Abstract
The dwarf Sardinian mammoth, Mammuthus lamarmorai, is a well-known species frequently cited in the literature; however, the fossil record of the Pleistocene Sardinian mammoths mainly consists of isolated remains (an incomplete skeleton from Guardia Pisano Hill, isolated teeth and a largely incomplete tibia from different localities, and some footprints from Funtana Morimenta), which have been found in sites presumably ranging in age from the late Middle to the Late Pleistocene. All of the remains have been ascribed to a single species of an endemic mammoth, Mammuthus lamarmorai, which is moderately reduced in size. The paucity of remains increases uncertainties about the chronological range of Sardinian mammoth remains, some of which are currently missing, while others lack sound information about their exact provenance or were removed and collected without contextual information. As a result, the different sizes of molariform teeth from different localities, the lack of chewing tooth remains at Guardia Pisano Hill, and the doubtful chronology of some remains hamper any attempt to infer whether one or more species that originated from an anagenetic or radiative evolutionary process or through multiple arrivals from the mainland inhabited the island. Therefore, the continental ancestor or ancestors of Sardinian mammoth populations and the time and number of dispersals of the ancestral taxon or taxa have long been debated, and the persistence through time of mammoth endemic populations still remains an unsolved matter. This research summarizes and critically reviews our knowledge about the Sardinian endemic mammoth, provides new evidence about the Sardinian mammoth’s ancestor and the possible time of its dispersal from the mainland to the island, gives new information about the Sardinian mammoth’s histology and physical characteristics, and highlights some focal, unsolved questions (e.g., morphological and dimensional differences in dentition, number of Sardinian mammoth species, population dynamics, decline, and disappearance). Further research and increasing data, which will enable taphonomic, spectrometric, and dating studies, will provide better results to solve the remaining questions.
1. Introduction
Insular endemic proboscideans (stegodonts, mammoths, and the most noticeable examples of straight-tusked elephant miniaturization) are a noticeable example of one of the most intriguing phenomena in nature. In particular, the reduction in size with respect to their mainland ancestors is distinctive of the evolution of insular large mammals. Pleistocene proboscideans colonized both continental and a few oceanic-like islands across wide temporal and geographic ranges, thus becoming one of the most common taxa of the disharmonic insular fauna. Their overseas dispersal was likely facilitated by their remarkable swimming abilities, their peculiar bauplan, and their physiology (e.g., a trunk serving as a snorkel device, an excellent sense of smell that allowed them to detect distant vegetation, a digestive system that produced gases that aided buoyancy, and elephants’ propensity to swim in a herd) (e.g., [1,2,3,4,5]).
Diminutive descendants of continental stegodonts and straight-tusked elephants have been reported from the Indonesian Archipelago to the Mediterranean and the California Channel Islands. On islands, they evolved, giving rise to variously sized endemic species [6,7,8,9]. Palaeoloxodon dwarf representatives were especially abundant on the eastern (Crete, Cyclades, Dodecanese, and Cyprus) and central Mediterranean islands (Malta, Sicily, and Favignana), while dwarf Mammuthus representatives were definitely less abundant. Pygmy populations originated from Mammuthus columbi and inhabited the Channel Islands during the Late Pleistocene and Early Holocene; a dwarf mammoth (Mammuthus creticus) that originated from Mammuthus meridionalis is recorded from a single Early Pleistocene locality in Crete, and a few specimens apparently belonging to dwarf mammoths with rather different sizes, currently ascribed to Mammuthus lamarmorai, are known in a few Sardinian late Middle to Late Pleistocene sites (e.g., [7,9,10,11,12,13,14,15]). The Siberian Wrangel Island (Arctic Ocean) was inhabited until about 4000–3500 years ago by some relict woolly mammoth populations believed to belong to a moderately dwarfed subspecies, Mammuthus primigenius vrangeliensis [16,17]. However, their average long bone size, although rather smaller than that of typical M. primigenius, falls within the dimensional variation range of the last woolly mammoth continental populations, and the Wrangel mammoths cannot be regarded as dwarfs [18].
Sardinia is the single example of a Mediterranean island never colonized by straight-tusked elephants, and M. lamarmorai is the only insular mammoth living at the time when Palaeoloxodon antiquus was present on the neighboring mainland. The Sardinian mammoth fossil record consists of a few remains presumably retrieved from late Middle to Late Pleistocene deposits in western Sardinia, while no elephant remains are known to date in other parts of the island (Figure 1). Some remains are old specimens removed and collected without contextual information and lacking information about their exact provenance and stratigraphic position, while others are currently missing, hampering their exhaustive study. Their attribution to the Mammuthus genus is substantially based on (i) the taxonomically informative characters shown by the molariform teeth found at Campu Giavesu (Giave, SS) (CG hereinafter) and San Giovanni di Sinis (Cabras, OR) (SGS hereinafter); (ii) the Schreger line patterns shown by the tusk’s large fragment from Guardia Pisano Hill (Gonnesa, SU) (GPH hereinafter); and (iii) some less-compelling features of the limb bones of the incomplete postcranial skeleton. The latter was found by the end of the 19th century on the GPH slope [19], a few meters southward from Funtana Morimenta spring (Gonnesa, SU) (FM). Two years later, Major [20] described it as a new species, Elephas lamarmorae (recte M. lamarmorai) (see below). Assuming that only one species inhabited the island, it has been presumed that even the specimens without firm diagnostic features may belong to the same species (e.g., the molariform tooth from Tramariglio and the incomplete tibia from the southern coast of Alghero) [12,21,22].
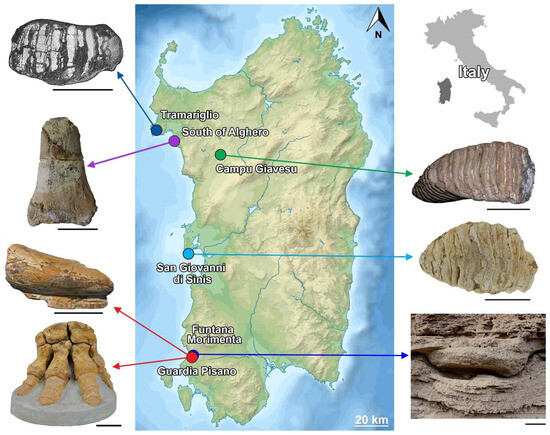
Figure 1.
Map of Sardinia showing the localities that yielded mammoth remains and footprints. Scale bar = 5 cm.
The paucity of remains, some tooth dimensional differences, and uncertainties about the chronology of most remains make the Sardinian mammoth a problematic and intriguing taxon. The nature of remain samples poses a number of still unanswered questions, such as the range of morphological and dimensional variation in Sardinian mammoth populations, the number and identity of the putative ancestor(s), the time and number of island colonization(s), the possible causes of the populations’ decline, and the time of the endemic Sardinian mammoth extinction.
This research aims to summarize and critically review our knowledge about the Sardinian endemic mammoth, provide new information about the Sardinian mammoth’s histology and physical characteristics, and discuss some focal, unsolved questions (e.g., the actual number of Sardinian mammoth species, the time, the identity and the number of dispersals of the ancestral taxon or taxa, the morphological and dimensional differences shown by tooth remains, the dynamics of Sardinian mammoth populations and their persistence through time, the possible causes of the populations’ decline and disappearance, as well as preliminary data on the histological characteristics of the Sardinian mammoth’s long bones), while waiting for the availability of more sound data.
2. Methods
According to the terminology herein adopted, upper-case letters and lower-case letters, respectively, indicate upper and lower premolars and molars (i.e., Dp/dp and M/m, respectively).
Regarding elephant molars, we use the term “platelet” to indicate the small pseudoplate that extends to the crown base on the posterior side of the last molars (M3/m3) rather than fusing with the last plate, as the talon (x) does [23]. We measured premolar and molar specimens with a digital caliper according to Aguirre, Maglio, and Lister [24,25,26]. However, we calculated the lamellar frequency by averaging the values taken on the occlusal surface and the labial and lingual sides. We took the measurements of the skeleton from GPH on the casts stored at the Museo dei Paleoambienti Sulcitani—“E.A. Martel” (Carbonia, SU) (MPAS). Among the diverse predictive formulas recommended by researchers for estimating the proboscidean body mass (BM), we chose to use the regression equations proposed for dwarf elephants (Table 9.1 in [27] (p. 158)) and some proboscidean species [28] and for tetrapods [29], which are all based on specific linear dimensions of the forelimb and hindlimb long bones of the stylopodium and zeugopodium.
We applied multivariate analysis (similarity and principal component analysis, PCA, ordination method) to compare the M2 and M3 data with those of some specimens of Mammuthus meridionalis and Mammuthus trogontherii and mammoth specimens showing a mix of both species’ characteristics to scrutinize their major affinity (if any) with one or the other potential ancestor of the Sardinian dwarf mammoth. We performed the hierarchical cluster analysis by using the unweighted pair group average method (UPGMA) and Euclidean index. Analyses were executed with the PAST (PAleontological STatistics) 4.01 software [30]. We processed the samples for histological analysis according to the following method. We removed a bone sample of approximately 2 mm thick from the incomplete tibia of M. cf. M. lamarmorai (from the southern coast of Alghero) by means of a steel cutting disk mounted in a mini portable electrical screwdriver. The small bone fragment was embedded with a bicomponent epoxy resin (Araldite 2020, Huntsman, Basel, Switzerland) using a degassing vacuum chamber (Ablaze1, Ablaze, Hong Kong) to avoid bubbles. It was cut and grinded by means of a thin section cutting and grinding machine (Geoform102, Metkon Instruments, Bursa, Turkey) and an abrasive cutting machine (Metacut302, Metkon Instruments) by using a diamond disk (Dimos Ø 250, Metkon Instruments). The obtained 35 μm thick sections were mounted onto glass slides with Eukitt (Merck, Darmstadt, Germany) and coverslipped. We observed and photographed the sections using a Zeiss Axiophot microscope at 2.5×, 10×, and 20× magnifications. The bone specimens and the slides are currently deposited in the paleontological slide collection (PSC) of the Section of Anatomy (Department of Veterinary Medicine, University of Sassari, Sassari, Italy), where they are available for repeatability. We observed, photographed, and digitalized all sections, measured the maximum and minimum diameters, the perimeter, and the eccentricity of both osteons and Haversian canals, and calculated the ratio between each osteon’s diameter and that of its Haversian canal.
3. Historical Background
The first report of a possible fossil proboscidean from Sardinia was by General Alberto La Marmora [31], who briefly mentioned some elephant remains collected near Cagliari. At that time, those remains were part of the original collection of the Viceroy of Sardinia, Carlo Felice di Savoia, of the archaeological and geological museum of Cagliari. Unfortunately, they are currently lost; no drawings or descriptions are available, and no further details confirming their identification are known.
The first remains surely referable to a Pleistocene elephant are those found at the end of the 19th century at GPH, near the village of Gonnesa (SW Sardinia), during the construction of a railway. These remains consist of an incomplete skeleton belonging to an adult individual, and they have been collected in late Middle Pleistocene aeolian or alluvial deposits [19,21]. A German engineer, who coordinated the construction of the railway, sent part of the bone elements to Pisa. Luigi Acconci referred them to a small representative of the genus Elephas larger than the dwarf elephant of Malta, “Elephas melitensis” [19]. Most of these remains are currently housed at the Natural History Museum of Pisa University (Calci, Pisa) (NHMP), while others, such as two molars, were sent to Germany and probably became part of a private collection [32].
Acconci [19] provided the list of the skeleton’s bones he determined (i.e., a right mandible fragment, incomplete right and left scapulae, a right humerus and a distal portion of a left one, an incomplete right radius and ulna, a right pelvic bone portion, a left femur distal epiphysis and part of the diaphysis, a left tibia, a right distal fibula, carpal and metacarpal bones, and a complete left tarsus). Some of these bones are not present in the NHMP, suggesting a possible misidentification, while others, including acropodium bones (metacarpals, tarsals, and metatarsals), are currently housed at the Naturhistorisches Museum Basel (Major’s Collection) [21,22] (Figure 2).
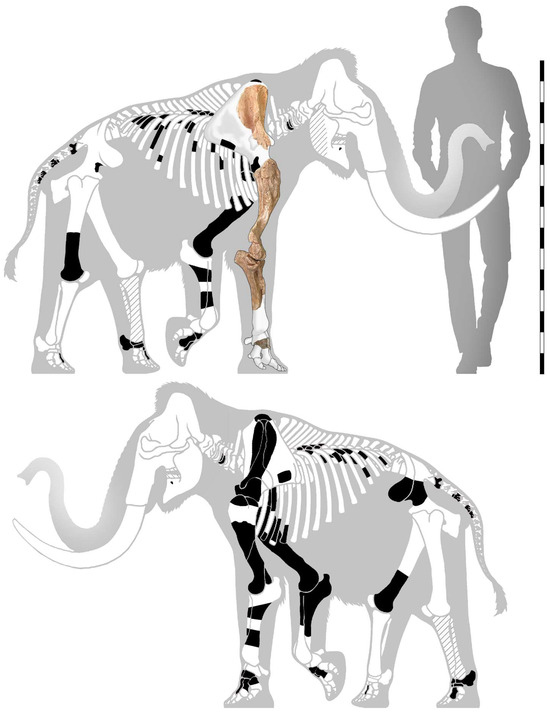
Figure 2.
Mammuthus lamarmorai from Guardia Pisano Hill (Gonnesa); schematic drawing of the skeleton showing the main identified bones (black) housed at Museo di Storia Naturale e del Territorio (Pisa), Naturhistorisches Museum Basel, and Museo Sardo di Geologia e Paleontologia “D. Lovisato” (Cagliari); in oblique lines are the bones cited in the literature but not found in the examined collections (lost and/or misidentified elements); scale bar = 150 cm (redrawing from [22]).
In 1883, Forsyth Major [20] created a new species, “Elephas lamarmorae”, for the elephant remains from GPH, but without providing a diagnosis or illustrating them. Between the late 19th and early 20th centuries, the Scottish scientist conducted a series of excavations at the discovery site and collected about 200 remains that almost surely belong to the same skeleton (e.g., numerous rib fragments, vertebrae, and some unidentifiable bone fragments) [20,32]. Zoboli and colleagues [22] provided the complete list of the material in the Major’s Collection, while Palombo and colleagues [21] briefly analyzed for the first time the material housed in NHMP. Three incomplete thoracic vertebrae possibly belonging to the same individual, as their preservation state suggests, are currently housed at the Museo Sardo di Geologia e Paleontologia “D. Lovisato” (Cagliari) (MDLCA), but no information is available about the collector or the date of their arrival at the Museum. In the 1980s, a fragment of tusk was collected at GPH, but there is not enough evidence to state that it belongs to the same individual [21,22].
The taxonomic attribution of the skeleton was subject to different interpretations, as inferable from the available literature. In fact, some authors questioned the validity of the taxon established by Forsyth Major and deemed, on the basis of its small size, that the Sardinian proboscidean was similar to the dwarf elephant from Malta and Sicily, naming it “Elephas” cf. melitensis [33,34]; “Elephas sp.” [34]; “Elephas melitensis” [32,35]; and “Elephas cf. mnaidriensis” [36].
Other authors considered the Major’s species valid, confirming that it belongs to the genus Elephas [37,38], while [39] referred it to the genus Palaeoloxodon, which, in his opinion, also includes the other insular straight-tusked elephants. Subsequently, various authors correctly referred “Elephas lamarmorae” to the genus Mammuthus (e.g., [21,40]). Finally, since the early 2000s, one amendment has been made to the name following the International Code of Zoological Nomenclature (ICZN), and Mammuthus lamarmorai is currently the valid species name [21,41,42].
In the 1950s and 1960s, two other elephant remains were discovered at two western Sardinia sites. In the Tramariglio Tower area (Alghero, NW Sardinia), Malatesta [37] identified as belonging to “Elephas lamarmorae” an upper molar (perhaps an M1) of a proboscidean in breccia deposits that the authors considered “post-Tyrrhenian” (i.e., post-MIS 5e). A second molar (an M3 or perhaps an M2), currently stored in MSGPL, was found in the 1960s in a late Middle Pleistocene deposit along the coast of SGS [40,43]. Maxia and Pecorini [43] illustrated for the first time the molar. Ambrosetti [38] described it four years later.
Other remains consisting of an almost complete upper right third molar and a fragment of a very worn molar attributable to two adult individuals with different ontogenetic ages were found in the CG area (Giave, northern Sardinia). These molars come from a marsh deposit that, on the basis of geomorphological evidence, was referred to the late Middle Pleistocene. Based on the more archaic morphology and the larger dimensions than those of the molar found at SGS, it has been hypothesized that the fossils from CG could belong to the ancestor of Mammuthus lamarmorai or, alternatively, testify to different immigration phases of proboscideans from the mainland [44].
In more recent times, Palombo and colleagues [12] described a tibia’s distal fragment found in aeolian deposits outcropping along the coast south of Alghero. The bone fragment was tentatively reported as belonging to Mammuthus cf. M. lamarmorai and referred to MIS 4-MIS 3.
Pillola and Zoboli [45] reported the presence of small proboscidean footprints in a stratigraphic section of a late Middle Pleistocene aeolian deposit at FM. The authors assigned the footprints to the ichnospecies Proboscipeda panfamilia and suggested M. lamarmorai as a possible trackmaker [45,46,47,48,49].
4. The Sardinian Mammoth Remains: A Critical Synopsis
In the following paragraphs, we critically scrutinize the data available for the Sardinian mammoth fossil record and provide some additional information about the size of the individual to which the GPH skeleton reported belongs.
4.1. The Mammoth Skeleton and Other Remains from Guardia Pisano Hill, Funtana Morimenta, and the Surrounding Area
The bout one square kilometer area, from which the rich remains were gathered, which is less than, is located southwest of the Gonnesa village. The remains consist of the well-known skeleton, currently regarded as the holotype of the M. lamarmorai species, a tusk fragment, three vertebrae still articulated in physiological position, and some footprints found in three distinct localities, GPH, Cuccu de Cori, and FM spring, far away from each other by a few hundred meters (Figure 5, p. 162 in [21], [22]). The chronology of the four remains is uncertain. The incomplete skeleton (Figure 2) was probably retrieved from the aeolian deposit outcropping on the northeast top of the GPH slope. The tusk’s large fragment was retrieved from the eastern slope of GPH, whereas the three thoracic vertebras would come from the near Cuccu de Cori site, even if their provenance from GPH cannot be rejected (see [22] for a discussion) (Figure 3 and Figure 4). The footprints were impressed on the aeolian sandstone cliff exposed at the Funtana Morimenta spring [45,46]. The footprints are clearly detectable along the 10 m high stratigraphic section due to the deformations of the underlying laminae of the dune deposits.
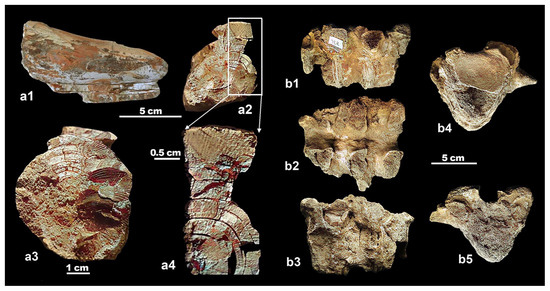
Figure 3.
Mammuthus lamarmorai from Guardia Pisano Hill (Gonnesa) (Museo dei Paleoambienti Sulcitani—“E.A. Martel” of Carbonia); large fragment of tusk in lateral view (a1); distal (a2) and proximal (a3) natural transversal sections; particular of the distal natural section where the Schreger lines and angles are clearly visible (a4). Mammoth remains found at “Cuccu de Cori”, at least according to [21] (Museo Sardo di Geologia e Paleontologia “D. Lovisato” of Cagliari); largely incomplete thoracic vertebrae in lateral (b1,b3), dorsal (b2), cranial, and caudal (b4,b5) view.
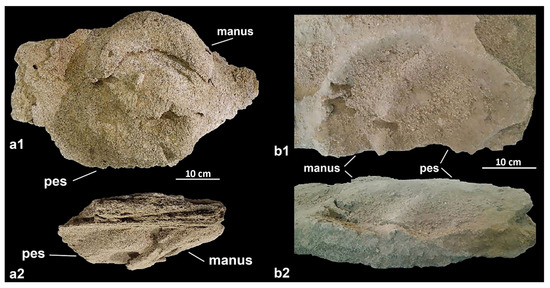
Figure 4.
Proboscipeda panfamilia footprints from Funtana Morimenta spring (Gonnesa); manus-pes hyporelief in inferior (a1) and lateral (a2) view; incomplete epirelief of the manus-pes couple in superior (b1) and lateral (b2) view (modified from [45]).
The aeolian deposits exposed in the Gonnesa area, which most likely yielded the mammoth remains, have been correlated with the Funtana Morimenta Formation (FMF), for which the age is a matter of debate. Orrù and Ulzega [50] supposed that the FMF predates the onset of the MIS 5e climatic event. Indeed, a “Tyrrhenian” conglomerate (MIS 5e) lies on the erosional surface that cuts the aeolianites ascribed to the FMF. The latter are part of several generations of dunes exposed at Plag’e Mesu beach and elsewhere along the coast of the Gonnesa gulf. Successively, Quaternary stratigraphers argued the age of the coastal aeolian deposits and, in turn, that of the correlated landward aeolianites exposed in the GPH/FM area. The disagreement among specialists was mainly related to some inconsistencies in Optically Stimulated Luminescence (OSL) dates and to the scantiness of either reliable isotopic data or precise biostratigraphic constraints of coastal deposits (see [21] and references therein).
Preliminary dating of a few FM samples indicated an age not older than 130 ka for the footprint levels (fide [45]). The date gives some support to a hypothetical correlation of the FM dunal deposit with MIS 6, as proposed by [51]. The authors analyzed the aeolian and alluvial deposits of the stratigraphic section that were at that time exposed in a now-worked-out quarry at GPH. Melis and colleagues [51] described, from the bottom to the top, Units A, B, and C. Based on stratigraphic evidence and correlations, they hypothesized that the aeolian cross-bedded deposits at the bottom of Unit A might have been deposited during the MIS 6, in spite of the 14C age (43,000 ± 1400 BP) obtained for charcoal remains found in the alluvial deposits of Unit B. Indeed, the actual age of the alluvial deposits might be older because the 14C dating falls close to the lower limit of the radiocarbon dating method. Accordingly, the available evidence supports the hypothesis of a pre-“Tyrrhenian” (MIS 5e) age for the Gonnesa mammoth remains as the most suitable. More specifically, the FM dunes probably formed during MIS 6 (Andreucci personal communication, 2003).
The remains have a different taxonomic and biological significance. The tusk’s large fragment shows firm diagnostic characteristics that confirm some taxonomic hints provided by the skeleton’s long bones and enable us to support the identification of other remains as those of a diminutive Mammuthus representative.
Indeed, the Schreger angles visible on the flat surfaces of the naturally fractured, almost transverse distal and proximal sections of the tusk (Figure 3) run from the tusk axis to the cementum with a nearly constant bend radius, which slightly diverges from the section’s radius. As a result, the maximum (58.7°) of the values of the “outer” (near the dentine–cementum junction) and “inner” (near the proximal–distal tusk axis) angles falls in the range of the Schreger angles reported for M. primigenius, M. meridionalis, and M. trogontherii and is definitely narrower than those known in extant elephants and fossil straight-tusked elephants (e.g., [52,53] and references therein). The skeleton provides little information about taxonomy or the morphofunctional modification triggered in some limb bones to adapt to peculiar environmental conditions, and it gives rather intriguing hints about the size reduction of the GPH individual, as it can be estimated from long bone dimensions. Palombo and colleagues [44] considered the morphology of the carpal and tarsal bones of the Sardinian mammoth to be more similar to that of Mammuthus than Palaeoloxodon. However, a comparative metric analysis among the carpal bones of M. lamarmorai, Palaeoloxodon ex gr. P. mnaidriensis, Palaeoloxodon antiquus, and M. meridionalis [21] gave contrasting results. In particular, the M. lamarmorai unciform is wider and lower than that of M. meridionalis, a difference that seems to correspond to that observed in the Sicilian dwarf elephant with respect to its putative ancestor, P. antiquus, and might suggest some possible, but unproved, similarities in the morphofunctional modification of the acropodium related to these elephants’ gaits. Moreover, in the astragalus of M. lamarmorai, the lateral articular facet for the calcaneus is laterally expanded with respect to that of continental mammoths, as it has been observed in at least three separate dwarf straight-tusked elephant lineages (P. ex gr. P. mnaidriensis, Palaeoloxodon melitensis, and Palaeoloxodon ex gr. falconeri) [54]. Therefore, the “Palaeoloxodon-like” morphology of M. lamarmorai unciform might be due to either a change in proportions related to the dwarfing process or functional requirements shared with other dwarf insular elephants, such as the shifting of body mass to the lateral side of the hind foot [31,54]. The preservation status of most limb bones, particularly the femur, prevents us from confirming whether the Sardinian mammoth dwarfing process might have led to a reduction in graviportal posture, as observed in other endemic proboscideans (and, in turn, to a more agile gait permitted by slender limb bones and more flexible articular joints, thus bringing the limbs closer to the sagittal plane) (e.g., [55,56]).
The dimension and the body mass estimate (BM, the best proxy of body size) of the GPH skeleton indicate a rather significant size reduction of this individual with respect to the average size of Mammuthus adult males (BM of about 10–11 tons, flesh stature of about 4 m) [57,58,59]. An estimation of M. lamarmorai body mass for inferring the species evolution in the insular, peculiar environment has some relevance because a number of physiological variables and ecologically significant characteristics of mammals correlate with body size (e.g., metabolic rates, growth and reproduction, population density, home range, life history, and body traits).
Accordingly, we have estimated the M. lamarmorai BM by comparing the range of the values obtained by using the regression equations proposed for dwarf elephants, proboscideans, and quadrupedal terrestrial tetrapods [27,28,29]. The last two authors proposed the universal regression equation as the best proxy for calculating body mass in extinct tetrapods, including the largest mammals. Roth [27] (Table 9.1, p. 158) considered the length and circumference of the humerus and femur to be the best mass estimates in elephants weighing less than 2000 kg, while for Christiansen [28] (p. 529), “the five best osteological predictors of body mass are humerus anteroposterior diameter, ulna least circumference, humerus least circumference, ulna length, and fibula least circumference, despite the last of these not being a primary weight-bearing bone” in extant Asian and African elephants.
Finally, the nature of the mammoth footprints found at FM (Figure 4)—isolated manus-pes couples preserved as convex hyporeliefs or concave epireliefs or visible in transverse sections [45]—prevents us from estimating the BM from the manus circumference (cf. [60,61]). The BM estimates returned by the equation proposed by Roth [27] (342.12 kg for the humerus length, 807.45 kg for its circumference, and 1033.23 kg for the femur circumference) indicate a BM of roughly 700 kg (average value of 727.60 kg). The values that result from applying the Campione and Evans formulas [29] are slightly higher at 516.27 kg and 858.33 kg for the humerus length and circumference, respectively, and 995.56 kg for the femur circumference, with an average BM of 944.48 kg, which is identical to that obtained using the universal formula (LogBM = 2.754 × log Ch+f − 1.097, where C = circumference; h = humerus; and f = femur). The BM value obtained using the M. lamarmorai available measurements among those proposed by Christiansen [28] shows a wide variation (Table 1). The BM values obtained from the left (incomplete) and right humerus measurements range from 241 kg to 2829 kg, with an average value of 1252.08 kg. By removing the anomalously high and lowest estimates (i.e., those obtained from the humerus’ antero-posterior and latero-medial diameters of the diaphysis and the width of the humerus lateral condyles, respectively), the BM value variation sensibly reduces. The BM values range from 538 kg (width of the right humerus medial condyle) to 1220 kg (right humerus’ least circumference), with an average value of 876.85 kg. The BM values obtained from the incomplete right femur range from 333 kg (femur lateral condyle length) to 1648 kg (femur lateromedial diaphysial diameter), with an average value of 848.16 kg. The BM average value increases (951.26 kg), becoming similar to that obtained by applying the universal regression equation, if the lowest measurement (lateral condyle length) is removed. The results obtained suggest great caution in inferring BM from estimates based on single or fragmentary bones. Indeed, BM estimates vary, for example, because mass estimation equations can yield different estimates for the same individual and even for the same bone due to the fluctuating asymmetry, and also because of the variation in bone proportions during proboscidean growth and, of course, the intra-population variability (e.g., [27,59]).

Table 1.
Body mass (BM) estimates of Mammuthus lamarmorai from Pisano Hill calculated using the following Christiansen’s formula: log(bM) = a + b (logX), where X = bone measurement (variable) [23,28].
The results obtained, though only indicative, suggest that the mammoth individual from GPH might have had a weight of about 750–950 kg. The alleged BM would imply a size reduction of more than 90% with respect to the size of its putative ancestor’s large males (about 10 tons). However, the size reduction suggested by the GPH humerus of M. lamarmorai is roughly consistent with that of the smallest Mammuthus exilis from the Channel Islands (Southern California) (e.g., [27,62]). Much more data are needed to confidently infer the size of the Sardinian dwarf mammoth.
Finally, M. lamarmorai has frequently been described in the literature as a dwarf mammoth of about 1.5 m tall (Figure 2). However, its stature cannot be estimated because the height at the shoulder of the GPH skeleton cannot be positively estimated because the available material prevents applying one or another of the methods currently used for inferring this physical parameter (cf. [58] for a discussion).
4.2. Molar from San Giovanni di Sinis
The molar from SGS provides some taxonomic information and chronological data and gives some hints about the putative ancestor of the endemic Sardinian mammoth.
The SGS molar is the only one available among the small-sized elephant remains; G. Pecorini discovered it at the bottom of the stratigraphic sequence exposed near the SGS village [38,40,43]. Although most of the levels present in the area were deposited during MIS 5e (see [63] and references therein), the mammoth molar was retrieved from a calcrete that is overlain by pedogenized beach sediments truncated by an erosional surface (unconformity) related to the MIS 5e transgressive sea level rise [40,63,64,65] (Figure 5). Therefore, these levels predate MIS 5e and might have been deposited during MIS 6 or even during MIS 7, for which the levels are locally exposed in this sector of the SGS coast at different heights above sea level (Figure 5c) [66].
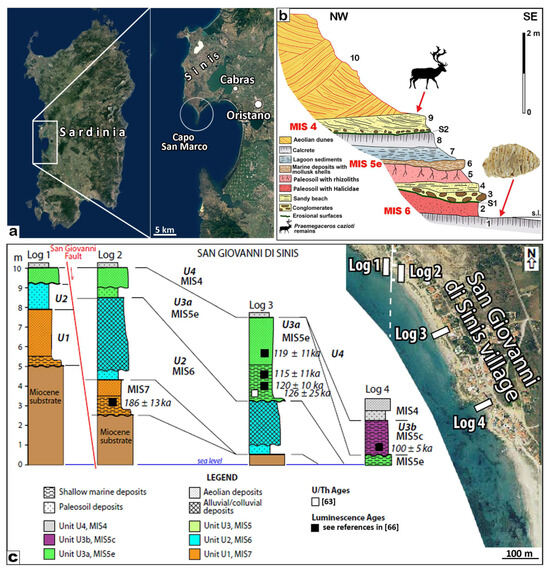
Figure 5.
San Giovanni di Sinis. (a) Location of the outcrops of the San Giovanni di Sinis area. (b) Stratigraphic sketch from which the Mammuthus lamarmorai molar was retrieved. 1: calcrete; 2: palaeosol developed on beach sediments rich in Helicidae remains; 3: conglomerate containing pebbles from the erosion of the underlying level as well as basalt pebbles; 4: beach sediments; 5: palaesols with rhizoliths; 6: marine deposits rich in Mytilidae and Ostreidae shells (MIS 5e); 7: lagoonal deposits with mollusks; 8: calcrete; 9: beach deposits overlying a conglomerate with remains of Ostrea shells; 10: dunes; asterisk: mammoth molar; the arrow indicates the coastal marsh deposit with Praemegaceros cazioti remains; S1 and S2 erosional surfaces (modified from [40]). (c) Stratigraphic logs measured along the San Giovanni di Sinis coast close to the stratigraphic sequence. The bold line indicates the unconformities bounding the units (modified from [63,66]).
After [43] published a picture of the SGS molar, the tooth was damaged before [38] described and illustrated it. Moreover, old restorations have obliterated some morphological features (Figure 6). For instance, it is challenging to detect the potential presence of the first root, above which there are three or four plates in M. trogontherii, while in M. meridionalis, the number varies from two to three [67]. This hampers the chance to know the total number of plates, though the molar seems to be almost complete and the mesial plates are missing due to wear, as the anterior half of the first preserved plate, worn down to the root, is lost. The other functional plates are at a quite advanced wear stage, and the dot-line-dot arrangement typical of Mammuthus is only detectable on the enamel loop of the most proximal, less worn plate. Most of the undulating enamel loops are unfolded at their medial and lateral edges and show rather fine to rather large wrinkles in their middle parts, similarly to most M. trogontherii molars. The lamellar frequency, the thin enamel, and the functional hypsodonty index support the hypothesis of a possible phylogenetic relationship with the steppe mammoth.
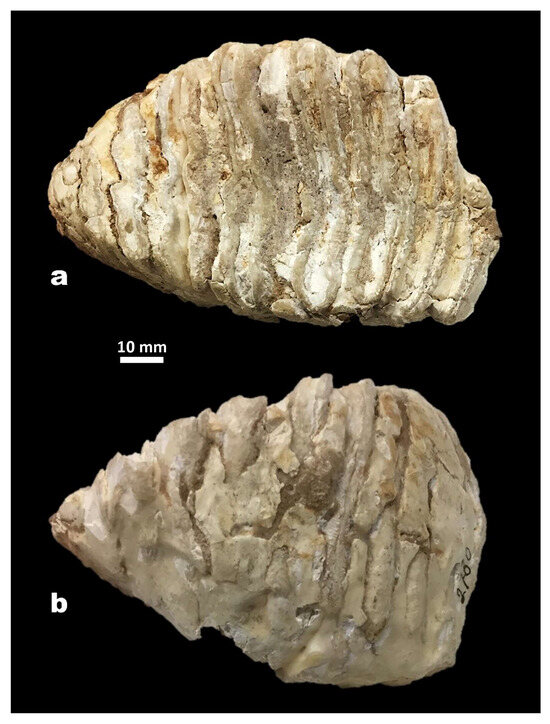
Figure 6.
Mammuthus lamarmorai from San Giovanni di Sinis (Cabras, Western Sardinia) (Museo Sardo di Geologia e Paleontologia “D. Lovisato” of Cagliari). Ultimate, or maybe penultimate, right upper molar in occlusal (a) and buccal (b) view.
Ambrosetti [38] described the molar as a last right upper molar (M3) because, in his opinion, the posterior surface of the crown, where a small platelet seems to be present, is apparently not flattened by the pressure of the following tooth. However, the molar shows some contradictory features that make it challenging to firmly identify its position within the molar succession. The oval occlusal surface shows a progressive narrowing typical of the last molars, but the crown posterior outline is moderately angulated and slightly rounded close to the occlusal surface, but almost straight towards the crown base. Moreover, the tooth lacks the gradual height decrease of the posterior side, which is generally detectable in the last molars at an advanced wear stage.
For the purpose of obtaining some clues, we carried out a similarity analysis of the data reported in Table 2, using as variables the M2 and M3 measurements (Figure 7). In the dendrogram obtained for M2, two clusters are present. One gathers mostly M. trogontherii specimens; the other includes M. meridionalis, M. trogontherii-like, and a couple of M. trogontherii molars. The SGS molar is set as a separate ramus of the M. trogontherii cluster. In the dendrogram obtained for M3, the separation between M. meridionalis and M. trogontherii is less clear. SGS still falls in a separate ramus, but in a large cluster that includes two subclusters: a smaller one with some steppe mammoths and the CG molar, and a larger one with M. meridionalis, M. trogontherii-like, and specimens showing a mosaic of advanced and primitive characteristics. Therefore, the results of the analysis prevent us from asserting with absolute certainty whether the SGS tooth is an ultimate (M3) or a penultimate molar (M2). However, considering the molar size reduction, the maximum crown width is proportionally more similar to that of M3 than that of M2 (Table 2), which could lean towards an assignment to M3.

Table 2.
Comparison among biometric data of selected Mammuthus meridionalis, M. trogontherii, and mammoth molars showing a mixture of dental characteristics of both species. * Number of plates and occlusal lamellar frequency inferred on the basis of the picture and schematic drawing published by [32], who conversely reported 6 plates and a frequency of 4 plates per 5 cm, thus extrapolating a frequency of 8 plates per 10 cm. Data from [25,37,40,41,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85].
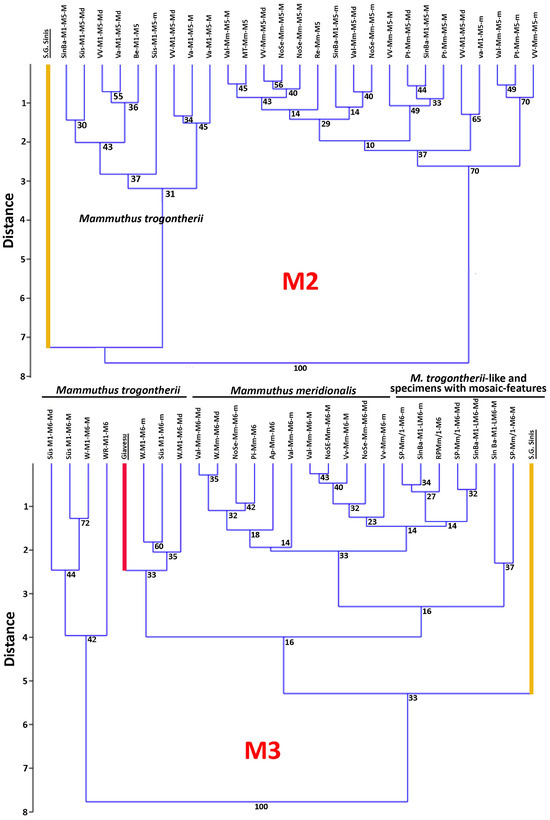
Figure 7.
Q-mode dendrogram showing the hierarchical ordering of the Early and Middle Pleistocene for the molars of the main continental representatives of the Mammuthus genus and the Sardinian Mammuthus lamarmorai cluster according to their basic measurements (crown length, width, and height, number of plates, average plate frequency index and enamel thickness, and hypsodonty index). Data from Table 2 (selected average values).
4.3. The Molars from Campu Giavesu
About 20 years ago, one of us (M.R.P.) had the chance to examine two mammoth molars belonging to a private collection that were found in the first half of the last century somewhere in the Campu Giavesu area (northwestern Sardinia, Sassari). The molars—an almost complete last upper molar and a fragment of a very worn one—are currently unavailable. According to [44], who performed a geological survey of the area, the mammoth teeth might have been retrieved from marsh sediments deposited after the end of M. Annaru volcanic activity (dated about 0.2 Ma) [86], during which lava flows prevented river drainage. Later, colluvial sediments filled the Campu Giavesu valley. Marsh environments may have characterized the first filling phases; thus, the molars have been supposed to be late Middle Pleistocene in age, but there is not compelling evidence supporting this hypothesis.
The almost complete tooth (Figure 8) is the only one providing useful taxonomic information due to the too-advanced wear stage of the other, largely incomplete molar. It is definitely the last right upper molar (M3), as the typical reduced height of the posterior unworn plates and the lack of any evidence of pressure by a posterior tooth clearly indicate. Only half of the first plate is preserved due to a molar break. However, the first three plates join together above the first root, so the total number of plates should be 16, as the breaking pattern seems to suggest, or not more than 17 [67] (Table 2). The molar is high-crowned, and the hypsodonty index falls in the variation range of M. trogontherii. The enamel is rather thick; the enamel loops are unfolded on the medial and lateral edges but show rather fine wrinkles, especially in the middle parts. The plates are not densely packed. On the occlusal surface, the loops range from oval to moderately proximally curved in the most worn and the averagely worn plates, respectively. At an incipient wear stage, the plate divides into sub-circular loops that decrease in size from the center to the lingual and buccal edges of the plate. When the wear increases, the lateral and buccal loops merge together in transversely elongated islets, while the central ones are partially fused to each other. The enamel is rather thick, crenulated, and almost unwrinkled. Although the thickness falls in the overlapping variation range of M. meridionalis and M. trogontherii, the enamel thickness is close to the maximum values of M. trogontherii, and the enamel is thinner than in the majority of M. meridionalis specimens. The molar biometric characteristics suggest some major affinities with M. trogontherii compared with M. meridionalis, as supported by the results of the similarity analysis (Figure 7).
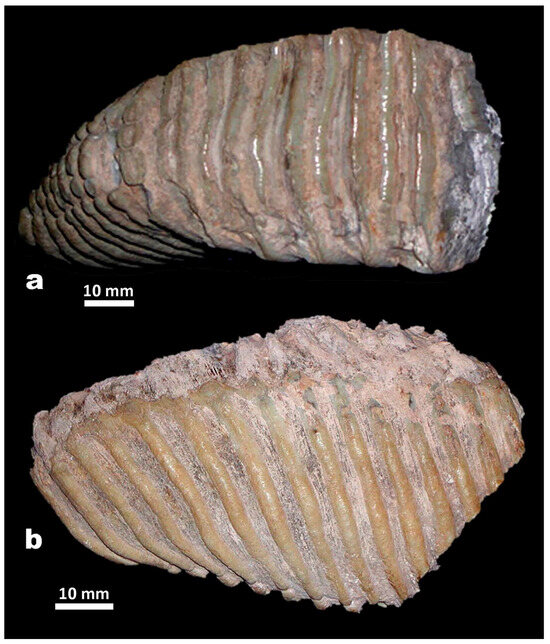
Figure 8.
Mammuthus lamarmorai from Campu Giavesu (Giave, SS). Right upper last molar (M3) in occlusal (a) and buccal (b) view.
4.4. The Problematic Lost Molar from the Tramariglio Bone Breccia
The first mammoth molar recorded in Sardinia was discovered during a geological survey along Porto Conte Bay (Alghero) [37]. For a long time, it has been and is unavailable, as it is not present in the Malatesta collection of the Earth Science Museum (MUST, Sapienza University, Rome) and, apparently, it is also not found in the Sardinian fossil material stored at ISPRA (previously Italian Geological Service) (Rome), where the fossils belonging to the Malatesta’s collection are stored. Malatesta [37] stated that the molar was retrieved from a well-cemented post-Tyrrhenian breccia exposed at Tramariglio on the eastern side of the Capo Caccia promontory. However, some breccia older than MIS 5e may be present along the coast from the promontory to Tramariglio Tower [87]. In particular, at “Punta del Quadro”, not far from Tramariglio Tower, both pre- and post-Tyrrhenian breccias have been detected [71] (plate 1) (Figure 9). Because Malatesta [37] did not indicate the exact location where the molar was found, the actual chronology of the elephant molar cannot be confidently established.
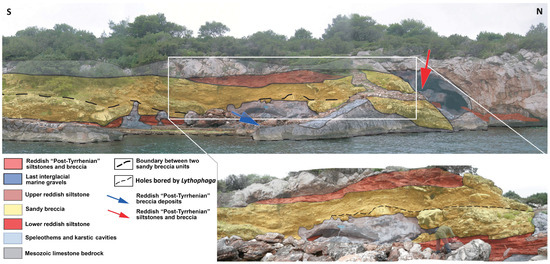
Figure 9.
Panoramic view of the deposits exposed at “Punta del Quadro”, not far from Tramariglio Tower (modified from [61] according to Palombo and Ibba’s unpublished data).
Although the molar is unavailable, we can make a few comments regarding its morphological traits and the alleged attribution to M. lamarmorai on the basis of the tooth’s published description and illustrations [37] (Figure 10).
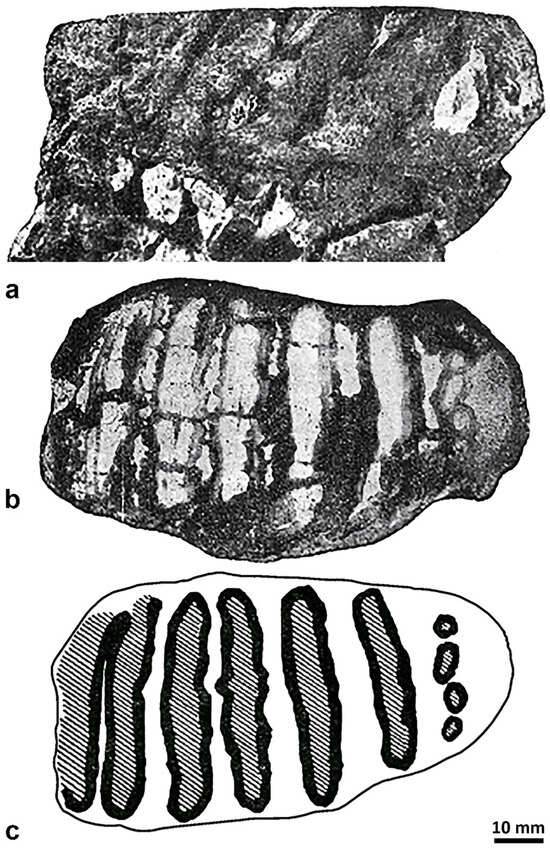
Figure 10.
Mammuthus cf. M. lamarmorai from the Tramariglio area (Porto Conte Bay, Alghero, SS): molar in labial (a) and occlusal (b) view; schematic drawing of the occlusal surface (c) (modified from [37]).
Malatesta [37] asserted that the rather worn upper molar (possibly an M1 according to its dimensions (Table 2)) has six plates (i.e., the most anterior, which lacks the anterior enamel band, five worn plates, and the posterior talon). However, according to the tooth pictures in buccal and occlusal views and the schematic drawing of its occlusal surface cut for better detection of the enamel figures, the number of plates is seven because the posterior one, which is at an initial wear stage, is apparently followed by the talon (Figures 2 and 3, pp. 97,98 in [37]). The enamel loops, which are elliptical in shape, run rather parallel and are outdistanced from each other, and only two are weakly sinuous (Figure 10c). The loop of the first posterior plate consists of four enamel islets. No loops of plates show just a partial fusion of the lateral enamel, such as the dot-line-dot or line-dot-lines (most common in straight-tusked elephants and mammoths, respectively), likely due to the combined effect of the rather advanced wear stage and the distance separating the last, most posterior plates. The lamellar frequency, which falls in the variation range of the continental Eurasian Palaeoloxodon and between the maximum and minimum values reported for M. meridionalis and M. trogontherii, respectively [25], and the moderately thick enamel with few, rather large wrinkles suggest that the molar might belong to a mammoth less advanced than that from SGS. However, some conflicting points, such as the fact that, on the one hand, proportionally smaller molars have more closely spaced plates [88], and, on the other hand, the fact that dwarf insular proboscideans have a reduced number of plates and thicker enamel than their continental ancestors, suggest that the hypothesis should be considered with great caution.
4.5. The Mammoth Remains from the Alghero Aeolian Deposits
A distal portion of a left tibia (Figure 11), referred to Mammuthus cf. M. lamarmorai, was found in the 1980s embedded in a well-cemented sandy block in an unrecorded trait along the coast south of Alghero (NW Sardinia) [12]. The sandy beach and aeolian dune deposits, exposed along the coast from Porto Conte Bay to about 30 km south of Alghero, were OSL dated from MIS 6 to MIS 3 [89,90]. Shallow marine sediments and paleosoils, referred to MIS 5.5 (MIS 5e), overlay the older aeolian deposits [89]. The composition of the sediment embedding the mammoth’s incomplete tibia is similar to that of sandy MIS 6 or MIS 4 costal dunes. However, the sediment contains some rare red pedorelicts likely derived from paleosoils present in MIS 5e as well as in MIS 3 deposits. Accordingly, it is reasonable to suppose that the tibia was originally embedded in the sandy aeolian sediments deposited during MIS 4 or MIS 3, about 57–29 ka BP [12] (Figure 12).

Figure 11.
Mammuthus cf. M. lamarmorai from Alghero: distal part of the left tibia in lateral (a), anterior (b), and posterior (c) views (modified from [12]).
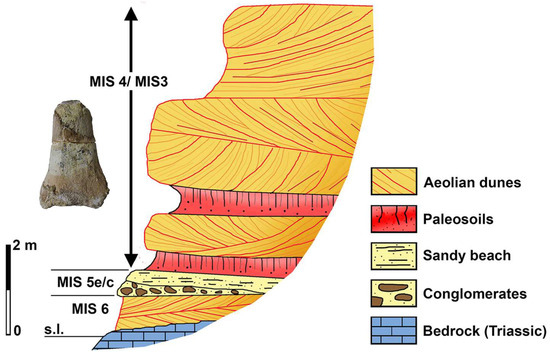
Figure 12.
Stratigraphic sketch of the Pleistocene deposits exposed along the coast sector south of the Alghero coast (modified from [12]).
The distal epiphysis of the large fragment of the rather robust tibia from Alghero is somewhat damaged, but a few morphological traits are still detectable, which might provide some useful clues for its identification (Figure 11). For instance, on the one hand, the lateral outline of the joint with the fibula and the shape of the medial malleolus conform to the morphology of M. exilis from the Channel Islands, which has, however, a less concave epiphyseal inferior outline. On the other hand, they differ from the sinuous inferior outline and the more robust and rounded medial malleolus. However, such characteristics have weak taxonomic significance due to the great variability characterizing the morphology of the tibia distal epiphysis in Elephantidae and the changes in shape during the dwarfing process of insular species.
Because tibias are missing in the GPH skeleton, a comparison between the Alghero tibia and the M. lamarmorai holotype is not possible. Therefore, a hypothetical similarity in size between the two individuals [12] cannot be demonstrated. Moreover, a BM estimate based on the tibia circumference (about 1600 kg, according to our analysis) might be overestimated because the incompleteness of the Alghero tibia hampers knowing what the real value of the last circumference is. However, the size might be comparable with that of the largest Palaeoloxodon ex gr. P. mnaidriensis from the Puntali Cave (Sicily) [91], with a height at the shoulder of about 2 m, and the smallest Palaeoloxodon tiliensis from Tilos (Greece), with a height at the shoulder of about 1.5 m [92,93].
Recently, [94] reported an incomplete external imprint of a tusk distal portion found in a sandstone deposit exposed close to the shore and the high tide line in the small Las Tronas bay (Alghero village coast). However, successive surveys failed to find any evidence of a tusk imprint at Las Tronas.
4.6. A Few Taxonomic Notes
On the whole, the morphologic and biometric characters of the molars from SGS and CG (shape of the enamel loops, enamel folding and thickness, plate frequency, and hypsodonty index) roughly conform to those of moderately advanced continental mammoth representatives, especially when the morpho-biometric changes shown by the dwarf elephant molars are considered (e.g., proportionally reduced number of plates, thicker enamel, and more primitive pattern of enamel folding).
The results of the multivariate analyses, including, in particular, the PCA, indicate that the affinity of these specimens with M. trogontherii is greater than that with M. meridionalis, giving support to this hypothesis. In the PCA biplot (Figure 13), the score position highlights the nearly complete separation between M. meridionalis and M. trogontherii. Moreover, the dispersion area of the first indicates that they are less variable than those of the latter, which, on average, are higher-crowned with a more densely packed plate (higher frequency index) and moderately thinner enamel. The position of SGS confirms, on the one hand, some major affinity with the steppe mammoth and, on the other hand, the peculiarity and small size of the Sardinian specimen. Indeed, the score falls outside of the variation ranges of both groups but closer to those of M. trogontherii small M2. Conversely, the score of the larger CG molar falls within the variation range of the M. trogontherii scores in an intermediate position between the M2 and M3 ranges.
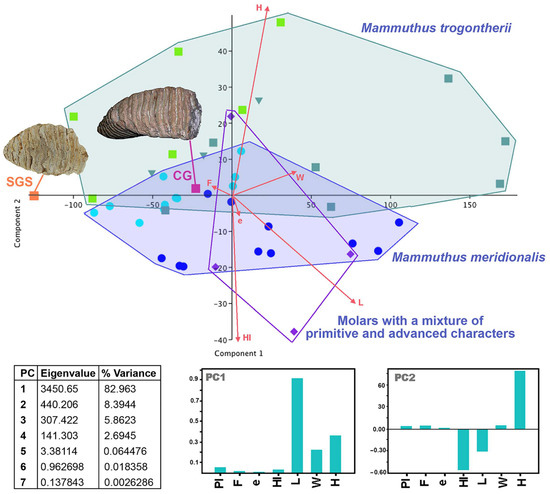
Figure 13.
Biplot diagram produced through the principal components analysis (PCA) of the basic molar measurements (crown length, width, and height, number of plates, average plate frequency index and enamel thickness, and hypsodonty index) (variables) of Early and Middle Pleistocene continental Mammuthus the Sardinian Mammuthus lamarmorai specimens (cases).
All things considered, even assuming as true that the molar from SGS is actually an M3, the morpho-biometrical traits of the CG M3 (e.g., plate packing, enamel thickness, hypsodonty index, lamellar frequency) might suggest that the latter belongs to one that is less advanced and reduced in size compared to the SGS one. Although the unproven older age of the CG molars could support the hypothesis of a progressive reduction in size of the Sardinian mammoth populations throughout time, this idea has to be considered just speculative due to the uncertainties about the position of the SGS molar within the tooth sequence and the well-known variability that characterizes elephant populations.
5. Ongoing and Planned Research
5.1. Histological Analysis
The bone tissue is a plastic tissue that adapts to the mechanical stresses occurring during locomotion. In recent years, an increasing number of articles have underlined the close relationships between the bone microstructure of any vertebrate and its lifestyle [95,96,97,98]. Histomorphometrical analysis based on some measurements of the features of the bone tissue (e.g., osteon density, maximum and minimum diameter of osteons and corresponding Haversian canals, as well as their ellipticity) could facilitate inferring the intensity of locomotion’s stresses and, in turn, some features of the environment the animal inhabits. Paleohistology, i.e., the application of this method to fossil remains of extinct species, permits the acquisition of significant biological information about their life history and lifestyle and provides useful hints for scrutinizing the adaptive shifts in body size on islands among dwarf island settlers [99,100,101], including, in particular, proboscideans [102,103,104,105,106,107].
The results we have obtained by analyzing the bone histology of M. lamarmorai, summarized below, are largely preliminary and merely indicative, because only the shape and dimensions of the osteons and Haversian canals of a single specimen (the distal tibia’s portion from Alghero [12]) have been analyzed.
5.1.1. Preliminary Results
Despite numerous cracks in the microstructure, the osteon structure is still clearly visible, as is the presence of the Haversian canals. The bone tissue of M. lamarmorai is characterized by a high number of osteons, which are crammed and closely packed with each other. As a result, there is almost no space for the extra-osteon area; thus, the dwarf mammoth bone tissue can be classified as dense Haversian tissue (Figure 14a). More specifically, the arrangement of osteons, which are H2 and H3 subtypes of dense Haversian tissue, conforms to the arrangement of the osteons regarded as the most resistant to biomechanical stress [108] (Figure 14a). Osteons are often formed by more than eight bone lamellae, which surround the central Haversian canal (Figure 14b).
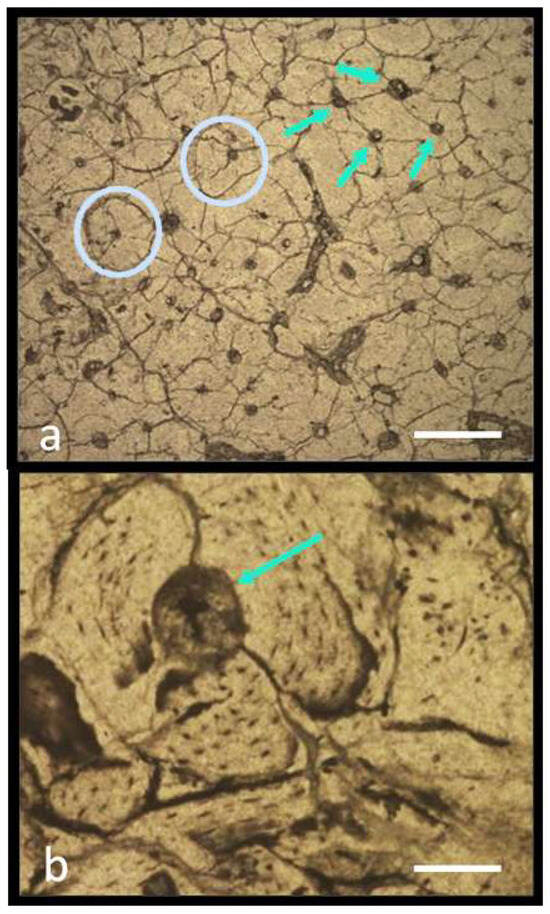
Figure 14.
Mammuthus cf. M. lamarmorai: Bone tissue from the tibia’s distal portion from Alghero. (a) Dense Haversian tissue with numerous osteons close to each other. Bar scale = 200 µm. (b) Osteon formed by 8 bone lamellae surrounding the Haversian canal. Scale bar = 50 µm. The green arrows indicate some Haversian canals, and the light blue circles indicate two osteons.
The osteons and the Haversian canal are rather small and narrow, respectively. The mean diameter of the osteons is 205 ± 28 µm, the perimeter of the osteons is 684 ± 145 µm, and the osteon eccentricity is 0.38. As regards the Haversian canals, the mean diameter is 41 ± 12 µm, the perimeter 138 ± 36 µm, and the average eccentricity is 0.34. The main histomorphometrical data are given in Table 3.

Table 3.
Histomorphometrical data from the tibia’s bone tissue of Mammuthus cf. M. lamarmorai from Alghero.
5.1.2. Notes
The results of the morphological analysis show that the main characteristics of the osteons and the Haversian canals seen in the mammoth tibia’s bone tissue roughly correspond to those reported in the literature of both extinct and living Elephantinae species, including M. primigenius [104,109], Loxodonta africana [103], and Palaeoloxodon falconeri [106]. However, the osteons of the dwarf Sardinian mammoth are slightly smaller than those of species of continental Eurasian elephants. The diameter average values of the Sardinian mammoth osteons and Haversian canals are inferior by about 20% and 45% compared to those of E. maximus (data from [107,110]) and by 30% and 20% compared to those of M. primigenius, respectively (data from [104]).
The size of osteons only reduces by up to 25%, which is interesting given that insular dwarfism causes M. lamarmorai to lose nearly 90% of its body mass with respect to its continental forerunner. A number of factors, including variations in environmental conditions and lifestyle, may have acted together to prevent a more drastic diameter reduction. These factors may have contributed to triggering some variations in the biomechanical stress that the limb bones could have experienced.
Moreover, we have, for now at least, limited our analysis to a single bone, while we have made the comparison with the average values obtained by different skeletal elements. Moreover, body and osteon size are not correlated in other mammals belonging to the same lineage. For instance, some ruminant species with different body masses do not show a parallel difference in the osteon’s size [111].
The histomorphometric data provide some clues to infer the locomotory aptitude of the Sardinian dwarf mammoth. For instance, in the examined elephants, the value of the ratio of the osteon diameters (On.Dm) against those of their Haversian canals (HC.Dm) increases from E. maximus (3.2) to M. primigenius (4.9) and M. lamarmorai (5.1) (Table 4). Because the Haversian canals are usually large in sedentary individuals [112,113], it is probable that M. lamarmorai, due to their small osteons with narrow Haversian canals, had a high locomotor aptitude and were, maybe, able to move fast. However, more data are necessary to support or reject this hypothesis.

Table 4.
Histomorphometrical data of the Sardinian dwarf mammoth compared with M. primigenius and E. maximus.
5.2. Planned Research
5.2.1. Geochronology
Although the little available stratigraphic evidence supports the Mammuthus presence in Sardinia from the latest Middle to the last glacial period, no dating is available for some sites that could give firm chronologic information, such as aeolianite deposits. Some research and luminescence dating, which we have planned to conduct at FM in collaboration with researchers from Sassari University, could add new hints to the debate about the age of the well-known dune sediments exposed at the site.
Moreover, it would be desirable to acquire new data, possibly from new ichnosites. In fact, Pleistocene ichnosites with large mammal (mainly deer) footprints are relatively common in aeolian deposits along the entire coast of western Sardinia, and, given the paucity of proboscidean bone remains, their study could be a useful way to confirm the presence of M. lamarmorai in the most advanced phases of the Pleistocene.
5.2.2. Paleoecology
On islands, a new way of life causes morphofunctional changes that are related to the changed environmental conditions to which insular mammalian communities have to adapt. Using the information obtained from the morphofunctional analysis of limb bones, we may evaluate the changes that the island settlers underwent with respect to the characteristics of their continental progenitors. A species’ suitability to move on a certain type of substrate can be inferred, for example, by the gait and agility of its movements, which can be determined, in turn, by the functionality of its limbs and the proportion among limb bone segments (epiphysis morphology, joint firmness in the latero-medial direction and flexibility in the antero-posterior one, limb position with respect to the sagittal plane, muscles’ and tendons’ impression scars, etc.). We could enhance our knowledge about the Sardinian mammoth’s lifestyle and its habitat by applying the most appropriate method among those developed to use the postcranial bones for predicting a species’ paleohabitat.
Moreover, it could be useful to infer the feeding behavior of the SGS elephant, the only one for which chewing teeth are available. The study of tooth enamel microwear defects produced on tooth enamel by attrition with food particles (abrasion) and tooth/tooth (attrition) contacts during chewing, provides useful information for species paleodiet reconstruction, especially if performed using the three-dimensional dental microwear texture analysis (DMTA) method (e.g., [114] and references therein). Some authors (e.g., [115] and references therein) consider the combination of both quantitative and qualitative methods to be the most suitable for obtaining compelling results. This approach is particularly useful for inferring the feeding habits of elephants, which have peculiar occlusal surface features with enamel figures that change progressively as the wear of the tooth increases (e.g., [116] and references therein).
The results obtained by applying the microwear analysis could be compared and integrated with those of the mesowear angles’ method. The latter was developed by [117] to study the shape modifications in sharpness and height that the masticatory processes produce in the upper molar cusps of herbivore ungulates. Some authors successively applied the method to infer the food habits of different herbivore groups, including elephants. The analysis of tooth enamel micro-defects in the herbivores belonging to a palaeocommunity would mirror the local vegetation cover and herbivore species’ niche separation. Both methods would be potentially useful to determine the feeding habits of each elephant population of the Sardinian mammoth and, in turn, to infer the local vegetation patterns and their potential changes, but the results obtained have some significance only if based on several specimens. Accordingly, the results of the mesowear analysis that we executed on the SGS molar, which suggest a feeding habit close to that of mixed feeders, have merely an indicative significance.
Pending the chance to collect and analyze other samples, a few additional clues about the dietary preferences and habitat of this mammoth might come from the analysis of the relative abundance of stable isotopes (e.g., δ13C and δ18O) in its tooth enamel. The carbon isotopic composition depends on the photosynthetic route used by plants. C3 plants (most trees, bushes, herbs, temperate shrubs, and grasses), the only ones largely widespread in Europe, are characterized by an enrichment of light carbon isotopes (12C). Because C3 plants in close and forested vegetation cover have lower δ13C values than those in open and arid environments [118] and in extant elephants, the percentage of browsing aptitude varies depending on the availability of different kinds of plants and on climatic conditions (i.e., it increases during arid periods when grasses dry out), and the δ13C value gives information about both the feeding habit and the habitat preferred by the analyzed elephant individuals [61]. The oxygen isotopic (δ18O) composition shown by herbivores depends on the type of ingested plants and the temperature of the drank water, which relates to the geographic position of the territory they inhabit and the water source (e.g., [119]).
As a result, stable isotope analysis can be a valid support for inferring the diet, habitat, and microclimatic conditions of the territory in which the animal lived.
6. Open Questions
This critical revision of the Sardinian mammoth remains enables us to offer some hints, if not definitive answers, to some questions, such as those regarding its phyletic relationships with the continental species and when the putative ancestor entered the island. However, the rarity of the remains limits the chance to clarify some other important issues regarding the mammoth’s evolutionary pattern on the island.
For instance, even when the peculiar characteristics displayed by the chewing teeth of dwarf elephants in comparison to their progenitors are taken into consideration, the reassessment of the morphology and biometry of SGS and CG molars suggests that the mainland ancestor was probably the steppe mammoth. However, the time of the arrival of the pioneering population in Sardinia cannot be defined with certainty because authors disagree about both the time and mode of the M. meridionalis’s replacement by M. trogontherii (see [22,120] for a discussion). The species was first reported in Italy shortly after the beginning of the Middle Pleistocene (MIS 17) at Ponte Galeria [69,121] and, with doubt, at Sassa [122] and in France at L’Escale [123]. The species possibly survived in Europe until about 200 ka [23], though it might have disappeared from the northwestern Mediterranean region (Iberian Peninsula, France, and Italy) after MIS 9, when it is reported, for example, in Spain at la Solana del Zamborino (Guadiz-Baza Basin), a site with a rather controversial age, which supposedly ranges from about 480 ka to 300 ka (cf. [124]). Therefore, we could hypothesize that the dispersal from the mainland occurred roughly between 650 ka and 300–200 ka.
Even if we suppose the hypothesis is correct, the uncertainties regarding the actual chronology of the majority of the remains and their extremely small number prevent us from chronologically ordering them and, as a result, from knowing whether some differences shown by the molars fall within the intra- and inter-population variation of the morpho-biometric characters or not. As a result, it is just a speculative exercise to try to answer some key questions, such as how many elephant species inhabited Sardinia, how long each population or species persisted on the island, and, in turn, how many times the putative ancestor entered Sardinia. Moreover, even assuming that the ancestor entered only once, it is challenging to say whether the hypothetical subspecies arose from an anagenetic or a radiative evolutionary process. Furthermore, we know that M. lamarmorai was most likely still present during MIS 4-MIS 3 and that no mammoth remains have been reported in more recent deposits; thus, we might suppose that M. lamarmorai disappeared in the postglacial period, but the hypothesis that it disappeared at the transition to the Holocene, if not in the Early Holocene, cannot be ruled out. Whenever the extinction occurred, what were the factors that caused the decline and disappearance of the Sardinian mammoth? The combined actions of several factors likely contributed to the megafauna extinction at the end of the Pleistocene and were similarly responsible for the definitive decline and disappearance of residual individuals in large mammalian populations of endemic island settlers (e.g., [125,126] and references therein). Some of these causal factors contributed to the definite demise of the Sardinian mammoth populations, which we could speculate were few and composed of a limited number of individuals, perhaps close to the survival limit. Among other factors, we could consider the postglacial climatic improvement and the change in the vegetation cover, the dominant presence of more competitive herbivores, i.e., the large deer Praemegaceros cazioti, as well as the arrival of alien, more competitive competitors introduced by Neolithic pioneering populations, or even some specialized hunting human activity if some mammoth individuals survived until the time of the human pioneering population.
Finally, if, on the one hand, the scarcity of the mammoth remains in Sardinia and the rarity of alluvial and aeolian deposits in Corse might account for its apparent absence on the island, on the other hand, it is challenging to presume the reason for the absence of P. antiquus on the Corso-Sardinian Massif. Indeed, the straight-tusked elephant was widely spread and a common component of the late Middle and Late Pleistocene mammalian fauna of southern Europe, and particularly of Italy, while, at that time, the steppe mammoth was little documented.
7. Concluding Remarks
This critical overview of Sardinian mammoth remains and new studies provides some additional pieces of information and sheds some light on the problematic history of this endemic elephant. Available data concur to indicate M. trogontherii as the putative ancestor but do not specify the number of its possible arrivals on the island, the period of dispersal, or the source area because the dwarf mammoth is not currently reported in Corsica. Dispersal probably occurred one or more times during most of the Middle Pleistocene, i.e., more generally from 650 ka to 200 ka, or from 650 ka to 300 ka if the pioneer populations dispersed from southwestern Europe, while endemic mammoth populations inhabited Sardinia at least from MIS 6 to MIS 4-MIS 3 (Figure 15).
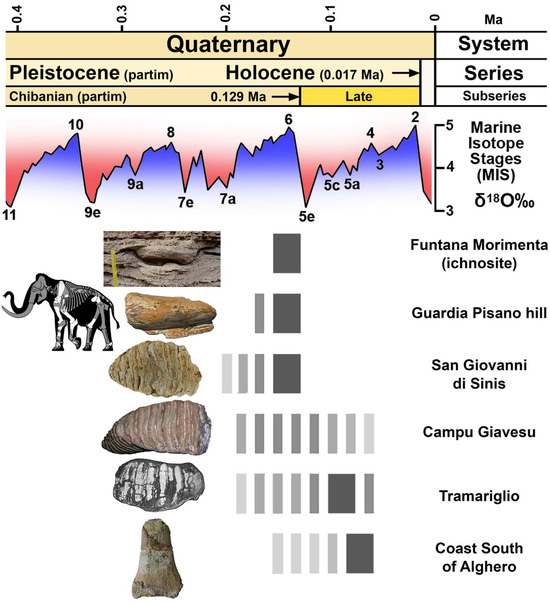
Figure 15.
Chronological range of the Middle and Late Pleistocene dwarf mammoth remains recorded in Sardinia. Dark grey = almost certain chronological range; light grey = uncertain chronological range.
The estimated adult weight of the GPH individual (between 750 and 950 kg) roughly equates to 9.5% of the mean adult male weight of M. trogontherii. Therefore, M. lamarmorai seems to have sensibly reduced its body mass in relation to its mainland ancestor, though we cannot know if the stature might be less reduced due to the apparently slender limbs of the Sardinian mammoth. Additionally, preliminary histological data indicate that the dwarf mammoth had some limb maneuverability, good locomotory aptitude, and perhaps a fast gait. However, these results are only indicative and need to be supported, at least, by the histological and morphofunctional analyses of the GPH limb bones.
Many shadows therefore still obscure the evolutionary history of the Sardinian mammoth with regard primarily to the relative chronology of the sites yielding its remains and the morpho-dimensional variability of the species. Based on available data, the mammoth was present in Sardinia from the late Middle to Late Pleistocene (maybe to MIS 3), while there is not any evidence of its persistence during the Last Glacial Maximum. The age of most sites is unknown, hypothetical, or debated (e.g., GPH, FM, CG, and Tramariglio). Therefore, the impossibility of knowing the temporal relationships between the populations to which the few known remains belong prevents us from evaluating the real meaning of the alleged dimensional differences shown by SGS and CG molars and hypothesizing whether the endemic mammoth underwent an anagenetic evolutionary process with a progressive size reduction, a radiative evolution, or, perhaps more likely, whether the differences fit the intra- and inter-population variability or not.
Although the results coming from further studies could give some information about the chronology of the known sites and some paleoecological items, such as mammoth feeding behavior and gait, they fail to have a convincing value given the scarcity of material on which they could currently be based.
As a result, without the acquisition of new material, lights and shadows will persist in the evolutionary history of the dwarf Sardinian mammoth, which remains one of the most intriguing insular proboscideans. Much more data are needed (e.g., improved dating results and spectrometric and isotopic methods of analysis, as well as new material providing sound chronostratigraphic data and permitting a detailed taphonomic analysis of the fossils to confirm the origin of the site formation) to obtain more precise chronological and palaeoecological interpretations of the Sardinian mammoth remains.
Author Contributions
M.R.P. conceived the manuscript, critically revised the mammoth Sardinian record, and wrote the manuscript with the contribution and assistance of M.Z. and D.Z.; D.Z. collected the historical data and information about the historical background and measured the long bones casts of the GPH skeleton stored at the MPAS; M.Z. executed the histological analyses and wrote the corresponding paragraph. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the academic editor and the anonymous reviewers for critically reading the manuscript and for their suggestions. We are grateful to acknowledge the editorial staff for the advice provided during the preparation of the manuscript.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
| CG | Campu Giavesu (Giave, SS) |
| FM | Funtana Morimenta spring (Gonnesa, SU) |
| GPH | Guardia Pisano Hill (Gonnesa, SU) |
| MPAS | Museo dei Paleoambienti Sulcitani—“E.A. Martel” (Carbonia) |
| MDLCA | Museo Sardo di Geologia e Paleontologia “D. Lovisato” (Cagliari) |
| NHMP | Natural History Museum of Pisa University (Calci, Pisa) |
| SGS | San Giovanni di Sinis (Cabras, OR) |
| HC.Dm | Haversian canal diameter |
| On.Dm | Osteon diameter |
References and Notes
- Christy, C. The African Elephant: Part II. J. R. Afr. Soc. 1922, 21, 187–198. [Google Scholar] [CrossRef]
- Sondaar, P.Y. Insularity and Its Effect on Mammal Evolution. In Major Patterns in Vertebrate Evolution; Hecht, M., Goody, P.L., Hecht, B.M., Eds.; Plenum Press: New York, NY, USA, 1977; pp. 671–707. [Google Scholar]
- Johnson, D.L. Problems in the land vertebrate zoogeography of certain islands and the swimming powers of elephants. J. Biogeogr. 1980, 7, 383–398. [Google Scholar] [CrossRef]
- Van Hoven, W.; Prins, R.A.; Lankhorst, A. Fermentative digestion in the African elephant. S. Afr. J. Wildl. Res. 1981, 11, 78–88. [Google Scholar]
- Plotnik, J.M.; Shaw, R.C.; Brubaker, D.L.; Tiller, L.N.; Clayton, N.S. Thinking with their trunks: Elephants use smell but not sound to locate food and exclude nonrewarding alternatives. Anim. Behav. 2014, 88, 91–98. [Google Scholar] [CrossRef]
- Palombo, M.R. How can endemic proboscideans help us understand the “island rule”? A case study of Mediterranean islands. Quat. Int. 2007, 169, 105–124. [Google Scholar] [CrossRef]
- Herridge, V.L. Dwarf Elephants on Mediterranean Islands: A Natural Experiment in Parallel Evolution. Ph.D. Thesis, London University College, London, UK, 2010; pp. 1–480. [Google Scholar]
- Van der Geer, A.; Lyras, G.; de Vos, J. History of Island. In Evolution of Island Mammals: Adaptation and Extinction of Placental Mammals on Islands; Wiley-Blackwell: Oxford, UK, 2021. [Google Scholar]
- Palombo, M.R.; Moncunill-Solé, B. Dwarfing and Gigantism in Quaternary Vertebrates. In Encyclopedia of Quaternary Science, 3rd ed.; Elias, S.A., Mock, C., Eds.; Elsevier: Oxford, UK, 2023; pp. 1–25. [Google Scholar] [CrossRef]
- Herridge, V.L.; Lister, A.M. Extreme insular dwarfism evolved in a mammoth. Proc. R. Soc. B 2012, 279, 3193–3200. [Google Scholar] [CrossRef] [PubMed]
- Rick, T.C.; Hofman, C.A.; Braje, T.J.; Maldonado, J.E.; Sillett, T.S.; Danchisko, K.; Erlandson, J.M. Flightless ducks, giant mice and pygmy mammoths: Late Quaternary extinctions on California’s Channel Islands. World Archaeol. 2012, 44, 3–20. [Google Scholar] [CrossRef]
- Palombo, M.R.; Zedda, M.; Melis, R.T. A new elephant fossil from the late Pleistocene of Alghero: The puzzling question of Sardinian dwarf elephants. C. R. Palevol 2017, 16, 841–849. [Google Scholar] [CrossRef]
- Agenbroad, L.D. Giants and pygmies: Mammoths of Santa Rosa island, California (USA). Quat. Int. 2012, 255, 2–8. [Google Scholar] [CrossRef]
- Athanassiou, A.; Van der Geer, A.A.; Lyras, G.A. Pleistocene insular Proboscidea of the Eastern Mediterranean: A review and update. Quat. Sci. Rev. 2019, 218, 306–321. [Google Scholar] [CrossRef]
- Palombo, M.R.; Antonioli, F.; Di Patti, C.; Valeria, L.P.; Scarborough, M.E. Was the dwarfed Palaeoloxodon from Favignana Island the last endemic Pleistocene elephant from the western Mediterranean islands? Hist. Biol. 2021, 33, 2116–2134. [Google Scholar] [CrossRef]
- Garutt, V.E.; Avenanov, A.O.; Vartanyan, S.L. On the systematic position of Holocene Dwarf Mammoths, Mommuthus primigenius (Blumenbach, 1799) from Wrangel Island (North East Siberia). Dokl. Akad. Nauk 1993, 332, 799–801. (In Russian) [Google Scholar] [PubMed]
- Vartanyan, S.L.; Garutt, V.E.; Sher, A.V. Holocene dwarf mammoths from Wrangel Island in the Siberian. Arct. Nat. 1993, 362, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Vartanyan, S.L.; Arslanov, K.A.; Karhu, J.A.; Possnert, G.; Sulerzhitsky, L.D. Collection of radiocarbon dates on the mammoths (Mammuthus primigenius) and other genera of Wrangel Island, northeast Siberia, Russia. Quat. Res. 2008, 70, 51–59. [Google Scholar] [CrossRef]
- Acconci, L. Sopra alcune ossa fossili di elefanti rinvenute nel Quaternario di Morimenta in Sardegna. Atti Soc. Sci. Nat. Processi Verbali 1881, 2, 266–267. [Google Scholar]
- Major, F.C.J. Die Tyrrhenis: Studien über geographische Verbreitung von Tieren und Pflanzen im westlich Mittelmeergebiet. Kosmos 1883, 13, 81–106. [Google Scholar]
- Palombo, M.R.; Ferretti, M.P.; Pillola, G.L.; Chiappini, L. A reappraisal of the dwarfed mammoth Mammuthus lamarmorai (Major, 1883) from Gonnesa (south-western Sardinia, Italy). Quat. Int. 2012, 255, 15–170. [Google Scholar] [CrossRef]
- Zoboli, D.; Pillola, G.L.; Palombo, M.R. The remains of Mammuthus lamarmorai (Major, 1883) housed in the Naturhistorisches Museum of Basel (Switzerland) and the complete “Skeleton-Puzzle”. Boll. Soc. Paleontol. Ital. 2018, 57, 45–57. [Google Scholar]
- Lister, A.M.; Sher, A.V.; van Essen, H.; Wei, G. The pattern and process of mammoth evolution in Eurasia. Quat. Int. 2005, 126, 49–64. [Google Scholar] [CrossRef]
- Aguirre, E. Revision Sistematica de los Elephantidae, por su morfologia y morfometria dentaria. Estud. Geol. 1968, 24, 109–167. [Google Scholar]
- Maglio, V.J. Origin and evolution of the Elephantidae. Trans. Am. Philos. Soc. 1973, 63, 1–149. [Google Scholar] [CrossRef]
- Lister, A.M. Sexual Dimorphism in the Mammoth Pelvis: An Aid to Gender Determination. In The Proboscidea; Shoshani, J., Tassy, P., Eds.; Oxford University Press: Oxford, UK, 1966; pp. 254–259. [Google Scholar]
- Roth, V.L. Insular Dwarf Elephants: A case Study in Body Mass Estimation and Ecological Inference. In Body Size in Mammalian Paleobiology: Estimation and Biological Implications; Damuth, J., MacFadden, B., Eds.; Cambridge University Press: Cambridge, UK, 1990; pp. 151–179. [Google Scholar]
- Christiansen, P. Body size in proboscideans, with notes on elephant metabolism. Zool. J. Linn. Soc. 2004, 140, 523–549. [Google Scholar] [CrossRef]
- Campione, N.E.; Evans, D.C. A universal scaling relationship between body mass and proximal limb bone dimensions in quadrupedal terrestrial tetrapods. BMC Biol. 2012, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- La Marmora, A. Voyage en Sardaigne. Troisième Partie. Description Géologique et Paléontologique; Delaforest: Paris, France, 1857; pp. 651–704. [Google Scholar]
- Comaschi Caria, I. L’elefante nano del Quaternario di Gonnesa (Sardegna sudoccidentale). Rend. Sem. Fac. Sci. Univ. Cagliari 1965, 35, 1–11. [Google Scholar]
- De Stefani, C. Cenni preliminari sui terreni cenozoici della Sardegna. Rend. Accad. Lincei 1891, 7, 464–467. [Google Scholar]
- Caterini, F. Catalogo dei proboscidiani Pliocenici e Quaternari conservati nel Museo di Geologia dell’Università di Pisa. Mem. Soc. Toscana Sci. Nat. 1923, 35, 195–210. [Google Scholar]
- Vaufrey, R. Les éléphants nains des ȋles méditerranéennes et la question des isthmes Pléistocènes. Arch. L’Inst. Paléontol. Hum. Mémoire 1929, 6, 1–220. [Google Scholar]
- Joleaud, L. Le peuplement de la Corse: Les mammifères. Bull. Soc. Sci. Hist. Nat. Corse 1926, 45, 35–107. [Google Scholar]
- Malatesta, A. Primo dente di elefante fossile rinvenuto in Sardegna. Quaternaria 1954, 1, 97–105. [Google Scholar]
- Ambrosetti, P. L’elefante fossile della Sardegna. Boll. Soc. Geol. Ital. 1972, 91, 127–131. [Google Scholar]
- Osborn, H.F. Proboscidea. A monograph of the discovery, evolution, migration and extinction of the mastodonts and elephants of the world. Vol. II. Stegodontoidea, Elephantoidea. Am. Mus. Press 1942, 27, 805–1676. [Google Scholar]
- Melis, R.T.; Palombo, M.R.; Mussi, M. Mammuthus lamarmorae (Major, 1883) Remains in the Pre-Tyrrhenian Deposits of S. Giovanni di Sinis (Western Sardinia, Italy). In The World of Elephants; Cavarretta, G., Gioia, P., Mussi, M., Palombo, M.R., Eds.; CNR: Rome, Italy, 2001; pp. 481–485. [Google Scholar]
- Palombo, M.R.; Ginesu, S.; Sias, S. Mammuthus lamarmorai (Major, 1883) Remains in the Middle Pleistocene Alluvial Deposits of Campu Giavesu Plain (North-Western Sardinia; Italy). In AAVV, Occasional Paper in Earth Science, Abstracts, Proceedings of the 3rd International Mammoth Conference, Dawson City, YT, Canada, 25–29 May 2003; Paleontology Program, Government of Yukon: Whitehorse, YT, Canada, 2003; pp. 122–125. [Google Scholar]
- Palombo, M.R. Biochronology of the Plio-Pleistocene terrestrial mammals of Sardinia: The state of the art. Hell. J. Geosci. 2006, 41, 47–66. [Google Scholar]
- Maxia, C.; Pecorini, G. Il Quaternario della Sardegna. In Proceedings of the Atti del X Congresso Internazionale di Studi Sardi–Simposio sul Quaternario della Sardegna (1968), Cagliari, Italy, 29 December 1966–6 January 1967; pp. 59–69. [Google Scholar]
- Palombo, M.R.; Ginesu, S.; Melis, R.T.; Sias, S. The endemic elephants from Sardinia: An unsolved problem. Monogr. Soc. Hist. Nat. Balear. 2005, 12, 245–254. [Google Scholar]
- Pillola, G.L.; Zoboli, D. Dwarf mammoth footprints from the Pleistocene of Gonnesa (southwestern Sardinia, Italy). Boll. Soc. Paleontol. Ital. 2017, 56, 57–64. [Google Scholar]
- Pillola, G.L.; Palombo, M.R.; Panarello, A.; Zoboli, D. Tetrapod ichnology in Italy: The state of the art. J. Mediterr. Earth Sci. 2020, 12, 193–212. [Google Scholar]
- Zoboli, D.; Pillola, G.L. New evidences of mammal tracks from the Pleistocene of Gonnesa area (southwestern Sardinia, Italy). J. Mediterr. Earth Sci. 2018, 10, 173–175. [Google Scholar]
- Zoboli, D.; Pillola, G.L. The Funtana Morimenta ichnosite (Sardinia, Italy): A potential geotourist attraction. Geoheritage 2021, 13, 30. [Google Scholar] [CrossRef]
- Zoboli, D. The rich palaeontological heritage of SW Sardinia (Italy), a possible resource for geotourism development. Geoheritage 2023, 15, 41. [Google Scholar] [CrossRef]
- Orrù, P.; Ulzega, A. Geomorfologia costiera e sottomarina della Baia di Funtanamare (Sardegna sud-occidentale). Geogr. Fis. Din. Quat. 1986, 9, 56–67. [Google Scholar]
- Melis, R.; Palombo, M.R.; Mussi, M. The stratigraphic sequence of Gonnesa (SW Sardinia): Palaeoenvironmental, palaeontological and archaeological evidence. BAR Int. Ser. 2002, 1095, 445–453. [Google Scholar]
- Palombo, M.R.; Villa, P. Schreger Lines as Support in the Elephantinae Identification. In The World of Elephants; Cavaretta, G., Gioia, P., Mussi, M., Palombo, M.R., Eds.; Consiglio Nazionale Ricerche: Rome, Italy, 2001; pp. 656–660. [Google Scholar]
- Palombo, M.R.; Villa, P. L’utilità Dell’analisi delle Linee di Schreger nello Studio dei Proboscidati. Atti 3° Conv. Intern. Archeozoologia. In Atti 3° Convegno Nazionale di Archeozoologia (Siracusa, 2000) Collana Boll. Paleontol. It.; Fiore, I., Malerba, G., Chilardi, S., Eds.; Studi di Paleontologia: Rome, Italy, 2007; pp. 35–44. [Google Scholar]
- Scarborough, M.E. Insular Adaptations in the Appendicular Skeleton of Sicilian and Maltese Dwarf Elephants. Unpublished Ph.D. Dissertation, University of Cape Town, Cape Town, South Africa, 2020; 232p. [Google Scholar]
- Agenbroad, L.D. Channel Island (USA) Pygmy Mammoths (Mammuthus exilis) Compared and Contrasted with M. columbi, Their Continental Stock. In The World of Elephants; Cavaretta, G., Gioia, P., Mussi, M., Palombo, M.R., Eds.; Consiglio Nazionale Richerche: Rome, Italy, 2001; pp. 473–475. [Google Scholar]
- Palombo, M.R. Elephas? Mammuthus? Loxodonta? The question of the true ancestor of the smallest dwarfed elephant of Sicily. Deinsea 2003, 9, 273–291. [Google Scholar]
- Larramendi, A. Skeleton of a Late Pleistocene steppe mammoth (Mammuthus trogontherii) from Zhalainuoer, Inner Mongolian Autonomous Region, China. Paläontol. Z. 2015, 89, 229–250. [Google Scholar] [CrossRef]
- Larramendi, A. Shoulder height, body mass, and shape proboscideans. Acta Palaeontol. Pol. 2016, 61, 537–574. [Google Scholar] [CrossRef]
- Romano, M.; Manucci, F.; Antonelli, M.; Rossi, M.A.; Agostini, S.; Palombo, M.R. In vivo restoration and volumetric body mass estimate of Mammuthus meridionalis from Madonna della Strada (Scoppito, Aquila). Riv. Ital. Paleontol. Stratigr. 2022, 128, 559–573. [Google Scholar]
- Palombo, M.R.; Giovinazzo, C. Elephas falconeri from Spinagallo Cave (South-Eastern Sicily, Hyblean Plateau, Siracusa): A preliminary report on brain to body weight comparison. Monogr. Soc. Hist. Nat. Balear. 2005, 12, 255–264. [Google Scholar]
- Palombo, M.R.; Ibba, A.; Fanelli, F. Porto Conte Bay. In Fossil Mammalian Biotas of Sardinia Fieldtrip Guide-Book; Palombo, M.R., Pillola, G.L., Kotsakis, T., Eds.; EuroMam; PubliedilService, Pirri (CA): Cagliari, Italy, 2008; pp. 47–57. [Google Scholar]
- Roth, V.L. Dwarfism and Variability in the Santa Rosa Island Mammoth: An Interspecific Comparison of Limb Bone Sizes and Shapes in Elephants. In Recent Advances in Research on the California Islands; Hochberg, F.G., Ed.; 3rd California Islands Symp.; Santa Barbara Museum of Natural History: Santa Barbara, CA, USA, 1993; pp. 433–442. [Google Scholar]
- Carboni, S.; Lecca, L.; Hillaire-Marcel, C.; Ghaleb, B. MIS 5e at San Giovanni di Sinis (Sardinia, Italy): Stratigraphy, U/Th dating and “eustatic” inferences. Quat. Int. 2014, 328, 21–30. [Google Scholar] [CrossRef]
- Chesi, F.; Delfino, M.; Abbazzi, L.; Carboni, S.; Lecca, L.; Rook, L. New Fossil Vertebrate remains from San Giovanni di Sinis (Late Pleistocene, Sardinia): The last Mauremys (Reptilia, Testudines) in the Central Mediterranean. Riv. Ital. Paleontol. Stratigr. 2007, 113, 287–297. [Google Scholar]
- Lecca, L.; Carboni, S. The Tyrrhenian section of San Giovanni di Sinis (Sardinia): Stratigraphic record, of an irregular single high stand. Riv. Ital. Paleontol. Stratigr. 2007, 113, 509–523. [Google Scholar]
- Cocco, F.; Andreucci, S.; Sechi, D.; Cossu, G.A. Upper Pleistocene tectonics in western Sardinia (Italy): Insights from the Sinis peninsula structural high. Terra Nova 2019, 31, 485–493. [Google Scholar] [CrossRef]
- Sher, A.V.; Garutt, V.E. New data on the morphology of elephant molars. Trans. USSR Acad. Sci. Earth Sci. Sect. 1987, 285, 195–199. [Google Scholar]
- Agenbroad, L.D. New absolute dates and comparisons for California’s Mammuthus exilis. Deinsea 2003, 9, 1–16. [Google Scholar]
- Palombo, M.R.; Ferretti, M.P. Elephant fossil record from Italy: Knowledge, problems, and perspectives. Quat. Int. 2005, 126–128, 107–136. [Google Scholar] [CrossRef]
- Ferretti, M.P. Mammuthus meridionalis (Mammalia, Proboscidea, Elephantidae) from the ‘‘Sabbie Gialle’’ of Oriolo (Cava La Salita, Faenza, Northern Italy) and other European late populations of southern mammoth. Eclogae Geol. Helv. 1999, 92, 503–515. [Google Scholar]
- Maccagno, A.M. L’Elephas meridionalis Nesti di Contrada “Madonna della Strada” Scoppito (L’Aquila). Atti Accad. Sci. Fis. E Mat. Napoli Mem. 1962, 4, 1–132. [Google Scholar]
- van Essen, J.A. Tracing Transitions: An Overview of the Evolution and Migrations of the Genus Mammuthus Brookes, 1828 (Mammalia, Proboscidea). Ph.D. Dissertation, Leiden University, Leiden, The Netherlands, 2011. [Google Scholar]
- Konidaris, G.E.; Kostopoulos, D.S.; Koufos, G.D. Mammuthus meridionalis (Nesti, 1825) from Apollonia-1 (Mygdonia Basin, Northern Greece) and its importance within the Early Pleistocene mammoth evolution in Europe. Geodiversitas 2020, 42, 69–91. [Google Scholar] [CrossRef]
- Albayrak, E.; Lister, A.M. Dental remains of fossil elephants from Turkey. Quat. Int. 2012, 276, 198–211. [Google Scholar] [CrossRef]
- Agostini, S.; Palombo, M.R.; Rossi, M.A.; Di Canzio, E.; Tallini, M. Mammuthus meridionalis (Nesti, 1825) from Campo di Pile (L’Aquila, Abruzzo, Central Italy). Quat. Int. 2012, 276, 42–52. [Google Scholar] [CrossRef]
- Coppens, Y.; Beden, M.; Piveteau, J. Mammuthus meridionalis depereti n. subsp. (mammalia, elephantidae): Nouveau mammouth du Pléistocene inférieur d’Europe occidentale. C. R. Acad. Sci. 1982, 294, 291–294. [Google Scholar]
- Dubrovo, I.A.; Palombo, M.R. Personal unpublished data collected in the last decade of the 20th century by Dubrovo, I.A. and Palombo, M.R. during their collaboration founded by CNR and Russian Academy of Sciences.
- Lister, A.M.; Dimitrijević, V.; Marković, Z.; Knežević, S.; Mol, D. A skeleton of ‘steppe’ mammoth (Mammuthus trogontherii (Pohlig)) from Drmno, near Kostolac, Serbia. Quat. Int. 2012, 276, 129–144. [Google Scholar] [CrossRef]
- Dubrovo, I.A. A history of elephants of the Archidiskodon-Mammuthus phylogenetic line on the territory of the USSR. J. Palaeontol. Soc. India 1977, 20, 33–40. [Google Scholar]
- Lister, A.M.; Stuart, A.J. The West Runton mammoth (Mammuthus trogontherii) and its evolutionary significance. Quat. Int. 2010, 228, 180–209. [Google Scholar] [CrossRef]
- Athanassiou, A. A skeleton of Mammuthus trogontherii (Proboscidea, Elephantidae) from NW Peloponnese, Greece. Quat. Int. 2012, 255, 9–28. [Google Scholar] [CrossRef]
- Baygusheva, V.S.; Titov, V.V.; Timonina, G.I. Two skeletons of Mammuthus trogontherii from the Sea of Azov Region. Quat. Int. 2012, 276, 242–252. [Google Scholar] [CrossRef]
- Pawłowska, K.; Greenfield, H.; Czubla, P. Steppe’mammoth (Mammuthus trogontherii) remains in their geological and cultural context from Bełchatów (Poland): A consideration of human exploitation in the Middle Pleistocene. Quat. Int. 2014, 326, 448–468. [Google Scholar] [CrossRef]
- Lenardić, J. Description and morphometric analysis of the Mammuthus armeniacus (Falconer) skull from Slavonski Brod (Croatia). Geol. Croat. 1994, 47, 157–166. [Google Scholar]
- Garutt, V.E.; Foronova, A.V. Issledovanie zubov vmershih slonov (metodicheskie rekomendacii). Akacl. Nauk SSSR, ibirskoe otdelenie, lnstitut geologii i geofiziki press. Novosibirsk 1976, 35, 1–93. [Google Scholar]
- Beccaluva, L.; Deriu, M.; Macciotta, G.P.; Savelli, C. Carta Geopetrografica del Vulcanismo Plio-Pleistocenico della Sardegna nordoccidentale, Scala 1:50.000; L.A.C. Firenze: Florence, Italy, 1981. [Google Scholar]
- Malatesta, A. Cynotherium sardous Studiati, an extinct canid from the Pleistocene of Sardinia. Mem. Ist. Ital. Paleontol. Um. 1970, 1, 1–72. [Google Scholar]
- Lister, A.M.; Ioysey, K.A. Scaling Effects in Elephant Dental Evolution—The Example of Eurasian Mammuthus. In Structure, Function and Evolution of Teeth; Smith, P., Tchernov, E., Eds.; Freund Publishing House Ltd.: Jerusalem, Israel; London, UK; Tel Aviv, Israel, 1992; pp. 185–213. [Google Scholar]
- Andreucci, S.; Clemmensen, L.B.; Murray, A.S.; Pascucci, V. Middle to late Pleistocene coastal deposits of Alghero, Northwest Sardinia (Italy): Chronology and evolution. Quat. Int. 2012, 222, 3–16. [Google Scholar] [CrossRef]
- Sechi, D. Reconstruction of the Sardinia the North-West Coast Evolution: A Chronologic and Sedimentologic Approach. Ph.D. Dissertation, University of Sassari, Sassari, Italy, 2013. [Google Scholar]
- Ferretti, M.P. The dwarf elephant Palaeoloxodon mnaidriensis from Puntali Cave, Carini (Sicily; late middle Pleistocene): Anatomy, systematics and phylogenetic relationships. Quat. Int. 2008, 182, 90–108. [Google Scholar] [CrossRef]
- Theodorou, G.E. The Dwarf Elephants of the Charkadio Cave on the Island of Tilos (Dodekanese, Greece). Ph.D. Dissertation, University of Athens, Athens, Greece, 1983. [Google Scholar]
- Theodorou, G.; Symeonidis, N.; Stathopoulou, E. Elephas tiliensis n. sp. from Tilos island (Dodecanese, Greece). Hell. J. Geosci. 2007, 42, 19–32. [Google Scholar]
- Palombo, M.R.; Zedda, M. New evidence for the presence of endemic elephants from the late Pleistocene of Alghero (Nortwestern Sardinia, Italy). Alp. Mediterr. Quat. 2020, 33, 107–114. [Google Scholar]
- Skedros, J.G.; Knight, A.N.; Clark, G.C.; Crowder, C.M.; Dominguez, V.M.; Qiu, S.; Mulhern, D.M.; Donahue, S.W.; Busse, B.; Hulsey, B.I.; et al. Scaling of Haversian canal surface area to secondary osteon bone volume in ribs and limb bones. Am. J. Phys. Anthropol. 2013, 151, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Giua, S.; Farina, V.; Cacchioli, A.; Ravanetti, F.; Carcupino, M.; Novas, M.M.; Zedda, M. Comparative histology of the femur between mouflon (Ovis aries musimon) and sheep (Ovis aries aries). J. Biol. Res. 2014, 87, 74–77. [Google Scholar] [CrossRef]
- Zedda, M.; Brits, D.; Giua, S.; Farina, V. Distinguishing domestic pig femora and tibiae from wild boar through microscopic analyses. Zoomorphology 2019, 138, 159–170. [Google Scholar] [CrossRef]
- Babosová, R.; Zedda, M.; Belica, A.; Golej, M.; Chovancová, G.; Kalaš, M.; Vondráková, M. The enrichment of knowledge about the microstructure of brown bear compact bone tissue. Eur. Zool. J. 2022, 89, 615–624. [Google Scholar] [CrossRef]
- Kolb, C.; Scheyer, T.M.; Veitschegger, K.; Forasiepi, A.M.; Amson, E.; Van deer Geer, A.A.E.; Van den Hoek Ostende, L.W.; Hayashi, S.; Sánchez-Villagra, M.R. Mammalian bone palaeohistology: A survey and new data with emphasis on island forms. PeerJ 2015, 10, e1358. [Google Scholar] [CrossRef] [PubMed]
- Zedda, M.; Sathé, V.; Chakraborty, P.; Palombo, M.R.; Farina, V. A first comparison of bone histomorphometry in extant domestic horses (Equus caballus) and a Pleistocene Indian wild horse (Equus namadicus). Integr. Zool. 2020, 15, 448–460. [Google Scholar] [CrossRef]
- Palombo, M.R.; Zedda, M. The intriguing giant deer from the Bate cave (Crete): Could paleohistological evidence question its taxonomy and nomenclature? Integr. Zool. 2022, 17, 54–77. [Google Scholar] [CrossRef]
- Ezra, H.C.; Cook, S.F. Histology of Mammoth Bone. Science 1959, 20, 465–466. [Google Scholar] [CrossRef]
- Curtin, A.J.; Macdowell, A.A.; Schaible, E.G.; Roth, V.L. Noninvasive histological comparison of bone growth patterns among fossil and extant neonatal elephantids using synchrotron radiation X-ray microtomography. J. Vertebr. Paleontol. 2012, 32, 939–955. [Google Scholar] [CrossRef]
- Rogoz, A.; Sawlowicz, Z.; Wojtal, P. Diagenetic history of wolly mammoth (Mammuthus primigenius) skeletal remains from the archeological site Cracow Spadzista street B, Southern Poland. Palaios 2012, 27, 541–549. [Google Scholar] [CrossRef]
- Papageorgopoulou, C.; Link, K.; Rühli, F.J. Histology of a Woolly Mammoth (Mammuthus primigenius) Preserved in Permafrost, Yamal Peninsula, Northwest Siberia. Anat. Rec. 2015, 298, 1059–1071. [Google Scholar] [CrossRef]
- Köhler, M.; Herridge, V.; Nacarino-Meneses, C.; Fortuny, J.; Moncunill-Solé, B.; Rosso, A.; Sanfilippo, R.; Palombo, M.R.; Moyà-Solà, S. Palaeohistology reveals a slow pace of life for the dwarfed Sicilian elephant. Sci. Rep. 2021, 11, 22862. [Google Scholar] [CrossRef]
- Basilia, P.; Miszkiewicz, J.; Nganvongpanit, K.; Zaim, J.; Rizal, Y.; Aswan, A.; Puspaningrum, M.R.; Trihascaryo, A.; Price, G.J.; van der Geer, A.A.E.; et al. Bone histology in a fossil elephant (Elephas maximus) from Pulau Bangka, Indonesia. Hist. Biol. 2022, 35, 1356–1367. [Google Scholar] [CrossRef]
- Zedda, M. The Arrangement of the Osteons and Kepler’s Conjecture. Appl. Sci. 2023, 13, 5170. [Google Scholar] [CrossRef]
- Cook, S.F.; Brooks, S.T.; Ezra-Cohn, H.E. Histological studies on fossil bones. J. Paleontol. 1962, 36, 483–494. [Google Scholar]
- Nganvongpanit, K.; Siengdee, P.; Buddhachat, K.; Brown, J.L.; Klinhom, S.; Pitakarnnop, T.; Angkawanish, T.; Thitaram, C. Anatomy, histology and elemental profile of long bones and ribs of the Asian elephant (Elephas maximus). Anat. Sci. Int. 2017, 92, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Zedda, M.; Babosovà, R. Does the osteon morphology depend on the body mass? A scaling study on macroscopic and histomorphometric differences between cow (Bos taurus) and sheep (Ovis aries). Zoomorphology 2021, 140, 169–181. [Google Scholar] [CrossRef]
- Pfeiffer, S.; Crowder, C.; Harrington, L.; Brown, M. Secondary osteons and Haversian canals dimensions as behavioral indicators. Am. J. Phys. Anthropol. 2006, 131, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Felder, A.A.; Phillips, C.; Cornish, H.; Cooke, M.; Hutchinson, J.R.; Doube, M. Secondary osteons scale allometrically in mammalian humerus and femur. R. Soc. Open Sci. 2017, 4, 170431. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Janis, C.M.; Goodall, R.H.; Purnell, M.A. An examination of feeding ecology in Pleistocene proboscideans from southern China (Sinomastodon, Stegodon, Elephas), by means of dental microwear texture analysis. Quat. Int. 2017, 445, 60–70. [Google Scholar] [CrossRef]
- Mainland, I.L. Dental microwear in grazing and browsing Gotland sheep (Ovis aries) and its implications for dietary reconstruction. J. Archaeol. Sci. 2003, 30, 1513–1527. [Google Scholar] [CrossRef]
- Palombo, M.R.; Albayrak, E.; Marano, F. The Straight-Tusked Elephants from Neumark-Nord, a Glance into a Lost World. In Elefantenreich. Eine Fossilwelt in Europa. Landesamt für Denkmalpflege und Archaologie Sachsen; Meller, H., Ed.; Anhalt Landesmuseum für Vorgeschichte: Halle (Saale), Germany, 2010; pp. 218–251. [Google Scholar]
- Fortelius, M.; Solounias, N. Functional characterization of ungulate molars using the abrasion-attrition wear gradient: A new method for reconstructing paleodiets. Am. Mus. Novit. 2000, 3301, 1–36. [Google Scholar] [CrossRef]
- Bocherens, H.; Drucker, D.G. Carbonate Stable Isotopes. Terr. Teeth Bones 2013, 10, 30–44. [Google Scholar]
- Sponheimer, M.; Lee-Thorp, J.A. Oxygen isotopes in enamel carbonate and their ecological significance. J. Archaeol. Sci. 1999, 26, 723–728. [Google Scholar] [CrossRef]
- Van Essen, H. Tooth morphology of Mammuthus meridionalis from the southern bight of the North Sea and from several localities in the Netherlands. Deinsea 2003, 9, 453–511. [Google Scholar]
- Palombo, M.R.; Milli, S. Mammalian fossil record, depositional setting, and sequence stratigraphy in the Middle and Upper Pleistocene of Roman basin. Quat. Ital. J. Quat. Sci. 2011, 23, 243–248. [Google Scholar]
- Palombo, M.R.; Mussi, M.; Agostini, S.; Barbieri, M.; Di Canzio, E.; Di Rita, F.; Fiore, I.; Iacumin, P.; Magri, D.; Speranza, F.; et al. Human peopling of Italian intramontane basins: The early Middle Pleistocene site of Pagliare di Sassa (L’Aquila, central Italy). Quat. Int. 2010, 223, 170–178. [Google Scholar] [CrossRef]
- Bonifay, M.F. Les Praemegaceros du Pléistocène moyen de la Grotte de ’’Escale à Saint-Estève-Janson (Bouches-du-Rhône). Leur intérêt dans le contexte biostratigraphique européen. Bull. Assoc. Franç. Etude Quat. 1981, 18, 109–120. [Google Scholar]
- Álvarez-Posada, C.; Parés, J.M.; Sala, R.; Viseras, C.; Pla-Pueyo, S. New magnetostratigraphic evidence for the age of Acheulean tools at the archaeo-palaeontological site “Solana del Zamborino” (Guadix–Baza Basin, S Spain). Sci. Rep. 2017, 7, 13495. [Google Scholar] [CrossRef] [PubMed]
- Kouvari, M.; van der Geer, A.A. Biogeography of extinction: The demise of insular mammals from the Late Pleistocene till today. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 505, 295–304. [Google Scholar] [CrossRef]
- Kouvari, M.; van der Geer, A.A. Corrigendum to “Biogeography of Extinction: The demise of insular mammals from the Late Pleistocene till today”[PALAEO 505 (15 September 2018) pages 295–304]. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 518, 232–233. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).