Abstract
Background/Objectives: The diagnostic approach to prostate cancer (PCa) has evolved from systematic biopsies to imaging-guided strategies that improve detection of clinically significant PCa (csPCa) while reducing overdiagnosis. Multiparametric magnetic resonance imaging (mpMRI) has emerged as the gold standard for pre-biopsy evaluation, while micro-ultrasound (MicroUS) offers a promising alternative with real-time imaging capabilities. Methods: We examined the principles, image interpretation frameworks (Prostate Imaging Reporting and Data System (PI-RADS) and Prostate Risk Identification using Micro UltraSound (PRI-MUS)), and clinical applications of mpMRI and MicroUS, comparing their diagnostic accuracy in biopsy-naïve patients, repeat biopsy scenarios, active surveillance, and staging. Results: mpMRI improves csPCa detection, reduces unnecessary biopsies, and enhances risk stratification. Landmark studies such as PRECISION (Prostate Evaluation for Clinically Important Disease: Sampling Using Image Guidance or Not?) and PRIME (Prostate Imaging Using MRI±Contrast Enhancement) confirm its superiority over systematic biopsy. However, mpMRI remains resource-intensive, with limitations in accessibility and interpretation variability. Conversely, MicroUS, with its high-resolution real-time imaging, shows non-inferiority to mpMRI and potential advantages in magnetic resonance imaging (MRI)-ineligible patients. It improves lesion visualization and biopsy targeting, with ongoing trials such as OPTIMUM (Optimization of prostate biopsy—Micro-Ultrasound versus MRI) evaluating its standalone efficacy. Conclusions: mpMRI and MicroUS are complementary modalities in PCa diagnosis. While mpMRI remains the preferred imaging standard, MicroUS offers an alternative, particularly in patients with MRI contraindications. Combining these techniques could enhance diagnostic accuracy, reduce unnecessary interventions, and refine active surveillance strategies. Future research should focus on integrating both modalities into standardized diagnostic pathways for a more individualized approach.
1. Introduction
The diagnostic landscape for prostate cancer (PCa) has evolved significantly, transitioning from random systematic biopsies—long considered the gold standard—to more targeted imaging-driven approaches. Multiparametric magnetic resonance imaging (mpMRI) has emerged as the diagnostic gold standard for detecting clinically significant PCa (csPCa) while minimizing overdiagnosis of clinically insignificant cases. Additionally, alternative modalities like micro-ultrasound (MicroUS) have shown promise with diagnostic accuracy comparable to mpMRI. Despite these advancements, challenges remain in fully integrating these imaging technologies into standard clinical practice. Variability in accessibility, cost, operator dependency, and the need for standardized interpretation limit their widespread adoption. Furthermore, there is an ongoing need to refine patient selection criteria and optimize the diagnostic workflow to balance sensitivity, specificity, and clinical utility. We aim to summarize the evidence supporting these imaging tools and their roles in optimizing the diagnostic workflow for PCa.
2. Magnetic Resonance Imaging (MRI)
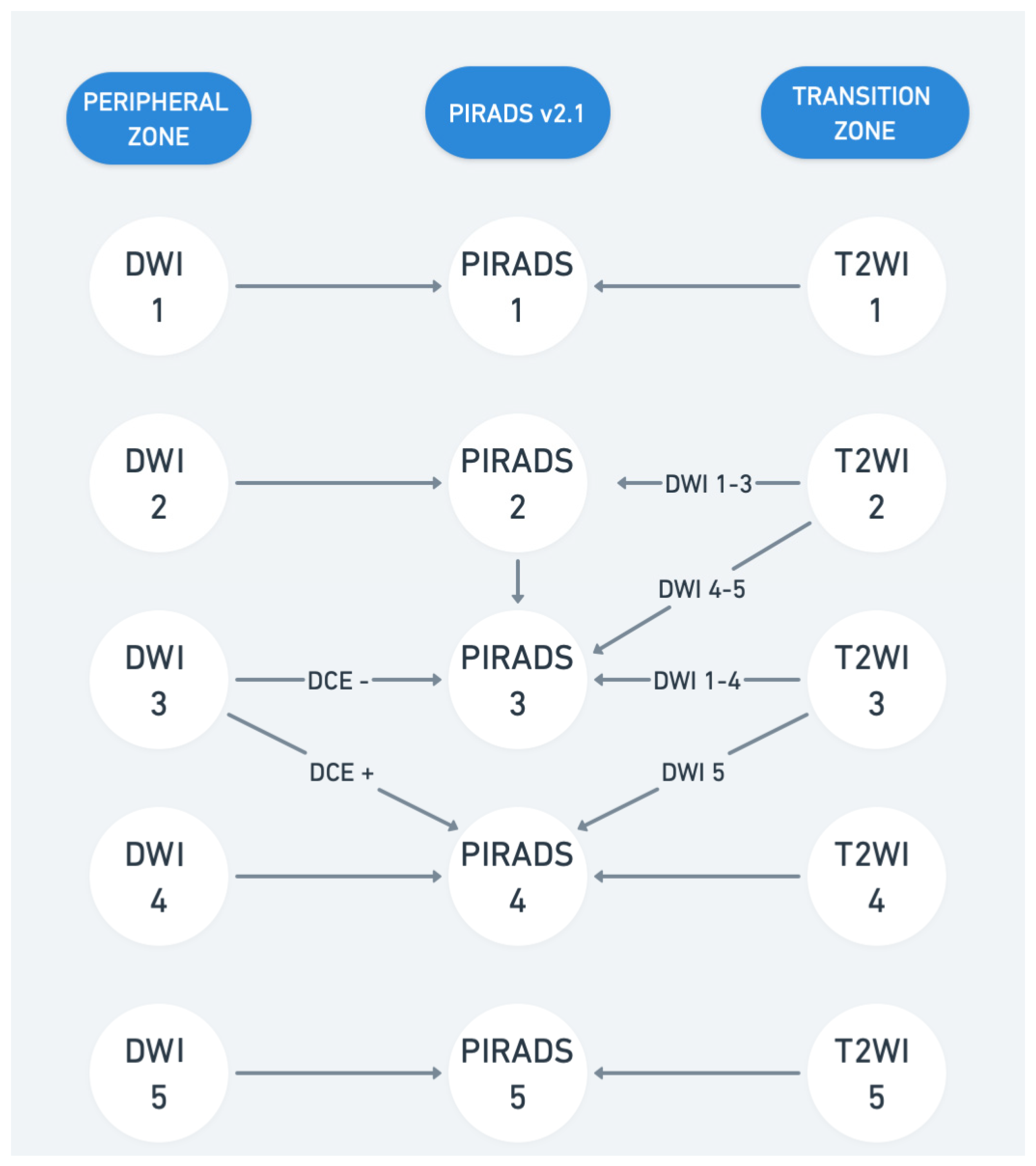
Multiparametric MRI of the prostate includes various anatomical and functional imaging parameters, each focusing on specific aspects of the prostate gland. MRI combines T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and dynamic contrast-enhanced imaging (DCE). These sequences provide high-resolution anatomical and functional details, distinguishing indolent from significant cancer and supporting risk stratification and image-guided biopsies. T1WI is particularly useful for identifying hemorrhages post-biopsy, while T2WI delineates prostate zonal anatomy. T2WI offers precise anatomical details, differentiating the peripheral and transition zones. Prostate cancer typically appears hypointense due to high cellularity and low water content, though conditions like prostatitis or post-biopsy hemorrhage may mimic malignancy. DWI evaluates water diffusion, with cancerous tissue showing restricted diffusion—hyperintense on high b-value images and hypointense on apparent diffusion coefficient (ADC) maps. Optimal protocols include high b-values (1400–2000 s/mm2). Quantitative analysis of apparent diffusion coefficient (ADC) values can further enhance differentiation between aggressive and indolent tumors [1,2]. DCE uses gadolinium-based contrast to assess tissue perfusion. PCa shows early contrast wash-in and wash-out patterns, useful in ambiguous cases or recurrence evaluation despite debates about cost and gadolinium safety [3,4]. Prostate Imaging Reporting and Data System (PI-RADS) assigns lesion scores (1–5) based on the likelihood of csPCa. PI-RADS 4 and 5 often warrant biopsy, while scores ≤2 usually do not. Although widely adopted, flexibility exists in applying PI-RADS vs. the Likert scoring system [5]. Current updates to PI-RADS emphasize structured reporting, and integration with clinical parameters like PSA density as such does not provide management recommendations. At present, PI-RADS v.2.1 (Figure 1) is widely used, though some experienced radiologists prefer the subjective Likert scoring system, emphasizing flexibility in considering additional parameters beyond the rigid PI-RADS criteria [6,7,8]. Biparametric MRI (bpMRI) and mpMRI differ in the imaging techniques used to evaluate the prostate. MpMRI employs three sequences—T2-weighted imaging, diffusion-weighted imaging (apparent diffusion coefficient (ADC)), and dynamic contrast-enhanced imaging—while bpMRI uses only the first two, omitting contrast agents. Studies suggest that bpMRI is faster, cheaper, and safer than mpMRI while maintaining comparable accuracy, sensitivity, and specificity for detecting csPCa [9,10,11,12]. However, mpMRI may still be preferable in certain scenarios, such as in patients with PSA levels of 10–20 ng/mL, and current guidelines generally recommend mpMRI for broader clinical use [13]. Further research, including the upcoming Prostate Imaging Using MRI±Contrast Enhancement (PRIME) trial, will help clarify the best imaging approach for csPCa.
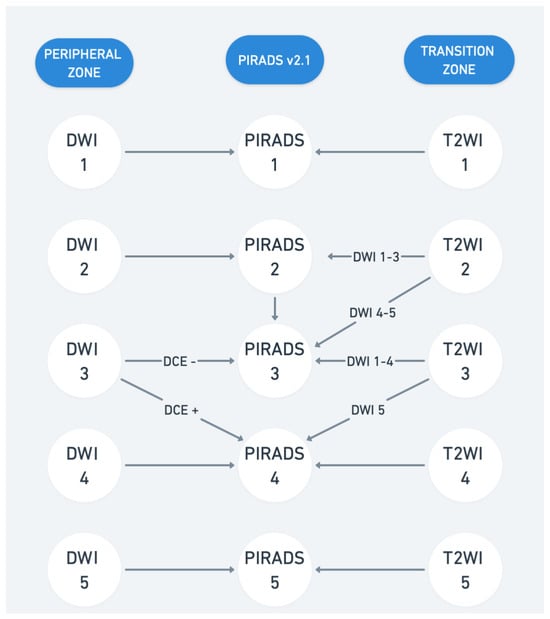
Figure 1.
Prostate Imaging Reporting and Data System (PI-RADS) v2.1. T2WI represents T2-weighted imaging and DWI represents diffusion-weighted imaging. DCE represents dynamic contrast-enhanced imaging.
3. MRI PCa Diagnosis in Biopsy-Naïve Patients
Systematic transrectal ultrasound-guided (TRUS) biopsy has historically been shown to underestimate the Gleason grade of prostate tumors, leading to inaccurate risk assessment and suboptimal treatment decisions. Additionally, TRUS biopsy often detects a higher proportion of low-risk, clinically insignificant PCa, contributing to overdiagnosis, psychological burden, and potential overtreatment. In contrast, mpMRI has proven effective in diagnosing, localizing, risk-stratifying, and staging csPCa, including in patients undergoing their first biopsy. Several studies highlight the advantages of mpMRI over systematic biopsy. For example, Panebianco et al. [1] reported that 52% of men with a prior negative TRUS biopsy were found to have csPCa on mpMRI-targeted biopsy. Similarly, Haffner et al. [2] showed higher detection rates of csPCa in the MRI-targeted biopsy arm (63%) compared to systematic biopsy (54%). Van der Leest’s 2018 multicenter study also found that mpMRI-guided biopsy detected similar rates of csPCa (25% vs. 23% with systematic biopsy) while significantly reducing the detection of clinically insignificant PCa (14% vs. 25%). However, this study noted that combining systematic and MRI-targeted biopsies could have introduced bias in assessing each method independently [3]. More recent high-quality evidence supports the use of mpMRI. The Prostate Evaluation for Clinically Important Disease: Sampling Using Image Guidance or Not? (PRECISION) trial demonstrated that mpMRI with targeted biopsy detected more csPCa (≥Gleason Grade (GG)2) with fewer cores than standard systematic biopsy while reducing the detection of clinically insignificant PCa [4]. Notably, over 25% of men in the mpMRI arm avoided a biopsy altogether. Similarly, a Canadian randomized controlled trial found that 38% of men in the mpMRI arm avoided biopsy due to negative MRI results (PI-RADS ≤ 2), while csPCa detection was higher in the mpMRI arm (35% vs. 30% with systematic biopsy) and detection of clinically insignificant PCa was lower (10% vs. 22%) [5]. A follow-up study confirmed no significant difference in csPCa detection between the arms, despite fewer biopsies in the MRI group [6]. These findings, supported by multiple studies, have led to guideline recommendations favoring mpMRI before biopsy. This approach improves the detection and localization of csPCa, reduces unnecessary biopsies, and minimizes overdiagnosis of low-risk PCa.
4. MRI PCa Diagnosis in Repeat Biopsy Settings
Detecting csPCa remains a key quality-of-care concern, with the diagnostic pathway differing significantly from other solid organ cancers [7]. The current standard, TRUS biopsy, relies on semi-random [8]. In this context, mpMRI has emerged as a valuable tool for evaluating the risk of csPCa, particularly in men with elevated prostate-specific antigen (PSA) levels or abnormal digital rectal exams. A Cochrane [9] review reported mpMRI’s sensitivity for detecting csPCa (International Society of Urological Pathology (ISUP) grade ≥ 2) at 0.91, though specificity was lower at 0.37. Detection rates for csPCa based on PI-RADS scores increase with higher scores: 6% for PI-RADS 2, 12% for PI-RADS 3, 48% for PI-RADS 4, and 72% for PI-RADS 5. The Likert scoring system, based on subjective radiologist assessments, demonstrated high sensitivity (0.94) and specificity (0.77) for prostate cancer diagnosis [10,11]. Grivas et al. [12] conducted a systematic review focusing on detection rates of PCa and csPCa in patients with negative initial biopsies but positive mpMRI findings. Their analysis revealed:
- For PI-RADS 3, csPCa detection ranged from 2.5% to 22%.
- For PI-RADS 4, csPCa detection ranged from 7.7% to 45%.
- For PI-RADS 5, csPCa detection reached up to 50%.
These findings suggest tailored follow-up strategies: monitoring with repeat mpMRI and prostate-specific antigen (PSA) testing for PI-RADS 3 lesions, guiding biopsy decisions with imaging for PI-RADS 4, and standard repeat biopsies for PI-RADS 5. This approach underscores the importance of re-evaluating initial mpMRI results to optimize outcomes. In addition to imaging, biomarkers offer potential for noninvasive risk assessment. While biomarkers like the prostate health index and PCA3 were found to be less accurate and cost-effective compared to imaging-based pathways, others, such as the 4-kallikrein panel, show promise and are undergoing further validation [13].
5. MRI and Active Surveillance
mpMRI plays a pivotal role in selecting and monitoring active surveillance (AS) candidates, offering high negative predictive value and reducing unnecessary biopsies. The Prostate Cancer Radiological Estimation of Change in Sequential Evaluation (PRECISE) score assesses progression risk during AS, though robust trials are needed to refine imaging intervals and progression thresholds. Combining mpMRI with biomarkers like PSA velocity enhances surveillance accuracy [14].
6. MRI and Staging
Precise locoregional staging of PCa at the time of diagnosis is crucial for optimizing treatment strategies and avoiding both overtreatment and undertreatment. The TNM classification system is used to determine the extent of PCa spread. Currently, PCa staging is primarily based on serum PSA levels, digital rectal examination (DRE), and Gleason score, which can sometimes underestimate the true extent of disease. Extracapsular extension and seminal vesicle invasion are associated with an increased risk of biochemical recurrence following definitive treatment due to a greater likelihood of positive surgical margins and lymph node metastases. The ability to accurately identify extraprostatic disease using mpMRI allows for more precise surgical planning, potentially reducing the incidence of positive margins in final pathology. For patients with T3a disease, standard treatment options include radical prostatectomy or brachytherapy. However, managing T3b disease often necessitates a multimodal approach incorporating long-term androgen deprivation therapy along with definitive treatment. The ability to localize seminal vesicle and regional invasion with mpMRI facilitates tailored therapeutic decisions. A study involving 532 patients who underwent mpMRI prior to MRI-ultrasound fusion biopsy (MRUSFBx) and radical prostatectomy demonstrated that incorporating mpMRI into clinical nomograms improved the predictive accuracy for organ-confined disease, extraprostatic extension, seminal vesicle invasion, and lymph node involvement. A meta-analysis of 75 studies assessing the accuracy of mpMRI in PCa staging reported sensitivity and specificity values of 0.57 and 0.91 for detecting extraprostatic disease and 0.58 and 0.96 for seminal vesicle invasion. These findings highlight mpMRI’s high specificity for locoregional staging, though a negative scan does not necessarily rule out extraprostatic disease. Various scoring systems have been developed to assess the likelihood of extraprostatic extension on mpMRI, including the European Society of Urogenital Radiology score, the Likert scale, tumor contact length, and extraprostatic extension grade. A retrospective study comparing these criteria emphasized the value of extraprostatic extension grade, which integrates both quantitative and qualitative mpMRI parameters while being less dependent on the radiologist’s experience. Additionally, recent research has identified the capsular enhancement sign on DCE MRI as a highly specific marker for extraprostatic extension. Further studies are needed to refine the most sensitive and specific parameters for identifying extraprostatic spread. A hypointense signal on T2WI, restricted diffusion on DWI, and early enhancement on DCE MRI may indicate seminal vesicle invasion and infiltration of adjacent structures. However, the ability of mpMRI to detect nodal metastases remains limited due to its restricted field of view compared to whole-body imaging techniques. Relying solely on lymph node size to distinguish between malignant and benign nodes can lead to both false positives (e.g., inflammatory lymphadenopathy) and false negatives (e.g., micro metastases below the detection threshold). Traditionally, PCa staging has included computed tomography (CT) and bone scintigraphy to assess nodal and distant metastases. However, novel imaging techniques such as prostate-specific membrane antigen positron emission tomography (PSMA PET) have demonstrated improved accuracy. In a study of 764 men with intermediate- to high-risk PCa undergoing PSMA PET before radical prostatectomy, the sensitivity and specificity for detecting pelvic nodal metastases were 0.40 and 0.95, respectively. This suggests that PSMA PET provides high specificity for detecting nodal disease, though small metastases may still go undetected, meaning a negative scan does not entirely exclude nodal involvement. The role of MRI in detecting local recurrence after radical prostatectomy is also being increasingly recognized. Furthermore, functional MRI with diffusion and perfusion imaging has shown potential in identifying local recurrence even at low PSA levels. This capability is clinically significant, as early detection of recurrence can alter management strategies, particularly in the context of salvage radiation therapy (SRT). MRI has been shown to outperform CT in delineating local recurrences and defining target volumes for radiation therapy, potentially enabling dose escalation while minimizing toxicity. Furthermore, dynamic contrast-enhanced (DCE) MRI and DWI have been identified as effective tools in distinguishing between recurrent tumor tissue and post-surgical fibrosis [15,16,17,18,19,20,21,22].
7. Benefits and Pitfalls of MRI
MRI’s superior tissue contrast improves csPCa detection, facilitates targeted biopsies, and supports precise treatment planning (e.g., radiotherapy targeting). It also aids in surveillance and early recurrence detection. Its use in pre-biopsy settings can stratify patients more effectively, reducing invasive procedures [23,24]. MRI is resource-intensive, with access limitations due to cost, patient incompatibilities (e.g., implants, claustrophobia), or interpretation variability. Overdiagnosis of insignificant lesions remains a concern, highlighting the need for careful clinical judgment. Long-term benefits of MRI-guided pathways require further validation [4,25,26].
8. Micro-Ultrasound
Conventional transrectal ultrasound (TRUS) of the prostate typically employs a 6–9 MHz curved linear endocavity transducer, which provides real-time imaging crucial for procedures such as local nerve blocks and systematic biopsy guidance [27]. However, while it can detect internal prostatic tissue, its sensitivity for targeted sampling is limited, with an axial resolution of 210 μm insufficient to visualize the prostatic ductal architecture. To address this limitation, a new MicroUS system was developed, offering a resolution of 70 μm—optimal for visualizing the prostate ducts, which average 100 μm in size. This system allows imaging of the entire prostate while maintaining high sensitivity and specificity. Clinical testing in patients undergoing radical prostatectomy demonstrated superior accuracy compared to conventional TRUS, with further validation by Pensa et al., showing that MicroUS resolves approximately 95% of prostatic ducts compared to 15% with conventional ultrasound. The success of MicroUS is attributed to advanced transducer designs featuring densely packed elements with wide bandwidth, enabling improved penetration depth and a full field of view while maintaining high resolution. Unlike traditional ultrasound, MicroUS effectively visualizes tissue architecture across all prostate zones, including the anterior, transition, and peripheral zones. New clinical interpretation protocols, such as the PRI-MUS (Prostate Risk Identification using Micro-UltraSound) system, first described by Ghai et al., have been developed to identify cancer-specific patterns on MicroUS, demonstrating robust cancer detection across all prostate regions (Figure 2). This technology represents a significant advancement in prostate imaging and cancer detection [28,29,30,31,32].
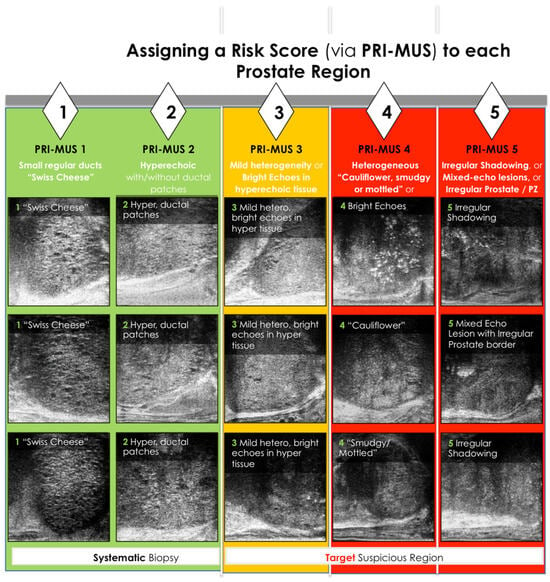
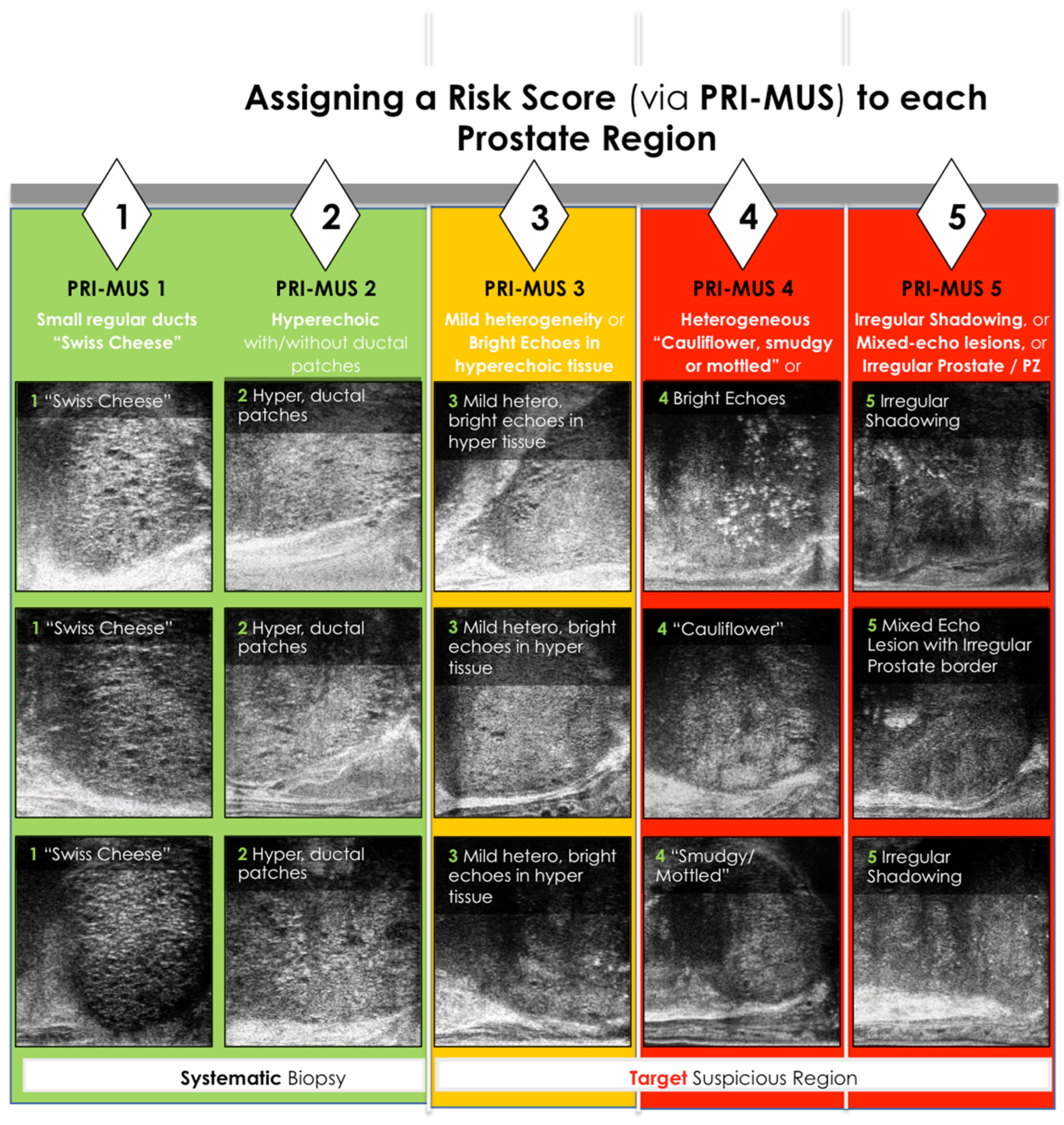
Figure 2.
PRI—MUS Score. PZ represents peripheral zone [33]
9. MicroUS Image Interpretation and PRI-MUS Scoring
MicroUS enables high-resolution visualization of prostate tissue, offering imaging detail comparable to low-magnification microscopy. This precision allows differentiation of prostate zones (peripheral, transition, central, and anterior fibromuscular stroma) and detection of PCa based on distinct acoustic features. Cancer, most found in the peripheral zone (77%), transition zone (20%), and less frequently in the central zone (3%), arises as dense, hypoechoic areas where benign ducts condense into tumor tissue [34,35].
The PRI-MUS protocol assigns risk scores to lesions based on acoustic patterns. In practice, interpreting these patterns involves systematically examining the prostate zones:
- Anterior Prostate: Anterior tumors lack ducts, have a hypoechoic stroma, and are longer than tall. Larger lesions may show irregular borders or loss of surrounding fat. Benign prostatic hyperplasia (BPH) nodules, in contrast, appear as taller-than-long structures with smooth, hyperechoic capsules and occasional ducts or cysts.
- Central Zone: Rarely affected by cancer (3%), this zone serves as a reference for evaluating echogenicity. Identifying midline landmarks, such as the verumontanum, helps differentiate normal ducts from cancerous areas.
- Peripheral Zone: Large, visible ducts indicate no cancer (PRI-MUS 1). As ducts shrink or disappear, the lesion progresses to PRI-MUS 2 or higher. A mixed or mottled echogenic background suggests PRI-MUS 4, while a hypoechoic or isoechoic background relative to the central zone suggests PRI-MUS 5. Larger cancers may disrupt adjacent tissue or the prostate capsule.
Additional features, such as the “starry night” pattern caused by calcifications, can mimic cancer but may also indicate benign conditions like prior inflammation or urethral calcifications [31,32,36,37].
10. MicroUS and PCa Diagnosis in Biopsy-Naïve Patients
MicroUS offers real-time, high-resolution imaging (70 μm), enabling precise visualization of prostate lesions and supporting both biopsy decisions and targeted sampling. While most studies on MicroUS performance have included men preselected with mpMRI (PI-RADS ≥ 3), the data highlight its potential as an adjunct to or alternative for mpMRI. In a multicenter trial by Hofbauer et al., MicroUS demonstrated non-inferiority to mpMRI, detecting 97% of csPCa in men with a PI-RADS ≥3 lesion undergoing biopsy. Similarly, Lughezzani et al. reported high sensitivity (86.5%) and negative predictive value (71.4%) for PRI-MUS scoring in an Italian cohort. Studies combining targeted biopsies guided by MicroUS and mpMRI showed no additional csPCa detection from systematic biopsies, indicating the effectiveness of these approaches together. Two meta-analyses involving over 2800 men confirmed that MicroUS achieves cancer detection rates comparable to mpMRI, including cases of repeat biopsy. Furthermore, Wiemer et al. found that MicroUS identified an additional 17% of csPCa and 9% of high-risk cancers missed by mpMRI alone, suggesting that MicroUS could reduce the need for systematic biopsies while maintaining diagnostic accuracy. Notably, in men with PI-RADS 3 lesions, MicroUS helped avoid unnecessary biopsies in 27% of cases without missing csPCa. The ongoing Optimization of prostate biopsy - Micro-Ultrasound versus MRI (OPTIMUM) trial is evaluating MicroUS as a standalone or complementary tool to mpMRI, with a primary goal of establishing its non-inferiority in detecting csPCa. This trial will also explore its potential as a screening tool and its ability to reduce unnecessary biopsies, aiming to surpass the 30% reduction seen in the mpMRI pathway. Early results suggest that MicroUS could play a key role in prostate cancer diagnostics, offering a real-time, high-resolution alternative or adjunct to mpMRI while potentially improving biopsy precision and patient outcomes [27,38,39,40,41,42].
11. Microus and PCa Diagnosis in Repeat Biopsy Settings
MicroUS effectively identifies residual lesions post-negative MRI-targeted biopsy, with sensitivity (91.2%) and NPV (66.7%) exceeding mpMRI [43]. Emerging data suggest its utility in guiding repeat biopsies and minimizing overdiagnosis. Visualization of prior biopsy channels aids in targeting unsampled areas.
12. Microus and Active Surveillance
Active surveillance is now the preferred approach for managing men with low-risk prostate cancer, with its adoption significantly increasing over the past decade. When patients are carefully selected and monitored, prostate cancer metastases and mortality are rare. For example, a study of 1818 men on AS reported only four prostate cancer-related deaths, though the rates of definitive treatment reached 36% at five years and 48% at ten years. MRI-guided prostate biopsy has become the standard for identifying men who may require active treatment upfront and monitoring disease progression during AS. Emerging evidence suggests that high-resolution MicroUS is a promising alternative. Studies have shown that MicroUS detects clinically significant cancers (Gleason Grade Group ≥ 2) with sensitivity rates of 94–97% for PRI-MUS ≥3 lesions, comparable to MRI. Additionally, the concept of a “double negative” (negative findings on both MRI and MicroUS) has demonstrated strong predictive value, with no upgrades to Gleason Grade Group ≥2 in one study. To further validate MicroUS’s role in AS, ongoing trials like the MUSIC-AS study are comparing its accuracy in detecting clinically significant cancers during confirmatory biopsies [28,29,34,35,44,45,46,47].
13. Microus and Staging
Recent studies have evaluated the utility of MicroUS for local staging in men undergoing radical prostatectomy, comparing pre-operative imaging to whole-mount pathology. Key MicroUS features associated with non-organ-confined disease include:
- Visible breach of the prostate capsule;
- Capsular bulging;
- Obliteration of the prostatic–seminal vesicle angle;
- Presence of a hypoechoic halo;
- Capsular contact length ≥ 15 mm.
The likelihood of T3 disease increases with the number of these factors present. These findings have been used to develop a nomogram for predicting extracapsular extension (ECE) based on MicroUS imaging. However, MicroUS has limitations in staging pelvic lymph nodes, bony structures, and detecting recurrence, areas where MRI remains superior [30,31,32].
14. Benefits and Pitfalls of MicroUS
MicroUS offers unique advantages and specific challenges in prostate imaging, making it a valuable tool in certain clinical scenarios. Understanding these benefits and limitations is crucial for effective use. MicroUS enables immediate identification of suspicious prostate lesions during the same clinical visit, unlike other imaging technologies requiring separate appointments. It has demonstrated diagnostic accuracy comparable to mpMRI in several studies. MicroUS provides high-resolution visualization of the prostate and surrounding structures, including the capsule, neurovascular bundles, facial planes, and ejaculatory ducts. This level of detail aids in precise lesion localization and planning for focal therapy or surgical procedures. Features like needle scars from prior biopsies are visible even years after the procedure, offering additional diagnostic context [32,36,37,38,40,41,48]. MicroUS could be useful in several scenarios. For men unable to undergo mpMRI due to contraindications (e.g., pacemakers, metal implants, impaired kidney function, or claustrophobia), MicroUS provides an alternative with high diagnostic accuracy, reducing reliance on systematic biopsy. In cases where MRI results are inconclusive or negative but clinical suspicion remains (e.g., elevated PSA or abnormal DRE), MicroUS offers independent diagnostic insights. Studies suggest that MicroUS detects csPCa 47% more effectively than conventional ultrasound in MRI-negative cases. MicroUS supports real-time guidance for treatments like laser ablation, cryotherapy, High-Intensity Focused Ultrasound (HIFU), and steam therapy. Its fast imaging of lesion borders and critical structures enhances control over treatment margins, improving precision and safety. Although useful in clinical practice, MicroUS faces several limitations. Operator-dependent variability, artifacts (e.g., calcifications), and limited field-of-view may challenge accuracy. Continued refinement of protocols (e.g., PRI-MUS updates) is needed to standardize usage. A key consideration in the adoption of MicroUS is the potential for inter-observer variability, as image interpretation is operator-dependent. Unlike MRI, which has standardized reporting systems such as PI-RADS, MicroUS interpretation relies on the PRI-MUS protocol, which requires dedicated training for consistent application. Studies suggest that proficiency improves with experience, and structured training programs can enhance diagnostic accuracy while minimizing variability. Given that urologists, rather than radiologists, are often the primary users of MicroUS for biopsy guidance, hands-on training and validation studies remain essential to ensure reproducibility and reliability across different clinical settings. Further research should explore standardization efforts and learning curves to optimize its integration into routine practice. Studies to assess inter-reader variability and training requirements are critical for broader adoption [31,32].
15. Conclusions
MRI and MicroUS represent complementary modalities for localized PCa diagnosis. While mpMRI remains the gold standard, MicroUS offers a valuable, real-time alternative, particularly in patients contraindicated for MRI. Combining these technologies could further optimize detection, reduce unnecessary interventions, and refine active surveillance strategies. Future studies should aim to validate their integration in diagnostic pathways and explore their long-term clinical benefits. These advancements promise a more individualized, patient-centered approach to prostate cancer care.
Author Contributions
Conceptualization: C.S., W.G.B., H.C., S.G., F.G., A.K., D.M. and G.L.; Methodology: C.S.; Software: C.S.; Validation: C.S., W.G.B. and G.L.; Formal analysis: C.S.; Investigation: C.S.; Resources: G.L.; Data curation: C.S.; Writing—original draft preparation: C.S.; Writing—review and editing: C.S., W.G.B., H.C., S.G., F.G., A.K., D.M. and G.L.; Visualization: C.S.; Supervision: G.L.; Project administration: G.L.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Panebianco, V.; Barchetti, F.; Sciarra, A.; Ciardi, A.; Indino, E.L.; Papalia, R.; Gallucci, M.; Tombolini, V.; Gentile, V.; Catalano, C. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: A randomized study. Urol. Oncol. Semin. Orig. Investig. 2015, 33, e1–e17. [Google Scholar] [CrossRef]
- Haffner, J.; Lemaitre, L.; Puech, P.; Haber, G.-P.; Leroy, X.; Jones, J.S.; Villers, A. Role of magnetic resonance imaging before initial biopsy: Comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int. 2011, 108, E171–E178. [Google Scholar] [CrossRef] [PubMed]
- van der Leest, M.; Cornel, E.; Israël, B.; Hendriks, R.; Padhani, A.R.; Hoogenboom, M.; Zamecnik, P.; Bakker, D.; Setiasti, A.Y.; Veltman, J.; et al. Head-to-Head Comparison of Transrectal Ultrasound-Guided Prostate Biopsy Versus Multiparametric Prostate Resonance Imaging with Subsequent Magnetic Resonance-Guided Biopsy in Biopsy-Naïve Men with Elevated Prostate-Specific Antigen: A Large Prospective Multicenter Clinical Study. Eur. Urol. 2019, 75, 570–578. [Google Scholar] [CrossRef]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Chin, J.; Black, P.C.; Finelli, A.; Anidjar, M.; Bladou, F.; Mercado, A.; Levental, M.; Ghai, S.; Chang, S.D.; et al. Comparison of Multiparametric Magnetic Resonance Imaging–Targeted Biopsy with Systematic Transrectal Ultrasonography Biopsy for Biopsy-Naive Men at Risk for Prostate Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 534–542. [Google Scholar] [CrossRef]
- Klotz, L.; Chin, J.; Black, P.C.; Finelli, A.; Anidjar, M.; Machado, A.; Levental, M.; Ghai, S.; Chang, S.D.; Patel, C.; et al. Magnetic Resonance Imaging–Targeted Versus Systematic Prostate Biopsies: 2-Year Follow-Up of a Prospective Randomized Trial (PRECISE). Eur. Urol. Oncol. 2024, 7, 456–461. [Google Scholar] [CrossRef]
- Shaw, G.L.; Thomas, B.C.; Dawson, S.N.; Srivastava, G.; Vowler, S.L.; Gnanapragasam, V.J.; Shah, N.C.; Warren, A.Y.; Neal, D.E. Identification of pathologically insignificant prostate cancer is not accurate in unscreened men. Br. J. Cancer 2014, 110, 2405–2411. [Google Scholar] [CrossRef]
- Abraham, N.E.; Mendhiratta, N.; Taneja, S.S. Patterns of Repeat Prostate Biopsy in Contemporary Clinical Practice. J. Urol. 2015, 193, 1178–1184. [Google Scholar] [CrossRef]
- Drost, F.-J.H.; Osses, D.; Nieboer, D.; Bangma, C.H.; Steyerberg, E.W.; Roobol, M.J.; Schoots, I.G. Prostate Magnetic Resonance Imaging, with or Without Magnetic Resonance Imaging-targeted Biopsy, and Systematic Biopsy for Detecting Prostate Cancer: A Cochrane Systematic Review and Meta-analysis. Eur. Urol. 2020, 77, 78–94. [Google Scholar] [CrossRef]
- Barkovich, E.J.; Shankar, P.R.; Westphalen, A.C. A Systematic Review of the Existing Prostate Imaging Reporting and Data System Version 2 (PI-RADSv2) Literature and Subset Meta-Analysis of PI-RADSv2 Categories Stratified by Gleason Scores. Am. J. Roentgenol. 2019, 212, 847–854. [Google Scholar] [CrossRef]
- Zawaideh, J.P.; Sala, E.; Pantelidou, M.; Shaida, N.; Koo, B.; Caglic, I.; Warren, A.Y.; Carmisciano, L.; Saeb-Parsy, K.; Gnanapragasam, V.J.; et al. Comparison of Likert and PI-RA DS version 2 MRI scoring systems for the detection of clinically significant prostate cancer. Br. J. Radiol. 2020, 93, 20200298. [Google Scholar] [CrossRef] [PubMed]
- Grivas, N.; Lardas, M.; Espinós, E.L.; Lam, T.B.; Rouviere, O.; Mottet, N.; Van Den Bergh, R.C.N. Prostate Cancer Detection Percentages of Repeat Biopsy in Patients with Positive Multiparametric Magnetic Resonance Imaging (Prostate Imaging Reporting and Data System/Likert 3–5) and Negative Initial Biopsy. A Mini Systematic Review. Eur. Urol. 2022, 82, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Diagnosing Prostate Cancer: PROGENSA PCA3 Assay and Prostate Health Index |Guidance| NICE. Available online: https://www.nice.org.uk/guidance/dg17 (accessed on 18 June 2024).
- Rajwa, P.; Pradere, B.; Quhal, F.; Mori, K.; Laukhtina, E.; Huebner, N.A.; D’Andrea, D.; Krzywon, A.; Shim, S.R.; Baltzer, P.A.; et al. Reliability of Serial Prostate Magnetic Resonance Imaging to Detect Prostate Cancer Progression During Active Surveillance: A Systematic Review and Meta-Analysis. Eur. Urol. 2021, 80, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Boesen, L.; Nørgaard, N.; Løgager, V.; Balslev, I.; Bisbjerg, R.; Thestrup, K.-C.; Winther, M.D.; Jakobsen, H.; Thomsen, H.S. Assessment of the Diagnostic Accuracy of Biparametric Magnetic Resonance Imaging for Prostate Cancer in Biopsy-Naive Men: The Biparametric MRI for Detection of Prostate Cancer (BIDOC) Study. JAMA Netw. Open 2018, 1, e180219. [Google Scholar] [CrossRef]
- Oliveira, T.; Ferreira, L.A.; Marto, C.M.; Marques, C.; Oliveira, C.; Donato, P. The Role of Multiparametric MRI in the Local Staging of Prostate Cancer. FBE 2023, 15, 21. [Google Scholar] [CrossRef]
- Bossi, A.; Dariane, C.; Sargos, P. How To Manage T3b Prostate Cancer in the Contemporary Era: Referee Position. Eur. Urol. Open Sci. 2023, 53, 58–59. [Google Scholar] [CrossRef]
- Rayn, K.N.; Bloom, J.B.; Gold, S.A.; Hale, G.R.; Baiocco, J.A.; Mehralivand, S.; Czarniecki, M.; Sabarwal, V.K.; Valera, V.; Wood, B.J.; et al. Added Value of Multiparametric Magnetic Resonance Imaging to Clinical Nomograms for Predicting Adverse Pathology in Prostate Cancer. J. Urol. 2018, 200, 1041–1047. [Google Scholar] [CrossRef]
- de Rooij, M.; Hamoen, E.H.J.; Witjes, J.A.; Barentsz, J.O.; Rovers, M.M. Accuracy of Magnetic Resonance Imaging for Local Staging of Prostate Cancer: A Diagnostic Meta-analysis. Eur. Urol. 2016, 70, 233–245. [Google Scholar] [CrossRef]
- Baco, E.; Rud, E.; Vlatkovic, L.; Svindland, A.; Eggesbø, H.B.; Hung, A.J.; Matsugasumi, T.; Bernhard, J.-C.; Gill, I.S.; Ukimura, O. Predictive Value of Magnetic Resonance Imaging Determined Tumor Contact Length for Extracapsular Extension of Prostate Cancer. J. Urol. 2015, 193, 466–472. [Google Scholar] [CrossRef]
- Mehralivand, S.; Shih, J.H.; Harmon, S.; Smith, C.; Bloom, J.; Czarniecki, M.; Gold, S.; Hale, G.; Rayn, K.; Merino, M.J.; et al. A Grading System for the Assessment of Risk of Extraprostatic Extension of Prostate Cancer at Multiparametric MRI. Radiology 2019, 290, 709–719. [Google Scholar] [CrossRef]
- Renard-Penna, R.; Zhang-Yin, J.; Montagne, S.; Aupin, L.; Bruguière, E.; Labidi, M.; Latorzeff, I.; Hennequin, C. Targeting Local Recurrence After Surgery with MRI Imaging for Prostate Cancer in the Setting of Salvage Radiation Therapy. Front. Oncol. 2022, 12, 775387. [Google Scholar] [CrossRef] [PubMed]
- Westphalen, A.C.; McCulloch, C.E.; Anaokar, J.M.; Arora, S.; Barashi, N.S.; Barentsz, J.O.; Bathala, T.K.; Bittencourt, L.K.; Booker, M.T.; Braxton, V.G.; et al. Variability of the Positive Predictive Value of PI-RADS for Prostate MRI across 26 Centers: Experience of the Society of Abdominal Radiology Prostate Cancer Disease-focused Panel. Radiology 2020, 296, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.; Tree, A.C. Prostate cancer—Advantages and disadvantages of MR-guided RT. Clin. Transl. Radiat. Oncol. 2019, 18, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.U.; El-Shater Bosaily, A.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; Freeman, A.; et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef]
- Rosenkrantz, A.B.; Taneja, S.S. Radiologist, Be Aware: Ten Pitfalls That Confound the Interpretation of Multiparametric Prostate MRI. Am. J. Roentgenol. 2014, 202, 109–120. [Google Scholar] [CrossRef]
- Avolio, P.P.; Lughezzani, G.; Paciotti, M.; Maffei, D.; Uleri, A.; Frego, N.; Hurle, R.; Lazzeri, M.; Saita, A.; Guazzoni, G.; et al. The use of 29 MHz transrectal micro-ultrasound to stratify the prostate cancer risk in patients with PI-RADS III lesions at multiparametric MRI: A single institutional analysis. Urol. Oncol. Semin. Orig. Investig. 2021, 39, e1–e832. [Google Scholar] [CrossRef]
- Albers, P.; Wang, B.; Broomfield, S.; Medina Martín, A.; Fung, C.; Kinnaird, A. Micro-ultrasound Versus Magnetic Resonance Imaging in Prostate Cancer Active Surveillance. Eur. Urol. Open Sci. 2022, 46, 33–35. [Google Scholar] [CrossRef]
- Maffei, D.; Fasulo, V.; Avolio, P.P.; Saitta, C.; Paciotti, M.; De Carne, F.; Colombo, P.; Pasini, L.; De Zorzi, S.Z.; Saita, A.; et al. Diagnostic performance of microUltrasound at MRI-guided confirmatory biopsy in patients under active surveillance for low-risk prostate cancer. Prostate 2023, 83, 886–895. [Google Scholar] [CrossRef]
- Regis, F.; Casale, P.; Persico, F.; Colombo, P.; Cieri, M.; Guazzoni, G.; Buffi, N.M.; Lughezzani, G. Use of 29-MHz Micro-ultrasound for Local Staging of Prostate Cancer in Patients Scheduled for Radical Prostatectomy: A Feasibility Study. Eur. Urol. Open Sci. 2020, 19, 20–23. [Google Scholar] [CrossRef]
- Fasulo, V.; Buffi, N.M.; Regis, F.; Paciotti, M.; Persico, F.; Maffei, D.; Uleri, A.; Saita, A.; Casale, P.; Hurle, R.; et al. Use of high-resolution micro-ultrasound to predict extraprostatic extension of prostate cancer prior to surgery: A prospective single-institutional study. World J. Urol. 2022, 40, 435–442. [Google Scholar] [CrossRef]
- Pedraza, A.M.; Parekh, S.; Joshi, H.; Grauer, R.; Wagaskar, V.; Zuluaga, L.; Gupta, R.; Barthe, F.; Nasri, J.; Pandav, K.; et al. Side-specific, Microultrasound-based Nomogram for the Prediction of Extracapsular Extension in Prostate Cancer. Eur. Urol. Open Sci. 2023, 48, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.exactimaging.com/primus-protocol (accessed on 18 June 2024).
- Tosoian, J.J.; Mamawala, M.; Epstein, J.I.; Landis, P.; Macura, K.J.; Simopoulos, D.N.; Carter, H.B.; Gorin, M.A. Active Surveillance of Grade Group 1 Prostate Cancer: Long-Term Outcomes from a Large Prospective Cohort. Eur. Urol. 2020, 77, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Pond, G.; Loblaw, A.; Sugar, L.; Moussa, M.; Berman, D.; Van der Kwast, T.; Vesprini, D.; Milot, L.; Kebabdjian, M.; et al. Randomized Study of Systematic Biopsy Versus Magnetic Resonance Imaging and Targeted and Systematic Biopsy in Men on Active Surveillance (ASIST): 2-Year Postbiopsy Follow-up. Eur. Urol. 2020, 77, 311–317. [Google Scholar] [CrossRef]
- Claros, O.R.; Tourinho-Barbosa, R.R.; Fregeville, A.; Gallardo, A.C.; Muttin, F.; Carneiro, A.; Stabile, A.; Moschini, M.; Macek, P.; Cathala, N.; et al. Comparison of Initial Experience with Transrectal Magnetic Resonance Imaging Cognitive Guided Micro-Ultrasound Biopsies Versus Established Transperineal Robotic Ultrasound Magnetic Resonance Imaging Fusion Biopsies for Prostate Cancer. J. Urol. 2020, 203, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, V.; Kabre, B.; Pignot, G.; Branger, N.; Pacchetti, A.; Thomassin-Piana, J.; Brunelle, S.; Gregori, A.; Salem, N.; Musi, G.; et al. Comparison Between Micro-Ultrasound and Multiparametric MRI Regarding the Correct Identification of Prostate Cancer Lesions. Clin. Genitourin Cancer 2022, 20, e339–e345. [Google Scholar] [CrossRef]
- Lughezzani, G.; Saita, A.; Lazzeri, M.; Paciotti, M.; Maffei, D.; Lista, G.; Hurle, R.; Buffi, N.M.; Guazzoni, G.; Casale, P. Comparison of the Diagnostic Accuracy of Micro-Ultrasound and Magnetic Resonance Imaging/Ultrasound Fusion Targeted Biopsies for the Diagnosis of Clinically Significant Prostate Cancer. Eur. Urol. Oncol. 2019, 2, 329–332. [Google Scholar] [CrossRef]
- Ghai, S.; Perlis, N.; Atallah, C.; Jokhu, S.; Corr, K.; Lajkosz, K.; Incze, P.F.; Zlotta, A.R.; Jain, U.; Fleming, H.; et al. Comparison of Micro-US and Multiparametric MRI for Prostate Cancer Detection in Biopsy-Naive Men. Radiology 2022, 305, 390–398. [Google Scholar] [CrossRef]
- Cotter, F.; Perera, S.; Sathianathen, N.; Lawrentschuk, N.; Murphy, D.; Bolton, D. Comparing the Diagnostic Performance of Micro- Ultrasound-Guided Biopsy Versus Multiparametric Magnetic Resonance Imaging-Targeted Biopsy in the Detection of Clinically Significant Prostate Cancer: A Systematic Review and Meta-Analysis. Société Int. D’urologie J. 2023, 4, 465–479. Available online: https://www.mdpi.com/2563-6499/4/6/465 (accessed on 18 June 2024).
- Sountoulides, P.; Pyrgidis, N.; Polyzos, S.A.; Mykoniatis, I.; Asouhidou, E.; Papatsoris, A.; Dellis, A.; Anastasiadis, A.; Lusuardi, L.; Hatzichristou, D. Micro-Ultrasound–Guided vs Multiparametric Magnetic Resonance Imaging-Targeted Biopsy in the Detection of Prostate Cancer: A Systematic Review and Meta-Analysis. J. Urol. 2021, 205, 1254–1262. [Google Scholar] [CrossRef]
- Klotz, L.; Andriole, G.; Cash, H.; Cooperberg, M.; Crawford, E.D.; Emberton, M.; Gomez-Sancha, F.; Klein, E.; Lughezzani, G.; Marks, L.; et al. Optimization of prostate biopsy—Micro-Ultrasound versus MRI (OPTIMUM): A 3-arm randomized controlled trial evaluating the role of 29 MHz micro-ultrasound in guiding prostate biopsy in men with clinical suspicion of prostate cancer. Contemp. Clin. Trials 2022, 112, 106618. [Google Scholar] [CrossRef]
- Beatrici, E.; Frego, N.; Chiarelli, G.; Sordelli, F.; Mancon, S.; Saitta, C.; De Carne, F.; Garofano, G.; Arena, P.; Avolio, P.P.; et al. A Comparative Evaluation of Multiparametric Magnetic Resonance Imaging and Micro-Ultrasound for the Detection of Clinically Significant Prostate Cancer in Patients with Prior Negative Biopsies. Diagnostics 2024, 14, 525. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L. Active surveillance in intermediate-risk prostate cancer. BJU Int. 2020, 125, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Vesprini, D.; Sethukavalan, P.; Jethava, V.; Zhang, L.; Jain, S.; Yamamoto, T.; Mamedov, A.; Loblaw, A. Long-Term Follow-Up of a Large Active Surveillance Cohort of Patients with Prostate Cancer. J. Clin. Oncol. 2015, 33, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.C.; Rumble, R.B.; Loblaw, D.A.; Finelli, A.; Ehdaie, B.; Cooperberg, M.R.; Morgan, S.C.; Tyldesley, S.; Haluschak, J.J.; Tan, W.; et al. Active Surveillance for the Management of Localized Prostate Cancer (Cancer Care Ontario Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. JCO 2016, 34, 2182–2190. [Google Scholar] [CrossRef]
- Mahal, B.A.; Butler, S.; Franco, I.; Spratt, D.E.; Rebbeck, T.R.; D’Amico, A.V.; Nguyen, P.L. Use of Active Surveillance or Watchful Waiting for Low-Risk Prostate Cancer and Management Trends Across Risk Groups in the United States, 2010–2015. JAMA 2019, 321, 704–706. [Google Scholar] [CrossRef]
- Ghai, S.; Eure, G.; Fradet, V.; Hyndman, M.E.; McGrath, T.; Wodlinger, B.; Pavlovich, C.P. Assessing Cancer Risk on Novel 29 MHz Micro-Ultrasound Images of the Prostate: Creation of the Micro-Ultrasound Protocol for Prostate Risk Identification. J. Urol. 2016, 196, 562–569. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Société Internationale d’Urologie. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).