Defining Fragmentation Patterns of Archaeological Bone Remains without Typologies: A Landmark-Based Approach on Rodent Mandibula
Abstract
:1. Introduction
2. Materials
3. Methods
4. Results
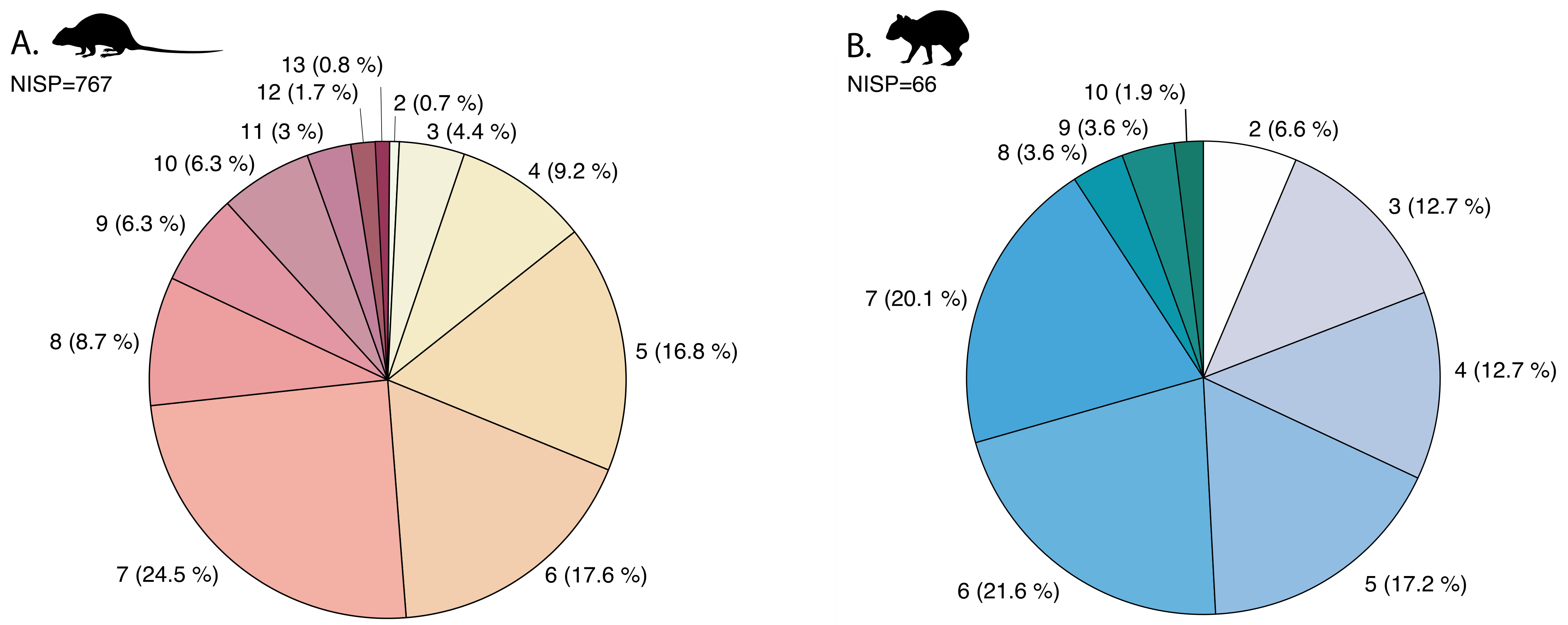
4.1. Mandibular Preservation
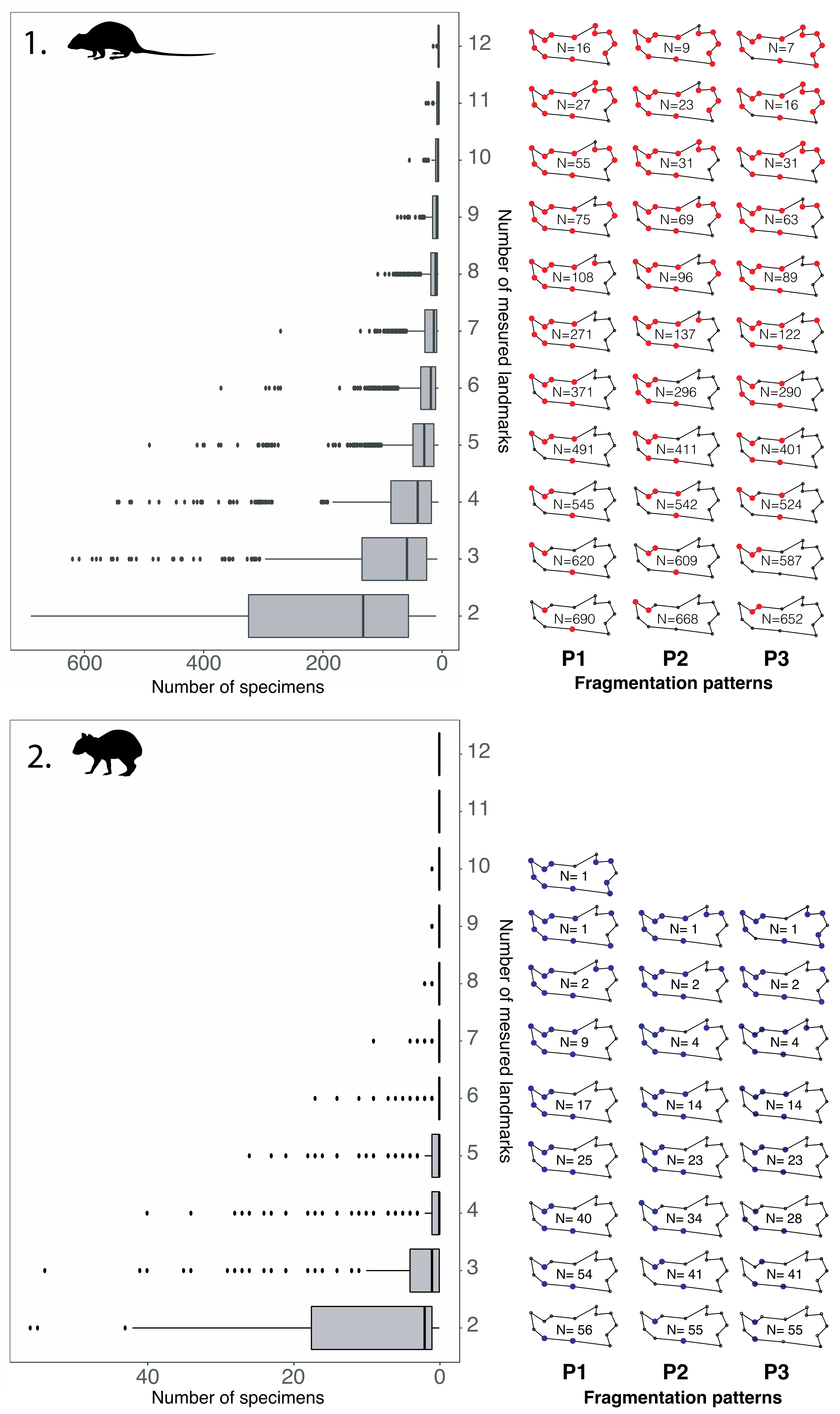
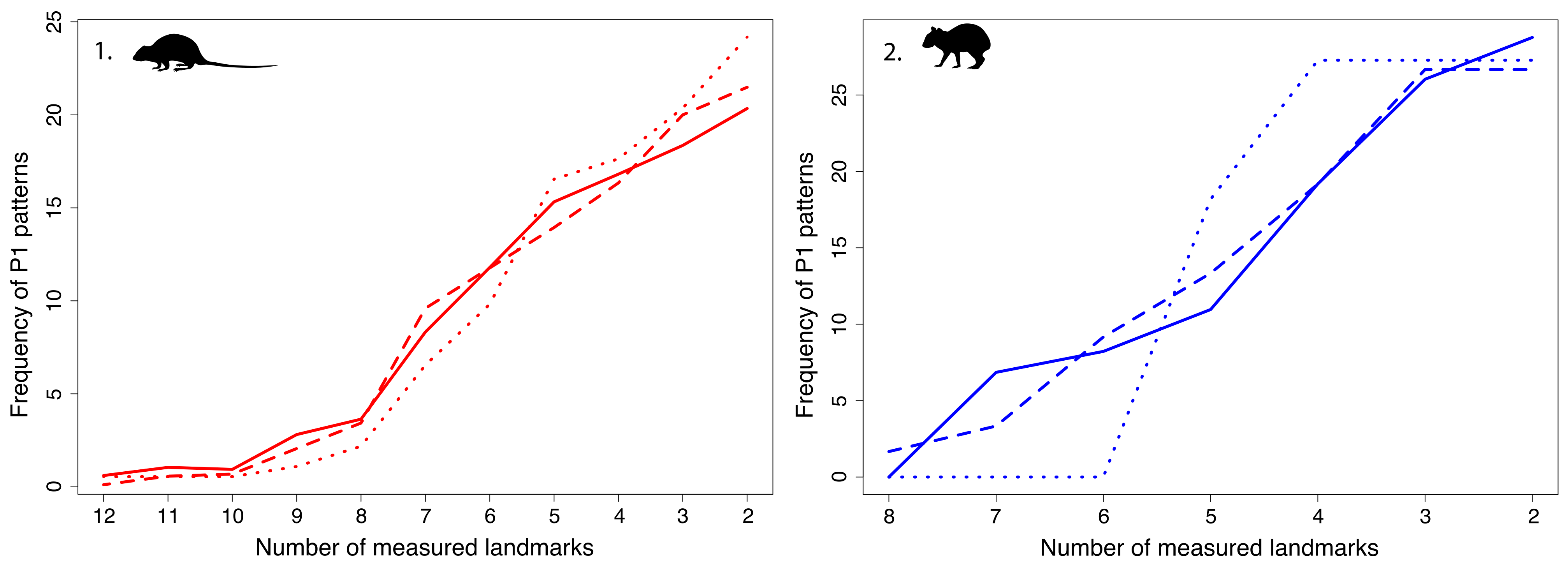
4.2. Fragmentation Patterns
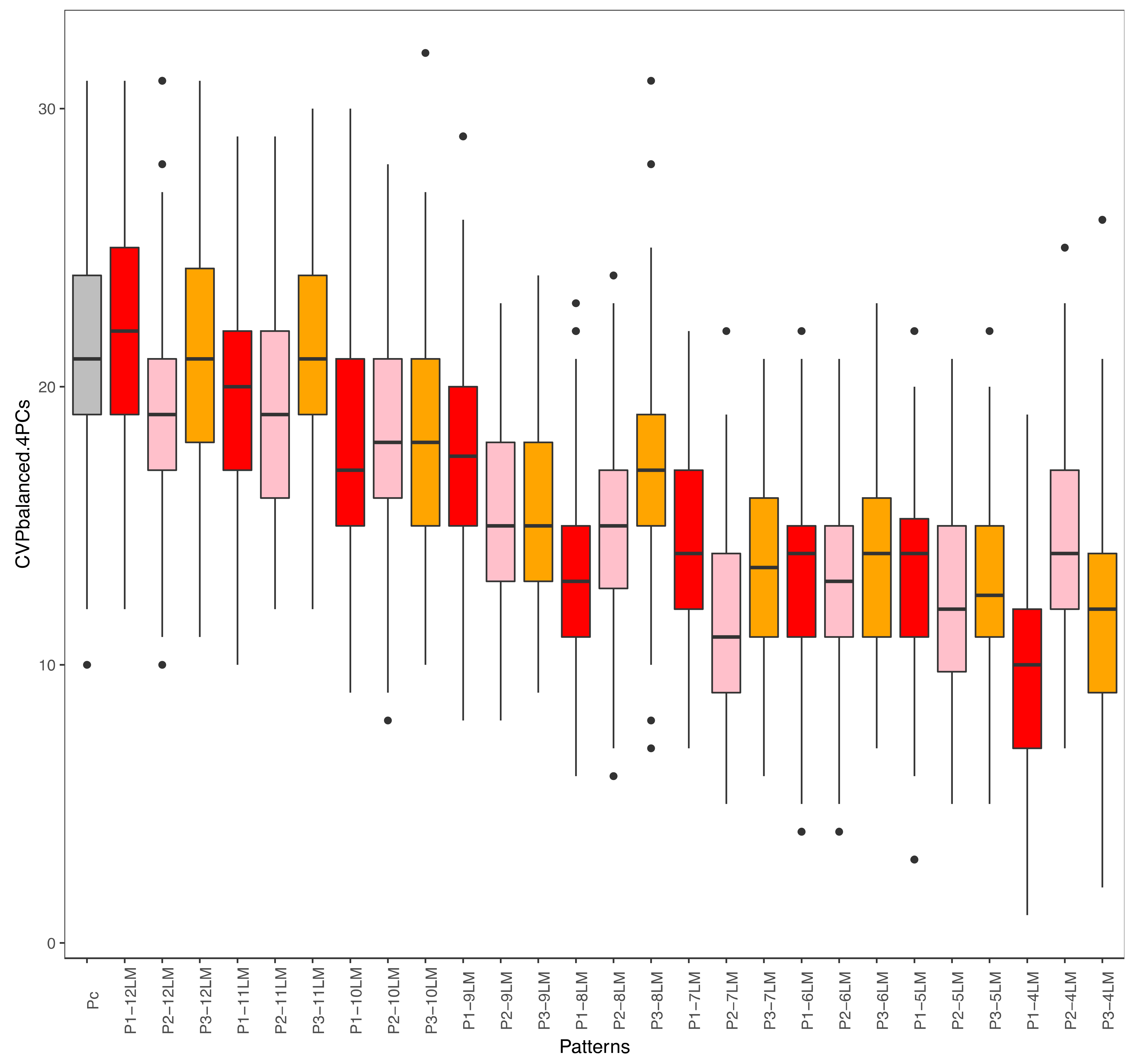
4.3. Which Pattern to Select for a Geometric Morphometrics Study? The Case of Oryzomyini
5. Discussion
5.1. Impact of Mandibular Properties on Preservation
5.2. Application to Zooarchaeological Studies
5.3. Interpretation of Differential Preservation between Taxa
5.4. Future Geometric Morphometrics Application
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Behrensmeyer, A.K.; Kidwell, S.M.; Gastaldo, R.A. Taphonomy and Paleobiology. Paleobiology 2000, 26, 103–147. [Google Scholar] [CrossRef]
- Brugal, J.-P. TaphonomieS; Archives Contemporaines: Paris, France, 2017. [Google Scholar]
- Davis, S.J. The Archaeology of Animals; Routledge: London, UK, 2012. [Google Scholar]
- Grouard, S. Première Contribution à La Détermination de l’origine Des Assemblages Fossiles de Prolagus Sardus (Sur Le Massif Corso-Sarde). In Utilisation Des Statistiques En Recherche Taphonomique; Mémoire de DEA, Université Paris X Nanterre: Paris, France, 1994. [Google Scholar]
- Horard-Herbin, M.-P.; Lepetz, S.; Vallet, C.; Clavel, B.; Corbellini, J.-P.; Guintard, C. Des Traces Observées Aux Gestes Anthropiques: Le Projet D. Coupes. Nouv. Archéologie 2017, 148, 16–22. [Google Scholar] [CrossRef]
- Pickering, T.R. Taphonomy of the Swartkrans hominid postcrania and its bearing on issues of meat-eating and fire management. In Meat-Eating and Human Evolution; Oxford University Press Oxford: Oxford, UK, 2001; pp. 33–51. [Google Scholar]
- Pickering, T.R.; Clarke, R.J.; Moggi-Cecchi, J. Role of Carnivores in the Accumulation of the Sterkfontein Member 4 Hominid Assemblage: A Taphonomic Reassessment of the Complete Hominid Fossil Sample (1936–1999). Am. J. Phys. Anthropol. Off. Publ. Am. Assoc. Phys. Anthropol. 2004, 125, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.R.K. Carnivore Damage to Antelope Bones and Its Archaeological Implications. Palaeontologia Africana 1980, 23, 109–125. [Google Scholar]
- Val, A.; Taru, P.; Steininger, C. New Taphonomic Analysis of Large-Bodied Primate Assemblage from Cooper’s D, Bloubank Valley, South Africa. S. Afr. Archaeol. Bull. 2014, 199, 49–58. [Google Scholar]
- Vigne, J. Découpe Du Cerf (Cervus Elaphus) Au Mésolithique Moyen, à Noyen-Sur-Seine (Seine-et-Marne): Analyses Tracéologique et Expérimentale. Rev. Paléobiol. 2006, 24, 69. [Google Scholar]
- Vigne, J.-D.; Marinval-Vigne, M.-C.; De Lanfranchi, F.; Weiss, M.-C. Consommation Du Lapin-Rat (Prolagus Sardus Wagner) Au Néolithique Ancien Méditerranéen Abri d’Araguina-Sennola (Bonifacio, Corse). Bull. Société Préhistorique Fr. 1981, 78, 222–224. [Google Scholar]
- Vigne, J.-D.; Marinval-Vigne, M.-C. Méthode pour la mise en évidence de la consommation du petit gibier. In Animals and Archaeology: Hunters and Their Prey; BAR International Series: Oxford, UK, 1983; Volume 163, pp. 239–242. [Google Scholar]
- Gautier, A. L’apport de l’étude Des Vestiges Animaux à La Reconstitution Archéologique: Une Introduction à l’archéozoologie. Rev. Nord 1988, 70, 23–37. [Google Scholar] [CrossRef]
- Brain, C.K. Hottentot Food Remains and Their Bearing on the Interpretation of Fossil Bone Assemblages. Sci. Pap. Namib Desert Res. Stn. 1967, 1967, 1–11. [Google Scholar]
- Pavao, B.; Stahl, P.W. Structural Density Assays of Leporid Skeletal Elements with Implications for Taphonomic, Actualistic and Archaeological Research. J. Archaeol. Sci. 1999, 26, 53–66. [Google Scholar] [CrossRef]
- Marean, C.W. Measuring the Post-Depositional Destruction of Bone in Archaeological Assemblages. J. Archaeol. Sci. 1991, 18, 677–694. [Google Scholar] [CrossRef]
- Gifford-Gonzalez, D. Calibrating Bone Durability. In An Introduction to Zooarchaeology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 453–474. [Google Scholar]
- Andrews, P.; Cook, J. Owls, Caves and Fossils: Predation, Preservation and Accumulation of Small Mammal Bones in Caves, with an Analysis of the Pleistocene Cave Faunas from Westbury-Sub-Mendip, Somerset, UK; University of Chicago Press: Chicago, IL, USA, 1990. [Google Scholar]
- Denys, C. Palaeoenvironmental and Palaeobiogeographical Significance of the Fossil Rodent Assemblages of Laetoli (Pliocene, Tanzania). Palaeogeogr. Palaeoclimatol. Palaeoecol. 1985, 52, 77–97. [Google Scholar] [CrossRef]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Mitteroecker, P.; Gunz, P. Advances in Geometric Morphometrics. Evol. Biol. 2009, 36, 235–247. [Google Scholar] [CrossRef] [Green Version]
- Rohlf, F.J.; Slice, D. Extensions of the Procrustes Method for the Optimal Superimposition of Landmarks. Syst. Biol. 1990, 39, 40–59. [Google Scholar] [CrossRef] [Green Version]
- Clavel, J.; Merceron, G.; Escarguel, G. Missing Data Estimation in Morphometrics: How Much Is Too Much? Syst. Biol. 2014, 63, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.E. When Is a Wolf a Dog? Combined Geometric Morphometrics and Stable Isotope Analyses for Differentiating Wild from Domestic Canids on the Northern Plains. Plains Anthropol. 2019, 64, 316–349. [Google Scholar] [CrossRef]
- Neeser, R.; Ackermann, R.R.; Gain, J. Comparing the Accuracy and Precision of Three Techniques Used for Estimating Missing Landmarks When Reconstructing Fossil Hominin Crania. Am. J. Phys. Anthropol. Off. Publ. Am. Assoc. Phys. Anthropol. 2009, 140, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cornette, R.; Herrel, A.; Stoetzel, E.; Moulin, S.; Hutterer, R.; Denys, C.; Baylac, M. Specific Information Levels in Relation to Fragmentation Patterns of Shrew Mandibles: Do Fragments Tell the Same Story? J. Archaeol. Sci. 2015, 53, 323–330. [Google Scholar] [CrossRef]
- Comay, O.; Dayan, T. Taphonomic Signatures of Owls: New Insights into Micromammal Assemblages. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 492, 81–91. [Google Scholar] [CrossRef]
- Dodson, P.; Wexlar, D. Taphonomic Investigations of Owl Pellets. Paleobiology 1979, 5, 275–284. [Google Scholar] [CrossRef]
- Giovas, C.M. Pre-Columbian Amerindian Lifeways at the Sabazan Site, Carriacou, West Indies. J. Isl. Coast. Archaeol. 2018, 13, 161–190. [Google Scholar] [CrossRef]
- Grouard, S. Variation Des Stratégies de Subsistance Des Précolombiens à Hope Estate, Saint-Martin (Petites Antilles), d’après l’analyse Des Restes Des Petits Vertébrés. In Petits Animaux et Sociétés Humaines; APDCA: Antibes, France, 2004; pp. 441–467. [Google Scholar]
- Newsom, L.A.; Wing, E.S. On Land and Sea: Native American Uses of Biological Resources in the West Indies; University of Alabama Press: Tuscaloosa, AL, USA, 2004; ISBN 0-8173-1315-X. [Google Scholar]
- Wing, E.S. Native American use of animals in the Caribbean. In Biogeography of the West Indies: Patterns and Perspectives, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2001; pp. 481–518. [Google Scholar]
- Wing, E.S. The Sustainability of Resources Used by Native Americans on Four Caribbean Islands. Int. J. Osteoarchaeol. 2001, 11, 112–126. [Google Scholar] [CrossRef]
- Durocher, M.; Nicolas, V.; Perdikaris, S.; Bonnissent, D.; Robert, G.; Debue, K.; Evin, A.; Grouard, S. Archaeobiogeography of Extinct Rice Rats (Oryzomyini) in the Lesser Antilles during the Ceramic Age (500 BCE–1500 CE). Holocene 2020, 31, 433–445. [Google Scholar] [CrossRef]
- Maestri, R.; Luza, A.L.; Barros, L.; Maria Hartz, S.; Ferrari, A.; Freitas, T.; Duarte, L. Geographical Variation of Body Size in Sigmodontine Rodents Depends on Both Environment and Phylogenetic Composition of Communities. J. Biogeogr. 2016, 43, 1192–1202. [Google Scholar] [CrossRef]
- Maestri, R.; Monteiro, L.R.; Fornel, R.; Upham, N.S.; Patterson, B.D.; de Freitas, T.R.O. The Ecology of a Continental Evolutionary Radiation: Is the Radiation of Sigmodontine Rodents Adaptative? Evolution 2016, 71, 610–632. [Google Scholar] [CrossRef] [PubMed]
- Rohlf, F.J. TpsDig, Ver. Acta Ichthyol. Piscat. 2010, 43, 219. [Google Scholar]
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.; Takahashi, K.; Wilke, C.; Woo, K. R Package ‘Ggplot2’v. 3. Cran R 2019, 11, 154. [Google Scholar]
- Murrell, P. R Graphics; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Barone, R. Anatomie Comparée Des Mammifères Domestiques. In Tome 1: Ostéologie; Vigot Freres: Paris, France, 1986; p. 761. [Google Scholar]
- Cornette, R.; Herrel, A.; Cosson, J.-F.; Poitevin, F.; Baylac, M. Rapid Morpho-Functional Changes among Insular Populations of the Greater White-Toothed Shrew. Biol. J. Linn. Soc. 2012, 107, 322–331. [Google Scholar] [CrossRef] [Green Version]
- Chaix, L.; Méniel, P. Archéozoologie: Les Animaux et L’archéologie; Errance: Paris, France, 2001. [Google Scholar]
- Cucchi, T.; Barnett, R.; Martínková, N.; Renaud, S.; Renvoisé, E.; Evin, A.; Sheridan, A.; Mainland, I.; Wickham-Jones, C.; Tougard, C.; et al. The Changing Pace of Insular Life: 5000 Years of Microevolution in the Orkney Vole (Microtus Arvalis Orcadensis). Evolution 2014, 68, 2804–2820. [Google Scholar] [CrossRef] [Green Version]
- Evin, A.; Cucchi, T.; Cardini, A.; Vidarsdottir, U.S.; Larson, G.; Dobney, K. The Long and Winding Road: Identifying Pig Domestication through Molar Size and Shape. J. Archaeol. Sci. 2013, 40, 735–743. [Google Scholar] [CrossRef]
- Evin, A.; Flink, L.G.; Bălăşescu, A.; Popovici, D.; Andreescu, R.; Bailey, D.; Mirea, P.; Lazăr, C.; Boroneanţ, A.; Bonsall, C. Unravelling the Complexity of Domestication: A Case Study Using Morphometrics and Ancient DNA Analyses of Archaeological Pigs from Romania. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20130616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neaux, D.; Sansalone, G.; Lecompte, F.; Noûs, C.; Haruda, A.; Schafberg, R.; Cucchi, T. Examining the Effect of Feralization on Craniomandibular Morphology in Pigs, Sus Scrofa (Artiodactyla: Suidae). Biol. J. Linn. Soc. 2020, 131, 870–879. [Google Scholar] [CrossRef]
- Harbers, H.; Neaux, D.; Ortiz, K.; Blanc, B.; Laurens, F.; Baly, I.; Callou, C.; Schafberg, R.; Haruda, A.; Lecompte, F.; et al. The Mark of Captivity: Plastic Responses in the Ankle Bone of a Wild Ungulate (Sus Scrofa). R. Soc. Open Sci. 2020, 7, 192039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ameen, C.; Hulme-Beaman, A.; Evin, A.; Germonpré, M.; Britton, K.; Cucchi, T.; Larson, G.; Dobney, K. A Landmark-Based Approach for Assessing the Reliability of Mandibular Tooth Crowding as a Marker of Dog Domestication. J. Archaeol. Sci. 2017, 85, 41–50. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durocher, M.; Grouard, S.; Nicolas, V.; Maestri, R.; Evin, A. Defining Fragmentation Patterns of Archaeological Bone Remains without Typologies: A Landmark-Based Approach on Rodent Mandibula. Quaternary 2022, 5, 14. https://doi.org/10.3390/quat5010014
Durocher M, Grouard S, Nicolas V, Maestri R, Evin A. Defining Fragmentation Patterns of Archaeological Bone Remains without Typologies: A Landmark-Based Approach on Rodent Mandibula. Quaternary. 2022; 5(1):14. https://doi.org/10.3390/quat5010014
Chicago/Turabian StyleDurocher, Marine, Sandrine Grouard, Violaine Nicolas, Renan Maestri, and Allowen Evin. 2022. "Defining Fragmentation Patterns of Archaeological Bone Remains without Typologies: A Landmark-Based Approach on Rodent Mandibula" Quaternary 5, no. 1: 14. https://doi.org/10.3390/quat5010014
APA StyleDurocher, M., Grouard, S., Nicolas, V., Maestri, R., & Evin, A. (2022). Defining Fragmentation Patterns of Archaeological Bone Remains without Typologies: A Landmark-Based Approach on Rodent Mandibula. Quaternary, 5(1), 14. https://doi.org/10.3390/quat5010014





