Early Clinical Outcomes of the Novel Hinotori Robotic System in Urological Surgery—A Review of Existing Literature
Abstract
1. Introduction
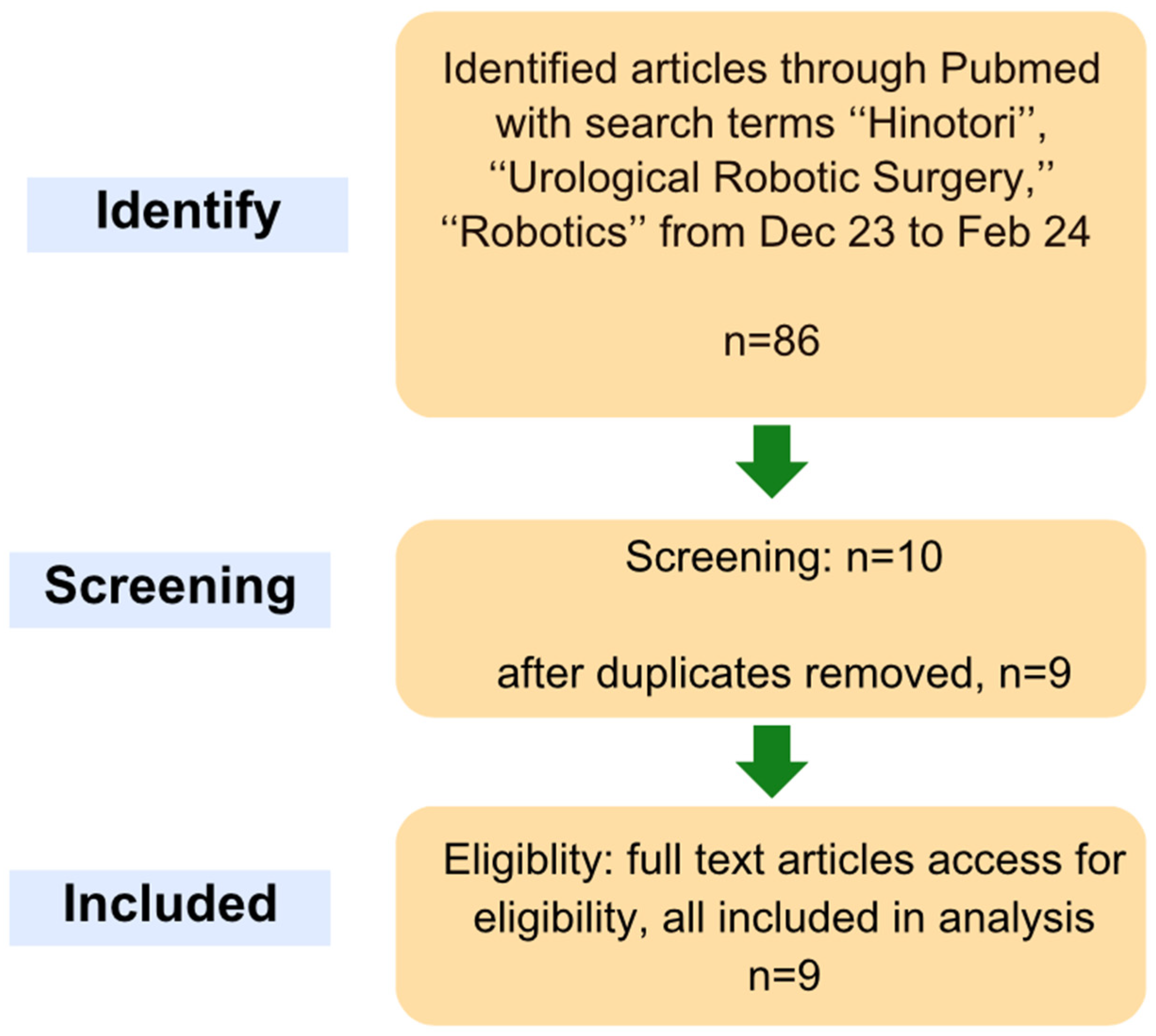
2. Materials and Methods
3. Results
3.1. Robotic-Assisted Partial Nephrectomy (RAPN)
3.2. Robotic-Assisted Radical Nephrectomy (RARN)
3.3. Robotic-Assisted Nephroureterectomy (RANU)
3.4. Robotic-Assisted Adrenalectomy (RAA)
3.5. Robotic-Assisted Radical Prostatectomy (RARP)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cisu, T.; Crocerossa, F.; Carbonara, U.; Porpiglia, F.; Autorino, R. New robotic surgical systems in urology: An update. Curr. Opin. Urol. 2021, 31, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Koliakos, N.; Denaeyer, G.; Willemsen, P.; Schatteman, P.; Mottrie, A. Failure of a robotic arm during da Vinci prostatectomy: A case report. J. Robot. Surg. 2008, 2, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Ham, W.S.; Park, S.Y.; Yu, H.S.; Choi, Y.D.; Hong, S.J.; Rha, K.H. Malfunction of da Vinci Robotic System—Disassembled Surgeon’s Console Hand Piece: Case Report and Review of the Literature. Urology 2009, 73, 209.e7–209.e8, ISSN 0090-4295. [Google Scholar] [CrossRef]
- Pandolfo, S.D.; Cerrato, C.; Wu, Z.; Franco, A.; Del Giudice, F.; Sciarra, A.; Verze, P.; Lucarelli, G.; Imbimbo, C.; Perdonà, S.; et al. A systematic review of robot-assisted partial nephrectomy outcomes for advanced indications: Large tumors (cT2-T3), solitary kidney, completely endophytic, hilar, recurrent, and multiple renal tumors. Asian J. Urol. 2023, 10, 390–406. [Google Scholar] [CrossRef]
- Gul, Z.G.; Tam, A.; Badani, K.K. Robotic partial nephrectomy: The current status. Indian J. Urol. 2020, 36, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, D.; Matsushita, Y.; Watanabe, H.; Tamura, K.; Otsuka, A.; Fujisawa, M.; Miyake, H. Perioperative outcomes of robot-assisted partial nephrectomy using hinotori versus da Vinci surgical robot system: A propensity score-matched analysis. J. Robot. Surg. 2023, 17, 2435–2440. [Google Scholar] [CrossRef] [PubMed]
- Hinata, N.; Yamaguchi, R.; Kusuhara, Y.; Kanayama, H.; Kohjimoto, Y.; Hara, I.; Fujisawa, M. Hinotori Surgical Robot System, a novel robot-assisted surgical platform: Preclinical and clinical evaluation. Int. J. Urol. 2022, 29, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Miyake, H.; Motoyama, D.; Matsushita, Y.; Watanabe, H.; Tamura, K.; Otsuka, A.; Fujisawa, M. Initial Experience of Robot-Assisted Partial Nephrectomy Using Hinotori Surgical Robot System: Single Institutional Prospective Assessment of Perioperative Outcomes in 30 Cases. J. Endourol. 2023, 37, 531–534. [Google Scholar] [CrossRef]
- Motoyama, D.; Matsushita, Y.; Watanabe, H.; Tamura, K.; Otsuka, A.; Fujisawa, M.; Miyake, H. Robot-assisted radical nephrectomy using novel surgical robot platform, hinotori: Report of initial series of 13 cases. Int. J. Urol. 2023, 30, 1175–1179. [Google Scholar] [CrossRef]
- Motoyama, D.; Matsushita, Y.; Watanabe, H.; Tamura, K.; Otsuka, A.; Fujisawa, M.; Miyake, H. Robot-assisted radical nephrectomy and inferior vena cava tumor thrombectomy using the novel surgical robot platform, hinotori: Initial experience with two cases. IJU Case Rep. 2024, 7, 96–99. [Google Scholar] [CrossRef]
- Zuluaga, L.; Rich, J.M.; Razdan, S.; Ucpinar, B.; Okhawere, K.E.; Saini, I.; Badani, K.K. Robotic nephroureterectomy supplanting open and laparoscopic approach for upper tract urothelial carcinoma management: A narrative review. Transl. Androl. Urol. 2023, 12, 1740–1752. [Google Scholar] [CrossRef]
- Kim, K.H.; Ahn, H.K.; Kim, M.; Yoon, H. Technique and perioperative outcomes of single-port robotic surgery using the da Vinci SP platform in urology. Asian J. Surg. 2023, 46, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Morizane, S.; Yumioka, T.; Iwamoto, H.; Hikita, K.; Honda, M.; Takenaka, A. Initial Experience of Robot-Assisted Laparoscopic Nephroureterectomy in Japan: A Useful Technique Using a Vessel Sealing Device for Securing a Good Surgical Field and Efficient Sealing. Asian J. Endosc. Surg. 2022, 15, 458–462. [Google Scholar] [CrossRef]
- Jacobs, J.K.; Goldstein, R.E.; Geer, R.J. Laparoscopic adrenalectomy. A new standard of care. Ann. Surg. 1997, 225, 495–501; discussion 501–492. [Google Scholar] [CrossRef]
- Francis, A.; Mellert, L.; Parekh, N.; Pozsgay, M.; Dan, A. Robotic Adrenalectomy: A 10-Year Clinical Experience at a Tertiary Medical Center. J. Soc. Laparosc. Robot. Surg. 2022, 26, e2021-00083. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.M.; Talamini, M.A.; Stanfield, C.L.; Chang, D.C.; Hundt, J.D.; Dackiw, A.P.; Campbell, K.A.; Schulick, R.D. Thirty robotic adrenalectomies: A single institution’s experience. Surg. Endosc. 2006, 20, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Hemal, A.K.; Menon, M. Robotic renal and adrenal surgery: Present and future. BJU Int. 2005, 96, 244–249. [Google Scholar] [CrossRef]
- Sforza, S.; Minervini, A.; Tellini, R.; Ji, C.; Bergamini, C.; Giordano, A.; Lu, Q.; Chen, W.; Zhang, F.; Ji, H.; et al. Perioperative outcomes of robotic and laparoscopic adrenalectomy: A large international multicenter experience. Surg. Endosc. 2021, 35, 1801–1807. [Google Scholar] [CrossRef]
- Agrusa, A.; Romano, G.; Navarra, G.; Conzo, G.; Pantuso, G.; Di Buono, G.; Citarrella, R.; Galia, M.; Monte, A.L.; Cucinella, G.; et al. Innovation in endocrine surgery: Robotic versus laparoscopic adrenalectomy. Meta-analysis and systematic literature review. Oncotarget 2017, 8, 102392–102400. [Google Scholar] [CrossRef]
- Motoyama, D.; Matsushita, Y.; Watanabe, H.; Tamura, K.; Otsuka, A.; Fujisawa, M.; Miyake, H. Robot-assisted adrenalectomy using a hinotori surgical robot system: Report of first series of six cases. Asian J. Endosc. Surg. 2023, 16, 489–495. [Google Scholar] [CrossRef]
- Menon, M.; Tewari, A.; Peabody, J.O.; Shrivastava, A.; Kaul, S.; Bhandari, A.; Hemal, A.K. Vattikuti Institute prostatectomy, a technique of robotic radical prostatectomy for management of localized carcinoma of the prostate: Experience of over 1100 cases. Urol. Clin. North Am. 2004, 31, 701–717. [Google Scholar] [CrossRef]
- Cathelineau, X.; Rozet, F.; Vallancien, G. Robotic radical prostatectomy: The European experience. Urol. Clin. North Am. 2004, 31, 693–699. [Google Scholar] [CrossRef]
- Kohjimoto, Y.; Yamashita, S.; Iwagami, S.; Muraoka, S.; Wakamiya, T.; Hara, I. hinotoriTM vs. da Vinci®: Propensity score-matched analysis of surgical outcomes of robot-assisted radical prostatectomy. J. Robot. Surg. 2024, 18, 130. [Google Scholar] [CrossRef]
- Zorn, K.C.; Gofrit, O.N.; Orvieto, M.A.; Mikhail, A.A.; Galocy, R.M.; Shalhav, A.L.; Zagaja, G.P. Da Vinci robot error and failure rates: Single institution experience on a single three-arm robot unit of more than 700 consecutive robot-assisted laparoscopic radical prostatectomies. J. Endourol. 2007, 21, 1341–1344. [Google Scholar] [CrossRef]
- Chang, K.D.; Raheem, A.A.; Choi, Y.D.; Chung, B.H.; Rha, K.H. Retzius-sparing robot-assisted radical prostatectomy using the Revo-i robotic surgical system: Surgical technique and results of the first human trial. BJU Int. 2018, 122, 441–448. [Google Scholar] [CrossRef]
- Zahid, A.; Ayyan, M.; Farooq, M.; Cheema, H.A.; Shahid, A.; Naeem, F.; Ilyas, M.A.; Sohail, S. Robotic surgery in comparison to the open and laparoscopic approaches in the field of urology: A systematic review. J. Robot. Surg. 2023, 17, 11–29. [Google Scholar] [CrossRef]
- Azhar, R.A.; Gill, I.S.; Aron, M. Robotic nephron-sparing surgery for renal tumors: Current status. Indian J. Urol. 2014, 30, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, A.; Izumi, K.; Ikezoe, E.; Inoue, M.; Tsujioka, H.; Nirazuka, A.; Hasegawa, K.; Osaka, A.; Yasuda, Y.; Fukuda, Y.; et al. Robot-assisted radical prostatectomy using the novel hinotoriTM surgical robot system: Initial experience and operation learning curve at a single institution. Transl. Cancer Res. 2024, 13, 57–64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miyamoto, S.; Hatayama, T.; Shikuma, H.; Yukihiro, K.; Iwane, K.; Tasaka, R.; Kohada, Y.; Fukushima, T.; Takemoto, K.; Naito, M.; et al. Robotic urologic applications of the hinotoriTM surgical robot system. Asian J. Urology. 2024, 12, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, L.; Man, J.; Yang, J.; Wang, H.; Zhang, Y.; Yang, L. Comparison of perioperative outcomes and prognosis between Da Vinci surgical system and Hinotori system in urologic tumor surgery: Evidence from controlled trials. J. Robot. Surg. 2025, 19, 1–12. [Google Scholar] [CrossRef]
- Noshiro, H.; Ide, T.; Nomura, A.; Yoda, Y.; Hiraki, M.; Manabe, T. Introduction of a new surgical robot platform “hinotoriTM” in an institution with established da Vinci surgeryTM for digestive organ operations. Surg. Endosc. 2024, 38, 3929–3939. [Google Scholar] [CrossRef] [PubMed]
- Lavery, H.J.; Thaly, R.; Albala, D.; Ahlering, T.; Shalhav, A.; Lee, D.; Fagin, R.; Wiklund, P.; Dasgupta, P.; Costello, A.J.; et al. Robotic equipment malfunction during robotic prostatectomy: A multi-institutional study. J. Endourol. 2008, 22, 2165–2168. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Société Internationale d’Urologie. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, S.M.; Peng, H.M.; So, W.Z.; Tiong, H.Y. Early Clinical Outcomes of the Novel Hinotori Robotic System in Urological Surgery—A Review of Existing Literature. Soc. Int. Urol. J. 2025, 6, 56. https://doi.org/10.3390/siuj6040056
Ong SM, Peng HM, So WZ, Tiong HY. Early Clinical Outcomes of the Novel Hinotori Robotic System in Urological Surgery—A Review of Existing Literature. Société Internationale d’Urologie Journal. 2025; 6(4):56. https://doi.org/10.3390/siuj6040056
Chicago/Turabian StyleOng, Simone Meiqi, Hong Min Peng, Wei Zheng So, and Ho Yee Tiong. 2025. "Early Clinical Outcomes of the Novel Hinotori Robotic System in Urological Surgery—A Review of Existing Literature" Société Internationale d’Urologie Journal 6, no. 4: 56. https://doi.org/10.3390/siuj6040056
APA StyleOng, S. M., Peng, H. M., So, W. Z., & Tiong, H. Y. (2025). Early Clinical Outcomes of the Novel Hinotori Robotic System in Urological Surgery—A Review of Existing Literature. Société Internationale d’Urologie Journal, 6(4), 56. https://doi.org/10.3390/siuj6040056





