Abstract
In western Europe, the Middle Pleistocene is marked by Acheulean settlement and their diversification after the MIS 12. The Arago cave recovery of numerous human settlements correlate to MIS 14, 13 and MIS 12 making it an important site for the understanding of the Lower Palaeolithic in Southwestern Europe. It is also an important site for the understanding of palaeoenvironments and palaeobiodiversity as it has yielded rich faunal associations. The faunal associations allow us to observe three climatic stages within this study: two cold ones and a mild one. Small vertebrates, with their abundance and their diversity, are particularly useful for observing these periods, which historically have been correlated to glacial or interglacial stages. If the first cold phase, dated 438 ± 31 ka, is correlated to the Marine Isotopic Stage 12 (MIS 12), the correlation of the following phases to isotopic stages can be discussed. They may correspond to climatic variations of the MIS 12. Indeed, the latest studies about palaeoclimatic reconstitution which allow us to define the evolution of the palaeo-temperature show that these differences are relatively small. Therefore, instead of a correlation to MIS 12, 13 and 14, the medium complex of the Arago cave could belong solely to MIS 12. The correlation of these environmental changes to other global data, notably the isotopic curve, is challenging because there are various local factors influencing faunal association. We propose here both hypotheses and discuss the various factors which influence the distribution and the representation of the small vertebrate species present on the site.
1. Introduction
Climate dynamics during the Quaternary are well documented by extra-site proxies such as deep-sea cores or ice cores, providing a clear view of climatic oscillations during this period [1,2]. These climate changes have had an important impact on human populations in Europe, modifying their environment, their ecosystems and also their resource distributions [3]. Whereas continental-scale climate change is well understood, it is the local environment that directly affects human groups by altering their ecosystem and resource availability. However, the correlation between the local environment and global changes can be delicate due to the particularities of the local context, this context is needed to analyse environmental responses during climatic oscillations.
Small mammals serve as reliable indicators due to their abundance and diversity in archaeological sites, their limited migration, and their ability to establish stable populations in small areas, acting as climatic refuges allowing a local scale approach to studying environmental variations [4,5].
Based on this background, the present study uses several methods to characterize different climatic variations and tries to incorporate these into a more global context. To this end, we use different methods to carry out tests at the Arago cave. This cave provides a good foundation for palaeoenvironmental reconstructions of the Middle Pleistocene in the western Mediterranean area. Alternations of cold and temperate periods are traceable, and the numerous layers are correlated to MIS 14, 13 and 12 [6]. This site has a good small vertebrates record that allows us to follow these climatic changes. The location of the site is also important because it lies within the climatic influence of the Pyrenees and the Mediterranean Sea, which may have a significant impact on local environments.
The Arago cave is a key site for understanding the Acheulean occupations in southern Europe because it has provided recurrent occupation layers with Acheulean industry from MIS 14 to MIS 12 [7,8,9]. This period is important for an appreciation of the diversity of the early Acheulean, as the end of the MIS 12 glaciation marks a threshold after which the archaeological records show an increasing number of occupations, evidence of new subsistence behaviours, and numerous technical innovations [10]. Furthermore, early Acheulean sites witness occasional occupations [11,12,13,14,15]. The recurrence of occupations in the Arago cave allows us to observe technical developments during phases of climatic variation in a similar geographical context. Understanding the environment that enabled human settlement to continue during phases of climatic transition is key to studying the diversity and evolution of human settlements in southwestern Europe.
2. Site Presentation
The Arago cave is a vast karstic cavity located in the valley of Tautavel, in southern France (Figure 1a). The site was occupied several times by groups of hominins during different climatic phases. These phases have been determined through variations in the abundance of faunal species and pollen. The main sedimentary sequence (the Middle Complex) is subdivided into three ensembles, comprising stratigraphic units (SU) ranging from D to R, the last excavated layer (Figure 1b). Ensemble I (SU K to Q) is correlated to MIS 14, with SU P dated to 532 ± 106 ka [16]. In this ensemble, Rangifer tarandus is predominant, followed by Ovis ammon antiqua and Cervus elaphus [17]. Ensemble II (SU H, I and J) is correlated to a temperate phase (MIS 13) mainly on the basis of changes in the representation of the faunal community. Indeed, Cervus elaphus is the predominant species, along with Dama cf. clactoniana. It is noteworthy that Rangifer tarandus and Praeovibos priscus are still present [17,18,19]. Ensemble III (SU D to G) is correlated to MIS 12, with SU F dated to 392 ± 43 ka and SU G to 438 ± 31 ka [16]. The predominant species for this ensemble are Ovis ammon antiqua and Equus ferus mosbachensis [17]. The filling of the Middle Complex of the Arago cave yielded many Acheulean occupations that are among the oldest in Europe, as well as many human remains (Homo erectus tautavelensis) [20].
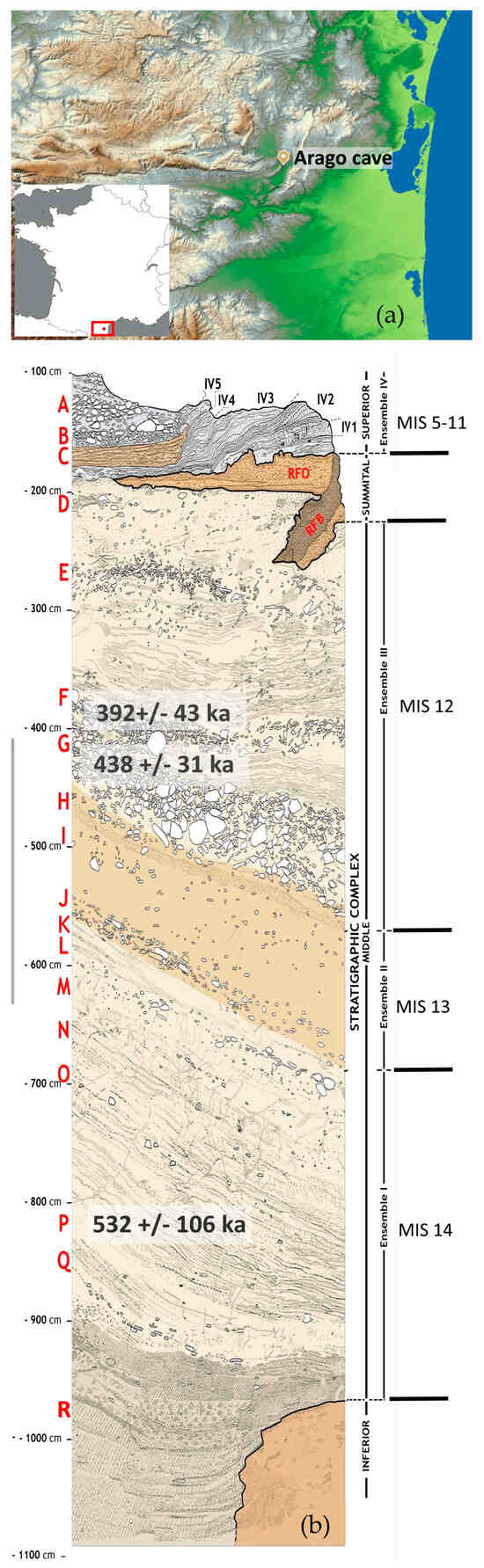
Figure 1.
(a) Location of the Arago cave. (b) Synthetic stratigraphic column of the Arago cave [21], dating and correlation with the MIS come from [16].
Small vertebrates are abundant in the site. These have been recovered by systematic sieving through 1.2 mm and 0.6 mm mesh. The remains have shown great diversity and highlighted different climatic phases based on variations in the abundance of different species [21,22,23,24,25,26,27,28,29,30,31].
3. Materials and Methods
The material used for this study has been previously published in several papers [21,24,25,27,28,29].
Multivariate analysis makes it possible to use the abundance of each taxon (MNI) by archaeological level to highlight possible associations between rodent species and different archaeological levels. This method enables us to observe associations between levels without attributing any ecological value to the species, dispensing with the principle of actualism. Multivariate analyses are often used to characterize associations of micromammals according to their ecological and climatic affinities [21,24,25,27,32,33,34]. Correspondence analyses (CA) were carried out using R 3.1.2 software (with the Rcmdr package), with rodent data from Table 1. In order to have a better vision of the grouping between levels, we apply a cluster analysis (UPGMA, Euclid distance) based on the data from Axes 1–3 of the CA with consideration of the stratigraphic position of the layers. This analysis is carried out using Past 4.06 software.

Table 1.
Minimum number of individuals (MNI) of the small-mammal species for the different stratigraphic units of the Arago cave. Data come from [21,27,28,29].
To obtain palaeoclimatic parameters, we used the bioclimatic model (BM) and the quantified ecology method (QEM).
The bioclimatic model, first developed by Hernández-Fernández [35] and further developed in subsequent papers [36,37,38,39], is based on the assumption that there is a correlation between climate and mammalian communities. This method (using R software, version 3.1.2) allows for an initial approach to climatic parameters, in particular temperature (mean annual temperature, mean temperature of the hottest month, and mean temperature of the coldest month) and precipitation (mean annual precipitation).
The values were adapted for some species. One case was Marmota sp. and as there was no single value for the genus Marmota, we used the data for Marmota baibacina, which is the most ubiquitous species. For Microtus vaufreyi, the ecological values of Microtus atapuerquensis were attributed to it. For the genus Pliomys, the values assigned to subtropical environments were not considered. The genus Hystrix was not considered.
The quantified ecology method developed by Jeannet [40] assigns quantitative climatic values (average annual temperature, minimum annual temperature, etc.) to each species according to its geographical area. One of the limitations of this method is that it only considers current species. For some species, ecological data from related species or their descendants can be used. Ecological data from Microtus cabrerae are used for M. (Iberomys) mediterraneus, and data from Cricetulus migratorius for Allocricetus bursae. However, this method is based on a large database, and a comparison with the values obtained by the other methods seems interesting.
To compare our local data with global variations we rely on the ‘Oscillayer’ data. This dataset builds upon interpolated anomalies (Δ layers) between bioclim layers of the present and the Last Glacial Maximum (LGM) that are scaled relative to the Plio-Pleistocene global mean temperature curve, derived from benthic stable oxygen isotope ratios [41]. These data allow us to display the evolution of the climate variables in a global way at 10 ka intervals. In this case, we are interested in the variations in the mean annual temperature. These data were processed using QGIS software (version 3.28.0).
4. Results
4.1. Correspondence Analysis
The correspondence analysis (CA) (Figure 2) sets on axis 1 (50.3% inertia) the species with affinities to open environments in cold and dry climates (Alexandromys oeconomus, Lasiopodomys gregalis, Chionomys nivalis) against the species associated with closed environments in temperate climates (Microtus (Iberomys) brecciensis, Pliomys episcopalis, Glis glis, Apodemus sp.). The species contributing most to this axis are L. gregalis (33.5%) and A. oeconomus (5.9%) in the negative part, and M. (I.) brecciensis (33.5%) and Apodemus sp. (11%) in the positive part. However, we can see that it is more of a general trend and some taxa like Dicrostonyx sp., which has an affinity for cold environments, are seen in the positive part of the axis. This is related to the fact that other variables have an influence on the grouping of species, as suggested by the 50% inertia of axis 1. Axis 2 (23.2% inertia) is more difficult to interpret because there does not seem to be a link between the different species that are divided by it.
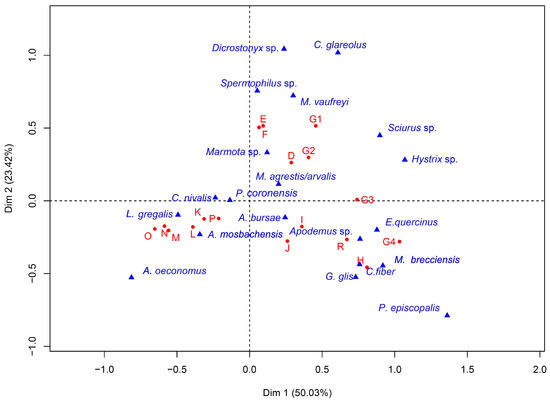
Figure 2.
Correspondence analysis (CA) between rodent species and layers of the Arago cave. Axis 1 represents 50.3% inertia and axis 2 represents 23.2% inertia.
On this factorial level, the clustering of the major stratigraphic units can be distinguished, in much the same way as in the previous study [27]. Nevertheless, the subdivisions of SU G reveal a convergence of SU G3 and G4 with SU H, I and J of Ensemble II. The same is true for SU R, which joins the levels of Ensemble II attributed to a temperate episode. SU G1 and G2 are grouped with the other stratigraphic units of Ensemble III. The dendrogram produced via the cluster analysis gives us a better view of the groupings of levels. (Figure 3). We can observe a grouping that corresponds to the stratigraphic ensemble except for the clustering of SU G3 and G4 with the other layers of Ensemble II. This leads us to assume that the climatic transition between Ensemble II and Ensemble III occurs between SU G2 and G3 and not between SU G4 and H.
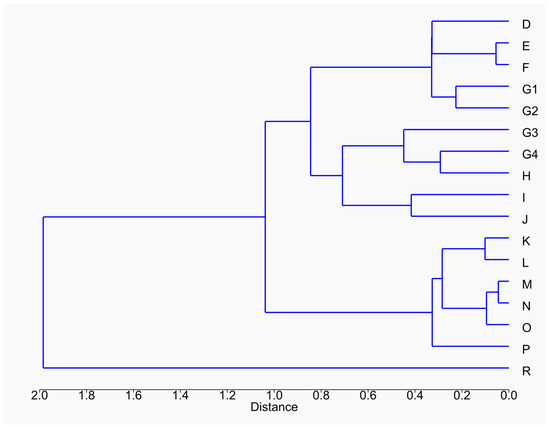
Figure 3.
Dendrogram plot obtained from the cluster analysis based on the first three CA axes.
This dendrogram also illustrates the isolation of US R from the rest of the other layers of the Arago cave. This isolation could be a consequence of a sampling bias or of a very different taxonomic composition, notably related to the presence of P. episcopalis.
4.2. Climatic Parameters
The bioclimatic model reveals that the mean annual temperature ranges from 6 °C for the coldest stratigraphic unit (O) to 9.5 °C for SU R (Table 2). Apart from SU R, all the mean annual temperatures are around 7 ± 1 °C. The high mean temperature for SU R is related to the absence of species associated with cold environments, such as L. gregalis, A. oeconomus, and Dicrostonyx torquatus. Since L. gregalis is ultra-dominant in the overlying stratigraphic units, the absence of this species in SU R is probably not the result of a recording bias. Furthermore, taphonomic studies have shown that the accumulation of small vertebrates in this level is produced by the Eurasian eagle-owl, which is a species with a broad prey spectrum, whose accumulations are a good reflection of the palaeocommunities [42].

Table 2.
Climatic parameters obtained by the bioclimatic model (BM, standard error in parentheses) and the quantified ecology method (QEM) for the different stratigraphic units of the Arago cave. MAT = mean annual temperature, T°max = mean temperature of the warmest month, T°min = mean temperature of the coldest month, expressed in °C.
The mean temperature values of the warmest and coldest months follow the variations in mean annual temperature. There are no phases with a more temperate or continental climate. The difference between the mean of the warmest and coldest month is around 26 °C. The only stratigraphic unit that stands out is the SU R, which has a higher mean temperature of the coldest month than the others (−2.3 °C), even if this value remains fairly low. For each layer, the summer/gap is still above the 25 °C mark which is the limit of the continental climate [40].
The quantified ecology method shows that the mean annual temperature values range from 6.3 °C (for SU E) to 9.5 °C for SU R. SU G3, G4, and I (8.4 °C, 8.3 °C and 8.1 °C) are among the stratigraphic units with the highest temperatures. SU E, F, and K (6.3 °C, 6.7 °C, and 6.4 °C) have the lowest temperatures (Table 2).
The temperatures of the warmest month show little variation among the stratigraphic units. They range from 20.7 °C for SU G1 to 21.9 °C for SU N. For the temperatures of the coldest month, the variations are more marked. The lowest values are observed for SU E, F, and K (−8.5 °C, −7.8 °C, and −8.2 °C). The highest values are observed for SU G3, G4, and R (−4.9 °C, −4.9 °C, and −3 °C). The summer/winter temperature difference is around 27 °C for the different SU except for SU R with a summer/winter gap of 24.9 °C.
4.3. Synthesis
An initial observation of the fauna list highlights the presence of species with different ecological affinities in each SU. This reflects the potentially great diversity of habitats around the Arago cave. Three groups of stratigraphic units and an isolated layer can be defined using climate reconstruction methods.
The first is composed of SU D to G2. This group is well individualised in the CA projection and the dendrogram (Figure 2 and Figure 3). The lemming (Dicrostonyx sp.), which is a characteristic species of the tundra, is present. The stratigraphic units that make up this group have some of the lowest values obtained using the BM and the QEM, particularly for SU E and F. They correspond to a cold phase, the average temperature being between 6.1 °C and 7.2 °C according to the BM and the QEM.
The second group is made up of SU G3 to J. This group stands out from the other stratigraphic units in Ensembles I and III, and is found in the positive part of the CA axis. The stratigraphic units in this group have some of the highest values determined via palaeotemperature reconstruction methods. They seem to correspond to a milder phase than SU D to G2. The calculated mean annual temperature is between 7.2 °C and 8.4 °C according to the BM and QEM. This climate is still colder and more continental than the present climate.
The third group is made up of SU K to P, corresponding to the stratigraphic units of Ensemble I. This group is quite distinct from the other stratigraphic units, and is found in the negative part of axis 1 of the CA. This group presents median palaeotemperature values compared to the other units of the Arago Cave. The average palaeotemperatures calculated for them range from 6 °C to 7.8 °C according to the BM and the QEM. The climate for these stratigraphic units is a little colder than that of SU G3 to J and is similar to SU D to G2
On the CA, the SU R is projected in the positive part of axis 1 and is found with SU G3 to J, which are those whose climate reconstruction is least cold. The dendrogram allows us to clearly separate the SU R from the other layer. This stratigraphic unit represents the warmest and most temperate climatic episode observed at the Arago Cave.
5. Discussion
The reconstructions of the climatic parameters show a similar value for the MAT (mean annual temperature). If they are comparable for the T°max (mean temperature of the warmest month), they are more variable for the T°min (mean temperature of the coldest month). We can also observe that the standard error with the BM is significant for the MAT and the T°max and higher for the T°min. These high values require us to step back from the data. We prefer to base our comparisons only on the MAT, as the standard error of the T°min data is too high. In addition, the values of T°min obtained via the two methods are low for a site located on the Mediterranean coast. These outliers may be intrinsic to the method, which is based on current ecological data for species that may have evolved during the Pleistocene. Furthermore, climatic parameters do not have the same impact on rodent species, as in the case of T°min, which will have less impact on burrowing and hibernating species [40]. However, despite these limits these methods remain the preferred tools for quantifying climatic variation between different layers.
Statistical analyses based on MNI highlight three groups of SU and isolate the SU R from the others. With the reconstruction of the climatic parameters, we can observe slight differences between these groups. These differences are less pronounced with climate reconstruction methods, as only the presence or absence of species is considered. In fact, if we look at each stratigraphic grouping, we see little change in the communities, as the species are broadly similar throughout the stratigraphy, except for the SU R. This is also true for the large mammals, where species associated with cold environments (Rangifer tarandus, Praeovibos priscus) and species associated with temperate environments (Dama cf. clactoniana, Cervus elaphus) are present in the three ensembles [17].
The presence of non-analogous communities, characterized by the cohabitation of species with different ecological affinities that are not currently associated, has been identified at the Arago cave [21,43]. Such communities may vary in their origins, which are not mutually exclusive. They may correspond to a palaeoecological reality and are in this case associated with a palaeoenvironment that is not currently found [44]. They may also correspond to a contact zone between two climatic influences. The Arago cave is located between two zones with a strong climatic influence. The Pyrenees, which correspond to a continental zone located at altitude, are likely to have served as a refuge for “cold” species during temperate phases. The Mediterranean coastline, on the other hand, corresponds to an area with a milder climate and may have served as a refuge for “temperate” species during cold periods [34,45,46,47]. They may also generate a time-averaging effect, which is thought to have increased palaeobiodiversity significantly [48,49]. This phenomenon is increased by the proximity of the communities, which allows for rapid recolonization phases.
The local environment has an important impact on the palaeobiodiversity observed at the Arago cave and therefore on the human groups that occupied it. The position of the site offers a definite advantage in terms of access to the resources stretching between the Mediterranean coast and the Pyrenees, highlighted by the wide variety of species exploited [50]. The diversity of ecosystems that can be exploited over a short distance is an argument for a preferential location for settlement. Human groups can exploit different ecological niches and these will react differently to climatic variations. This diversity endowed human settlements with greater resistance and resilience to Pleistocene climate change. This helps to justify the large number of occupations present at the Arago cave.
If the local environment has an impact on diversity, it is also necessary to understand how global changes have influenced palaeocommunities. However, temperature variations can be seen to be very limited among the layers of the Middle Complex (US D to P), and the differences between Ensemble II, interpreted until now as being correlated to MIS 13, and Ensembles I and III are small, of the order of 1 °C for the mean annual temperature. One possible explanation is the relatively low amplitude of global temperature variations along the oxygen isotope series for this period [51]. This could justify less warming during MIS 13 than observed during other interglacials [52]. However, it is complicated to link global data (on benthic record) to our on-site data.
The use of the Oscillayers method allows us to consider the regional context for reconstructing the mean annual temperature. Compared to our data, those one modelled by the Oscillayers method [41] show strong variation between MIS 14, 13, and 12 (Figure 4). Caution must be exercised in interpreting the temperature values at a given point using this type of global model, but it can give us an idea of the scale of climatic variations. It can be seen that the average temperature at the location of the Arago cave varies from 8.7 °C to 13.2 °C (Table 3), a difference of 4.5 °C. These variations are much more marked than those observed in our layers. The variations observed with our data at the Arago cave were more in line with those observed in MIS12.
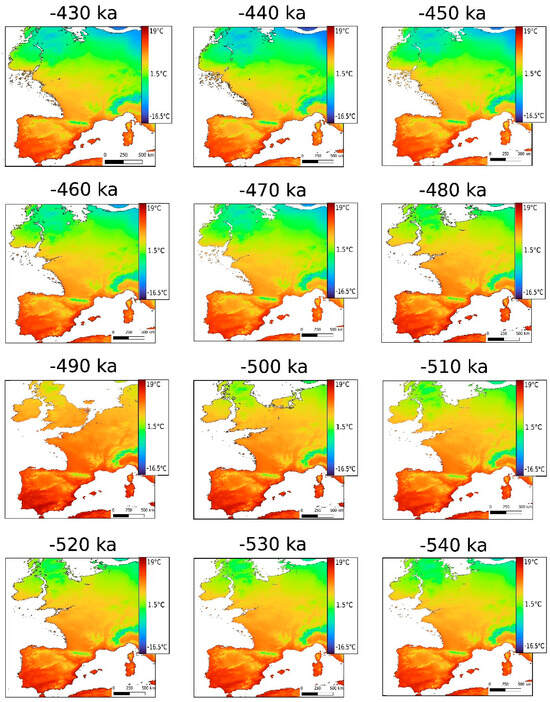
Figure 4.
Reconstructed palaeocoastlines and mean annual temperature estimates between −433 ka and −540 ka using the 10 ka stepped outputs from Gamisch [41]. Colour scale based on minimum (−16.5 °C, dark blue) and maximum values (19 °C, dark red).

Table 3.
Mean annual temperature (MAT in °C) observed at the location of the Arago cave on each Oscillayer modelled using the Oscillayers method [41].
In order to better correspond to the global variations, it is necessary to rethink the correlations between the ensembles in the Arago cave. As we have fairly precise dates for SU G, it makes sense to correlate Ensemble III with MIS 12. Previous studies correlate Ensembles II and I with MIS 13 and 14, respectively [16,17]. This applies in particular to the dates from level P, which could correspond to MIS 14. However, the dates obtained for level P are less accurate, as the age given is 532 ± 106 ka [17]. These environmental variations have been interpreted as a change in MIS, whereas there is little change in species and the variations are mainly variations in MNI. However, variations in species representation alone are not necessarily synonymous with climatic variations, particularly for small vertebrates [40]. If the low amplitude of the oxygen isotope series was used as an argument for correlating Ensemble II to the MIS 13, there is no clear argument against correlating Ensemble II with a phase of MIS 12. On the contrary, SU R represents a real discontinuity in the small-vertebrate communities and can be considered a marked environmental change that corresponds better with a change in stage. Variations between Ensembles I, II, and III may then be considered intra-stage variations. This is not in conflict with the dates, which have a very wide confidence interval. Thus, Ensembles I, II, and III of the Arago cave can be correlated to MIS 12, and SU R would mark a stage change and fall within MIS 13.
This calls into question the interpretation of the timing of the filling of the Arago cave. This gives a new perspective to our perceptions of the pace of technical evolution in the Middle Pleistocene.
6. Conclusions
The small-mammal palaeocommunities of the Arago cave indicate the presence of a cold climate throughout the stratigraphic sequence, but with a clear warming in the deepest layer (SU R). The composition of the small-mammal communities is strongly influenced by the local context of the site, with an influence from the Mediterranean coast and the Pyrenees. This double influence has allowed the coexistence of different ecological niches within a restricted space, which may have favored the establishment and maintenance of human groups, endowing the ecosystem with greater resilience and resistance to climatic variations. Environmental reconstruction methods display fairly low climatic variations within the Middle Complex (Ensembles I, II, and III). These variations may correspond better to those observed within MIS 12 than to interstage variations as previously interpreted. Thus, the Middle Complex of the Arago Cave could be correlated to MIS 12 in accordance with the dating, and SU R would mark the possible transition to MIS 13. This has implications for our understanding of Acheulean development in this region, because SU R would then be the only unit that would be correlated with MIS 13. The chronology of the filling of the Arago cave deserves further study in order to gain a better understanding of the evolution of Acheulean behaviour in southwestern Europe.
Author Contributions
Conceptualization, L.L. and J.M.L.-G.; methodology, L.L. and J.M.L.-G.; formal analysis, L.L.; investigation, L.L. and J.M.L.-G.; resources, L.L. and J.M.L.-G.; data curation, L.L. and J.M.L.-G.; writing—original draft preparation, L.L.; writing—review and editing, L.L. and J.M.L.-G.; visualization, L.L. and J.M.L.-G.; supervision, J.M.L.-G.; funding acquisition, L.L. and J.M.L.-G. All authors have read and agreed to the published version of the manuscript.
Funding
The Institut Català de Paleoecologia Humana i Evolució Social (IPHES-CERCA) has received financial support from the Spanish Ministry of Science and Innovation through the “María de Maeztu” program for Units of Excellence (CEX2019-000945-M). This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 101034349 and the State Research Agency of the Spanish Ministry of Science and Innovation through the Program Maria de Maeztu Unit of Excellence (CEX2019-000945-M). This project was supported by the English correction of Rupert Glasgow. This manuscript is part of a Knowledge Generation project PID2021-122533NB-I00 of the Spanish Ministry of Science and Innovation (MICIN) and project SGR2021-01238 of the Generalitat de Catalunya-AGAUR.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
We also want to thank the two anonymous reviewers for their suggestions and comments that improved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Berger, W.H.; Jansen, E. Mid-pleistocene climate shift—The Nansen Connection. In The Polar Oceans and Their Role in Shaping the Global Environment; Johannessen, O.M., Muench, R.D., Overland, J.E., Eds.; American Geophysical Union: Washington, DC, USA, 2013; Volume 85, pp. 295–311. [Google Scholar]
- Head, M.J.; Gibbard, P.L. Early-Middle Pleistocene transitions: An Overview and Recommendation for the Defining Boundary. In Early-Middle Pleistocene Transitions: The Land-Ocean Evidence; Head, M.J., Gibbard, P.L., Eds.; Geological Society of London: London, UK, 2005; pp. 1–18. [Google Scholar]
- Discamps, E.; Royer, A. Reconstructing palaeoenvironmental conditions faced by Mousterian hunters during MIS 5 to 3 in southwestern France: A multi-scale approach using data from large and small mammal communities. Quat. Int. 2017, 433, 64–87. [Google Scholar]
- Denys, C. Des référentiels en taphonomie des petits vertébrés: Bilan et perspectives. In Taphonomie des Petits Vertébrés: Référentiels et Transferts Aux Fossiles; Laroulandie, V., Mallye, J.-B., Denys, C., Eds.; British Archaeological Reports; International Series 2269; Archaeopress: Oxford, UK, 2011; pp. 7–22. [Google Scholar]
- Puzachenko, A.Y.; Markova, A.K. The Scandinavian ice sheet against the Atlantic ocean: How the scandinavian ice sheet affected European small mammal assemblage during the Greenland stadial GS-2.1. Quat. Sci. Rev. 2023, 305, 108013. [Google Scholar]
- Lumley, H.; Fournier, A.; Park, Y.C.; Yokoyama, Y.; Demouy, A. Stratigraphie du remplissage Pléistocène moyen de la Caune de l’Arago à Tautavel. Etude de huit carottages effectués de 1981 à 1983. L’Anthropologie 1984, 88, 5–18. [Google Scholar]
- Barsky, D.; Lumley, H. Early European Mode 2 and the stone industry from the Caune de l’Arago’s archeostratigraphical levels “P”. Quat. Int. 2010, 223, 71–86. [Google Scholar]
- Barsky, D.; Moigne, A.M.; Pois, V. The shift from typical Western European Late Acheulian to microproduction in unit ‘D’of the late Middle Pleistocene deposits of the Caune de l’Arago (Pyrénées-Orientales, France). J. Hum. Evol. 2019, 135, 102650. [Google Scholar] [PubMed]
- Viallet, C.; Grégoire, S.; Perrenoud, C. Break to Rebuild—The First European Evidence of a Fragmented Chaine Opératoire for Handaxe Production (OIS 14, Caune de l’Arago, France). J. Paleolit. Archaeol. 2022, 5, 1. [Google Scholar]
- Blain, H.A.; Fagoaga, A.; Ruiz-Sánchez, F.J.; Garcia-Medrano, P.; Ollé, A.; Jiménez-Arenas, J.M. Coping with arid environments: A critical threshold for human expansion in Europe at the Marine Isotope Stage 12/11 transition? The case of the Iberian Peninsula. J. Hum. Evol. 2021, 153, 102950. [Google Scholar] [PubMed]
- Connet, N.; Soriano, S.; Bertran, P.; Lhomme, V.; Debenham, N. A 400,000 years old milestone of the Acheulian technocomplex in Central-Western France at Londigny (Charente). J. Archaeol. Sci. Rep. 2020, 30, 102225. [Google Scholar]
- Moncel, M.H.; Despriée, J.; Voinchet, P.; Tissoux, H.; Moreno, D.; Bahain, J.J.; Courcimault, G.; Falguères, C. Early evidence of Acheulean settlement in northwestern Europe-La Noira Site, a 700 000 year-old occupation in the center of France. PLoS ONE 2013, 8, e75529. [Google Scholar]
- Moncel, M.H.; Ashton, N. From 800 to 500 ka in Western Europe. The oldest evidence of Acheuleans in their technological, chronological, and geographical framework. In The Emergence of the Acheulean in East Africa, 1st ed.; Mussi, M., Gallotti, R., Eds.; Springer: New York, NY, USA, 2018; pp. 215–235. [Google Scholar]
- Nicoud, E.; Aureli, D.; Pagli, M.; Villa, V.; Chaussé, C.; Agostini, S.; Zupancich, A. Preliminary data from Valle Giumentina Pleistocene site (Abruzzo, Central Italy): A new approach to a Clactonian and Acheulian sequence. Quat. Int. 2016, 409, 182–194. [Google Scholar]
- Ollé, A.; Lombao, D.; Asryan, L.; García-Medrano, P.; Arroyo, A.; Fernández-Marchena, J.L.; Vallverdú, J. The earliest European Acheulean: New insights into the large shaped tools from the late Early Pleistocene site of Barranc de la Boella (Tarragona, Spain). Front. Earth Sci. 2023, 11, 1188663. [Google Scholar]
- Falguères, C.; Shao, Q.; Han, F.; Bahain, J.J.; Richard, M.; Perrenoud, C.; Moigne, A.M.; Lumley, H. New ESR and U-series dating at Caune de l’Arago, France: A key-site for European Middle Pleistocene. Quat. Geochronol. 2015, 30, 547–553. [Google Scholar]
- Moigne, A.M.; Palombo, M.R.; Belda, V.; Heriech-Briki, D.; Kacimi, S.; Lacombat, F.; Lumley, M.A.; Moutoussamy, J.; Rivals, F.; Quilès, J.; et al. Les faunes de grands mammifères de la Caune de l’Arago (Tautavel) dans le cadre biochronologique des faunes du Pléistocène moyen italien. L’Anthropologie 2006, 110, 788–831. [Google Scholar]
- Magniez, P.; Moigne, A.M.; Testu, A.; Lumley, H. Biochronologie des mammifères quaternaires. Apport des cervidae du site pléistocène moyen de la Caune de l’Arago (Tautavel, Pyrénées-Orientales, France). Quaternaire 2013, 24, 477–502. [Google Scholar]
- Lumley, H. Caune de l’Arago, Tautavel-en-Roussillon, Pyrénées-Orientales, France: Individualisation des Unités Archéostratigraphiques; CNRS Editions: Paris, France, 2015; p. 642. [Google Scholar]
- Lumley, M.A. L’homme de Tautavel. Un Homo erectus européen évolué. Homo erectus tautavelensis. L’Anthropologie 2015, 119, 303–348. [Google Scholar]
- Lebreton, L.; Desclaux, E.; Hanquet, C.; Moigne, A.M.; Perrenoud, C. Environmental context of the Caune de l’Arago Acheulean occupations (Tautavel, France), new insights from microvertebrates in Q-R levels. Quat. Int. 2016, 411, 182–192. [Google Scholar]
- Chaline, J. L’âge des Hominiens de la Caune de l’Arago à Tautavel (Pyrénées-Orientales), d’après l’étude des Rongeurs. Comptes Rendus Hebd. Des Séances De L’académie Des Sci. 1971, 272, 1743–1746. [Google Scholar]
- Brunet-Lecomte, P. Statut des campagnols souterrains (Rodentia, Arvicolidae) du gisement du Pléistocène moyen de l’Arago à Tautavel (Pyrénées-Orientales, France). Bull. Mens. De La Société Linnéenne De Lyon 1990, 59, 100–104. [Google Scholar]
- Desclaux, E. Les petits vertébrés de la Caune de l’Arago (Tautavel, Pyrénées-orientales): Paléontologie, paléoécologie et taphonomie. Ph.D. Thesis, University of Perpignan, Perpignan, France, 1992. [Google Scholar]
- Paunescu, A.C. Les Rongeurs du Pléistocène Inférieur et Moyen de trois grottes du sud-est de la France (Vallonnet, Caune de l’Arago, Baume Bonne). Implications systématiques, biostratigraphiques et paléoenvironnementales. Ph.D. Thesis, University of Perpignan, Perpignan, France, 2001. [Google Scholar]
- Brunet-Lecomte, P.; Paunescu, A. Morphologie comparée de la première molaire inférieure du campagnol Microtus (Terricola) vaufreyi tautavelensis (Rodentia, Arvicolidae) du gisement pléistocène moyen de l’Arago (Pyrénées, France) et inférences paléoclimatiques. Quaternaire 2004, 15, 263–268. [Google Scholar] [CrossRef]
- Hanquet, C. Evolution des paléoenvironnements et des paléoclimats au Pléistocène moyen, en Europe méridionale, d’après les faunes de micromammifères. Ph.D. Thesis, University of Perpignan, Perpignan, France, 2011. [Google Scholar]
- Lebreton, L.; Desclaux, E.; Hanquet, C.; Cuenca-Bescós, G.; Moigne, A.M.; Perrenoud, C.; Grégoire, S. Variations paléoenvironnementales au sein de l’Unité Archéostratigraphique G (UA G) de la Caune de l’Arago (Tautavel, France): Apport des paléocommunautés de rongeurs. Quaternaire 2017, 28, 313–321. [Google Scholar] [CrossRef]
- Lebreton, L.; Moigne, A.M.; Filoux, A.; Perrenoud, C. A specific small game exploitation for Lower Paleolithic: The beaver (Castor fiber) exploitation at the Caune de l’Arago (Pyrénées-Orientales, France). J. Archaeol. Sci. Rep. 2017, 11, 53–58. [Google Scholar]
- Manzano, A. Les Amphibiens et les Reptiles des Sites du Pléistocène Moyen et Supérieur du Pourtour Méditérranéen (Caune de l’Arago, Grotte du Lazaret, Baume Moula-Guercy). Etude D’herpétofaunes et Reconstitutions Paléoclimatiques et Paléoenvironnementales. Ph.D. Thesis, University of Perpignan, Perpignan, France, 2015. [Google Scholar]
- Lebreton, L. Approche taphonomique multi-taxons des accumulations de petits vertébrés, implication pour les reconstitutions paléoenvironnementales au Pléistocène. Ph.D. Thesis, University of Perpignan, Perpignan, France, 2018. [Google Scholar]
- Marquet, J.C. Paléoenvironnement et Chronologie des Sites du Domaine Atlantique Français d’âge Pléistocène Moyen et Supérieur D’après L’étude des Rongeurs. Ph.D. Thesis, University of Dijon, Dijon, France, 1989. [Google Scholar]
- Chaline, J.; Brunet-Lecomte, P.; Campy, M. The last glacial/interglacial record of rodent remains from the Gigny karst sequence in the French Jura used for palaeoclimatic and palaeoecological reconstructions. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1995, 117, 229–252. [Google Scholar]
- Foury, Y.; Desclaux, E.; Daujeard, C.; Defleur, A.; Moncel, M.H.; Raynal, J.P. Evolution des faunes de rongeurs en moyenne vallée du Rhône (rive droite, Ardèche, France) au cours du pléistocène moyen final et du pléistocène supérieur ancien, du mis 6 au mis 4. Quaternaire 2016, 27, 55–79. [Google Scholar]
- Hernández Fernández, M. Bioclimatic discriminant capacity of terrestrial mammal faunas. Glob. Ecol. Biogeogr. 2001, 10, 189–204. [Google Scholar]
- Hernández Fernández, M.; Peláez-Campomanes, P. The bioclimatic model: A method of palaeoclimatic qualitative inference based on mammal associations. Glob. Ecol. Biogeogr. 2003, 12, 507–517. [Google Scholar]
- Hernández Fernández, M.; Peláez-Campomanes, P. Quantitative palaeoclimatic inference based on terrestrial mammal faunas. Glob. Ecol. Biogeogr. 2005, 14, 39–56. [Google Scholar]
- Fernández, M.H.; Sierra, M.Á.; Peláez-Campomanes, P. Bioclimatic analysis of rodent palaeofaunas reveals severe climatic changes in Southwestern Europe during the Plio-Pleistocene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 251, 500–526. [Google Scholar]
- Royer, A.; Yelo, B.A.G.; Laffont, R.; Fernández, M.H. New bioclimatic models for the quaternary palaearctic based on insectivore and rodent communities. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 560, 110040. [Google Scholar]
- Jeannet, M. L’écologie quantifiée. Essai de description de l’environnement continental à l’aide des microvertébrés. Préhistoires méditerranéennes 2010, 1, 1–26. [Google Scholar]
- Gamisch, A. Oscillayers: A dataset for the study of climatic oscillations over Plio-Pleistocene time-scales at high spatial-temporal resolution. Glob. Ecol. Biogeogr. 2019, 28, 1552–1560. [Google Scholar]
- Lebreton, L.; Bailon, S.; Guillaud, E.; Testu, A.; Perrenoud, C. Multi-taxa referential of a modern Eurasian Eagle-Owl (Bubo bubo) aerie. J. Archaeol. Sci. Rep. 2020, 32, 102417. [Google Scholar]
- Hanquet, C.; Desclaux, E. Analyse paléoécologique des communautés de micromammifères de la Caune de l’Arago (Tautavel, France) dans le contexte des migrations de faunes en Europe méridionale au cours du Pléistocène moyen. Quaternaire 2011, 22, 34–45. [Google Scholar]
- Guthrie, D.; van Kolfschoten, T. Neither warm and moist, nor cold and arid: The ecology of the Mid Upper Palaeolithic. In Hunters of the Golden Age; Roebroeks, W., Mussi, M., Svoboda, J., Fennema, K., Eds.; Leiden University: Leiden, The Netherlands, 2000; pp. 3–20. [Google Scholar]
- Bennett, K.D.; Provan, J. What do we mean by ‘refugia’? Quat. Sci. Rev. 2008, 27, 2449–2455. [Google Scholar]
- Stewart, J.R.; Lister, A.M.; Barnes, I.; Dalén, L. Refugia revisited: Individualistic responses of species in space and time. Proc. Royal Soc. B 2010, 277, 661–671. [Google Scholar]
- Royer, A.; Montuire, S.; Legendre, S.; Discamps, E.; Jeannet, M.; Lécuyer, C. Investigating the influence of climate changes on rodent communities at a regional-scale (MIS 1-3, Southwestern France). PLoS ONE 2016, 11, e0145600. [Google Scholar]
- Fürsich, F.T.; Aberhan, M. Significance of time-averaging for palaeocommunity analysis. Lethaia 1990, 23, 143–152. [Google Scholar]
- Denys, C. Rodent faunal lists in karstic and open-air sites of Africa: An attempt to evaluate predation and fossilization biases on paleodiversity. Cuad. De Geol. Ibérica 1997, 23, 73–94. [Google Scholar]
- Moigne, A.M.; Grégoire, S.; Lumley, H.D. Les territoires de chasse et d’exploitation des matières premières des hommes préhistoriques de la Caune de l’Arago entre 600 000 ans et 400 000 ans. Actes Des Congrès Natx. Des Sociétés Hist. Et Sci. 2005, 126, 17–31. [Google Scholar]
- Lisiecki, L.E.; Raymo, M.E. A Pliocene-Pleistocene stack of 57 globally distributed benthic δ18O records. Paleoceanography 2005, 20, 1–17. [Google Scholar]
- López-García, J.M.; Fagoaga, A.; Nabais, M.; Póvoas, L.; Zilhão, J. Late Quaternary (MIS 5a-5b) climate and environments of western Iberia inferred from the small-mammal assemblage of Gruta da Oliveira, Torres Novas, Portugal. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2022, 603, 111194. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).