SIU-ICUD: Screening and Early Detection of Prostate Cancer
Abstract
1. Introduction
2. Individual Versus Population Perspective
3. Completed Screening Trials
3.1. The ERSPC Trial
3.2. The Gothenburg-1 Trial
3.3. The Prostate, Lung, Colorectal and Ovarian (PLCO) Trial
3.4. The Cluster Randomised Trial of PSA Testing for Prostate Cancer (CAP) Trial
4. Diagnostic Improvements
4.1. Use of PSA for Risk Stratifying Testing Intervals
4.2. PSA Density
4.3. MRI and Lesion-Targeted Biopsy
4.4. Risk Calculators
4.5. Biomarkers
5. Ongoing Screening Trials
5.1. The ProScreen Trial
5.2. The Gothenburg-2 Trial
5.3. The Prostate Cancer Early Detection Study Based on a Baseline PSA Value in Young Men (PROBASE) Trial
5.4. The Stockholm3-MRI Trial
5.5. Screening Trials in High-Risk Populations
6. Prostate Cancer Screening Policies
6.1. Africa
6.2. The Americas
6.3. Asia
6.4. Europe
6.5. Oceania
7. Information to Men Invited to Screening
8. Conclusions and Future Directions
- How are men best informed about the potential advantages and disadvantages?
- What are the optimal PSA cut-off values in different age groups?
- Which are the optimal start and stop ages?
- What is the outcome of repeated screening rounds with modern diagnostics?
- What are the optimal screening intervals for men with a raised PSA and negative further investigations?
- What is the value of an ancillary test to select men for MRI or biopsy in repeated screening?
- What are the cost-effectiveness and health economics of different screening algorithms?
- What are the long-term mortality and overdiagnosis outcomes after modern screening?
- How transferable are results from ongoing screening trials to other populations and ethnic groups?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hugosson, J.; Roobol, M.J.; Mansson, M.; Tammela, T.L.J.; Zappa, M.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Carlsson, S.V.; Talala, K.M.; et al. A 16-yr follow-up of the European randomized study of screening for prostate cancer. Eur. Urol. 2019, 76, 43–51. [Google Scholar] [CrossRef]
- Tsodikov, A.; Gulati, R.; Heijnsdijk, E.A.M.; Pinsky, P.F.; Moss, S.M.; Qiu, S.; de Carvalho, T.M.; Hugosson, J.; Berg, C.D.; Auvinen, A.; et al. Reconciling the effects of screening on prostate cancer mortality in the ERSPC and PLCO trials. Ann. Intern. Med. 2017, 167, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Bergengren, O.; Pekala, K.R.; Matsoukas, K.; Fainberg, J.; Mungovan, S.F.; Bratt, O.; Bray, F.; Brawley, O.; Luckenbaugh, A.N.; Mucci, L.; et al. 2022 update on prostate cancer epidemiology and risk factors—A systematic review. Eur. Urol. 2023, 84, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Vaccarella, S.; Li, M.; Bray, F.; Kvale, R.; Serraino, D.; Lorenzoni, V.; Auvinen, A.; Dal Maso, L. Prostate cancer incidence and mortality in europe and implications for screening activities: Population based study. BMJ 2024, 386, e077738. [Google Scholar] [CrossRef]
- Arnsrud Godtman, R.; Holmberg, E.; Lilja, H.; Stranne, J.; Hugosson, J. Opportunistic testing versus organized prostate-specific antigen screening: Outcome after 18 years in the Göteborg randomized population-based prostate cancer screening trial. Eur. Urol. 2015, 68, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Bokhorst, L.P.; Bangma, C.H.; van Leenders, G.J.; Lous, J.J.; Moss, S.M.; Schroder, F.H.; Roobol, M.J. Prostate-specific antigen-based prostate cancer screening: Reduction of prostate cancer mortality after correction for nonattendance and contamination in the rotterdam section of the European randomized study of screening for prostate cancer. Eur. Urol. 2014, 65, 329–336. [Google Scholar] [CrossRef]
- Hugosson, J.; Carlsson, S.; Aus, G.; Bergdahl, S.; Khatami, A.; Lodding, P.; Pihl, C.G.; Stranne, J.; Holmberg, E.; Lilja, H. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010, 11, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Franlund, M.; Mansson, M.; Godtman, R.A.; Aus, G.; Holmberg, E.; Kollberg, K.S.; Lodding, P.; Pihl, C.G.; Stranne, J.; Lilja, H.; et al. Results from 22 years of followup in the Göteborg randomized population-based prostate cancer screening trial. J. Urol. 2022, 208, 292–300. [Google Scholar] [CrossRef]
- Carlsson, S.V.; Arnsrud Godtman, R.; Pihl, C.G.; Vickers, A.; Lilja, H.; Hugosson, J.; Mansson, M. Young age on starting prostate-specific antigen testing is associated with a greater reduction in prostate cancer mortality: 24-year follow-up of the Göteborg randomized population-based prostate cancer screening trial. Eur. Urol. 2023, 83, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, P.F.; Prorok, P.C.; Yu, K.; Kramer, B.S.; Black, A.; Gohagan, J.K.; Crawford, E.D.; Grubb, R.L.; Andriole, G.L. Extended mortality results for prostate cancer screening in the PLCO trial with median follow-up of 15 years. Cancer 2017, 123, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Turner, E.L.; Young, G.J.; Metcalfe, C.; Walsh, E.I.; Lane, J.A.; Sterne, J.A.C.; Noble, S.; Holding, P.; Ben-Shlomo, Y.; et al. Prostate-specific antigen screening and 15-year prostate cancer mortality: A secondary analysis of the cap randomized clinical trial. JAMA 2024, 331, 1460–1470. [Google Scholar] [CrossRef]
- Vickers, A.J.; Cronin, A.M.; Bjork, T.; Manjer, J.; Nilsson, P.M.; Dahlin, A.; Bjartell, A.; Scardino, P.T.; Ulmert, D.; Lilja, H. Prostate specific antigen concentration at age 60 and death or metastasis from prostate cancer: Case-control study. BMJ 2010, 341, c4521. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, S.; Assel, M.; Sjoberg, D.; Ulmert, D.; Hugosson, J.; Lilja, H.; Vickers, A. Influence of blood prostate specific antigen levels at age 60 on benefits and harms of prostate cancer screening: Population based cohort study. BMJ 2014, 348, g2296. [Google Scholar] [CrossRef]
- Ola, I.O.; Talala, K.; Tammela, T.; Taari, K.; Murtola, T.; Kujala, P.; Raitanen, J.; Auvinen, A. Raitanen and A. Auvinen. Prostate cancer incidence in men with prostate-specific antigen below 3 ng/ml: The Finnish randomized study of screening for prostate cancer. Int. J. Cancer 2023, 152, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Krilaviciute, A.; Kaaks, R.; Seibold, P.; de Vrieze, M.; Lakes, J.; Radtke, J.P.; Kuczyk, M.; Harke, N.N.; Debus, J.; Fink, C.A.; et al. Risk-adjusted screening for prostate cancer—Defining the low-risk group by data from the PROBASE trial. Eur. Urol. 2024, 86, 493–500. [Google Scholar] [CrossRef]
- Nordstrom, T.; Akre, O.; Aly, M.; Gronberg, H.; Eklund, M. Prostate-specific antigen (PSA) density in the diagnostic algorithm of prostate cancer. Prostate Cancer Prostatic Dis. 2018, 21, 57–63. [Google Scholar] [CrossRef]
- Fazekas, T.; Shim, S.R.; Basile, G.; Baboudjian, M.; Koi, T.; Przydacz, M.; Abufaraj, M.; Ploussard, G.; Kasivisvanathan, V.; Rivas, J.G.; et al. Magnetic resonance imaging in prostate cancer screening: A systematic review and meta-analysis. JAMA Oncol. 2024, 10, 745–754. [Google Scholar] [CrossRef]
- Bass, E.J.; Pantovic, A.; Connor, M.; Gabe, R.; Padhani, A.R.; Rockall, A.; Sokhi, H.; Tam, H.; Winkler, M.; Ahmed, H.U. A systematic review and meta-analysis of the diagnostic accuracy of biparametric prostate MRI for prostate cancer in men at risk. Prostate Cancer Prostatic Dis. 2021, 24, 596–611. [Google Scholar] [CrossRef] [PubMed]
- Eklund, M.; Jaderling, F.; Discacciati, A.; Bergman, M.; Annerstedt, M.; Aly, M.; Glaessgen, A.; Carlsson, S.; Gronberg, H.; Nordstrom, T.; et al. MRI-targeted or standard biopsy in prostate cancer screening. N. Engl. J. Med. 2021, 385, 908–920. [Google Scholar] [CrossRef]
- Bratt, O.; Godtman, R.A.; Jiborn, T.; Wallstrom, J.; Akre, O.; Carlsson, S.; Nordstrom, T.; Thimansson, E.; Alterbeck, M.; Zackrisson, S.; et al. Population-based organised prostate cancer testing: Results from the first invitation of 50-year-old men. Eur. Urol. 2023, 85, 207–214. [Google Scholar] [CrossRef]
- Hugosson, J.; Godtman, R.A.; Wallstrom, J.; Axcrona, U.; Bergh, A.; Egevad, L.; Geterud, K.; Khatami, A.; Socratous, A.; Spyratou, V.; et al. Results after four years of screening for prostate cancer with PSA and MRI. N. Engl. J. Med. 2024, 391, 1083–1095. [Google Scholar] [CrossRef]
- Zattoni, F.; Fasulo, V.; Kasivisvanathan, V.; Kesch, C.; Marra, G.; Martini, A.; Falagario, U.; Soeterik, T.; van den Bergh, R.; Rajwa, P.; et al. Enhancing prostate cancer detection accuracy in magnetic resonance imaging-targeted prostate biopsy: Optimizing the number of cores taken. Eur. Urol. Open Sci. 2024, 66, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Nam, R.; Patel, C.; Milot, L.; Hird, A.; Wallis, C.; Macinnis, P.; Singh, M.; Emmenegger, U.; Sherman, C.; Haider, M.A. Prostate MRI versus PSA screening for prostate cancer detection (the MVP study): A randomised clinical trial. BMJ Open 2022, 12, e059482. [Google Scholar] [CrossRef] [PubMed]
- Eldred-Evans, D.; Tam, H.; Sokhi, H.; Padhani, A.R.; Connor, M.; Price, D.; Gammon, M.; Klimowska-Nassar, N.; Burak, P.; Day, E.; et al. An evaluation of screening pathways using a combination of magnetic resonance imaging and prostate-specific antigen: Results from the IP1-PROSTAGRAM study. Eur. Urol. Oncol. 2023, 6, 295–302. [Google Scholar] [CrossRef]
- Marsden, T.; McCartan, N.; Brown, L.; Rodriguez-Justo, M.; Syer, T.; Brembilla, G.; Van Hemelrijck, M.; Coolen, T.; Attard, G.; Punwani, S.; et al. The reimagine prostate cancer risk study protocol: A prospective cohort study in men with a suspicion of prostate cancer who are referred onto an MRI-based diagnostic pathway with donation of tissue, blood and urine for biomarker analyses. PLoS ONE 2022, 17, e0259672. [Google Scholar] [CrossRef]
- Denijs, F.B.; van Harten, M.J.; Meenderink, J.J.L.; Leenen, R.C.A.; Remmers, S.; Venderbos, L.D.F.; van den Bergh, R.C.N.; Beyer, K.; Roobol, M.J. Risk calculators for the detection of prostate cancer: A systematic review. Prostate Cancer Prostatic Dis. 2024, 27, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Kawada, T.; Shim, S.R.; Quhal, F.; Rajwa, P.; Pradere, B.; Yanagisawa, T.; Bekku, K.; Laukhtina, E.; von Deimling, M.; Teoh, J.Y.; et al. Diagnostic accuracy of liquid biomarkers for clinically significant prostate cancer detection: A systematic review and diagnostic meta-analysis of multiple thresholds. Eur. Urol. Oncol. 2024, 7, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Nordstrom, T.; Discacciati, A.; Bergman, M.; Clements, M.; Aly, M.; Annerstedt, M.; Glaessgen, A.; Carlsson, S.; Jaderling, F.; Eklund, M.; et al. Prostate cancer screening using a combination of risk-prediction, MRI, and targeted prostate biopsies (STHLM3-MRI): A prospective, population-based, randomised, open-label, non-inferiority trial. Lancet Oncol. 2021, 22, 1240–1249. [Google Scholar] [CrossRef]
- Agnello, L.; Vidali, M.; Giglio, R.V.; Gambino, C.M.; Ciaccio, A.M.; Lo Sasso, B.; Ciaccio, M. Prostate health index (PHI) as a reliable biomarker for prostate cancer: A systematic review and meta-analysis. Clin. Chem. Lab. Med. 2022, 60, 1261–1277. [Google Scholar] [CrossRef] [PubMed]
- Josefsson, A.; Mansson, M.; Kohestani, K.; Spyratou, V.; Wallstrom, J.; Hellstrom, M.; Lilja, H.; Vickers, A.; Carlsson, S.V.; Godtman, R.; et al. Performance of 4Kscore as a reflex test to prostate-specific antigen in the GÖTEBORG-2 prostate cancer screening trial. Eur. Urol. 2024, 86, 223–229. [Google Scholar] [CrossRef]
- Auvinen, A.; Tammela, T.L.J.; Mirtti, T.; Lilja, H.; Tolonen, T.; Kenttamies, A.; Rinta-Kiikka, I.; Lehtimaki, T.; Natunen, K.; Nevalainen, J.; et al. Prostate cancer screening with PSA, Kallikrein Panel, and MRI The ProScreen randomized trial. JAMA 2024, 331, 1452–1459. [Google Scholar] [CrossRef]
- Nordstrom, T.; Annerstedt, M.; Glaessgen, A.; Carlsson, S.; Clements, M.; Abbadi, A.; Gronberg, H.; Jaderling, F.; Eklund, M.; Discacciati, A. Repeated prostate cancer screening using prostate-specific antigen testing and magnetic resonance imaging: A secondary analysis of the STHLM3-MRI randomized clinical trial. JAMA Netw. Open 2024, 7, e2354577. [Google Scholar] [CrossRef]
- Chiu, P.K.; Liu, A.Q.; Lau, S.Y.; Teoh, J.Y.; Ho, C.C.; Yee, C.H.; Hou, S.M.; Chan, C.K.; Tang, W.L.; Bangma, C.H.; et al. A 2-year prospective evaluation of the Prostate Health Index in guiding biopsy decisions in a large cohort. BJU Int. 2024, 135, 71–77. [Google Scholar] [CrossRef]
- Moller, F.; Mansson, M.; Wallstrom, J.; Hellstrom, M.; Hugosson, J.; Arnsrud Godtman, R. Prostate cancers in the prostate-specific antigen interval of 1.8-3 ng/ml: Results from the Göteborg-2 prostate cancer screening trial. Eur. Urol. 2024, 86, 95–100. [Google Scholar] [CrossRef]
- Arsov, C.; Albers, P.; Herkommer, K.; Gschwend, J.; Imkamp, F.; Peters, I.; Kuczyk, M.; Hadaschik, B.; Kristiansen, G.; Schimmoller, L.; et al. A randomized trial of risk-adapted screening for prostate cancer in young men-results of the first screening round of the PROBASE trial. Int. J. Cancer 2022, 150, 1861–1869. [Google Scholar] [CrossRef]
- Graham, N.J.; Souter, L.H.; Salami, S.S. A systematic review of family history, race/ethnicity, and genetic risk on prostate cancer detection and outcomes: Considerations in PSA-based screening. Urol. Oncol. 2025, 43, 29–40. [Google Scholar] [CrossRef]
- Makau-Barasa, L.K.; Manirakiza, A.; Carvalho, A.L.; Rebbeck, T.R. Prostate cancer screening, diagnostic, treatment procedures and costs in sub-Saharan Africa: A situational analysis. Cancer Control 2022, 29, 10732748221084932. [Google Scholar] [CrossRef]
- Baratedi, W.M.; Tshiamo, W.B.; Mogobe, K.D.; McFarland, D.M. Barriers to prostate cancer screening by men in Sub-Saharan Africa: An integrated review. J. Nurs. Scholarsh. 2020, 52, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Tourinho-Barbosa, R.R.; Pompeo, A.C.; Glina, S. Prostate cancer in Brazil and Latin America: Epidemiology and screening. Int. Braz. J. Urol. 2016, 42, 1081–1090. [Google Scholar] [CrossRef]
- Force, U.S.P.S.T.; Grossman, D.C.; Curry, S.J.; Owens, D.K.; Bibbins-Domingo, K.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Ebell, M.; Epling, J.W., Jr.; et al. Screening for prostate cancer: US preventive services task force recommendation statement. JAMA 2018, 319, 1901–1913. [Google Scholar] [CrossRef]
- Ramesar, N.S.; Hosein, A.; Samaroo, K.; Ali, J. Prostate cancer in the Caribbean. Cureus 2023, 15, e50150. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, S.; Okinaka, Y.; Nishizawa, K.; Yoshida, T.; Ishitoya, S.; Shichiri, Y.; Kim, C.J.; Iwata, T.; Yokokawa, R.; Arai, Y.; et al. Population-based prostate-specific antigen screening for prostate cancer may have an indirect effect on early detection through opportunistic testing in Kusatsu City, Shiga, Japan. Mol. Clin. Oncol. 2023, 18, 3. [Google Scholar] [CrossRef]
- Ruan, X.; Zhang, N.; Wang, D.; Huang, J.; Huang, J.; Huang, D.; Chun, T.T.S.; Ho, B.S.H.; Ng, A.T.; Tsu, J.H.; et al. The impact of prostate-specific antigen screening on prostate cancer incidence and mortality in china: 13-year prospective population-based cohort study. JMIR Public Health Surveill. 2024, 10, e47161. [Google Scholar] [CrossRef]
- Council of the European Union. Council recommendation of 9 December 2022 on strengthening prevention through early detection: A new EU approach on cancer screening replacing council recommendation 2003/878/EC 2022/C 473/01. Off. J. Eur. Union 2022. Available online: https://eu.vlex.com/vid/raccomandazione-consiglio-9dicembre-2022-916324729 (accessed on 19 November 2024).
- Patasius, A.; Krilaviciute, A.; Smailyte, G. Prostate cancer screening with PSA: Ten years’ experience of population based early prostate cancer detection programme in lithuania. J. Clin. Med. 2020, 9, 3826. [Google Scholar] [CrossRef]
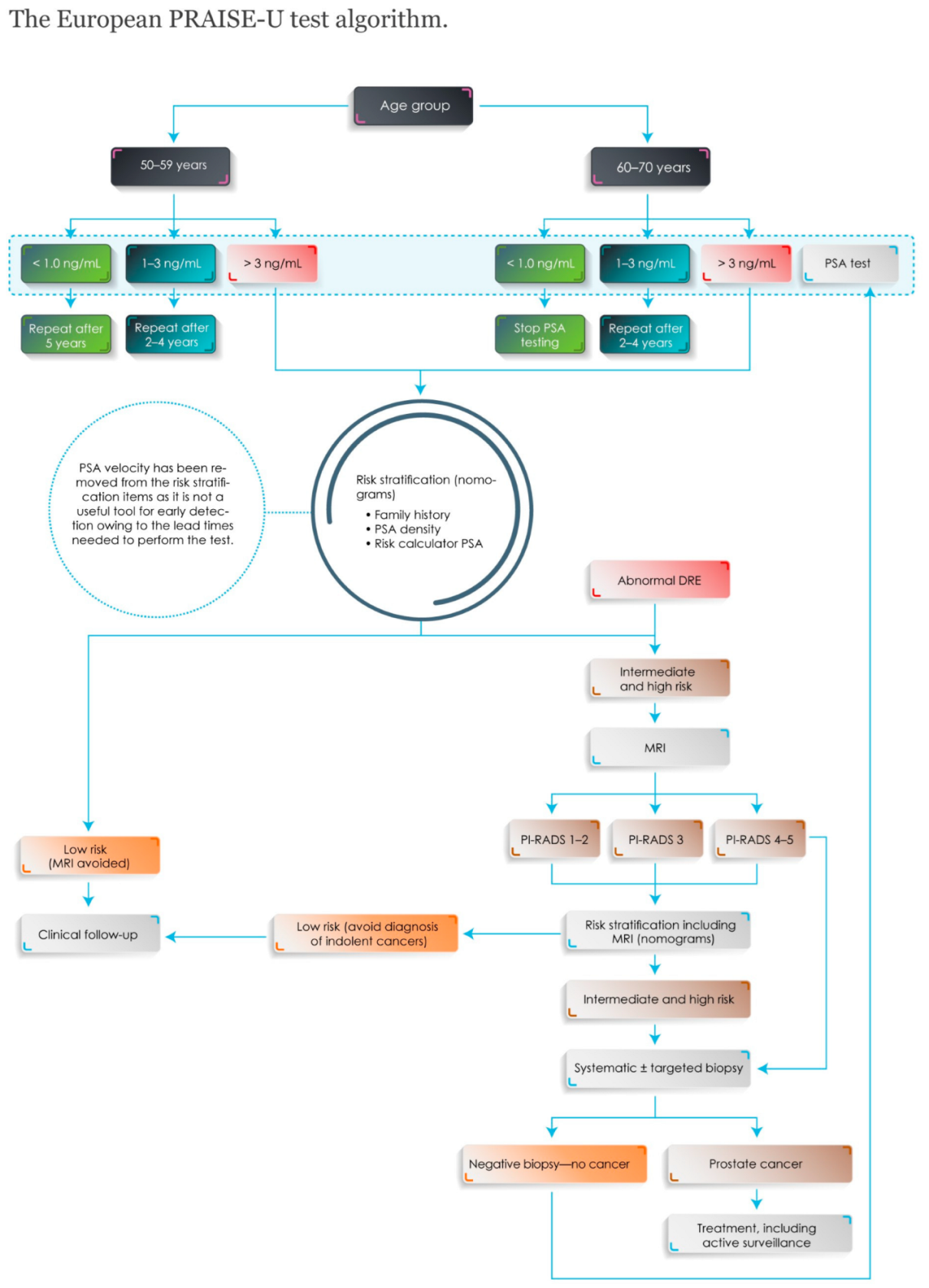
- Van Poppel, H.; Roobol, M.J.; Chandran, A. Early detection of prostate cancer in the European Union: Combining forces with PRAISE-U. Eur. Urol. 2023, 84, 519–522. [Google Scholar] [CrossRef]
- Pekala, K.R.; Shill, D.K.; Austria, M.; Langford, A.T.; Loeb, S.; Carlsson, S.V. Shared decision-making before prostate cancer screening decisions. Nat. Rev. Urol. 2024, 21, 329–338. [Google Scholar] [CrossRef]
- Riikonen, J.M.; Guyatt, G.H.; Kilpelainen, T.P.; Craigie, S.; Agarwal, A.; Agoritsas, T.; Couban, R.; Dahm, P.; Jarvinen, P.; Montori, V.; et al. Decision aids for prostate cancer screening choice: A systematic review and meta-analysis. JAMA Intern. Med. 2019, 179, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Pathirana, T.I.; Pickles, K.; Riikonen, J.M.; Tikkinen, K.A.O.; Bell, K.J.L.; Glasziou, P. Including information on overdiagnosis in shared decision making: A review of prostate cancer screening decision aids. MDM Policy Pract. 2022, 7, 23814683221129875. [Google Scholar] [CrossRef] [PubMed]
- Pasanen, N.; Talala, K.; Remmers, S.; Tammela, T.L.J.; Hugosson, J.; Roobol, M.J.; Taari, K.; Arnsrud Godtman, R.; Bangma, C.; Auvinen, A. Which men benefit from prostate cancer screening? Prostate cancer mortality by subgroup in the European randomised study of screening for prostate cancer. BJU Int. 2024, 134, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Alberts, A.R.; Schoots, I.G.; Bokhorst, L.P.; Drost, F.H.; van Leenders, G.J.; Krestin, G.P.; Dwarkasing, R.S.; Barentsz, J.O.; Schroder, F.H.; Bangma, C.H.; et al. Characteristics of prostate cancer found at fifth screening in the European randomized study of screening for prostate cancer Rotterdam: Can we selectively detect high-grade prostate cancer with upfront multivariable risk stratification and magnetic resonance imaging? Eur. Urol. 2018, 73, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Wallstrom, J.; Geterud, K.; Kohestani, K.; Maier, S.E.; Pihl, C.G.; Socratous, A.; Stranne, J.; Arnsrud-Godtman, R.; Mansson, M.; Hellstrom, M.; et al. Prostate cancer screening with magnetic resonance imaging: Results from the second round of the Göteborg prostate cancer screening 2 trial. Eur. Urol. Oncol. 2022, 5, 54–60. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; Tannock, I.; N’Dow, J.; Feng, F.; Gillessen, S.; Ali, S.A.; Trujillo, B.; Al-Lazikani, B.; Attard, G.; Bray, F.; et al. The Lancet Commission on prostate cancer: Planning for the surge in cases. Lancet 2024, 403, 1683–1722. [Google Scholar] [CrossRef]
- Straat, K.R.V.; Hagens, M.J.; Cools Paulino Pereira, L.J.; van den Bergh, R.C.N.; Mazel, J.W.; Noordzij, M.A.; Rynja, S.P. Risk calculator strategy before magnetic resonance imaging stratification for biopsy-naive men with suspicion for prostate cancer: A cost-effectiveness analysis. Eur. Urol. Open Sci. 2024, 70, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Wagensveld, I.M.; Osses, D.F.; Groenendijk, P.M.; Zijta, F.M.; Busstra, M.B.; Rociu, E.; Barentsz, J.O.; Michiel Sedelaar, J.P.; Arbeel, B.; Roeleveld, T.; et al. A prospective multicenter comparison study of risk-adapted ultrasound-directed and magnetic resonance imaging-directed diagnostic pathways for suspected prostate cancer in biopsy-naive men. Eur. Urol. 2022, 82, 318–326. [Google Scholar] [CrossRef] [PubMed]
| Descriptive Element | Individual Perspective (Early Detection) | Governmental Population Perspective (Screening) |
|---|---|---|
| Definition | Diagnostic testing in individuals who are asymptomatic or have non-specific symptoms | Organised programme inviting specific age groups to undergo screening test(s) |
| Initiation | Personal request or provider recommendation | Healthcare authorities by repeated programme invitations |
| Perspective | Individuals’ priority | Public health priority |
| Purpose | To diagnose before symptoms for individual benefit: reduce risks of advanced cancer and death from prostate cancer | To reduce prostate cancer-specific mortality in a population |
| Considerations | Balance of benefits versus harms for the individual | Balance of benefits versus costs, harms and other priorities in the population |
| Statistical assessment of study results | Per-protocol analysis | Intention-to-screen analysis |
| Guidelines | Often issued or adapted by professional societies | Issued by national or regional healthcare authorities |
| Communication | Individualised discussion and shared decision-making | Standardised materials for the population |
| Testing strategy | Single time-point assessments and safety net monitoring | Protocolised repeated testing |
| Test performance | Varies based on the clinical setting | Optimised for the population |
| Risk stratification | Based on individual risk factors and preferences | Based on average risks |
| Emphasis on specificity vs. sensitivity | Emphasis often on higher sensitivity because of individual preference | Emphasis on high specificity as missed cancers can be detected at repeat screening |
| Overdiagnosis tolerance | Higher, because of individual preferences related to cancer worry, family history, and/or symptoms | Low, because harms affect an entire population |
| Preferred biopsy strategy | Targeted and systematic biopsy to maximise detection sensitivity | Targeted biopsy after imaging (if available) to minimise overdiagnosis |
| MRI cut-offs for biopsy | Lower threshold to minimise missed significant cancer | Higher threshold to minimise over-diagnosis |
| Resources | Uses existing clinical resources | Requires organised infrastructure |
| Access | Depends on clinical access which may vary | Promotes equal access through programme outreach |
| Quality assurance | Variable based on clinical setting; multi-disciplinary team meetings preferred | Accreditation programme with systematic monitoring of outcomes |
| Trial | Primary Outcome Measure | Target Ages (yrs) | Population Size | Participation Rate | Detection of GG ≥ 2 Cancer # | Detection of GG 1 Cancer # |
|---|---|---|---|---|---|---|
| ProScreen | Prostate cancer mortality | 50–63 | 65,000 enrolled 115,000 target size | 51% | 1.7% vs. 0.6% | 0.4% vs. 0.1% |
| Gothenburg-2 | Detection of clinically insignificant (GG 1) cancer | 50–60 | 58,225 | 46% | 0.9% vs. 1.1% | 0.6% vs. 1.2% |
| PROBASE | Metastatic prostate cancer at age 60 | 45 vs. 50 | 46,495 | 11% | 0.06% | 0.14% |
| STHLM3-MRI | Detection of GG ≥ 2 cancer | 50–74 | 12,750 enrolled, 1532 randomised | 26% | 1.0% vs. 1.2% * (21% vs. 18%) | 0.5% vs. 1.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Société Internationale d’Urologie. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bratt, O.; Jalloh, M.; Padhani, A.R.; Pinsky, P.F.; Van Poppel, H.; Ranasinghe, W.; Zargar-Shoshtari, K.; Zhang, K.; Auvinen, A. SIU-ICUD: Screening and Early Detection of Prostate Cancer. Soc. Int. Urol. J. 2025, 6, 36. https://doi.org/10.3390/siuj6030036
Bratt O, Jalloh M, Padhani AR, Pinsky PF, Van Poppel H, Ranasinghe W, Zargar-Shoshtari K, Zhang K, Auvinen A. SIU-ICUD: Screening and Early Detection of Prostate Cancer. Société Internationale d’Urologie Journal. 2025; 6(3):36. https://doi.org/10.3390/siuj6030036
Chicago/Turabian StyleBratt, Ola, Mohamed Jalloh, Anwar R. Padhani, Paul F. Pinsky, Hein Van Poppel, Weranja Ranasinghe, Kamran Zargar-Shoshtari, Kai Zhang, and Anssi Auvinen. 2025. "SIU-ICUD: Screening and Early Detection of Prostate Cancer" Société Internationale d’Urologie Journal 6, no. 3: 36. https://doi.org/10.3390/siuj6030036
APA StyleBratt, O., Jalloh, M., Padhani, A. R., Pinsky, P. F., Van Poppel, H., Ranasinghe, W., Zargar-Shoshtari, K., Zhang, K., & Auvinen, A. (2025). SIU-ICUD: Screening and Early Detection of Prostate Cancer. Société Internationale d’Urologie Journal, 6(3), 36. https://doi.org/10.3390/siuj6030036






