Contextual, Taphonomic, and Paleoecological Insights from Anurans on Tiwanaku Sites in Southern Peru
Abstract
:1. Introduction
Anurans and Taphonomy
2. Anurans in the Andes
2.1. Cultural Entanglements
2.2. Anurans Species and Ecology in the Western Andes
3. Moquegua Valley
3.1. The Environmental Setting
3.2. The Cultural Setting
4. Materials and Methods
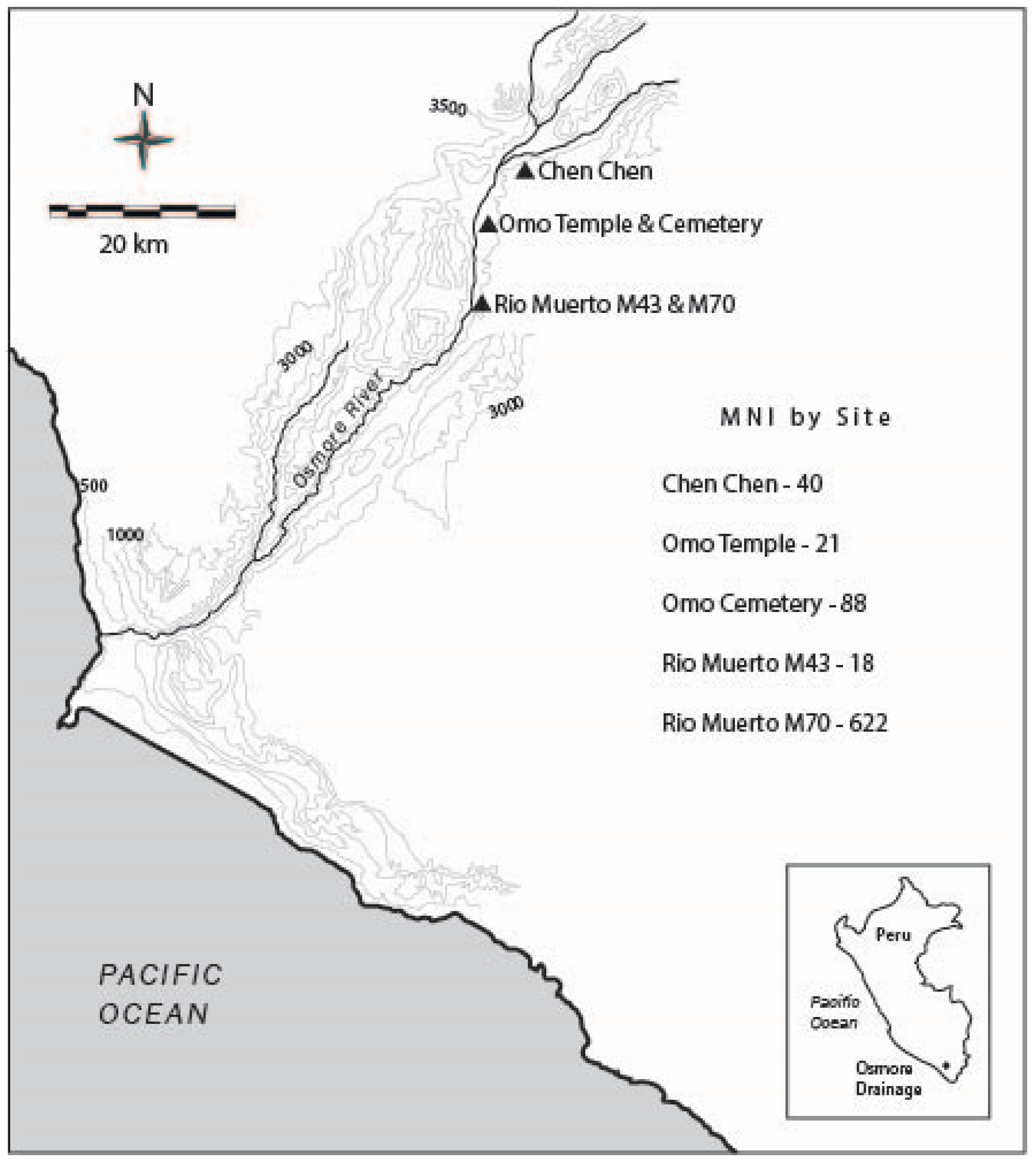
4.1. Archaeological Sites
4.1.1. Chen Chen (M1)
4.1.2. Omo Temple and Cemeteries (M10), and Domestic Sectors (M10, M12)
4.1.3. Rio Muerto (M43 and M70)
4.2. Methods
5. Results
5.1. Taxonomic Identifications of Anurans and Non-Anuran Fauna
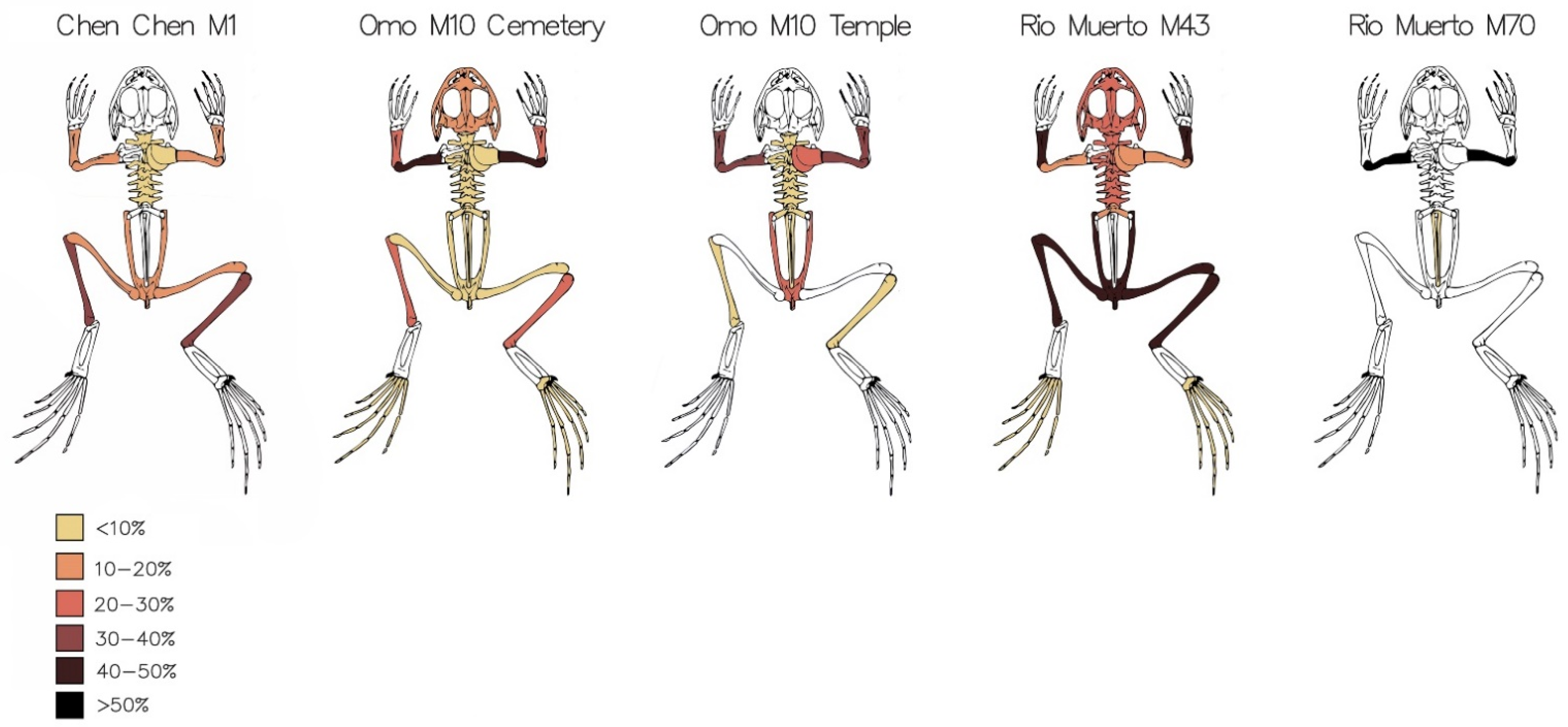
5.2. Abundance and Ubiquity of Anuran Remains
6. Discussion
6.1. Anurans and Agents of Accumulation
6.2. Anurans and Palaeoecology
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moore, K.M.; Steadman, D.; Defrance, S. Herds, Fish, and Fowl in the Domestic and Ritual Economy of Formative Chiripa. In Early Settlement at Chiripa; Hastorf, C.A., Ed.; Research of the Taraco Archaeological Project: La Paz, Bolivia, 1999; pp. 105–116. ISBN 1-882744-01-1. [Google Scholar]
- Aguayo, R. Anfibios. In Libro rojo de la fauna de vertebrados de Bolivia; Aguirre, L., Balderrama, J., Aguayo, R., Cortez, C., Tarifa, T., Damme, P., Arteaga, L., Eds.; Ministerio del Medio Ambiente y Agua: La Paz, Bolivia, 2009; ISBN 978-99954-0-699-8. [Google Scholar]
- Vellard, J. Associated Animal Communities: The Amphibia. In Lake Titicaca: A Synthesis of Limnological Knowledge; Dejoux, C., Iltis, A., Eds.; Monographiae Biologicae; Springer: Dordrecht, The Netherlands, 1992; pp. 449–457. ISBN 978-0-7923-1663-3. [Google Scholar]
- Tschopik, H. The Aymara of Chucuito, Peru; Anthropological Papers of the American Museum of Natural History; AMNH Division of Anthropology: New York, NY, USA, 1951; Volume 44, Pt. 2. [Google Scholar]
- Warwick, M.C. In the Shadow of the Peñon: A Zooarchaeological Study of Formative Diet, Economy, and Sociopolitics in the Río Pukara Valley, Peru; The University of Wisconsin-Milwaukee: Milwaukee, WI, USA, 2012. [Google Scholar]
- La Barre, W. The Aymara Indians of the Lake Titicaca Plateau, Bolivia. Memoirs of the American Anthropological Association; Pan American Institute of Geography and History: Bloomington, IN, USA, 1948. [Google Scholar]
- Chávez, K.L.M. The Significance of Chiripa in Lake Titicaca Basin Developments. Exped. Mag. Univ. Pa. 1988, 30, 17–26. [Google Scholar]
- Korpisaari, A.; Pärssinen, M. Pariti: The Ceremonial Tiwanaku Pottery of An Island in Lake Titicaca; Finnish Academy of Science and Letters: Helsinki, Finland, 2011; ISBN 978-951-41-1081-8. [Google Scholar]
- Catenazzi, A.; Von May, R. Conservation Status of Amphibians in Peru1. Herpetol. Monogr. 2014, 28, 1–23. [Google Scholar] [CrossRef]
- Whyte, T.R.; Compton, J.M. Explaining Toad Bones in Southern Appalachian Archaeological Deposits. Am. Antiq. 2020, 85, 305–330. [Google Scholar] [CrossRef]
- Andrews, P. Owls, Caves, and Fossils: Predation, Preservation, and Accumulation of Small Mammal Bones in Caves, with An Analysis of the Pleistocene Cave Faunas from Westbury-Sub-Mendip, Somerset, UK; University of Chicago Press: Chicago, IL, USA, 1990; ISBN 978-0-226-02037-2. [Google Scholar]
- Hockett, B.; Haws, J. Taphonomic and Methodological Perspectives of Leporid Hunting During the Upper Paleolithic of the Western Mediterranean Basin. J. Archaeol. Method Theory 2002, 9, 269–302. [Google Scholar] [CrossRef]
- De Cupere, B.; Thys, S.; Van Neer, W.; Ervynck, A.; Corremans, M.; Waelkens, M. Eagle owl (Bubo bubo) pellets from Roman Sagalassos (SW Turkey): Distinguishing the prey remains from nest and roost sites. Int. J. Osteoarchaeol. 2008, 19, 1–22. [Google Scholar] [CrossRef]
- Lloveras, L.; Moreno-García, M.; Nadal, J. The eagle owl (Bubo bubo) as a leporid remains accumulator: Taphonomic analysis of modern rabbit remains recovered from nests of this predator. Int. J. Osteoarchaeol. 2008, 19, 573–592. [Google Scholar] [CrossRef]
- Serra, A.S.; Margalef, C.R.; Pérez, J.V.M.; Ripoll, M.P.; Cuñat, C.T.; Marco, Y.C.; Jordá, G.P.; Gómez, A.R.; Marqués, J.B.; Bonilla, V.V. Towards the identification of a new taphonomic agent: An analysis of bone accumulations obtained from modern Egyptian vulture (Neophron percnopterus) nests. Quat. Int. 2014, 330, 136–149. [Google Scholar] [CrossRef] [Green Version]
- Hockett, B. Taphonomic comparison of bones in mountain lion scats, coyote scats, golden eagle pellets, and great-horned owl pellets. Quat. Int. 2018, 466, 141–144. [Google Scholar] [CrossRef]
- Llona, A.C.P.; Andrews, P.J. Amphibian taphonomy and its application to the fossil record of Dolina (middle Pleistocene, Atapuerca, Spain). Palaeogeogr. Palaeoclim. Palaeoecol. 1999, 149, 411–429. [Google Scholar] [CrossRef]
- Stoetzel, E.; Denys, C.; Bailon, S.; El Hajraoui, M.A.; Nespoulet, R. Taphonomic Analysis of Amphibian and Squamate Remains from El Harhoura 2 (Rabat-Témara, Morocco): Contributions to Palaeoecological and Archaeological Interpretations. Int. J. Osteoarchaeol. 2011, 22, 616–635. [Google Scholar] [CrossRef]
- Blain, H.-A.; Villa, P. Amphibians and squamate reptiles from the early Upper Pleistocene of Bois Roche Cave (Charente, southwestern France). Acta Zoöl. Cracoviensia–Ser. A Vertebr. 2006, 49, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Blain, H.-A. Contribution de La Paléoherpétofaune (Amphibia & Squamata) à La Connaissance de l’Evolution Du Climat et Du Paysage Du Pliocène Supérieur Au Pléistocène Moyen d’Espagne. Treb. Del Mus. De Geol. De Barc. 2009, 16, 39–170. [Google Scholar]
- Martin, C.; Szyndlar, Z.; Sanchiz, B. Herpetofauna Del Pleistoceno Superior de La Cueva de Cobrante Upper Pleistocene Herpetofauna from Cueva de Cobrante. Sautuola 2009, XV, 107–114. [Google Scholar]
- Biton, R.; Bailon, S.; Birkenfeld, M.; Bridault, A.; Khalaily, H.; Valla, F.R.; Rabinovich, R. The anurans and squamates assemblage from Final Natufian Eynan (Ain Mallaha, Israel) with an emphasis on snake-human interactions. PLoS ONE 2021, 16, e0247283. [Google Scholar] [CrossRef]
- Bisbal-Chinesta, J.F.; Bañuls-Cardona, S.; Fernández-García, M.; Cáceres, I.; Blain, H.-A.; Vergès, J.M. Elucidating anuran accumulations: Massive taphocenosis of tree frog Hyla from the Chalcolithic of El Mirador cave (Sierra de Atapuerca, Spain). J. Archaeol. Sci. Rep. 2020, 30, 102277. [Google Scholar] [CrossRef]
- Cooke, A.G. American University, Washington, DC, USA. Personal Communication, 2020. [Google Scholar]
- Kidder, A., II. Two Peruvian Frogs; Expedition Magazine–Penn Museum: Philadelphia, PA, USA, 1968. [Google Scholar]
- Jarvis, L.E.; Angulo, A.; Catenazzi, A.; von May, R.; Brown, J.L.; Lehr, E.; Lewis, J. A Re-Assessment of Priority Amphibian Species of Peru. Trop. Conserv. Sci. 2015, 8, 623–645. [Google Scholar] [CrossRef]
- Aguilar, C.; Ramírez, C.; Rivera, D.; Siu-Ting, K.; Suárez, J.; Torres, C. Anfibios andinos del Perú fuera de Áreas Naturales Protegidas: Amenazas y estado de conservación. Rev. Peru. Biol. 2011, 17, 005–028. [Google Scholar] [CrossRef] [Green Version]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Janzen, D.H. Why Mountain Passes are Higher in the Tropics. Am. Nat. 1967, 101, 233–249. [Google Scholar] [CrossRef]
- Polato, N.R.; Gill, B.A.; Shah, A.A.; Gray, M.M.; Casner, K.L.; Barthelet, A.; Messer, P.W.; Simmons, M.P.; Guayasamin, J.M.; Encalada, A.C.; et al. Narrow thermal tolerance and low dispersal drive higher speciation in tropical mountains. Proc. Natl. Acad. Sci. USA 2018, 115, 12471–12476. [Google Scholar] [CrossRef] [Green Version]
- MINAM Situación Actual de Las Especies de Anfibios y Reptiles Del Perú. 2018. Available online: https://cdn.www.gob.pe/uploads/document/file/355102/Publicacion_Anfibios_y_Reptiles_final_15.08.19.pdf (accessed on 1 July 2021).
- Oficina Nacional de Evaluación de Recursos Naturales (ONERN) Mapa ecológico del Perú: Guía explicativa. Autoridad Nacional del Agua 1976. Available online: https://sinia.minam.gob.pe/mapas/mapa-perfil-ambiental-peru (accessed on 1 July 2021).
- Magilligan, F.J.; Goldstein, P.S. El Niño Floods and Culture Change: A Late Holocene Flood History for the Rio Moquegua, Southern Peru. Geology 2001, 29, 431–434. [Google Scholar] [CrossRef]
- Williams, P.R. Disaster in the Development of Agriculture and the Evolution of Social Complexity in the South-Central Andes. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 1997. [Google Scholar]
- Caviedes, C.N.; Waylen, P.R. El Niño y Crecidas Anuales En Los Ríos Del Norte Del Perú. Bull. Inst. Fr. Études Andines 1987, 16, 1–19. [Google Scholar]
- Keefer, D.K.; Defrance, S.D.; Moseley, M.E.; Richardson, J.B.; Satterlee, D.R.; Day-Lewis, A. Early Maritime Economy and El Niño Events at Quebrada Tacahuay, Peru. Science 1998, 281, 1833–1835. [Google Scholar] [CrossRef] [PubMed]
- Manners, R.B.; Magilligan, F.J.; Goldstein, P.S. Floodplain Development, El Niño, and Cultural Consequences in a Hyperarid Andean Environment. Ann. Assoc. Am. Geogr. 2007, 97, 229–249. [Google Scholar] [CrossRef]
- Caramanica, A.; Mesia, L.H.; Morales, C.R.; Huckleberry, G.; Quilter, J. El Niño resilience farming on the north coast of Peru. Proc. Natl. Acad. Sci. USA 2020, 117, 24127–24137. [Google Scholar] [CrossRef]
- Nesbitt, J. Ancient agriculture and climate change on the north coast of Peru. Proc. Natl. Acad. Sci. USA 2020, 117, 24617–24619. [Google Scholar] [CrossRef]
- Goldstein, P.S.; Magilligan, F.J. Hazard, risk and agrarian adaptations in a hyperarid watershed: El Niño floods, streambank erosion, and the cultural bounds of vulnerability in the Andean Middle Horizon. CATENA 2011, 85, 155–167. [Google Scholar] [CrossRef]
- Satterlee, D.R.; Moseley, M.E.; Keefer, D.K.; Tapia A., J.E. The Miraflores El Niño Disaster: Convergent Catastrophes and Prehistoric Agrarian Change in Southern Peru. Andean Past 2000, 6, 8. [Google Scholar]
- Satterlee, D.R. Impact of a Fourteenth Century El Niño Flood on an Indigenous Population near Ilo, Peru. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 1993. [Google Scholar]
- Moseley, M.E.; Defrance, S.D.; Vining, B.R. Droughts, floods, and farming at Quebrada Tacahuay from late prehispanic to colonial times. Ñawpa Pacha 2017, 37, 25–37. [Google Scholar] [CrossRef]
- Reycraft, R.M. The Terminal Chiribaya Project: The Archaeology of Human Response to Natural Disaster in South Coastal Peru. Ph.D. Thesis, The University of New Mexico, Albuquerque, NM, USA, 1998. [Google Scholar]
- Goldstein, P.S. Andean Diaspora: The Tiwanaku Colonies and the Origins of South American Empire, 1st ed.; University Press of Florida: Gainesville, FL, USA, 2005; ISBN 978-0-8130-2774-6. [Google Scholar]
- Goldstein, P.S. Multiethnicity, pluralism, and migration in the south central Andes: An alternate path to state expansion. Proc. Natl. Acad. Sci. USA 2015, 112, 9202–9209. [Google Scholar] [CrossRef] [Green Version]
- Stanish, C.; de la Vega, E.; Moseley, M.; Williams, P.R.; Chávez J., C.; Vining, B.; LaFavre, K. Tiwanaku trade patterns in southern Peru. J. Anthr. Archaeol. 2010, 29, 524–532. [Google Scholar] [CrossRef]
- Vargas, V.B. Informe Final Del Proyecto: “Rescate Arqueológico Del Cementerio de Chen Chen”; Instituto Nacional de Cultura, Sucursal Departamental Moquegua: Moquegua, Peru, 1988. [Google Scholar]
- Sanchiz, B. Salientia; Handbuch der Paläoherpetologie/begr. von Oskar Kuhn. Hrsg. von Peter Wellnhofer. Unter Mitarb. von R. M. Appleby; Pfeil: München, Germany, 1998; ISBN 978-3-931516-27-7. [Google Scholar]
- Shine, R. Sexual Selection and Sexual Dimorphism in the Amphibia. Copeia 1979, 1979, 297–306. [Google Scholar] [CrossRef]
- Keeffe, R.; Blackburn, D.C. Comparative morphology of the humerus in forward-burrowing frogs. Biol. J. Linn. Soc. 2020, 131, 291–303. [Google Scholar] [CrossRef]
- Lynch, J.D. Evolutionary Relationships, Osteology, and Zoogeography of Leptodactyloid Frogs. Misc. Publ.–Univ. Kans. Mus. Nat. Hist. 1971, 53, 1–238. [Google Scholar]
- Reitz, E.J.; Wing, E.S. Zooarchaeology; Cambridge manuals in archaeology; Cambridge University Press: Cambridge, NY, USA, 1999; ISBN 978-0-521-48069-7. [Google Scholar]
- Miranda, P.M. Rhinella Spinulosa. Available online: https://calphotos.berkeley.edu/cgi/img_query?seq_num=904525&one=T (accessed on 4 June 2021).
- Kosch, T. Telmatobius Marmoratus. Available online: https://calphotos.berkeley.edu/cgi/img_query?seq_num=328239&one=T (accessed on 4 June 2021).
- Kosch, T. Pleurodema Marmoratum. Available online: https://calphotos.berkeley.edu/cgi/img_query?seq_num=364994&one=T (accessed on 4 June 2021).
- Park, J.E. Food from the Heartland: The Iwawi Site and Tiwanaku Political Economy from A Faunal Perspective. Master’s Thesis, Simon Fraser University, Vancouver, BC, Canada, 2001. [Google Scholar]
- Vallières, C. A Taste of Tiwanaku: Daily Life in an Ancient Andean Urban Center as Seen through Cuisine. Ph.D. Thesis, McGill University, Toronto, ON, Canada, 2012. [Google Scholar]
- Webster, A.D. The Role of Camelid in the Development of the Tiwanaku State. Unpublished. Ph.D. Thesis, University of Chicago, Chicago, IL, USA, 1993. [Google Scholar]
- Delaere, C.; Capriles, J.M.; Stanish, C. Underwater ritual offerings in the Island of the Sun and the formation of the Tiwanaku state. Proc. Natl. Acad. Sci. USA 2019, 116, 8233–8238. [Google Scholar] [CrossRef] [Green Version]
- Montalvo, C.I.; Fernández, F.J.; Tomassini, R.L.; Mignino, J.; Kin, M.S.; Santillán, M.A. Spatial and temporal taphonomic study of bone accumulations of the burrowing owl (Athene cunicularia) in central Argentina. J. Archaeol. Sci. Rep. 2020, 30. [Google Scholar] [CrossRef]
- Montalvo, C.I.; Tejerina, P. Análisis tafonómico de los huesos de anfibios y roedores depredados por Athene cunicularia (Strigiformes, Strigidae) en La Pampa, Argentina. Mamül Mapu Pasado Y Presente Desde La Arqueol. Pampeana 2009, 1, 323–334. [Google Scholar]
- Burrowing Owl Conservation Network Burrowing Owl Facts. Burrowing Owl Conservation Network. Available online: http://burrowingowlconservation.org/ (accessed on 4 June 2021).
- Arana, M.; Ruíz-Luna, M.L.; María, S.S.; Ramirez, O. Population fluctuations of the house mouse in a Peruvian loma and the functional response of burrowing owls. Austral Ecol. 2006, 31, 956–963. [Google Scholar] [CrossRef]
- Cavalli, M.; Isacch, J.P.; Baladrón, A.V.; Martínez, G.; Bó, M.S. Prey selection and food habits of breeding Burrowing Owls (Athene cunicularia) in natural and modified habitats of Argentine pampas. Emu–Austral Ornithol. 2013, 114, 184–188. [Google Scholar] [CrossRef]
- Andrade, A.; Nabte, M.J.; Kun, M.E. Diet of the Burrowing Owl (Athene cunicularia) and its seasonal variation in Patagonian steppes: Implications for biodiversity assessments in the Somuncurá Plateau Protected Area, Argentina. Stud. Neotropical Fauna Environ. 2010, 45, 101–110. [Google Scholar] [CrossRef]
- Cadena-Ortiz, H.; Garzón, C.; Villamarín-Cortéz, S.; Pozo-Zamora, G.M.; Echeverría-Vaca, G.; Yánez, J.; Brito, J. Diet of the Burrowing Owl Athene cunicularia, in two locations of the inter-Andean valley Ecuador. Rev. Bras. Ornitol. 2016, 24, 122–128. [Google Scholar] [CrossRef]
- Zilio, F. Diet of Falco Sparverius (Aves: Falconidae) and Athene Cunicularia (Aves: Strigidae) in a Dune Region on North Coast of Rio Grande Do Sul, Brazil. Rev. Bras. Ornitol. 2006, 14, 379–392. [Google Scholar]
- Peru Aves Burrowing Owl (Athene Cunicularia). Available online: https://www.peruaves.org/strigidae/burrowing-owl-athene-cunicularia/ (accessed on 30 May 2021).
- Johnston, D. Breakfast with the Burrowing Owls. Available online: https://dinascitywildlife.com/2012/04/21/breakfast-with-the-burrowing-owls/ (accessed on 4 June 2021).
- Schulenberg, T.S.; Stotz, D.F.; Rico, L. Distribution Maps of the Birds of Peru, Version 1.0. Environment, Culture & Conservation (ECCo). Available online: http://fm2.fieldmuseum.org/uw_test/birdsofperu/ (accessed on 4 June 2021).
- The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/en (accessed on 4 June 2021).
- eBird EBird Homepage. Available online: https://ebird.org/home (accessed on 9 November 2021).
- Nabte, M.J.; Pardiñas, U.J.F.; Saba, S.L. The Diet of the Burrowing Owl, Athene Cunicularia, in the Arid Lands of Northeastern Patagonia, Argentina. J. Arid Environ. 2008, 72, 1526–1530. [Google Scholar] [CrossRef]
- Schlatter, R.P.; Yáñez, J.L.; Núñez, H.; Jaksić, F.M. The Diet of the Burrowing Owl in Central Chile and Its Relation to Prey Size. Am. Ornithol. Soc. 1980, 97, 616–619. [Google Scholar]
- Carevic, F.; Carmona, E.; Muñoz-Pedreros, A. Seasonal diet of the burrowing owl Athene cunicularia Molina, 1782 (Strigidae) in a hyperarid ecosystem of the Atacama desert in northern Chile. J. Arid. Environ. 2013, 97, 237–241. [Google Scholar] [CrossRef]
- Catenazzi, A.; Vargas, V.; Lehr, E. A new species of Telmatobius (Amphibia, Anura, Telmatobiidae) from the Pacific slopes of the Andes, Peru. ZooKeys 2015, 480, 81–95. [Google Scholar] [CrossRef] [Green Version]
- Faivovich, J.; Ferraro, D.P.; Basso, N.G.; Haddad, C.F.; Rodrigues, M.T.; Wheeler, W.C.; Lavilla, E.O. A phylogenetic analysis of Pleurodema (Anura: Leptodactylidae: Leiuperinae) based on mitochondrial and nuclear gene sequences, with comments on the evolution of anuran foam nests. Cladistics 2012, 28, 460–482. [Google Scholar] [CrossRef]
- De La Riva, I.; Aparicio, J.; Ríos, J.N. New Species of Telmatobius (Anura: Leptodactylidae) from Humid Paramo of Peru and Bolivia. South Am. J. Herpetol. 2005, 39, 409–416. [Google Scholar] [CrossRef]
- Sousa, J.G.; Ávila, R. Body size, reproduction and feeding ecology of Pleurodema diplolister (Amphibia: Anura: Leiuperidae) from Caatinga, Pernambuco state, Northeastern Brazil. Acta Herpetol. 2015, 10, 129–134. [Google Scholar] [CrossRef]
- Duellman, W.E.; Trueb, L. Biology of Amphibians; Johns Hopkins University Press: Baltimore, MD, USA, 1994; ISBN 978-0-8018-4780-6. [Google Scholar]
- Seimon, T.A.; Seimon, A.; Yager, K.; Reider, K.; Delgado, A.; Sowell, P.; Tupayachi, A.; Konecky, B.; McAloose, D.; Halloy, S. Long-term monitoring of tropical alpine habitat change, Andean anurans, and chytrid fungus in the Cordillera Vilcanota, Peru: Results from a decade of study. Ecol. Evol. 2017, 7, 1527–1540. [Google Scholar] [CrossRef]
- Mendoza, R. Moquegua: Puente Montalvo Colapsó Por Intensas Lluvias. Available online: https://diariocorreo.pe/peru/moquegua-puente-montalvo-colapso-por-intensas-lluvias-869564/ (accessed on 30 May 2021).
- Holmgren, M.; Stapp, P.; Dickman, C.; Gracia, C.; Graham, S.; Gutiérrez, J.R.; Hice, C.; Jaksic, F.; Kelt, D.A.; Letnic, M.; et al. Extreme climatic events shape arid and semiarid ecosystems. Front. Ecol. Environ. 2006, 4, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Carey, C.; Alexander, M.A. Climate Change and Amphibian Declines: Is There a Link? Divers. Distrib 2003, 9, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Jensen, J.B.; Bailey, M.A.; Blankenship, E.L.; Camp, C.D. The Relationship between Breeding by the Gopher Frog, Rana capito (Amphibia: Ranidae) and Rainfall. Am. Midl. Nat. 2003, 150, 185–190. [Google Scholar] [CrossRef]
- Pérez-Granados, C.; Schuchmann, K.-L.; Ramoni-Perazzi, P.; Marques, M.I. Calling behaviour of Elachistocleis matogrosso (Anura, Microhylidae) is associated with habitat temperature and rainfall. Bioacoustics 2019, 29, 670–683. [Google Scholar] [CrossRef]
- Alexander, M.A.; Eischeid, J.K. Climate Variability in Regions of Amphibian Declines. Conserv. Biol. 2001, 15, 930–942. [Google Scholar] [CrossRef] [Green Version]
- Carey, C.; Heyer, W.R.; Wilkinson, J.; Alford, R.A.; Arntzen, J.W.; Halliday, T.; Hungerford, L.; Lips, K.R.; Middleton, E.M.; Orchard, S.A.; et al. Amphibian Declines and Environmental Change: Use of Remote-Sensing Data to Identify Environmental Correlates. Conserv. Biol. 2001, 15, 903–913. [Google Scholar] [CrossRef]
- Kiesecker, J.M.; Blaustein, A.R.; Belden, L. Complex causes of amphibian population declines. Nature 2001, 410, 681–684. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Walls, S.C.; Bancroft, B.; Lawler, J.J.; Searle, C.; Gervasi, S.S. Direct and Indirect Effects of Climate Change on Amphibian Populations. Diversity 2010, 2, 281. [Google Scholar] [CrossRef]
- Cannon, M.D. A Mathematical Model of the Effects of Screen Size on Zooarchaeological Relative Abundance Measures. J. Archaeol. Sci. 1999, 26, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Lyman, R.L. Quantitative Paleozoology; Cambridge University Press: Cambridge, UK, 2008; ISBN 978-0-511-38463-9. [Google Scholar]
- Lyman, R.L. The influence of screen mesh size, and size and shape of rodent teeth on recovery. J. Archaeol. Sci. 2012, 39, 1854–1861. [Google Scholar] [CrossRef]








| Site | NISP | Total MNI | MNI from Tombs | Percentage MNI from Tombs | MNI from Domestic Contexts | Percentage MNI from Domestic Contexts | MNI from Ceremonial Contexts | Percentage MNI from Ceremonial Contexts | MNI from Midden | Percentage MNI from Midden |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen Chen M1 | 60 | 40 | 30 | 75.0 | 8 | 20.0 | 2 | 5.0 | n/a | n/a |
| Omo Cemetery | 541 | 88 | 85 | 96.6 | n/a | n/a | n/a | n/a | 3 | 3.4 |
| Omo Temple | 80 | 21 | n/a | n/a | n/a | n/a | 21 | 100.0 | n/a | n/a |
| Rio Muerto M43 | 158 | 18 | 11 | 61.1 | 7 | 38.9 | 0 | 0.0 | n/a | n/a |
| Rio Muerto M70 | 4808 | 622 | 352 | 56.6 | 1 | 0.2 | n/a | n/a | 269 | 43.2 |
| TOTAL | 5647 | 789 | 478 | 60.6 | 16 | 2 | 23 | 2.9 | 272 | 34.5 |
| Image | Taxon | Probable species | NISP | % of NISP | MNI | % of MNI |
|---|---|---|---|---|---|---|
 | Rhinella sp. | Rhinella limensis or spinulosa | 58 | 38.9 | 32 | 36.8 |
| Rhinella sp. (possible) | https://calphotos.berkeley.edu/cgi/img_query?seq_num=904525&one=T | 10 | 6.7 | 9 | 10.3 | |
 | Telmatobius sp. | Telmatobius arequipensis, marmoratus or peruvianus | 17 | 11.4 | 11 | 12.6 |
| Telmatobius sp. (possible) | https://calphotos.berkeley.edu/cgi/img_query?enlarge=0000+0000+0810+0233 | 8 | 5.4 | 8 | 9.2 | |
 | Pleurodema sp. | Pleurodema cinereum or marmoratum | 10 | 6.7 | 5 | 5.7 |
| Pleurodema sp. (possible) | https://calphotos.berkeley.edu/cgi/img_query?seq_num=364994&one=T | 4 | 2.7 | 2 | 2.3 | |
| unidentified | 42 | 28.2 | 20 | 23 | ||
| All | 149 | 100 | 87 | 100 |
| Site\Taxa | Vertebrata | Mammalia | Camelidae | Rodentia | Carnivora | Chiroptera | Artiodactyla and Cervidae | Aves | Squamata | Osteichthyes | Crustacean | Mollusca | Coral |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen Chen M1 | X | X | X | X | X | X | X | ||||||
| Omo Cemetery | X | X | X | X | X | X | X | X | X | X | X | ||
| Omo Temple | X | X | X | X | X | X | X | X | X | ||||
| Rio Muerto M43 | X | X | X | X | X | X | X | X | X | X | X | X | |
| Rio Muerto M70 | X | X | X | X | X | X | X | X |
| Site | Anura MNI | Rodentia MNI | Chiroptera MNI | Squamata MNI |
|---|---|---|---|---|
| Chen Chen | 40 | 15 | 0 | 0 |
| Omo Cemetery | 88 | 43 | 2 | 0 |
| Omo Temple | 19 | 13 | 1 | 1 |
| RM M43 | 17 | 10 | 0 | 1 |
| RM M70 | 622 | 144 | 11 | 1 |
| TOTAL | 786 | 225 | 14 | 3 |
| Site | Total Contexts Analyzed | Total Contexts with Anurans | Ubiquity Index (%) | Total Tombs Analyzed | Total Tombs with Anurans | Tomb Ubiquity Index (%) | Total Domestic Contexts Analyzed | Total Domestic with Anurans | Domestic Ubiquity Index (%) | Total Ceremonial Contexts Analyzed | Total Ceremonial with Anurans | Ceremonial Ubiquity Index (%) | Total Midden Contexts Analyzed | Total Midden with Anurans | Ceremonial Ubiquity Index (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen Chen | 144 | 22 | 15.3 | 36 | 12 | 33.3 | 90 | 8 | 8.9 | 18 | 2 | 11.1 | n/a | n/a | n/a |
| Omo Cem. | 142 | 29 | 20.4 | 65 | 26 | 40.0 | n/a | n/a | n/a | n/a | n/a | n/a | 80 | 3 | |
| Omo Tem. | 304 | 13 | 4.3 | n/a | n/a | n/a | n/a | n/a | n/a | 304 | 13 | 4.3 | n/a | n/a | n/a |
| Rio M43 | 153 | 14 | 9.2 | 34 | 8 | 23.5 | 100 | 6 | 6.0 | 4 | 0 | 0 | n/a | n/a | n/a |
| Rio M70 | 64 | 28 | 43.8 | 27 | 23 | 85.2 | 31 | 1 | 3.2 | n/a | n/a | n/a | 6 | 4 | n/a |
| TOTAL | 807 | 106 | 13.1 | 162 | 69 | 42.6 | 221 | 15 | 6.8 | 326 | 15 | 4.6 | 86 | 7 | 8.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubinatto Serrano, J.; Vallejo-Pareja, M.C.; deFrance, S.D.; Baitzel, S.I.; Goldstein, P.S. Contextual, Taphonomic, and Paleoecological Insights from Anurans on Tiwanaku Sites in Southern Peru. Quaternary 2022, 5, 16. https://doi.org/10.3390/quat5010016
Rubinatto Serrano J, Vallejo-Pareja MC, deFrance SD, Baitzel SI, Goldstein PS. Contextual, Taphonomic, and Paleoecological Insights from Anurans on Tiwanaku Sites in Southern Peru. Quaternary. 2022; 5(1):16. https://doi.org/10.3390/quat5010016
Chicago/Turabian StyleRubinatto Serrano, Juliana, Maria Camila Vallejo-Pareja, Susan D. deFrance, Sarah I. Baitzel, and Paul S. Goldstein. 2022. "Contextual, Taphonomic, and Paleoecological Insights from Anurans on Tiwanaku Sites in Southern Peru" Quaternary 5, no. 1: 16. https://doi.org/10.3390/quat5010016
APA StyleRubinatto Serrano, J., Vallejo-Pareja, M. C., deFrance, S. D., Baitzel, S. I., & Goldstein, P. S. (2022). Contextual, Taphonomic, and Paleoecological Insights from Anurans on Tiwanaku Sites in Southern Peru. Quaternary, 5(1), 16. https://doi.org/10.3390/quat5010016






