Abstract
Two zoogenic deposits from the central part of the Negev Desert (Israel) were investigated by stable isotopes (carbon 13C/12C and nitrogen 15N/14N) and pollen analyses. The merger of these data and results of radiocarbon dating of Atzmaut and Ramon I deposits enabled us to reconstruct climate and vegetation changes in the Negev Desert over the past 8500 years. Decrease of the δ13C value in plant remains is a sensitive indicator of paleoclimatic conditions in the region. The decline of the δ13C value over the past 8500 years almost fully coincides with an increase of the total pollen concentration, the proportion of Poaceae pollen in the profile of zoogenic deposits and wetter periods. Thus, four humid periods are identified, from the middle of the 4th millennium to the end of 3rd millennium BC, the 1st half of the 2nd millennium BC, from the 2nd half of the 1st millennium BC to the 1st half of the 1st millennium AD and the middle of the 2nd millennium AD. The δ15N value of plant remains is a less sensitive indicator of climate dynamics and represents the most significant change of precipitation in the region by the end of the 3rd millennium BC.
1. Introduction
Vegetation is an essential component of most terrestrial ecosystems. It changes under the influence of various factors, the main factors of which are climatic and anthropogenic. In arid areas, the relationship of vegetation dynamics with the history of settlement and climatic changes is the closest; therefore, the study of changes in the vegetation cover of deserts in the Holocene is extremely interesting and important.
The main method of vegetation reconstruction is pollen analysis. One of the first works on the study of the desert vegetation history using pollen analysis was carried out in the late 1950s–1960s of the twentieth century in the Sahara (for example [1]) and in the southwestern United States (for example [2]). However, it was only since the 1980s that palynologists began to study deserts systematically. The main problem associated with the study of the arid ecosystems’ history is the small number of preserved biological remains and, in this regard, a small number of sources of palaeoinformation. Lake and marine sediments, palaeosoils in archaeological sites and zoogenic deposits can be such sources. But the use of lake and marine sediments to reconstruct vegetation in arid conditions is not always possible due to the frequent absence of the latter. In addition, the pollen spectra of such deposits in treeless regions reflect the vegetation of a large area, and the proportion of the desert pollen spectrum may be only a small part of them. This is due to the low pollen productivity of desert vegetation because of the sparse vegetation cover and the high proportion of insect-pollinated species. It should also be noted that pollen transport in open areas is hundreds of kilometers [3]. The study of palaeosoils for the reconstruction of vegetation dynamics in the desert is also a difficult task. The pollen concentration in such soils is very low and does not persist as well as in soils of temperate latitudes [3].
One of the most promising lines of palaeoenvironmental investigation is research of zoogenic deposits accumulated at the bottom of rock shelters, niches and caves over thousands of years. They are mainly formed by activities of different animal species. When shelters are used by herbivores, deposits consist mainly of dung. Research on such deposits is still less common for reconstruction of the palaeoenvironment than analysis of lake deposits and peat bogs. But these sediments are unique material for the study of the dynamics of various ecosystems, particularly in areas where there are no other sources of palaeoinformation.
Pollen and macrofossils analyses are one of the main methods for studying plant remains from herbivore dung. However, for about 20 years, stable isotope (especially carbon and nitrogen isotopes) analysis has also been used by researchers to study dung [4,5,6,7]. Carbon and nitrogen are two of six the main components of biological organisms. Stable isotopes of these elements in natural objects are present in different proportions that allow the use of stable isotope analysis in ecological and palaeoecological investigations.
Enrichment in the carbon-isotope ratio (13C/12C) with each trophic level is minimal (0–2‰) [8,9], but the isotopic fractionation between diet and faeces is consistent regardless of the carbon isotopic content of the food and the relative digestibility [7]. Thus, variations in the δ13C values of herbivore animals dung deposits potentially can reflect diet of these animals and also indicate vegetation changes that may have occurred over thousands of years [4]. This is possible because there are three different types of photosynthesis in higher plants: C3, C4 and CAM. The δ13C values of modern C3 plants range from about −37‰ to −20‰. In C4 species, most values are concentrated in a narrow band between −11 and −14‰, with a global average of −12.5‰ [10,11,12,13,14]. CAM plants have isotope values ranging between those of C3 and C4 plants. Moreover, ecologists have identified clear isotopic signatures based not only on different photosynthetic pathways but also on ecophysiological differences, such as photosynthetic water-use efficiency [15]. Stable carbon isotope concentrations in C3 plants are influenced by environmental factors like temperature, evaporation and precipitation. B.M. Chase with co-authors [16,17] established that the δ13C records from dung deposits (hyrax middens) in C3 ecosystems may provide an independent proxy for past hydrologic conditions.
There is a little research on the study of 15N abundance in dung deposits of herbivore animals. Their dung is enriched with 15N relative to the vegetation the animals had been feeding on. This presumably occurs due to fractionation in the animals’ digestive system where 14N is preferentially assimilated in the feeding animal tissues, while the heavier, 15N-enriched organic waste (i.e., dung) is excreted [18]. On the other hand, the dung of herbivorous animals contains undigested plants. The isotopic compositions of the light elements of these remains are not subject to change under the influence of metabolism. The latter allows reconstructing of the climate based on the knowledge of the change in the plants’ isotopic composition in dry and wet conditions. The natural ratio of 15N/14N isotopes in plants is not as well established as that of C isotope ratios. Many studies have determined that the isotopic composition of plants’ nitrogen is determined by the complex influence of a number of factors, such as the contributions of various N sources to plant N uptake in the field, including symbiotic nitrogen fixation and atmospheric deposition, the role of mycorrhizal infection, the uptake of dissolved N and the interpretation of δ15N profiles in the soils [15]. However, in spite of these difficulties, some work on hyrax middens have shown that plants’ foliar δ15N is positively correlated with faecal δ15N [19], and 15N abundance in dung deposits is influenced by climate and may provide an estimation of past water availability [16,17,20,21,22,23,24,25,26,27,28].
The results of the most recent studies indicate negative correlations between both carbon and nitrogen isotope values and rainfall in C3 plant communities on global [29,30,31,32] and regional scales, especially in arid and semi-arid conditions [33,34,35,36,37,38].
There are a large number of climate reconstructions for Israel. But the main part of the objects is located in the north of Israel due to a small number of possible palaeoinformation sources in the desert. Recent work on dung deposits from central part of the Negev Desert may provide an estimation of water availability directly in this area. The focus of this study is stable isotopes (carbon 13C/12C and nitrogen 15N/14N) analyses of two zoogenic deposits: Atzmaut (N 30°36′27,48″ E 34°48′26,28″ 820 masl) and Ramon I (N 30°35′1,32″ E 34°44′0,36″ 850 masl). They are formed in rock shelters. The deposits are disclosed in central part of the Negev Desert at the top of the northern cliff erosion crater known as Makhtesh Ramon (Figure 1) [39]. The rock shelters are located close to each other (about 5 km) under the same climatic conditions: the average annual precipitation is about 100 mm [40]. The central part of the Negev Desert is covered with sparse vegetation. Artemisia herba-alba (Asteraceae), Zigophyllum dumosum (Zigophyllaceae) and Reaumuria negevensis (Tamaricaceae) are abundant on the slopes, while Anabasis siriaca and Hammada scoparia (Amaranthaceae) dominate in the valleys [41].
Figure 1.
The central part of the Negev Desert and the location of rock shelters.
The main purpose of this research is to reconstruct past water availability in the central Negev Desert by δ13C, δ15N of Ramon I and Atzmaut dung deposits compared to pollen and climate records and to assess the possibility of using isotope analysis for sheep and goat dung to study climate dynamics.
When comparing δ13C and δ15N records with the dynamics of the climate in Israel, it is necessary to consider the published palaeoclimatic data. The sources of palaeoinformation are a wide range of natural objects: the sediments of the Mediterranean, the Red Sea, the Dead Sea and the Sea of Galilee [42,43,44,45,46,47,48,49,50,51,52], palaeosoils [53], speleothems [54,55,56], land snail shells [57], corals [58], cave deposits [59], wood, which is preserved in the archaeological sites and caves [60,61] and the rings of juniperus [62]. According to this literature, the climate in Israel was not stable. Four main humid periods (I—the middle of the 4th millennium–the end of the 3rd millennium BC, II—the 1st half of the 2nd millennium BC, III—the 2nd half of the 1st millennium BC–the 1st half of the 1st millennium AD and IV—the middle of the 2nd millennium AD) and some driest events can be distinguished on the basis of palaeoclimatic data over the past 8500 years (at about 6200, 5700, 2200–1900, 1500, 200 cal yr BC and 500 and 800 cal yr AD and nowdays).
2. Materials and Methods
The niche Atzmaut was destroyed during the construction of a road through the Makhtesh Ramon erosion crater in the 1950s of the last century, so its size is unknown (Figure 2). Only a small part of the zoogenic deposits has been preserved. The Ramon I niche is 20 m long and 2 m wide (Figure 2). The studied deposits are about one meter in depth. They consist mainly of alternating pressed dung, ash and mineral layers [39]. In arid conditions, dung keeps very well and may appear in the deposit as unbroken faeces, and it even sometimes retains a specific smell. Identification of the preserved faeces showed that ibexes (Capra ibex) as well as domestic sheep and goats used the rock shelters [63].
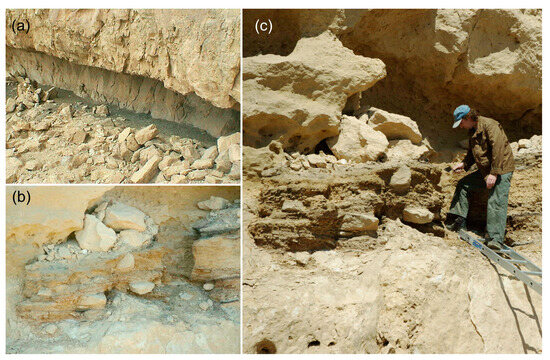
Figure 2.
The Ramon I rock shelter (a) and the Atzmaut zoogenic deposit (b) with sampling (c).
Usually, the depth of the layers is measured starting from the deposit surface. If the surface is uneven, a zero level should be defined for convenience in sampling. The zero levels for the Ramon I and Atzmaut deposits were taken to be 7 and 17 cm (respectively) above their upper boundary. Samples from the studied zoogenic deposits were taken layer by layer and used for radiocarbon dating, pollen and stable isotope analysis. The thickness of the samples depends upon how friable the material is, and it usually lies between 1–5 cm. Samples are taken in an uninterrupted column.
The plant remains and charcoal from the samples were treated with 5% HCl for radiocarbon dating. The radiocarbon was measured in the Group of Historical Ecology (IEE, RAS). The dating was obtained using the scintillation method.
Dung deposits can have rather complex stratigraphy due to the intricate history of their accumulation. Thus, the same cave or niche could at times have been used by ancient shepherds as a shelter for their stock, by wild animals in other periods, as campsites in some periods, and have also undergone periods of abandonment. The deposits would thus consist of alternating layers of different origin, rapidly accrued layers of domestic animals’ dung, less dense deposits that more slowly accumulated from wild animals, and complex deposits from the other uses. To make things even more complicated, shepherds frequently used fire, resulting in the formation of ash layers. Such layers indicate not only the presence of hearths; people often burned or cleared out accumulated dung deposits in order to clean the shelter floor. These events could cause the interruption of sedimentation processes or (and) the formation of thick ash layers [39,64]. Many other circumstances may also affect the accumulation rate of dung deposits and result in their complex stratigraphy and non-uniform growth. That is why the robust absolute chronology of dung deposits can be constructed only with adequate age-depth models.
Age-depth models of zoogenic deposits were constructed with the Bchron 4.7.6 program [65], using the Intcal20 calibration curve [66]. The package Bchron is based only on the assumption that the upper layers are more recent than the lower ones. While modeling a growth of a deposit, it generates a curve restricted by calibrated radiocarbon dates attributed to certain depths during each of the multiple iterations. The set of generated curves constitutes a range covered by the age-depth model. The algorithm implemented in the Bchron program can be applied to all types of deposits [67].
Materials (25 samples from Atzmaut and 18 samples from Ramon I) were sampled for pollen and stable isotope analyses. Standard pollen concentration methods were used according to laboratory methods suggested in Faegri K. and Iversen J. [68]. One of the samples from the Atzmaut deposit was lost during preparation (45–51 cm). The minimum count was 500 pollen grains per sample. Sample weights were recorded and Lycopodium clavatum spores (batch # 938934) added as markers to facilitate absolute counting [69] at ×400 or ×1000 magnification. Pollen diagrams were drawn, and a subdivision of pollen sequences into zones was conducted with computer programs TILIA 2.6.1 [70].
The sampled materials for stable isotope analysis were subjected to chemical treatment. Samples were boiled in hydrochloric acid (5% HCl) to remove the carbonate and then thoroughly rinsed with distilled water. After that, samples were washed through a fine sieve (0.25 mm) to remove the mineral grains and large plant remains. A small fraction of plant remains is the best averaged material. Thus, the results reflect the content of the stable isotopes directly in the homogenized samples of plant remains. Further, the acid insoluble fraction was dried. A large fraction of plant remains was homogenized using a pestle, and it was also used for nitrogen isotopic analysis. The isotopic composition of the samples was determined at the common data center “Instrumental methods in ecology» (A.N. Severtsov Institute of Ecology and Evolution of RAS, Moscow, Russia) using a Thermo-Finnigan Delta V Plus («Thermo Electron», Bremen, Germany) continuous-flow IRMS coupled to an elemental analyzer (Thermo Flash 1112). Two replicates of each sample were analyzed. The N and C isotopic composition was measured in one run (using IRMS “jump”). The mass of analyzed samples for C and N isotopes was about 1500 μg for dung samples and from 2500 to 4900 μg for others. Weighted samples were wrapped in tin capsules. Carbon and nitrogen ratios were calibrated relative to Vienna Pee Dee Belemnite (VPDB) and atmospheric nitrogen international standards (respectively). The analytical measurement error (standard deviation of the laboratory standard, n = 4–8) was <0.2% for δ13C and <0.25% for δ15N.
The Shapiro–Wilk test was used to examine the normality distribution of 15N records of small (<0.25 mm) and large (>0.25 mm) fractions of the Atzmaut samples. The same records were checked by Wilcoxon Matched Pairs Test (p < 0.05) [71].
3. Results
3.1. Chronology
Ten radiocarbon dates were obtained for Atzmaut and four for Ramon I deposits (Table 1). The Atzmaut and Ramon I deposits have accumulated for about the last 6000 and 8500 years, respectively (Figure 3). The models allow us to determine the age and accumulation duration of each sample and to build graphs taking into consideration these parameters. The bottom layers of the Ramon I deposit were formed about 2500 years earlier than those of Atzmaut. The merger of the results of analysis of the two deposits enable us to reconstruct the palaeoenvironment of the region for the last 8500 years.

Table 1.
Conventional radiocarbon dates of the Atzmaut and Ramon I zoogenic deposits, Israel.
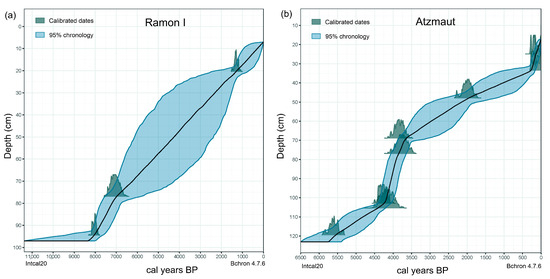
Figure 3.
Sediment accumulation models of the Ramon I (a) and the Atzmaut (b) zoogenic deposits.
Only four samples from the bottom part (approximately 6400–4700 cal yr BC) and four samples from the upper part (approximately 400–the mid-20th century cal yr AD) of Ramon I deposit were used for pollen analyses. And only five samples (from the bottom parts, approximately 6400–4200 cal yr BC) were used for stable isotope analysis. The middle part of the Ramon I deposit consists of burned layers without enough organic materials for analysis. There were not enough pollen grains there (10 samples) because of burning [72]. The thickness of the samples was 4–6 cm, so the material in them was very averaged. The upper dated part was accumulated from about the last fifteen hundred years. From this part, only four samples were taken; therefore, each sample contains information from about 350 years. The bottom five samples are averaged even more, each having been accumulated for about five hundred years. For this reason, the data obtained from the Ramon I deposit can only be considered as additional data.
3.2. Pollen Analysis
The results of the pollen analysis have already been presented in detail in the articles [73,74]. In this paper, the data are presented briefly in order to be able to compare the results of the isotope analysis with them. The following palynomorphs are dominant in spectra of both deposits: Amaranthaceae, Asteraceae, Poaceae and Artemisia (Figure 4). The bottom parts of the Ramon I and Atzmaut (zone A) deposits are characterized by larger proportions of Liliaceae and Poaceae than the upper layers. The total pollen concentration (TPC) varies greatly. It is high in dung layers (140 thousand–4.3 million grains/cm3) and low in ash and gravel layers (7–230 thousand grains/cm3).
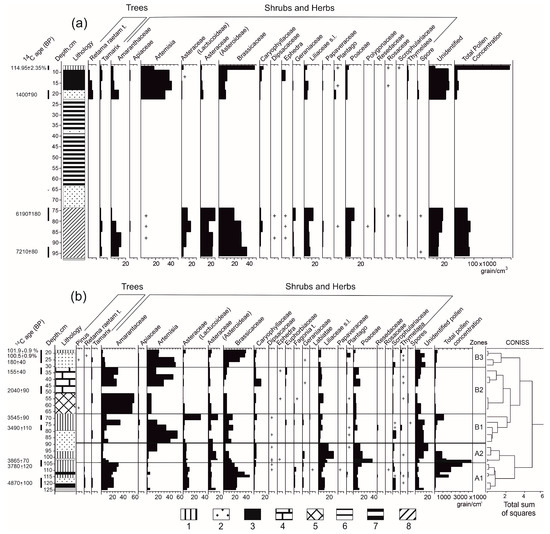
Figure 4.
The profile and the pollen diagram of the Ramon I (a) and Atzmaut (b) zoogenic deposits (+ specified percentage of taxa ≤ 1%). Layers: 1—pressed dung; 2—friable ash; 3—burned dung with inclusions of charcoal; 4—consolidated sediment of gravel and dung; 5—consolidated sediment of gravel and fine-grained sediments; 6—gravel layer; 7—consolidated sediment of ash and gravel; 8—gravel with ash and charcoal.
Ramon I: bottom part (97–74 cm, approximately 6400–4700 cal yr BC). These layers are characterized by a high proportion of Brassicaceae and Asteraceae pollen (Figure 4a). Although Brassicaceae pollen decreases towards the end of the part, its share still remains high (20%). Near the top of the bottom part, Poaceae and Liliaceae pollen increases, and Amaranthaceae pollen decreases. The TPC varies slightly from 69 to 83 thousand grains/cm3.
Ramon I: upper part (23–7 cm, approximately 400–the mid-20th century cal yr AD). Artemisia pollen dominates in three samples (33–42%), but the proportion of Brassicaceae pollen increases towards the top of these layers (48%). The share of Poaceae pollen is low. The Amaranthaceae pollen varies to the top from 10 to 3%. The TPC is low (10–20 thousand grains/cm3), but towards the top, it reaches maximum values (300 thousand grains/cm3).
Atzmaut: Zone A (125–89 cm, approximately 3900–2000 cal yr BC). The palynological spectra of the lowest layers (subzone A1, 125–104 cm, 3900–2300 cal yr BC) are dominated by the pollen of Brassicaceae (22–47%) and Amaranthaceae (27–31%) (Figure 4b). Poaceae is more abundant in the layers 104–101 cm (subzone A2, 2300–2200 cal yr BC) than in the succeeding subzones. The TPC also reaches its highest value here (4.3 million grains/cm3), but towards the end of the zone (subzone A2, 101–89 cm, 2200–2000 BC), it, as well as Poaceae and Liliaceae pollen, rapidly decreases. Artemisia pollen is dominant in the upper part of this zone.
Atzmaut: Zone B (89–17 cm, approximately 2000 cal yr BC–the mid-20th century cal yr AD). Zone B was divided into three subzones. In the bottom part (subzone B1, 89–67 cm, 2000–1500 cal yr BC), Artemisia and Amaranthaceae pollen are prevalent. The proportions of Poaceae are low except for one layer (75–79 cm, 1900–1800 cal yr BC). The TPC sharply increases in the 16th–18th centuries BC (67–71 cm, 1530–1770 BC). The relative content of Asteraceae pollen became higher, and by the middle of the second millennium BC, its share reaches 48%. In subzone B2 (67–31 cm, 1500 cal yr BC–1700 cal yr AD, as in subzone B1, Artemisia and Amaranthaceae pollen are dominant. The proportion of Asteraceae pollen gradually decreased throughout the corresponding period. Brassicaceae and Poaceae pollen are low, but the last one shows an increase in the layers of 45–40 cm (300–900 cal yr AD) and 36–31 cm (1400–1700 cal yr AD) to 12 and 9%, respectively. The TPC does not exceed 85 thousand grains/cm3. Subzone B3 (31–17 cm, 1700–the mid-20th century cal yr AD) is marked by a relatively lower share of Asteraceae and Poaceae pollen. Artemisia and Brassicaceae are dominant. The proportion of Amaranthaceae pollen decreases, whereas the TPC increases by the end of this period.
3.3. Stable Isotope Analysis
The stable isotope analyses of dung deposits demonstrate that sheep and goats’ diets are dominated by C3 vegetation. The δ13C value varies from −24.6 to −20.8 ‰ (Table 2). A decrease of the δ13C value is observed in the following periods: approximately 3300–2200 cal yr BC, 1700–1500 cal yr BC, 300–900 cal yr AD and 1400–1700 cal yr AD.

Table 2.
δ13C and δ15N of Atzmaut and Ramon I zoogenic deposits, Israel.
The Shapiro–Wilk test showed that 15N records of small fraction do not have a normal distribution (p = 0.04). The Wilcoxon Pairs Test indicated that the δ15N value in different fractions is similar (p-level 0.13). In this regard, the mean values were used to plot it. The nitrogen isotope signature of the Ramon I and Atzmaut zoogenic deposits has a wide range (from 8.92 to 20.65‰) and reaches maximum values in the lowest layers of the Atzmaut deposit that formed before 2200 cal yr BC. The δ15N value decreased from this time until the 20th century AD, except for the increase in nitrogen isotope signatures in 1700–1800 cal yr AD. The 15N of the top layers of the Atzmaut deposit are similar to the modern vegetation [37,38].
4. Discussion
The results of the zoogenic deposits study should be interpreted carefully due to the difficulty of forming the latter. To date, only hyrax dung deposits have been studied using isotope analysis to reconstruct climate dynamics. The isotope analysis of ibexes (Capra ibex) and domestic sheep and goats’ dung for this purpose is being carried out for the first time. In this regard, to assess the possibility of using this analysis, it is necessary to compare the data obtained with the results of analysis done on the same samples. Since plant residues extracted from animal dung were used for isotope analysis, it should be necessary to at least briefly consider the vegetation changes of the central Negev Desert, features of using rock shelters and the dynamics of some taxa on pollen diagrams.
The proportion of pollen in the spectra does not always reflect the role of a particular family in the vegetation. This is especially evident when studying dung deposits. Herbivore faeces contain both the pollen of plants eaten by the animals and the pollen coming from the atmosphere. Their palynological spectra can reflect the vegetation composition of the animals’ habitat (or larger region), dietary composition or a combination of both [75,76,77]. To reconstruct the dynamics of vegetation in the central Negev Desert from palynological data from the zoogenic deposit, it is necessary to reveal which spectra are largely indicated the composition of ungulates’ diets and which are not.
The majority of Brassicaceae, Liliaceae and Asteraceae are entomophilous and produce small amount of pollen, compared to wind-pollinated (anemophilous) species of Amaranthaceae, Poaceae and Artemisia. Most species of the Poaceae, Brassicaceae, Asteraceae and Liliaceae growing in the Negev Desert bloom between February and May, whereas the Amaranthaceae and Artemisia bloom between May and November [78,79,80]. Thus, the palynological spectra rich in the pollen of the entomophilous species indicate that the corresponding layers were formed mainly from winter–spring, while the prevalence of the anemophilous species pollen in a certain layer is indicative of its year-round formation.
High proportions (40–72%) of the pollen of entomophilous species coincide with the peaks of pasture disturbance indicators (Plantago and Thymelaea pollen), layers of ash and rapidly accumulating dung in deposits and with the presence of pastoralists in the central part of the Negev Desert. A significant share of Brassicaceae, Liliaceae and Asteraceae (excluding Artemisia) pollen in the spectra apparently largely reflects the composition of livestock feed [73,74].
Wind-pollinated Poaceae, Artemisia and Amaranthaceae in winter–spring palynological spectra account for only 10–30%, but these taxa are the basis of the central Negev Desert vegetation. During three periods, Amaranthaceae dominated the vegetation of the desert: the approximately 6400–2300 cal yr BC, 1900–300 cal yr BC and 1400–1700 cal yr AD. For only about one hundred years (2300–2200 cal yr BC), grasses (Poaceae) played a major role in the vegetation of the desert. As in the last 350 years, (Artemisia) dominated in other periods (Figure 4).
Poaceae pollen is the main sensitive indicator of moisture in the arid ecosystems [3,81]. The lower layers of the two dung deposits differ from the upper ones by a greater proportion of grasses (Poaceae); therefore, the results obtained suggest wetter conditions in the central Negev Desert from 6400 until 2200 cal yr BC than after that. This does not contradict climatic reconstructions [49,51]. The period after the first dry event (6200 cal yr 325 BC until 3600 cal yr BC) was unstable and dry but wetter than nowadays. Wetter conditions lasted for about fifteen hundred years (3600–2200 cal yr BC). Poaceae in the Atzmaut pollen diagram increases in approximately 2600–2200 cal yr BC, 1900–1800 cal yr BC, 300–900 cal yr AD and 1400–1700 cal yr AD, which coincided with humid periods (Figure 5). Unfortunately, dry events are not well reflected in the dynamics of Poaceae pollen due to insufficiently frequent sampling, especially in the Ramon I deposit.
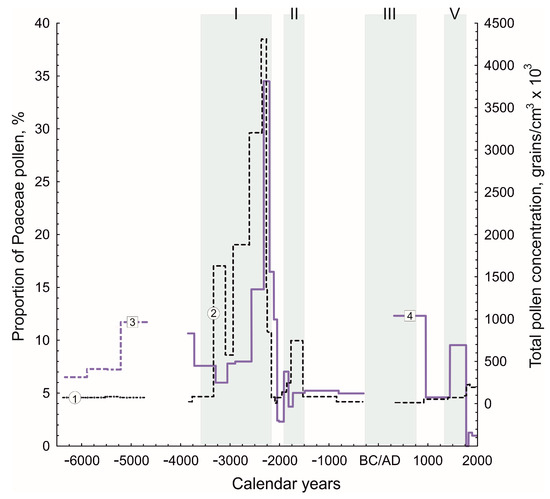
Figure 5.
Changes in the total pollen concentration of Ramon I (1) and Atzmaut (2) and Poaceae pollen proportions in the Ramon I (3) and Atzmaut (4) dung deposits relative to periods with moist conditions in the study region (I–IV).
The changes of the δ13C value over the past 8500 years largely coincide with the pollen and palaeoclimatic data. The results of stable isotope analysis of the Ramon I dung deposit and the lowest layers of Atzmaut deposit (Figure 6) allow us to suggest that climatic conditions for about three thousand years (6400–3600 cal yr BC) were unstable (during this period wetter and drier conditions alternated), but it was comparatively drier than the next period. The δ13C value varies between −22.52 and −21.13‰. These data do not contradict the palaeoclimatic data [52].
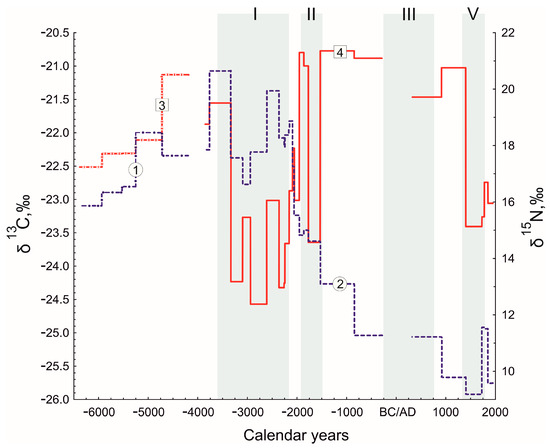
Figure 6.
The δ15N value of Ramon I (1) and Atzmaut (2) and the δ13C value of the Ramon I (3) and Atzmaut (4) zoogenic deposits. I–IV—periods with moist conditions in the study region.
The periods of δ13C value decreasing closely correspond with increase of the total pollen concentration within the uniform dung layers (two peaks: approximately 3300–2200 and 1800–1500 cal yr BC) and the proportion of the Poaceae pollen (approximately 2600–2200, 1900–1800 cal yr BC, 300–900 and 1400–1700 cal yr AD) in the profile of the Atzmaut zoogenic deposit and with wetter conditions (Figure 5 and Figure 6). The minimum values of δ13C were achieved in the layers of sediment, which were accumulated over the 3rd millennium BC before 2200 cal yr BC. At the same time, the proportion of the Poaceae pollen and total pollen concentration in the Atzmaut zoogenic deposit are the highest. The considered data correspond closely with the first period of the more humid conditions in the region (Figure 5) [52,54,73,82]. However, perhaps the pollen profile hints at the possibility of episodes of not only higher precipitation but summer precipitation for which we have no analogues in modern times, when rain falls exclusively in the winter months. The TPC and proportion of Poaceae pollen is 14 and 26 times higher than the concentration and grasses pollen in the similar dung layers (not burnt) of the deposits’ upper part. Grasses and other annuals with a short growing spring season have low pollen production due to reproductive strategies related to the dry summer climate. Plants, which bloom during summer, have much higher pollen production [81]. Therefore, the observed changes in the vegetation may be related not only to changes in the precipitation amount but also to the seasonal shift. Note that the results of study corals (Porites spp.) in the north of the Gulf of Eilat showed the presence of summer precipitation and wetter climatic conditions at least during to the Middle Holocene [58]. At the same time, precipitation in southern Israel came from the north, as the influence of the Afro-Asian monsoon did not reach the northern Red Sea and thus the territory of Israel [50]. Lake data from the Mediterranean region also indicate that precipitation over the Early and the Middle Holocene could have occurred during the summer months. The transition from such conditions to modern ones in the Mediterranean region occurred in a short period by about 2000 BC [83].
A sharp increase in the δ13C value, a decrease in the total pollen concentration and the proportion of the Poaceae pollen (Figure 5 and Figure 6) at about 2200–1900 cal yr BC indicate climate aridization, which is confirmed by palaeoclimatic data: the amount of precipitation in the region decreased in this period by 20–30% [52,54]. These climatic changes were not local but occurred in many regions of the Mediterranean, Asia and Africa [84,85]. A sharp decrease in precipitation entailed significant changes in the plant cover.
The decline of dung deposits 13C in the 1st half of the 2nd millennium BC (to −23.6‰) corresponds to the increase of the total pollen concentration and the proportion of the Poaceae pollen (Figure 5) and to a wet period [52]. The same climatic changes were also evident in the Levant region [86].
The 13C record and palynological spectra of samples formed over the past 3000 years are characterized by a high δ13C value and a low total pollen concentration, which is indicative of more stable climatic conditions. The climate of the region became arid, similar to the present-day climate [52,83,87]. Changes in humidity during the last 3000 years have been less significant than in previous periods [52,54].
Two periods of δ13C value decrease and Poaceae pollen increase (approximately 300–900 cal yr AD, 1400–1700 cal yr AD) correspond closely with wet conditions (Figure 5 and Figure 6). The last changes in the 2nd millennium AD may reflect the Little Ice Age [54,62,88].
Interpretation of the nitrogen isotope signature of the Ramon I and Atzmaut zoogenic deposits has been a more difficult problem. Changes of the δ 15N value in profiles would seem to contradict the results of most studies about the negative correlation between nitrogen isotope values and rainfall. Thus, the δ15N value reaches a maximum in the lower layers of the Atzmaut deposit that formed before the end of the 3rd millennium BC, when the climatic conditions in the region were more humid [52,54,82]. After that, the δ 15N value sharply decreases during an aridization event (Figure 6).
One of the reasons for the 15N enrichment in the low layers of the Atzmaut dung deposit may be related to the old age of the layers and a result of a greater degree of microbial organic matter decomposition [89]. However, the study of the older layers of the Ramon I deposit, which are preservation similar, have lower value of δ15N. Moreover, 15N enrichments may be greater in small than large fractions due to isotopic fractionation during microbial nitrogen transformations [90,91]. However, the result of the Wilcoxon Test indicates no differences between nitrogen isotope signatures of small and large fractions. Therefore, it can be concluded that 15N enrichment in the low layers of the Atzmaut deposit is not related to organic matter decomposition. We can assume that the maximum δ15N values may reflect wet climatic conditions. J. Aranibar [35] with co-authors showed that the 15N enrichment with aridity really could be enhanced during wet years. The authors suggest that the unusually high-water availability could have enhanced soil microbial activity, such as N mineralization of old, heavy organic N pools and gaseous emissions by denitrification. All these processes result in more 15N-enriched soil nitrogen, which could have been absorbed by C3 plants [35].
The negative correlation between nitrogen isotope values and rainfall is observed only in the last millennium. The δ15N value decreases in the 2nd millennium AD during more humid period [54,62,88] and increases in the 18th century AD with dry conditions [92].
5. Conclusions
Zoogenic deposits are unique palaeoclimatic and palaeoenvironmental archives. They are of particular value in arid zones, where there are almost no classical objects (lakes and peat bogs) for research. The palynological analysis of the Ramon I and the Atzmaut zoogenic deposits allowed us to determine the seasonality in the use of these rock shelters, reconstruct the dynamics of vegetation over the past 8500 years and reveal the effect of climatic changes and livestock grazing on the vegetation of the central Negev Desert [39,73,74]. Amaranthaceae and Artemisia were dominant almost throughout this period, yielding this status to Poaceae only between 2600–2200 cal yr BC. The proportion of Poaceae pollen is a very good indicator of wet conditions in arid zones. The total pollen concentration can be used as an additional indicator of moisture. The highest values of pollen concentration and Poaceae pollen most likely reflect vegetation changes associated with summer precipitation in the central Negev Desert.
The stable carbon (13C/12C) and nitrogen (15N/14N) isotope analysis of zoogenic (dung) deposits has been first conducted for the Negev Desert. The δ13C values of the studied deposits show an inverse relation between δ13C and precipitation. The decrease of δ13C values over the past 8500 years almost fully coincides with more humid conditions and an increase in the total pollen concentration and Poaceae pollen in profile, which happened approximately 3300–2200 cal yr BC, 1700–1500 cal yr BC, 300–900 cal yr AD and 1400–1700 cal yr AD. The relation between the δ15N value and the climatic conditions is indicated only in some cases. This relationship can be not only negative but also positive. The δ15N value well represents the most significant changes of precipitation in the region in the 3rd millennium BC.
The carbon and nitrogen isotopes’ signature of the zoogenic deposits from the Negev Desert coincides with the palaeoclimatic data of the region. Thus, the zoogenic deposits are an important source of unique information for palaeoecological reconstruction in arid regions where there are no other sources of palaeoinformation. Therefore, the sampling of zoogenic deposits should be done as fractionally as possible. The thickness of one sample should not exceed 2–3 cm to avoid the large averaging of data and, consequently, the loss of information.
Funding
This research was funded by the state assignment No. 122011200264-9 (Institute of Archaeology of the RAS, Moscow, Russia) «Exploring the Formation and Development of Ancient and Medieval Anthropogenic Ecosystems: An Interdisciplinary Approach».
Data Availability Statement
Data are available upon request to the author.
Acknowledgments
I am much indebted to B. Khassanov (IEE RAS, Moscow, Russia) for fruitful discussions and radiocarbon dates and to A. Tiunov (IEE RAS, Moscow, Russia) for the help of stable isotope analysis.
Conflicts of Interest
The author declare no conflicts of interest.
References
- Pons, A.; Quézel, P. Premieres remarques sur l’etude palynologique d’un guano fossile du Hoggar. Comptes-Rendus Seances L’Academie Sci. 1958, 246, 2290–2292. [Google Scholar]
- Potter, L.D.; Rowley, J. Pollen Rain and Vegetation, San Augustin Plains, New Mexico. Bot. Gaz. 1960, 122, 1–25. [Google Scholar] [CrossRef]
- Horowitz, A. Palynology of Arid Lands; Elsevier: Amsterdam, The Netherlands, 1992; p. 546. [Google Scholar]
- Scott, L.; Vogel, J.C. Evidence for environmental conditions during the last 20 000 years in Southern Africa from 13C in fossil hyrax dung. Glob. Planet. Change 2000, 26, 207–215. [Google Scholar] [CrossRef]
- Choi, W.-J.; Lee, S.-M.; Ro, H.-M.; Kim, K.-C.; Yoo, S.-H. Natural 15N abundances of maize and soil amended with urea and composted pig manure. Plant and Soil. 2002, 245, 223–232. [Google Scholar] [CrossRef]
- Sørensen, P.; Weisbjerg, M.R.; Lund, P. Dietary effects on the composition and plant utilization of nitrogen in dairy cattlemanure. J. Agric. Sci. 2003, 141, 79–91. [Google Scholar] [CrossRef]
- Codron, D.; Codron, J.; Lee-Thorp, J.A.; Sponheimer, M.; de Ruiter, D. Animal diets in the Waterberg based on stable isotopic composition of faeces. S. Afr. J. Wildl. Res. 2005, 35, 43–52. [Google Scholar] [CrossRef]
- Kelly, J.F. Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can. J. Zool. 2000, 78, 1–27. [Google Scholar] [CrossRef]
- Bocherens, H.; Drucker, D. Trophic level isotopic enrichment of carbon and nitrogen in bone collagen: Case studies from recent and ancient terrestrial ecosystems. Int. J. Osteoarchaeol. 2003, 13, 46–53. [Google Scholar] [CrossRef]
- O`Leary, M.H. Carbon isotopes in photosynthesis. BioScience 1988, 38, 328–336. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discriminationand photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Cerling, T.E.; Harris, J.M.; MacFadden, B.J.; Leakey, M.G.; Quade, J.; Eisenmann, V.; Ehleringer, J.R. Global vegetation change through the Miocene/Pliocene boundary. Nature 1997, 389, 153–158. [Google Scholar] [CrossRef]
- Kohn, M.J. Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo)ecology and (paleo)climate. Proc. Natl. Acad. Sci. USA 2010, 107, 19691–19695. [Google Scholar] [CrossRef] [PubMed]
- Cernusak, L.A.; Ubierna, N.; Winter, K.; Holtum, J.A.M.; Marshall, J.D.; Farquhar, G.D. Environmental and physiological determinants of carbon isotope discrimination in terrestrial plants. New Phytol. 2013, 200, 950–965. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.D.; Brooks, J.R.; Lajtha, K. Sources of variation in the stable isotopic composition of plants. In Stable Isotopes in Ecology and Environmental Science, 2nd ed.; Michener, R., Lajtha, K., Eds.; Blackwell Publishing Ltd.: Malden, MA, USA; Oxford, UK; Carlton, Australia, 2007; pp. 22–60. [Google Scholar]
- Chase, B.M.; Quick, L.J.; Meadows, M.E.; Scott, L.; Thomas, D.S.G.; Reimer, P.J. Late glacial interhemispheric climate dynamics revealed in South African hyrax middens. Geology 2011, 39, 19–22. [Google Scholar] [CrossRef]
- Chase, B.M.; Boom, A.; Carr, A.S.; Carré, M.; Chevalier, M.; Meadows, M.E.; Pedro, J.B.; Stager, J.C.; Reimer, P.J. Evolving southwest African response to abrupt deglacial North Atlantic climate change events. Quat. Sci. Rev. 2015, 121, 132–136. [Google Scholar] [CrossRef]
- Shahack-Gross, R.; Simons, A.; Ambrose, S.H. Identification of pastoral sites using stable nitrogen and carbon isotopes from bulk sediment samples: A case study in modern and archaeological pastoral settlements in Kenya. J. Archaeol. Sci. 2008, 35, 983–990. [Google Scholar] [CrossRef]
- Carr, A.S.; Chase, B.M.; Boom, A.; Medina-Sanchez, J. Stable isotope analyses of rock hyrax faecal pellets, hyraceum and associated vegetation in southern Africa: Implications for dietary ecology and palaeoenvironmental reconstructions. J. Arid. Environ. 2016, 134, 33–48. [Google Scholar] [CrossRef]
- Chase, B.M.; Meadows, M.E.; Carr, A.S.; Reimer, P.J. Evidence for progressive Holocene aridification in southern Africa recorded in Namibian hyrax middens: Implications for African Monsoon dynamics and the “African Humid Period”. Quat. Res. 2010, 74, 36–45. [Google Scholar] [CrossRef]
- Chase, B.M.; Scott, L.; Meadows, M.E.; Gil-Romera, G.; Boom, A.; Carr, A.S.; Reimer, P.J.; Truc, L.; Valsecchi, V.; Quick, L.J. Rock hyrax middens: A palaeoenvironmental archive for southern African drylands. Quat. Sci. Rev. 2012, 56, 107–125. [Google Scholar] [CrossRef]
- Chase, B.M.; Niedermeyer, E.M.; Boom, A.; Carr, A.S.; Chevalier, M.; He, F.; Meadows, M.E.; Ogle, N.; Reimer, P.J. Orbital controls on Namib Desert hydroclimate over the past 50,000 years. Geology 2019, 47, 867–871. [Google Scholar] [CrossRef]
- Chase, B.M.; Boom, A.; Carr, A.S.; Quick, L.J.; Reimer, P.J. High-resolution record of Holocene climate change dynamics from southern Africa’s temperate-tropical boundary, Baviaanskloof, South Africa. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 539, 109518. [Google Scholar] [CrossRef]
- Chase, B.M.; Boom, A.; Carr, A.S.; Reimer, P.J. Climate variability along the margin of the southern African monsoon region at the end of the African Humid Period. Quat. Sci. Rev. 2022, 291, 107663. [Google Scholar] [CrossRef]
- Chase, B.M.; Boom, A.; Carr, A.S.; Meadows, M.E.; Lima, S. A ca. 39,000-year record of vegetation and climate change from the margin of the Namib Sand Sea. Quat. Res. 2023, 116, 1–11. [Google Scholar] [CrossRef]
- Horisk, K.; Ivory, S.; McCorriston, J.; McHale, M.; Al Mehri, A.; Anderson, A.; Scott, R.A.; Al Kathiri, A. Vegetation dynamics in Dhofar, Oman, from the Late Holocene to present inferred from rock hyrax middens. Quat. Res. 2023, 116, 12–29. [Google Scholar] [CrossRef]
- Ivory, S.J.; Cole, K.L.; Anderson, R.S.; Anderson, A.; McCorriston, J.; Williams, J. Human landscape modification and expansion of tropical woodland in southern Arabia during the mid-Holocene from rock hyrax middens. J. Biogeogr. 2021, 48, 2588–2603. [Google Scholar] [CrossRef]
- Meadows, M.E.; Seliane, M.; Chase, B.M. Holocene palaeoenvironments of the Cederberg and Swartruggens mountains, Western Cape, South Africa: Pollen and stable isotope evidence from hyrax dung middens. J. Arid. Environ. 2010, 74, 786–793. [Google Scholar] [CrossRef]
- Handley, L.L.; Austin, A.T.; Robinson, D.; Scrimgeour, C.M.; Raven, J.A.; Heaton, T.H.E.; Schmidt, S.; Stewart, G.R. The 15N natural abundance (δ15N) of ecosystem samples reflects measures of water availability. Australian J. Plant Physiol. 1999, 26, 185–199. [Google Scholar] [CrossRef]
- Robinson, D. δ15N as an integrator of the nitrogen cycle. Trends Ecol. Evol. 2001, 16, 153–162. [Google Scholar] [CrossRef]
- Amundson, R.; Austin, A.T.; Schuur, E.A.G.; Yoo, K.; Matzek, V.; Kendall, C.; Uebersax, A.; Brenner, D.; Baisden, W.T. Global patterns of the isotopic composition of soil and plant nitrogen. Glob. Biogeochem. Cycles 2003, 17, 1031. [Google Scholar] [CrossRef]
- Craine, J.M.; Elmore, A.J.; Aidar, M.P.M.; Bustamante, M.; Dawson, T.E.; Hobbie, E.A.; Kahmen, A.; Mack, M.C.; McLauchlan, K.K.; Michelsen, A.; et al. Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol. 2009, 183, 980–992. [Google Scholar] [CrossRef]
- Heaton, T.H.E. The 15N/14N ratios of plants in South Africa and Namibia: Relationship to climate and coastal/saline environments. Oecologia 1987, 74, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Swap, R.J.; Aranibar, J.N.; Dowty, P.R.; Gilhooly, W.P.; Macko, S.A. Natural abundance of 13C and 15N in C3 and C4 vegetation of southern Africa: Patterns and implications. Glob. Change Biol. 2004, 10, 350–358. [Google Scholar] [CrossRef]
- Aranibar, J.N.; Otter, L.; Macko, S.A.; Feral, C.J.W.; Epstein, H.E.; Dowty, P.R.; Eckardt, F.; Shugart, H.H.; Swap, R.J. Nitrogen cycling in the soil–plant system along a precipitation gradient in the Kalahari sands. Glob. Change Biol. 2004, 10, 359–373. [Google Scholar] [CrossRef]
- Murphy, B.P.; Bowman, D.M.J.S. The carbon and nitrogen isotope composition of Australian grasses in relation to climate. Funct. Ecol. 2009, 23, 1040–1049. [Google Scholar] [CrossRef]
- Hartman, G.; Danin, A. Isotopic values of plants in relation to water availability in the Eastern Mediterranean region. Oecologia 2010, 162, 837–852. [Google Scholar] [CrossRef]
- Hartman, G. Are elevated δ15N values in herbivores in hot and arid environments caused by diet or animal physiology? Funct. Ecol. 2011, 25, 122–131. [Google Scholar] [CrossRef]
- Babenko, A.N.; Khassanov, B.F. The absolute chronology of the zoogenic deposits from the Negev Desert (Israel). Geochronometria 2007, 28, 47–53. [Google Scholar] [CrossRef]
- Kahana, R.; Baruch, Z.; Enzel, Y.; Dayan, U. Synoptic climatology of major floods in the Negev desert, Israel. Int. J. Climatol. 2002, 22, 867–882. [Google Scholar] [CrossRef]
- Danin, A. Desert Vegetation of Israel and Sinai; Cana Publishing House: Jerusalem, Israel, 1983; p. 133. [Google Scholar]
- Frumkin, A. The Holocene History of Dead Sea Levels. In The Dead Sea: The Lake and Its Setting; Ben-Avraham, Z., Gat, Y., Niemi, T.M., Eds.; Oxford University Press: Oxford, MS, USA, 1997; pp. 237–248. [Google Scholar]
- Rossignol-Strick, M. The Holocene climatic optimum and pollen records of sapropel 1 in the eastern Mediterranean, 9000–6000 BP. Quat. Sci. Rev. 1999, 18, 515–530. [Google Scholar] [CrossRef]
- Frumkin, A.; Magaritz, M.; Carmi, I.; Zak, I. The Holocene climatic record of the salt caves of Mount Sedom, Israel. Holocene 1991, 1, 191–200. [Google Scholar] [CrossRef]
- Frumkin, A.; Carmi, I.; Zak, I.; Magaritz, M. Middle Holocene environmental change determined from the Salt Caves of Mount Sodom, Israel. In Late Quaternary Chronology and Paleoclimates of the Eastern Mediterranean; Bar-Yosef, O., Kra, R.S., Eds.; RADIOCARBON: Tucson, AZ, USA; American School of Prehistoric Research: Cambridge, MA, USA, 1994; pp. 315–332. [Google Scholar]
- Frumkin, A.; Kadan, G.; Enzel, Y.; Eyal, Y. Radiocarbon Chronology of the Holocene Dead Sea: Attempting a regional Correlation. Radiocarbon 2001, 43, 1179–1189. [Google Scholar] [CrossRef]
- Frumkin, A.; Elitzur, Y. Historic Dead Sea Level Fluctuations Calibrated with Geological and Archaeological Evidence. Quat. Res. 2002, 57, 334–342. [Google Scholar] [CrossRef]
- Bookman (Ken-Tor), R.; Enzel, Y.; Agnon, A.; Stein, M. Late Holocene lake levels of the Dead Sea. GSA Bull. 2004, 116, 555–571. [Google Scholar] [CrossRef]
- Hazan, N.; Stein, M.; Agnon, A.; Marco, S.; Nadel, D.; Negendank, J.F.W.; Schwab, M.J.; Neev, D. The late Quaternary limnological history of Lake Kinneret (Sea of Galilee), Israel. Quat. Res. 2005, 63, 60–77. [Google Scholar] [CrossRef]
- Arz, H.W.; Lamy, F.; Patzold, J.; Muller, P.J.; Prins, M. Mediterranean Moisture Source for an Early-Holocene Humid Period in the Northern Red Sea. Science 2003, 300, 118–121. [Google Scholar] [CrossRef]
- Arz, H.W.; Lamy, F.; Patzold, J. A pronounced dry event recorded around 4.2 ka in brine sediments from the northern Red Sea. Quat. Res. 2006, 66, 432–441. [Google Scholar] [CrossRef]
- Migowski, C.; Stein, M.; Prasad, S.; Negendank, J.F.W.; Agnon, A. Holocene climate variability and cultural evolution in the Near East from the Dead Sea sedimentary record. Quat. Res. 2006, 66, 421–431. [Google Scholar] [CrossRef]
- Gvirtzman, G.; Wieder, M. Climate of the last 53,000 years in the eastern Mediterranian, based on the soil-sequence stratigraphy in the coastal plain of Israel. Quat. Sci. Rev. 2001, 20, 1827–1849. [Google Scholar] [CrossRef]
- Bar-Matthews, M.; Ayalon, A.; Kaufman, A. Middle to Late Holocene (6500 Yr. period) paleoclimate in the Eastern Mediterranian region from stable isotopic composition of speleothems from Soreq Cave, Israel. In Water, Environment and Society in Times of Climatic Change; Issar, A.S., Brown, N., Eds.; Water Science and Technology Library; Springer: Dordrecht, The Netherlands, 1998; Volume 31, pp. 203–214. [Google Scholar] [CrossRef]
- Orland, I.J.; Bar-Matthews, M.; Kita, N.T.; Ayalon, A.; Matthews, A.; Valley, J.W. Climate deterioration in the Eastern Mediterranean as revealed by ion microprobe analysis of a speleothem that grew from 2.2 to 0.9 ka in Soreq Cave, Israel. Quat. Res. 2009, 71, 27–35. [Google Scholar] [CrossRef]
- Bar-Matthews, M.; Ayalon, A. Mid-Holocene climate variations revealed by high-resolution speleothem records from Soreq Cave, Israel and their correlation with cultural changes. Holocene 2011, 21, 163–171. [Google Scholar] [CrossRef]
- Goodfriend, G.A. Terrestrial stable isotope records of Late Quaternary paleoclimates in the eastern Mediterranean region. Quat. Sci. Rev. 1999, 18, 501–513. [Google Scholar] [CrossRef]
- Moustafa, Y.A.; Patzold, J.; Loya, Y.; Wefer, G. Mid-Holocene stable isotope record of corals from the northern Red Sea. Int. J. Earth Sci. 2000, 88, 742–751. [Google Scholar] [CrossRef]
- Lisker, S.; Porat, R.; Davidovich, U.; Eshel, H.; Lauritzen, S.E.; Frumkin, A. Late Quaternary environmental and human events at En Gedi, reflected by the geology and archaeology of the Moringa Cave (Dead Sea area, Israel). Quat. Res. 2007, 68, 203–212. [Google Scholar] [CrossRef]
- Yakir, D.; Issar, A.; Gat, J.; Adar, E.; Trimborn, P.; Lipp, J. 13C and 18O of wood from the Roman siege rampart in Masada, Israel (AD 70-73): Evidence for a less arid climate for the region. Geochim. Cosmochim. Acta 1994, 58, 3535–3539. [Google Scholar] [CrossRef]
- Frumkin, A. Stable isotopes of a subfossil Tamarix tree from the Dead Sea region, Israel, and their implications for the Intermediate Bronze Age environmental crisis. Quat. Res. 2009, 71, 319–328. [Google Scholar] [CrossRef]
- Waisel, Y.; Liphschitz, N. Dendrochronological investigations in Israel: II Juniperus phoenica of West and Central Sinai. La-Yarran 1968, 18, 63–67. [Google Scholar]
- Rosen, S.A.; Savinetsky, A.B.; Plakht, Y.; Kisseleva, N.K.; Khassanov, B.F.; Pereladov, A.M.; Haiman, M. Dung in the Desert: Preliminary Results of the Negev Holocene Ecology Project. Curr. Anthropol. 2005, 46, 317–327. [Google Scholar] [CrossRef]
- Simms, S.R.; Russell, K.W. Tur Imdai Rockshelter: Archaeology of Recent Pastoralists in Jordan. J. Field Archaeol. 1997, 24, 459–472. [Google Scholar] [CrossRef]
- Haslett, J.; Parnell, A. A simple monotone process with application to radiocarbon-dated depth chronologies. J. R. Stat. Soc. Ser. C (Appl. Stat.) 2008, 57, 399–418. [Google Scholar] [CrossRef]
- Reimer, P.; Austin, W.; Bard, E.; Bayliss, A.; Blackwell, P.; Ramsey Bronk, C.; Butzin, M.; Cheng, H.; Edwards, R.L.; Friedrich, M.; et al. The IntCal20 Northern Hemisphere Radiocarbon Age Calibration Curve (0–55 cal kBP). Radiocarbon 2020, 62, 725–757. [Google Scholar] [CrossRef]
- Parnell, A.C.; Buck, C.E.; Doan, T.K. A review of statistical chronology models for high-resolution, proxy-based Holocene palaeoenvironmental reconstruction. Quat. Sci. Rev. 2011, 30, 2948–2960. [Google Scholar] [CrossRef]
- Faegri, K.; Iversen, J. Textbook of Pollen Analysis, 4th ed.; John Wiley & Sons: Chichester, UK, 1989; p. 328. [Google Scholar]
- Stockmarr, J. Determination of spore concentration with an electronic particle counter. Dan. Geol. Undersøgelse Årbog 1973, 1972, 87–89. [Google Scholar]
- Grimm, E.C. Computer Software, TILIA 2.6.1 Version; State Museum: Springfield, IL, USA, 2019. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson: London, UK, 2010; p. 960. [Google Scholar]
- Brooks, J.; Shaw, G. Geochemistry of sporopollenin. Chem. Geol. 1972, 10, 69–87. [Google Scholar] [CrossRef]
- Babenko, A.N.; Kiseleva, N.K.; Plakht, I.; Rosen, S.; Savinetskii, A.B.; Khasanov, B.F. Reconstruction of the Holocene Vegetation in the Central Negev Desert, Israel, on the Basis of Palynological Data on the Atzmaut Zoogenic Deposit. Russ. J. Ecol. 2007, 38, 388–397. [Google Scholar] [CrossRef]
- Babenko, A.N.; Kuzmicheva, E.A.; Khasanov, B.F.; Rosen, S.; Kiseleva, N.K.; Savinetsky, A.B. Dung deposits as archives of environmental change. In Soils and Sediments as Archives of Environmental Change; Lucke, B., Bäumler, R., Schmidt, M., Eds.; Geoarchaeology and Landscape Change in the Subtropics and Tropics; Chapter 15; Fränkische Geographische Gesellschaft: Erlanger, German, 2015; Volume 42, pp. 201–217. [Google Scholar]
- Moe, D. Palynology of sheep’s faeces: Relationship beetween pollen content, diet and local pollen rain. Grana 1983, 22, 105–113. [Google Scholar] [CrossRef]
- Akeret, O.; Haas, N.J.; Leuzinger, U.; Jacomet, S. Plant macrofossils and pollen in goat/sheep faeces from the Neolithic lake-shore settlement Arbon Bleiche 3, Switzerland. Holocene 1999, 9, 175–182. [Google Scholar] [CrossRef]
- Hunt, C.O.; Rushworth, G.; Gilbertson, D.D.; Mattingly, D.J. Romano-Libyan Dryland Animal Husbandry and Landscape: Pollen and Palynofacies Analyses of Coprolites from a Farm in the Wadi el-Amud, Tripolitania. J. Archaeol. Sci. 2001, 28, 351–363. [Google Scholar] [CrossRef]
- Feinbrun-Dothan, N. Flora Palaestina; Part III; Israel Academy of Sciences and Humanities: Jerusalem, Israel, 1978; p. 481. [Google Scholar]
- Feinbrun-Dothan, N. Flora Palaestina; Part IV; Israel Academy of Sciences and Humanities: Jerusalem, Israel, 1986; p. 463. [Google Scholar]
- Zohary, M. Flora Palaestina; Part 1; Israel Academy of Sciences and Humanities: Jerusalem, Israel, 1981; p. 367. [Google Scholar]
- El-Moslimany, A.P. Ecological significance of common nonarboreal pollen: Examples from drylands of the Middle East. Rev. Palaeobot. Palynol. 1990, 64, 343–350. [Google Scholar] [CrossRef]
- Finne, M.; Holmgren, K.; Sundqvis, H.S.; Weiberg, E.; Lindblom, M. Climate in the eastern Mediterranean, and adjacent regions, during the past 6000 years—A review. J. Archaeol. Sci. 2011, 38, 3153–3173. [Google Scholar] [CrossRef]
- Harrison, S.P.; Yu, G.; Tarasov, P.E. Late Quaternary Lake-Level Record from Northern Eurasia. Quat. Res. 1996, 45, 138–159. [Google Scholar] [CrossRef]
- Staubwasser, M.; Weiss, H. Holocene climate and cultural evolution in late prehistoric-early historic West Asia. Quat. Res. 2006, 66, 372–387. [Google Scholar] [CrossRef]
- Weninger, B.; Alram-Stern, E.; Bauer, E.; Clare, L.; Danzeglocke, U.; Joris, O.; Kubatzki, C.; Rollefson, G.; Todorova, H.; Van Andel, T. Climate forcing due to the 8200 cal yr BP event observed at Early Neolithic sites in the eastern Mediterranean. Quat. Res. 2006, 66, 401–420. [Google Scholar] [CrossRef]
- Verheyden, S.; Nader, F.H.; Cheng, H.J.; Edwards, L.R.; Swennen, R. Paleoclimate reconstruction in the Levant region from the geochemistry of a Holocene stalagmite from the Jeita cave, Lebanon. Quat. Res. 2008, 70, 368–381. [Google Scholar] [CrossRef]
- Gat, J.; Magaritz, M. Climatic variations in the eastern Mediterranean Sea area. Naturwissenschaften 1980, 67, 80–87. [Google Scholar] [CrossRef]
- Roberts, N.; Moreno, A.; Valero-Garces, B.L.; Corella, J.P.; Jones, M.; Allcock, S.; Woodbridge, J.; Morellon, M.; Luterbache, J.; Xoplaki, E.; et al. Palaeolimnological evidence for an east–west climate see-saw in the Mediterranean since AD 900. Glob. Planet. Chang. 2012, 84–85, 23–34. [Google Scholar] [CrossRef]
- Connin, S.L.; Feng, X.; Virginia, R.A. Isotopic discrimination during long-term decomposition in an arid land ecosystem. Soil Bioogy Biochem. 2001, 33, 41–51. [Google Scholar] [CrossRef]
- Tiessen, H.; Stewart, J.W.B.; Hunt, H.W. Concepts of soil organic matter transformations in relation to organo-mineral particle size fractions. Plant Soil 1984, 76, 287–295. [Google Scholar] [CrossRef]
- Huygens, D.; Denef, K.; Vandeweyer, R.; Godoy, R.; Van Cleemput, O.; Boeckx, P. Do nitrogen isotope patterns reflect microbial colonization of soil organic matter fractions? Biol. Fertil. Soils 2008, 44, 955–964. [Google Scholar] [CrossRef]
- Tsoar, H. Desertification in Northern Sinai in the eighteenth century. Clim. Chang. 1995, 29, 429–438. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).