Cassini, G.H.; Toledo, N.; Bargo, M.S. On the evolution of large size in mammalian herbivores of Cenozoic. In Bones, Clones and Biomes: The History and Geography of Recent Neotropical Mammals; Patterson, B.D., Costa, L.P., Eds.; University of Chicago Press: Chicago, IL, USA, 2012; pp. 76–101. [Google Scholar]
- Salemme, M.; Miotti, L. Archaeological Hunter-Gatherer Landscapes since the Latest Pleistocene in Fuego-Patagonia. In The Late Cenozoic of Patagonia and Tierra del Fuego; Rabassa, J., Ed.; Elsevier: New York, NY, USA, 2008; pp. 437–483. [Google Scholar]
- Bargo, M.S. Biomechanics and palaeobiology of the Xenarthra: The state of the art. Senck. Biol. 2003, 83, 41–50. [Google Scholar]
- Prado, J.L.; Alberdi, M.T. Fossil Horses of South America. Phylogeny, Systemics and Ecology; Springer: Cham, Switzerland, 2017; p. 150. [Google Scholar]
- Villavicencio, N.A.; Corcoran, D.; Marquet, P.A. Assessing the causes behind the Late Quaternary extinction of horses in South America using species distribution models. Front. Ecol. Evol. 2019, 7, 226. [Google Scholar] [CrossRef]
- Labarca, R. La meso y megafauna terrestre extinta del Pleistoceno de Chile. Publicaciόn Ocas. Mus. Nac. Hist. Nat. Chile 2015, 63, 401–465. [Google Scholar]
- Chichkoyan, K.V.; Tessone, A.; Lanata, J.L.; Lezcano, M.; Hajduk, A.; Palmqvist, P. Paleoenvironment from the Nahuel Huapi region (Argentina) based in δ13C and δ15N and AMS dates from extinct and extant species of the last 20.000 cal bp. J. Iber. Geol. 2024, 50, 449–465. [Google Scholar] [CrossRef]
- Dillehay, T.D.; Ocampo, C.; Saavedra, J.; Sawakuchi, A.O.; Vega, R.M.; Pino, M.; Collins, M.B.; Scott Cummings, L.; Arregui, I.; Villagran, X.S.; et al. New archaeological evidence for an early human presence at Monte Verde, Chile. PLoS ONE 2015, 10, e0141923. [Google Scholar]
- Dillehay, T.D.; Ocampo, C.; Saavedra, J.; Pino, M.; Scott-Cummings, L.; Kovácik, P.; Silva, C.; Alvar, R. New excavations at the late Pleistocene site of Chinchihuapi I, Chile. Quat. Res. 2019, 92, 70–80. [Google Scholar] [CrossRef]
- Domingo, L.; Tomassini, R.L.; Montalvo, C.I.; Sanz-Perez, D.; Alberdi, M.T. The Great American Biotic Interchange revisited: A new perspective from the stable isotope record of Argentine Pampas fossil mammals. Sci. Rep. 2020, 10, 1608. [Google Scholar] [CrossRef]
- González-Guarda, E.; Domingo, L.; Tornero, C.; Pino, M.; Hernandez Fernandez, M.; Sevilla, P.; Villavicencio, N.; Agusti, J. Late Pleistocene ecological, environmental and climatic reconstruction based on megafauna stable isotopes from northwestern Chilean Patagonia. Quat. Sci. Rev. 2017, 170, 188–202. [Google Scholar] [CrossRef]
- Sanz-Pérez, D.; Fernández, M.H.; Tomassini, R.L.; Montalvo, C.I.; Beilinson, E.; Gasparini, G.M.; Domingo, L. The Pampean region (Argentina) underwent larger variation in aridity than in temperature during the late Pleistocene: New evidence from the isotopic analysis of mammalian taxa. Quat. Sci. Rev. 2022, 286, 107555. [Google Scholar] [CrossRef]
- Bellinzoni, J.E.; Valenzuela, L.O.; Prado, J.L. Feeding habits of a latest Pleistocene megamammal community. A synecological perspective by stable isotopes analysis. J. S. Am. Earth Sci. 2023, 129, 104496. [Google Scholar]
- Czerwonogora, A.; Fariña, R.A.; Tonni, E.P. Diet and isotopes of Late Pleistocene ground sloths: First results for Lestodon and Glossotherium (Xenarthra, Tardigrada). N. Jb. Geol. Paläont. Abh 2011, 262, 257–266. [Google Scholar] [CrossRef]
- Prevosti, F.J.; Martin, F.M. Paleoecology of the mammalian predator guild of Southern Patagonia during the latest Pleistocene: Ecomorphology, stable isotopes, and taphonomy. Quat. Int. 2013, 305, 74–84. [Google Scholar] [CrossRef]
- Bocherens, H.; Cotte, M.; Bonini, R.; Scian, D.; Straccia, P.; Soibelzon, L.; Prevosti, F.J. Paleobiology of Sabretooth Cat Smilodon populator in the Pampean Region (Buenos Aires Province, Argentina) Around the Last Glacial Maximum: Insights from Carbon and Nitrogen Stable Isotopes in Bone Collagen. Palaeogeogr Palaeoclimatol. Palaeoecol. 2016, 449, 463–474. [Google Scholar] [CrossRef]
- Rabanus-Wallace, T.M.; Wooller, M.J.; Zazula, G.D.; Shute, E.; Jahren, A.H.; Kosintsev, P.; Burns, J.A.; Breen, J.; Llamas, B.; Cooper, A. Megafaunal isotopes reveal role of increased moisture on rangeland during late Pleistocene extinctions. Nat. Ecol. Evol. 2017, 1, 0125. [Google Scholar] [CrossRef]
- Tessone, A. An isotopic perspective of the Alero el Puesto 1 zooarchaeology: Environmental changes, extinct fauna and the first human occupations of southern Patagonia. In Archaeology of Piedra Museo Locality. An Open Window to the Early Population of Patagonia; Miotti, L., Monica, S., Hermo, D., Eds.; Springer: Cham, Switzerland, 2022; pp. 291–307. [Google Scholar]
- Tessone, A.; Srur, A.; Aranibar, J.N. δ13C and δ15N of plants in a longitudinal transect from the Andes to the Atlantic coast: Terrestrial baseline for paleodietary and paleocological studies. J. Archaeol. Sci. Rep. 2023, 47, 103787. [Google Scholar] [CrossRef]
- Varela, L.; Clavijo, L.; Tambusso, P.S.; Fariña, R.A. A window into a late Pleistocene megafauna community: Stable isotopes show niche partitioning among herbivorous taxa at the Arroyo del Vizcaíno site (Uruguay). Quat. Sci. Rev. 2023, 317, 15. [Google Scholar] [CrossRef]
- Kohn, M.J. Carbon Isotope Compositions of Terrestrial C3 Plants as Indicators of (Paleo) Ecology and (Paleo) Climate. Proc. Natl. Acad. Sci. USA 2010, 107, 19691–19695. [Google Scholar] [CrossRef]
- Drucker, D.G. The isotopic ecology of the mammoth steppe. Annu. Rev. Earth Planet. Sci. 2022, 50, 395–418. [Google Scholar] [CrossRef]
- Heaton, T.H.E.; Vogel, J.C.; von la Chevallerie, C.; Collet, G. Climatic influence on the isotopic composition of bone nitrogen. Nature 1986, 322, 822–823. [Google Scholar] [CrossRef]
- Pradeiro, A.; Gil, A.; Forasiepi, A.M. El registro de Megatherium (Xenarthra, Tardigrada) en Mendoza (Argentina): Aspectos taxonómicos, cronológicos y paleoecológicos. Mastozoología Neotrop. 2012, 19, 163–178. [Google Scholar]
- González-Guarda, E.; Petermann-Pichincura, A.; Tornero, C.; Domingo, L.; Agustí, J.; Pino, M.; Abarzúa, A.M.; Capriles, J.M.; Villavicencio, N.; Labarca, R.; et al. Multiproxy evidence for leaf-browsing and closed habitats in extinct proboscideans (Mammalia, Proboscidea) from Central Chile. Proc. Natl. Acad. Sci. USA 2018, 115, 9258–9263. [Google Scholar] [CrossRef]
- Metcalf, J.L.; Turney, C.; Barnett, R.; Martin, F.; Bray, S.C.; Vilstrup, J.T.; Orlando, L.; Salas-Gismondi, R.; Loponte, D.; Medina, M.; et al. Synergistic roles of climate warming and human occupation in Patagonian megafaunal extinctions during the Last Deglaciation. Sci. Adv. 2016, 2, e1501682. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.R. Principles of Geoarchaeology: A North American Perspective; University of Arizona Press: Tucson, AZ, USA, 1992. [Google Scholar]
- Alberdi, M.T.; Prieto, A. Hippidion (Mammalia, Perissodactyla) de las cuevas de las provincias de Magallanes y Tierra del Fuego. An. Inst. Patagon. 2000, 28, 147–171. [Google Scholar]
- Martínez, G.A.; Gutiérrez, M.A. Paso Otero 5: A summary of the interdisciplinary lines of evidence for reconstructing early human occupation and paleoenvironment in the Pampean region, Argentina. In Peuplements et Préhistoire en Amériques; Vialou, D., Ed.; Éditions du Comité des Travaux Historiques et Scientifiques: Paris, France, 2011; pp. 271–284. [Google Scholar]
- Chichkoyan, K.V.; Martínez-Navarro, B.; Moigne, A.M.; Belinchón, M.; Lanata, J.L. Description and interpretation of a Megatherium americanum atlas with evidence of human intervention. Riv. Ital. Pal. Strat. 2017, 123, 51–64. [Google Scholar]
- Marchionni, L.; Vázquez, M.; Miotti, L. The Archaeofaunas of Piedra Museo. Zooarchaeological and Taphonomic Study of the AEP-1 Site (Argentine Patagonia). In Archaeology of Piedra Museo Locality. An Open Window to the Early Population of Patagonia; Miotti, L., Monica, S., Hermo, D., Eds.; Springer: Cham, Switzerland, 2022; pp. 199–256. [Google Scholar]
- Miotti, L. Piedra Museo, A Place and a History of the Peopling of Patagonia. An Open Window to the Early Population of Patagonia. In Archaeology of Piedra Museo Locality. An Open Window to the Early Population of Patagonia; Miotti, L., Monica, S., Hermo, D., Eds.; Springer: Cham, Switzerland, 2022; pp. 1–56. [Google Scholar]
- Gasparini, G.M.; Tonni, E.P. Quaternary fossil vertebrates of Tierra del Fuego and southernmost Patagonia. In Archaeology of Piedra Museo Locality. An Open Window to the Early Population of Patagonia; Miotti, L., Monica, S., Hermo, D., Eds.; Springer: Cham, Switzerland, 2022; pp. 127–157. [Google Scholar]
- Rindel, D.D.; Moscardi, B.F.; Perez, S.I. The distribution of the guanaco (Lama guanicoe) in Patagonia during Late Pleistocene–Holocene and its importance for prehistoric human diet. Holocene 2021, 31, 644–657. [Google Scholar] [CrossRef]
- Davis, C.V.; Rindel, D.D. Final Pleistocene faunal diversity, human processing and consumption in the central plateau of Santa Cruz, Argentina: Contributions from Cueva Túnel site. J. Archaeol. Sci. Rep. 2024, 57, 104638. [Google Scholar]
- Steele, J.; Politis, G. AMS 14C dating of early human occupation of southern South America. J. Archaeol. Sci. 2009, 36, 419–429. [Google Scholar] [CrossRef]
- Bryan, A.L.; Casamiquela, R.M.; Cruxent, J.M.; Gruhn, R.; Ochsenius, C. An El Jobo mastodon kill at Taima-taima Venezuela. Science 1978, 200, 1275–1277. [Google Scholar] [CrossRef]
- Dillehay, T.D. Probing Deeper into First American Studies. Proc. Natl. Acad. Sci. USA 2009, 106, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.R.; Forman, S.L.; Jennings, T.A.; Nordt, L.C.; Driese, S.G.; Feinberg, J.M.; Keene, J.L.; Halligan, J.; Lindquist, A.; Pierson, J.; et al. The Buttermilk Creek Complex and the origins of Clovis at the Debra L. Friedkin Site, Texas. Science 2011, 331, 1599–1603. [Google Scholar] [CrossRef]
- Waters, M.R.; Keene, J.L.; Forman, S.L.; Prewitt, E.R.; Carlson, D.L.; Wiederhold, J.E. Pre-Clovis projectile points at the Debra L. Friedkin site, Texas-Implications for the Late Pleistocene peopling of the Americas. Sci. Adv. 2018, 4, eaat4505. [Google Scholar] [CrossRef]
- Carlini, A.A.; Carrillo-Briceño, J.D.; Jaimes, A.; Aguilera, O.; Zurita, A.E.; Iriarte, J.; Sánchez-Villagra, M.R. Damaged glyptodontid skulls from Late Pleistocene sites of northwestern Venezuela: Evidence of hunting by humans? Swiss J. Palaeontol. 2022, 141, 11. [Google Scholar] [CrossRef]
- Del Papa, M.; De Los Reyes, M.; Poiré, D.G.; Rascovan, N.; Jofré, G.; Delgado, M. Anthropic cut marks in extinct megafauna bones from the Pampean region (Argentina) at the last glacial maximum. PLoS ONE 2024, 19, e0304956. [Google Scholar] [CrossRef]
- Jaimes, A.; Carrillo-Briceño, J.D.; De Jesús, I.; Martín La Riva, C.A.; Sánchez-Villagra, M.R. Diversidad tecnológica en proyectiles del cuaternario en el norte de Venezuela. In Contribuciones en Venezuela Arqueológica; Sánchez-Villagra, M.R., Carrillo-Briceño, J.D., Jaimes, A., Arvelo, L., Eds.; Scidinge Hall Verlag: Tübingen, Germany, 2024; pp. 11–33. [Google Scholar]
- Coronato, A.; Mazzoni, E.; Vázquez, M.; Coronato, F. Patagonia: Una Síntesis de su Geografía Física; Universidad Nacional de la Patagonia Austral: Río Gallegos, Argentina, 2017; p. 218. [Google Scholar]
- Gil, A.F.; Sugrañes, N.; Acevedo, A.; Neme, G.; Salgán, L.; Giardina, M.; Tucker, H.; Fiore, D.; Seitz, V.P.; Pompei, M.; et al. Biogeografía Humana y Tendencia Demográfica en el Monte Nordpatagónico. Una aproximación arqueológica desde El Corcovo (SE de Mendoza). Rev. Mus. Antr. 2019, 12, 23–40. [Google Scholar] [CrossRef]
- Long, A.; Martin, P.S.; Lagiglia, H.A. Ground sloth extinction and human occupation at Gruta del Indio, Argentina. Radiocarbon 1998, 40, 693–700. [Google Scholar] [CrossRef]
- Neme, G.; Gil, A. Biogeografía humana en los Andes meridionales: Tendencias arqueológicas en el sur de Mendoza. Chungará 2008, 40, 5–18. [Google Scholar]
- Hogg, A.; Heaton, T.; Hua, Q.; Palmer, J.; Turney, C.; Southon, J.; Bayliss, A.; Blackwell, P.; Boswijk, G.; Bronk Ramsey, C.; et al. SHCal20 Southern Hemisphere calibration, 0–55,000 years cal BP. Radiocarbon 2020, 62, 759–778. [Google Scholar] [CrossRef]
- Varela, L.; Tambusso, P.S.; Patiño, S.J.; Di Giacomo, M.; Fariña, R.A. Potential distribution of fossil xenarthrans in South America during the late Pleistocene: Co-occurrence and provincialism. J. Mamm. Evol. 2018, 25, 539–550. [Google Scholar] [CrossRef]
- Paunero, R.; Davis, C.V.; Rindel, D.; Tessone, A. La fauna pleistocénica: Evidencias zooarqueológicas en la Meseta Central de Santa Cruz, los sitios de La María. Magallania 2017, 45, 181–198. [Google Scholar] [CrossRef]
- Vizcaíno, S.F.; Bargo, M.S.; Cassini, G.H. Dental occlusal surface area in relation to body mass, food habits and other biological features in fossil xenarthrans. Ameghiniana 2006, 43, 11–26. [Google Scholar]
- Bargo, M.S.; Vizcaíno, S.F. Paleobiology of Pleistocene ground sloths (Xenarthra, Tardigrada): Biomechanics, morphogeometry and ecomorphology applied to the masticatory apparatus. Ameghiniana 2008, 45, 175–196. [Google Scholar]
- van Geel, B.; van Leeuwen, J.F.N.; Nooren, K.; Mol, D.; den Ouden, N.; van der Knaap, P.W.O.; Seersholm, F.V.; Rey-Iglesia, A.; Lorenzen, E.D. Diet and environment of Mylodon darwinii based on pollen of a Late-Glacial coprolite from the Mylodon Cave in southern Chile. Rev. Palaeobot. Palynol. 2022, 296, 104549. [Google Scholar] [CrossRef]
- Brandoni, D.; Ferrero, B.S.; Brunetto, E. Mylodon darwini Owen (Xenarthra, Mylodontinae) from the Late Pleistocene of Mesopotamia, Argentina, with remarks on individual variability, paleobiology, paleobiogeography, and paleoenvironment. J. Vertebr. Paleontol. 2010, 30, 1547–1558. [Google Scholar] [CrossRef]
- Hajduk, A.; Albornoz, A.M.; Lezcano, M.J. Levels with Extinct Fauna in the Forest Rockshelter El Trébol (Northwest Patagonia, Argentina). Cur. Res. Pleist. 2006, 23, 55–57. [Google Scholar]
- Vizcaíno, S.F.; Cassini, G.H.; Fernicola, J.C.; Bargo, M.S. Evaluating habitats and feeding habits through ecomorphological features in glyptodonts (Mammalia, Xenarthra). Ameghiniana 2011, 48, 305–319. [Google Scholar] [CrossRef]
- Emperaire, J.; Laming, A. La grotte du Mylodon (Patagonie occidentale). J. Soc. Am. 1954, 43, 173–206. [Google Scholar] [CrossRef]
- Saxon, E.C. La Prehistoria de Fuego-Patagonia. Colonización de un Hábitat Marginal. An. Inst. Pat. 1976, 7, 63–73. [Google Scholar]
- Sutcliffe, A.J. On the Track of Ice Age Mammals; Harvard University Press: Cambridge, CA, USA, 1986; p. 224. [Google Scholar]
- Menegaz, A.; Nami, H.G. Análisis de los materiales faunísticos del sitio arqueológico “Cueva del Medio”, Seno de Ultima Esperanza, Chile. In VI Jornadas Argentinas de Paleontología de Vertebrados; Instituto y Museo de Ciencias Naturales; Universidad Nacional de San Juan: San Juan, Argentina, 1989; pp. 80–81. [Google Scholar]
- Menegaz, A.; Nami, H.G. Late Pleistocene Faunal Diversity in Ultima Esperanza (Chile). Further Data from Cueva del Medio. Curr. Res. Pleistocene 1994, 11, 93–94. [Google Scholar]
- Nami, H.G.; Menegaz, A. Cueva del Medio: Aportes para el conocimiento de la diversidad faunística hacia el Pleistoceno Holoceno en Patagonia Austral. An. Inst. Patagon. 1991, 21, 117–132. [Google Scholar]
- Menegaz, A.N.; Nami, H.G.; Senatore, M.X. Alteraciones de los restos faunísticos óseos de Cueva del Medio: Un análisis preliminar, Actas y Memorias del XI Congreso Nacional de Arqueología Argentina. Resúm. Rev. Mus. Hist. Nat. San Rafael 1994, 13, 35–36. [Google Scholar]
- Borrero, L.A.; Lanata, J.L.; Borella, F. Reestudiando huesos: Nuevas consideraciones sobre sitios de Ultima Esperanza. An. Inst. Patagon. 1988, 18, 133–156. [Google Scholar]
- Martin, F.M.; Todisco, D.; Rodet, J.; San Román, M.; Morello, F.; Prevosti, F.; Stern, C.; Borrero, L.A. Nuevas excavaciones en Cueva del Medio. Procesos de formación de la cueva y avances en los estudios de interacción entre cazadores recolectores y fauna extinta (Pleistoceno final, Patagonia Meridional). Magallania 2015, 43, 165–189. [Google Scholar] [CrossRef]
- Nami, H.G. Paleoamerican Occupation, Stone Tools from the Cueva del Medio, and Considerations for the Late Pleistocene Archaeology in Southern South America. Quaternary 2019, 2, 28. [Google Scholar] [CrossRef]
- Nami, H.G. Reseña sobre los avances de la arqueología finipleistocénica del extremo sur. Chungará 1994, 26, 145–163. [Google Scholar]
- Nami, H.G. New Assessments of Early Human Occupations in the Southern Cone. In Prehistoric Mongoloid Dispersals; Akazawa, T., Szathmáry, E.J.E., Eds.; Oxford University Press: Oxford, UK, 1996; pp. 254–269. [Google Scholar]
- Labarca, R. La Subsistencia de Los Cazadores Recolectores de Patagonia Meridional Chilena Durante la Transición Pleistoceno-Holoceno: Un Enfoque Integrador Desde la Zooarqueología. Ph.D. Thesis, Facultad de Ciencias Sociales, Universidad Nacional del Centro, Olavarria, Argentina, 2016. [Google Scholar]
- López Mendoza, P.; Rojas Mondaca, O. El Pleistoceno de la Cuenca de Calama. Evidencias Paleontológicas y Arqueológicas; Monografías del Museo de Historia Natural y Cultural del Desierto de Atacama; Gráfica LOM: Santiago, Chile, 2018; p. 156. [Google Scholar]
- Nami, H.G. Las excavaciones arqueológicas y los hallazgos de fauna extinta en el Seno de Ultima Esperanza, Chile Explotación de Recursos Faunísticos en Sistemas Adaptativos Americanos. Arq. Cont. 1993, 4, 123–133. [Google Scholar]
- Martin, F.; Borrero, L.A. Climate change, availability of territory, and Late Pleistocene human exploration of Última Esperanza, South Chile. Quat. Int. 2017, 428, 86–95. [Google Scholar] [CrossRef]
- Kaltwasser, J.; Medina, A.; Aspillaga, E.; Cáceres, I. Punta Cola de Pescado encontrada en Chile Central. Rev. Chil. Antropol. 1986, 5, 11–16. [Google Scholar] [CrossRef][Green Version]
- Jackson, D.; Aspillaga, E.; Pedro Rodríguez, X.; Jackson, D.; Santana, F.; Méndez, C. Las ocupaciones humanas del sitio arqueológico de Santa Inés, Laguna de Tagua Tagua, Chile central. Rev. Chil. Antropol. 2012, 26, 151–168. [Google Scholar] [CrossRef][Green Version]
- Nami, H.G. Late Pleistocene Technology in the New World: Bone Artifacts from Cueva del Medio and other Sites in the Southern Cone of South America. In Ancient and Modern Bone Artefacts from America to Russia Cultural, Technological and Functional Signature; Legrand-Pineau, A., Sidéra, I., Buc, N., David, E., Scheinsohn, V., Eds.; BAR International Series; Archeopress: Oxford, UK, 2010; pp. 279–286. [Google Scholar]
- Nuñez, L.; Varela, J.; Casamiquela, R.; Schiappacasse, V.; Niemeyer, H.; Villagrán, C. Mastodon Kill in Central Chile. In The First World Conference on Prehistoric Mongoloid Dispersals, Book Abstracts, Tokyo, Japan, 16–21 November 1992; The University Museum-University of Tokyo: Tokyo, Japan, 1992; p. 28. [Google Scholar]
- Méndez, C. Los Primeros Andinos: Tecnología Lítica de los habitantes de Chile Trece Mil Años Atrás; Fondo Editorial, Pontificia Universidad Católica del Perú: Lima, Perú, 2015; p. 251. [Google Scholar]
- Cornejo Bustamente, L.E. El Pais de los grande Valles. Prehistoria de Chile central. In Chile Antes de Chile. Prehistoria; Catalog of the exposition, November 1997–December 1998; Museo Chileno de Arte Precolombino: Santiago, Chile, 1997; pp. 44–58. [Google Scholar]
- Jackson, D.; Méndez, C.; Núñez, L.; Jackson, F. Procesamiento de fauna extinta durante la transición Pleistoceno-Holoceno en el centro-norte de Chile. Boletín Arqueol. PUCP 2011, 15, 315–336. [Google Scholar] [CrossRef]
- Lizama-Catalán, Á.; Labarca, R. Who eats What: Unravelling a complex taphonomic scenario in the lacustrine deposits of the late Pleistocene archaeological site, Taguatagua 1, central Chile. Quat. Sci. Rev. 2023, 300, 107831. [Google Scholar] [CrossRef]
- Pino, M.; Dillehay, T.D. Monte Verde II: An assessment of new radiocarbon dates and their sedimentological context. Antiquity 2023, 97, 524–540. [Google Scholar] [CrossRef]
- Callahan, E. The Basics of Biface Knapping in the Eastern Fluted Point Tradition. A Manual for flintknappers and lithic analysts. Arch. East. N. Am. 1979, 7, 1–180. [Google Scholar]
- Nami, H.G. Experimentos para explorar la secuencia de reducción Fell de la Patagonia Austral. Magallania 2003, 30, 107–138. [Google Scholar]
- Nami, H.G. Experiments to Understand North and South American Late Pleistocene Lithic Reduction Sequences: An Actualistic and Comparative Study. In Experiment and Interpretation of Traditional Technologies: Essays in Honor of Errett Callahan; Nami, H.G., Ed.; Ediciones de Arqueología Contemporánea: Buenos Aires, Argentina, 2010; pp. 203–225. [Google Scholar]
- Schick, N.; Toth, P. Making Silent Stones Speak: Human Evolution and the Dawn of Technology; Simon and Schuster: New York, NY, USA, 1994; p. 352. [Google Scholar]
- Gingerich, J.A.M.; Stanford, D.J. Lessons from Ginsberg: An analysis of elephant butchery tools. Quat. Int. 2016, 466, 269–283. [Google Scholar] [CrossRef]
- Starkovich, B.M.; Cuthbertson, P.; Kitagawa, K.; Thompson, N.; Konidaris, G.E.; Rots, V.; Münzel, S.C.; Giusti, D.; Schmid, V.C.; Blanco-Lapaz, A.; et al. Minimal Tools, Maximum Meat: A Pilot Experiment to Butcher an Elephant Foot and Make Elephant Bone Tools Using Lower Paleolithic Stone Tool Technology. Ethnoarchaeology 2020, 12, 118–147. [Google Scholar] [CrossRef]
- Nami, H.G. Transcending Projectile Points: Unifacial Technology in the South of Southamerica during the Last Millennium of the Pleistocene and Supra-Regional Considerations. 2024; Submitted for publication. [Google Scholar]
- Nami, H.G.; Loponte, D.; Carbonera, M. Additional Records to Deepening the Knowledge of Fell Points from South-Eastern South America. PaleoAmerica 2022, 8, 330–339. [Google Scholar] [CrossRef]
- Loponte, D.; Carbonera, M.; Nami, H.G. Projectile Point Stem Repairs. An Insight from Southeastern South America. Lithic Technol. 2024, 50, 23–39. [Google Scholar] [CrossRef]
- Nami, H.G. Theoretical Reflections on Experimental Archaeology and Lithic Technology. In Experiment and Interpretation of Traditional Technologies: Essays in Honor of Errett Callahan; Nami, H.G., Ed.; Ediciones de Arqueología Contemporánea: Buenos Aires, Argentina, 2010; pp. 91–168. [Google Scholar]
- Nami, H.G. A Glimpse into Advances in Archaeological Research in North-Central Uruguay. Archaeol. Discov. 2020, 8, 147–187. [Google Scholar] [CrossRef]
- Nami, H.G.; Nakamura, T. Cronologia radiocarbonica con AMS sobre muestras de huesos procedentes del sitio Cueva del Medio (Ultima Esperanza, Chile). An. Inst. Patagon. 1995, 23, 125–133. [Google Scholar]
- Taimagambetov, Z.K. Early Human Expansion into Kazakhstan and Subsequent Paleolithic Migrations. In Mobility and Ancient Society in Asia and the Americas; Franchetti, M.D., Spengler, R.N., III, Eds.; Springer: Cham, Switzerland, 2015; pp. 17–21. [Google Scholar]
- Stanford, D.; Bradley, B. Across Atlantic Ice: The Origin of America’s Clovis Culture; University of California Press: Berkeley, CA, USA, 2012; p. 336. [Google Scholar]
- Chauchat, C. Limaces and Unifaces in the Paiján Industry, Perú, and the Early Prehistory of America. Lithic Technol. 2022, 47, 231–242. [Google Scholar] [CrossRef]
- Miotti, L. La fachada atlántica como puerta de ingreso alternativa de la colonización humana de América del Sur durante la transición Pleistoceno Holoceno. In 2do Simposio Internacional del Hombre Temprano en América; Jiménez López, J.C., González, S., Eds.; INAH: México City, Mexico, 2006; pp. 155–188. [Google Scholar]
- Palmqvist, P.; Rodríguez-Gómez, G.; Bermúdez de Castro, J.M.; García-Aguilar, J.M.; Espigares, M.P.; Figueirido, B.; Ros-Montoya, S.; Granados, A.; Serrano, F.J.; Martínez-Navarro, B.; et al. Insights on the Early Pleistocene Hominin Population of the Guadix-Baza Depression (SE Spain) and a Review on the Ecology of the First Peopling of Europe. Front. Ecol. Evol. 2022, 10, 881651. [Google Scholar] [CrossRef]
- Aschero, C.A.; Bozzuto, D.; Civalero, T.; De Nigris, M.; Di Vruno, A.; Dolce, V.; Fernández, N.; González, L.; Sacchi, M. Nuevas evidencias sobre las ocupaciones tempranas en Cerro Casa de Piedra 7. In Arqueología de Fuego-Patagonia. Levantando Piedras, Desenterrando Huesos y Develando Arcanos; Morello, F., Martinic, M., Prieto, A., Bahamonde, G., Eds.; CEQUA: Punta Arenas, Chile, 2007; pp. 569–576. [Google Scholar]
- Iriarte, J.; Ziegler, M.J.; Outram, A.K.; Robinson, M.; Roberts, P.; Aceituno, F.J.; Morcote-Ríos, G.; Keesey, T.M. Ice Age megafauna rock art in the Colombian Amazon? Philos. Trans. R. Soc. B 2022, 377, 20200496. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).