The “Footprint” Sign in Voiding Cystourethrography Indicates Poor Renal Function in Vesicoureteral Reflux: Is It a Pop-Off Mechanism?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Statistical Analysis
2.3. Ethical Considerations
3. Results
3.1. Patients’ Characteristics
3.2. VUR Grade
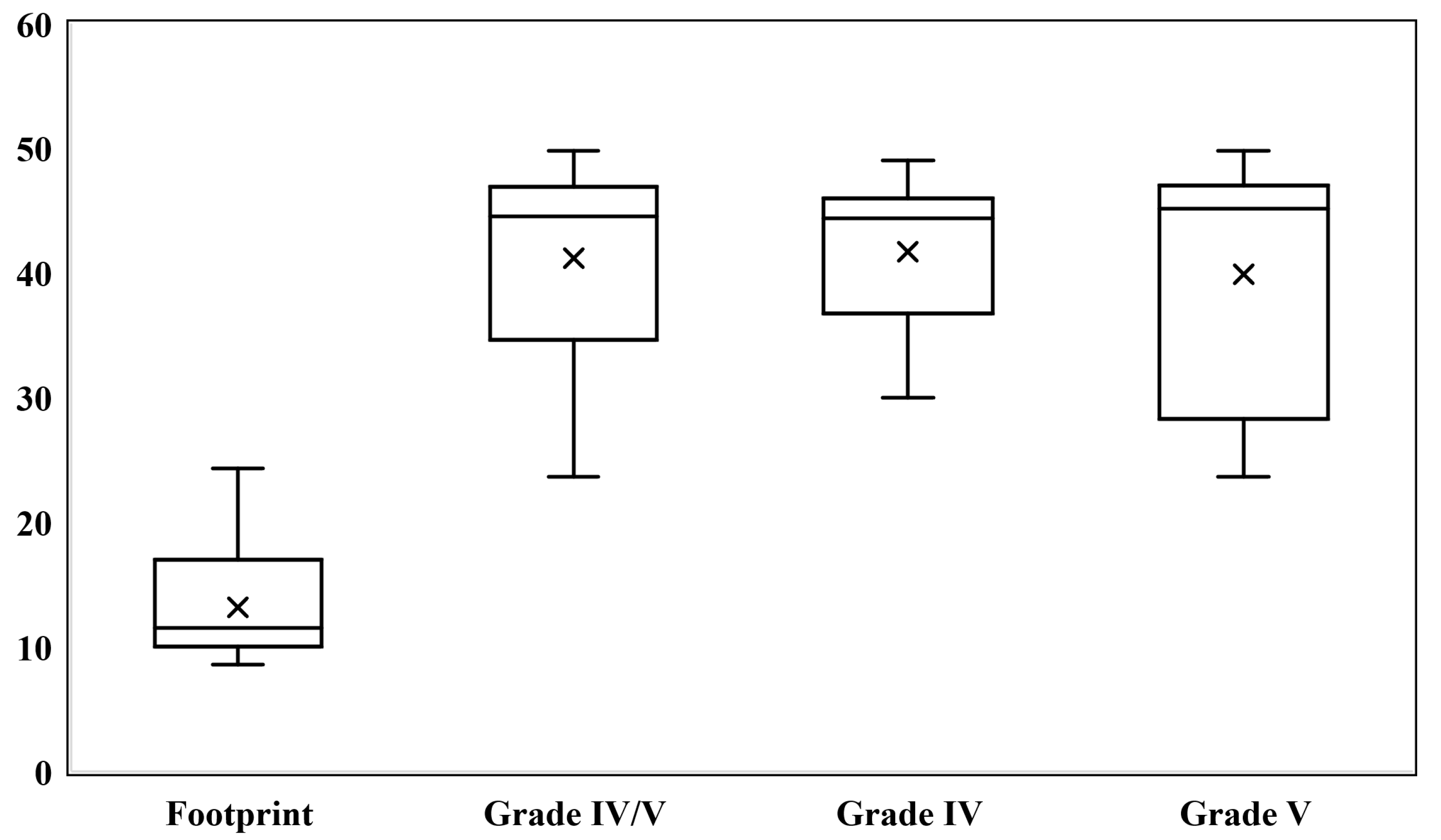
3.3. Tc99m-DMSA
3.4. Clinical Information at Follow-Up
4. Discussion
Limitations and Suggestions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Arapović, A.; Punda, A.; Brdar, D.; Čapkun, V.; Bajo, D.; Veljačić, D.; Punda, H.; Simičić-Majce, A.; Saraga-Babić, M.; Vukojević, K.; et al. Types of Parenchymal Changes Diagnosed on DMSA Scans of Kidneys Affected by Different Grades of Vesicoureteral Reflux. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e929617. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, T.K. Vesicoureteral reflux and reflux nephropathy. Adv. Chronic Kidney Dis. 2011, 18, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Darge, K. Voiding urosonography with US contrast agents for the diagnosis of vesicoureteric reflux in children. II. Comparison with radiological examinations. Pediatr. Radiol. 2008, 38, 54–63, quiz 126-127. [Google Scholar] [CrossRef] [PubMed]
- Koyle, M.A.; Elder, J.S.; Skoog, S.J.; Mattoo, T.K.; Pohl, H.G.; Reddy, P.P.; Abidari, J.M.; Snodgrass, W.T. Febrile urinary tract infection, vesicoureteral reflux, and renal scarring: Current controversies in approach to evaluation. Pediatr. Surg. Int. 2011, 27, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Unver, T.; Alpay, H.; Biyikli, N.K.; Ones, T. Comparison of direct radionuclide cystography and voiding cystourethrography in detecting vesicoureteral reflux. Pediatr. Int. Off. J. Jpn. Pediatr. Soc. 2006, 48, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Lebowitz, R.L.; Olbing, H.; Parkkulainen, K.V.; Smellie, J.M.; Tamminen-Möbius, T.E. International system of radiographic grading of vesicoureteric reflux. International Reflux Study in Children. Pediatr. Radiol. 1985, 15, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.D.; Gupta, K.; Swords, K.A.; Belman, A.B.; Majd, M.; Rushton, H.G.; Pohl, H.G. The “flowerpot” sign: Inference of poor renal function in high grade vesicoureteral reflux by calyceal orientation. J. Pediatr. Urol. 2015, 11, 31.e1–31.e4. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef] [PubMed]
- Weitz, M.; Licht, C.; Müller, M.; Haber, P. Renal ultrasound volume in children with primary vesicoureteral reflux allows functional assessment. J. Pediatr. Urol. 2013, 9 Pt B, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Rossleigh, M.A. Renal infection and vesico-ureteric reflux. Semin. Nucl. Med. 2007, 37, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.A.; Skoog, S.J.; Arant, B.S., Jr.; Copp, H.L.; Elder, J.S.; Hudson, R.G.; Khoury, A.E.; Lorenzo, A.J.; Pohl, H.G.; Shapiro, E.; et al. Summary of the AUA Guideline on Management of Primary Vesicoureteral Reflux in Children. J. Urol. 2010, 184, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, M.; Colhoun, E.; Puri, P. Prevalence and predictors of renal functional abnormalities of high grade vesicoureteral reflux. J. Urol. 2013, 190, 1490–1494. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Rushton, H.G. Vesicoureteral reflux associated renal damage: Congenital reflux nephropathy and acquired renal scarring. J. Urol. 2010, 184, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.; Diniz, J.S.; Silva, A.C.; Azevedo, M.V.; Pimenta, M.R.; Oliveira, E.A. Predictive factors of chronic kidney disease in severe vesicoureteral reflux. Pediatr. Nephrol. 2006, 21, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.K.; Sreedhar, B.; Sihoe, J.D.; Sit, F.K. Renal and bladder functional status at diagnosis as predictive factors for the outcome of primary vesicoureteral reflux in children. J. Urol. 2006, 176, 1152–1156, discussion 1156–1157. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Ni, J.; Li, Y.; Jin, J.; Zhu, Y. Renal function damage in children with duplex kidneys. Int. Urol. Nephrol. 2023, 55, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hoover, D.L.; Duckett, J.W., Jr. Posterior urethral valves, unilateral reflux and renal dysplasia: A syndrome. J. Urol. 1982, 128, 994–997. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, S.P.; Hensle, T.W.; Berdon, W.E.; Wigger, H.J. Unilateral vesicoureteral reflux and unilateral nonfunctioning kidney associated with posterior urethral valves--a syndrome? J. Urol. 1983, 130, 733–738. [Google Scholar] [CrossRef] [PubMed]
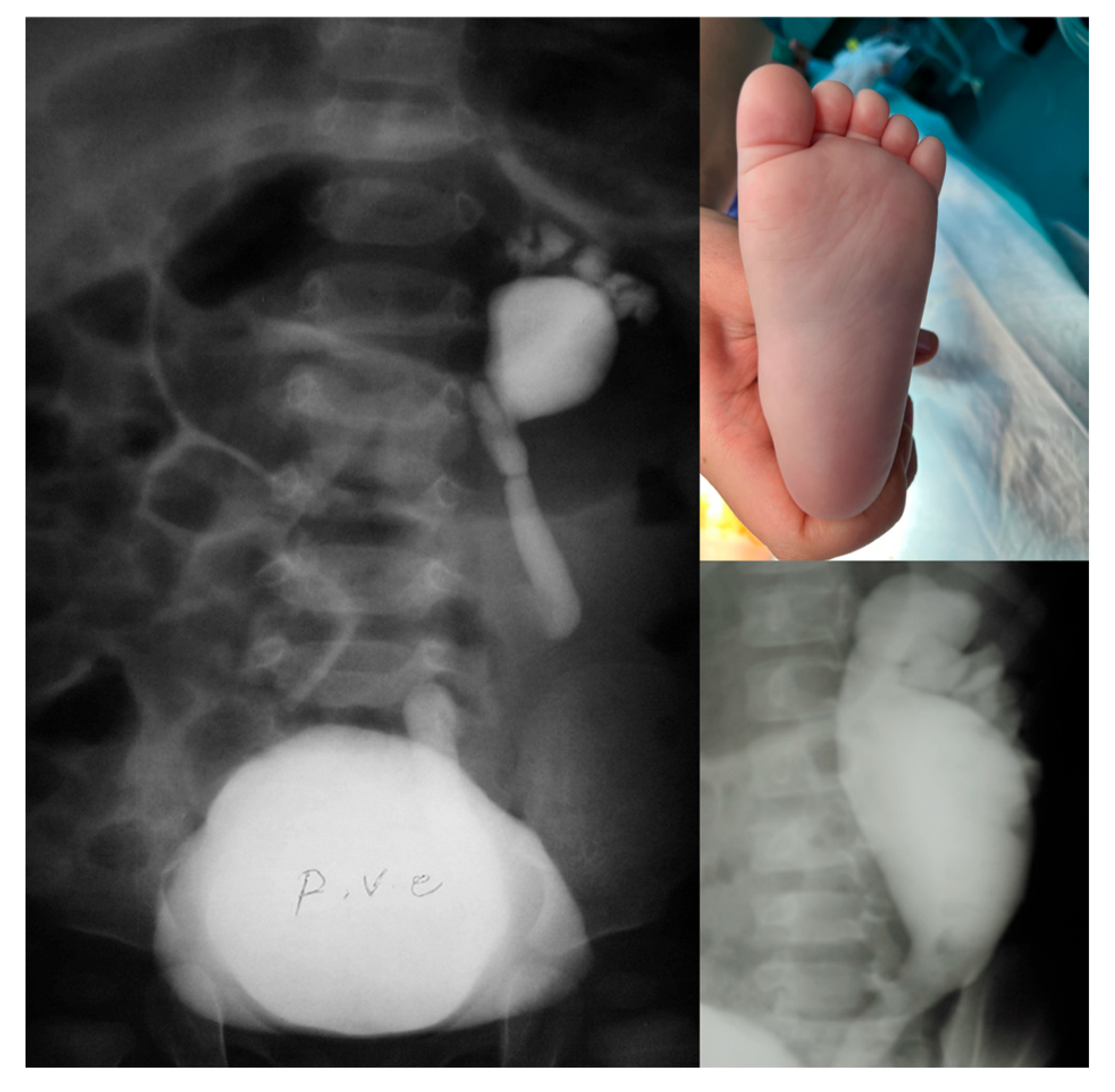

| Variable | Patients | |
|---|---|---|
| Group A (n = 18) | Group B (n = 37) | |
| Gender; male/female | 16:2 | 12:25 |
| Age, months; median [IQR] | 28.2 [66.5] | 13.0 [42.0] |
| Bilateral VUR; n (%) | 7 (38.9%) | 19 (51.4%) |
| Side of “footprint” or high-grade VUR; right: left | 4: 14 | 18: 19 |
| DRF; median [IQR] | 11.5 [7.0] | 44.5 [12.3] |
| Presentation; n (%) | ||
| Antenatal hydronephrosis | 6 (33.3%) | 13 (35.1%) |
| Recurrent UTIs | 9 (50.0%) | 23 (62.2%) |
| Other/Incidental | 3 (16.7%) | 1 (2.7%) |
| Neurological and other related disorders; n (%) | ||
| Spina bifida occulta | 4 (22.2%) | 3 (8.1%) |
| Sacral abnormality | 1 (5.6%) | 5 (13.5%) |
| History of ureterovesical junction obstruction | 0 (0.0%) | 4 (10.8%) |
| Clinical information at follow-up | ||
| Hypertension; n (%) | 0 (0.0%) | 0 (0.0%) |
| Significant proteinuria in urinalysis; n (%) | 0 (0.0%) | 0 (0.0%) |
| Nephrectomy; n (%) | 0 (0.0%) | 0 (0.0%) |
| Creatinine, mg/dL; median [IQR] | 0.6 [0.18] | 0.6 [0.21] |
| VUR Grade | Renal Units | |
|---|---|---|
| Group A (n = 25) | Group B (n = 56) | |
| I | 1 | 2 |
| II | 2 | 4 |
| III | 2 | 12 |
| IV | 2 | 27 |
| V | - | 11 |
| Footprint | 18 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Société Internationale d’Urologie. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamran, H.; Mohammadi Ganjaroudi, N.; Tafazoli, N.; Mehdizadeh, M.; Kajbafzadeh, A.-M. The “Footprint” Sign in Voiding Cystourethrography Indicates Poor Renal Function in Vesicoureteral Reflux: Is It a Pop-Off Mechanism? Soc. Int. Urol. J. 2025, 6, 55. https://doi.org/10.3390/siuj6040055
Kamran H, Mohammadi Ganjaroudi N, Tafazoli N, Mehdizadeh M, Kajbafzadeh A-M. The “Footprint” Sign in Voiding Cystourethrography Indicates Poor Renal Function in Vesicoureteral Reflux: Is It a Pop-Off Mechanism? Société Internationale d’Urologie Journal. 2025; 6(4):55. https://doi.org/10.3390/siuj6040055
Chicago/Turabian StyleKamran, Hooman, Negar Mohammadi Ganjaroudi, Nooshin Tafazoli, Mehrzad Mehdizadeh, and Abdol-Mohammad Kajbafzadeh. 2025. "The “Footprint” Sign in Voiding Cystourethrography Indicates Poor Renal Function in Vesicoureteral Reflux: Is It a Pop-Off Mechanism?" Société Internationale d’Urologie Journal 6, no. 4: 55. https://doi.org/10.3390/siuj6040055
APA StyleKamran, H., Mohammadi Ganjaroudi, N., Tafazoli, N., Mehdizadeh, M., & Kajbafzadeh, A.-M. (2025). The “Footprint” Sign in Voiding Cystourethrography Indicates Poor Renal Function in Vesicoureteral Reflux: Is It a Pop-Off Mechanism? Société Internationale d’Urologie Journal, 6(4), 55. https://doi.org/10.3390/siuj6040055







