Extreme Body Size Variation in Pleistocene Dwarf Elephants from the Siculo-Maltese Palaeoarchipelago: Disentangling the Causes in Time and Space
Abstract
:1. Introduction
1.1. Phylogeny and Palaeogeography
1.2. The Causes of Dwarfism in Insular Proboscideans
2. Materials and Methods
3. Results
4. Discussion
4.1. Palaeoloxodon falconeri
4.2. Possible Late Phyletic Ancestors of P. ex gr. P. falconeri
4.3. Probable Early Phyletic Ancestors of P. ex gr. P. falconeri
4.4. Earliest Insular Palaeoloxodont Ghost Lineage
4.5. P. ex gr. P. mnaidriensis from Puntali Cave, Sicily
4.6. The Possibility of a Late Middle Pleistocene Colonization of Sicily by P. antiquus
4.7. Palaeoloxodon sp. from Favignana Island
4.8. The Problems of Disentangling Ontogenetic Stage, Phylogeny and Sexual Size Dimorphism
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van der Geer, A.A.E.; van den Bergh, G.D.; Lyras, G.A.; Prasetyo, U.W.; Due, R.A.; Setiyabudi, E.; Drinia, H. The effect of area and isolation on insular dwarf proboscideans. J. Biogeogr. 2016, 43, 1656–1666. [Google Scholar] [CrossRef] [Green Version]
- Roth, V.L. Inferences from allometry and fossils: Dwarfing of elephants on islands. In Oxford Surveys in Evolutionary Biology; Futuyma, D., Antonovics, J., Eds.; Oxford University Press: Oxford, UK, 1992; pp. 259–288. [Google Scholar]
- Palombo, M.R. How can endemic proboscideans help us understand the “island rule”? A case study of Mediterranean islands. Quat. Int. 2007, 169, 105–124. [Google Scholar] [CrossRef]
- Herridge, V.L. Dwarf Elephants on Mediterranean Islands: A Natural Experiment in Parallel Evolution. Ph.D. Thesis, University College London, London, UK, 2010. Available online: http://discovery.ucl.ac.uk/133456/1/133456_Vol.1.pdf (accessed on 5 January 2022).
- Mangano, G.; Bonfiglio, L. First finding of a partially articulated elephant skeleton from a Late Pleistocene hyena den in Sicily (San Teodoro Cave, North Eastern Sicily, Italy). Quat. Int. 2012, 276–277, 53–60. [Google Scholar] [CrossRef]
- Romano, M.; Manucci, F.; Palombo, M.R. The smallest of the largest: New volumetric body mass estimate and in vivo restoration of the dwarf elephant Palaeoloxodon ex gr. P. falconeri from Spinagallo Cave (Sicily). Hist. Biol. 2019, 33, 340–353. [Google Scholar] [CrossRef]
- Mangano, G.; Insacco, G.; Bonfiglio, L.; Mazza, P.P.A. New finds from San Teodoro Cave: An updating of the Middle Pleistocene fossil record from Acquedolci (north-eastern Sicily). Paleobiodivers Paleoenviron. 2020, 100, 1065–1076. [Google Scholar] [CrossRef]
- Scarborough, M.E. Insular Adaptations in the Appendicular Skeleton of Sicilian and Maltese Dwarf Elephants. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 2020. Available online: https://open.uct.ac.za/handle/11427/32747 (accessed on 30 November 2021).
- Bonfiglio, L.; Mangano, G.; Marra, A.C.; Masini, F.; Pavia, M.; Petruso, D. Pleistocene Calabrian and Sicilian bioprovinces. Geobios 2002, 24, 29–39. [Google Scholar] [CrossRef]
- Palombo, M.R. Insular mammalian fauna dynamics and Paleogeography: A lesson from the Western Mediterranean islands. Integr. Zool. 2018, 13, 2–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedley, M. The Calabrian Stage, Pleistocene highstand in Malta: A new marker for unravelling the Late Neogene and Quaternary history of the islands. J. Geol. Soc. Lond. 2011, 168, 913–925. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 1967; 203p. [Google Scholar]
- Furlani, S.; Antonioli, F.; Biolchi, S.; Gambin, T.; Gauci, R.; Lo Presti, V.; Anzidei, M.; Devoto, S.; Palombo, M.R.; Sulli, A. Holocene Sea Level Change in Malta. Quat. Int. 2013, 288, 146–157. [Google Scholar] [CrossRef]
- Benítez-López, A.; Santini, L.; Gallego-Zamorano, J.; Milá, B.; Walkden, P.; Huijbregts, M.A.J.; Tobias, J.A. The island rule explains consistent patterns of body size evolution across terrestrial vertebrates. Nat. Ecol. Evol. 2021, 5, 768–786. [Google Scholar] [CrossRef]
- Foster, J.B. The evolution of mammals on islands. Nature 1964, 202, 234–235. [Google Scholar] [CrossRef]
- Waldren, W.H.; Ensenyat, J.A. (Eds.) World Islands in Prehistory: International Insular Investigations; BAR International Series; BAR International: Oxford, UK, 2002; pp. 445–453. [Google Scholar]
- van der Geer, A.; Lyras, G.; de Vos, J. Evolution of Island Mammals: Adaptation and Extinction of Placental Mammals on Islands, 2nd ed.; Wiley-Blackwell: Oxford, UK, 2021. [Google Scholar]
- Palombo, M.R. Body size structure of Pleistocene mammalian communities: What support is there for the “island rule”? Integr. Zool. 2009, 4, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Raia, P.; Meiri, S. The Island Rule in Large Mammals: Paleontology Meets Ecology. Evolution 2006, 60, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Lomolino, M.V.; Sax, D.F.; Palombo, M.R.; van der Geer, A.A. Of mice and mammoths: Evaluations of causal explanations for body size evolution in insular mammals. J. Biogeogr. 2012, 39, 842–854. [Google Scholar] [CrossRef]
- Lomolino, M.V.; van der Geer, A.A.; Lyras, G.A.; Palombo, M.R.; Sax, D.F.; Rozzi, R. Of mice and mammoths: Generality and antiquity of the island rule. J. Biogeogr. 2013, 40, 1427–1439. [Google Scholar] [CrossRef]
- van der Geer, A.; Lyras, G.A.; Lomolino, M.V.; Palombo, M.R.; Sax, D.F. Body size evolution of palaeoinsular mammals: Temporal variations and interspecific interactions. J. Biogeogr. 2013, 40, 1440–1450. [Google Scholar] [CrossRef]
- Clauss, M.; Streich, W.J.; Schwarm, A.; Ortmann, S.; Hummel, J. The relationship of food intake and ingesta passage predicts feeding ecology in two different megaherbivore groups. Oikos 2007, 116, 209–216. [Google Scholar] [CrossRef]
- McKay, G.M. Behavior and ecology of the Asiatic elephant in southeastern Ceylon. Smithson. Contr. Zool. 1973, 125, 1–113. [Google Scholar] [CrossRef] [Green Version]
- Viljoen, P.J. Spatial distribution and movements of elephants (Loxodonta africana) in the northern Namib Desert region of the Kaokoveld, South West Africa/Namibia. J. Zool. 1989, 219, 1–19. [Google Scholar] [CrossRef]
- Christiansen, P. Body size in proboscideans, with notes on elephant metabolism. Zool. J. Linn. Soc. 2004, 140, 523–549. [Google Scholar] [CrossRef] [Green Version]
- Viljoen, P.J.; Bothma, J.P. Daily movements of desert-dwelling elephants in the northern Namib Desert. S. Afr. J. Wildl. Res. 1990, 20, 69–72. [Google Scholar]
- Garland, T. Scaling the Ecological Cost of Transport to Body Mass in Terrestrial Mammals. Am. Nat. 1983, 121, 571–587. [Google Scholar] [CrossRef] [Green Version]
- Thouless, C.R. Long distance movements of elephants in northern Kenya. Afr. J. Ecol. 1995, 33, 321–334. [Google Scholar] [CrossRef]
- Thouless, C.R. Home ranges and social organization of female elephants in northern Kenya. Afr. J. Ecol. 1996, 34, 284–297. [Google Scholar] [CrossRef]
- Nowak, R.M. Walker’s Mammals of the World, 6th ed.; Johns Hopkins University Press: Baltimore, MD, USA, 1999; 1919p. [Google Scholar]
- Suc, J.P. Origin and evolution of the Mediterranean vegetation and climate in Europe. Nature 1984, 307, 429–432. [Google Scholar] [CrossRef]
- Suc, J.P.; Bertini, A.; Combourieu-Nebout, N.; Diniz, F.; Leroy, S.; Russo-Ermolli, E.; Zheng, Z.; Bessais, E.; Ferrier, J. Structure of West Mediterranean vegetation and climate since 5.3 ma. Acta. Zool. Cracov. 1995, 38, 3–16. [Google Scholar]
- Palkovacs, E.P. Explaining adaptive shifts in body size on islands: A life history Approach. OIKOS 2003, 103, 37–44. [Google Scholar] [CrossRef]
- Raia, P.; Barbera, C.; Conte, M. The fast life of a dwarfed giant. Evol. Ecol. 2003, 17, 293–312. [Google Scholar] [CrossRef]
- Dirks, W.; Bromage, T.G.; Agenbroad, L.D. The duration and rate of molar plate formation in Palaeoloxodon cypriotes and Mammuthus columbi from dental histology. Quat. Int. 2012, 255, 79–85. [Google Scholar] [CrossRef]
- Larramendi, A.; Palombo, M.R. Body Size, Biology and Encephalization Quotient of Palaeoloxodon ex gr. P. falconeri from Spinagallo Cave (Hyblean plateau, Sicily). Hystrix 2015, 26, 102–109. [Google Scholar]
- Long, E.S.; Courtney, K.L.; Lippert, J.C.; Wall-Scheffler, C.M. Reduced body size of insular black-tailed deer is caused by slowed development. Oecologia 2019, 189, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Clauss, M.; Dittmann, M.T.; Dennis, W.H.; Zerbe, P.; Codron, D. Low scaling of a life history variable: Analysing eutherian gestation periods with and without phylogeny informed statistics. Mamm. Biol. 2014, 79, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Ambrosetti, P. The Pleistocene dwarf elephants of Spinagallo (Siracusa, southeastern Sicily). Geol. Romana 1968, 7, 277–398. [Google Scholar]
- Haynes, G. Age profiles in Elephant and Mammoth Bone Assemblages. Quat. Res. 1985, 24, 333–345. [Google Scholar] [CrossRef]
- Haynes, G. Proboscidean die-offs and die-outs: Age profiles in fossil collections. J. Archaeol. Sci. 1987, 14, 659–668. [Google Scholar] [CrossRef]
- Haynes, G. Mammoths, Mastodonts, and Elephants; Cambridge University Press: Cambridge, UK, 1991; 395p. [Google Scholar]
- Simonelli, C. Un Approccio Biometrico alla Morfologia Dentaria per la Revisione della Specie Elephas Mnaidriensis; Fisiche e Naturali; Università degli Studi di Palermo: Palermo, Italy, 1995. [Google Scholar]
- Guenther, E.W. Auf Mittelmeer-Inseln während des Pleistozäns lebende Säuger und ihre morphologischen Abänderungen. Schr. Naturwiss. Ver. Schleswig-Holstein 1988, 57, 91–108. [Google Scholar]
- Marano, F.; Palombo, M.R. Dimorphic traits in the dwarf elephant “Palaeoloxodon falconeri” from Spinagallo Cave (Siracusa, south-eastern Sicily). In Proceedings of the 6th International Conference on Mammoths and their Relatives, Siatista, Greece, 5–12 May 2014; Volume 102, pp. 112–113. [Google Scholar]
- KÖhler, M.; Herridge, V.; Nacarino-Meneses, C.; Fortuny, J.; Moncunill-Solé, B.; Rosso, A.; Sanfilippo, R.; Palombo, M.R.; Moyà-Solà, S. Palaeohistology Reveals a Slow Pace of Life for the Dwarfed Sicilian Elephant. Sci. Rep. 2021, 11. Available online: https://www.nature.com/articles/s41598-021-02192-4 (accessed on 18 January 2022). [CrossRef]
- Larramendi, A. Shoulder height, body mass and shape of proboscideans. Acta Palaeontol. Pol. 2016, 61, 537–574. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.H.; Marquet, P.A.; Taper, M.L. Evolution of body size: Consequences of an energetic definition of fitness. Am. Nat. 1993, 142, 573. [Google Scholar] [CrossRef] [Green Version]
- Damuth, J. Cope’s Rule, the Island Rule and the scaling of mammalian population-density. Nature 1993, 365, 748–750. [Google Scholar] [CrossRef]
- Lomolino, M.V. Body size evolution in insular vertebrates: Generality of the island rule. J. Biogeogr. 2005, 32, 1683–1699. [Google Scholar] [CrossRef]
- Meiri, S.; Simberloff, D.; Dayan, T. Insular carnivore biogeography: Island area and mammalian optimal body size. Am. Nat. 2005, 165, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Lister, A.M. Dwarfing in island elephants and deer: Processes in relation to time of isolation. Symp. Zool. Soc. Lond. 1996, 69, 277–292. [Google Scholar]
- Tikhonov, A.; Agenbroad, L.; Vartanyan, S. Comparative analysis of the mammoth populations on Wrangel Island and the Channel Islands. Deinsea 2003, 9, 415–420. [Google Scholar]
- Meiri, S.; Dayan, T.; Simberloff, D. Body Size of Insular Carnivores: Little Support for the Island Rule. Am. Nat. 2004, 163, 469–479. [Google Scholar] [CrossRef] [Green Version]
- Meiri, S.; Dayan, T.; Simberloff, D. The generality of the island rule reexamined. J. Biogeogr. 2006, 33, 1571–1577. [Google Scholar] [CrossRef]
- Meiri, S.; Raia, P.; Phillimore, A.B. Slaying dragons: Limited evidence for unusual body size evolution on islands. J. Biogeogr. 2011, 38, 89–100. [Google Scholar] [CrossRef]
- Herridge, V.; Nita, D.; Schwenninger, J.; Mangano, G.; Bonfiglio, L.; Lister, A.; Richards, D. A new chronology for Spinagallo Cave (Sicily): Implications for the evolution of the insular dwarf elephant Palaeoloxodon falconeri. In Proceedings of the VIth International Conference on Mammoths and Their Relatives S.A.S.G., Siatista, Greece, 5–12 May 2014; Volume 102, p. 70. [Google Scholar]
- Bonavia, C.G. The early stages of the Maltese Pleistocene mammalian sequence, evidence from the Maghlaq Quaternary deposits. In Facets on Maltese Prehistory; Mifsud, A., Savonia-Ventura, C., Eds.; Prehistory Society of Malta: Mosta, Malta, 1999; pp. 33–40. [Google Scholar]
- Ferretti, M.P. The dwarf elephant Palaeoloxodon mnaidriensis from Puntali Cave, Carini (Sicily; late Middle Pleistocene): Anatomy, systematics and phylogenetic relationships. Quat. Int. 2008, 182, 90–108. [Google Scholar] [CrossRef]
- Mania, D. Die Umwelt der Elefanten von Neumark-Nord. In Elefantenreich: Eine Fossilwelt in Europa; Höhne, D., Schwarz, W., Eds.; Landesamt für Denkmalpflege und Archäologie Sachsen-Anhalt: Halle (Saale), Germany, 2010; pp. 187–194. [Google Scholar]
- Mania, D.; Mai, D.H. Der Klimacharakter der Warmzeit von Neumark-Nord 1. In Elefantenreich: Eine Fossilwelt in Europa; Höhne, D., Schwarz, W., Eds.; Landesamt für Denkmalpflege und Archäologie Sachsen-Anhalt: Halle (Saale), Germany, 2010; pp. 175–185. [Google Scholar]
- Palombo, M.R. Intra-specific variation of stylohyoid bones in Palaeoloxodon: A case study of Neumark-Nord 1 (Geiseltal, Sachsen-Anhalt, Germany) straight-tusked elephants. Quat. Int. 2012, 276, 77–92. [Google Scholar] [CrossRef]
- Mania, D. Der Fossilbericht von den Waldelefanten im Seebecken von Neumark-Nord. In Elefantenreich: Eine Fossilwelt in Europa; Höhne, D., Schwarz, W., Eds.; Landesamt für Denkmalpflege und Archäologie Sachsen-Anhalt: Halle (Saale), Germany, 2010; pp. 201–218. [Google Scholar]
- Palombo, M.R.; Allbayrak, E.; Marano, F. The straight-tusked elephants from Neumark-Nord. A glance into a lost world. In Elefantenreich: Eine Fossilwelt in Europa; Hohne, D., Schwarz, W., Eds.; Landesamt fur Denkmalpflege und Archaologie Sachsen-Anhalt: Halle (Saale), Germany, 2010; pp. 219–247. [Google Scholar]
- van der Geer, A.; Lyras, G.; de Vos, J.; Dermitzakis, M. Evolution of Island Mammals: Adaptation and Extinction of Placental Mammals on Islands; Wiley-Blackwell: Oxford, UK, 2010. [Google Scholar]
- Marra, A.C. Evolution of Endemic Species, Ecological Interactions and Geographical Changes in an Insular Environment: A Case Study of Quaternary Mammals of Sicily (Italy, EU). Geoscience 2013, 3, 114–139. [Google Scholar] [CrossRef] [Green Version]
- Baleka, S.; Herridge, V.L.; Catalano, G.; Lister, A.M.; Dickinson, M.R.; Di Patti, C.; Barlow, A.; Penkman, K.E.H.; Hofreiter, M.; Paijmans, J.L.A. Estimating the dwarfing rate of an extinct Sicilian elephant. Curr. Biol. 2021, 31, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.; Lister, A. Palaeoloxodon cypriotes, the dwarf elephant of Cyprus: Size and scaling comparisons with P. falconeri (Sicily Malta) and mainland P. antiquus. In Proceedings of the 1st International Congress of the World of Elephants, Rome, Italy, 6–20 October 2001; pp. 479–480. [Google Scholar]
- Herridge, V.L.; Lister, A.M. Extreme Insular Dwarfism Evolved in a Mammoth. Proc. R. Soc. B 2012, 279, 3193–3200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogiatzakis, I.N.; Pungetti, G.; Mannion, A.M. (Eds.) Mediterranean Island Landscapes: Natural and Cultural Approaches; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Patton, M. Islands in Time: Island Sociogeography and Mediterranean Prehistory; Routledge: Abingdon, UK, 1996. [Google Scholar]
- Antonioli, F.; Presti, V.L.; Morticelli, M.G.; Bonfiglio, L.; Mannino, M.A.; Palombo, M.R.; Sannino, G.; Ferranti, L.; Furlani, S.; Lambeck, K.; et al. Timing of the emergence of the Europe-Sicily bridge (40–17 cal ka BP) and its implications for the spread of modern humans. In Geology and Archaeology: Submerged Landscapes of the Continental Shelf; Harff, J., Bailey, G., Luth, F., Eds.; Special Publications; Geological Society: London, UK, 2016; Volume 411, pp. 111–144. [Google Scholar]
- Scarborough, M.E.; Palombo, M.R.; Chinsamy, A. Insular adaptations in the astragalus-calcaneus of Sicilian and Maltese dwarf elephants. Quat. Int. 2016, 406, 111–122. [Google Scholar] [CrossRef]
- Accordi, B.; Colacicchi, R. Excavations in the Pygmy Elephants Cave of Spinagallo (Siracusa). Geol. Romana 1962, 1, 217–229. [Google Scholar]
- Palombo, M.R. Endemic elephants of the Mediterranean Islands: Knowledge, problems and perspectives. In Proceedings of the 1st International Congress of the World of Elephants, Rome, Italy, 6–20 October 2001; pp. 486–491. [Google Scholar]
- Micallef, A.; Foglini, F.; Le Bas, T.; Angeletti, L.; Maselli, V.; Pasuto, A.; Taviani, M. Submerged Palaeolandscape of the Maltese islands: Morphology, evolution and relation to quaternary environmental change. Mar. Geol. 2012, 335, 129–147. [Google Scholar] [CrossRef]
- Foglini, F.; Prampolini, M.; Micallef, A.; Angeletti, L.; Vandelli, V.; Deidun, A.; Soldati, M.; Taviani, M. Late Quaternary coastal landscape morphology and evolution of the Maltese Islands (Mediterranean Sea) reconstructed from high-resolution seafloor data. In Geology and Archaeology: Submerged Landscapes of the Continental Shelf; Harff, J., Bailey, G., Luth, F., Eds.; Special Publications; Geological Society: London, UK, 2016; Volume 411, pp. 77–95. [Google Scholar]
- Prampolini, M.; Foglini, F.; Biolchi, S.; Devoto, S.; Angelini, S.; Soldati, M. Geomorphological mapping of terrestrial and marine areas, northern Malta and Comino (central Mediterranean Sea). J. Maps 2017, 13, 457–469. [Google Scholar] [CrossRef]
- Bonfiglio, L.; Insacco, G. Palaeoenvironmental, paleontologic and stratigraphic significance of vertebrate remains in Pleistocene limnic and alluvial deposits from southeastern Sicily. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1992, 95, 195–208. [Google Scholar] [CrossRef]
- Bonfiglio, L.; Marra, A.C.; Masini, F. The Contribution of Quaternary Vertebrates to Palaeoenvironmental and Palaeoclimatological Reconstructions in Sicily; Special Publications; Geological Society: London, UK, 2000; Volume 181, pp. 171–184. [Google Scholar]
- Bonfiglio, L.; Marra, A.C.; Masini, F.; Petruso, D. Depositi a vertebrati e ambienti costieri pleistocenici della Sicilia e della Calabria meridionale. Biogeographia 2001, 22, 29–43. [Google Scholar] [CrossRef] [Green Version]
- Stuart, A.J. The extinction of woolly mammoth (Mammuthus primigenius) and straight-tusked elephant (Palaeoloxodon antiquus) in Europe. Quat. Int. 2005, 126, 171–177. [Google Scholar] [CrossRef]
- Palombo, M.R. Discrete dispersal bioevents of large mammals in Southern Europe in the post-Olduvai Early Pleistocene: A critical overview. Quat. Int. 2017, 431 Pt B, 3–19. [Google Scholar] [CrossRef]
- Palombo, M.R. Gli Elefanti del Pliocene Superiore e del Pleistocene dell’Italia Centrale Peninsulare, alcune Considerazioni; Studi Geologici Camerti (Special Volume, Biostratigrafia dell’Italia Central); Università di Camerino: Camerino, Italy, 1994; pp. 447–457. [Google Scholar]
- Lister, A.M. Rapid dwarfing of red deer on Jersey in the Last Interglacial. Nature 1989, 342, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Sondaar, P.Y.; van der Geer, A.A.E. Plio-Pleistocene terrestrial vertebrate faunal evolution on Mediterranean islands, compared to that of the Palearctic mainland. Annales Géologiques des Pays Helléniques 2002, 39A, 165–180. [Google Scholar]
- Rozzi, R.; Lomolino, M.V. Rapid dwarfing of an insular mammal—The Feral Cattle of Amsterdam Island. Nat. Sci. Rep. 2017, 7, 8820. [Google Scholar] [CrossRef]
- Diniz-Filho, J.A.F.; Santos, A.M.C.; Barreto, E.; Naves, F.; Santos, W.; Souza, K.S.; Santos-Silva, R.; Dobrovolski, R.; Soares, T.N.; Tidon, R.; et al. Quantitative genetics of extreme insular dwarfing: The case of red deer on Jersey. J. Biogeogr. 2021, 48, 1720–1730. [Google Scholar] [CrossRef]
- Diniz-Filho, J.A.F.; Jardim, L.; Rangel, T.F.; Holden, P.B.; Edwards, N.R.; Hortal, J.; Santos, A.M.C.; Raia, P. Quantitative genetics of body size evolution on islands: An individual-based simulation approach. Biol. Lett. 2019, 15. [Google Scholar] [CrossRef]
- Theodorou, G.; Symeonidis, N.; Stathopoulou, E. Elephas tiliensis n. sp. from Tilos island (Dodecanese, Greece). Hellenic J. Geosci. 2007, 42, 19–32. [Google Scholar]
- Hardin, G. The Competitive Exclusion Principle. Science 1960, 131, 1292–1297. [Google Scholar] [CrossRef] [Green Version]
- Lister, A.M. Ecological interactions of elephantids in Pleistocene Eurasia: Palaeoloxodon and Mammuthus. In Human Palaeoecology in the Levantine Corridor; Goren-Inbar, N., Spelth, J.D., Eds.; Oxbow: Oxford, ME, USA, 2004; pp. 53–60. [Google Scholar]
- Stuart, A.J. Pleistocene Vertebrates in the British Isles; Longman: London, UK, 1982; 212p. [Google Scholar]
- Rivals, F.; Semprebon, G.; Lister, A. An examination of dietary diversity patterns in Pleistocene proboscideans (Mammuthus, Palaeoloxodon, and Mammut) from Europe and North America as revealed by dental microwear. Biol. Conserv. 2012, 255, 188–195. [Google Scholar] [CrossRef]
- Palombo, M.R.; Ferretti, M.P. Elephant fossil record from Italy: Knowledge, problems, and perspectives. Quat. Int. 2005, 126–128, 107–136. [Google Scholar] [CrossRef]
- Chilardi, S. Large-sized and middle-sized elephants from the Pleistocene of Sicily: The case of Contrada Fusco (Siracusa, Southeastern Sicily). In The World of Elephants: Proceedings of the First International Congress; Cavaretta, G., Gioia, P., Mussi, M., Palombo, M.R., Eds.; CNR: Rome, Italy, 2001; pp. 476–478. [Google Scholar]
- Rhodes, E.J. ESR dating of tooth enamel. In Siracusa, le ossa dei Giganti: Lo Scavo Paleontologico di Contrada Fusco; Basile, B., Chilardi, S., Eds.; Arnaldo Lombardi: Siracusa, Italy, 1996; pp. 39–44. [Google Scholar]
- Bonfiglio, L.; Esu, D.; Mangano, G.; Masini, F.; Petruso, D.; Soligo, M.; Tuccimei, P. Late Pleistocene vertebrate-bearing deposits at San Teodoro Cave (Northe-Eastern Sicily): Preliminary data on faunal diversification and chronology. Quat. Int. 2008, 190, 26–37. [Google Scholar] [CrossRef]
- Palombo, M.R.; Di Patti, C.; Lo Presti, V.; Scarborough, M.E. Was the dwarfed Palaeoloxodon from Favignana Island the last endemic Pleistocene elephant from the western Mediterranean islands? Hist. Biol. 2020. [Google Scholar] [CrossRef]
- Kuhlemann, J.; Rohling, E.J.; Krumrei, I.; Kubik, P.; Ivy-Ochs, S.; Kucera, M. Regional synthesis of Mediterranean atmospheric circulation during the last glacial maximum. Science 2008, 321, 1338–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, J.R.M.; Watts, W.A.; Huntley, B. Weichselian palynostratigraphy, palaeovegetation and palaeoenvironment; the record from Lago Grande di Monticchio, southern Italy. Quat. Int. 2000, 74, 91–110. [Google Scholar] [CrossRef]
- Yll, R.; Carrión, J.S.; Marra, A.C.; Bonfiglio, L. Vegetation reconstruction on the basis of pollen in Late Pleistocene hyena coprolites from San Teodoro Cave (Sicily, Italy). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 237, 32–39. [Google Scholar] [CrossRef]
- Symeonidis, N.; Bachmeyer, F.; Zapfe, H. Grabungen in der Zwergelefanten-Höhle ‘Charkadio’ auf der Insel Tilos (Dodekanes, Griechenland). Ann. Naturhist. Mus. Wien. 1973, 77, 133–139. [Google Scholar]


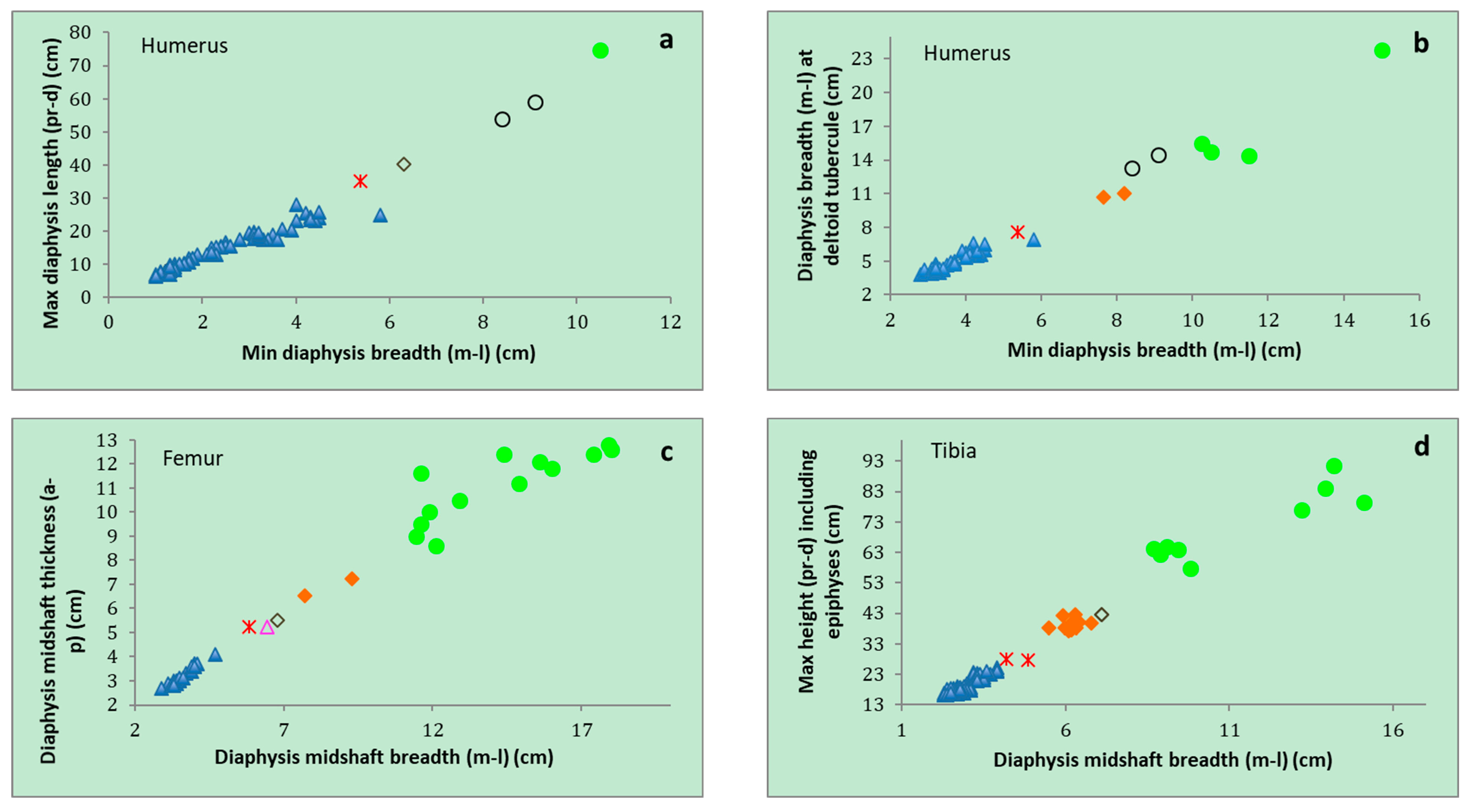
| Taxon | Shoulder Height (m) | Provenance | Chronology | Collections |
|---|---|---|---|---|
| Palaeoloxodon antiquus | 3.0–4.0 | Neumark-Nord 1, Germany | late Middle or early Late Pleistocene | Landesmuseum für Vorgeschichte, Halle, Landesamt für Denkmalpflege und Archäologie Sachsen-Anhalt |
| Riano, Italy | Middle Pleistocene | Museo di Paleontologia, Sapienza, University of Rome | ||
| Blanzac, France | Pleistocene | Naturhistorisches Museum, Basel | ||
| Palaeoloxodon ex gr. P. mnaidriensis | 2.0 | Puntali Cave, Sicily | late Middle—early Late Pleistocene | Museo di paleontologia e geologia Gaetano Giorgio Gemmellaro, Università di Palermo; Naturhistorisches Museum, Basel |
| Palaeoloxodon sp. | ~1.7 | Grotta dell’Ucceria, Favignana | Late Pleistocene | Museo di paleontologia e geologia Gaetano Giorgio Gemmellaro, Università di Palermo |
| Palaeoloxodon mnaidriensis | ~1.7 | Mnaidra Gap, Malta | Pleistocene | Natural History Museum, London |
| Palaeoloxodon ‘melitensis’ | - | Benghisa Gap, Malta | Middle Pleistocene | Natural History Museum, London |
| Palaeoloxodon sp. 1 | ~1.8 | Luparello Fissure, Sicily | late Early or early Middle Pleistocene | Insitut de Paléontologie Humaine, Paris; Museo di paleontologia e geologia Gaetano Giorgio Gemmellaro, Università di Palermo |
| Palaeoloxodon falconeri | 1.0–1.2 | Luparello Fissure, Sicily | Middle Pleistocene | Insitut de Paléontologie Humaine, Paris; Museo di paleontologia e geologia Gaetano Giorgio Gemmellaro, Università di Palermo |
| Spinagallo Cave, Sicily | Middle Pleistocene | Geological Museum, University of Catania; Museo di Paleontologia, Sapienza, University of Rome; Naturhistorisches Museum, Basel |
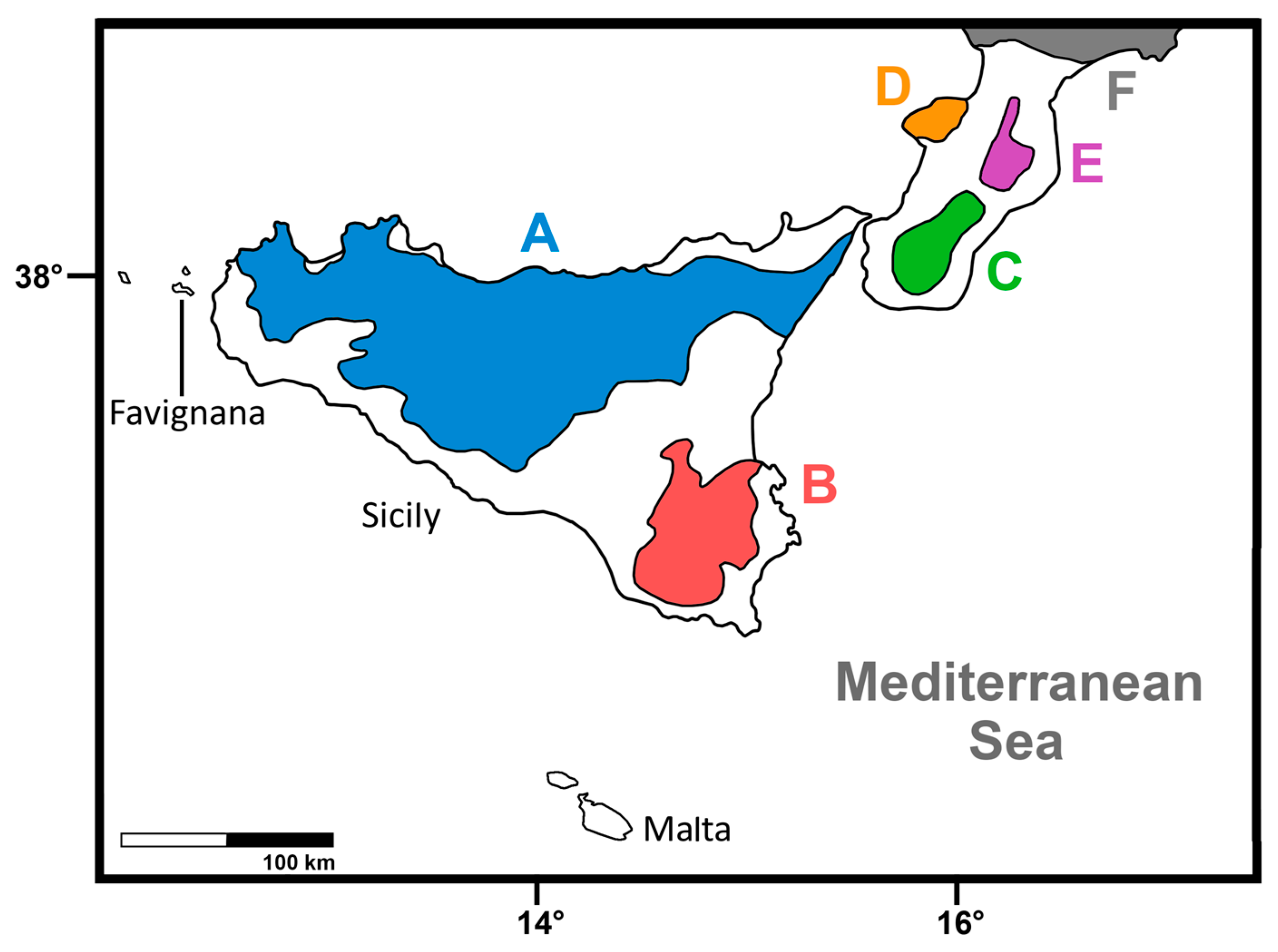
| Taxon | Island | Figures | Locality | Age | Predators | Competitors | Duration of Isolation (ka) |
|---|---|---|---|---|---|---|---|
| P. antiquus | Sicily | Figure 1 | Unknown | Pleistocene | ? | ? | ? |
| P. ex gr. P. mnaidriensis | Sicily | Figure 3a,c and Figure 4b–d | Puntali Cave | late Middle-early Late Pleistocene | Panthera leo, Crocuta crocuta spelaea, Canis lupus, Ursus cf. arctos | Hippopotamus pentlandi, Cervus elaphus siciliae, Dama carburangelensis, Bos primigenius siciliae, Bison priscus siciliae | ? |
| Palaeoloxodon sp. | Sicily | Figure 4a,b | Contrada Fusco | MIS5? (130–80 ka) | Likely all taxa in cell above | Likely all taxa in cell above | Likely relatively brief (tens of ka) |
| Palaeoloxodon sp. | Sicily | Figure 4a,c,d | San Teodoro Cave | Younger than 32 ± 4 ka | Likely all taxa in cell above | Likely all taxa in cell above; also Equus hydruntinus | Likely > 50 ka |
| Palaeoloxodon sp. | Favignana | Figure 3d | Grotta dell’Ucceria | 20.350–19.840 cal. BP | None? | ?Dama carburangelensis | Likely ca. 10 ka longer than at San Teodoro |
| Palaeoloxodon sp. 1 | Northern-Central Sicily * and/or Sicily | Figure 3a,d | Luparello Fissure | Late Early or early Middle Pleistocene | None | None | # Likely > 340 ka |
| P. falconeri | Sicily | Figure 3a,c,d and Figure 4a–d | Spinagallo Cave | Middle Pleistocene (ca. 350–230 ka) | None | None | # Likely > 460 ka |
| P. mnaidriensis | Malta | Figure 3a | Mnaidra Gap | ?Middle Pleistocene | None | None? | ? |
| P. ‘melitensis’ | Malta | Figure 3c,d | Benghisa Gap | Pleistocene | None? | None? | ? |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarborough, M.E. Extreme Body Size Variation in Pleistocene Dwarf Elephants from the Siculo-Maltese Palaeoarchipelago: Disentangling the Causes in Time and Space. Quaternary 2022, 5, 17. https://doi.org/10.3390/quat5010017
Scarborough ME. Extreme Body Size Variation in Pleistocene Dwarf Elephants from the Siculo-Maltese Palaeoarchipelago: Disentangling the Causes in Time and Space. Quaternary. 2022; 5(1):17. https://doi.org/10.3390/quat5010017
Chicago/Turabian StyleScarborough, Matthew Edward. 2022. "Extreme Body Size Variation in Pleistocene Dwarf Elephants from the Siculo-Maltese Palaeoarchipelago: Disentangling the Causes in Time and Space" Quaternary 5, no. 1: 17. https://doi.org/10.3390/quat5010017
APA StyleScarborough, M. E. (2022). Extreme Body Size Variation in Pleistocene Dwarf Elephants from the Siculo-Maltese Palaeoarchipelago: Disentangling the Causes in Time and Space. Quaternary, 5(1), 17. https://doi.org/10.3390/quat5010017





