Renal Decompression for Malignant Ureteric Obstruction: A Tertiary Hospital Cohort Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Data Collection
2.4. Statistical Analysis
3. Results
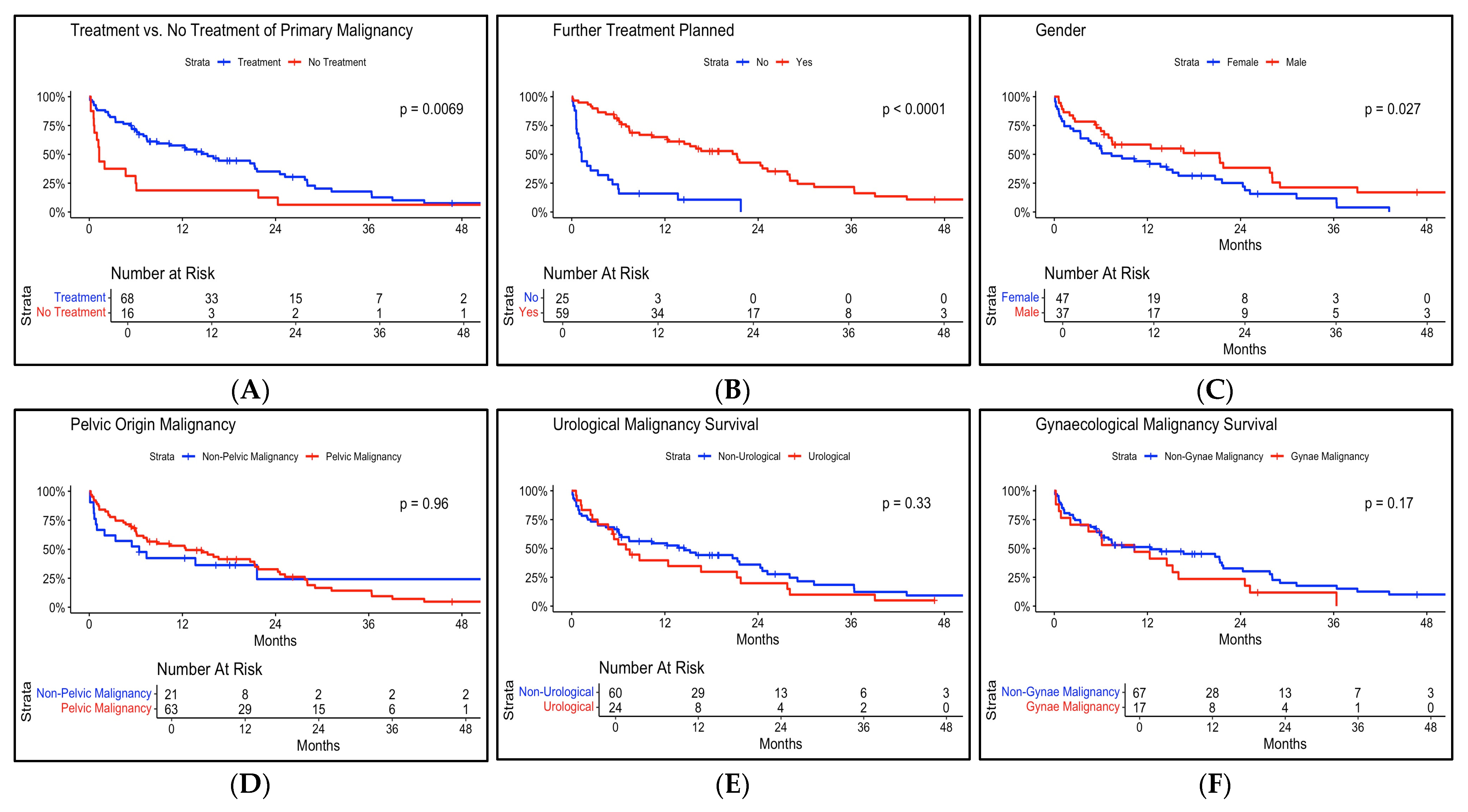
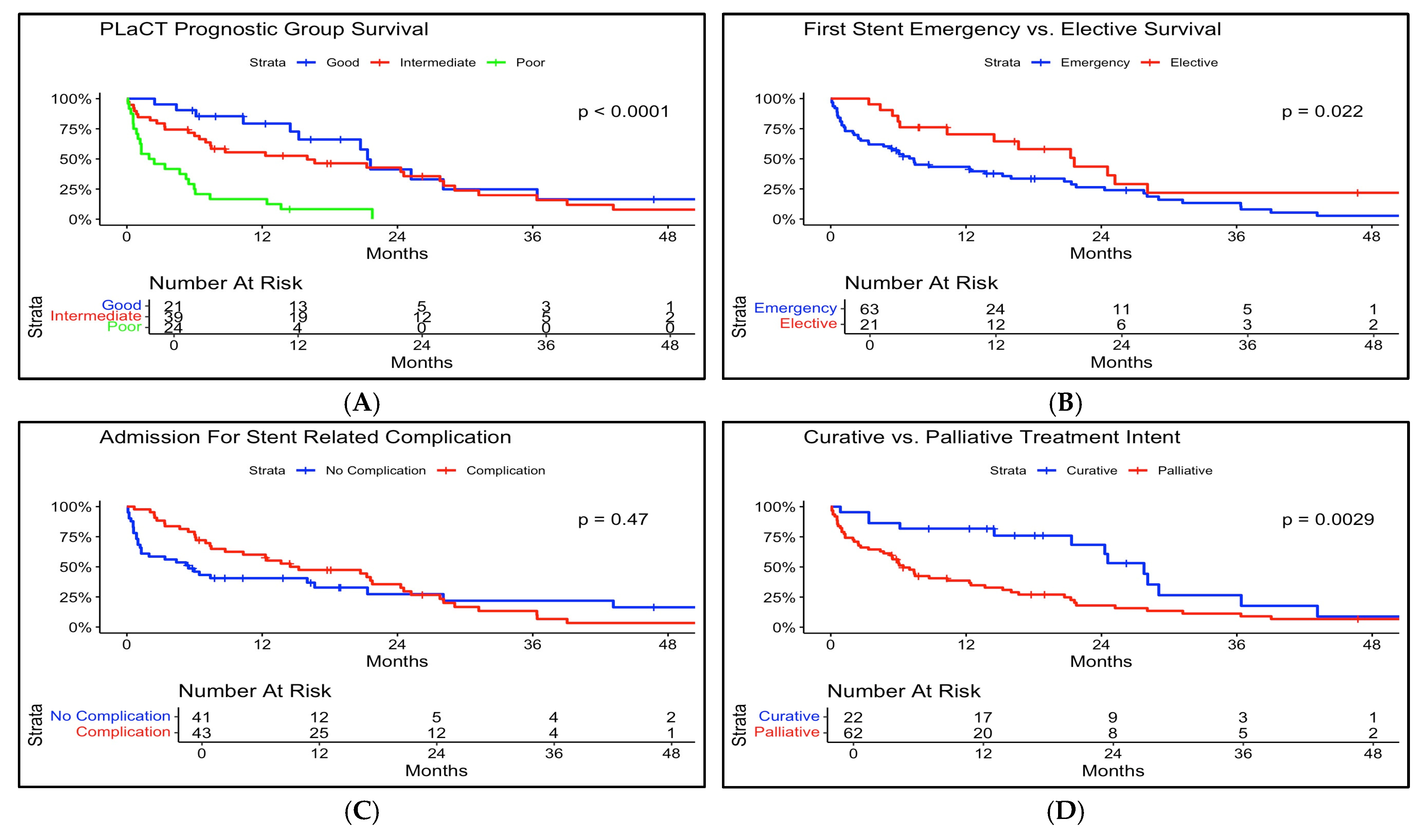
3.1. Survival Outcomes
3.2. Renal Function Outcomes
3.3. Complications
3.4. PLaCT Prognostic Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUA | American Urological Association |
| EAU | European Association of Urology |
| ECOG | European Cooperative Oncology Group |
| HR | Hazard Ratio |
| MUO | Malignant Ureteric Obstruction |
| NALHN | Northern Adelaide Local Health Network |
| PLaCT | Primary site, Laterality, serum Creatinine level, and Treatment |
| RPF | Retroperitoneal Fibrosis |
References
- Lomas, D.J.; Ziegelmann, M.J. Update on the Management of Malignant Ureteral Obstruction. Available online: https://auanews.net/issues/articles/2023/may-2023/update-on-the-management-of-malignant-ureteral-obstruction (accessed on 1 October 2023).
- Kouba, E.; Wallen, E.M.; Pruthi, R.S. Management of ureteral obstruction due to advanced malignancy: Optimizing therapeutic and palliative outcomes. J. Urol. 2008, 180, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Artiles Medina, A.; Laso García, I.; González Tello, F.; Álvarez Rodríguez, S.; Hevia Palacios, M.; Mata Alcaraz, M.; Mínguez Ojeda, C.; Arias Funez, F.; Gómez Dos Santos, V.; Burgos Revilla, F.J. The challenging management of malignant ureteral obstruction: Analysis of a series of 188 cases. Curr. Urol. 2024, 18, 34–42. [Google Scholar] [CrossRef]
- Blackmur, J. Management of malignant ureteric obstruction with ureteric stenting or percutaneous nephrostomy. Br. J. Surg 2024, 111, znae035. [Google Scholar] [CrossRef]
- Gauhar, V.; Pirola, G.M.; Scarcella, S.; De Angelis, M.V.; Giulioni, C.; Rubilotta, E.; Gubbiotti, M.; Lim, E.J.; Law, Y.X.T.; Wroclawski, M.L.; et al. Nephrostomy tube versus double J ureteral stent in patients with malignant ureteric obstruction. A systematic review and meta-analysis of comparative studies. Int. Braz. J. Urol. 2022, 48, 903–914. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shah, M.; Blest, F.; Blackmur, J.; Laird, A.; Dawson, S.; Aning, J. Malignant upper urinary tract obstruction in cancer patients: A systematic review. BJUI Compass 2024, 5, 405–416. [Google Scholar] [CrossRef]
- van der Heijden, A.G.; Rink, M.; Bruins, H.M.; Carrion, A.; Cathomas, R.; Compérat, E.M.; Dimitropoulos, K.; Efstathiou, J.A.; Fietkau, R.; Lorch, A.; et al. Muscle-Invasive and Metastatic Bladder Cancer. In EAU Guidelines. Edn. Presented at the EAU Annual Congress Paris 2024; EAU Guidelines Office: Arnhem, The Netherlands, 2024; ISBN 978-94-92671-23-3. [Google Scholar]
- Izumi, K.; Shima, T.; Shigehara, K.; Sawada, K.; Naito, R.; Kato, Y.; Ofude, M.; Kano, H.; Iwamoto, H.; Yaegashi, H.; et al. A novel risk classification score for malignant ureteral obstruction: A multicenter prospective validation study. Sci. Rep. 2021, 11, 4455. [Google Scholar] [CrossRef]
- Sountoulides, P.; Mykoniatis, I.; Dimasis, N. Palliative management of malignant upper urinary tract obstruction. Hippokratia 2014, 18, 292–297. [Google Scholar]
- Prentice, J.; Amer, T.; Tasleem, A.; Aboumarzouk, O. Malignant ureteric obstruction decompression: How much gain for how much pain? A narrative review. J. R. Soc. Med. 2018, 111, 125–135. [Google Scholar] [CrossRef]
- Hyams, E.S.; Shah, O. Malignant extrinsic ureteral obstruction: A survey of urologists and medical oncologists regarding treatment patterns and preferences. Urology 2008, 72, 51–56. [Google Scholar] [CrossRef]
- Hsu, L.; Li, H.; Pucheril, D.; Hansen, M.; Littleton, R.; Peabody, J.; Sammon, J. Use of percutaneous nephrostomy and ureteral stenting in management of ureteral obstruction. World J. Nephrol. 2016, 5, 172–181. [Google Scholar] [CrossRef]
- De Lorenzis, E.; Lievore, E.; Turetti, M.; Gallioli, A.; Galassi, B.; Boeri, L.; Montanari, E. Ureteral Stent and Percutaneous Nephrostomy in Managing Malignant Ureteric Obstruction of Gastrointestinal Origin: A 10 Years’ Experience. Gastrointest. Disord. 2020, 2, 456–468. [Google Scholar] [CrossRef]
- Ghannam, E.; Musleh, H.; Ahmad, T.; Mustafa, M.; Odeh, R.; Shawahna, R. Outcomes of nephrostomy and double J stent in malignant ureteral obstruction in the Palestinian practice. BMC Urol. 2024, 24, 245. [Google Scholar] [CrossRef]
- Izumi, K.; Mizokami, A.; Maeda, Y.; Koh, E.; Namiki, M. Current outcome of patients with ureteral stents for the management of malignant ureteral obstruction. J. Urol. 2011, 185, 556–561. [Google Scholar] [CrossRef]
- Ishioka, J.; Kageyama, Y.; Inoue, M.; Higashi, Y.; Kihara, K. Prognostic model for predicting survival after palliative urinary diversion for ureteral obstruction: Analysis of 140 cases. J. Urol. 2008, 180, 618–621; discussion 621. [Google Scholar] [CrossRef]
- Cordeiro, M.D.; Coelho, R.F.; Chade, D.C.; Pessoa, R.R.; Chaib, M.S.; Colombo-Júnior, J.R.; Pontes-Júnior, J.; Guglielmetti, G.B.; Srougi, M. A prognostic model for survival after palliative urinary diversion for malignant ureteric obstruction: A prospective study of 208 patients. BJU Int. 2016, 117, 266–271. [Google Scholar] [CrossRef]
- Lapitan, M.C.; Buckley, B.S. Impact of palliative urinary diversion by percutaneous nephrostomy drainage and ureteral stenting among patients with advanced cervical cancer and obstructive uropathy: A prospective cohort. J. Obs. Gynaecol. Res. 2011, 37, 1061–1070. [Google Scholar] [CrossRef]
- Tatenuma, T.; Tsutsumi, S.; Yasui, M.; Noguchi, G.; Umemoto, S.; Kishida, T. Outcome of Palliative Urinary Diversion and Observation for Malignant Extrinsic Ureteral Obstruction. J. Palliat. Med. 2019, 23, 254–258. [Google Scholar] [CrossRef]
- Folkard, S.S.; Banerjee, S.; Menzies-Wilson, R.; Reason, J.; Psallidas, E.; Clissold, E.; Al-Mushatat, A.; Chaudhri, S.; Green, J.S.A. Percutaneous nephrostomy in obstructing pelvic malignancy does not facilitate further oncological treatment. Int. Urol. Nephrol. 2020, 52, 1625–1628. [Google Scholar] [CrossRef]
- Cartapatti, M.; Machado, R.D.; Mesquita, J.C.; Freua, R.; Cáceres, D.; dos Reis, R.B. Urinary Diversion Can Improve the Chance of Implementing New Therapeutic Lines in Patients with Malignant Ureteral Obstruction: A Multicenter Study. Curr. Oncol. 2024, 31, 7107–7116. [Google Scholar] [CrossRef]
- Yoon, J.H.; Park, S.; Park, S.; Moon, K.H.; Cheon, S.H.; Kwon, T. Renal function is associated with prognosis in stent-change therapy for malignant ureteral obstruction. Investig. Clin. Urol. 2018, 59, 376–382. [Google Scholar]
- Heo, J.E.; Jeon, D.Y.; Lee, J.; Ham, W.S.; Choi, Y.D.; Jang, W.S. Clinical Outcomes After Urinary Diversion for Malignant Ureteral Obstruction Secondary to Non-urologic Cancer: An Analysis of 778 Cases. Ann. Surg. Oncol. 2021, 28, 2367–2373. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Points |
|---|---|
| Primary Cancer Site (P) | |
| Gynaecological | 0 |
| Lower Digestive Tract or Urinary Tract | 1 |
| Others | 2 |
| Laterality (La) | |
| Unilateral | 0 |
| Bilateral | 1 |
| Serum Creatinine (C) | |
| <1.2 mg/mL | 0 |
| >1.2 mg/mL | 2 |
| Treatment of Primary Cancer (T) | |
| In process or on schedule | 0 |
| No intention | 2 |
| PLaCT Risk Group | |
| Good | 0–2 |
| Intermediate | 3–4 |
| Poor | 5–7 |
| Characteristic | N = 84 1 |
|---|---|
| Age | |
| Median (Q1, Q3) | 64.50 (55, 76) |
| Min, Max | 27, 95 |
| Gender | |
| Female | 47/84 (56%) |
| Male | 37/84 (44%) |
| Charleston Comorbidity Score | |
| 1. <4 | 6/84 (7%) |
| 2. 4–6 | 24/84 (29%) |
| 3. 7–9 | 33/84 (39%) |
| 4. >10 | 21/84 (25%) |
| Primary Cancer | |
| Appendiceal | 1/84 (1%) |
| Bladder | 13/84 (15%) |
| Breast | 4/84 (5%) |
| Cervical | 12/84 (14%) |
| Colorectal | 23/84 (27%) |
| Endometrial | 1/84 (1%) |
| Gastric | 5/84 (6.0%) |
| Jejunal | 1/84 (1%) |
| Lymphoma | 6/84 (7%) |
| Oesophageal | 1/84 (1%) |
| Ovarian | 4/84 (5%) |
| Prostate | 10/84 (12%) |
| Renal Cell | 1/84 (1%) |
| Testicular | 2/84 (2%) |
| Cancer Stage | |
| 2 | 7/84 (8%) |
| 3 | 18/84 (21%) |
| 4 | 59/84 (70%) |
| Treatment Intent | |
| Curative | 22/84 (26%) |
| Palliative | 62/84 (74%) |
| Further Oncological Treatment Planned | |
| No | 25/84 (30%) |
| Yes | 59/84 (70%) |
| Never had Treatment For Malignancy | |
| No | 68/84 (81%) |
| Yes | 16/84 (19%) |
| Total Length of Follow-up (Days) | |
| Median (Q1, Q3) | 233 (78, 641) |
| Min, Max | 3, 2077 |
| Characteristic | N = 84 1 |
|---|---|
| Emergency vs. Elective Initial Stent Insertion | |
| Emergency | 63/84 (75%) |
| Elective | 21/84 (25%) |
| Method of First Stent Insertion | |
| Antegrade | 14/84 (17%) |
| Retrograde | 64/84 (76%) |
| Retrograde and Antegrade | 6/84 (7%) |
| Level of Ureteric Obstruction | |
| Distal | 58/84 (69%) |
| Mid | 16/84 (19%) |
| Proximal | 10/84 (12%) |
| Cause of Obstruction | |
| Ischaemic | 2/84 (2%) |
| Nodal | 25/84 (30%) |
| Radiation | 3/84 (4%) |
| RPF | 5/84 (6.0%) |
| Tumour | 49/84 (58%) |
| Unilateral vs. Bilateral Obstruction | |
| Bilateral | 39/84 (46%) |
| Unilateral | 45/84 (54%) |
| First Stent Insertion Failure | |
| No | 66/84 (79%) |
| Yes | 18/84 (21%) |
| Characteristic | N = 84 1 |
|---|---|
| Mortality | |
| No | 18/84 (21%) |
| Yes | 66/84 (79%) |
| Time to Death from MUO Treatment (Days) | |
| Median (Q1, Q3) | 197 (67, 651) |
| Min, Max | 3, 1549 |
| Total Follow-up Days | |
| Median (Q1, Q3) | 233 (78, 641) |
| Min, Max | 3, 2077 |
| Survived 30 Days Post-MUO Treatment | |
| No | 12/84 (14%) |
| Yes | 72/84 (86%) |
| Survived 90 Days Post-MUO Treatment | |
| No | 22/84 (26%) |
| Yes | 62/84 (74%) |
| Survived 6 Months Post MUO Treatment | |
| No | 33/84 (39%) |
| Yes | 51/84 (61%) |
| Survived 12 Months Post-MUO Treatment | |
| No | 47/84 (56%) |
| Yes | 37/84 (44%) |
| PLaCT Prognostic Group Survival (Days) | |
| Poor (Med) (Min, Max) | 67 (6, 765) |
| Intermediate (Med) (Min, Max) | 235 (3, 1549) |
| Good (Med) (Min, Max) | 555 (75, 1162) |
| Baseline (Pre-Morbid) Serum Creatinine (umol/L) | |
| Median (Q1, Q3) | 73 (64, 95) |
| Min, Max | 38, 231 |
| Pre-Stent Serum Creatinine (umol/L) | |
| Median (Q1, Q3) | 149 (98, 333) |
| Min, Max | 50, 1049 |
| Serum Creatinine 6 Months Post MUO Treatment (umol/L) | |
| Median (Q1, Q3) | 103 (82, 146) |
| Min, Max | 58, 672 |
| Change in Serum Creatinine from Pre-Stent at 6 Months (umol/L) | |
| Median (Q1, Q3) | −16 (−72, 4) |
| Min, Max | −446, 262 |
| Improvement in Serum Creatinine at 6 Months from Pre-Stent | |
| No | 13/47 (28%) |
| Yes | 34/47 (72%) |
| Missing data | 4 |
| Worsening Serum Creatinine at 6 Months from Pre-Morbid Baseline | |
| No | 8/48 (17%) |
| Yes | 40/48 (83%) |
| Missing data | 3 |
| Serum Creatinine 12 Months Post MUO Treatment (umol/L) | |
| Median (Q1, Q3) | 105 (80, 145) |
| Min, Max | 58, 762 |
| Change in Serum Creatinine from Pre-Stent at 12 Months (umol/L) | |
| Median (Q1, Q3) | −27 (−55, −1) |
| Min, Max | −264, 53 |
| Improvement in Serum Creatinine at 12 Months from Pre-Stent | |
| No | 8/36 (22%) |
| Yes | 28/36 (78%) |
| Missing data | 1 |
| Worsening Serum Creatinine at 12 Months from Pre-Morbid Baseline | |
| No | 6/37 (16%) |
| Yes | 31/37 (84%) |
| Variable | Hazard Ratio (95% CI) | p-Value |
|---|---|---|
| Age | 1.01 (0.98–1.05) | 0.38 |
| Gender (Male) | 0.53 (0.28–1.00) | 0.05 |
| Charlson Comorbidity Index | 1.04 (0.86–1.26) | 0.70 |
| Stage (3) | 1.08 (0.31–3.76) | 0.90 |
| Stage (4) | 1.46 (0.45–4.79) | 0.53 |
| No cancer treatment (Yes) | 0.84 (0.36–1.94) | 0.68 |
| Treatment Intent (Palliative) | 1.81 (0.87–3.74) | 0.11 |
| Hydronephrosis (Unilateral) | 0.92 (0.44–1.89) | 0.81 |
| Cancer Treatment Planned (Yes) | 0.24 (0.07–0.80) | 0.02 |
| PLaCT Group (Intermediate) | 1.2 (0.55–2.6) | 0.65 |
| PLaCT Group (Poor) | 1.25 (0.3–5.23) | 0.76 |
| Elective MUO Treatment | 0.69 (0.32–1.5) | 0.35 |
| Gynaecological Malignancy (Yes) | 1.18 (0.55–2.52) | 0.68 |
| Harrell’s C Statistic: 0.76 (0.71–0.82) Proportional Hazards Assumption: Global p-value = 0.13 | ||
| Characteristic | N = 84 1 |
|---|---|
| Admitted with Stent Complication | |
| No | 41/84 (49%) |
| Yes | 43/84 (51%) |
| Total Number of Stent-Related Complications | 143 |
| Urinary Tract Infections | 67 |
| Migrated Stents | 4 |
| Occluded Stents | 31 |
| Encrusted Stents | 2 |
| Stent-Related Pain | 15 |
| Urinary Frequency and Urgency | 2 |
| Haematuria | 22 |
| Length of Stay for Stent Complications (Days) | 966 |
| Failed Stent Procedures | 34 |
| Planned Stent Exchanges | 77 |
| Unplanned Stent Exchanges | 44 |
| Stents Removed | 20 |
| Ureteric Reconstructions Performed | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Société Internationale d’Urologie. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buckby, A.; David, R.; Kahokehr, A. Renal Decompression for Malignant Ureteric Obstruction: A Tertiary Hospital Cohort Analysis. Soc. Int. Urol. J. 2025, 6, 62. https://doi.org/10.3390/siuj6050062
Buckby A, David R, Kahokehr A. Renal Decompression for Malignant Ureteric Obstruction: A Tertiary Hospital Cohort Analysis. Société Internationale d’Urologie Journal. 2025; 6(5):62. https://doi.org/10.3390/siuj6050062
Chicago/Turabian StyleBuckby, Alex, Rowan David, and Arman Kahokehr. 2025. "Renal Decompression for Malignant Ureteric Obstruction: A Tertiary Hospital Cohort Analysis" Société Internationale d’Urologie Journal 6, no. 5: 62. https://doi.org/10.3390/siuj6050062
APA StyleBuckby, A., David, R., & Kahokehr, A. (2025). Renal Decompression for Malignant Ureteric Obstruction: A Tertiary Hospital Cohort Analysis. Société Internationale d’Urologie Journal, 6(5), 62. https://doi.org/10.3390/siuj6050062






