Abstract
Background/Objectives: Bulkamid® (Axonics, Irvine, CA, USA) is a non-particulate polyacrylamide hydrogel used in the treatment of urinary incontinence. While its effectiveness is well-documented in female stress urinary incontinence (SUI), there is limited data on its role in male stress urinary incontinence, particularly post-prostatectomy incontinence (PPI). This study evaluates the efficacy of Bulkamid as a primary or adjunctive treatment for male PPI. Methods: A retrospective chart review was conducted on male patients who developed PPI and underwent Bulkamid injections between 2016 and 2021. Data collected included pre- and post-procedure pad usage, the volume of Bulkamid injected, prior and subsequent incontinence treatments, and patient-reported satisfaction. Bulkamid was injected transurethrally in four quadrants near the vesicourethral anastomosis using a rigid cystoscope. Results: Twenty-one men with a history of radical prostatectomy (six open and fifteen robotic), including four who received adjuvant radiotherapy, were included. Fifteen underwent Bulkamid injection as a primary treatment, with five (33%) requiring repeat injections due to initial improvement. Eight (54%) subsequently underwent an AdVance XP® sling placement, while two (13%) required no further treatment. Six patients received Bulkamid as an adjunct to prior incontinence surgery, with 80% of post-sling patients reporting improved continence. Bulkamid was less effective in men with detrusor overactivity or prior radiation. Conclusions: Bulkamid demonstrated a higher success rate as an adjunct to the AdVance XP sling, with 80% of men experiencing improved continence. As a primary treatment for PPI, success was modest, with only 33% achieving improvement, often requiring repeat injections or conversion to a sling. Bulkamid presents a low-risk option for select male PPI patients, particularly those with prior sling placement, but durability and long-term effectiveness remain concerns.
1. Introduction/Objectives
Bulkamid® (Axonics, Irvine, CA, USA) is the only non-particulate bulking agent available worldwide for the treatment of urinary incontinence. It is injected transurethrally and aims to improve coaptation of the urethra to increase urethral resistance and minimise urinary leakage. It is composed of 2.5% polyacrylamide hydrogel and 97.5% water and its effect is achieved by the volume injected and a subsequent mesh network which anchors the gel in place. To date there is a lack of evidence in the literature on the effectiveness of bulking agents in the setting of male incontinence. The aim of this study is to evaluate the impact of Bulkamid injections in the treatment of our patients with post-prostatectomy incontinence (PPI).
2. Material and Methods
A retrospective chart review was conducted from all male patients who had developed incontinence post prostatectomy and subsequently underwent Bulkamid injections over a 5-year period (2016–2021). Patients were identified by the item number used for the procedure. Data was collected on their pre-operative pads-per-day (PPD) usage, urodynamics, the volume of Bulkamid used, other incontinence procedures, post-operative PPD usage, and patient-reported satisfaction. Success was defined as a decrease in PPD usage after bulking.
Bulkamid was injected in 4 quadrants, just distal to vesicourethral anastomosis and at the level of the external urethral sphincter, using a Williams needle (Cook Medical, Bloomington, IN, USA) and a rigid 22Fr cystoscope (see Figure 1). All patients received preoperative antibiotics as per their urine microscopy results and local antibiogram guidelines. Cystoscopy and urethral bulking were performed under a general anaesthetic for all patients.
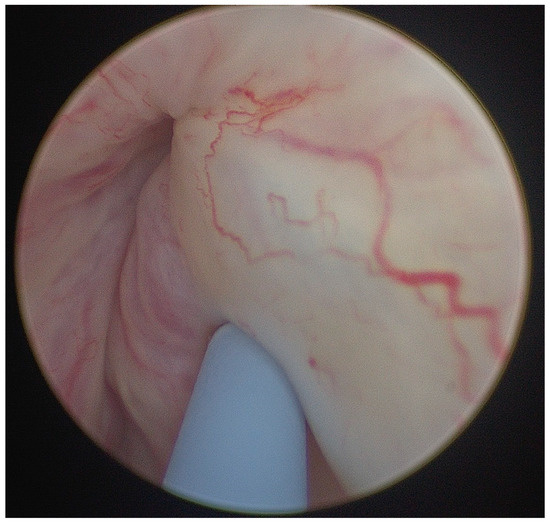
Figure 1.
Image of injection of Bulkamid in male post radical retropubic prostatectomy (RRP) patient at level of external urethral sphincter with Williams needle.
The protocol for this research project was approved by the Concord Repatriation Hospital Ethics Committee CH62/6/2018-062, and it conforms to the provisions of the 1995 Declaration of Helsinki (and as revised in Brazil in 2013). Informed consent was not applicable.
3. Results
Twenty-one male patients were recruited using the above methodology (see Table 1. Demographics of cohort). All patients had prior radical prostatectomy (6 open and 15 robotic) and 4 had received adjuvant radiotherapy. All patients underwent urodynamics and cystoscopy to investigate their incontinence prior to undergoing incontinence surgery. The timeline between radical prostatectomy and incontinence surgery ranged from 2 years to 20 years. The average age of our patients was 67 years old (range 58–74). The patients were followed up to the year 2022, which gave a follow-up range from 1 to 6 years.

Table 1.
Demographics of cohort.
All patients were treated with 1–2 mL of Bulkamid in total, based on their surgeon’s preference and the achievement of visual coaptation. Most patients used 1–2 pads per day to manage their incontinence, so this was a low volume leakage cohort. Whilst the numbers are small, the surgeons are considered a tertiary referral service for continence and many of those who had received an AdVance XP® sling or artificial urinary sphincter prior were their own patients. The decision to administer Bulkamid was a joint decision between the surgeon and the patient, it was not offered in the context of a trial, and the decision was based on the low volume of leakage, patient appetite for major vs. minor surgery, and the ability to repeat or upgrade procedures based on outcome.
Pad usage decreased from a mean of 2.1 pads/day pre Bulkamid to 1.3 pads/day post Bulkamid (p = 0.005, paired t-test). Median pad use fell from 2 (IQR X–Y) to 1 (IQR X–Y) pad/day, which was also significant on Wilcoxon signed-rank testing (p = 0.005).
Five of the 21 men had mixed incontinence on urodynamics and were treated with oral anticholinergics or beta 3 agonists prior to the injection of the bulking agent. They used an average of 2 pads per day with only one patient using up to 6 per day. Only one of these patients was a recipient of prior radiotherapy. Two patients also received intravesical Botox injections to treat overactive bladder which predated their bulking agent injections by several years. In most cases, treatment for detrusor overactivity occurred prior to treatment for stress incontinence. Urodynamics was not repeated routinely in all patients prior to offering Bulkamid; this decision was surgeon- and patient-led, and was supported by the patient’s symptoms and prior urodynamics supporting mixed pathology.
In total, 15 of the 21 patients underwent bulking as their primary procedure for treatment of stress urinary incontinence with an average of two pads used per day prior to treatment (see Figure 2 Flowchart and Table 2 Outcomes). Five (33%) of these patients proceeded to repeat injections due to subjective and objective improvement (one less pad used per day) in continence after the first injection. These repeat injections occurred at annual intervals to a total of three treatments. Eight of these fifteen men (54%) proceeded to receiving an AdVance XP® sling (Boston Scientific ©, Marlborough, MA, USA) and two (13%) patients had no further treatment for their incontinence.
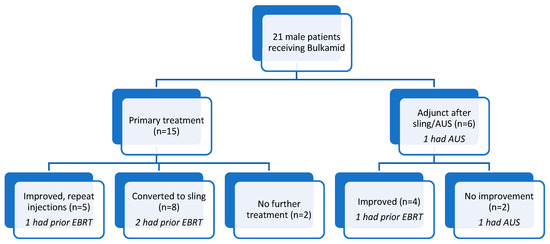
Figure 2.
Flowchart of our cohort. AUS: artificial urinary sphincter, EBRT: external beam radiation therapy.

Table 2.
Overall outcomes and subgroup results are shown according to treatment type, radiotherapy history, and detrusor overactivity.
All 21 patients had at least 12 months of follow-up after their initial Bulkamid injection. The median interval between repeat Bulkamid injections was 1 year (range 1–2 years). For men who subsequently underwent a sling procedure, the median time from Bulkamid to sling was 1 year (range 0–4 years). One patient trialled Bulkamid after artificial urinary sphincter (AUS) insertion; this did not result in improvement.
Of the two men who had no further stress incontinence treatment, both subjectively felt they were not improved by the Bulkamid injection and both had detrusor overactivity noted on urodynamics with a variety of treatments trialled. One had a very low volume of leakage before (30 mL/day) and after (10 mL/day) the procedure. The other decreased his pad use from 2 to 1 per day post Bulkamid. Objectively there was improvement in their leakage, but the patients did not feel improved.
Six of the patients in the cohort were given Bulkamid injections as an adjunct to prior stress urinary incontinence surgery. Five had previously received an AdVance XP® sling and four of these patients (80%) noted an improvement in their continence after the Bulkamid injection. The one patient who was not improved did trial three doses of Bulkamid in total without subjective or objective improvement.
One man had a functioning artificial urinary sphincter in situ, inserted 4 years prior. This patient trialled Bulkamid injection distal to his cuff twice without improvement. Ultimately his continence was improved with a downsizing of his sphincter cuff.
Four of our cohort had radiotherapy. Only one was treated with Bulkamid alone and noted improvement. He was the most delayed in our cohort in terms of time from radical prostatectomy to incontinence surgery, at 20 years, and was 71 years of age at the time of bulking. Two of the men proceeded to AdVance XP sling insertion after no improvement with bulking and were satisfied with their post-sling continence. One man received his bulking agent after his sling and was much improved.
There were no significant post-operative complications in this cohort.
4. Discussion
In the female population, there is ample data supporting the use of bulking agents in the treatment of stress urinary incontinence (SUI) and confirming the non-inferiority of Bulkamid compared to bulking agents which have been in longer use. Bulkamid is unique in that it is a non-particulate hydrogel whereas other agents, which have also been in common use, are particulate and become solid after injection, such as Macroplastique and Durasphere. These agents are associated with product-related complications such as erosion, migration, and abscess formation, unlike Bulkamid which has a low risk of injection-site rupture alone. All bulking agents do carry the risk of urinary retention, failure, infection, and de novo overactive bladder, which is common to all stress urinary incontinence surgery.
A systematic review by Hoe et al. concluded that with a total of 17 available studies, there continues to be a wide range of success reported in the short and long term (29–83% vs. 40–70%) for Bulkamid, which is comparable with other bulking agents [1]. It has been shown to be inferior to transvaginal tape overall but still boasts that 59.8% patients are satisfied at 12 months with 66.4% of patients having negative stress tests. For a low-risk procedure that allows for reintervention with minimal additional morbidity, it is not an unreasonable treatment choice.
There is a paucity of data in the literature regarding the use of transurethral bulking agents for male incontinence. A Cochrane review in 2011 found only a single randomised controlled trial comparing artificial urinary sphincters with the use of Macroplastique [2]. Whilst there was an understandably lower success rate with treating severe incontinence with Macroplastique, the success rate was still reported to be 46% compared with 83% for the artificial sphincter. The Cochrane review could not draw any conclusions given the single available study in this area.
Another study of bulking agents in male patients was a pilot study published in 2018 looking at the use of Opsys® (Promedon, Cordoba, Argentina) bulking agent for ten men with <30 g of urine loss per day post radical prostatectomy [3]. Opsys is a non-absorbable polyacrylate polyalcohol copolymer, used in children with vesicoureteric reflux. Only one out of 10 injected patients remained improved at 3 months post procedure and overall there was an increase in incontinence noted on pad weighs. It was deemed a failed and unsafe intervention.
With bulking agents, the surgeon and patient need to be prepared to accept a repeat injection in many cases to prolong effectiveness, and this is worth noting from the outset. An interesting American study from 2016 analysed Medicare data for the treatment of stress urinary incontinence in men [4]. It found that those who underwent injection of a bulking agent required longer follow up than those who had a sling or artificial sphincter inserted (6 years vs. 3.5 years vs. 5 years). A total of 52.9% of bulking patients required a repeat procedure, which was much higher than in the other cohorts, 21% required more than two extra procedures, and 40% of the original cohort required another device implanted. Interestingly, regarding the choice of device, 35.9% had more bulking performed, 27.9% had a sling, and 18.5% had a sphincter, which would indicate that the original bulking was successful but short lived in terms of its effect, thus prompting a repeat of the same procedure. Overall, bulking was associated with fewer short- and long-term complications than the other procedures.
Only one systematic review assessing the issue of bulking agents and male incontinence exists and, apart from the abovementioned studies, it reports significant bias and heterogeneity of the populations used to study Macroplastique, Durasphere, Opsys, and Urolastic [5]. Some studies have reported 82% of men being dry after multiple procedures compared with other studies reporting success in only 32% after more than one injection. The key conclusion appeared to be that multiple injections were required to achieve success, but that the durability of this success is not clearly reported. Furthermore, there is no standardisation of the injection technique employed, the volume used, etc.
There are no other studies, however, involving the use of Bulkamid in male patients, as used in this study, and which is a non-particulate bulking agent. It is composed of a polyacrylamide hydrogel and its effect is achieved by the volume injected and a subsequent mesh network which anchors the gel in place.
Although the absolute reduction in pad use in our cohort was modest, the difference reached statistical significance on both parametric and non-parametric analysis. This supports our observation that Bulkamid can provide measurable but limited improvement in continence, particularly for a cohort of men with mild, low-volume incontinence, rather than all-comers.
We feel that the success seen in our post-sling patients receiving Bulkamid reflects the plausible explanation that, after successful continence treatment with a sling, with the passage of time, the urethra naturally atrophies and the external urinary sphincter deteriorates, particularly with prior radiation therapy. With these changes, coaptation is affected and the degree of dynamic and passive compression from the sling is no longer adequate, resulting in recurrent incontinence that is more likely to respond to the lower risk intervention of the bulking agent. This mirrors the idea of the adjustable transobturator male sling whereby, as the urethra changes with time, the addition of extra volume into the cushion improves the effect. Whilst the Advance XP sling itself works by creating both passive and dynamic compression of the bulbar urethra for males, the addition of the Bulkamid increases the maximum urethral pressure and provides a central filler to support the length of the urethra, as discovered in cadaveric studies [6,7].
5. Conclusions
In our small cohort, there was improvement in continence in 80% of men who underwent Bulkamid injection after their Advance XP sling. By contrast, only 33% of men who chose Bulkamid as a primary treatment for the PPI were improved and all of these men had subsequent repeat injections to maintain the effect. Of those men in whom Bulkamid was a primary intervention, 54% ultimately proceeded to the insertion of a sling. These numbers are in keeping with international data regarding the reintervention rates in men with PPI. The remaining 13% had objective improvements in continence with reduced pad usage post bulking but did not feel improved.
Our series confirms that Bulkamid provides only temporary improvement for many men, with repeat injections typically required within 1–2 years, and conversion to sling usually occurring within 1 year of the first injection. Importantly, all patients were followed for at least 12 months, strengthening the durability assessment of our outcomes.
Bulkamid is a reasonable, low-risk option for improving continence in PPI, particularly in men who have an AdVance XP sling in situ. It is only modestly successful in men as a primary treatment and men should be counselled regarding the potential need repeat for injections or upgrading to an alternate product to produce a long-lasting result.
Author Contributions
Conceptualisation, S.P., T.K., L.C. and V.T.; methodology, S.P. and L.C.; software, S.P. and J.M.; validation, S.P.; formal analysis, S.P. and J.M.; investigation, S.P. and J.M.; resources, V.T. and L.C.; data curation, S.P., and J.M.; writing—original draft preparation, S.P.; writing—review and editing, S.P., V.T. and L.C.; visualisation, S.P., V.T. and L.C.; supervision, L.C. and V.T.; project administration, V.T. and L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Concord Hospital Research Ethics Committee (Local reference number: CH62/6/2018-062, HREC reference number: LNR/18/CRGH/79) on 15 April 2018.
Informed Consent Statement
The HREC granted a waiver of the usual requirement for the consent of the individual for the use of their health information in a research project, in accordance with the Health Records and Information Privacy Act 2002 (NSW) and the NSW Privacy Commissioner’s Statutory guidelines on research.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors would like to acknowledge the support of the staff at the Department of Urology, Concord Repatriation Hospital, for their assistance with data collection and patient coordination. We also extend our gratitude to colleagues who provided valuable insights and feedback during the preparation of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hoe, V.; Haller, B.; Yao, H.H.; O’Connell, H.E. Urethral bulking agents for the treatment of stress urinary incontinence in women: A systematic review. Neurourol. Urodyn. 2021, 40, 1349–1388. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.; Andriolo, R.B.; Atallah, Á.N.; da Silva, E.M.K. Surgery for stress urinary incontinence due to presumed sphincter deficiency after prostate surgery. Cochrane Database Syst. Rev. 2011, CD008306, Erratum in Cochrane Database Syst. Rev. 2014, CD008306. [Google Scholar] [CrossRef]
- van Uhm, J.I.M.; Vermeer, M.; Elzevier, H.W.; Noordzij, J.W.; Koldewijn, E.L.; Cornel, E.B. Injectable Bulking Agent to Treat Postprostatectomy Urinary Incontinence: A Safety and Effectiveness Pilot Study. BioMed Res. Int. 2018, 2018, 2796967. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, B.; Sedrakyan, A.; Isaacs, A.J.; Mao, J.; Lee, R.; Te, A.; Kaplan, S. National study of utilization of male incontinence procedures. Neurourol. Urodyn. 2016, 35, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Toia, B.; Gresty, H.; Pakzad, M.; Hamid, R.; Ockrim, J.; Greenwell, T. Bulking for stress urinary incontinence in men: A systematic review. Neurourol. Urodyn. 2019, 38, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Toia, B.; O’Connor, E.; Greenwell, T.J.; Ockrim, J.L.; Solomon, E. Bulkamid® injection in men: Cadaver study of operative technique and putative mechanism of action. J. Clin. Urol. 2021, 15, 470–475. [Google Scholar] [CrossRef]
- Kahokehr, A.A.; Selph, J.P.; Belsante, M.J.; Bashir, M.; Sofue, K.; Tausch, T.J.; Brand, T.C.; Lloyd, J.C.; Goldsmith, Z.G.; Walter, J.R.; et al. Mechanism of Action of the Transobturator Sling for Post-Radical Prostatectomy Incontinence: A Multi-institutional Prospective Study Using Dynamic Magnetic Resonance Imaging. Urology 2018, 116, 185–192. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Société Internationale d’Urologie. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).